Abstract
Terminalia arjuna is a tree having an extensive medicinal potential in cardiovascular disorders. Triterpenoids are mainly responsible for cardiovascular properties. Alcoholic and aqueous bark extracts of T. arjuna, arjunic acid, arjunetin and arjungenin were evaluated for their potential to inhibit CYP3A4, CYP2D6 and CYP2C9 enzymes in human liver microsomes. We have demonstrated that alcoholic and aqueous bark extract of T. arjuna showed potent inhibition of all three enzymes in human liver microsomes with IC50 values less than 50 μg/mL. Arjunic acid, arjunetin and arjungenin did not show significant inhibition of CYP enzymes in human liver microsomes. Enzyme kinetics studies suggested that the extracts of arjuna showed reversible non-competitive inhibition of all the three enzymes in human liver microsomes. Our findings suggest strongly that arjuna extracts significantly inhibit the activity of CYP3A4, CYP2D6 and CYP2C9 enzymes, which is likely to cause clinically significant drug–drug interactions mediated via inhibition of the major CYP isozymes.
Abbreviations: CYP, cytochrome P450; DMSO, dimethyl sulfoxide; HDI, herb–drug interactions; HLM, human liver microsomes; NADPH, nicotinamide adenine dinucleotide phosphate reduced tetrasodium salt
Keywords: CYP enzyme, Terminalia arjuna, Human liver microsomes, CYP inhibition, Herb–drug interactions, Toxicity
1. Introduction
About 70% of the world population currently uses medicinal herbs as complementary or alternative medicine. Herb–drug interactions (HDI) are one of the most important clinical concerns in the concomitant consumption of herbs and prescription drugs. The necessity of polypharmacy in the management of most diseases further increases the risk of HDI in patients [8].
In the Indian system of medicine, Ayurveda, the bark of the tree Terminalia arjuna (T. arjuna), which is popularly known as ‘Arjuna’ in many Indian languages, is used as a cardioprotective agent [12]. As a whole T. arjuna, i.e., stem bark, fruit, leaves and roots is associated with therapeutic properties. Particularly, T. arjuna bark extract has been reported to play a significant role as a cardiac stimulant for its beneficial effects in angina, i.e., “hritshool”, and is stipulated in the treatment of hypercholesterolemia, heart failure and atherosclerosis [4].
It is believed that the saponin glycosides in T. arjuna may be responsible for its inotropic effects, while the flavonoids/phenolics may provide antioxidant activity as well as vascular strengthening activity, thereby confirming the multiple activities of this medicinal plant for its cardioprotective role [5].
It is well documented that the ability of intestinal and hepatic cytochrome P450 to metabolize numerous structurally unrelated compounds, apart from being responsible for the poor oral bioavailability of numerous drugs, is responsible for the large number of documented drug–drug and drug–food interactions [8]. A notable example of this is the inhibition of CYP3A by grapefruit juice, which can result in elevations of systemic exposure to CYP3A-cleared compounds [15].
Three P450 gene families (i.e., CYPl, CYP2, and CYP3) of the 27 families identified are currently thought to be responsible for the majority of hepatic drug metabolism. Six principle enzymes (CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6 and CYP3A4) appear to be the most commonly responsible for the metabolism of most drugs and the associated drug–drug interactions in humans [9].
Despite the understanding of the immense potential of T. arjuna as a cardioprotective agent, there is a lack of information on its interaction with cytochrome enzymes. Previously, we have reported the CYP1A interaction of arjuna extracts, arjunic acid and arjungenin in rat and human liver microsomes [24].
Here we report the in vitro inhibition potential of alcoholic and aqueous bark extracts of arjuna, arjunic acid, arjunetic and arjungenin to inhibit CYP3A4, CYP2D6 and CYP2C9 enzyme activities in human liver microsomes (HLM).
2. Materials and methods
2.1. Chemical and reagents
Testosterone and 6β-hydroxy testosterone were gift samples from Avik Pharma, Vapi, Gujarat and Piramal Life Sciences Ltd., Mumbai, respectively. Dextrometorphan and dextrorphan were gift samples from Aarti Drugs Pvt. Ltd. and Advinus Therapeutics Pvt. Ltd., respectively. 4′-Hydroxy diclofenac was a gift sample from Advinus Therapeutics Pvt. Ltd. Diclofenac, caffeine and serotonin were purchased from Sigma–Aldrich Ltd. Nicotinamide adenine dinucleotide phosphate reduced tetrasodium salt (NADPH) was purchased from Sisco Research Laboratories (SRL) Pvt. Ltd. Arjunic acid (Fig. 1; 95.1% purity), arjunetin (Fig. 1; 98.2% purity) and arjungenin (Fig. 1; 96.2% purity) were purchased from Natural Remedies, Bangalore, India. Pooled human liver microsomes (HLM) of 20 individual male donors (Batch No. 0710112) were purchased from Krishgen Biosystems. All solvents were of high performance liquid chromatography (HPLC) grade and were purchased from Thermo Fischer Scientific India Pvt. Ltd. Double distilled water filtered through 0.45 μ filter was used for the study.
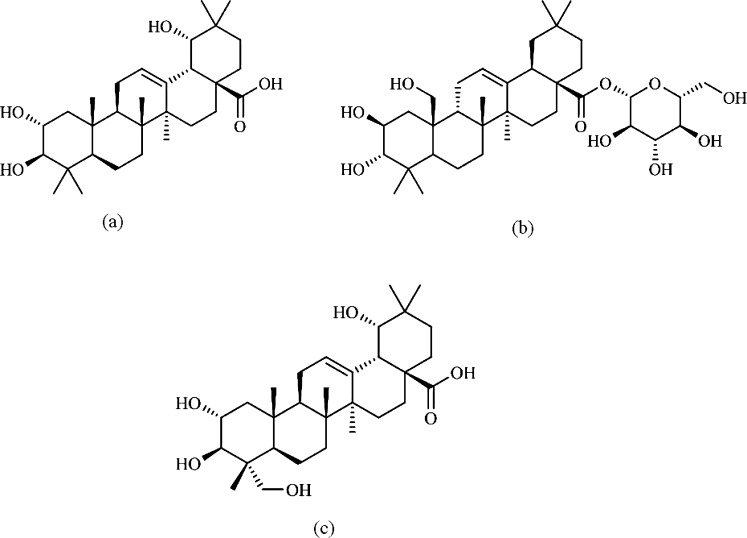
Fig. 1.
Structures of triterpenoids studied. (a) Arjunic acid; (b) arjunetin; and (c) arjungenin.
2.2. Preparation of alcoholic and aqueous bark extract of T. arjuna
Fresh bark of T. arjuna specimens sourced from different trees was purchased from Zandu Foundation, Gujarat, India. Each of the specimens were identified visually for typical arjuna bark characteristics by Dr. Naik, Senior Research Scientist, Piramal Life Sciences Ltd. and were further authenticated by Agharkar Research Institute, Pune with the voucher specimen (S/B 104) deposited for future reference. The fresh bark was dried in vacuum oven at temperature not exceeding 55 °C. Initially, 500 g (approximately) of dried powdered bark was defatted with 2 L of petroleum ether for 2 days with intermittent shaking. Following which the defatted sample was subjected to cold maceration using 2 L absolute ethanol and water, respectively to yield ethanolic and aqueous bark extracts. Thereafter, the alcoholic extract was evaporated to dryness at 40 °C using rotary vacuum evaporator resulting in a brownish crystalline powder. Similarly, the aqueous extract was prepared by lyophilization resulting in a whitish brown free flowing powder. The prepared extracts were dried and stored in a vacuum dessicator till use.
2.3. Preparation of stock solutions
An accurately weighed amounts of alcoholic and aqueous extracts of T. arjuna were solubilized in dimethyl sulfoxide (DMSO) to result in solutions of final concentration 20 mg/mL. Similarly, accurately weighed amounts of arjunic acid, arjunetin and arjungenin were solubilized individually in methanol to yield solutions of 10 mM final concentrations each. For in vitro assays, stock solutions of probe substrates, testosterone, dextromethorphan and diclofenac were prepared in acetonitrile at final concentrations of 40 mM, 1.6 mM and 10 mM, respectively. Phosphate buffer (100 mM, pH 7.4) was used to make NADPH solution (10 mM).
2.4. Instrument
The high pressure thin layer chromatography (HPTLC) system consisted of the applicator AS 30, 230V, with Densitometer CD 60, 230V, with Windows® software ProQuant®. Analysis of in vitro microsomal assays was performed using LC-2010HT High Pressure Liquid Chromatography system (Shimadzu Corporation, Japan). The HPLC was equipped with a CBM-20A communication module, a SPD-M20A diode array detector, RF-20A fluorescence detector and LC Solutions® software.
2.5. Estimation of content of arjunic acid, arjunetin and arjungenin in alcoholic and aqueous extracts of T. arjuna by HPTLC
The percent w/w content of arjunic acid, arjunetin and arjungenin in alcoholic and aqueous extracts of T. arjuna were quantified using a reported HPTLC method [3]. HPTLC was performed on 10 cm × 100 cm coated with 0.2 mm layer of silica gel 60 F254 aluminium backed plates coated. Stock solutions of arjunic acid, arjunetin and arjungenin were spotted on the TLC plate to yield spots of concentrations in the range of 100–1000 ng/spot each using the HPTLC DESAGA applicator. The analysis (n = 3) was performed by comparing and interpolating the extract peak area (response) with that of the standard arjunic acid, arjunetin and arjungenin from the calibration curve. Reported mobile phase (toluene:ethyl acetate:acetic acid in the ratio of 7:3:0.5) was used to develop the plates [3]. The developed plates were derivatized using anisaldehyde in sulphuric acid solution. Post derivatization the plates were dried and scanned with an HPTLC Densitometer CD 60, 230V, with ProQuant Software at 365 nm.
Similarly, accurately 50 mg of alcoholic and aqueous extracts were solubilized in 1 mL methanol and 1 mL distilled water, respectively. Each of these resulting solutions were filtered through 0.45 μ syringe filter and 20 μL of the solutions (n = 3) were analyzed as mentioned earlier.
2.6. In vitro cytochrome P450 assays
The probe substrates were incubated at their reported Km values in HLM [9]. Triplicate samples were run to generate the IC50 values by incubating probe substrates at a concentration approaching Km in the presence of different concentrations of test compound in HLM. Alcoholic and aqueous extracts were evaluated in the range of 1–100 μg/mL final concentration, arjunic acid, arjunetin and arjungenin were evaluated in the range of 1–50 μM final concentrations. Triplicate negative and positive control incubations were run simultaneously. In all the incubations organic content was not more than 1% (v/v).
2.6.1. CYP3A4 testosterone 6β-hydroxylase assay in human liver microsomes
CYP3A4 enzyme activity in human liver microsomes was measured on the basis of the probe reaction, 6β-hydroxylation of testosterone by CYP3A4 [9]. Briefly, a standard 100 μL incubation mixture contained liver microsomes (0.25 mg/mL protein concentration), testosterone in 0.1 M sodium phosphate buffer pH 7.4 at 37 °C was incubated for 30 min (n = 3) at 100 μM [9]. The reactions were initiated using NADPH (final concentration 1 mM) and then terminated with 50 μL of internal standard caffeine (50 μg/mL) in acetonitrile at the end of 30 min. The samples were centrifuged at 4000 rpm for 10 min at 4 °C and the supernatant were subjected to RP-HPLC analysis. A modified RP-HPLC method was used for simultaneous estimation of 6β-hydroxy testosterone and testosterone at a wavelength of 245 nm [2]. Samples were run on a Kromasil C18 column 5μ (4.6 mm × 150 mm) and mobile phase used was (A) 1 mM Potassium dihydrogen phosphate (KH2PO4) buffer, pH 3.0 and (B) HPLC grade acetonitrile at a flow rate of 1 ml/min. The gradient programme used was time: %B – 0/20; 12/45; 14/55; 16/20; 22/20. The retention times of caffeine (IS), 6β-hydroxy testosterone and testosterone was 2.8, 8.5 and 16.1 min, respectively. Ketoconazole incubated at a concentration of 0.1 μM was run parallel in triplicate as a positive control [9].
2.6.2. CYP2D6 dextromethorphan O-demethylation assay in human liver microsomes
CYP2D6 enzyme activity in human liver microsomes was measured on the basis of the probe reaction, O-demethylation of dextromethorphan by CYP2D6 [9]. Briefly, a standard 100 μL incubation mixture contained liver microsomes (0.25 mg/mL protein concentration), Dextromethorphan in 0.1 M sodium phosphate buffer pH 7.4 at 37 °C was incubated for 30 min (n = 3) at 8 μM [9]. The reactions were initiated using NADPH (final concentration 1 mM) and then terminated with 50 μL of internal standard serotonin (1 μg/mL) in methanol at the end of 30 min. The samples were centrifuged at 4000 rpm for 10 min at 4 °C and the supernatant were subjected to RP-HPLC analysis. A modified RP-HPLC method was used for simultaneous estimation of dextromethorphan, dextrorphan and serotonin by fluorescence detection [1]. Samples were run on a Kromasil C18 column 5μ (4.6 mm × 150 mm) and mobile phase used was (A) 1 mM Potassium dihydrogen phosphate (KH2PO4) buffer, pH 3.0 and (B) HPLC grade acetonitrile and was pumped at a flow rate of 1 mL/min. The gradient programme used was time: %B – 0/10, 8/55, 12/55, 15/10, 17/10. Emission and excitation wavelengths of dextrorphan, dextromethorphan and serotonin used were 320 nm and 280 nm, respectively. The retention times of serotonin (IS), dextrorphan and dextromethorphan was 4.1, 7.6 and 9.6 min, respectively. Quinidine was incubated at a concentration of 0.4 μM was run parallel in triplicate as a positive control [9].
2.6.3. CYP2C9 diclofenac 4′-hydroxylation assay in human liver microsomes
CYP2C9 enzyme activity in human liver microsomes was measured on the basis of the probe reaction, 4′-hydroxylation of diclofenac by CYP2C9 [9]. Briefly, a standard 100 μL incubation mixture contained liver microsomes (0.5 mg/mL protein concentration), diclofenac sodium in 0.1 M sodium phosphate buffer pH 7.4 at 37 °C was incubated for 1 h (n = 3) at 50 μM [9]. The reactions were initiated with NADPH (final concentration 1 mM) and then terminated with 50 μL of internal standard serotonin (1 μg/mL) in methanol at the end of an hour. The samples were centrifuged at 4000 rpm for 10 min at 4 °C and the supernatant were subjected to RP-HPLC analysis. A modified RP-HPLC method was used for simultaneous estimation of diclofenac, 4′-hydroxy diclofenac and phenacetin by UV detection at 280 nm. [23]. Samples were run on a Kromasil C18 column 5μ (4.6 mm × 150 mm) and mobile phase used was (A) 1 mM potassium dihydrogen phosphate (KH2PO4) buffer, pH 3.0 and (B) HPLC grade acetonitrile and was pumped at a flow rate of 1 ml/min. The gradient programme used was time: %B – 0/10, 5/40, 12/98, 14/10, 15/10. The retention times of phenacetin (IS), 4′-hydroxy diclofenac and diclofenac was 7.3, 9.4 and 11.2 min, respectively. Sulphaphenazole incubated at a concentration of 0.3 μM was run parallel in triplicate as a positive control [9].
2.7. Data analysis for CYP activities in human liver microsomes
Percentage inhibition of extracts and T. arjuna standards to inhibit CYP3A4, CYP2D6 and CYP2C9 enzyme activity was calculated by the formula mentioned below:
AR control, area ratio of metabolite/internal standard in negative control (solvent); AR sample, area ratio of metabolite/internal standard in presence of extract/standard; IC50 values were calculated using Graph Pad Prism®.
2.8. Assessment of type of inhibition, inhibition constant and time-dependent inhibition studies
The inhibition constant (Ki) values of the arjuna extracts were determined only if their IC50 values were found to be lower than 100 μg/mL.
Typically, Ki experiments include a matrix of substrate and inhibitor concentrations spanning a range of at least ∼0.5–5× expected Ki (inhibitor) and Km (substrate) concentrations, respectively. Resulting data is usually represented in the form of Dixon plots of 1/reaction rate versus inhibitor concentration [7].
In this study, Ki values and mechanism of inhibition of alcoholic and aqueous extracts of T. arjuna in HLM for CYP3A4, CYP2D6 and CYP2C9 were determined by using testosterone, dextromethorphan and diclofenac at concentrations bracketing their reported Km values.
Briefly, testosterone concentrations ranged from 20 to 200 μM, dextromethorphan concentrations ranged from 2 to 16 μM and diclofenac concentrations ranged from 20 to 80 μM.
Alcoholic and aqueous extracts were incubated in the range of 2.5–75 μg/mL. Ki values were also determined for selected activities by non-linear regression analysis of untransformed data to the mixed competitive–non-competitive, uncompetitive, or competitive inhibition models using Graph Pad Prism®. Goodness of fit criteria (Akaike's Information Criterion) was used to determine the inhibition model that best described the data. The mechanism of inhibition was determined by visual inspection of the data using Dixon (1/V versus extracts [I]) and Lineweaver–Burk (1/V versus 1/testosterone [S]) plots [11].
The IC50 shift assay is one of the most efficient and convenient methods of evaluating time-dependent inhibitory effects of test compounds. When the inhibition curve is shifted to a lower IC50 value by 30-min pre-incubation in the presence of NADPH, it is an indication of time-dependent inhibition [19]. Time dependent inhibition potential of alcoholic and aqueous extracts of T. arjuna was determined by preincubating the inhibitor at various concentrations, surrounding the IC50 values, with protein for 30 min in presence and absence of NADPH. The inactivator was diluted into another incubation tube (final incubation volume 100 μL) containing NADPH generating system and respective substrates (testosterone – 100 μM, dextromethorphan – 8 μM and diclofenac – 50 μM) and NADPH (1 mM). This reaction mixture was incubated for 30 min at 37 °C in a shaking incubator. The reactions were terminated, processed and analyzed using HPLC procedures as mentioned in Sections 2.6.1, 2.6.2, 2.6.3. From the analysis, percentage inhibition of CYP3A4, CYP2D6 and CYP2C9 enzyme activity by alcoholic and aqueous extracts of T. arjuna after dilution was computed (n = 3).
3. Results
3.1. Percent content of arjunic acid, arjunetin and arjungenin in alcoholic and aqueous extracts of T. arjuna by HPTLC
Linearity of the calibration curves of arjunic acid, arjunetin and arjungenin was tested by linear regression analysis and found to be linear in the concentration range 100–1000 ng/spot with good correlation between concentration and peak area with a correlation coefficient (r2) of more than 0.9980. Average retention factor (Rf) values of arjunic acid, arjungenin and arjunetin in the given mobile phase was 0.6, 0.5 and 0.3, respectively, indicating they were well resolved. The bands of arjunic acid, arjunetin and arjungenin in the extracts were identified by comparing Rf values of reference arjunic acid, arjunetin and arjungenin. The amount of arjunin acid in alcoholic and aqueous extracts was estimated to be about 0.44 ± 0.05% (w/w) and 0.17 ± 0.02% (w/w), respectively. The amount of arjunetin in alcoholic and aqueous extracts was estimated to be about 0.33 ± 0.02% (w/w) and 0.07 ± 0.01% (w/w), respectively. The amount of arjungenin in alcoholic and aqueous extracts was estimated to be about 0.23 ± 0.02% (w/w) and 0.16 ± 0.001% (w/w), respectively. The percent content of triterpenoids, arjunic acid, arjunetin and arjungenin was found to be more in the alcoholic extract of T. arjuna.
3.2. Inhibition of CYP3A4, CYP2D6 and CYP2C9 enzyme in HLM by alcoholic and aqueous extracts of T. arjuna, arjunic acid, arjunetin and arjungenin
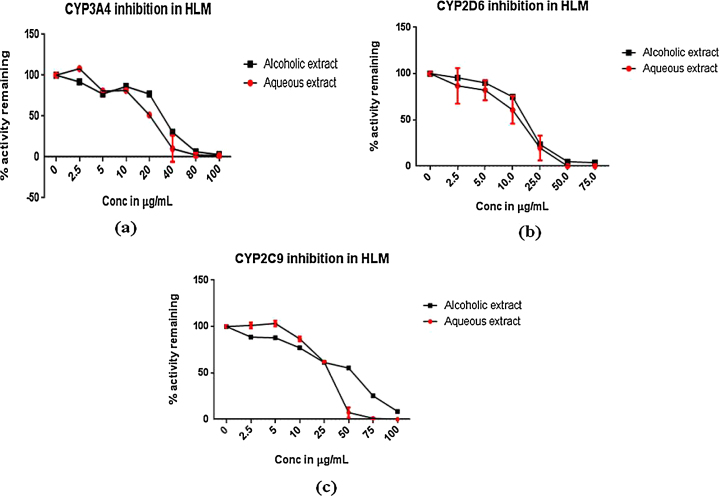
To investigate whether the arjuna extracts and triterpenoids affect the catalytic activity of CYP3A4, CYP2D6 and CYP2C9, the probe reaction assay was conducted with various concentrations of arjuna extracts and triterpenoids in HLM. Ketoconazole, quinidine and sulphaphenazole at final individual concentrations of 0.1, 0.4 and 0.3 μM, respectively, lead to a 50% inhibition of CYP3A4, CYP2D6 and CYP2C9 enzyme activity in human liver microsomes as compared to negative solvent control. Results of the study summarized in Table 1 and Fig. 2 revealed that both alcoholic and aqueous extracts of T. arjuna showed potent CYP3A4, CYP2D6 and CYP2C9 inhibition in HLM with their IC50 values less than 50 μg of extract/mL. However, arjunic acid, arjunetin and arjungenin showed no significant inhibition of CYP3A4, CYP2D6 and CYP2C9 in HLM even upto 50 μM concentrations. Among both of the extracts, aqueous extract was found to show a relatively more potent inhibitory effect than alcoholic extract of T. arjuna on enzyme activities of all the three isozymes in HLM.
Table 1.
IC50 values of alcoholic and aqueous extracts of T. arjuna, arjunic acid, arjunetin and arjungenin for CYP3A4, CYP2D6 and CYP2C9 enzymes in HLM.
| Enzyme | IC50 values |
||||
|---|---|---|---|---|---|
| Alcoholic extract of T. arjuna (μg of extract/mL) | Aqueous extract of T. arjuna (μg of extract/mL) | Arjunic acid | Arjunetin | Arjungenin | |
| CYP3A4 | 16.6 ± 6.5 | 17.4 ± 2.7 | >50 μM (>25 μg/ml) | >50 μM (>33 μg/ml) | >50 μM (>25 μg/ml) |
| CYP2D6 | 15.28 ± 4.16 | 11.97 ± 14.6 | >50 μM (>25 μg/ml) | >50 μM (>33 μg/ml) | >50 μM (>25 μg/ml) |
| CYP2C9 | 34.52 ± 3.2 | 27.78 ± 2.4 | >50 μM (>25 μg/ml) | >50 μM (>33 μg/ml) | >50 μM (>25 μg/ml) |
IC50 values, 50% inhibitory concentration. Data represent the mean of triplicate.
Fig. 2.
Plot of percent enzyme activity remaining versus concentration of extracts of T. arjuna in HLM. (a) Inhibition of CYP3A4 enzyme activity. (b) Inhibition of CYP2D6 enzyme activity. (c) Inhibition of CYP2C9 enzyme activity by alcoholic and aqueous extracts of T. arjuna.
3.3. Inhibition kinetic analysis
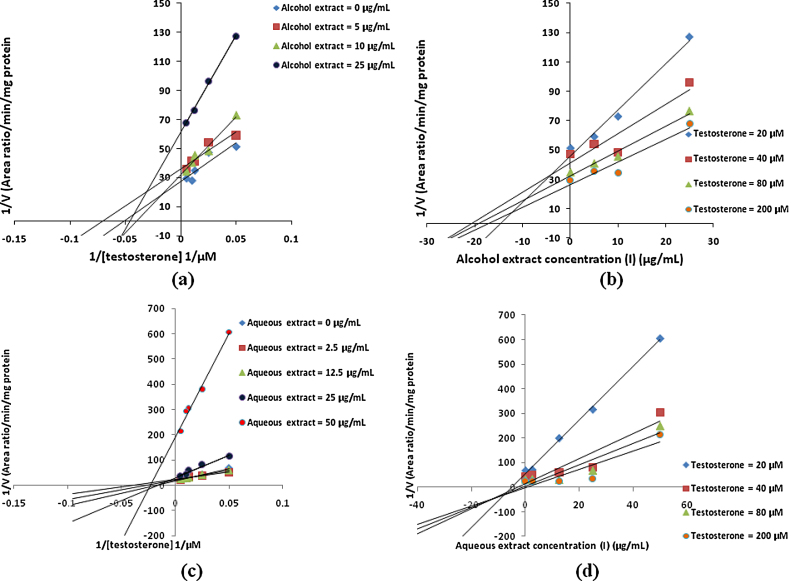
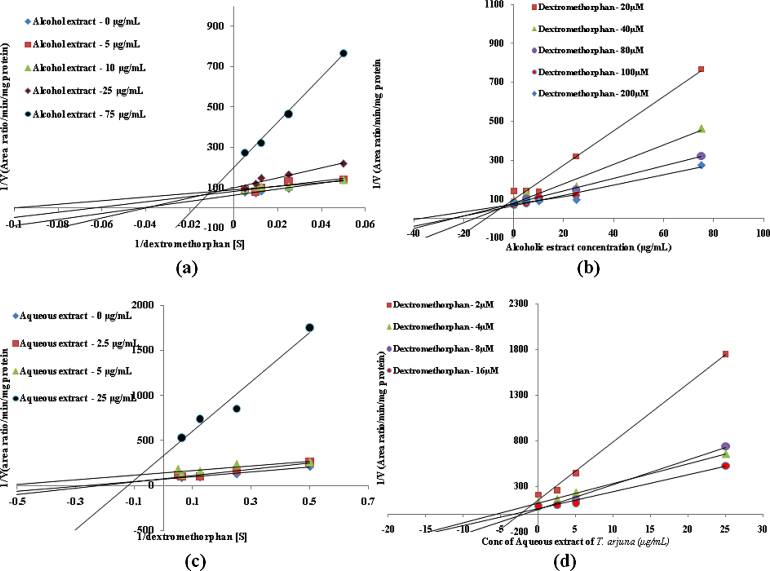
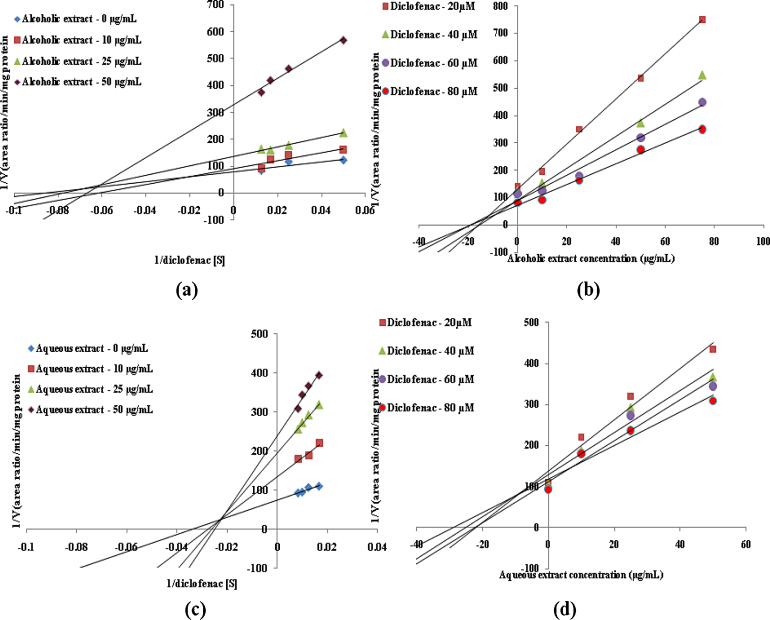
In vitro CYP inhibition assay results suggested that both alcoholic and aqueous extracts of T. arjuna showed potent inhibition of CYP3A4, CYP2D6 and CYP2C9 enzymes in human liver microsomes with IC50 values less than 35 μg/mL. However arjunic acid, arjunetin and arjungenin, did not show any significant inhibition of CYP3A4, CYP2D6 and CYP2C9 enzymes in human liver microsomes upto final concentrations of 50 μM. Inhibition constants (Ki values) and inhibition modes for those with IC50 less than 100 μg/mL (for extracts) or 100 μM (for active constituents) need to be determined [25]. Hence mode of inhibition and Ki values of alcoholic and aqueous extract of T. arjuna was determined for CYP3A4, CYP2D6 and CYP2C9 enzymes in human liver microsomes. Since arjunic acid, arjunetin and arjungenin failed to show any inhibition their Ki values were not determined. Inhibition constants and mode of inhibition determined for alcoholic and aqueous extracts of T. arjuna in human liver microsomes for CYP3A4, CYP2D6 and CYP2C9 enzymes is summarized in Table 2. Lineweaver–Burk plots and Dixon plots for alcoholic and aqueous extracts for CYP3A4, CYP2D6 and CYP2C9 inhibition in HLM are shown in Fig. 3, Fig. 4, Fig. 5, respectively.
Table 2.
Inhibition constants, mode of inhibition and effect of preincubation of T. arjuna extracts on CYP3A4, CYP2D6 and CYP2C9 activities in human liver microsomes (n = 3; values ± standard deviation).
| Enzyme | Alcoholic extract of T. arjuna (μg of extract/mL) |
Aqueous extract of T. arjuna (μg of extract/mL) |
||||||
|---|---|---|---|---|---|---|---|---|
| Ki value | Mode of inhibition | Shifted IC50 (μg/mL) – 30 min preincubation with NADPHa,b | Shifted IC50 (μg/mL) – 30 min preincubation without NADPHa,b | Ki value (μg of extract/mL) | Mode of inhibition | Shifted IC50 (μg/mL) – 30 min preincubation with NADPHa,b | Shifted IC50 (μg/mL) – 30 min preincubation without NADPHa,b | |
| CYP3A4 | 24.05 ± 1.82 | Non-competitive | >75 | >75 | 27.06 ± 0.219 | Non-competitive | >75 | >75 |
| CYP2D6 | 17.01 ± 1.28 | Non-competitive | >75 | >75 | 8.33 ± 0.44 | Non-competitive | >75 | >75 |
| CYP2C9 | 23.72 ± 4.7 | Non-competitive | >75 | >75 | 20.56 ± 2.8 | Non-competitive | >75 | >75 |
Each data represents the average of triplicate measurements ± standard deviation.
Ki value, mode of inhibition and effect of preincubation was determined only for alcoholic and aqueous extract, since they were potent inhibitors and also reported to be used therapeutically.
Fig. 3.
Representative Lineweaver–Burk plot (a) and Dixon plot (b) of effect of alcoholic extract of T. arjuna on 6β-hydroxytestosterone formation in human liver microsomes. Representative Lineweaver–Burk plot (c) and Dixon plot (d) of effect of aqueous extract of T. arjuna on 6β-hydroxytestosterone formation in human liver microsomes.
Fig. 4.
Representative Lineweaver–Burk plot (a) and Dixon plot (b) of effect of alcoholic extract of T. arjuna on dextrorphan formation in human liver microsomes. Representative Lineweaver–Burk plot (c) and Dixon plot (d) of effect of aqueous extract of T. arjuna on dextrorphan formation in human liver microsomes.
Fig. 5.
Representative Lineweaver–Burk plot (a) and Dixon plot (b) of effect of alcoholic extract of T. arjuna on 4′-hydroxy diclofenac formation in human liver microsomes. Representative Lineweaver–Burk plot (c) and Dixon plot (d) of effect of aqueous extract of T. arjuna on 4′-hydroxy diclofenac formation in human liver microsomes.
For inhibitors of substrates which follow Michaelis–Menten kinetics tested at the Km concentration of substrate, Ki values fall between IC50/2 (competitive inhibitors) and IC50 (non-competitive inhibitors). Goodness of fit criteria (Akaike's Information Criterion) was used to determine the best model for inhibition. Based on Graph Pad Prism® analysis and the plots it was concluded that the alcoholic and aqueous extracts of T. arjuna were non-competitive inhibitors of CYP3A4, CYP2D6 and CYP2C9 enzymes in human liver microsomes.
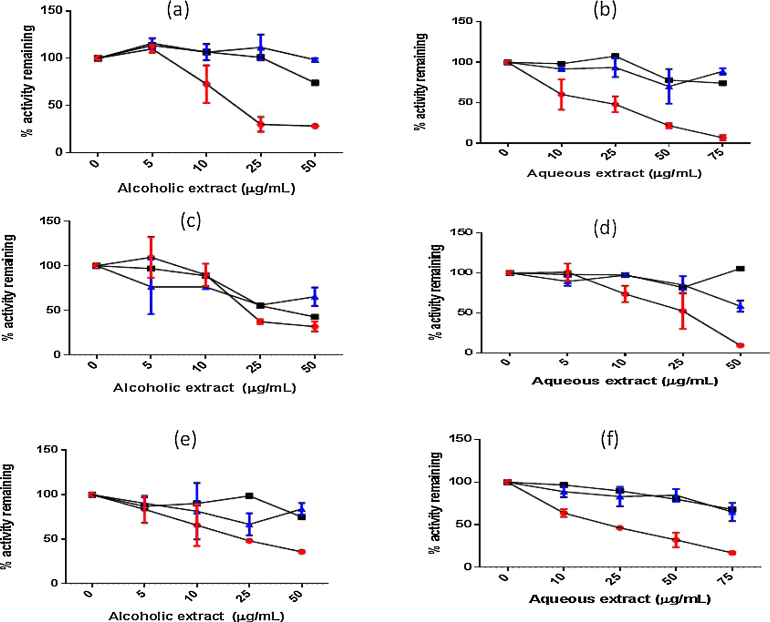
As shown in Fig. 6 on preincubation of alcoholic and aqueous extracts for 30 min in presence and absence of NAPDH and diluting the inactivator, there was no shift in IC50 values indicating the interaction to be of a reversible type. Based on these evidence it could be postulated that both the extracts of T. arjuna are rapidly reversible non-competitive inhibitors.
Fig. 6.
Effect of 30 min preincubation (with and without NADPH regenerating system) of extent of inhibition of CYP3A4 mediated testosterone 6′-hydroxylation by alcoholic (a) and aqueous extract (b) of T. arjuna; inhibition of CYP2D6 mediated dextromethorphan O-demethylation by alcoholic (c) and aqueous (d) extract of T. arjuna; inhibition of CYP2C9 mediated diclofenac 4′-hydroxylation by alcoholic (e) and aqueous (f) extract of T. arjuna ( – preincubation for 30 min with NADPH regenerating system, ■ – preincubation for 30 min without NADPH regenerating system,
– preincubation for 30 min with NADPH regenerating system, ■ – preincubation for 30 min without NADPH regenerating system,  – no preincubation).
– no preincubation).
4. Discussion
The stem bark of T. arjuna (Roxb.) is used by the Ayurvedic physicians in India for the treatment of various cardiovascular diseases, collectively referred to as hritroga [12]. Many important biologically active chemical compounds have been isolated from T. arjuna. These include triterpenoids (arjunolic acid, arjunic acid, arjungenin, etc.), tannins (ellagic acid, gallic acid), flavonoids (leucocyanidin, luteolin) and minerals (magnesium, calcium, zinc, etc.) [13]. Various extracts (water, hydroalcohol, ethanol, etc.) of the stem bark of T. arjuna and active compounds present in these extracts have been investigated in many experimental studies and have been reported to exhibit blood pressure-lowering effects [21], direct cardioprotective effects [6], antioxidant activities [16], antiplatelet effects [18], hypolipidemic [10], and anti-atherogenic effects [17]. Clinical studies have suggested that arjuna bark extracts at a human dose of 500 mg three times daily showed a therapeutic effect comparable to the benefits of isosorbide dinitrate therapy in chronic stable angina [13].
Although useful, there have been reports cited in the literature stating the potent effects of herbal drugs on metabolizing CYP enzymes. It is well documented that any alteration in the activity of one or several CYP enzymes could have pronounced effect in the plasma and tissue levels of a co-administered drug. This irregularity in drug levels could contribute to adverse side effects or potential toxicity. It could also alter metabolic pathways of other drugs, which may lead to inhibition, induction or activation of other drug metabolizing enzymes [22].
HPTLC quantification showed that the percent content levels of arjunic acid, arjunetin and arjungenin in alcoholic extract was approximately 5–15 times more than the contents in aqueous extract of T. arjuna. Arjunic acid is freely soluble in methanol and insoluble in water whereas arjungenin is freely soluble in methanol and insoluble in hexane. Arjunic acid and arjungenin are triterpenoid saponins, which make them non-polar in nature, thus explaining the difference in its levels in alcoholic and aqueous bark extracts.
Our previous study reported that alcoholic, hydroalcoholic and aqueous extracts of T. arjuna showed a potent inhibition of CYP1A enzyme in rat and human liver microsomes with IC50 values less than 50 μg/ml. While, arjunic acid and arjungenin did not show inhibition of CYP1A enzyme in rat and human liver microsomes [24].
Since CYP3A4, CYP2D6 and CYP2C9 are the three principle enzymes to be most commonly responsible for the metabolism of most drugs and the associated drug–drug interactions in humans [20]. Hence we investigated the in vitro inhibitory effect of alcoholic and aqueous extract of T. arjuna and triterpenoids, arjunic acid, arjunetin and arjungenin, on CYP3A4, CYP2D6 and CYP2C9 activity in human liver microsomes.
Of the three isoforms studied, based on the inhibition constant (Ki values) values, alcoholic and aqueous extracts of T. arjuna showed a more potent inhibition of CYP2D6 enzyme as compared to CYP3A4 and CYP2C9. Aqueous extract of T. arjuna showed more potent inhibition of CYP2D6 enzyme than alcoholic extract of T. arjuna in human liver microsomes. The alcoholic and aqueous bark extracts of T. arjuna inhibited both CYP3A4 and CYP2C9 to a similar extent.
Ki values and the Lineweaver–Burk plots, Dixon plots and analysis of data using Graph Pad Prism software for alcoholic and aqueous extracts of T. arjuna, revealed that the extracts can be classified as potent non-competitive inhibitors. Non-competitive inhibition is rarely seen in single-substrate reactions (multireactant systems) [14]. Based on our results, it is evident that there is significant inhibition of CYP enzymes by arjuna bark extracts, however there was no inhibition by individual triterpenoids. Preincubation of alcoholic and aqueous bark extract of T. arjuna in human liver microsomes in presence and absence of NADPH, followed by dilution of the inactivator did not cause significant reduction of CYP3A4, CYP2D6 and CYP2C9 enzyme activity. Thus, alcoholic and aqueous bark extract of T. arjuna could be classified as rapidly reversible non-competitive inhibitors of CYP3A4, CYP2D6 and CYP2C9 enzymes in rats.
As reiterated earlier, despite the difference in the levels of concentrations of triterpenoids in the alcoholic and aqueous extracts the IC50 values and Ki values of both the extracts for all the three enzymes were very close further confirming the absence of inhibitory activity by the triterpenoids correlating with our in vitro data. The literature suggests that stem bark of T. arjuna also shows presence of flavonoids like arjunone, luteolin, quercetin, kaempferol, etc. and tannins like gallic acid, ellagic acid, pyrocatechols, terchebulin, etc. [4]. It is reported that flavonoids and tannins are potent CYP inhibitors [26], [27]. Since arjuna bark shows presence of flavonoids and tannins, inhibition potential of the extracts may be due to these same constituents which needs to be investigated further. Though it is important to determine the constituents responsible for the interaction, it is also essential to note that the extracts are commonly prescribed as therapeutic agents than the individual constituents. Hence by studying the inhibition potential of therapeutic extracts with CYP3A4, CYP2D6 and CYP2C9 enzymes, safety potential of these therapeutic extracts could be indexed.
Based on these observations, in vitro inhibition of CYP3A4, CYP2D6 and CYP2C9 enzymes in human liver microsomes by extracts of T. arjuna, suggests that there is a possibility of T. arjuna to lead to clinically significant pharmacokinetic interactions, since CYP3A4, CYP2D6 and CYP2C9 are the main enzymes involved in metabolism of most of our drugs. However, effect of T. arjuna bark and extracts co-administered with other medications, clinically and preclinically need to be investigated. The potential for in vivo inhibition lies not only with the inhibitory potency (Ki) but also with the overall dispositional properties of the inhibitor (i.e., extent of absorption from the gastrointestinal tract, extent of plasma protein binding, uptake into the liver, rate of clearance, etc.) [15]. Also, due to lack of standardized extracts the relative abundance of each compound in the extracts would also have an impact on the drug–herb interactions. This, in vitro data can be used as a guide to design preclinical and clinical pharmacokinetic interactions with T. arjuna.
5. Conclusion
Based on our knowledge, inhibition potential of arjuna bark and its principle phytoconstituents of CYP3A4, CYP2D6 and CYP2C9 has not been reported. In this study, we demonstrated that alcoholic and aqueous extracts of T. arjuna, a plant used for cardiovascular disorders, have been identified to possess strong inhibitory potential of CYP3A4, CYP2D6 and CYP2C9 enzymes in human liver microsomes, which is involved in metabolism of many drugs. The triterpenoids, arjunic acid, arjunetin and arjungenin, did not show significant inhibition of CYP3A4, CYP2D6 and CYP2C9 enzymes in human liver microsomes. Enzyme kinetics and time dependent inhibition studies classified alcoholic and aqueous bark extracts as rapidly reversible non-competitive inhibitors. Until clinical pharmacokinetic drug interaction experiments are conducted, the co-administration of drugs, especially those primarily cleared by CYP3A4, CYP2D6 and CYP2C9 enzymes, with Terminalia arjuna bark extracts should be done with caution.
Conflict of interest
The authors have declared that there is no conflict of interest.
Transparency document
Acknowledgements
The authors would like to thank Department of Biotechnology, New Delhi, India for providing financial support through project grant [DBT Project no: BT/PR14460/PBD/17/703/2010] for the present research work.
Contributor Information
Alice Varghese, Email: alice.varghese@nmims.edu, alicevarghese02@gmail.com.
Jay Savai, Email: jaysavai08@gmail.com.
Nancy Pandita, Email: nancy.pandita@nmims.edu.
Ram Gaud, Email: RSGaud@nmims.edu.
References
- 1.Aiming Y.U., Haining R.L. Comparative contribution to dextromethorphan metabolism by cytochrome P450 isoforms in vitro: can dextromethorphan be used as a dual probe for both CYP2D6 and CYP3A activities. Drug Metab. Dispos. 2001;29(11):1514–1520. [PubMed] [Google Scholar]
- 2.Andrew J.S. Regulation of testosterone hydroxylation by rat liver microsomal cytochrome P-450. Arch. Biochem. Biophys. 1987;255:27–41. doi: 10.1016/0003-9861(87)90291-8. [DOI] [PubMed] [Google Scholar]
- 3.Dambal A.A., Bhagwat D.A., Khadbadi S.S. Pharmacognostic studies of the Terminalia arjuna bark. Int. J. Chem. Pharm. Sci. 2014;2(5):837–840. [Google Scholar]
- 4.Divya K. Terminalia arjuna in coronary artery disease: ethnopharmacology, pre-clinical, clinical and safety evaluation. J. Ethnopharmacol. 2014;155(2):1029–1045. doi: 10.1016/j.jep.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 5.Dwivedi S. Terminalia arjuna Wight and Arn. – a useful drug for cardiovascular disorders. J. Ethnopharmacol. 2007;114:114–129. doi: 10.1016/j.jep.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Dwivedi S. Cardioprotective effects of certain indigenous drugs in myocardial ischemia in rabbits. Indian J. Exp. Biol. 1988;26:969–975. [PubMed] [Google Scholar]
- 7.Fand S., Zhang H. In vitro evaluation of reversible and irreversible cytochrome P450 inhibition: current status on methodologies and their utility for predicting drug–drug interactions. AAPS J. 2008;10:410–424. doi: 10.1208/s12248-008-9042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fasinu P.S., Bouic P.J., Rosenkranz B. An overview of the evidence and mechanisms of herb–drug interactions. Front. Pharmacol. 2012;3:1–19. doi: 10.3389/fphar.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidance for Industry . 2006, September. Drug Interactions Studies: Study Design, Data Analysis and Implications for Dosing and Labeling. http://www.fda.gov/cder, http://www.fda.gov./cder/guidance/6695dft.pdf. [DOI] [PubMed] [Google Scholar]
- 10.Khanna A.K. Terminalia arjuna: an ayurvedic cardiotonic regulates lipid metabolism in hyperlipidemic rats. Phytother. Res. 1996;10:663–666. [Google Scholar]
- 11.Liu Y. Influence of ginsenoside Rh1 and F1 on human cytochrome P450 enzymes. Planta Med. 2006;72:126–131. doi: 10.1055/s-2005-873197. [DOI] [PubMed] [Google Scholar]
- 12.Maulik S.K., Katiyar C.K. Terminalia arjuna in cardiovascular diseases: making the transition from traditional to modern medicine in India. Curr. Pharm. Biotechnol. 2010;11:855–860. doi: 10.2174/138920110793262051. [DOI] [PubMed] [Google Scholar]
- 13.Maulik S.K., Talwar K.K. Therapeutic potential of Terminalia arjuna in cardiovascular disorders. Am. J. Cardiovasc. Drugs. 2012;12:157–163. doi: 10.2165/11598990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Michael M.J. Approaches to the rational design of enzyme inhibitors. Burg. Med. Chem. Drug Discov. 2001;1:715–779. [Google Scholar]
- 15.Obach S.R. Inhibition of human cytochrome P450 enzymes by constituents of St. John's Wort, an herbal preparation used in the treatment of depression. J. Pharmacol. Exp. Ther. 2000;294:88–95. [PubMed] [Google Scholar]
- 16.Pawar R.S. Effect of oleanane triterpenoids from Terminalia arjuna: a cardioprotective drug on the process of respiratory oxyburst. Phytomedicine. 2005;12:391–393. doi: 10.1016/j.phymed.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Subramaniam S. Anti-hyperlipidemic and antioxidant potential of different fractions of Terminalia arjuna Roxb. bark against PX-407 induced hyperlipidemia. Indian J. Exp. Biol. 2011;49:282–288. [PubMed] [Google Scholar]
- 18.Sumitra M. Experimental myocardial necrosis in rats: role of arjunolic acid on platelet aggregation, coagulation and antioxidant status. Mol. Cell Biochem. 2001;224:135–142. doi: 10.1023/a:1011927812753. [DOI] [PubMed] [Google Scholar]
- 19.Stephen F. In vitro evaluation of reversible and irreversible cytochrome P450 inhibition: current status on methodologies and their utility for predicting drug–drug interactions. AAPS J. 2008;10:410–423. doi: 10.1208/s12248-008-9042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steven A.W., Jeffrey C.S. The human hepatic cytochrome P450 involved in drug metabolism. Crit. Rev. Toxicol. 1992;22:1–21. doi: 10.3109/10408449209145319. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi S. Hypotensive effects in rats of hydrophilic extract from Terminalia arjuna containing tannin-related compounds. Phytother. Res. 1997;1:424–427. [Google Scholar]
- 22.Tang J. Inhibition of cytochrome P450 enzymes by rhein in rat liver microsomes. Phytother. Res. 2009;23:159–164. doi: 10.1002/ptr.2572. [DOI] [PubMed] [Google Scholar]
- 23.Ushijima K. Cranberry juice suppressed the diclofenac metabolism by human liver microsomes, but not in healthy human subjects. Br. J. Clin. Pharm. 2009;68(2):194–200. doi: 10.1111/j.1365-2125.2009.03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varghese A. In vitro and in vivo evaluation of CYP1A interaction potential of Terminalia arjuna bark. Indian J. Pharm. Sci. 2014;76:138–147. [PMC free article] [PubMed] [Google Scholar]
- 25.Yan P. In vitro modulatory effects of Andrographis paniculata, Centella asiatica and Orthosiphon stamineus on cytochrome P450 2C19 (CYP2C19) J. Ethnopharmacol. 2011;133:881–887. doi: 10.1016/j.jep.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 26.Ho P.C., Saville D.J., Wanvimolruk S. Inhibition of human CYP3A4 by grapefruit flavonoids, furanocoumarins and related compounds. J. Pharm. Pharm. Sci. 2001;4:217–227. [PubMed] [Google Scholar]
- 27.Ponnusankar S., Pandit S., Babu R. Cytochrome P450 inhibitory potential of Triphala—Rasayana from Ayurveda. J. Ethnopharmacol. 2011;133:120–125. doi: 10.1016/j.jep.2010.09.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.