Abstract
Background:
Cyclosporine A (CsA) is a commonly used clinical immunosuppressant. However, CsA exposure in rabbits during the gestation period was shown to cause a postnatal decrease in the number of nephrons, with the effects remaining unknown. In this study, we aimed to explore the effects of CsA on metanephros development in the pregnant BALB/c mice.
Methods:
Pregnant mice were randomly divided into two groups, and CsA (10 mg·kg−1·d−1) was subcutaneously injected from gestation day 10.5 to day 16.5 in the CsA group, whereas a comparable volume of normal saline was given to the control group. All of the mice were sacrificed on gestation day 17.5 and serum CsA concentration was measured. The fetuses were removed and weighed, and their kidneys were prepared for histological assessment and polymerase chain reaction assay. In an in vitro experiment, embryo kidneys of fetal mice on gestation day 12.5 were used, and CsA (10 μmol/L) was added in the culture of the CsA group. The growth pattern of the ureteric bud and nephrons was assessed by lectin staining.
Results:
No significant differences in the weight of embryo (4.54 ± 1.22 vs. 3.26 ± 1.09 mg) were observed between the CsA and control groups, the thickness of the cortical (510.0 ± 30.3 vs. 350.0 ± 29.7 μm, P < 0.05) and nephrogenic zone (272.5 ± 17.2 vs. 173.3 ± 24.0 μm, P < 0.05), and the number of glomeruli (36.5 ± 0.7 vs. 27.5 ± 2.1, P < 0.05) were reduced in the CsA group when compared to the control group. The cell proliferation of Ki-67 positive index between control and CsA group (307.0 ± 20.0 vs. 219.0 ± 25.0, P < 0.05) in the nephrogenic zone was decreased with the increase of apoptotic cells (17.0 ± 2.0 vs. 159.0 ± 33.0, P < 0.05). The mRNA expression of WT-1, Pax2, and Pax8 was downregulated by CsA treatment. As for the in vitro CsA group, the branch number of the ureteric bud was decreased in the CsA-treated group with the nephrons missing in contrast to control after the incubation for 24 h and 72 h (all P < 0.0001).
Conclusion:
Treatment of CsA suppressed metanephros development in the pregnant mice; however, the potential action of mechanism needs to be further investigated.
Keywords: Apoptosis, Cyclosporine A, Metanephros, Nephron, Ureteric Bud
INTRODUCTION
For nearly three decades, cyclosporine A (CsA) has been demonstrated to be a powerful immunosuppressive agent. CsA is commonly used for organ transplantation and the treatment of various autoimmune diseases,[1] and among patients undergoing CsA therapy, an increasing number of pregnant women have been reported. However, chronic maternal CsA treatment during pregnancy can cause fetal side effects,[2] and prenatal exposure to CsA has been shown to induce the vacuolation of proximal tubular cells and impair distal nephron differentiation in newborn rats.[3] Moreover, CsA exposure to embryonic kidneys can produce potential defect in nephrogenesis.[4]
During nephrogenesis, the kidney undergoes a succession of morphogenetic events driven by cell growth and differentiation. The embryonic kidney develops through three stages: the pronephros, the mesonephros, which both will regress throughout development, and the metanephros, which forms the final kidney.[5]
However, CsA, as an immunosuppressant, is a specific inhibitor of Calcineurin (Cn), which is a downstream effector molecule of calcium.[6] There is considerable evidences that CsA exposure in rabbits during the gestation period could cause a postnatal decrease in the number of nephrons, but the specific mechanism and effects remain unknown.[7] In our study, we aimed to explore the effect of CsA on the development of metanephros in the pregnant BALB/c mice.
METHODS
Animals
The BALB/c mice (6–8 weeks old), weighing 20 ± 2g, were from the Animal Center of Sichuan University. One male and three females were housed in one cage for mating. Gestation day 0.5 was confirmed by vaginal plug. The pregnant mice were randomly divided into two groups. The CsA group (n = 6) was subcutaneously injected with CsA (10 mg·kg−1·d−1) on gestation days 10.5–16.5. The control group (n = 6) received daily subcutaneous injection of saline at the same volume. The pregnant mice were sacrificed on gestation day 17.5, and blood samples were collected to determine serum CsA concentration with high-performance liquid chromatography assay (LC-2010CHT, Shimadzu Corporation, Japan). The fetuses were removed and weighed, and both kidneys were removed. The right kidneys were fixed with 4% paraformaldehyde for histological assessment and the left kidneys were prepared for mRNA expression testing. Animal experiments were conducted according to the guidelines of Animal Care and Use Committee of Sichuan University (IACUC number: 20100318).
Embryo kidney organ culture
In the in vitro experiment, embryo kidneys of fetal mice on gestation day 12.5 were isolated and cultured at 37°C with 5% CO2 on Transwell filters (Corning, USA). The culture was Dulbecco's modified Eagle's Medium (HyClone, USA) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% L-glutamine. CsA (10 μmol/L) was added into the culture in the experimental group. After incubation for 24, 48, and 72 h, the growth patterns of the ureteric bud and nephrons were assessed using lectin staining.
Histological studies
The right kidneys of fetuses were removed and fixed with 4% paraformaldehyde and made into paraffin block. Sections (3 μm) were made and stained with hematoxylin and eosin. The measurement of thickness of cortical and nephrogenic zone, the number of glomeruli/mm2, and the diameter of glomeruli were determined as described previously.[2]
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay
Rabbit monoclonal anti-Ki-67 antibody was purchased from Abcam (United Kingdom) and secondary reagent was purchased from Dako (Denmark). The single immunohistochemical staining was performed using the EnVision Detection System, Dako. The positive cells would display brownish yellow granules in the cell nucleus. Five high power fields under a microscope were randomly chosen on each section and two hundred cells were examined in each field. The Ki-67 positive index was defined as the percent of positive cells/1000 cells. Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay was conducted by the DeadEnd Fluorometric TUNEL system (Promega, USA) and the images were captured under fluorescence microscope.
Reverse transcription polymerase chain reaction assay
RNA was isolated from embryo kidneys by the TRIzol Kit (Invitrogen, USA) and cDNA was synthesized using the Revert Aid™ First Strand cDNA Synthesis Kit (Fermentas, Canada). TaqMan reverse transcription polymerase chain reaction (PCR) was conducted using the Taq DNA PCR Reaction Kit (Takara, Japan). ACTB was used as internal control, and the primer sequences are as follows: WT-1 F: 5’-CCCTACAGCAGTGACAAT-3’, R: 5’-CTCTGCCCTTCTGTCCAT-3’; Pax2 F: 5’-GGTCTGGACTTTAAGAGATGT-3’, R: 5’-TGCTGCTGGGTGAAGGTGT-3’; Pax8 F: 5’-AGCAAGATCCTTGGCAGGT-3’, R: 5’-GCAAACATGGTAGGGTTCTG-3’; Lim-1 F: 5’-GACTTCTTCCGATGTTTCGGT-3’, R: 5’-GAGAGCTGCTTGTTACACAT-3’; ACTB F: 5’-GAAGATCAAGATCATTGCTCCT-3’, R: 5’-TACTCCTGCTTGCTGATCCACA-3’.
Lectin histochemistry
Fluorescein-isothiocyanate (FITC)-labeled Dolichos biflorus agglutinin (DBA), which bonds to the ureteric bud/collecting duct epithelia, and rhodamine-labeled peanut agglutinin (PNA), which bonds to podocytes, were purchased from vector laboratories, USA. After the conclusion of culture, embryo kidneys were fixed in 2% paraformaldehyde at 4°C for 30 min and washed briefly in a phosphate-buffered saline (PBS). For FITC-DBA staining, metanephros were incubated in 0.1% Triton for 30 min following a brief wash and then incubated overnight with 10 μg/ml FITC-DBA at 4°C. To determine the nephrons, metanephros were incubated in 50 nmol/L NH4Cl overnight at 4°C following brief wash and then permeabilized with 0.1% Triton at 37°C for 30 min which was followed by incubation in 0.1 U/ml of neuraminidase at 37°C for 4 h. After being washed in PBS, the metanephros was incubated in 20 μg/ml PNA overnight at 4°C. The ureteric bud branches and nephrons were imaged by fluorescence microscope. The branch points were defined as the intersection of two connected branches.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Student's unpaired t-test was used for the comparison of mean differences between two different groups, and a two-sided P < 0.05 was considered statistically significant. We used SPSS version 19.0 (SPSS Inc., Chicago, USA) for statistical analyses.
RESULTS
Comparison of normal and cyclosporine A-treated embryo and embryo kidney development in vivo
Morphological data on gestation day 17.5 are summarized in Table 1. The number of embryos was comparable between the CsA and control groups (7.5 ± 1.0 vs. 6.83 ± 1.72, t = 0.8249, P = 0.4287). No significant differences in fetus weight (0.83 ± 0.1 vs. 0.74 ± 0.17 g) and embryo kidney weight (4.54 ± 1.22 vs. 3.26 ± 1.09 mg) between the two groups were observed (t = 0.7904, P = 0.4735). No reduced embryo kidney/fetus weight ratio was measured between the control and CsA groups (0.53 ± 0.15% vs. 0.39 ± 0.10%, t = 1.355, P = 0.2468). The residual value of CsA was 397 ± 46 ng/ml for the CsA group, which was collected on gestation day 17.5.
Table 1.
Comparison of normal and CsA-treated embryo and embryo kidney development on gestation 17.5 d
| Items | Control (n = 6) | CsA (n = 6) |
|---|---|---|
| Number of fetus of pregnant mouse | 7.50 ± 1.00 | 6.83 ± 1.72 |
| Fetus weight (g) | 0.83 ± 0.10 | 0.74 ± 0.17 |
| Embryo kidney weight (mg) | 4.54 ± 1.22 | 3.26 ± 1.09 |
| Embryo kidney weight/fetus weight (%) | 0.53 ± 0.15 | 0.39 ± 0.10 |
CsA: Cyclosporine A.
Comparison of normal and cyclosporine A-treated embryo renal morphologic features
The cortical zone (510.0 ± 30.3 vs. 350.0 ± 29.7 μm, t = 6.532, P = 0.0028) and nephrogenic zone (272.5 ± 17.2 vs. 173.3 ± 24.0 μm, t = 5.819, P = 0.0043) were significantly thicker in the control group than those of the CsA group. The number of glomeruli/mm2 in the experimental group was significantly decreased compared to that of the control group (36.5 ± 0.7 vs. 27.5 ± 2.1, t = 7.042, P = 0.0021), which was compensated by no significant hypertrophy [Figure 1 and Table 2].
Figure 1.
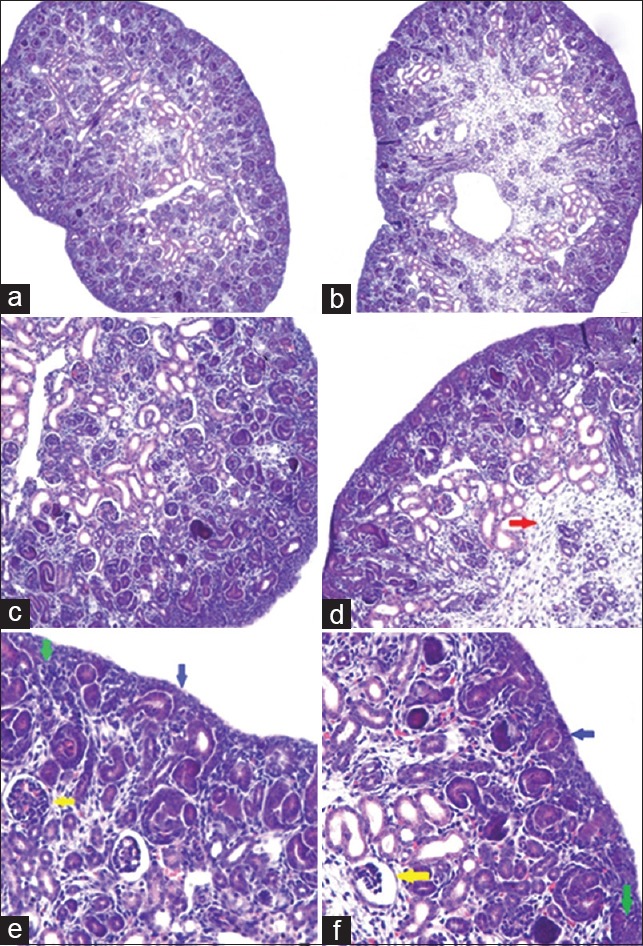
Immunohistochemical staining results of embryo kidney from pregnant BALB/c mice. (a) Control group, original magnification, ×100; (b) CsA group, original magnification, ×100; (c) Control group, original magnification, ×200; (d) CsA group, original magnification, ×200; (e) Control group, original magnification, ×400; (f) CsA group, original magnification, ×400 (Red arrow: Hyperplasia of interstitial cell; Yellow arrow: Mature glomeruli; Blue arrow: Renal capsule; Green arrow: Mesenchymal cells in nephrogenic zone). CsA: Cyclosporine A.
Table 2.
Comparison of normal and CsA-treated embryo renal morphologic features
| Items | Control (n = 6) | CsA (n = 6) | t | P |
|---|---|---|---|---|
| Thickness of cortex (μm) | 510.0 ± 30.3 | 350.0 ± 29.7* | 6.532 | 0.0028 |
| Thickness of nephrogenic zone (μm) | 272.5 ± 17.2 | 173.3 ± 24.0* | 5.819 | 0.0043 |
| Number of glomeruli/mm2 | 36.5 ± 0.7 | 27.5 ± 2.1* | 7.042 | 0.0021 |
| Diameter of glomeruli (μm) | 51.9 ± 8.9 | 63.3 ± 8.6 | 1.595 | 0.1858 |
*P<0.05, compared with control group. CsA: Cyclosporine A.
Comparison of cell proliferation and apoptosis
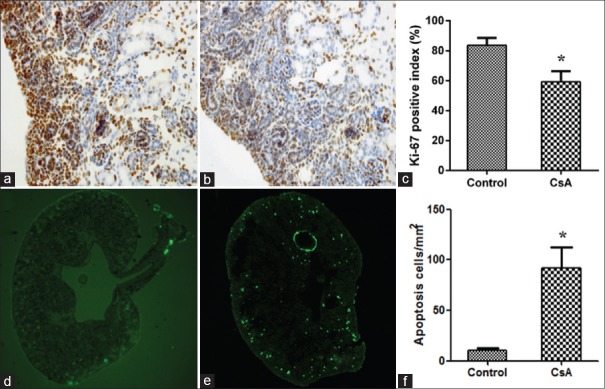
The Ki-67 nuclear positive cells were mainly located in the nephrogenic zone, which was just under the renal capsule. The Ki-67 positive index of the nephrogenic zone was higher in the control group than in the CsA group(307.0 ± 20.0 vs. 219.0 ± 25.0, P < 0.05, t = 4.761, P = 0.0089) [Figure 2a–2c]. The apoptosis cell count for the CsA group was increased (17.0 ± 2.0 vs. 159.0 ± 33.0, t = 7.439, P = 0.0017), and the apoptosis cells were also mainly located in the nephrogenic zone [Figure 2d–2f].
Figure 2.
Immunohistochemical staining for Ki-67 positive cells (a-c) and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling staining of cell apoptosis (d-f). *P < 0.05 versus control. (a) Control group, original magnification, ×400; (b) CsA group, original magnification, ×400; (c) Ki-67 positive cells index; (d) Control group, original magnification, ×100; (e) CsA group, original magnification, ×100; (f) The number of cell apoptosis. CsA: Cyclosporine A.
Comparison of normal and cyclosporine A-treated embryo kidney development in vitro
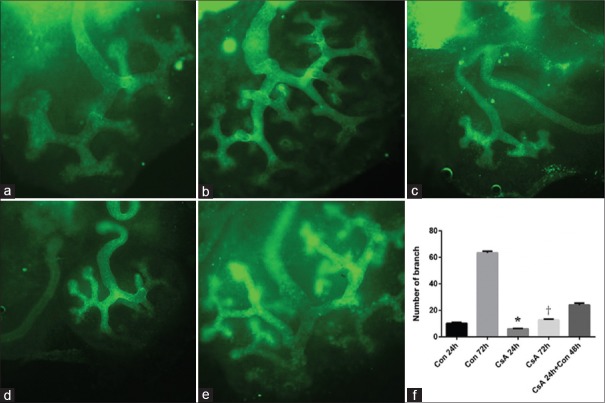
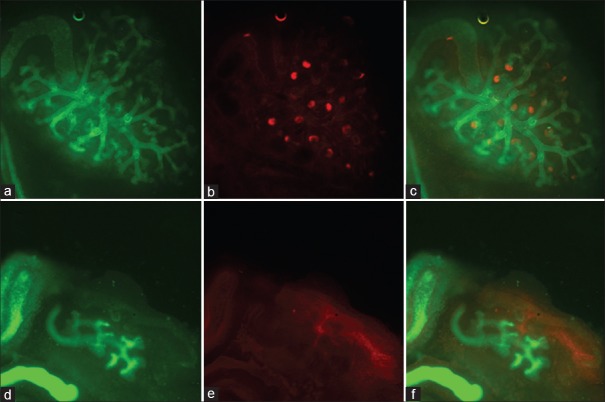
The branch number of the ureteric bud significantly increased in the control group with the extending of incubation time in vitro, and the branch number of the ureteric bud decreased in the CsA group after incubation for 24 h and 72 h, when compared with control group (t = 18.37, P < 0.0001 and t = 224.1, P < 0.0001). The ureteric bud branching could recover from incubation with CsA for 24 h when the culture was replaced by a normal one. After cultivating 72 h in normal culture, nephrons were observed surrounding the tips of the ureteric bud, which was missing in the CsA group [Figures 3 and 4].
Figure 3.
Comparison of cyclosporine A-induced embryo kidney development by DBA staining. (a) Control for 24 h; (b) control for 72 h; (c) CsA for 24 h; (d) CsA for 72 h; (e) CsA for 24 h + control for 48 h; (f) the branch number of the ureteric bud. a-e: Original magnification, ×100. *P < 0.05 versus control 24 h; †P < 0.05 versus control 72 h. DBA: Dolichos biflorus agglutinin; Con: Control; CsA: Cyclosporine A.
Figure 4.
Immunofluorescent staining of cyclosporine A-induced embryo kidney development. (a) Control for 72 h (DBA staining, original magnification, ×100); (b) Control for 72 h (PNA staining, original magnification, ×100); (c) Control for 72 h (double staining, original magnification, ×100); (d) CsA for 72 h (DBA staining, original magnification, ×100); (e) CsA for 72 h (PNA staining, original magnification, ×100); (f) CsA for 72 h (double staining, original magnification, ×100); CsA: Cyclosporine A; DBA: Dolichos biflorus agglutinin; PNA: Peanut agglutinin.
Comparison of gene expression associated with embryo kidney development
The mRNA expression of the WT-1 (0.99 ± 0.21 vs. 0.43 ± 0.20, t = 3.485, P = 0.0252), Pax-2 (1.05 ± 0.41 vs. 0.34 ± 0.13, t = 2.928, P = 0.0429), and Pax-8 genes(1.11 ± 0.53 vs. 0.18 ± 0.03, t = 3.134, P = 0.035) were significantly lower in the CsA group than in the control group, but the mRNA expression of the Lim1 gene was comparable in both groups, and no significant difference was observed (1.04 ± 0.34 vs. 0.93 ± 0.23, t = 0.4804, P = 0.6560) [Figure 5].
Figure 5.
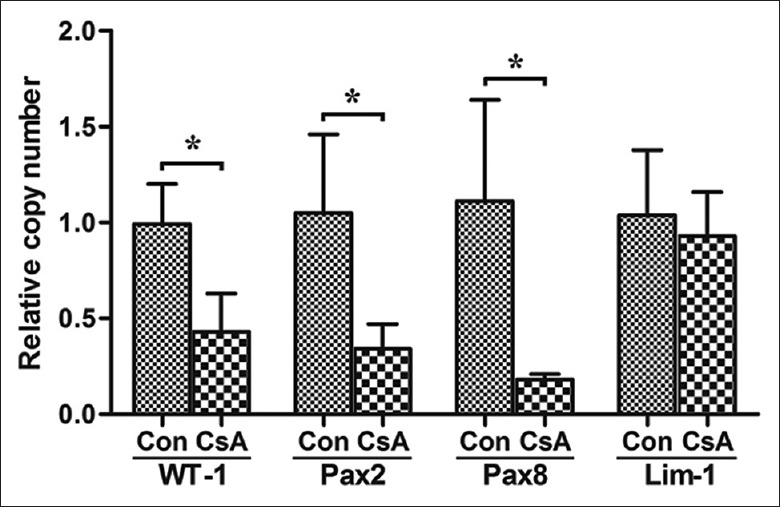
The mRNA expressions of relevant genes of embryo kidney from cyclosporine A treated pregnant BALB/c mice. *P < 0.05 versus control. Con: Control; CsA: Cyclosporine A; WT-1: Wilms’ tumor 1; Pax2: Paired box gene 2; Pax8: Paired box gene 8; Lim-1: Lim homeobox gene 1.
DISCUSSION
CsA is usually used as an effective immunosuppressive drug in the treatment of autoimmune diseases. According to the guidelines, CsA is allowed for use during pregnancy in some women with autoimmune diseases and organ transplantation. Nevertheless, CsA might cross the placenta and potentially influence fetal development with the long-term result of subtle defects as a consequence of in utero exposure to immunosuppressive agents. However, as the specific inhibitor of Cn, CsA suppresses the downstream activation of NFAT through inactivating Cn. The NFAT family includes five members, NFAT1 to NFAT5, which are all regulated by Ca2+/Cn signal except NFAT5.[8] Burn et al.'s study suggested that all five NFATs would express in the mouse metanephros from gestation day 11.5 to day 15.5.[9] Metanephros development in mice begins on gestation day 10.5 and about 85% of the METs are achieved on gestation day 16.5. Thus, in our in vivo study, the time period for the subcutaneous injection of CsA was chosen from gestation day 10.5 to day 16.5 to cover the important phase of metanephros development. The residual value of CsA was 397 ± 46 ng/ml in our study for the CsA group, which was collected on gestation day 17.5 which was similar to the values of human patients who received CsA for treatment. CsA exposure during gestation day 10.5 to day 16.5 in our study showed to reduce the embryo kidney weight, which was consistent with the decreased embryo weight. On histological appearance, the effect presented as attenuation of the cortical and nephrogenic zones. The compensatory hypertrophy of glomeruli accompanying with the decreased density might explain the relationship between congenital low nephron numbers with postnatal hypertension and kidney disease. Our findings were consistent with Tendron et al.'s study, in which the exposure of CsA to rabbit gestation led to low birth weight and reduced number of nephrons.[7] As a nuclear antigen, Ki-67 reflected the proliferation activity of cells.[10] In our study, the Ki-67 nuclear positive cells were mainly located in the nephrogenic zone, which was just under the renal capsule, and it was in accordance with the wide distribution of progenitor cells in the area. CsA exposure in our study suppressed the proliferation activity of cells in nephrogenic zone, which led to the decreased density of glomeruli. Michael et al.'s study also suggested that cell proliferation was an important mechanism of ureteric bud branching.[11] TUNEL assay detect cell apoptosis through labeling the 3’-OH end of fractured DNA.[12] The large amounts of apoptosis cells were also mainly located in the nephrogenic zone, which suggested that the inhibition of the Wnt signaling pathway resulted in apoptosis besides suppressing cell proliferation. Previous evidence also showed that cell survival played a key role in ureteric bud branching and nephron formation.[13]
WT-1, Pax2, Pax8, and Lim-1 were important genes which were associated with metanephros development. WT-1 gene mainly expresses in the progenitor cells of cap-stage mesenchymal and pronephric tubule aggregate during early metanephros development and then it will be confined to podocyte. Studies suggest that the pivotal role of WT-1 is to induce the ureteric bud and METs.[14,15,16] Only Pax2 and Pax8 of the Pax gene family express in the developing kidney.[17] The expression of the Pax2 and Pax8 genes is mainly distributed in the period of renal capsule body, coma-shaped body, and S-shaped body, which suggests their role in cell proliferation. In Narlis et al.'s study, severe renal dysplasia occurred in the Pax2/Pax8 knockout mouse.[18] Lim-1 gene expression is mainly distributed in the tips of ureteric bud, renal capsule body, coma-shaped body, and S-shaped body during early metanephros development. Considerable evidences also suggest that it has an important role in the development of the ureteric bud and nephron formation.[19,20,21] Dziarmaga et al.'s study indicated that the expression of Pax2 was associated with cell apoptosis and the inhibition of its expression could induce cell apoptosis.[22] In our study, CsA exposure suppressed the expression of WT-1, Pax2, and Pax8, but not Lim-1. A possible explanation for our study is that the inhibition of the Wnt signaling pathway might suppress the kidney development due to the expression inhibition of genes which are associated with metanephros development and cell proliferation and survival.[23,24]
We further studied the effect of CsA on metanephros development in an in vitro experiment. In the control group, the ureteric bud branched as incubation time went on and finally mature nephrons formed surrounding the tips of the ureteric bud. Both the ureteric bud branching morphogenesis and nephron formation were inhibited in the CsA-treated group. The fact that ureteric bud branching could recover from the incubation with CsA after replacing the culture in our study suggests that the suppression effect of CsA might be not from its direct cytotoxicity.
In conclusion, results in the present study showed that treatment of CsA suppressed metanephros development in the pregnant mice. Further studies are needed to clarify more details about the role of CsA in the regulation of the Wnt pathway in the metanephros development and its interaction with other signaling pathways needs be further explored.
Financial support and sponsorship
This study was supported by a grant from the National Basic Research Program of China (No. 2011CB944002).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Rezzani R. Cyclosporine A and adverse effects on organs: Histochemical studies. Prog Histochem Cytochem. 2004;39:85–128. doi: 10.1016/j.proghi.2004.04.001. doi: 10.1016/j.proghi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Bar Oz B, Hackman R, Einarson T, Koren G. Pregnancy outcome after cyclosporine therapy during pregnancy: A meta-analysis. Transplantation. 2001;71:1051–5. doi: 10.1097/00007890-200104270-00006. doi: 10.1097/00007890-200104270-00006. [DOI] [PubMed] [Google Scholar]
- 3.Papaccio G, Esposito V. Ciclosporin administration during pregnancy induces ultrastructural changes on pancreatic beta-cells of newborn rats. Acta Anat (Basel) 1990;137:336–41. doi: 10.1159/000146905. doi: 10.1159/000146905. [DOI] [PubMed] [Google Scholar]
- 4.Slabiak-Blaz N, Adamczak M, Gut N, Grajoszek A, Nyengaard JR, Ritz E, et al. Administration of cyclosporine A in pregnant rats – The effect on blood pressure and on the glomerular number in their offspring. Kidney Blood Press Res. 2015;40:413–23. doi: 10.1159/000368515. doi: 10.1159/000368515. [DOI] [PubMed] [Google Scholar]
- 5.Dihazi GH, Jahn O, Tampe B, Zeisberg M, Müller C, Müller GA, et al. Proteomic analysis of embryonic kidney development: Heterochromatin proteins as epigenetic regulators of nephrogenesis. Sci Rep. 2015;5:13951. doi: 10.1038/srep13951. doi: 10.1038/srep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De A. Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim Biophys Sin (Shanghai) 2011;43:745–56. doi: 10.1093/abbs/gmr079. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- 7.Tendron A, Decramer S, Justrabo E, Gouyon JB, Semama DS, Gilbert T. Cyclosporin A administration during pregnancy induces a permanent nephron deficit in young rabbits. J Am Soc Nephrol. 2003;14:3188–96. doi: 10.1097/01.asn.0000095637.13193.89. doi: 10.1097/01.ASN.0000095637.13193.89. [DOI] [PubMed] [Google Scholar]
- 8.Macian F. NFAT proteins: Key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–84. doi: 10.1038/nri1632. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 9.Burn SF, Webb A, Berry RL, Davies JA, Ferrer-Vaquer A, Hadjantonakis AK, et al. Calcium/NFAT signalling promotes early nephrogenesis. Dev Biol. 2011;352:288–98. doi: 10.1016/j.ydbio.2011.01.033. doi: 10.1016/j.ydbio.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heatley MK, Gatter KC. Ki67 protein: The immaculate deception? Histopathology. 2002;40:483. doi: 10.1046/j.1365-2559.2002.01390.x. doi: 10.1046/j.1365.2559.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 11.Michael L, Davies JA. Pattern and regulation of cell proliferation during murine ureteric bud development. J Anat. 2004;204:241–55. doi: 10.1111/j.0021-8782.2004.00285.x. doi: 10.1111/j.0021-8782.2004.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loo DT. In situ detection of apoptosis by the TUNEL assay: An overview of techniques. Methods Mol Biol. 2011;682:3–13. doi: 10.1007/978-1-60327-409-8_1. doi: 10.1007/978-1-60327-409-8_1. [DOI] [PubMed] [Google Scholar]
- 13.Dziarmaga A, Clark P, Stayner C, Julien JP, Torban E, Goodyer P, et al. Ureteric bud apoptosis and renal hypoplasia in transgenic PAX2-Bax fetal mice mimics the renal-coloboma syndrome. J Am Soc Nephrol. 2003;14:2767–74. doi: 10.1097/01.asn.0000094082.11026.ee. doi: 10.1097/01.ASN.0000094082.11026.EE. [DOI] [PubMed] [Google Scholar]
- 14.Menke AL, Schedl A. WT1 and glomerular function. Semin Cell Dev Biol. 2003;14:233–40. doi: 10.1016/s1084-9521(03)00026-0. doi: 10.1016/S1084-9521(03)00026-0. [DOI] [PubMed] [Google Scholar]
- 15.Kreidberg JA. WT1 and kidney progenitor cells. Organogenesis. 2010;6:61–70. doi: 10.4161/org.6.2.11928. doi: 10.4161/org.6.2.11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen X, Jiang H, Ying M, Xie Z, Li X, Wang H, et al. Calcineurin inhibitors cyclosporin A and tacrolimus protect against podocyte injury induced by puromycin aminonucleoside in rodent models. Sci Rep. 2016;6:32087. doi: 10.1038/srep32087. doi: 10.1038/srep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torban E, Goodyer P. What PAX genes do in the kidney. Exp Nephrol. 1998;6:7–11. doi: 10.1159/000020498. doi: 10.1159/000020498. [DOI] [PubMed] [Google Scholar]
- 18.Narlis M, Grote D, Gaitan Y, Boualia SK, Bouchard M. Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J Am Soc Nephrol. 2007;18:1121–9. doi: 10.1681/ASN.2006070739. doi: 10.1681/ASN.2006070739. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen A, Skjong C, Shawlot W. Lim 1 is required for nephric duct extension and ureteric bud morphogenesis. Dev Biol. 2005;288:571–81. doi: 10.1016/j.ydbio.2005.09.027. doi: 10.1016/j.ydbio.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y-T, Kobayashi A, Kwan KM, Johnson RL, Behringer RR. Gene expression profiles in developing nephrons using Lim1 metanephric mesenchyme-specific conditional mutant mice. BMC Nephrol. 2006;7:1. doi: 10.1186/1471-2369-7-1. doi: 10.1186/1471-2369-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto M, Cui L, Johkura K, Asanuma K, Okouchi Y, Ogiwara N, et al. Branching ducts similar to mesonephric ducts or ureteric buds in teratomas originating from mouse embryonic stem cells. Am J Physiol Renal Physiol. 2006;290:F52–60. doi: 10.1152/ajprenal.00001.2004. doi: 10.1152/ajprenal.00001.2004. [DOI] [PubMed] [Google Scholar]
- 22.Dziarmaga A, Eccles M, Goodyer P. Suppression of ureteric bud apoptosis rescues nephron endowment and adult renal function in Pax2 mutant mice. J Am Soc Nephrol. 2006;17:1568–75. doi: 10.1681/ASN.2005101074. doi: 10.1681/ASN.2005101074. [DOI] [PubMed] [Google Scholar]
- 23.Xue Y, Jiang L, Wan WG, Chen YM, Zhang J, Zhang ZC. Cytomegalovirus pneumonia in patients with rheumatic diseases after immunosuppressive therapy: A single center study in China. Chin Med J. 2016;129:267–73. doi: 10.4103/0366-6999.174490. doi: 10.4103/0366-6999.174490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao Z, Shan J, Li C, Luo L, Lu J, Li S, et al. Mechanisms of cyclosporine-induced renal cell apoptosis: A systematic review. Am J Nephrol. 2013;37:30–40. doi: 10.1159/000345988. doi: 10.1159/000345988. [DOI] [PubMed] [Google Scholar]