Abstract
Background:
Acute kidney injury (AKI) is the most common and life-threatening systemic complication of rhabdomyolysis. Inflammation plays an important role in the development of rhabdomyolysis-induced AKI. This study aimed to investigate the kidney model of AKI caused by rhabdomyolysis to verify the role of macrophage Toll-like receptor 4/nuclear factor-kappa B (TLR4/NF-κB) signaling pathway.
Methods:
C57BL/6 mice were injected with a 50% glycerin solution at bilateral back limbs to induce rhabdomyolysis, and CLI-095 or pyrrolidine dithiocarbamate (PDTC) was intraperitoneally injected at 0.5 h before molding. Serum creatinine levels, creatine kinase, the expression of tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6, and hematoxylin and eosin stainings of kidney tissues were tested. The infiltration of macrophage, mRNA levels, and protein expression of TLR4 and NF-κB were investigated by immunofluorescence double-staining techniques, reverse transcriptase-quantitative polymerase chain reaction, and Western blotting, respectively. In vitro, macrophage RAW264.7 was stimulated by ferrous myoglobin; the cytokines, TLR4 and NF-κB expressions were also detected.
Results:
In an in vivo study, using CLI-095 or PDTC to block TLR4/NF-κB, functional and histologic results showed that the inhibition of TLR4 or NF-κB alleviated glycerol-induced renal damages (P < 0.01). CLI-095 or PDTC administration suppressed proinflammatory cytokine (TNF-α, IL-6, and IL-1β) production and macrophage infiltration into the kidney (P < 0.01). Moreover, in an in vitro study, CLI-095 or PDTC suppressed myoglobin-induced expression of TLR4, NF-κB, and proinflammatory cytokine levels in macrophage RAW264.7 cells (P < 0.01).
Conclusion:
The pharmacological inhibition of TLR4/NF-κB exhibited protective effects on rhabdomyolysis-induced AKI by the regulation of proinflammatory cytokine production and macrophage infiltration.
Keywords: Acute Kidney Injury, Macrophages, Rhabdomyolysis, Toll-Like Receptor 4, NF-kappa B
INTRODUCTION
Rhabdomyolysis is a syndrome characterized by the breakdown of skeletal muscle and leakage of the muscle contents, including electrolytes, myoglobin, and other sarcoplasmic proteins, into the bloodstream.[1] Acute kidney injury (AKI) is the most common and life-threatening systemic complication of rhabdomyolysis, with rhabdomyolysis-induced AKI comprising 10% of all AKI cases.[2] Apart from classical supportive care strategies (e.g., intravenous fluid therapy and renal replacement therapy), effective therapies for rhabdomyolysis-induced AKI are limited.[3] Myoglobin-induced renal toxicity plays a key role in rhabdomyolysis-associated kidney damage,[4] and considerable evidence has demonstrated that significant inflammation is involved in the development and progression of rhabdomyolysis-induced AKI.[5,6,7]
Macrophages, one of the most dominant and widely distributed inflammatory cells, are involved in the initiation and maintenance of acute inflammatory response,[8] and macrophage infiltration into kidney has been found in rhabdomyolysis. Moreover, macrophage depletion ameliorates glycerol-induced AKI in mice.[5,6] Toll-like receptors (TLRs) are a family of pathogen recognition receptors that play a vital role in the innate immune system.[9,10] In addition, TLRs are activated by endogenous agonists of nonmicrobial origin and involved in noninfectious inflammatory conditions.[11] TLR4 activation results in the release of nuclear factor-kappa B (NF-κB), allowing NF-κB translocation from cytoplasm to nucleus, where it mediates the increase in cytokine gene expressions leading to proinflammatory responses.[12,13]
However, the role of TLR4/NF-κB in rhabdomyolysis-induced AKI is unclear, in addition to whether or not myoglobin activates the macrophage TLR4/NF-κB pathway. In this study, we used the glycerol-induced AKI model and myoglobin-stimulated macrophage cell line to address these questions and examine potential therapies for rhabdomyolysis-induced AKI.
METHODS
Animals and drug administration
The animal protocol was approved by the Animal Care and Use Committee of Sichuan University, and all the animal care and experimental procedures were conducted in accordance with The Guide for the Care and Use of Laboratory Animals. C57BL/6 male mice (8–10 weeks old; 25–30 g) were purchased from the Animal Laboratory Center of Sichuan University (Chengdu, China). TLR4 inhibitor CLI-095 and NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) were purchased separately from InvivoGen, Inc. (San Diego, CA, USA), and Tocris, Inc. (Minneapolis, MN, USA).
After 1 week of adaptation to the housing conditions, the mice were randomly divided into four groups (n = 6 per group): control group, glycerol-treated group, glycerol + CLI-095 group, and glycerol + PDTC group. The mice in the latter three groups were injected with 50% glycerol dissolved in 0.9% normal saline (10 ml/kg) at bilateral back limbs to stimulate the rhabdomyolysis-induced AKI model. The same volume of saline was injected in the mice of control group. To investigate the effect of TLR4 inhibitor and NF-κB inhibitor on rhabdomyolysis-induced AKI, in the glycerol + CLI-095 group, CLI-095 dissolved in dimethyl sulfoxide (DMSO) (Sigma Aldrich, China) at a dosage of 1 mg/ml was injected intraperitoneally 30 min before the injection of glycerol. In the glycerol + PDTC group, 100 mmol/L of PDTC was administered in the same way. The control group received the same volume of saline containing DMSO. The mice were sacrificed at 2 h, 8 h, 24 h, and 48 h after glycerol exposure. Terminal blood samples and kidney tissues were collected for further investigations.
Renal function
Serum creatinine levels (SCr) were evaluated by high-performance liquid chromatography, conducted by the Institute of Drug Clinical Trial and the GCP Center of West China Hospital, to assess renal function. Creatine kinase (CK) was measured in the same way.
Histology
Formalin-fixed, paraffin-embedded kidney sections (4 μm) were stained with hematoxylin and eosin (H&E). Tissue damage was scored on a scale of 0–4, with 0, 1, 2, 3, and 4 corresponding to 0%, <25%, 26–50%, 51–75%, and >76% of injured/damaged renal tubules, respectively. Ten fields of ×40 magnification were examined and averaged. To quantify macrophage infiltration, sections were stained with rat anti-mouse F4/80 antibody (Abcam, Inc., Cambridge, MA, USA) (1:50 dilution). Stained sections were photographed, and five ×40 fields of positive cells were examined for quantitation of macrophage.
Cell culture
Immortalized mouse macrophages (RAW264.7 cell) (ATCC, Rockville, MD, USA) were cultured in RPMI-1640 medium (Hyclone, Inc., Beijing, China), containing 10% fetal bovine serum (Hyclone, Inc., Beijing, China), and incubated at 37°C in a humidified atmosphere of air/CO2 (95:5). We divided the cells in exponential growth state into four groups: myoglobin-treated group, incubated with 25, 50, 100, and 200 μmol/L ferrous myoglobin for 24 h; myoglobin + CLI-095 group, incubated with CLI-095 at 10 μg/ml 30 min prior to myoglobin treatment; myoglobin + PDTC group, incubated with PDTC at 100 μmol/L 30 min before myoglobin treatment; and control group, cells were incubated with complete medium alone in the control group. Ferrous myoglobin was prepared as described previously.[14]
Western blotting analysis
Proteins were extracted from renal tissues or cultured cells with a radio immune precipitation lysis buffer (P0013B, Beyotime Biotechnology, China). Samples with equal amounts of total protein (30 mg/ml) were processed for Western blotting, as described previously,[14] using antibodies against TLR4 and NF-κB (Abcam, Cambridge, MA, USA), final dilution 1:500. The ratio of the protein examined was normalized against β-actin. The β-actin antibody and all secondary antibodies were purchased from R&D Systems (MI, USA).
Gene expression quantification
Total RNA was extracted from renal tissues and cultured cells using an RNeasy kit according to the manufacturer's instructions (Takara, Japan). The RNA concentration and purity were confirmed with Nanodrop 2000. The RNA quality was tested by agarose gel electrophoresis followed by cDNA synthesis. Quantitative polymerase chain reaction (qPCR) was performed with the CFX96TM Real-Time System (Bio-Rad, Hercules, USA) and SYBR Premix Ex TaqTM II (Tli RNase H Plus) (Takara). The primers were synthesized by Invitrogen and were as follows: TLR4 (upstream primer) 5’-GGCAGCAGGTCGAATTGTAT-3’, (downstream primer) 5’-TCAAGGCTTTTCCATCCAAC-3’; NF-κB (upstream primer) 5’-CGGAATGTGCAGATGCAT-3’, (downstream primer) 5’-ACCCCCACTACTCTTGCGGCA-3’; and GAPDH (endogenous control) forward (5’-AGAAGGCTGGGGCTCATTTG-3’), reverse (5’-AGG GGC CATCCACAGTCTTC-3’). Gene expression was analyzed using the 2−ΔΔCt method by normalizing with GAPDH gene expression in all experiments.
ELISA analysis
Serum and culture supernatants interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α levels were measured using the respective commercially available kits (R&D Systems, Inc., MI, USA) according to the manufacturers’ protocols.
Statistical analysis
Data were expressed as mean ± standard deviation. Multiple comparisons of means among groups were examined through one-way analysis of variance; a two-sided P < 0.05 was considered statistically significant. Between-group comparisons of means were analyzed by least significant difference t-tests, and P values were compared to a Bonferroni-corrected significance level of 0.0167 to control the overall significance level for the tests at a family-wise error rate of 0.05. We used SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) for statistical analyses.
RESULTS
Toll-like receptor4 inhibitor CLI-095 and nuclear factor-kappa B inhibitor pyrrolidine dithiocarbamate administration suppressed rhabdomyolysis-induced acute kidney dysfunction
As shown in Figure 1a, glycerol injection caused time-dependent kidney dysfunction with increased SCr, and the peak was observed at 24 h (1.09 ± 0.13 mg/dl). Glycerol-induced muscle damage was indicated by increased serum CK levels [t = 14.83, P < 0.0001; Figure 1b]. Respective administration of TLR4 inhibitor CLI-095 and NF-κB inhibitor PDTC significantly ameliorated kidney injury after glycerol injection (t = 15.7, P < 0.0001 and t = 16.11, P < 0.0001). The H&E-stained section showed less tubular necrosis, cast formation, and infiltration of inflammatory cells in the glycerol + CLI-095 and glycerol + PDTC groups as compared to the glycerol-treated group [Figure 1d], which was further supported by quantification of the kidney injury score [t = 12.19, P < 0.0001 and t = 11.91, P < 0.0001; Figure 1c].
Figure 1.
Effects of Toll-like receptor 4 inhibitor CLI-095 or nuclear factor-kappa B inhibitor pyrrolidine dithiocarbamate on rhabdomyolysis-induced AKI. (a) Serum creatinine levels at different time after various treatments. (b) Creatine kinase levels increased in response to glycerol injection. (c) Semiquantitative scoring of tubular injury. (d) Twenty-four hours after various treatment, kidney tissue was processed for H&E staining (overview: original magnification ×100; detail: original magnification ×400) *P < 0.01 versus control; †P < 0.01 versus glycerol treated (n = 3–6 per group). AKI: Acute kidney injury, CK: Creatine kinase.
CLI-095 and pyrrolidine dithiocarbamate downregulated renal Toll-like receptor 4 and nuclear factor-kappa B expression
Twenty-four hours after glycerol injection, the results of Western blotting and reverse transcriptase-qPCR (RT-qPCR) analysis indicated that the expression of TLR4 and NF-κB was significantly increased in glycerol-treated group [P < 0.01; Figure 2]. Respective administration of CLI-095 and PDTC reduced the expression of TLR4 and NF-κB in the glycerol + CLI-095 and glycerol + PDTC groups when compared to the glycerol-treated group (P < 0.01).
Figure 2.
CLI-095 or pyrrolidine dithiocarbamate downregulated TLR4 and NF-κB expression. Twenty-four hours after glycerol injection, TLR4 and NF-κB expression in kidney were quantified by Western blotting (a) and reverse transcriptase-quantitative polymerase chain reaction (d). Quantification of TLR4 (b) and NF-κB (c) expression in the kidney. *P < 0.01 versus control; †P < 0.05 (P = 0.015) versus glycerol-treated; ‡P < 0.01 versus glycerol treated (n = 4–6 per group). TLR4: Toll-like receptor 4; NF-κB: Nuclear factor-kappa B.
CLI-095 and pyrrolidine dithiocarbamate administration suppressed rhabdomyolysis-induced kidney inflammation
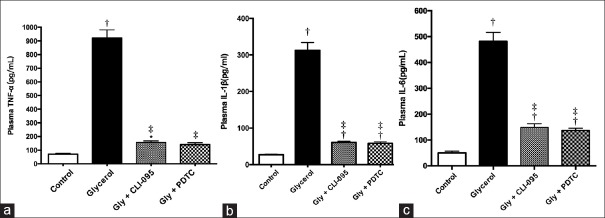
Since previous studies have shown that the TLR4/NF-κB signaling pathway regulates inflammation,[9,10,11] whether CLI-095 or PDTC treatment exhibited influence on glycerol-induced proinflammatory cytokine production and macrophage infiltration were investigated. As shown in Figure 3, glycerol injection significantly increased TNF-α, IL-6, and IL-1β levels in plasma, which was suppressed by CLI-095 or PDTC treatment (P < 0.01). Consistent with the plasma data, macrophage infiltration into the kidney was also inhibited by CLI-095 or PDTC as compared to glycerol treatment [t = 11.21, P < 0.0001; and t = 12.14, P < 0.0001; Figure 4].
Figure 3.
CLI-095 or pyrrolidine dithiocarbamate suppressed glycerol-induced increase in proinflammatory cytokine expression. Twenty-four hours after glycerol administration, cytokine levels in plasma were quantified by ELISA. IL-1β (a), IL-6 (b), tumor necrosis factor-α (c) levels in plasma. *P < 0.05 (P = 0.010) versus control; †P < 0.01 versus control; ‡P < 0.01 versus glycerol treated (n = 3–6 per group). IL: Interleukin.
Figure 4.
CLI-095 or pyrrolidine dithiocarbamate suppressed glycerol-induced macrophage infiltration into the kidney. (a-d) Immunohistochemical localization of macrophage infiltration (red arrows) at 24 h after different treatments (original magnification, ×400). (a) Saline-treated kidney. (b) Glycerol-treated kidney. (c) Glycerol + CLI-095-treated kidney. (d) Glycerol + pyrrolidine dithiocarbamate-treated kidney. (e) Quantification of infiltrated macrophage in the kidney. *P < 0.01 versus control; †P < 0.01 versus glycerol treated (n = 3–6 per group).
CLI-095 and pyrrolidine dithiocarbamate downregulated Toll-like receptor 4 and nuclear factor-kappa B expression in macrophage
As shown in Figure 5, Western blotting and RT-qPCR analysis indicated that myoglobin at 200 μmol/L for 24 h significantly upregulated TLR4 and NF-κB expression in RAW264.7 cells (P < 0.01). Pretreatment with CLI-095 or PDTC effectively lowered TLR4 and NF-κB expression (P < 0.01).
Figure 5.
CLI-095 or pyrrolidine dithiocarbamate down-regulated TLR4 and NF-κB expression in RAW264.7 cells. Twenty-four hours after ferrous myoglobin (200 μmol/L) administration, TLR4 and NF-κB expression in RAW264.7 cells were quantified by Western blotting (a) and reverse transcriptase-quantitative polymerase chain reaction (d). Quantification of TLR4 (b) and NF-κB (c) expression in RAW264.7 cells. *P < 0.01 versus control; †P < 0.01 versus glycerol treated. TLR4: Toll-like receptor 4; NF-κB: Nuclear factor-kappa B.
CLI-095 and pyrrolidine dithiocarbamate suppressed myoglobin-induced cytokine excretion in RAW264.7 cells
Ferrous myoglobin-induced proinflammatory cytokine excretion in RAW264.7 cells was suppressed by CLI-095 and PDTC, respectively (P < 0.01). ELISA results showed lower IL-1β, IL-6, and TNF-α levels in the myoglobin + CLI-095 and myoglobin + PDTC groups when compared to the myoglobin-treated group [P < 0.01; Figure 6].
Figure 6.
CLI-095 or pyrrolidine dithiocarbamate suppressed myoglobin-induced increase in proinflammatory cytokine expression in RAW264.7. Twenty-four hours after ferrous myoglobin administration, cytokine levels in supernatant were quantified by ELISA. IL-1β (a), IL-6 (b), tumor necrosis factor-α (c) levels in supernatant. *P < 0.05 (P = 0.014) versus control; †P < 0.01 versus control; ‡P < 0.01 versus glycerol treated. IL: Interleukin.
DISCUSSION
Myoglobin, a key pathogenic factor in rhabdomyolysis-induced AKI, leads to the development and progression of AKI via different mechanisms.[15] It is acknowledged that inflammation plays a major role in rhabdomyolysis-induced AKI. In the rhabdomyolysis-induced AKI mice model, using CLI-095 or PDTC to block TLR4/NF-κB, functional and histologic results showed that the inhibition of TLR4 or NF-κB alleviated glycerol-induced renal damages. CLI-095 or PDTC administration also suppressed proinflammatory cytokine (TNF-α, IL-6, and IL-1β) production and macrophage infiltration into the kidney. Moreover, in an in vitro study, CLI-095 or PDTC suppressed myoglobin-induced expression of TLR4, NF-κB, and proinflammatory cytokine levels in macrophage RAW264.7 cells.
Previous studies revealed that the depletion of the macrophage reduced NF-κB levels and restored the levels of other inflammatory markers.[6] In our study, the results showed that when TLR4/NF-κB was effectively inhibited, glycerol-induced macrophage infiltration into the kidney and proinflammatory cytokine expression was also suppressed. A previous study demonstrated that TLR4 levels were markedly downregulated in rhabdomyolysis-induced AKI kidneys,[16] which contradicted our results. However, as mentioned in that paper, their findings do not exclude increased TLR signaling, as it remained possible that the activity of residual TLR units in AKI kidneys could be enhanced.[16] Further, increased NF-κB expression in rhabdomyolysis-induced AKI kidneys has been observed in previous studies,[17,18,19] consistent with our findings, whereas it has been reported that in vitro gene expression of NF-κB showed no significant rise in kidney epithelium cells under 50 μmol/L myoglobin administration.[20] In our study, a higher myoglobin concentration and a different cell line were used. What's more, TLR4/NF-κB has also been found activated in ischemic kidney damage, glomerulonephritis, diabetes nephropathy, and renal allograft rejection.[21,22,23,24] The TLR4 inhibitor and NF-κB inhibitor have been applied to attenuate kidney injury in different models;[25,26,27] however, little is known about the effect of the TLR4/NF-κB antagonist on rhabdomyolysis-induced kidney injury, which was clarified by our study.
Both our study and other research have demonstrated that macrophage infiltration was involved in rhabdomyolysis-induced AKI.[5,6,9] It has been found that myoglobin prompted tubular cells to secrete macrophage recruiting chemokines, where in response to these chemoattractants, blood monocytes migrated to kidney. In the renal interstitium, myoglobin could directly activate macrophages with the overexpression of the inflammasome component (NLRP3) and proinflammatory factors (IL-1β),[5] which was consistent with our results. Except for macrophage depletion, there was no way reported to block myoglobin-induced proinflammatory cytokine production in macrophage, and our results indicated that TLR4/NF-κB played a role in proinflammatory cytokine production in macrophage.
Above all, in rhabdomyolysis-induced AKI, kidney inflammation is a vicious circle. Inflammatory cells and proinflammatory cytokine, together with intrinsic kidney cells, interacted with each other, thus resulting in the maintenance of a proinflammatory status. Our findings suggested that the pharmacological inhibition of TLR4/NF-κB provides a potential target to break the loop.
In summary, this study indicates that CLI-095 and PDTC exhibit renoprotective effects in the development of rhabdomyolysis-induced AKI, and importantly, pharmacological inhibition of TLR4/NF-κB suppresses macrophage infiltration and proinflammatory cytokine production. This might provide a new therapy for the treatment of rhabdomyolysis-induced AKI.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundation of China (No. 81570668, No. 81500524) and the Sichuan Science and Technology Support Program (No. 2015SZ0135).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Warren JD, Blumbergs PC, Thompson PD. Rhabdomyolysis: A review. Muscle Nerve. 2002;25:332–47. doi: 10.1002/mus.10053. doi: 10.1002/mus.10053. [DOI] [PubMed] [Google Scholar]
- 2.Bagley WH, Yang H, Shah KH. Rhabdomyolysis. Intern Emerg Med. 2007;2:210–8. doi: 10.1007/s11739-007-0060-8. doi: 10.1007/s11739-007-0060-8. [DOI] [PubMed] [Google Scholar]
- 3.Chavez LO, Leon M, Einav S, Varon J. Beyond muscle destruction: A systematic review of rhabdomyolysis for clinical practice. Crit Care. 2016;20:135. doi: 10.1186/s13054-016-1314-5. doi: 10.1186/s13054-016-1314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panizo N, Rubio-Navarro A, Amaro-Villalobos JM, Egido J, Moreno JA. Molecular mechanisms and novel therapeutic approaches to rhabdomyolysis-induced acute kidney injury. Kidney Blood Press Res. 2015;40:520–32. doi: 10.1159/000368528. doi: 10.1159/000368528. [DOI] [PubMed] [Google Scholar]
- 5.Belliere J, Casemayou A, Ducasse L, Zakaroff-Girard A, Martins F, Iacovoni JS, et al. Specific macrophage subtypes influence the progression of rhabdomyolysis-induced kidney injury. J Am Soc Nephrol. 2015;26:1363–77. doi: 10.1681/ASN.2014040320. doi: 10.1681/ASN.2014040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JH, Lee DW, Jung MH, Cho HS, Jeon DH, Chang SH, et al. Macrophage depletion ameliorates glycerol-induced acute kidney injury in mice. Nephron Exp Nephrol. 2014;128:21–9. doi: 10.1159/000365851. doi: 10.1159/000365851. [DOI] [PubMed] [Google Scholar]
- 7.Komada T, Usui F, Kawashima A, Kimura H, Karasawa T, Inoue Y, et al. Role of NLRP3 inflammasomes for rhabdomyolysis-induced acute kidney injury. Sci Rep. 2015;5:10901. doi: 10.1038/srep10901. doi: 10.1038/srep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–6. doi: 10.2174/1568010054022024. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Takeda K, Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 10.Gluba A, Banach M, Hannam S, Mikhailidis DP, Sakowicz A, Rysz J, et al. The role of toll-like receptors in renal diseases. Nat Rev Nephrol. 2010;6:224–35. doi: 10.1038/nrneph.2010.16. doi: 10.1038/nrneph.2010.16. [DOI] [PubMed] [Google Scholar]
- 11.Beutler B. Inferences, questions and possibilities in toll-like receptor signalling. Nature. 2004;430:257–63. doi: 10.1038/nature02761. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 12.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–58. doi: 10.1038/nri1630. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 13.O’Neill LA. How toll-like receptors signal: What we know and what we don’t know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Feng YY, Ma L, Liu LF, Hong HG, Zhang XM, Guo F, et al. Rhabdomyolysis induced AKI via the regulation of endoplasmic reticulum stress and oxidative stress in PTECs. Rsc Adv. 2016;6:109639–48. doi: 10.1039/c6ra18865f. [Google Scholar]
- 15.Holt SG, Moore KP. Pathogenesis and treatment of renal dysfunction in rhabdomyolysis. Intensive Care Med. 2001;27:803–11. doi: 10.1007/s001340100878. doi: 10.1007/s001340100878. [DOI] [PubMed] [Google Scholar]
- 16.Zager RA, Johnson AC, Lund S, Hanson S. Acute renal failure: Determinants and characteristics of the injury-induced hyperinflammatory response. Am J Physiol Renal Physiol. 2006;291:F546–56. doi: 10.1152/ajprenal.00072.2006. doi: 10.1152/ajprenal.00072.2006. [DOI] [PubMed] [Google Scholar]
- 17.Yang FL, Subeq YM, Chiu YH, Lee RP, Lee CJ, Hsu BG, et al. Recombinant human erythropoietin reduces rhabdomyolysis-induced acute renal failure in rats. Injury. 2012;43:367–73. doi: 10.1016/j.injury.2011.11.013. doi: 10.1016/j.injury.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Deng LL, Zhong L, Lei JR, Tang L, Liu L, Xie SQ, et al. Protective effect of lipoxin A4 against rhabdomyolysis-induced acute kidney injury in rats (in Chinese) Chin J Cell Mol Imm. 2012;28:907–10. doi: 10.13423/j.cnki.cjcmi.006567. [PubMed] [Google Scholar]
- 19.Subeq YM, Wu WT, Lee CJ, Lee RP, Yang FL, Hsu BG, et al. Pentobarbital reduces rhabdomyolysis-induced acute renal failure in conscious rats. J Trauma. 2009;67:132–8. doi: 10.1097/TA.0b013e318186253d. doi: 10.1097/TA.0b013e318186253d. [DOI] [PubMed] [Google Scholar]
- 20.Shanu A, Parry SN, Wood S, Rodas E, Witting PK. The synthetic polyphenol tert-butyl-bisphenol inhibits myoglobin-induced dysfunction in cultured kidney epithelial cells. Free Radic Res. 2010;44:843–53. doi: 10.3109/10715762.2010.485993. doi: 10.3109/10715762.2010.485993. [DOI] [PubMed] [Google Scholar]
- 21.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–59. doi: 10.1172/JCI31008. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown HJ, Lock HR, Wolfs TG, Buurman WA, Sacks SH, Robson MG, et al. Toll-like receptor 4 ligation on intrinsic renal cells contributes to the induction of antibody-mediated glomerulonephritis via CXCL1 and CXCL2. J Am Soc Nephrol. 2007;18:1732–9. doi: 10.1681/ASN.2006060634. doi: 10.1681/ASN.2006060634. [DOI] [PubMed] [Google Scholar]
- 23.Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, Au WS, et al. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol. 2012;23:86–102. doi: 10.1681/ASN.2010111210. doi: 10.1681/ASN.2010111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ducloux D, Deschamps M, Yannaraki M, Ferrand C, Bamoulid J, Saas P, et al. Relevance of toll-like receptor-4 polymorphisms in renal transplantation. Kidney Int. 2005;67:2454–61. doi: 10.1111/j.1523-1755.2005.00354.x. doi: 10.1111/j.1523-1755.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin M, Yiu WH, Li RX, Wu HJ, Wong DW, Chan LY, et al. The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int. 2013;83:887–900. doi: 10.1038/ki.2013.11. doi: 10.1038/ki.2013.11. [DOI] [PubMed] [Google Scholar]
- 26.Leon CG, Tory R, Jia J, Sivak O, Wasan KM. Discovery and development of toll-like receptor 4 (TLR4) antagonists: A new paradigm for treating sepsis and other diseases. Pharm Res. 2008;25:1751–61. doi: 10.1007/s11095-008-9571-x. doi: 10.1007/s11095-008-9571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coppo R. Proteasome inhibitors in progressive renal diseases. Nephrol Dial Transplant. 2014;29 Suppl 1:i25–30. doi: 10.1093/ndt/gft271. doi: 10.1093/ndt/gft271. [DOI] [PubMed] [Google Scholar]