Abstract
Background:
PM2.5 (aerodynamic diameter ≤ 2.5 μm) is a dominant and ubiquitous air pollutant that has become a global concern as PM2.5 exposure has been linked to many adverse health effects including cardiovascular and pulmonary diseases. Emerging evidence supports a correlation between increased air PM2.5 levels and skin disorders although reports on the underlying pathophysiological mechanisms are limited. Oxidative stress is the most common mechanism of PM2.5-induced adverse health effects. This study aimed to investigate PM2.5-induced oxidative damage and apoptosis in immortalized human keratinocyte (HaCaT) cells.
Methods:
HaCaT cells were exposed to 0, 25, 50, 100, or 200 μg/ml PM2.5 for 24 h. Reactive oxygen species (ROS) generation, lipid peroxidation products, antioxidant activity, DNA damage, apoptotic protein expression, and cell apoptosis were measured.
Results:
PM2.5 exposure (0–200 μg/ml) for 24 h resulted in increased ROS levels (arbitrary unit: 201.00 ± 19.28, 264.50 ± 17.91, 305.05 ± 19.57, 427.95 ± 18.32, and 436.70 ± 17.77) and malondialdehyde production (0.54 ± 0.05 nmol/mg prot, 0.61 ± 0.06 nmol/mg prot, 0.68 ± 0.05 nmol/mg prot, 0.70 ± 0.05 nmol/mg prot, and 0.76 ± 0.05 nmol/mg prot), diminished superoxide dismutase activity (6.47 ± 0.28 NU/mg prot, 5.97 ± 0.30 NU/mg prot, 5.15 ± 0.42 NU/mg prot, 4.08 ± 0.20 NU/mg prot, and 3.76 ± 0.37 NU/mg prot), and increased DNA damage and apoptosis in a dose-dependent manner in HaCaT cells. Moreover, cytochrome-c, caspase-3, and caspase-9 expression also increased proportionately with PM2.5 dosing.
Conclusion:
PM2.5 might elicit oxidative stress and mitochondria-dependent apoptosis that likely manifests as skin irritation and damage.
Keywords: Apoptosis, HaCaT Cells, Oxidative Stress, PM2.5, Skin Damage
INTRODUCTION
Dust-haze, a dominant and ubiquitous air pollution problem in China, has reached an alarming level and is now a global concern.[1] The emission of airborne particulate matter (PM), especially those with a mean aerodynamic diameter <2.5 μm (PM2.5), constitutes the primary causative agent that gives rise to haze. Owing to the rapid economic and urban development in China during the last few decades, the PM2.5 concentrations of fewer than 1% of China's 500 largest cities could meet the air quality standards (10 μg/m3 annual mean and 25 μg/m3 24 h mean) suggested by the World Health Organization (WHO) in accordance with the 2012 report of the Asian Development Bank.[2] Moreover, the concentrations of PM2.5 are higher in North than in South China because of the larger quantity of PM emissions and poorer pollution dispersion.[3] In particular, heavy metal, polyaromatic hydrocarbons, and other toxic substances are readily adsorbed to PM2.5 owing to its relatively small size and large surface area, which could reach some special biological sites and thereby lead to functional alteration, whereas larger PM cannot reach such sites.[4,5,6]
Specifically, a growing number of studies have investigated the effects of PM2.5 exposure on human health, the majority of which focused on respiratory and cardiovascular diseases as inhaled PM2.5 can diffuse into the blood through the microvasculature and then be transported throughout the body.[7,8] Large epidemiological studies have demonstrated that increasing hospital admissions, morbidity, and mortality from respiratory and cardiovascular diseases are associated with long-term exposure to high concentrations of PM2.5.[9,10,11,12] Similarly, accumulating evidence has linked PM2.5 exposure to an increased risk of cardiovascular diseases,[13,14] exacerbation of asthma,[15] chronic obstructive pulmonary disease,[13,16] and lung cancer.[17,18]
Along with the oral and respiratory routes, xenobiotics or chemicals can also enter into the body through dermal absorption.[19] Although the skin serves as the first barrier against harmful air pollutants, including PM2.5, its protective ability is limited. PM can penetrate the skin barrier and have a negative effect on human skin.[20,21] Krutmann et al.[20] have suggested that PM2.5 may induce or aggravate atopic dermatitis. In addition, recent epidemiological evidence demonstrated that long-term exposure to PM2.5 was significantly associated with premature skin aging and exacerbates preexistent skin diseases.[22,23] However, studies concerning the potential hazard of PM2.5 on skin are limited when compared to those on the respiratory and cardiovascular systems.
Previous studies have shown that oxidative stress is an important pathophysiological mechanism of PM2.5-induced respiratory and cardiovascular diseases.[24,25] Moreover, numerous studies have shown that exposure to air pollutants, including ultraviolet (UV), ozone (O3), cigarette smoke, and dust storm particles, could induce intracellular reactive oxygen species (ROS) accumulation, DNA damage, and apoptosis, which could manifest as skin damage and irritation.[26,27,28,29,30] Recently, exposure to concentrated ambient particles ranging in size from 0.1 to 2.5 μm was shown to induce ROS accumulation, lipid peroxidation, and keratinocyte apoptosis.[31] Thus, in this study, PM2.5 was collected from an atmospheric supersite in the Pearl River Delta region of China, which is the center of the mixture of primary pollutants and secondary pollutants conveyed from Guangfo region in the upwind area.
Oxidative stress is a common mechanism of PM2.5-induced adverse health effects and is known to play an important role in skin damage induced by other air pollutants. In addition, keratinocytes are important for the formation of a primary protective skin barrier against exogenous insults. As keratinocytes can produce numerous cytokines, conduct an immune response, and exhibit cell growth, they are commonly used as skin injury models.[32] Thus, this study focused on oxidative damage and apoptosis in an immortalized human keratinocyte (HaCaT) cell culture system using PM2.5 collected from an atmospheric supersite in the Pearl River Delta region of China, to assess the effect of PM2.5 exposure on cutaneous tissue damage. The key events involved in PM2.5-induced oxidative damage including ROS generation, lipid peroxidation products, antioxidant activity, DNA damage, apoptotic protein expression, and cell death were examined.
METHODS
PM2.5 sampling and preparation
PM2.5 samples were collected continuously at the atmospheric supersite in the Pearl River Delta region located in Taoyuan town, Heshan county, Jiangmen city of Guangdong province, China (112.929 °E, 22.728 °N) from December 4, 2014, to January 9, 2015.[33] The distance from Guangzhou, Foshan, and Jiangmen cities to the sampling site was 80, 50, and 30 km, respectively. The sampling site was located 3 km away from main roads and any industrial sources of pollution. During the monitoring period, the sampling site was downwind of the Guangfo region, a major source of PM2.5 emissions in the Pearl River Delta region, and was also subjected to the resulting secondary pollutants from atmospheric reactions. The major sources of PM2.5 were the mixture of primary pollutants and secondary pollutants that were conveyed from the Guangfo region in the upwind area. Daily PM2.5 samples were collected on Teflon filters (diameter = 47 mm; Whatman, Piscataway, NJ, USA) using a high-volume PM2.5 sampler (TH1000, Wuhan Tianhong Instruments Co., Ltd., Wuhan, China). The instrument was located at 15 m above ground level with a sampling flow rate of 1.05 m3/min.
After sampling, the Teflon filters were cut into 1 cm2 pieces, immersed in deionized water, and sonicated three times for 15 min at 40 kHz. The resulting suspensions were filtered through 6–8 pieces of sterile gauze, freeze-dried in a vacuum overnight, and then stored at −80°C in the dark. Blank, unexposed filters were processed with the same method for using as a negative control. Prior to addition in cell culture, PM2.5 particles were weighed and decontaminated with UV for 30 min as previously described.[34] The samples were then suspended in sterile phosphate-buffered saline (PBS) at 100 mg/ml. The solutions were treated with ultrasonic oscillation three times for 20 s each, and stored at −80°C. Prior to use, PM2.5 solutions were thawed, then sonicated for 20 min, and diluted with sterilized PBS.
Chemical characteristics of PM2.5
Six major ionic species (Na+, NH4+, K+, Cl−, NO3−, and SO42−) were measured by ion chromatography (DX90, Dionex, Sunnyvale, CA, USA). Organic carbon (OC) and elemental carbon (EC) contents were assessed by thermo-optical transmittance according to the National Institute for Occupational Safety and Health protocol with an aerosol OC/EC analyzer (Sunset Laboratory Inc., Tigard, OR, USA).
Cell culture
HaCaT cells (Guangzhou Jenniobio Biotechnology Co., Ltd., Guangzhou, China) are spontaneously immortalized keratinocytes derived from histologically normal human skin and have retained full differentiation capacity.[35] The cells were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco, Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Life Technologies, Carlsbad, CA, USA) and 1% penicillin/streptomycin (Gibco, Life Technologies, Carlsbad, CA, USA) at 37°C in a humidified atmosphere containing 5% CO2.
Cell viability
Cell viability was assessed with Cell Counting Kit-8 (CCK-8, Dojindo, Japan) and lactate dehydrogenase (LDH) release assays. Briefly, HaCaT cells were trypsinized, seeded at 5 × 104 cells/well in 96-well plates, and cultured for 26 h to allow for attachment. The cells were then treated with 0, 25, 50, 100, or 200 μg/ml PM2.5 for 24 h, at which point 20 μl CCK-8 solution was added to each well and the cells were incubated at 37°C for 1 h. Absorbance at 450 nm for each well was measured using a microplate reader (Synergy HT, BioTek, Winooski, VT, USA). Alternatively, LDH release was monitored with the Cytotoxicity Detection Kit LDH (Wuhan Biomed Biotechnology Limited Company, Wuhan, China) in cell-free culture supernatants.
Intracellular reactive oxygen species quantification
Intracellular ROS was measured with the fluorescent dye, 2, 7-dichlorofluorescein diacetate (DCFH-DA). HaCaT cells were seeded at 5 × 104 cells/well in 96-well plates and cultured to 80% confluency. Then, the media was discarded and 50 μl of DCFH-DA (10 μmol/L) was added to each well and incubated for 20 min. Stained cells were washed three times with DMEM and treated with 0, 25, 50, 100, or 200 μg/ml PM2.5 for 30 min. Green fluorescence intensity was measured using a fluorescence microplate reader (Synergy HT, BioTek) with excitation and emission wavelengths of 488 and 525 nm, respectively, and presented relative to intracellular ROS.
Superoxide dismutase activity
Superoxide dismutase (SOD) activity was measured 24 h after treatment with 0, 25, 50, 100, and 200 μg/ml PM2.5. Total SOD activity was assessed in cell lysates using the SOD reagent kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
Measurement of malondialdehyde
Oxygen radicals elicit membrane peroxidation and measurement of malondialdehyde (MDA) formation, which are detrimental to cellular function. MDA production was evaluated in cell lysates using the MDA reagent kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
Acridine orange/ethidium bromide double fluorescence staining
Acridine orange/ethidium bromide (AO/EB) staining assay was used to identify apoptotic and necrotic cells. Briefly, HaCaT cells were trypsinized and seeded at 1 × 106 cells/ml in 6-well plates (2 ml/well) and cultured for 24 h. Different concentrations of PM2.5 in serum-free culture medium (0, 25, 50, or 100 μg/ml) were added to each well in triplicate and incubated for 6 h. The cells were pelleted at 1000 rpm for 10 min and washed once with PBS. For staining, 5 μl each of 100 μg/ml AO and 100 μg/ml EB was added to 100 μl cell suspension. The labeled cell suspension was imaged using a fluorescent microscope (Axio Observer Z1, ZEISS, Oberkochen, Germany).
Annexin V/propidium iodide staining
Cell apoptosis rate was determined by annexin V/propidium iodide (PI) double staining and flow cytometry. HaCaT cells were exposed to PM2.5 at different concentrations (0, 25, 50, 100, and 200 μg/ml) for 6 h. The treated cells were trypsinized with 0.25% trypsin and collected by centrifugation at 800 r/min (centrifugal radius: 9.6 cm) for 3–4 min. The cell pellets were washed three times with prechilled PBS, resuspended in 100 μl binding buffer, and then incubated with 5 μl FITC-annexin V and 10 μl PI for 15 min at room temperature. Apoptosis was detected by flow cytometry (FACSCalibur™, Becton Dickinson, CA, USA), and the results were shown in two-dimensional plots. Total apoptosis rates were calculated as total apoptosis rate (%) = early apoptosis rate (%) + late apoptosis rate (%) when compared to untreated controls.
Comet assay
DNA damage and strand breaks were evaluated by comet assays. HaCaT cells were exposed to PM2.5 at different concentrations (0, 25, 50, and 100 μg/ml) for 24 h. The cells were then collected and washed twice by prechilled PBS. The treated cells (approximately 104 cells) were embedded in 0.7% low-melting point agarose on microscope slides coated with 0.5% normal melting point agarose and covered with a cover slip. After solidification, the cover slip was removed and a third layer of 0.7% low-melting point agarose was poured, covered with a cover slip, and allowed to solidify. The slides were then immersed in prechilled lysis buffer at 4°C for 1.5 h, washed three times with PBS, and submersed in an alkaline electrophoresis buffer at room temperature for 40 min. Electrophoresis was performed at 25 V for 25 min and the slides were then immersed in neutralization buffer (0.4 mmol/L Tris-HCl, pH 7.5) for 10 min at 4°C three times, and then stained with 10 μl PI for 10 min in dark. Images were obtained by fluorescence microscopy (Axio Observer Z1, ZEISS, Oberkochen, Germany), and the level of DNA damage was assessed based on the olive tail calculated by a computer-based image analysis system (IMI Comet Analysis software, version 1.0, China).
Western blot analysis
PM2.5-treated cells were harvested, lysed in RIPA buffer (Beyotime, Shanghai, China) on ice for 30 min, and centrifuged at 12,000 r/min (centrifugal radius: 9.6 cm) for 15 min at 4°C. The protein was quantified with a BCA protein assay kit (Dahui Bio, Guangzhou, China), separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech., England, UK) by semidry electrophoretic transfer at 300 mA for 30–90 min. The membranes were washed with Tris-buffered saline (TBS), blocked with 5% skim milk in TBS with Tween-20 (TBST) for 1 h at room temperature, and then incubated with primary antibodies (anti-β-actin, 1:400, anti-cytochrome-c, 1:5000, anti-caspase-3, 1:5000, anti-caspase-9, 1:2000, Abcam, Cambridge, UK) diluted in TBST for 2 h at room temperature. The probed membranes were washed three times with TBST for 10 min each, and then incubated with secondary antibodies for 2 h at room temperature. Immunoreactive bands were detected with ECL reagents (Beyotime, Shanghai, China) and exposed to X-ray film. β-actin served as a loading control for total protein and showed no differences between groups. Densitometric quantification was performed in Image J (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as mean ± standard deviation and analyzed using SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA). Differences among treatment groups were evaluated by one-way analysis of variance and two-tailed Student's t-test. A P < 0.05 was considered statistically significant.
RESULTS
Chemical composition of PM2.5
The chemical composition of PM2.5 in Heshan county from December 4, 2014, to January 9, 2015, is summarized in Table 1. The daily average concentration of PM2.5 during the collection period was 63.5 μg/m3.
Table 1.
The associated chemical components of PM2.5 at Heshan county from December 4, 2014, to January 9, 2015
| Component | EC | OM* | Na+ | NH4+ | K+ | Cl− | NO3− | SO42− |
|---|---|---|---|---|---|---|---|---|
| Concentration (µg/m3) | 4.28 | 25.12 | 1.01 | 8.21 | 1.27 | 2.02 | 11.16 | 13.20 |
*OM = OC × 1.6. OM: Organic matter; OC: Organic carbon; EC: Elemental carbon.
PM2.5-induced cytotoxicity in HaCaT cells
The cytotoxic effects of PM2.5 were monitored in HaCaT cells exposed to 0, 25, 50, 100, or 200 μg/ml PM2.5 for 24 h, and revealed a significant dose-dependent decrease in cell viability (1.41 ± 0.03 for 25 μg/ml, 1.32 ± 0.02 for 50 μg/ml, 1.05 ± 0.05 for 100 μg/ml, and 0.75 ± 0.04 for 200 μg/ml vs. 1.75 ± 0.09 for 0 μg/ml; t = 7.41, 9.54, 13.82, and 20.02, respectively, all P < 0.001; Figure 1). Correspondingly, LDH release was also significantly increased in cell-free culture supernatants in a dose-dependent manner (633.00 ± 63.05 U/L for 25 μg/ml, 828.00 ± 89.06 U/L for 50 μg/ml, 1139.00 ± 124.00 U/L for 100 μg/ml, and 1666.75 ± 157.88 U/L for 200 μg/ml vs. 536.50 ± 46.08 U/L for 0 μg/ml; t = −2.47, −5.81, −9.11, and −13.74; and P = 0.048, 0.001, <0.001, and <0.001, respectively; Figure 2).
Figure 1.
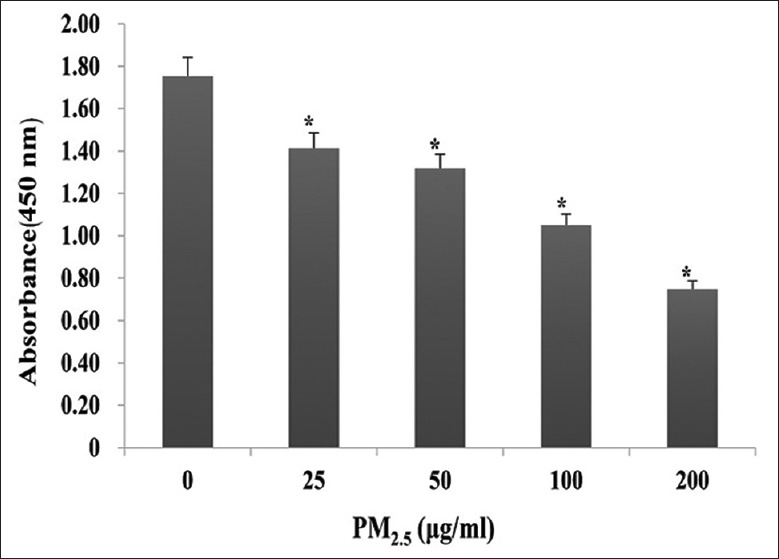
Effect of PM2.5 on HaCaT cell viability. HaCaT cells were exposed to 0, 25, 50, 100, or 200 μg/ml PM2.5 for 24 h. *P < 0.01 versus unexposed control cells.
Figure 2.
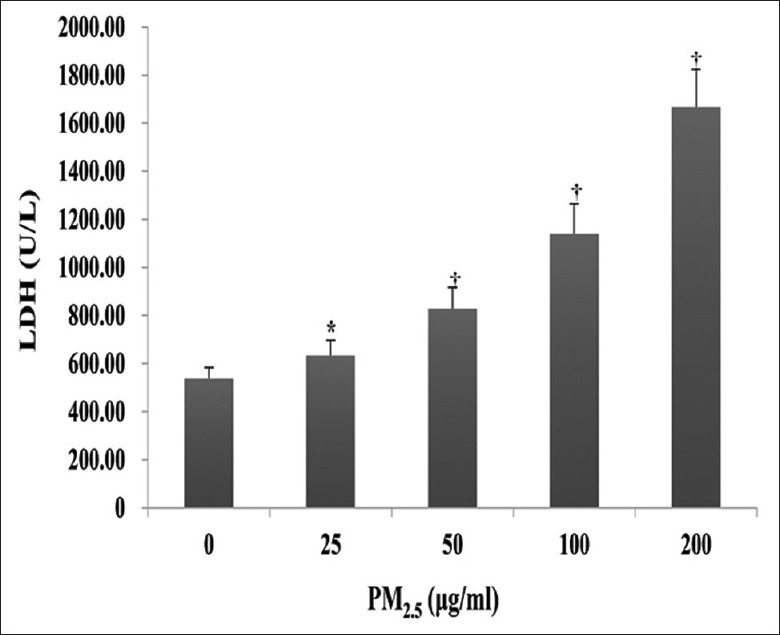
Effect of PM2.5 on lactate dehydrogenase activity in cell-free culture supernatants. HaCaT cells were exposed to 0, 25, 50, 100, or 200 μg/ml PM2.5 for 24 h. *P < 0.05, †P < 0.01 versus unexposed control cells.
PM2.5-induced reactive oxygen species generation
The potential of PM2.5 to induce intracellular ROS generation in HaCaT cells was evaluated by DCFH-DA fluorescence intensity. As shown in Figure 3, intracellular ROS levels were markedly increased in cells exposed to ≥25 μg/ml PM2.5 for 24 h, which peaked at a 2-fold increase in the presence of 100 and 200 μg/ml (arbitrary unit: 264.50 ± 17.91 for 25 μg/ml, 305.05 ± 19.57 for 50 μg/ml, 427.95 ± 18.32 for 100 μg/ml, and 436.70 ± 17.77 for 200 μg/ml vs. 201.00 ± 19.28 for 0 μg/ml; t = −4.83, −7.58, −17.06, and −17.98; and P = 0.012, 0.003, <0.001, and <0.001, respectively).
Figure 3.
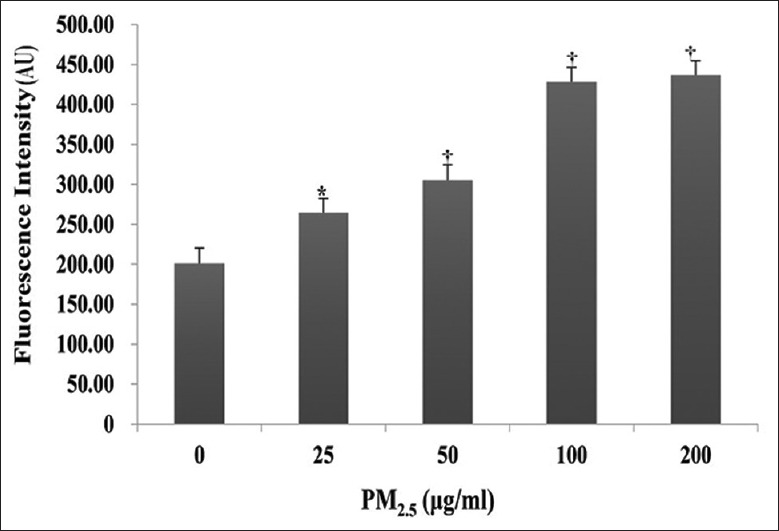
Effect of PM2.5 on reactive oxygen species generation in HaCaT cells. HaCaT cells were exposed to 0, 25, 50, 100, or 200 μg/ml PM2.5 for 24 h. Results were measured as mean fluorescence (AU). *P < 0.05, †P < 0.01 versus unexposed control cells. AU: Arbitrary unit.
Effect of PM2.5 on superoxide dismutase activity
Analysis of SOD activity in HaCaT cells after 24 h treatment with PM2.5 showed a slight decrease at 25 μg/ml (5.97 ± 0.30 NU/mg prot vs. 6.47 ± 0.28 NU/mg prot, t = 2.406, P = 0.082), which became significant at ≥50 μg/ml (5.15 ± 0.42 NU/mg prot for 50 μg/ml, 4.08 ± 0.20 NU/mg prot for 100 μg/ml, and 3.76 ± 0.37 NU/mg prot for 200 μg/ml vs. 6.47 ± 0.28 NU/mg prot for 0 μg/ml; t = 5.23, 11.65, and 13.81; and P = 0.008, <0.001, and <0.001, respectively; Figure 4).
Figure 4.
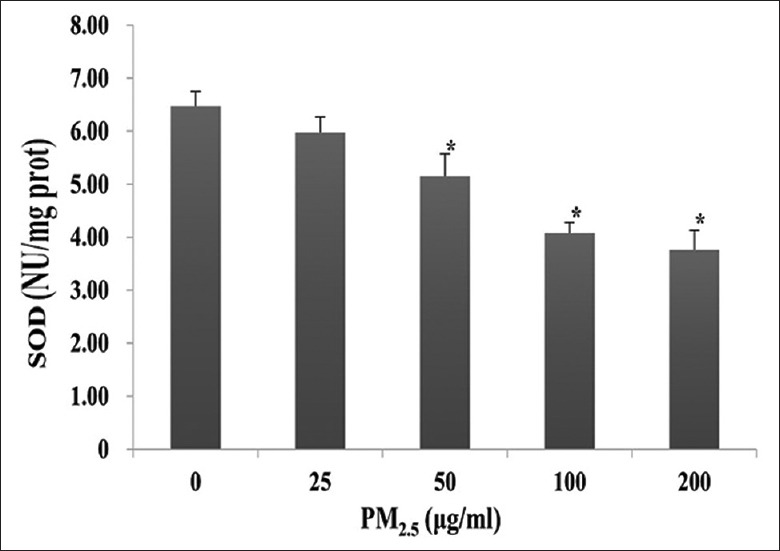
Superoxide dismutase (SOD) activity in PM2.5-treated HaCaT cells. HaCaT cells were exposed to 0, 25, 50, 100, or 200 μg/ml PM2.5 for 24 h. *P < 0.01 versus unexposed control cells.
Measurement of PM2.5-induced lipid peroxidation through malondialdehyde
As shown in Figure 5, increases in lipid peroxidation were observed in HaCaT cells after exposure to 25, 50, 100, or 200 μg/ml PM2.5 for 24 h, compared with untreated controls (0.61 ± 0.06 nmol/mg prot, 0.68 ± 0.05 nmol/mg prot, 0.70 ± 0.05 nmol/mg prot, and 0.76 ± 0.05 nmol/mg prot vs. 0.54 ± 0.05 nmol/mg prot; t = −1.80, −4.00, −4.58, and –6.23; and P = 0.122, 0.007, 0.004, and < 0.001, respectively). Notably, MDA production increased in a dose-dependent manner.
Figure 5.
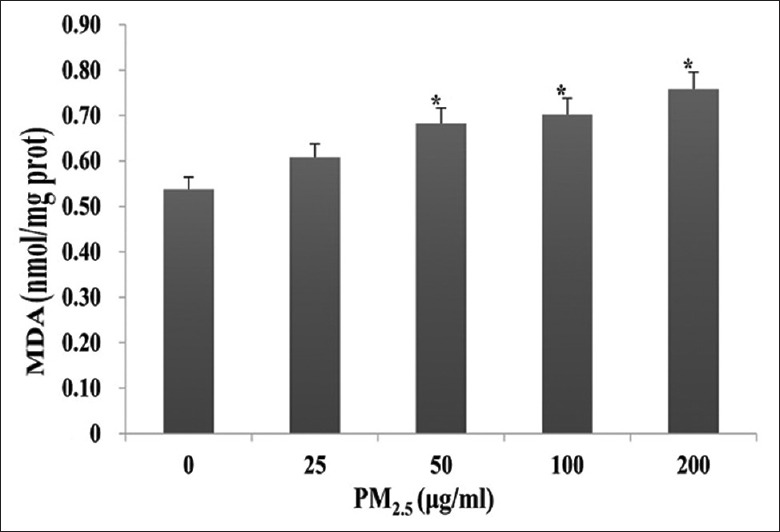
Effects of PM2.5 on oxidative stress-induced lipid peroxidation in HaCaT cells as determined by malondialdehyde production. HaCaT cells were exposed to 0, 25, 50, 100, or 200 μg/ml PM2.5 for 24 h. *P < 0.01 versus unexposed control cells.
Effect of PM2.5 on HaCaT cell apoptosis
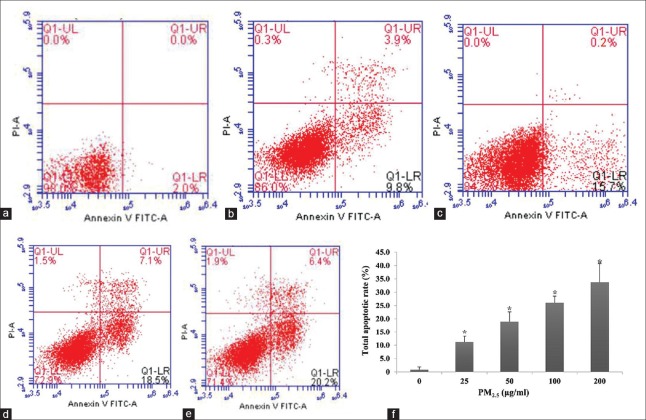
PM2.5-induced cell apoptosis was monitored by dual AO/EB staining [Figure 6]. As expected, untreated HaCaT cells displayed intact nuclei with uniform AO (green) staining; however, counterparts exposed to aqueous PM2.5 exhibited condensed and fragmented nuclei typically associated with apoptosis. Significantly, cell apoptosis rates increased in a dose-dependent manner with total apoptosis rates of 11.2%, 18.9%, 26.1%, and 33.7% observed at 25, 50, 100, and 200 μg/ml PM2.5, respectively (t = −7.47, −8.11, −8.05, −16.294; and P = 0.002, 0.001, 0.006, <0.001, respectively; Figure 7).
Figure 6.
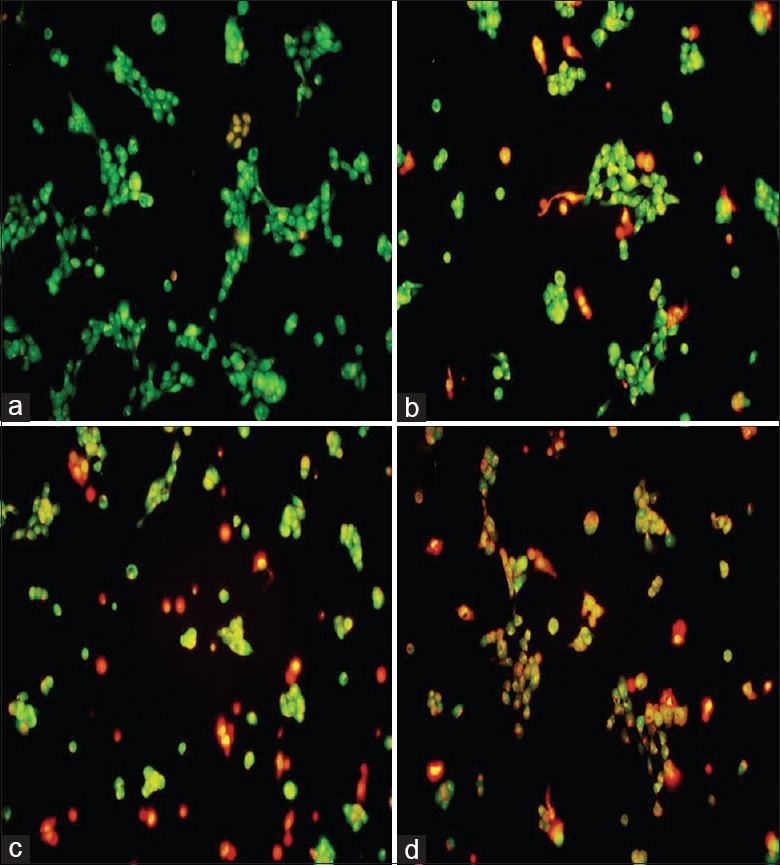
Effect of PM2.5 on apoptotic morphology in HaCaT cells assessed by acridine orange/ethidium bromide staining. Cells were exposed to 0 (a), 25 (b), 50 (c), or 100 μg/ml (d) PM2.5 for 24 h (original magnification, ×200).
Figure 7.
Effect of PM2.5 on HaCaT cell apoptosis. (a-e) Representative annexin V/propidium iodide flow cytometry plots. Cells were exposed with 0 (a), 25 (b), 50 (c), 100 (d), or 200 μg/ml (e) PM2.5 for 24 h. Early apoptotic (AV+ PI−) and late apoptotic/necrotic (AV+ PI+) cells were found in the lower and upper right quadrants, respectively. (f) Total apoptosis in each treatment group. *P < 0.01 versus unexposed control cells.
Cytochrome-c, caspase-3, and caspase-9 expression in HaCaT cells
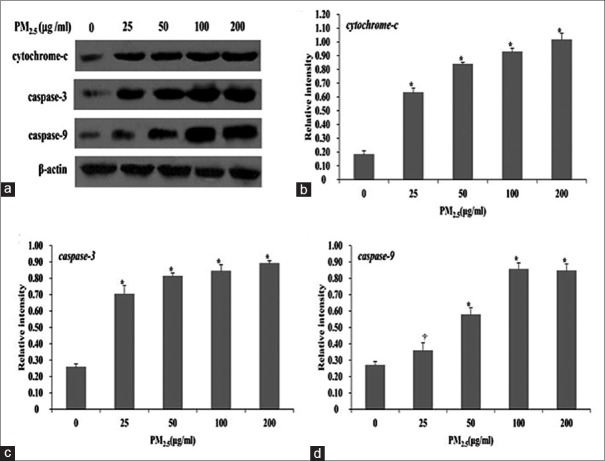
To explore the mechanism of apoptosis induced by PM2.5 exposure in HaCaT cells, the expression of apoptosis-associated proteins including cytochrome-c, caspase-3, and caspase-9 was examined. As shown in Figure 8, PM2.5 exposure significantly induced cytochrome-c (0.63 ± 0.03 for 25 μg/ml, 0.84 ± 0.02 for 50 μg/ml, 0.93 ± 0.03 for 100 μg/ml, and 1.02 ± 0.05 for 200 μg/ml vs. 0.18 ± 0.03 for 0 μg/ml; t = −18.10, −26.39, −33.91, and −36.92, respectively, all P < 0.001), caspase-3 (0.70 ± 0.05 for 25 μg/ml, 0.81 ± 0.02 for 50 μg/ml, 0.84 ± 0.04 for 100 μg/ml, 0.89 ± 0.02 for 200 μg/ml vs. 0.26 ± 0.02 for 0 μg/ml; t = −13.87, −22.10, −34.09, −42.17, and all P < 0.001), and caspase-9 expression (0.36 ± 0.05 for 25 μg/ml, 0.58 ± 0.04 for 50 μg/ml, 0.85 ± 0.04 for 100 μg/ml, and 0.84 ± 0.04 for 200 μg/ml vs. 0.27 ± 0.02 for 0 μg/ml, t = −2.84, −10.87, −21.92, and −20.54 and P = 0.047, <0.001, <0.001, and <0.001, respectively) in a dose-dependent manner.
Figure 8.
PM2.5-induced cytochrome-c, caspase-3, and caspase-9 expression changes in HaCaT cells. The image analysis of the protein levels of cytochrome-c, caspase-3, and caspase-9 (a) and the quantitative analysis of cytochrome-c (b), caspase-3 (c), and caspase-9 (d) expression in HaCaT cells exposed to 0, 25, 50, 100, or 200 μg/ml PM2.5 for 24 h by Western blot analysis. *P < 0.01, †P < 0.05 versus unexposed control cells.
Effects of PM2.5 on DNA damage in HaCaT cells
HaCaT cells were incubated with PM2.5 at different concentrations (0–100 μg/ml) for 24 h. Quantitative data and comet assays revealed progressive DNA damage that corresponded with increasing PM2.5 concentration (0.93 ± 0.06 for 25 μg/ml, 1.22 ± 0.11 for 50 μg/ml, and 1.76 ± 0.22 for 100 μg/ml vs. 0.42 ± 0.10 for 0 μg/ml; t = −6.69, −8.40, and −8.59; and P = 0.003, 0.001, and 0.001, respectively; Figure 9).
Figure 9.
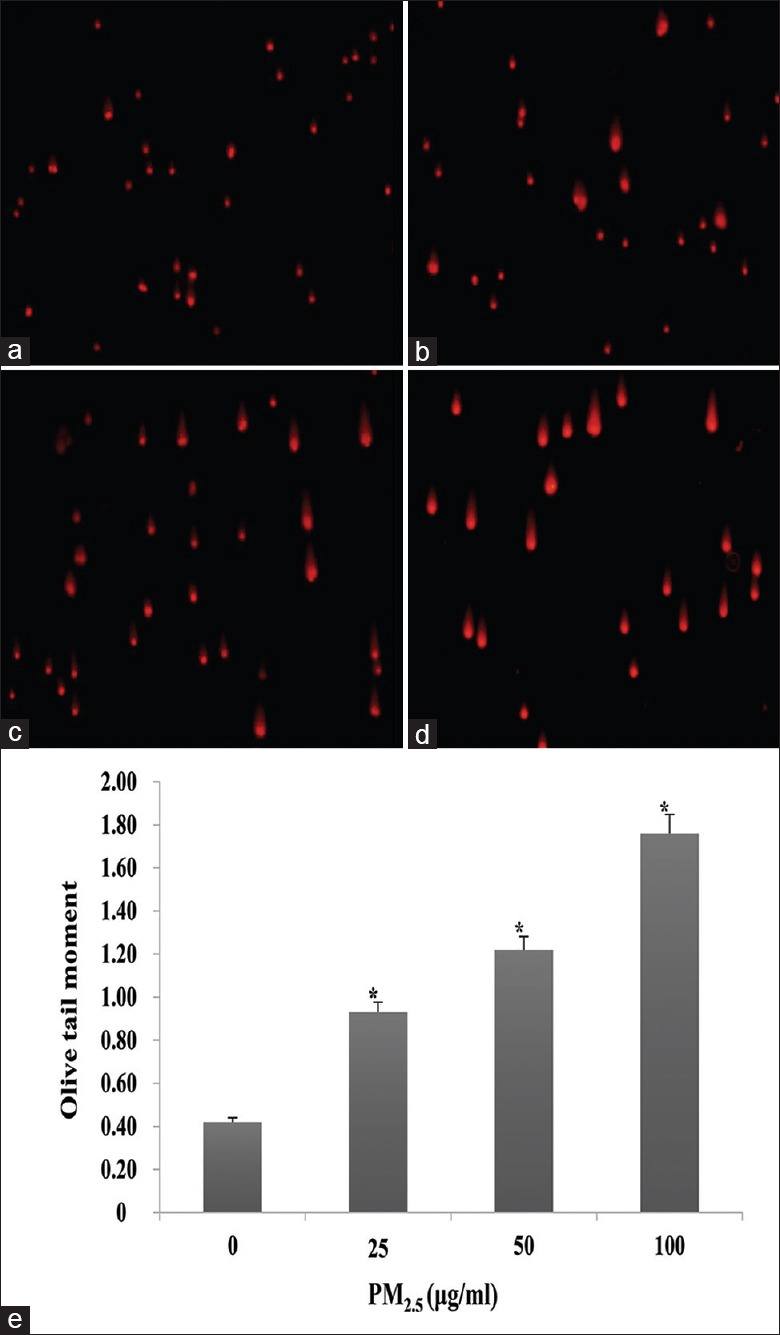
Effect of PM2.5 on DNA damage in HaCaT cells. (a-d) Representative comet images of cells exposed to 0 (a), 25 (b), 50 (c), or 100 μg/ml (d) PM2.5 for 24 h (original magnification, ×200). (e) Quantification of the olive tail moment in each group. *P < 0.01 versus unexposed control cells.
DISCUSSION
Recently, PM2.5 has received increased attention in China as studies have indicated that PM2.5 concentrations in China are much higher than the air quality guideline (25 μg/m3 for 24 h mean) suggested by the WHO.[2] For example, the population-weighted mean of PM2.5 in 190 Chinese cities was 61 μg/m3, ranging from 16 to 119 μg/m3.[3] Furthermore, Zhao et al.[36] have shown that the average concentration of 24 h individual PM2.5 for traffic police officers in China ranged from 86.48 to 116.98 μg/m3. PM2.5 is an “invisible killer” and has been linked to many adverse health effects including cardiovascular and pulmonary diseases. It can penetrate into the skin barriers to induce or aggravate atopic dermatitis, allergic dermatitis, and eczema deterioration,[20,21,37] although exposure can occur through inhalation or ingestion, as well as dermal contact. Recent epidemiological and clinical studies have established a correlation between increased atmospheric PM2.5 levels and skin disorders;[23,24] however, limited data are available to explain these effects. In human skin, epidermal keratinocytes play a role in the formation of a primary defensive skin barrier against environmental toxicants. Epidermal keratinocytes in the basal layer of the epidermis move upward and ultimately differentiate into cornified cells in the epidermal stratum corneum, thus forming the epidermal permeability barrier.[38,39] Therefore, in this report, we assessed the toxicity of PM2.5 sampled from the environment using an immortalized human keratinocyte HaCaT cell culture system and identified the cell apoptosis pathway triggered by exposure.
Notably, the data of this study have demonstrated that PM2.5 decreased HaCaT cell viability in a dose-dependent manner, similar to its observed effects on human bronchial epithelial, umbilical vein endothelial cells and lung epithelial cells.[40,41,42] A concentration-dependent increase in LDH activity was also found, indicative of a loss in membrane integrity and/or increased permeability. Taken together, these results supported the cytotoxic effect of PM2.5 in HaCaT cells. The cytotoxicity after PM2.5 contact has been suggested to be produced from the particles themselves and primarily from the chemicals coated on their surface. Our composition analysis of PM2.5 showed the presence of organic matter, EC, and other inorganic ions that may exert a toxic effect on HaCaT cells.
Although the underlying mechanisms responsible for cutaneous PM2.5-related disorders are inconclusive, evidence suggests that oxidative stress caused by the excessive production of ROS within affected cells is believed to play a role.[27,43] Consistently, this study observed that PM2.5 triggered a marked increase of ROS generation in HaCaT cells in a dose-dependent manner. High levels of ROS overwhelm the skin defenses by quickly depleting antioxidant activity, leading to deleterious effects.[44] SOD is an antioxidant enzyme that functions as a free radical scavenger. In the present study, we found that PM2.5 exposure resulted in a dose-dependent decrease in SOD activity, whereas a previous study demonstrated that SOD activity showed a change of early increases and/or late decreases in A549 cells exposed to rural, urban, or industrial PM.[45] This difference might be attributed to the different PM source or particle size, which could exhibit a different effect on cell damage. Superfluous free radicals and ROS generation can also interact with lipid-rich plasma membrane and initiate the lipid peroxidation reaction cascade.[46,47] Intracellular MDA is a sensitive and widely used marker of oxidative damage, particularly lipid peroxidation.[48] A recent study found that personal PM2.5 exposure increased MDA levels in human blood,[49] which was similar to that observed in our in vitro HaCaT culture model.
Elevated ROS levels alter the cellular redox status and can trigger inflammation and cell apoptosis at higher concentrations.[50,51] Apoptosis, an active process of cell death, is a physiological and pathogenic process with characteristic morphological and biochemical features, such as membrane blebbing, cell shrinkage, oligonucleosomal DNA fragmentation, chromatin condensation, and apoptotic body formation.[52] In our AO/EB double-staining assay, HaCaT cells showed a clear apoptotic morphology with condensed and fragmented nuclei following PM2.5 exposure. Further, annexin V/PI double staining and flow cytometry analysis revealed a dose-dependent increase in HaCaT cell apoptosis after PM2.5 exposure, consistent with that previously observed in some types of respiratory cells.[53,54] In addition, this study showed that PM2.5 induced DNA damage in treated cells as shown by increased olive tail moment in comet assay images. Hence, these data suggested that oxidative stress may facilitate PM2.5-induced DNA damage and apoptosis in HaCaT cells. However, the signal pathway of apoptosis induced by PM2.5 in HaCaT cells was not definite. In general, intrinsic apoptosis is a mitochondrion-centered cell death that is mediated by mitochondrial outer membrane permeabilization, and results in apoptosome formation, activation of caspase-9, and subsequent activation of effector caspases. The release of cytochrome-c from the inter-membrane space into the cytosol represents a critical event in the mitochondria-mediated cell apoptosis pathway; cytochrome-c can then bind apoptosis protease-activating factor-1, forming the apoptosome; this then recruits and converts the latent apoptosis-promoting pro-caspase-9 to its active form and activates an overlapping set of effector caspases including caspase-3.[55,56] Active effector caspases then induce DNA damage, resulting in the morphological features of apoptosis.[57]
To determine the apoptotic pathway induced by PM2.5 in HaCaT cells, this study evaluated the pro-apoptotic cytochrome-c, caspase-9, and caspase-3 expression and found a significant increase following exposure, suggesting that PM2.5 triggers apoptosis in a mitochondria-dependent manner. This result was similar to that previously observed in human L132 lung epithelial cells; however, apoptosis was also found to be partly caused by TNF-α pathway activation,[53] which will be further explored and investigated in our future studies. Collectively, these data indicate that PM2.5 exposure likely elicits HaCaT cell death through mitochondria-mediated apoptosis.
The evidence presented herein indicated that oxidative stress might be the critical mechanism underlying PM2.5-induced toxicity in HaCaT cells, which would likely manifest as cutaneous damage in vivo. This work further clarified the possible pathophysiological mechanisms underlying the adverse skin effects observed following PM2.5 exposure; however, as skin differs considerably from other organs owing to its structure and large area, further and comprehensive research should be conducted to explore the more precise mechanisms involved in PM2.5-induced skin damage, such as destruction of the skin barrier function induced by PM2.5 exposure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Leung PY, Wan HT, Billah MB, Cao JJ, Ho KF, Wong CK, et al. Chemical and biological characterization of air particulate matter 2.5, collected from five cities in China. Environ Pollut. 2014;194:188–95. doi: 10.1016/j.envpol.2014.07.032. doi: 10.1016/j.envpol.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Q, Crooks R. Toward an Environmentally Sustainable Future: Country Environmental Analysis of the People's Republic of China. Mandaluyong City, Philippines: Asian Development Bank; 2012. [Google Scholar]
- 3.Zhang YL, Cao F. Fine particulate matter (PM2.5) in China at a city level. Sci Rep. 2015;5:14884. doi: 10.1038/srep14884. doi: 10.1038/srep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gai HF, An JX, Qian XY, Wei YJ, Williams JP, Gao GL, et al. Ovarian damages produced by aerosolized fine particulate matter (PM2.5) pollution in mice: Possible protective medications and mechanisms. Chin Med J. 2017;130:1400–10. doi: 10.4103/0366-6999.207472. doi: 10.4103/0366-6999.207472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberdörster G, Utell MJ. Ultrafine particles in the urban air: To the respiratory tract – And beyond? Environ Health Perspect. 2002;110:A440–1. doi: 10.1289/ehp.110-1240959. doi: 10.1289/ehp.110-a440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hetland RB, Cassee FR, Refsnes M, Schwarze PE, Låg M, Boere AJ, et al. Release of inflammatory cytokines, cell toxicity and apoptosis in epithelial lung cells after exposure to ambient air particles of different size fractions. Toxicol In Vitro. 2004;18:203–12. doi: 10.1016/s0887-2333(03)00142-5. doi: 10.1016/S0887-2333(03)00142-5. [DOI] [PubMed] [Google Scholar]
- 7.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–42. doi: 10.1016/S0140-6736(02)11274-8. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 8.Pope CA., 3rd Particulate air pollution, C-reactive protein, and cardiac risk. Eur Heart J. 2001;22:1149–50. doi: 10.1053/euhj.2001.2593. doi: 10.1053/euhj.2001.2593. [DOI] [PubMed] [Google Scholar]
- 9.Kloog I, Coull BA, Zanobetti A, Koutrakis P, Schwartz JD. Acute and chronic effects of particles on hospital admissions in New-England. PLoS One. 2012;7:e34664. doi: 10.1371/journal.pone.0034664. doi: 10.1371/journal.pone.0034664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alfaro-Moreno E, Nawrot TS, Nemmar A, Nemery B. Particulate matter in the environment: Pulmonary and cardiovascular effects. Curr Opin Pulm Med. 2007;13:98–106. doi: 10.1097/MCP.0b013e328013f47e. doi: 10.1097/MCP.0b013e328013f47e. [DOI] [PubMed] [Google Scholar]
- 11.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American heart association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 12.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173:667–72. doi: 10.1164/rccm.200503-443OC. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–34. doi: 10.1001/jama.295.10.1127. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samoli E, Stafoggia M, Rodopoulou S, Ostro B, Alessandrini E, Basagaña X, et al. Which specific causes of death are associated with short term exposure to fine and coarse particles in Southern Europe? results from the MED-PARTICLES project. Environ Int. 2014;67:54–61. doi: 10.1016/j.envint.2014.02.013. doi: 10.1016/j.envint.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Evans KA, Halterman JS, Hopke PK, Fagnano M, Rich DQ. Increased ultrafine particles and carbon monoxide concentrations are associated with asthma exacerbation among urban children. Environ Res. 2014;129:11–9. doi: 10.1016/j.envres.2013.12.001. doi: 10.1016/j.envres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai SS, Chang CC, Yang CY. Fine particulate air pollution and hospital admissions for chronic obstructive pulmonary disease: A case-crossover study in Taipei. Int J Environ Res Public Health. 2013;10:6015–26. doi: 10.3390/ijerph10116015. doi: 10.3390/ijerph110505081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European study of cohorts for air pollution effects (ESCAPE) Lancet Oncol. 2013;14:813–22. doi: 10.1016/S1470-2045(13)70279-1. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 18.Hamra GB, Guha N, Cohen A, Laden F, Raaschou-Nielsen O, Samet JM, et al. Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environ Health Perspect. 2014;122:906–11. doi: 10.1289/ehp/1408092. doi: 10.1289/ehp.1408092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monteiro-Riviere NA, editor. Toxicology of the Skin. New York: Informa Healthcare; 2010. [Google Scholar]
- 20.Krutmann J, Liu W, Li L, Pan X, Crawford M, Sore G, et al. Pollution and skin: From epidemiological and mechanistic studies to clinical implications. J Dermatol Sci. 2014;76:163–8. doi: 10.1016/j.jdermsci.2014.08.008. doi: 10.1016/j.jdermsci.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Song S, Lee K, Lee YM, Lee JH, Lee SI, Yu SD, et al. Acute health effects of urban fine and ultrafine particles on children with atopic dermatitis. Environ Res. 2011;111:394–9. doi: 10.1016/j.envres.2010.10.010. doi: 10.1016/j.envres.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Vierkötter A, Schikowski T, Ranft U, Sugiri D, Matsui M, Krämer U, et al. Airborne particle exposure and extrinsic skin aging. J Invest Dermatol. 2010;130:2719–26. doi: 10.1038/jid.2010.204. doi: 10.1038/jid.2010.204. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Kim EH, Oh I, Jung K, Han Y, Cheong HK, et al. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol. 2013;132:495–8.e1. doi: 10.1016/j.jaci.2013.04.019. doi: 10.1016/j.jaci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Donaldson K, Mills N, MacNee W, Robinson S, Newby D. Role of inflammation in cardiopulmonary health effects of PM. Toxicol Appl Pharmacol. 2005;207:483–8. doi: 10.1016/j.taap.2005.02.020. doi: 10.1016/j.taap.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Deng X, Zhang F, Rui W, Long F, Wang L, Feng Z, et al. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol In Vitro. 2013;27:1762–70. doi: 10.1016/j.tiv.2013.05.004. doi: 10.1016/j.tiv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Valacchi G, Sticozzi C, Pecorelli A, Cervellati F, Cervellati C, Maioli E, et al. Cutaneous responses to environmental stressors. Ann N Y Acad Sci. 2012;1271:75–81. doi: 10.1111/j.1749-6632.2012.06724.x. doi: 10.1111/j.1749-6632.2012.06724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotovio J, Onno L, Justine P, Lamure S, Catroux P. Generation of oxidative stress in human cutaneous models following in vitro ozone exposure. Toxicol In Vitro. 2001;15:357–62. doi: 10.1016/s0887-2333(01)00036-4. doi: 10.1016/S0887-2333(01)00036-4. [DOI] [PubMed] [Google Scholar]
- 28.Valacchi G, van der Vliet A, Schock BC, Okamoto T, Obermuller-Jevic U, Cross CE, et al. Ozone exposure activates oxidative stress responses in murine skin. Toxicology. 2002;179:163–70. doi: 10.1016/s0300-483x(02)00240-8. doi: 10.1016/S0300-483X(02)00240-8. [DOI] [PubMed] [Google Scholar]
- 29.Avezov K, Reznick AZ, Aizenbud D. Oxidative damage in keratinocytes exposed to cigarette smoke and aldehydes. Toxicol In Vitro. 2014;28:485–91. doi: 10.1016/j.tiv.2014.01.004. doi: 10.1016/j.tiv.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Choi H, Shin DW, Kim W, Doh SJ, Lee SH, Noh M, et al. Asian dust storm particles induce a broad toxicological transcriptional program in human epidermal keratinocytes. Toxicol Lett. 2011;200:92–9. doi: 10.1016/j.toxlet.2010.10.019. doi: 10.1016/j.toxlet.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Magnani ND, Muresan XM, Belmonte G, Cervellati F, Sticozzi C, Pecorelli A, et al. Skin damage mechanisms related to airborne particulate matter exposure. Toxicol Sci. 2016;149:227–36. doi: 10.1093/toxsci/kfv230. doi: 10.1016/S0378-4274(03)00153-X. [DOI] [PubMed] [Google Scholar]
- 32.Yang GZ, Wang ZJ, Bai F, Qin XJ, Cao J, Lv JY, et al. Epigallocatechin-3-gallate protects HUVECs from PM2.5-induced oxidative stress injury by activating critical antioxidant pathways. Molecules. 2015;20:6626–39. doi: 10.3390/molecules20046626. doi: 10.3390/molecules20046626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ou Y, Yue D, Zhong L, Chen D, Zeng L. Establishment and operational management mechanism of Guangdong atmospheric supersite of China (in Chinese) Environ Sci Manag. 2013;38:63–67. [Google Scholar]
- 34.Peeters PM, Eurlings IM, Perkins TN, Wouters EF, Schins RP, Borm PJ, et al. Silica-induced NLRP3 inflammasome activation in vitro and in rat lungs. Part Fibre Toxicol. 2014;11:58. doi: 10.1186/s12989-014-0058-0. doi: 10.1186/s12989-014-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE, et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J, Gao Z, Tian Z, Xie Y, Xin F, Jiang R, et al. The biological effects of individual-level PM2.5 exposure on systemic immunity and inflammatory response in traffic policemen. Occup Environ Med. 2013;70:426–31. doi: 10.1136/oemed-2012-100864. doi: 10.1136/oemed-2012-100864. [DOI] [PubMed] [Google Scholar]
- 37.Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol. 2014;134:993–9. doi: 10.1016/j.jaci.2014.09.023. doi: 10.1016/j.jaci.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 38.Elias PM, Ahn SK, Denda M, Brown BE, Crumrine D, Kimutai LK, et al. Modulations in epidermal calcium regulate the expression of differentiation-specific markers. J Invest Dermatol. 2002;119:1128–36. doi: 10.1046/j.1523-1747.2002.19512.x. doi: 10.1046/j.1523-1747.2002.19512.x. [DOI] [PubMed] [Google Scholar]
- 39.Feingold KR, Schmuth M, Elias PM. The regulation of permeability barrier homeostasis. J Invest Dermatol. 2007;127:1574–6. doi: 10.1038/sj.jid.5700774. doi: 10.1038/sj.jid.5700774. [DOI] [PubMed] [Google Scholar]
- 40.Zhou B, Liang G, Qin H, Peng X, Huang J, Li Q, et al. P53-dependent apoptosis induced in human bronchial epithelial (16-HBE) cells by PM(2.5) sampled from air in Guangzhou, China. Toxicol Mech Methods. 2014;24:552–9. doi: 10.3109/15376516.2014.951814. doi: 10.3109/15376516.2014.951814. [DOI] [PubMed] [Google Scholar]
- 41.Rui W, Guan L, Zhang F, Zhang W, Ding W. PM2.5-induced oxidative stress increases adhesion molecules expression in human endothelial cells through the ERK/AKT/NF-κB-dependent pathway. J Appl Toxicol. 2016;36:48–59. doi: 10.1002/jat.3143. doi: 10.1002/jat.3143. [DOI] [PubMed] [Google Scholar]
- 42.Choi JH, Kim JS, Kim YC, Kim YS, Chung NH, Cho MH, et al. Comparative study of PM2.5-and PM10-induced oxidative stress in rat lung epithelial cells. J Vet Sci. 2004;5:11–8. [PubMed] [Google Scholar]
- 43.Soberanes S, Urich D, Baker CM, Burgess Z, Chiarella SE, Bell EL, et al. Mitochondrial complex III-generated oxidants activate ASK1 and JNK to induce alveolar epithelial cell death following exposure to particulate matter air pollution. J Biol Chem. 2009;284:2176–86. doi: 10.1074/jbc.M808844200. doi: 10.1074/jbc.M808844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–75. doi: 10.1038/sj.jid.5700340. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 45.Kouassi KS, Billet S, Garçon G, Verdin A, Diouf A, Cazier F, et al. Oxidative damage induced in A549 cells by physically and chemically characterized air particulate matter (PM2.5) collected in Abidjan, Côte d’ivoire. J Appl Toxicol. 2010;30:310–20. doi: 10.1002/jat.1496. doi: 10.1002/jat.1496. [DOI] [PubMed] [Google Scholar]
- 46.Valacchi G, Fortino V, Bocci V. The dual action of ozone on the skin. Br J Dermatol. 2005;153:1096–100. doi: 10.1111/j.1365-2133.2005.06939.x. doi: 10.1111/j.1749-6632.2012.06724.x. [DOI] [PubMed] [Google Scholar]
- 47.Pillai S, Oresajo C, Hayward J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation - A review. Int J Cosmet Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 48.de Zwart LL, Meerman JH, Commandeur JN, Vermeulen NP. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic Biol Med. 1999;26:202–26. doi: 10.1016/s0891-5849(98)00196-8. doi: 10.1016/S0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 49.Sørensen M, Daneshvar B, Hansen M, Dragsted LO, Hertel O, Knudsen L, et al. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ Health Perspect. 2003;111:161–6. doi: 10.1289/ehp.111-1241344. doi: 10.1289/ehp.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Upadhyay D, Panduri V, Ghio A, Kamp DW. Particulate matter induces alveolar epithelial cell DNA damage and apoptosis: Role of free radicals and the mitochondria. Am J Respir Cell Mol Biol. 2003;29:180–7. doi: 10.1165/rcmb.2002-0269OC. doi: 10.1165/rcmb.2002-0269OC. [DOI] [PubMed] [Google Scholar]
- 51.Gosset P, Garçon G, Casset A, Fleurisse L, Hannothiaux MH, Creusy C, et al. Benzo (a) pyrene-coated onto FE2O3 particles-induced apoptotic events in the lungs of Sprague-Dawley rats. Toxicol Lett. 2003;143:223–32. doi: 10.1016/s0378-4274(03)00153-x. doi: 10.1016/S0378-4274(03)00153-X. [DOI] [PubMed] [Google Scholar]
- 52.Carson DA, Ribeiro JM. Apoptosis and disease. Lancet. 1993;341:1251–4. doi: 10.1016/0140-6736(93)91154-e. doi: 10.1016/0140-6736(93)91154-E. [DOI] [PubMed] [Google Scholar]
- 53.Dagher Z, Garçon G, Billet S, Gosset P, Ledoux F, Courcot D, et al. Activation of different pathways of apoptosis by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. Toxicology. 2006;225:12–24. doi: 10.1016/j.tox.2006.04.038. doi: 10.1016/j.tox.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 54.Holian A, Hamilton RF, Jr, Morandi MT, Brown SD, Li L. Urban particle-induced apoptosis and phenotype shifts in human alveolar macrophages. Environ Health Perspect. 1998;106:127–32. doi: 10.1289/ehp.98106127. doi: 10.2307/3434313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skulachev VP. Cytochrome c in the apoptotic and antioxidant cascades. FEBS Lett. 1998;423:275–80. doi: 10.1016/s0014-5793(98)00061-1. doi: 10.1016/S0014-5793(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 56.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 57.Nuñez G, Benedict MA, Hu Y, Inohara N. Caspases: The proteases of the apoptotic pathway. Oncogene. 1998;17:3237–45. doi: 10.1038/sj.onc.1202581. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]