Abstract
In one event, Chinese male individuals accidentally exposed to unknown chemicals and emerged erythema or blisters on contacted organism derma, then hospitalized. To identify the causative agents, blood, urine and exudate samples were collected from the patients during the therapeutic course. Five established liquid chromatography–mass spectrometry (LC–MS) and gas chromatography (GC)–MS methods were employed to analyze the samples. Here, an overall analysis of four types of sulfur mustard biomarkers, including the hydrolysis/oxidation products, β-lyase metabolites, DNA adducts and hemoglobin adducts, was conducted toward the samples from exposed individuals. The results of all the four types of biomarkers in different biomedical matrices showed high relevance, and verified that this exposure is indeed originated from sulfur mustard. The concentrations of the biomarkers in specimens revealed a good correlation with the severity of the patient's symptom. The concentration-time profile demonstrated that most of the biomarkers quickly achieved maximum at the beginning of the course, and then decreased and kept a detectable level until the 7th day after exposure. The DNA adducts in urine samples still appeared on the 30th day, and the N-terminal valine adducts in hemoglobin could be monitored for over 90 days, which was meaningful for the concurrent study of clinical samples. To the best of our knowledge, this work provides the total analysis and profile of four categories of biomarkers in human specimens for the first time, and the good accordance between concentration and level of burns, between time course and biomarkers will be of great importance for early diagnosis and medical treatment monitoring of sulfur mustard exposure.
Chemical compounds studied in this article: Sulfur mustard (PubChem CID: 10461), Bis-β-chloroethyl sulfoxide (PubChem CID: 22070), Thiodiglycol (PubChem CID: 5447), Thiodiglycol sulfoxide (PubChem CID: 18330)
Keywords: Sulfur mustard, Biomarker, Total analysis, Urine, Blood, Exudate
1. Introduction
Sulfur mustard (SM, U.S. Code HD, chemical name 2,2′-dichloroethyl sulfide) is a powerful vesicant and a biological alkylating agent, which can function via inhalational, cutaneous and ocular routes of exposure [1], [2]. It has been employed as a chemical warfare agent since World War I, and has a continued strike in more recent Middle Eastern conflicts [3]. SM was also a predominant agent found in the chemical weapons abandoned in China by the Japanese imperialist army after World War II. The old shells or containers of SM are still a large threat to civilian health. To date, accidental human injuries by SM occurred from a variety of situations have been reported [4], [5], [6], [7], [8]. Because of its ease of preparation, SM also poses a potential threat to public safety if abused by terrorists.
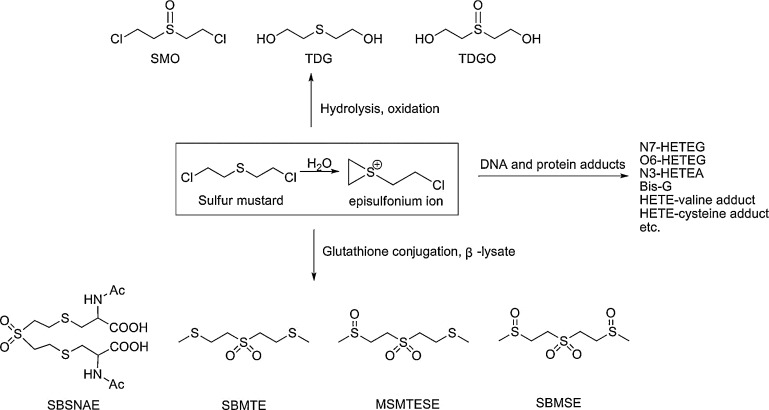
As a bifunctional alkylating agent, SM reacts rapidly with numerous nucleophiles, i.e., water, glutathione, DNA and proteins under physiological conditions via the intermediate episulfonium ion (Fig. 1). The corresponding four major metabolic routes have been identified in animal models (e.g., rat and mouse) [9], [10], [11], [12], [13]. The first pathway involves the direct oxidation product of SM, bis-β-chloroethyl sulfoxide (SMO), the directly hydrolyzed metabolite thiodiglycol (TDG), and its oxidation product thiodiglycol sulfoxide (TDGO). The second pathway involves a reaction with abundant glutathione and then undergoing oxidation to the sulfone followed by β-lyase cleavage, leading to the formation of 1,1′-sulfonylbis[2-S-(N-acetylcysteinyl) ethane] (SBSNAE), 1,1′-sulfonylbis[2-(methylthio) ethane] (SBMTE), 1-methylsulfinyl-2-[2-(methylthio)ethylsulfonyl]ethane (MSMTESE) and 1,1′-sulfonylbis [2-(methylsulfinyl) ethane] (SBMSE). The third way is the reaction on the critical nucleophilic sites in DNA to produce SM-DNA adducts. The major sites of DNA alkylation by SM include N7, O6 positions of guanine, N3 position of adenine, and interstrand or intrastrand crosslinks at the N7 position of guanine, and adducts of N7-[2-[(2-hydroxyethyl)thio]ethyl]-guanine (N7-HETEG), O6-[2-[(2-hydroxyethyl)thio]ethyl]-guanine (O6-HETEG), N3-[2-[(2-hydroxyethyl)thio]ethyl]-adenine (N3-HETEA), and bis[2-(guanin-7-yl)ethyl] sulfide (Bis-G) are correspondingly formed. The last pathway involves the reaction with various amino acid residues present in proteins, among which the HETE-valine (HETE-Val) adduct of hemoglobin and HETE-cysteine adduct of albumin are of the most attention now.
Fig. 1.
The four major metabolic routes of sulfur mustard. The main metabolic products studied in this paper corresponding to the four metabolite pathways were presented, including hydrolysis and oxidation products, β-lyase products, DNA adducts and protein adducts.
The biological fate of SM has been fully investigated in rats, while a complete understanding of SM in humans is lacking. This is due that, biological samples of alleged victims available for analysis are rare or difficult to be achieved, and new trace analytical methods to detect SM attached to long-lived proteins, DNAs, and other biomarkers are only vast improved in the last decade by virtue of booming chromatography–mass spectrometry technique. Basically, now researchers have the feasible tools to access the archived or freshly collected specimens, typical blood or urine samples, and find evidence after SM attack or accident.
For two subjects between 2 and 3 days after an accidentally cutaneous exposure to SM, free metabolites have been studied in the urine samples, the glutathione derived β-lyase metabolites SBMSE, MSMTESE, and hydrolysis products TDG, TDGO were identified in both individuals [14]. The β-lyase metabolites of SM in the urine of casualties are proposed as unequivocally diagnostic indicators of SM poisoning in human [15]. With regard to archived blood samples of four hospitalized Iranian casualties 5–10 days after exposure and one individual 2 days after an accidental exposure to SM, SM-alkylated hemoglobin adducts including HETE-histidine and HETE-Val adducts have been found for SM exposure [16]. In another case, archived blood samples of two hospitalized Iranians 22 and 26 days post a suspected SM exposure were tested positive for both the HETE-Val adduct and N7-HETEG [17]. For nine Iranian individuals exposed to SM, the archived blood specimens were tested positive for SM-alkylated albumin [18] and HETE-Val adduct of SM-hemoglobin [19]. In a most recent report, for two individuals 2–42 days after exposure, positive results of biomedical samples were obtained for SM poisoning. Notably, only in this case, two different biological matrices from an individual were used to mutually verify the SM exposure, in which the free metabolites of SM in urine and SM-albumin adduct in blood were tested positive [15], [20].
All above-mentioned reports indicated some similarity in metabolism of SM in human and rat. Generally, hydrolyzed/oxidation metabolites and β-lyase cleavage products formed in relatively high concentrations in early samples and persist only for several days due to the relatively rapid elimination, while DNA- and protein adducts existed for a relatively long time and thus offer valuable evidence for verification of SM exposure even weeks to months later. However, these researches only investigated one or up to two types of biomarkers in the clinic specimens, which cannot directly and comprehensively demonstrate the biological fate of SM in humans.
To address this issue, this paper provides a total analysis of four types of SM biomarkers in three different biomedical matrices collected from individuals who exposed to a vesicating agent in one accident. To identify the causative agent, blood, urine and blister exudate fluid samples collected from four representative cases with different extent of exposure symptoms were detected. A series of liquid chromatography–mass spectrometry (LC–MS) and gas chromatography (GC)–MS methods previously established and validated in our laboratory [13], [21], [22], [23], [24], [25], [26], [27] were employed to measure and profile the hydrolysis/oxidation products, β-lyase metabolites, DNA adducts and hemoglobin adducts in the samples. The correlation between concentration of biomarkers and level of burns were investigated. The detection windows of the four types of biomarkers were suggested, and thus a choice of suitable biomarker in certain biomedical samples were promoted. To the best of our knowledge, this is the first and detailed paper of four kinds of SM biomarkers in a typical exposure event in worldwide, it will be of great importance for early clinical diagnosis and medical treatment monitoring of SM exposure.
2. Materials and methods
2.1. Caution
SM is a highly reactive alkylation vesicant and cytotoxic agent. Handling of this agent should be carried out in the well-ventilated fume hood. The use of gloves and stringent protective measures must be adopted.
2.2. Chemicals and materials
SM (96% purity) was provided by the Institute of Chemical Defense of Chinese People's Liberation Army. Deuterated TDG (TDG-d8) and dibenzothiophene were purchased from the Cambridge Isotope Laboratories (Tewksbury, MA, USA). Heptafluorobutyryl imidazole (HFBI) was obtained from TCI (Tokyo, Japan). Analytical standards and isotopically labeled standards for TDGO, SMO, SBSNAE, SBMTE, MSMTESE, SBMSE, 1,1′-Sulfonylbis[2-(ethylsulfinyl) ethane] (SBESE), 1,1′-sulfonylbis[2-(ethylthio)ethane] (SBETE), N7-HETEG, Bis-G, N3-HETEA and O6-HETEG were synthesized in our laboratory, and the purities of these reference substances were greater than 95%, their deuterated ratios were over 99%, which were determined by nuclear magnetic resonance, LC–MS or GC–MS. Toluene, dichloromethane, ethyl acetate, methanol and acetonitrile were of HPLC grade and obtained from J.T. Baker (Phillipsburg, NJ, USA). TDG, 5-fluorobenzoyl chloride (PFBZ), pentafluorophenyl isothiocyanate (PFPITC), β-glucuronidase and all other chemicals, unless otherwise noted, were from Sigma-Aldrich (Louis, MO, USA). TiCl3 (20% solution in 2 M hydrochloric acid) was purchased from Acros (Morris Plains, NJ, USA). Ultrapure water was generated by a Milli-Q A10 water purification system (Bedford, MA, USA). Florisil with 60–100 meshes were from Alltech (Deerfield, IL, USA). The HLB columns were purchased from Waters (Milford, MA, USA).
2.3. Clinical patient samples
The four male individuals mentioned in this report accidentally contacted with an unknown viscous oily liquid contained in barrel at a construction site. After a latency period of 3–18 h, skin erythema, tingling sensation and blistering appeared in these individuals. Approximately 24 h after the chemical contact, patients were transferred to a burn center due to the aggravated symptoms. The signs and symptoms of these individuals were classified when hospitalized (Table 1). All patients received symptomatic treatment including local antibiotics, anti-inflammatory, hepatoprotection, kidney protection, myocardial and nerve nutrition, and microcirculation improvement. For patients 2–4, a wound debridement was performed to remove the affected areas and the big blisters on them were treated with vacuum sealing drainage. The eye injury of patient 3 was treated with soothing eye solution several times a day. After 4–6 weeks, satisfied wound healing was observed in these patients although with regular pigmentation and some scars and keloids.
Table 1.
The clinical diagnostic results of the four patients.
| Subject | Latency period | Injured regions | Degree of chemical burn |
|
|---|---|---|---|---|
| I° (%) | II° (%) | |||
| 1 | 18 h | Right wrist | 1 | – |
| 2 | 7 h | Hands, left forearm, left wrist and perineal region | 15 | 3 |
| 3 | 3 h | Right foot, right ankle, left leg, right wrist and face | 7 | 2 |
| 4 | 13 h | Hands, left wrist, right knee, root of right thigh and left toes | 8 | 2 |
With regard to the collection and analysis of clinical specimens, all four patients have signed the informed consent forms. Blood samples were collected from the four patients daily on days 3–32 after the exposure, and then weekly samples were taken up to 95 days after the exposure except 60 days for patient 1. The mid-morning urine samples were collected at the same time points. Blister exudate samples were collected from patients 2, 3, and 4 on days 3–6 after the exposure, but exudates with very small volume were collected from the blisters beyond day 6 due to the positive treatment effect. All samples were aliquot and stored at −70 °C on receipt.
2.4. GC–MS analysis of TDG or/and TDGO in urine samples
A highly-sensitive isotope dilution (ID)–negative chemical ionization (NCI)–GC–MS method was established on an Agilent 6890N GC-5975 MSD (Agilent, Palo Alto, CA, USA) in our laboratory [25]. The sample preparation procedures included solid-phase extraction (SPE) and derivatization. Briefly, when measuring sole TDG in the urine, each urine sample of 100 μL was spiked with 6 ng TDG-d8. Florisil silica SPE cartridges (500 mg/3 mL) were pre-conditioned with 4 mL ethyl acetate, and then samples were applied to the cartridges and eluted with 3.5 mL ethyl acetate and centrifuged to dryness at 50 °C. Aliquots of 80 μL pyridine, 50 μL ethyl acetate, and 20 μL PFBZ were added and allowed to react at 50 °C for 10 min. Next, 150 μL toluene was added and the solution was vortex mixed for 5 min. 0.1 mol/L of sodium acetate buffer (pH 5.0) was added for washing, the derivatized products were then reloaded to a new Florisil silica SPE column pre-equilibrated with 4 mL ethyl acetate and 4 mL CH2Cl2, consequently. Washing by 2 mL CH2Cl2 and 0.4 mL ethyl acetate, the product was eluted by 1.5 mL ethyl acetate. The obtained products were centrifuged to dryness at 50 °C, reconstituted in 50 μL of toluene, and analyzed via ID–NCI–GC–MS.
The GC equipment was mounted with a HP-5MS capillary column (25 m × 200 μm × 0.33 μm). The GC oven temperature was set at 100 °C and held for 1 min, increased to 290 °C by a linear ramp at 25 °C/min, and held for 5 min. The flow of helium carrier gas was set at 0.8 mL/min and injections were made in splitless mode for 30 s. The injection volume was 1 μL, with the injector and transfer line were both kept at a temperature of 280 °C, and methane was the reactant gas. The ion source and quadrupole temperatures were both 150 °C. Selective ion monitoring (SIM) mode was used for monitoring m/z 510 (target ion) and 518 (deuterated internal standard) of TDG and TDG-d8, respectively. In each analytical run, clinical samples, blank urine, quality control (QC) samples and a complete calibration standard set were all included.
For analysis of combined TDG and TDGO, firstly, a reduction step was conducted with 1 mL TiCl3 to the urine sample, and incubation at 75 °C for 1 h. Other pretreatment and analysis procedures were the same as the analysis of TDG.
2.5. GC–MS/MS analysis of the β-lyase metabolites in urine samples
In the analysis, the urine samples were first treated with TiCl3 to reduce the mono and di-sulfoxides (SBMSE, MSMTESE) to the disulfide (SBMTE) so that the three possible metabolites were traced from a single analyte [22]. Aliquots of 0.5 mL clinical urine specimens, blanks, QCs and standards were pipetted into 15 mL screw-cap culture tubes. The samples were added 50 μL of 1 μg/mL SBETE solution to give an internal standard concentration of 100 ng/mL. Aliquots of 0.5 mL TiCl3/20% HCl solution were added to the samples and mixed. The mixtures were then incubated for 1 h at 75 °C. After cooling to room temperature, 680 μL of 5 mol/L NaOH were added and vortex-mixed immediately until the precipitate turned white completely. Aliquots of 2 mL water were added, and the mixtures were well mixed and centrifuged at 5000 rpm for 20 min. The supernatants were transferred into the 3 mL HLB cartridges. The cartridges were pre-washed with 5% ammonia water, water, 2% formic acid and 10% acetonitrile in order, and the elutes containing SBMTE were collected by 3 mL acetonitrile. The solution of 2 μL was subjected to the GC–tandem MS (MS/MS) analysis.
The GC–MS/MS analysis was performed on a TSQ Quantum GC–triple-quadrupole MS/MS with an electroionzation source (Thermo-Fisher Scientific, San Jose, CA, USA). The GC column was a DB-17MS capillary column (30 m × 250 μm × 0.25 μm). The helium carrier gas flow was set at 1 mL/min. Sample injection time was 0.5 min in a splitless mode. The injection temperature was 250 °C and the transfer-line temperature was 260 °C. The source temperature was set at 250 °C. The initial GC oven temperature was 90 °C and held for 2 min, followed by a linear ramp at 20 °C/min to 290 °C, which was held for 4 min. Multiple-reaction monitoring (MRM) mode in MS was used, with m/z 140 → m/z 75 transition for SBMTE and m/z 154 → m/z 89 transition for internal standard SBETE.
2.6. LC–MS/MS analysis of the hydrolysis/oxidation products and β-lyase metabolites in urine, blood and blister exudates
A sensitive and rapid quantification method with ultra-high performance LC (UPLC)–MS/MS has been developed in our laboratory for simultaneous determination of seven SM biomarkers in samples including the oxidative/hydrolysis products (TDG, TDGO and SMO) and glutathione-derived metabolites (SBSNAE, SBMTE, MSMTESE and SBMSE) [21]. The method was fully validated to meet the bioanalytical requirements and was employed in this report to detection the metabolites in the specimens of blood, urine and blister exudate. Aliquots of 100 μL blood samples, 100 μL exudate sample or 1 mL urine samples were precipitated with acetonitrile–methanol (4:1, v/v), and analyzed by UPLC–MS/MS with a Waters ACQUITY UPLC system (Waters, Manchester, UK) and Qtrap 5500 tandem mass spectrometer (AB Sciex, Foster City, MA, USA). Clinic samples, blanks, QCs and standards were analyzed.
2.7. LC–MS/MS analysis of SM-DNA adducts in urine and blood samples
A simultaneous quantification method of N7-HETEG, O6-HETEG, N3-HETEA and Bis-G in the urine or blood samples exposed to SM was developed and validated with an ID–UPLC–MS/MS in a positive MRM mode (Waters UPLC- AB Sciex Qtrap 5500 tandem MS). The systematic validation of the method and its use in urine samples has been recently detailed discussed [27]. The brief procedures for the detection of four SM-DNA adducts in blood specimens were as following.
The human blood samples were processed to isolate DNA as the same procedures described previously [13]. The obtained DNA was digested by a formic acid hydrolysis at pH 3.5 with incubation at 90 °C for 1 h. After cooling down to room temperature, the mixture was centrifuged at 12,000 rpm for 15 min, supernatant of 0.3 mL was evaporated to dryness and redissolved in 70 μL water containing fixed concentration of deuterated ISs at 117 ng/mL for UPLC–MS/MS analysis.
In UPLC–MS/MS analysis, the HPLC column was a Waters ACQUITY UPLC BEH C18 (50 mm × 2.1 mm i.d., 1.7 μm) operated at 0.35 mL/min. Mobile phase A was deionized water and phase B was HPLC-grade methanol. The gradient profile started with 15% B and held 1 min, then linearly increased to 60% B over 1 min and held for 0.5 min, then B% decreased to 15% in 0.1 min and held for 1.4 min, the total run time was 4 min. An injection of 3 μL sample extract was used for all analyses. The quantification precursor/product ion pairs monitored for N7-HETEG, O6-HETEG, N3-HETEA and Bis-G were m/z 256 → 105, m/z 256 → 105, m/z 240 → 105 and m/z 389 → 210, and for corresponding deuterated ISs were m/z 260 → 109, m/z 260 → 109, m/z 244 → 109 and m/z 393 → 214, respectively.
2.8. GC–MS analysis of HETE-valine adduct of hemoglobin in blood samples
The exposed blood samples for N-terminal valine adducts in hemoglobin was analyzed by the method developed in our laboratory [23], [24]. Briefly, the globin was firstly isolated according to the procedure described by Bailey et al. [28]. The obtained globin was dissolved in formamide (10 mg/mL) and incubated with 8 μL pyridine and 8 μL PFPITC along with 50 μL deuterated IS solution of 10 mg/mL at 60 °C for 2 h. After cooling to room temperature, the mixture was extracted for three times using toluene (of 1 mL each) and the organic layers were merged and washed with water (of 0.5 mL each, for 2 times), 0.1 mol/L Na2CO3 solution (of 0.5 mL), and water (of 0.5 mL). The toluene layer was dried with anhydrous MgSO4, then evaporated to dryness under nitrogen, and dissolved in 100 μL toluene. The samples were applied onto a Florisil cartridge (500 mg, 3 mL) which precondition with 4 mL ethyl acetate/dichloromethane at 1:9 (v/v) ratio followed by 2 mL dichloromethane. The cartridges were washed with 2 mL dichloromethane and 1 mL ethyl acetate/dichloromethane at 1:9 (v/v) ratio and the analytes were then eluted with 2 mL ethylacetate/dichloromethane at 1:9 (v/v) and evaporated to dryness. The elute was dissolved in 200 μL toluene and derivated with HFBI of 14 mg at 60 °C for 30 min. The reaction mixture was washed with 200 μL water, 200 μL of 0.1 mol/L Na2CO3 and again with water before vaporization to dryness. It was redissolved in 50 μL toluene and analyzed by ID–NCI–GC–MS.
GC–MS analysis was performed on a 6890N GC-5975 MS detector, together with a 7683B autoinjector (Aglient, Palo Alto, CA, USA). The GC column was a HP-5MS capillary column (25 m × 200 μm × 0.33 μm). Helium was used as the carrier gas in the constant flow of 0.8 mL/min. The oven temperature was initially held at 120 °C for 2 min, increased to 280 °C at a rate of 20 °C/min, and finally held at 280 °C for 5 min. Splitless injections of 1 μL were made with an autosampler for 30 s. The injector and transfer-line temperatures were 260 °C and 280 °C, respectively. An MS analysis was conducted using NCI with methane as the reactant gas. The source and quadrupole temperatures were both set at 150 °C. Anions at m/z 564 (M−-3 HF, analyte) and 568(M−-3HF, IS) were monitored in SIM mode.
3. Results and discussion
According to the symptoms and contact histories of the patients, it is largely suspected that they have intimately contacted with one barrel containing WWII abandoned chemical weapons in China, and the most responsible causative agent might be SM. Urine, blood and blister exudate samples collected from four patients who had developed various degrees of blistering were tested for SM related breakdown products or adducts, i.e., hydrolysis/oxidation products, β-lyase metabolites, DNA adducts and protein adducts, with an aim to provide full evidence from mutual corroboration.
3.1. Hydrolysis/oxidation products in urine, blood and exudate samples
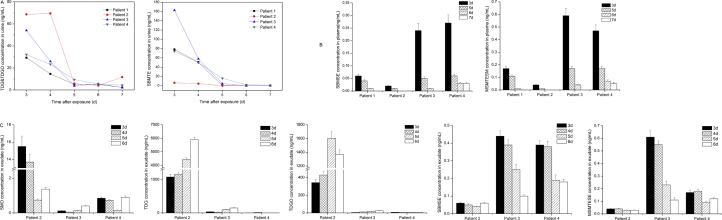
It should be noted both TDG and TDGO are endogenous substances, their background values in vivo were 0–1 ng/mL and 2–8 ng/mL, respectively [29], [30]. Only data with statistical difference can be considered for the influence of administrated exogenous matter. The hydrolysis/oxidation products of SM were initially analyzed in urine samples by the established ID–NCI–GC–MS method. As shown in Table 2, TDG or TDG plus TDGO in the urine samples from four patients collected from day 3 to 7 can be positively detected, and all values are greater than the limits of detection (LLOD) of 0.1 ng/mL for both metabolites, but only part of data are larger than the background values. TDG plus TDGO were found in much higher concentration than TDG, meaning that the concentration of TDGO in exposed humans was higher than that of TDG, as previously reported [14], [31]. The concentration-time profile of TDG, and TDG plus TDGO is similar; both of them were higher on days 3–4 after exposure and then gradually decreased from day 5 to day 7 (Fig. 2A).
Table 2.
Amount of hydrolysis/oxidation products and β-lyase metabolites of sulfur mustard in samples from four individual patients.a
| Patient (burn degree) | Day after exposure | Urined |
Bloode |
Exudatef |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TDGc | TDG + TDGOb,c | SBMTEb,c | SBMSE | MSMTESE | SMO | SBMSE | MSMTESE | SMO | TDG | TDGO | SBMSE | MSMTESE | ||
| 1 (I° 1%) | 3 | 0.48 | 29.3 | 78.2 | 12.7 | 44.9 | D | 0.06 | 0.17 | |||||
| 4 | 1.30 | 14.6 | 50.7 | 64.7 | 26.8 | NA | NA | |||||||
| 5 | 1.13 | 5.11 | 0.39 | 7.74 | 4.93 | 0.04 | 0.11 | NA | ||||||
| 6 | 0.26 | 5.02 | 0.03 | ND | 0.58 | 0.01 | 0.01 | |||||||
| 7 | 0.30 | 2.30 | 0.14 | ND | 0.08 | ND | ND | |||||||
| 2 (I° 15% II° 3%) | 3 | 2.30 | 68.6 | 6.30 | 3.76 | 2.22 | D | 0.02 | 0.04 | 15.5 | 1080 | 343 | 0.06 | 0.04 |
| 4 | 2.25 | 69.5 | 4.44 | 14.1 | 8.73 | NA | NA | 13.7 | 1165 | 428 | 0.05 | 0.04 | ||
| 5 | 0.44 | 5.78 | 0.02 | ND | 0.14 | 0.01 | 0.01 | 0.74 | 4430 | 1605 | 0.04 | 0.03 | ||
| 6 | 0.30 | 4.05 | 0.02 | ND | 0.08 | ND | ND | 1.37 | 5900 | 1370 | 0.06 | 0.03 | ||
| 7 | 0.30 | 11.7 | 0.12 | ND | 0.08 | ND | ND | NA | NA | NA | NA | NA | ||
| 3 (I° 7% II° 2%) | 3 | 1.65 | 54.1 | 163 | 183 | 47.0 | D | 0.24 | 0.59 | 0.12 | 33.8 | 4.94 | 0.44 | 0.61 |
| 4 | 4.34 | 25.9 | 58.0 | 151 | 51.6 | NA | NA | 0.05 | 24.9 | 11.2 | 0.39 | 0.55 | ||
| 5 | 1.05 | 3.68 | 4.53 | 14.7 | 19.8 | 0.05 | 0.17 | 0.13 | 100 | 18.7 | 0.25 | 0.23 | ||
| 6 | 1.04 | 5.58 | 0.16 | ND | 1.55 | 0.01 | 0.04 | 0.40 | 148 | 25.1 | 0.10 | 0.11 | ||
| 7 | 0.30 | 2.20 | 0.06 | ND | 0.06 | ND | ND | NA | NA | NA | NA | NA | ||
| 4 (I° 8% II° 2%) | 3 | 1.00 | 32.2 | 74.3 | 128 | 138 | D | 0.27 | 0.47 | 0.85 | 2.81 | 7.22 | 0.39 | 0.17 |
| 4 | 0.90 | 23.3 | 49.6 | 158 | 76.6 | NA | NA | 0.73 | 20.2 | 7.58 | 0.38 | 0.18 | ||
| 5 | 1.62 | 9.44 | 15.9 | 32.5 | 23.8 | 0.06 | 0.17 | 0.16 | ND | 6.38 | 0.19 | 0.09 | ||
| 6 | 0.30 | 5.47 | 2.0 | 13.5 | 10.5 | 0.03 | 0.17 | 0.90 | ND | 7.08 | 0.18 | 0.12 | ||
| 7 | 0.30 | 4.32 | 1.22 | 2.14 | 1.87 | 0.03 | 0.05 | NA | NA | NA | NA | NA | ||
Abbreviations: ND, not detected; NA, not applicable; D, detected but the amount is below LLOQ which cannot be accurately measured.
Results are showed for determinations performed in duplicate or triplicate. The unit of measurement is ng/mL.
After reduction with TiCl3.
detected by GC–MS methods.
SMO, TDG, TDGO, SBSNAE and SBMTE were not detected or found in the urine samples by HPLC–MS/MS method.
TDG, TDGO, SBSNAE and SBMTE were not detected or found in the blood samples by HPLC–MS/MS method.
SBSNAE and SBMTE were not found in the exudate samples by HPLC–MS/MS method.
Fig. 2.
Hydrolysis/oxidation products and β-lyase metabolites of sulfur mustard in urine, blood and exudate samples from four individual patients. Results are showed for determinations performed in duplicate or triplicate. (A) The TDG plus TDGO concentration and SBMTE concentration in urine samples respectively determined by GC–MS based method. (B) The SBMSE and MSMTESE concentration in blood samples determined by UPLC–MS/MS method. (C) The hydrolysis/oxidation products and β-lyase metabolites including SMO, TDG, TDGO, SBMSE and MSMTESE were detected in exudate samples from patients 2–4 using UPLC–MS/MS method.
The concentration of TDG and TDG plus TDGO in urine samples of patient 2 maintained at a relative high level of ca. 2.3 ng/mL and 69 ng/mL on the beginning two sampling days (days 3 and 4), respectively, which was in accordance with his relative severe clinical symptom. The TDG level in the other three patients exhibited a sudden rise on day 4 or 5, which may result from the further hydrolysis of SM that becomes sequestered in adipose tissue because of its lipophilic nature and from the hydrolysis of unstable SM-macromolecule covalent complex [32]. The highest concentration of TDG was 4.34 ng/mL in the samples of patient 3 on day 4, who exhibited an exception of facial injury, indicating that an inhalational exposed route probably occurred and resulted in a more severe and complex symptom. Moreover, the concentration of TDG of patient 3 was higher than patient 4, although the chemical burn areas of the two individuals were almost same. The TDG level of patient 1 was the lowest among the exposed patients corresponding to the longest asymptomatic latency period and the smallest chemical burn area. All TDG in urine samples kept at a relative low level of no more than 5 ng/mL in case of no sampling time point within 48 h after exposure. Our previous results of TDG in samples of SM dermal exposed rabbit achieved maximum at the first two days and then decreased quickly over 2 days after exposure [25]. Here we can rationally suppose that for these patients, their TDG level in day 1 and 2 would be higher than the values of following days.
The maximum levels of TDG plus TDGO in urine samples collected on day 3 were 54.1, 32.2 and 29.3 ng/mL for patients 3, 4 and 1, respectively, with a positive correlation with the severity of the symptoms. On days 5 to 7, the concentrations of TDG plus TDGO for these four patients were well above the lower limit of quantification (LLOQ) of 0.3 ng/mL, but were lower than the background values, indicating an elimination mechanism toward intoxicant in vivo has taken effect. β-Glucuronidase was also used to release the TDG and TDGO metabolites from their glucuronide or sulphate conjugates and the results revealed that there was little conjugated TDG or TDGO existed in exposed human (data not shown).
TDG and TDGO as two individual analytes were also directly detected in blood and exudate samples with the validated UPLC–MS/MS method developed in our laboratory [21]. TDG and TDGO were not detected in the blood samples of patients after 48 h of exposure. According to our previous results, which showed that for SD rat dermal exposure model, the detection time windows for quantification of TDG and TDGO in plasma were 4 and 12 h, respectively, while the detection windows of TDG and TDGO for human patients here must be less than 48 h. On the contrary, a remarkably high level of TDG and TDGO was interestingly observed in the exudate samples in the post-exposed days. For example, the concentration of TDG of patient 2 was 1080 ng/mL on day 3 and elevated to 5900 ng/mL on day 6, and the TDGO concentration also elevated from 343 ng/mL to 1370 ng/mL. The concentrations of TDG and TDGO in exudate samples were in good accordance with the extent of injury of the patients. After dermal administrated, SM was directly adsorbed into skin tissue, and would generate large amount of hydrolyted/oxidated products TDG and TDGO.
SMO, the direct oxidative metabolite of SM, was also tested in urine, blood and exudate samples from the suspected exposed patients. SMO was identified to be a very minor metabolite in rat urine and therefore it did not attract attention as a SM biomarker before [9]. Anyway, considering SMO is not an endogenous substance, its existence, even minor, is a direct evidence for SM exposure. In our recent work toward SM dermal exposed rats, we found that SMO was a predominant component in the circulation with a high level in the detection time window of 12 h [21]. In this work, for these four patients, we found no SMO in their urine samples, a low but detectable level of SMO in plasma samples with all values between LLOD (0.03 ng/mL) and LLOQ (0.05 ng/mL), and positive results in all exudate samples of patients 2, 3 and 4. Among these three patients, a highest level of SMO appeared in patient 2 with the most serious chemical burn symptom. The concentration of SMO on day 6, the last sampling day, was still kept eightfold greater than LLOQ.
Considering the apparently positive results of TDG, TDGO and SMO in exudates (Fig. 2C), it is indicated that the determination of hydrolysis/oxidation products of SM in exudate is especially useful for direct evidence of SM exposure and further rough estimation of exposure extent.
3.2. β-Lyase metabolites in urine, blood and exudate samples
Unlike TDG and TDGO but similar as SMO, β-lyase metabolites of SM are only produced upon exposure to SM. In GC–MS analysis, SBMTE, SBMSE and MSMTESE are usually reduced to one analyte, SBMTE, by treatment with TiCl3 [15], [33], [34], as the method we adopted here for urine specimens. SBMTE was detected in all samples from patients and the concentration profile showed a common pattern for non-persistent organic toxicants (Fig. 2A). The time-dependent profile shows a decreasing curve, with a sharp decline from day 4 to day 5. Among all patients, Patient 3 had the highest SBMTE level of 163 ng/mL and 58 ng/mL in urine on days 3 and 4, patients 1 and 4 had the similar but middle level, while patient 2, unexpectedly, had the lowest level of 6.30 ng/mL and 4.44 ng/mL, respectively (Table 2). This result presumably due to large and rapid depletion of glutathione in patient 2 with the most severe burn degree, maybe a quickly declined level of β-lyase metabolites was occurred beyond day 2. Another possible reason is that different medical treatments for individuals and varied glutathione detoxification ability among individuals may also lead to the difference of such results. Previous reports have concluded that glutathione related to the resistance of cells to alkylating agents and a significant loss of blood, hepatic and pulmonary glutathione observed in animals exposed to SM [35], [36]. This in turn suggested that prophylactic glutathione may help relieve the symptoms caused by SM, which was valuable for the treatment of exposed individuals.
The glutathione derived metabolites of SBSNAE, SBMTE, SBMSE and MSMTESE were simultaneously measured by our UPLC–MS/MS method. While SBSNAE and SBMTE were not detected, SBMSE and MSMTESE were positively detected in urine, blood and exudate samples, both LLOQs are 0.01 ng/mL for the latter two analytes. The fewest amounts of the two β-lyase metabolites were also observed in the body fluid samples from patient 2. For three sources of samples, high level at tens to hundreds ng/mL of SBMSE and MSMTESE was observed in urine samples due to the main kidney metabolite pathway of glutathione conjugates. The concentrations of the two β-lyase metabolites in blood or exudate samples were much lower, usually at sub ng/mL to ng/mL level (Fig. 2B and C). Since the β-lyase metabolites have been verified as an unequivocal diagnostic indicator of SM exposure, our positive results of such the β-lyase metabolites, together with hydrolysis/oxidation products, can clearly provide evidence of SM exposure to these four patients.
3.3. DNA adducts in urine and blood samples
Previous researches have demonstrated that the DNA alkylation and following inhibition of DNA replication were the molecular basis of the cytotoxicity caused by SM. Among the known SM-DNA adducts, N7-HETEG has the prominent abundance and has been proved to be a useful biomarker in vivo [37], [38], [39]. The methods including radio labeling, immunoassay and chromatography coupled with different detection techniques have been reported. For example, one immunoassay based on the monoclonal antibody against N7-HETEG was applied to blood samples from two Iranian victims of the Iran–Iraq conflict to confirm the exposure to SM [17]. However, there is no report of simultaneous detection and quantification of the four DNA adducts in blood, derma or urine specimen except UPLC–MS/MS methods recently developed in our laboratory [26], [27], the LLOQs of 0.005–0.010 ng/mL were achieved for these four SM-DNA adducts.
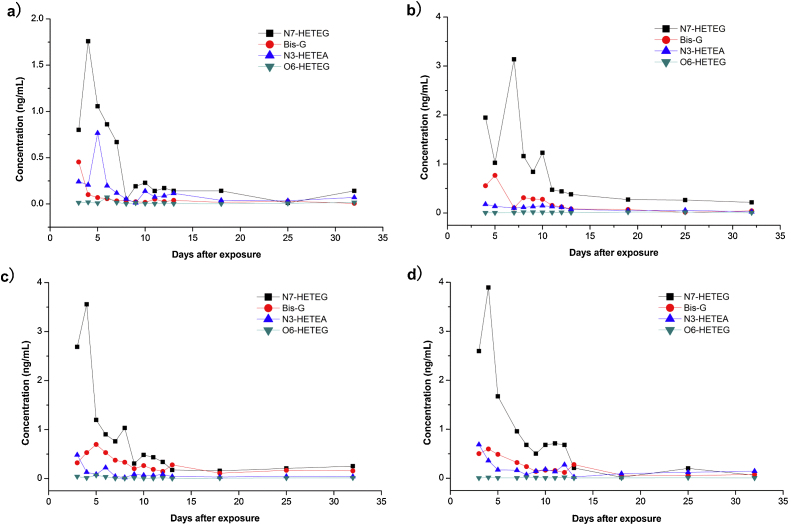
In urine samples, the kind of DNA adduct is free DNA adducts. They are directly removed from DNA strands during the process of DNA repair, delivered into the circulatory system and then passed out of the body through the urine. To some extent, the content of free SM-DNA adducts in urine can directly represent the inherent easy-to-be-repaired part of DNAs, and can be used to evaluate the damage of DNAs. The four free alkylated bases as the form of SM-DNAs adducts were positively detected from the first sampling day (day 3) up to day 32 after exposure, with a maximum peak on days 4–7 (Fig. 3). Of all four SM-DNA adducts, N7-HETEG was the most abundant one, Bis-G and N3-HETEA was less, O6-HETEG was the least. This is in accordance with the results observed in rabbits that approximately 67%, 23% and 10% of the DNA alkylation products were N7-HETEG, Bis-G and N3-HETEA respectively, while just ca. 0.1% of the total alkylation was O6-HETEG [27]. In spite of the minimum content, O6-HETEG has been noted for its interference of G-C Watson-Crick base pairs, also accepted as a most important biomarker of DNA damage by SM [40]. The results here initially revealed that all the four SM-DNA adducts could be used to determine the human exposure to SM, and the concentrations of DNA adducts are positively related to the exposure extent. Patients 2, 3 and 4 always have a higher level of the DNA adducts than patient 1, whose injury was the lightest. The time-dependent profiles of the patients are similar, especially for patient 3 and 4 due to their almost equal burn area and degree. Taking patient 3 as an example, N7-HETEG achieved the maximum of 3.55 ng/mL on day 4, decreased to 0.2 ng/mL on day 13 and then kept at the sub ng/mL level until day 32. Other three SM-DNAs have same trends.
Fig. 3.
Time-dependent profiles of four sulfur mustard-DNA adducts in urine samples from exposed individuals. An isotope-dilution ultrahigh performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) combining with solid phase extraction was performed to simultaneously detect the four DNA adducts which include N7-HETEG, Bis-G, N3-HETEG and O6-HETEG in urine samples. (a) Patient 1; (b) patient 2; (c) patient 3; (d) patient 4. Results are showed for determinations performed once due to the limited sample volume.
In blood samples, the adducts are covalently alkylated to DNAs, which need further heat or enzymatic cleavage procedures when processing the samples. In our experiment, the four SM-DNA adducts were detected after DNA extraction and digestion, and showed positive results for all patients on day 3 after exposure with concentrations of 0.03–0.09 ng/mL, and then decreased to a detectable level until day 14 (data not shown). N7-HETEG was still the most abundant one in the blood sample, Bis-G and N3-HETEA were the less, and few O6-HETEG was detected. The detected low concentrations of the SM-DNA adducts in plasma should be due to the sampling time point outside the detection window of 24 h for blood samples suffered to DNA adducts in vivo detection. Despite of the low level, the concentrations of the four SM-DNA adducts also demonstrated a good relationship with the exposure extent of the patients.
3.4. HETE-Val adducts in blood samples
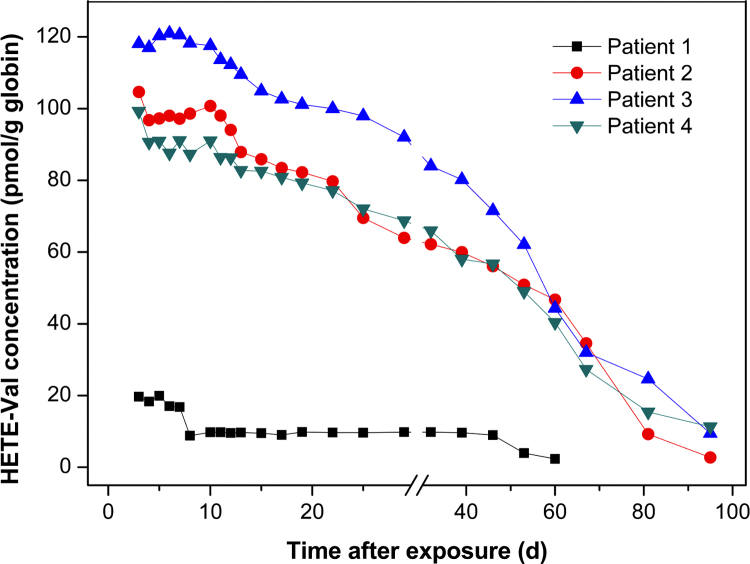
N-alkylated valine originated from the N-terminal valine of hemoglobin as a biological marker of exposure to alkylating agents has been previously reported [16], [17], [41]. Recent studies also showed that HETE-Val produced in the hemoglobin of blood treated with SM in vitro or in vivo. Blood samples of the four patients collected from days 3 to 95 after exposure were analyzed to profile HETE-Val (Fig. 4). High concentrations of HETE-Val were found on days 3–7 in patients, and continuously declined since the second week after exposure. The time-dependent relationships of patients 2, 3 and 4 were similar, except for slightly higher concentrations of patient 3. With regard to patient 3, the concentration of HETE-Val level was ca. 120 pmol/g hemoglobin in the first week, and decreased to 90 pmol/g hemoglobin one month later, further gradually decreased to 10 pmol/g hemoglobin three months after exposure (day 95), which was still above the LLOD (7 pmol/g). With regard to patient 1 of much lower HETE-Val concentration in his whole treated course, which is corresponding to a minimal chemical burn, the HETE-Val level was 20 pmol/g hemoglobin on day 3 and decreased to 9.8 pmol/g hemoglobin one month later, and to 2.3 pmol/g hemoglobin two months after exposure (day 60). Our results revealed a long persistence of SM-hemoglobin adduct in human plasma, which is in good accordance with the life-time of the erythrocyte of the human (approximate 120 days). It is indicated that HETE-Val adduct provides a valuable biomarker of SM exposure, especially for the retrospective analysis of the archived samples.
Fig. 4.
Persistence of N-terminal valine adduct in the blood of four patients after exposure to sulfur mustard. The blood samples were treated by solid phase extraction followed by modified Edman degradation and the extracted HETE-Val adducts were determined by established NCI–GC/MS method. Results are showed for determinations performed once due to the limited sample volume.
4. Conclusions
The accidental human exposure to chemical that caused blisters has provided a precious opportunity to comprehensively investigate the four types of SM biomarkers in urine, blood and exudate samples collected from four patients with different extent of exposure. LC–MS and GC–MS methods with high sensitivity and selectivity developed in our laboratory were used. Hydrolysis/oxidation products, glutathione derived β-lyase metabolites, DNA adducts and hemoglobin adducts were successfully detected in the samples, mutually verifying the SM exposure to the four individuals. The concentrations of the biomarkers in specimens revealed a good correlation with the severity of the patient's symptom.
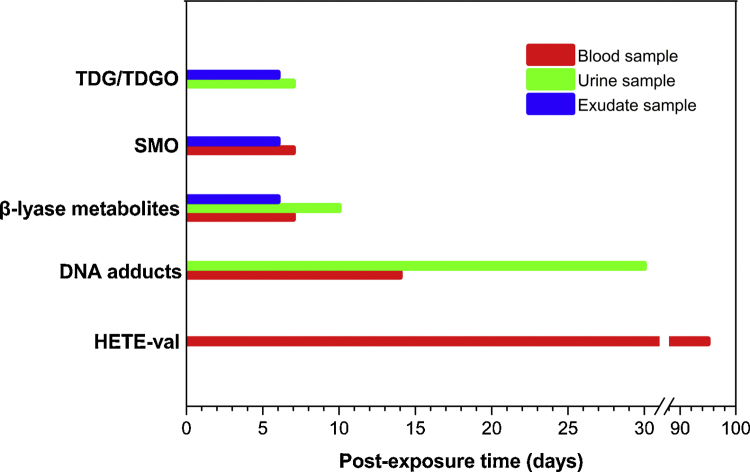
Most importantly, the data presented here also yield insights into the preliminary establishment of detection window of four kinds of metabolites or adducts for human, as shown in Fig. 5, which includes “double what” and “when”, i.e., to determine which kind of SM related biomarkers (“what”) in which time(“when”), and in which sample(“what”).
Fig. 5.
Time course of the biomarkers for the retrospective analysis of sulfur mustard exposure to human. The detection windows of the metabolites or adducts for clinical samples collected from sulfur mustard-exposed human were established on the basis of the detection results.
During the first week after a SM exposure, both urine and blister exudate samples are valuable biomedical samples, and the hydrolysis/oxidation products, TDG and TDGO, combining with the β-lyase metabolites, SBMTE, SBMSE and MSMTESE can be detected in the urine and exudate samples during the early period, as firstly noted here, direct oxidation product, SMO, can be also detected in exudate samples for early diagnosis of SM exposure. As for blood samples, a low level of SMO, SBMSE and MSMTESE were observed, and TDG, TDGO and SBMTE were not detected here due to the detection windows for them in plasma are always within 12 h.
From the first week to the end of one month, covalent binding adducts including four DNA adducts can be found excreted in urine from the first sampling day up to day 30. Although SM-DNA adducts are assumed to circulate in blood for a long period up to several months according to the long lifetime of DNAs, it is not suggested to continually determine such biomarkers in blood samples in one month, since they only show relative high concentration in the first 3 days after exposure. From the first week to the end of one quarter, the SM-hemoglobin adduct, here refers to HETE-Val, is likely to be a satisfied choice in blood samples to confirm SM exposure. Even over 90 days after exposure, HETE-Val is still persistent in the specimens, showing great importance to the retrospective researches and the clinical treatment monitoring of SM exposed humans.
Conflict of interest
None.
Transparency document
Acknowledgement
This paper was financially supported by National Science & Technology Major Project of the Ministry of Science and Technology of the People's Republic of China (Grant No. 2012ZX09301003-001-010).
Footnotes
Available online 13 August 2014
Contributor Information
Lei Guo, Email: guolei@bmi.ac.cn.
Jianwei Xie, Email: ammslta@163.com, xiejwbmi@163.com.
References
- 1.Ghabili K., Agutter P.S., Ghanei M., Ansarin K., Panahi Y., Shoja M.M. Sulfur mustard toxicity: history, chemistry, pharmacokinetics, and pharmacodynamics. Crit. Rev. Toxicol. 2011;41:384–403. doi: 10.3109/10408444.2010.541224. [DOI] [PubMed] [Google Scholar]
- 2.Kehe K., Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214:198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Kehe K., Thiermann H., Balszuweit F., Eyer F., Steinritz D., Zilker T. Acute effects of sulfur mustard injury – Munich experiences. Toxicology. 2009;263:3–8. doi: 10.1016/j.tox.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 4.Davis K.G., Aspera G. Exposure to liquid sulfur mustard. Ann. Emerg. Med. 2001;37:653–656. doi: 10.1067/mem.2001.114322. [DOI] [PubMed] [Google Scholar]
- 5.Newmark J., Langer J.M., Capacio B., Barr J., McIntosh R.G. Liquid sulfur mustard exposure. Mil. Med. 2007;172:196–198. doi: 10.7205/milmed.172.2.196. [DOI] [PubMed] [Google Scholar]
- 6.Ruhl C.M., Park S.J., Danisa O., Morgan R.F., Papirmeister B., Sidell F.R., Edlich R.F., Anthony L.S., Himel H.N. A serious skin sulfur mustard burn from an artillery shell. J. Emerg. Med. 1994;12:159–166. doi: 10.1016/0736-4679(94)90693-9. [DOI] [PubMed] [Google Scholar]
- 7.Thomsen A.B., Eriksen J., Smidt-Nielsen K. Chronic neuropathic symptoms after exposure to mustard gas: a long-term investigation. J. Am. Acad. Dermatol. 1998;39:187–190. doi: 10.1016/s0190-9622(98)70072-6. [DOI] [PubMed] [Google Scholar]
- 8.Weibrecht K., Rhyee S., Manuell M.E., Longo C., Boyer E.W., Brush E. Sulfur mustard exposure presenting to a community emergency department. Ann. Emerg. Med. 2012;59:70–74. doi: 10.1016/j.annemergmed.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Black R.M., Brewster K., Clarke R.J., Hambrook J.L., Harrison J.M., Howells D.J. Biological fate of sulphur mustard, 1,1′-thiobis(2-chloroethane): isolation and identification of urinary metabolites following intraperitoneal administration to rat. Xenobiotica. 1992;22:405–418. doi: 10.3109/00498259209046652. [DOI] [PubMed] [Google Scholar]
- 10.Black R.M., Hambrook J.L., Howells D.J., Read R.W. Biological fate of sulfur mustard, 1,1′-thiobis(2-chloroethane). Urinary excretion profiles of hydrolysis products and beta-lyase metabolites of sulfur mustard after cutaneous application in rats. J. Anal. Toxicol. 1992;16:79–84. doi: 10.1093/jat/16.2.79. [DOI] [PubMed] [Google Scholar]
- 11.Davison C., Rozman R.S., Smith P.K. Metabolism of bis-beta-chloroethyl sulfide (sulfur mustard gas) Biochem. Pharmacol. 1961;7:65–74. doi: 10.1016/0006-2952(61)90127-7. [DOI] [PubMed] [Google Scholar]
- 12.Fidder A., Noort D., de Jong A.L., Trap H.C., de Jong L.P., Benschop H.P. Monitoring of in vitro and in vivo exposure to sulfur mustard by GC/MS determination of the N-terminal valine adduct in hemoglobin after a modified Edman degradation. Chem. Res. Toxicol. 1996;9:788–792. doi: 10.1021/tx9502150. [DOI] [PubMed] [Google Scholar]
- 13.Wei Y., Yue L., Liu Q., Chen J., Xie J. A sensitive high performance liquid chromatography–positive electrospray tandem mass spectrometry method for N7-[2-[(2-hydroxyethyl)thio]-ethyl]guanine determination. J. Chromatogr. B: Analyt. Technol. Biomed. Life Sci. 2011;879:1707–1712. doi: 10.1016/j.jchromb.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 14.Black R.M., Read R.W. Biological fate of sulphur mustard, 1,1′-thiobis(2-chloroethane): identification of beta-lyase metabolites and hydrolysis products in human urine. Xenobiotica. 1995;25:167–173. doi: 10.3109/00498259509061842. [DOI] [PubMed] [Google Scholar]
- 15.Barr J.R., Pierce C.L., Smith J.R., Capacio B.R., Woolfitt A.R., Solano M.I., Wooten J.V., Lemire S.W., Thomas J.D., Ash D.H., Ashley D.L. Analysis of urinary metabolites of sulfur mustard in two individuals after accidental exposure. J. Anal. Toxicol. 2008;32:10–16. [PubMed] [Google Scholar]
- 16.Black R.M., Clarke R.J., Harrison J.M., Read R.W. Biological fate of sulphur mustard: identification of valine and histidine adducts in haemoglobin from casualties of sulphur mustard poisoning. Xenobiotica. 1997;27:499–512. doi: 10.1080/004982597240460. [DOI] [PubMed] [Google Scholar]
- 17.Benschop H.P., van der Schans G.P., Noort D., Fidder A., Mars-Groenendijk R.H., de Jong L.P. Verification of exposure to sulfur mustard in two casualties of the Iran–Iraq conflict. J. Anal. Toxicol. 1997;21:249–251. doi: 10.1093/jat/21.4.249. [DOI] [PubMed] [Google Scholar]
- 18.Noort D., Hulst A.G., de Jong L.P., Benschop H.P. Alkylation of human serum albumin by sulfur mustard in vitro and in vivo: mass spectrometric analysis of a cysteine adduct as a sensitive biomarker of exposure. Chem. Res. Toxicol. 1999;12:715–721. doi: 10.1021/tx9900369. [DOI] [PubMed] [Google Scholar]
- 19.Noort D., Fidder A., Hulst A.G., de Jong L.P., Benschop H.P. Diagnosis and dosimetry of exposure to sulfur mustard: development of a standard operating procedure for mass spectrometric analysis of haemoglobin adducts: exploratory research on albumin and keratin adducts. J. Appl. Toxicol. 2000;20:S187–S192. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat676>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 20.Smith J.R., Capacio B.R., Korte W.D., Woolfitt A.R., Barr J.R. Analysis for plasma protein biomarkers following an accidental human exposure to sulfur mustard. J. Anal. Toxicol. 2008;32:17–24. doi: 10.1093/jat/32.1.17. [DOI] [PubMed] [Google Scholar]
- 21.Li C., Chen J., Liu Q., Xie J., Li H. Simultaneous quantification of seven plasma metabolites of sulfur mustard by ultra high performance liquid chromatography–tandem mass spectrometry. Anal. Technol. Biomed. Life Sci. 2012;917–918:100–107. doi: 10.1016/j.jchromb.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Lin Y., Dong Y., Chen J., Li C.Z., Nie Z.Y., Guo L., Liu Q., Xie J.W. Gas chromatographic–tandem mass spectrometric analysis of β-lyase metabolites of sulfur mustard adducts with glutathione in urine and its use in a rabbit cutaneous exposure model. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2013;945–946:233–239. doi: 10.1016/j.jchromb.2013.11.058. [DOI] [PubMed] [Google Scholar]
- 23.Nie Z., Liu Q., Xie J. Improvements in monitoring the N-terminal valine adduct in human globin after exposure to sulfur mustard and synthesis of reference chemicals. Talanta. 2011;85:1154–1159. doi: 10.1016/j.talanta.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 24.Nie Z., Zhang Y., Dong Y., Wu B., Liu Q., Feng J., Xie J. Determination of the N-terminal valine adduct in rabbit hemoglobin after skin exposure to sulfur mustard. Sci. Sin. Vit. 2011;41:965–970. [Google Scholar]
- 25.Nie Z., Zhang Y., Chen J., Lin Y., Wu B., Dong Y., Feng J., Liu Q., Xie J. Monitoring urinary metabolites resulted from sulfur mustard exposure in rabbits based on highly sensitive isotope-dilution gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 2014 doi: 10.1007/s00216-014-7916-3. (in press) [DOI] [PubMed] [Google Scholar]
- 26.Yue L., Wei Y., Chen J., Shi Q., Liu Q., He J., Guo L., Zhang T., Xie J., Peng S. Abundance of four sulfur mustard-DNA adducts ex vivo and in vivo revealed by simultaneous quantification in stable isotope dilution-ultrahigh performance liquid chromatography–tandem mass spectrometry. Chem. Res. Toxicol. 2014;27:490–500. doi: 10.1021/tx4003403. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Yue L., Nie Z., Guo L., Wu B., Feng J., Liu Q., Xie J. Simultaneous determination of four sulfur mustard-DNA adducts in rabbit urine after dermal exposure by isotope-dilution liquid chromatography–tandem mass spectrometry. J. Chromatogr. B. 2014;961:29–35. doi: 10.1016/j.jchromb.2014.04.050. [DOI] [PubMed] [Google Scholar]
- 28.Bailey E., Brooks A.G., Dollery C.T., Farmer P.B., Passingham B.J., Sleightholm M.A., Yates D.W. Hydroxyethylvaline adduct formation in haemoglobin as a biological monitor of cigarette smoke intake. Arch. Toxicol. 1988;62:247–253. doi: 10.1007/BF00332482. [DOI] [PubMed] [Google Scholar]
- 29.Black R.M., Read R.W. Detection of trace levels of thiodiglycol in blood, plasma and urine using gas chromatography–electron-capture negative-ion chemical ionisation mass spectrometry. J. Chromatogr. 1988;449:261–270. doi: 10.1016/s0021-9673(00)94385-1. [DOI] [PubMed] [Google Scholar]
- 30.Black R.M., Read R.W. Methods for the analysis of thiodiglycol sulphoxide, a metabolite of sulphur mustard, in urine using gas chromatography–mass spectrometry. J. Chromatogr. 1991;558:393–404. doi: 10.1016/0021-9673(91)80006-3. [DOI] [PubMed] [Google Scholar]
- 31.Riches J., Read R.W., Black R.M. Analysis of the sulphur mustard metabolites thiodiglycol and thiodiglycol sulphoxide in urine using isotope-dilution gas chromatography–ion trap tandem mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2007;845:114–120. doi: 10.1016/j.jchromb.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 32.Drasch G., Kretschmer E., Kauert G., von Meyer L. Concentrations of mustard gas [bis(2-chloroethyl)sulfide] in the tissues of a victim of a vesicant exposure. J. Forensic Sci. 1987;32:1788–1793. [PubMed] [Google Scholar]
- 33.Daly J.D., O’Hehir C.M., Frame G.M. A sensitive method for quantitation of beta-lyase metabolites of sulfur mustard as 1,1′-sulfonylbis[2-(methylthio)ethane] (SBMTE) in human urine by isotope dilution liquid chromatography–positive ion-electrospray-tandem mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2007;850:120–127. doi: 10.1016/j.jchromb.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 34.Young C.L., Ash D., Driskell W.J., Boyer A.E., Martinez R.A., Silks L.A., Barr J.R. A sensitive method for quantitation of beta-lyase metabolites of sulfur mustard as 1,1′-sulfonylbis[2-(methylthio)ethane] (SBMTE) in human urine by isotope dilution liquid chromatography-positive ion-electrospray–tandem mass spectrometry. J. Anal. Toxicol. 2004;28:339–345. doi: 10.1093/jat/28.5.339. [DOI] [PubMed] [Google Scholar]
- 35.Gross C.L., Innace J.K., Hovatter R.C., Meier H.L., Smith W.J. Biochemical manipulation of intracellular glutathione levels influences cytotoxicity to isolated human lymphocytes by sulfur mustard. Cell Biol. Toxicol. 1993;9:259–267. doi: 10.1007/BF00755604. [DOI] [PubMed] [Google Scholar]
- 36.Maisonneuve A., Callebat I., Debordes L., Coppet L. Biological fate of sulphur mustard in rat: toxicokinetics and disposition. Xenobiotica. 1993;23:771–780. doi: 10.3109/00498259309166783. [DOI] [PubMed] [Google Scholar]
- 37.Fidder A., Moes G.W., Scheffer A.G., van der Schans G.P., Baan R.A., de Jong L.P., Benschop H.P. Synthesis, characterization, and quantitation of the major adducts formed between sulfur mustard and DNA of calf thymus and human blood. Chem. Res. Toxicol. 1994;7:199–204. doi: 10.1021/tx00038a013. [DOI] [PubMed] [Google Scholar]
- 38.Kehe K., Balszuweit F., Steinritz D., Thiermann H. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263:12–19. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Noort D., Benschop H.P., Black R.M. Biomonitoring of exposure to chemical warfare agents: a review. Toxicol. Appl. Pharmacol. 2002;184:116–126. [PubMed] [Google Scholar]
- 40.Ludlum D.B., Kent S., Mehta J.R. Formation of O6-ethylthioethylguanine in DNA by reaction with the sulfur mustard, chloroethyl sulfide, and its apparent lack of repair by O6-alkylguanine-DNA alkyltransferase. Carcinogenesis. 1986;7:1203–1206. doi: 10.1093/carcin/7.7.1203. [DOI] [PubMed] [Google Scholar]
- 41.Noort D., Fidder A., Degenhardt-Langelaan C.E., Hulst A.G. Retrospective detection of sulfur mustard exposure by mass spectrometric analysis of adducts to albumin and hemoglobin: an in vivo study. J. Anal. Toxicol. 2008;32:25–30. doi: 10.1093/jat/32.1.25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.