Abstract
BisphenolA (BPA) or 2,2-bis(4-hydroxyphenyl)propanepresent in polycarbonate baby bottles may have harmful effects for formula-fed infants. This study evaluated the risks associated with exposure to BPA among Iranian formula-fed infants in an urban society in Isfahan. New and used baby bottles (n = 7 and 8, respectively) as well as BPA-free marked bottles (n = 2) were collected from a retail outlet, and leaching of BPA was examined by conducting a migration test. Concentrations of BPA released from the new and used baby bottles were in the range of 0.49⿿8.58 and 0.63⿿2.47 μg/l, respectively. Next, probabilistic exposure estimation was performed. In all, 200 mothers registered with 11 health centres in Isfahan were interviewed. Data on feeding pattern, washing and sterilization practices, bottles types and manufacturers as well as the sex and weight of the infants were collected using a questionnaire. The results showed that majority of the surveyed infants were exposed to 0.1⿿0.3 μg/kg body weight (bw)/d of BPA, which corresponded to approximately 2⿿7.5% of the defined t-TDI (4 μg/kgbw/d). These results suggested that the risk of the adverse effects caused by exposure to BPA was very low in formula-fed Iranian infants even in the worst-case scenario.
Keywords: Bisphenol A, Risk assessment, Baby bottle, Iran
1. Introduction
Exposure to potentially migrating chemicals from food contact materials is inevitable [8], [20], [3], [29], [47], [17], [33], [2]. Bisphenol A (BPA), a synthetic chemical with identified adverse effects on health, is used as a key building block in the production of the most regular form of polycarbonates (PCs) [21], [38], [17], [18]. Several studies have highlighted the health hazards associated with BPA. Many animal and human studies have shown that BPA has oestrogenic activity [21], [26], [45], [47] and that it disrupts the endocrine functions of oestrogen [40]; therefore, it is also called a xeno-oestrogen [22], [41], [18]. Furthermore, an early exposure to BPA during gestational and neonatal periods can result in irreparable health effects [25], [24], [47], [6], [13]. Adverse health effects associated with BPA exposure include hormone disruption, prostate and breast cancer development, reproductive disorders, delayed puberty in females, poor semen quality and infertility in males [42], [30], [27], neuro-developmental impairment [24], [12], [4], [29], [47], [19] and neurological problems such as attention deficiency, autism and hyperactivity [44], [29]. Formula-fed infants aged <6 months are the most vulnerable to BPA-induced health hazards because they rely on plastic feeding bottles for food intake have immature detoxification system and have a high ratio of food intake per unit body weight (bw) than that adults [36], [45], [18], [43].
The safety of PC-containing baby bottles has been recently debated by safety authorities worldwide [9]. Moreover, Canada, some European countries and some states in the United State of America have banned the use of BPA in bottles, teats and accessories for infants. Individual studies have been performed to investigate the risk of exposure to BPA in baby bottles in infants [35], [31]. The European Food Safety Authority (EFSA) established 50 μg/kg/bw/d as the tolerable daily intake (TDI) of BPA based on non-observable adverse effect level (NOAEL) of 5000 μg/kgbw/d [10]. However, recently EFSA experts has reduced the previously settled TDI to 4 μg/kg bw/d as a temporary base due to the latest refined risk assessment and the uncertainties over the mechanism of BPA action to produce its adverse effects.
Specific migration limit of BPA into food or food stimulants has been established as 600 μg/kg [12]. Food is the main route of exposure to BPA in the general population [3], [37], [11], [27], [28]. But, BPA exposure in formula-fed babies depends on daily intake of formula and BPA migrated from their bottles; both, could be different from country to country.
In Iran no information is available on the concentration of BPA leached from commonly used baby bottles and its subsequent risk in formula-fed babies.
Therefore, the present study aimed to determine the risk of BPA exposure in formula-fed Iranian infants by measuring BPA concentration released from the commonly used brands of polymeric bottles and the current formula-feeding pattern in Iranian middle urban society.
2. Methods and materials
This study was performed in several phases. First, some new baby bottles belonging to best-selling brands were examined for BPA migration. Second, a survey on formula feeding pattern and baby bottles priorities was conducted among the mothers of infants less than 1 year through a questionnaire. Responded by the participants, the most popular polymeric baby bottles were identified, from which, seven samples belonging to the four most used baby bottle brands dedicated by the mothers and used in migration test as used baby bottles. Finally, risk of BPA intake was determined in formula-fed babies aged less than 1 year.
2.1. Chemicals and samples
BPA (99%) was purchased from Sigma⿿Aldrich. Methanol (HPLC grade) and methyl tert-butyl ether (MTBE, 99.9%) was purchased from Merck (Germany). Ultra-pure water (Millipore, Bedford, MA, USA) was used as a food simulant.
2.2. Baby bottles
Table 1 lists the tested bottles along with the name of producer country written on their labels. Selection of the branded samples was based on our preliminary field study results. Accordingly, seven of the best-selling polymer bottles in the market [32] were purchased. In addition, two BPA-free baby bottles were provided from the market. In order to provide used baby bottles, some mothers who had participated in the study were asked to provide us with their used⿿polymeric baby bottles. Hence, eight home-used bottles, which had been used for two to five months were provided by them and tested for BPA migration as the old baby bottles. Detailed information of one of the used samples was unclear because of excessive use. (Table 1). Volume of the bottles varied from 125 ml to 250 ml.
Table 1.
Baby bottle brands used in the current study.
| Sample⿿s category | Sample⿿s code | Commercial brands | Producers country |
|---|---|---|---|
| New bottles | 1 | Camro | Iran |
| 2 | My baby | Iran | |
| 3 | Panberiz | Iran | |
| Not marked BPA free | 4 | Camera | Taiwan |
| 5 | Potato | China | |
| 6 | Wee | Turkey | |
| 7 | Babynova | Germany | |
| Marked BPA free | 8 | Nuby | China |
| 9 | Babynova | Germany | |
| Used bottles | 10 | Camro | Iran |
| 11 | Camro | Iran | |
| 12 | Panberiz | Iran | |
| 13 | Panberiz | Iran | |
| 14 | Mybaby | Iran | |
| 15 | Wee | Turkey | |
| 16 | Mina baby | Iran | |
| 17 | No mark | China | |
2.3. BPA extraction from feeding bottles
BPA was extracted using a method described by Brede [7] with some modifications; Ultra-pure water from Milli-Q filter water (Millipore, Bedford, MA USA) was used as a food simulant. A 500-ml Erlenmeyer flask was filled with double distilled deionised water, and the water was boiled for one min. The boiled water was then poured into the feeding bottles. The bottles were tightly sealed, shaken for 10 min and left to stand in an ambient temperature for 30 min. In the original method, more vigorous condition was applied for migration test as the examined containers were kept at 100 °C for 1 h. The Solid-phase extraction was then performed. Briefly, 100 ml of water from the treated bottles was passed through C18 cartridge (500 mg; Chromabond, Germany) with silica-based bonded phase (4⿿5 inches/Hg), which was conditioned using 5 ml methanol (99%) and 10 ml ultra-pure water. The cartridge was washed with methanol under 1 inch/Hg pressure. The eluted methanol was collected and dried under nitrogen gas (with 99% purity) at room temperature. The dried extract was resolved in 100 μL MTBE from which three μL sampled and was injected to GC⿿MS for quantitative analysis in duplicated. Regarding the new bottles, for each bottle, the total procedure was replicated in two individual experiment runs and the results were expressed as the first and the second tests to compare the concentration of released BPA in the repeated experiment. Used baby bottles were examined once. In addition, upon the information acquired from the questioners regarding the washing and cleaning treatments of bottles in homes, one of the used baby bottles(Wee, 125 ml) was boiled in 2 l of pure water for 15 min and 100 ml of the boiled water was examined for BPA concentration.
2.4. Determination of BPA by gas chromatography
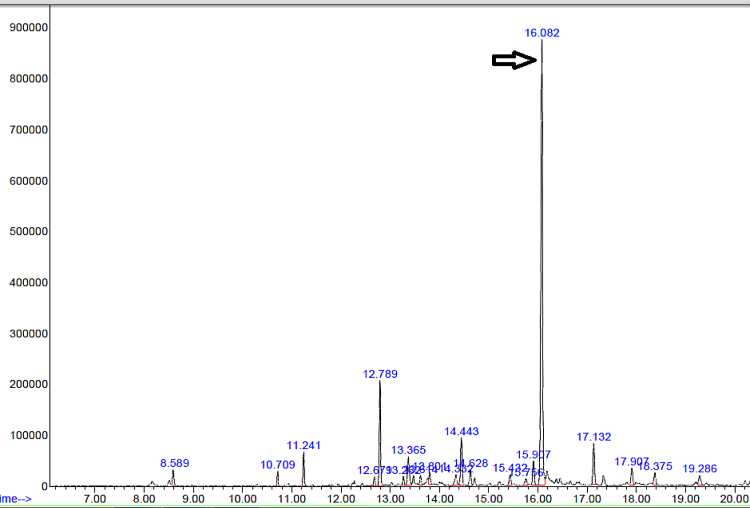
An Agilent Technologies system containing a 5975C inert MSD, a triple axis detector equipped with a 7890A gas chromatograph and a split/splitless injector was used for quantifying and confirming BPA. A fused silica column (HP-5 ms, 5% phenyl and 95% dimethylpolysiloxane, 30 m ÿ 0.25 mm I.D and 0.25-μm film thickness) was used with helium (99.995% purity) as the carrier gas at a flow rate of 1 ml/min. The column temperature was set according to that described by Rastkari et al. [37]: 100 °Cfor 1 min, increased to 210 °C at a rate of 10 °C/min, then to 250 °Cat a rate of 5 °C/min and finally to 280 °Cat a rate of 30 °C/min with holding for 1 min. The total running time was set as 25 min. Fig. 1 shows the BPA identification peak in 16.08 min at 213 and 228 m/z.
Fig. 1.
Representative chromatogram of BPA taken from a baby bottle (Camro).
2.5. Preparation of standard curve
A stock standard solution of BPA (99%) (Sigma⿿Alderich, USA) was prepared by accurate weighting of 0.01 g standard BPA powder in a 100 ml flask. Solved by 100 μl, methanol (HPLC grade), it was brought to the volume by ultra-pure water which was previously tested negative for BPA and was used as standard (0). The stock standard was diluted to desired concentrations using ultra-pure water. All working standard solutions were processed exactly the same as sample. A nine-point standard curve was plotted using BPA concentrations of 0, 0.1, 0.25, 0.5, 1, 5, 10, 25 and 50 μg/l. R2 linear curve was obtained as 0.994.
2.6. Quality control and quality assurance analysis
Regarding the quality control of the excretion procedure, recovery test was conducted using a couple of bottles from Camera and Camro brands. Each bottle filled with 100 ml of hot water (100 °C), of which 20 ml was used for BPA extraction and quantification and another 20 ml aliquot of this water was mixed with 1 ml stock solution of 50 μg/l and was processed similarly, both experiments was performed in duplicate for each of the tested bottles. Extraction recovery averaged as 75% with standard deviation as 16. Adsorption efficiency of solid phase extraction was ascertained in a replicated experiment, when testing 5 μg/l standards solutions of BPA, the eluted water (100 ml) was collected and re-tested for BPA concentration. Adsorption efficiency was ranged as (90.74⿿90.92%).
For BPA assay, reproducibility of the applied method was investigated by measuring BPA concentration of 5 μg/l solution for 5 successive days. Standard deviation and relative standard deviation were 0.433 and 7.36%, respectively.
The lowest concentration at which the BPA can not only be reliably detected but at which some predefined goals for bias and imprecision are met considered as LOQ (0.1 μg/l). LOD was obtained as 0.01 μg/l, using 3* signal/noise at LOQ.
2.7. Exposure assessment
A questionnaire was designed to determine the formula feeding pattern and BPA exposure distribution frequency among the investigated group of infants. The questionnaire intended to obtain three types of data: demographic information, types of bottles used, different methods of formula preparation and bottle cleaning practices. Table 2 lists the questions in the order that they appeared in the questioner. Sample size was determined by using the following formula.
where Z1⿿α/2:95% confidence interval, d: sampling error (0.5) and S: standard deviation (6.1).
Table 2.
The list of questions asked in the questionnaire.
| Demographic information | |
|---|---|
| 1) | What is the mother⿿s age?.................year |
| 2) | What is the mother⿿s education level? (1) Illiterate, (2) primary educated (3) high school (4) diploma (5) educated |
| 3) | What is the mother⿿s occupation? (1) Householder (2) Working in home (3) employer (4) others..... |
| 4) | What is the father⿿s age?...............year |
| 5) | What is the father⿿s education level? (1) Illiterate, (2) primary educated (3) high school (4) diploma (5) educated |
| 6) | What is the father⿿s occupation? (1) Unemployed (2) employed (3) Self-employed (4) others. |
| 7) | Which number of the family⿿s children is this baby? (1) first (2) Second (3) third 4) forth and more |
| Baby⿿s age, weight and gender and status | |
|---|---|
| 8) | What is the baby⿿s age?..............months. (1) less than six months (2) more than six months |
| 9) | What is the baby⿿s gender? (1) girl (2) boy |
| 10) | What is the weight of baby? (Filled after weighting by a health care centre consoler) |
| Formula feeding pattern and formal preparation | |
|---|---|
| 11) | How is the baby formula-fed? (1) exclusively formula feeding (2) mixed feeding (formula and breast milk feeding) |
| 12) | How long has the baby been bottle- feeding? ..months |
| 13) | Which types of bottles do mother use more often? (1) polymeric (2) glass ware (3) both |
| 14) | How often do family re-new the used bottles? (1) Every..months |
| 15) | Which baby bottle brands is the mother more familiar with? (1) Camro (2) My babay (3) Panberiz (4) Potato (5) Wee (6) Camera (7) Baby nova (9) Other |
| 16) | Which baby bottle brands is the mother more frequently buy? (1) Camro (2) My babay (3) Panberiz (4) Potato (5) Wee (6) Camera (7) Baby nova (8) Other |
| 17) | How does the mother treat the formal water? (1) Hot-boiled water is directly poured into bottle, kept in room temperature to get cold-enough for formula preparation. (2) Warm-boiled water is immediately mixed with dried formula in the bottle. (3) Other (explained) |
| 18) | How much milk does the baby have in a serving? (1) less than 125 ml (2) 125 (2) 125⿿250 ml (2) 250 ml |
| 19) | How many serving of milk dose the baby have in 24 h?........................ |
| 20) | How many time dose the family wash the baby bottle in 24 h? |
| 21) | How do the family usually clean the bottle? (1) Manual-hot water and detergent (2) Manual⿿hot water,detergent and boiling for at at least one minute (3) Dish washer (4) Other (explained) |
| 22) | If the family use boiling after cleaning, how often they do that? (explained) |
In all, 200 mothers participated in this study in 11 health-care centres in Isfahan. Where, Questionnaires were filled with the help of breastfeeding consultants. Mothers who fed formula to their infants were identified by formula coupon registration form at each health-care centre.
A two-stage cluster sampling was performed. Two main health centres in Isfahan were considered as the main clusters, and five to six health-care centres were chosen from the sub-branches as the second cluster. Inclusion criteria were as follow: mothers with babies aged between 0 and 1 years, mothers practising formula feeding or mixed feeding and mother using polymeric bottles for feeding their babies.
Daily exposure of babies to BPA via baby bottles was determined as (Daily intake of formula ÿ BPA migrated from bottles)/baby weight. In order to obtain the distribution frequency of exposure, daily intake of BPA for each infant was computed by multiplying the amount of daily milk intake of the that infant with the migration data of the relevant used bottle brand divided by the infant⿿s weight. This produces pretty realistic information on exposure to BPA via baby bottles among the investigated infants.
As another approach, point estimate exposure assessment was followed: daily milk intake was considered as the average milk intake of boys and girls in two age groups of below 6 months and higher than 6 months. Thenceforth, three choices of BPA migrated data were considered as the contaminant's concentration including the averaged value obtained from all tested baby bottles, averaged value, obtained from the most commonly used samples and the highest migration data obtained in the present study as the worst case condition.
2.8. Estimation of the risk.
The risk associated with ingestion of BPA in the surveyed population of infants was described as Ingestion Hazard Quotient (IHQ), calculated by the fallowing equation:
where the latest t-TDI of PBA set by EFSA [14] (4 μg/Kg bw) was considered as reference dose. Values more than one are regarded as high potential risk.
3. Results
3.1. Release of BPA
Results of BPA determination in the migration test are presented in Table 3. Concentration of BPA, released from the new and used bottles varied in the range of 0.49⿿8.58 and 0.63⿿2.47 μg/l, respectively. With reference to new baby bottles, there was no specific trend in the measured BPA, through the first and second experiments. Overall, in both new and used bottles the migrated amount of BPA in to water was quite below than the SML set for BPA (600 μg/l) by the relevant EU legislation. The BPA concentration of the water sample in which a used baby bottle was immersed and boiled for 15 min was determined as 243 μg/l that was still less than the regulated level.
Table 3.
BPA concentration (μg/l) released from tested baby bottles.
| New bottle samplesb |
Used samples |
|||||
|---|---|---|---|---|---|---|
| Concentration of bpa (μg/Kg) |
Concentration of bpa (μg/Kg) | |||||
| Sample⿿s code | 1st test | 2nd test | Sample⿿s code | |||
| 1 | Camro | 1.56(0.32)a | 4.15(1.82) | 10 | Camro | 0.83(0.09) |
| 2 | Panberiz | 0. 95(0.16) | 1.78(0.41) | 11 | Camro | 0.91(0.15) |
| 3 | My baby | 0.77(0.54) | 0. 88(0.10) | 12 | Panberiz | 1.42(0.32) |
| 4 | Camera | 13.58(0.46) | 1.05(0.62) | 13 | Panberiz | 1.81(0.04) |
| 5 | Wee | 0.58(0.27) | 0.49(0.02) | 14 | My baby | 2.47(0.08) |
| 6 | Potato | 0.89(0.30) | 0.61(0.07) | 15 | Wee | 1.01(0.21) |
| 7 | Babynova | 0.63(0.29) | 0.92(0.24) | 16 | Mina baby | 1.08(0.50) |
| 8 | Nuby | 0.78(0.19) | ⿿ | 17 | Un known | 0.63(0.43) |
| 9 | Babaynova | 1(0.180) | ⿿ | |||
Results are expressed as means (sd) of duplicate measurement of BPA extracted from each 100 ml pure water extract by GC⿿MS.
New bottles were examined in duplicate and the results of first and second run of experiments are reported separately.
3.2. Results of the survey on formula consumption and preparation
In all, 200 mothers of 103 baby boys and 97 baby girls filled the questionnaire. The infants were divided into two age groups: 0⿿6 and 6⿿12 months. Table 4 shows the average amount of daily formula intake per kilogram per body weight by each group of infants.
Table 4.
the average amounts of formula consumption by infants under one year in the presented study.
| Age group | Sex baby | Mean weight of infants (kg) | Mean daily milk consumption (ml) |
|---|---|---|---|
| 0⿿6 | Girl n = 57 |
a5.16 ± 1.4 | a518.94 ± 293.23 |
| Boy n = 75 |
5.8 ± 2.02 | 583.46 ± 385.92 | |
| 6⿿12 | Girl n = 40 |
7.73 ± 1.5 | 731.57 ± 484.93 |
| Boy n = 28 |
7.67 ± 1.4 | 702.67 ± 437.94 | |
Results are expressed as mean values ± SD.
Significant difference was observed in the weight of boys and girls aged below 6 months. Moreover, significant difference was observed in the mean daily formula intake between boys and girls aged below 6 months (P < 0.05). However, in the older group of infants, no significant difference (P > 0.05) was observed in weight and mean daily formula intake between boys and girls.
Of the 200 replies for the survey on the type of bottles used, only 117 were included in the analysis because (1) some mothers did not remember the brand of the bottles used, (2) bottles used by some mothers lacked brand information and (3) some mothers used brands that had not been examined in our laboratory.
Analysis of the results of our survey on the type of baby bottles used and their priority of use showed that 88% the included participants (n = 104) more often used Iranian brands. Three brands (Camro, Panberiz, My baby) out of the seven bestselling products that we previously determined and used as new bottles in our experiment were also recognised as the well-known and most used brands by the participants in the survey.
With respect to formula preparation, Although the majority of the respondents avoided filling their baby bottles with hot-boiled water; a few of them (6%) poured boiled-hot water directly into the bottle and kept it in room treatment to be cold enough to prepare the formula. Considering the baby bottles washing and cleaning habits, it was observed that about half of the respondents boiled the bottles after washing in frequent bases.
3.3. Estimated daily exposure and characterization of the risk
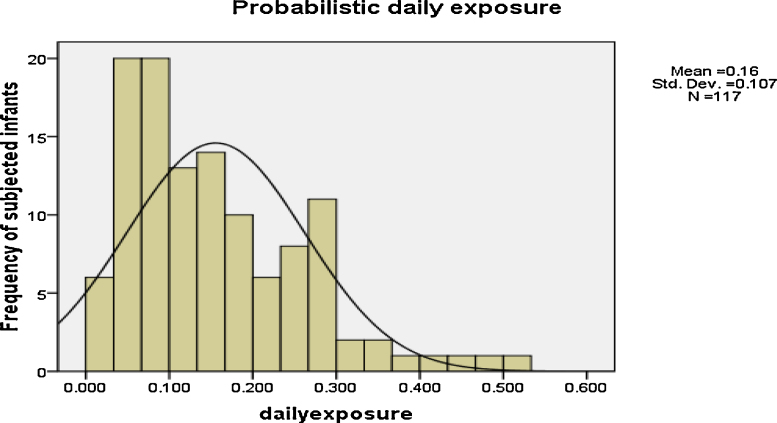
Fig. 2 illustrates the distribution frequency of daily exposure to BPA through polymeric baby bottles among formula-fed babies. Majority of the investigated infants were exposed to BPA concentration of 0.1⿿0.2 μg/kgbw/d, which was negligible compared to the temporary TDI of 4 μg/kgbw/d.
Fig. 2.
Distribution frequency of daily exposure to BPA among the investigated infants (μg/kg bw/d).
The results of estimated daily exposure to BPA for babies in the two age groups, considering the migration data acquired from all tested bottles, most commonly used brands and the highest concentration of BPA released in the migration test with their corresponding IHQ are listed in Table 5.
Table 5.
Daily exposure to BPA via formula consumption in two age groups of infants (μg/kg bw) and the corresponding IHQ.
| Age groups (month) | Gender | Exposure due to all sampled bottlesa | IHQ | Exposure due to most commonly used bottlesb | IHQ | Exposure in worse case conditionc | IHQ |
|---|---|---|---|---|---|---|---|
| 0⿿6 | Girl | 0.16 | 0.04 | 0.27 | 0.06 | 1.41 | 0.35 |
| Boy | 0.16 | 0.04 | 0.27 | 0.06 | 1.41 | 0.35 | |
| 6⿿12 | Girl | 0.15 | 0.03 | 0.25 | 0.06 | 1.32 | 0.33 |
| Boy | 0.14 | 0.03 | 0.24 | 0.73 | 1.28 | 0.32 | |
The average amounts of release from both new and old bottles was taken into account (1.57 μg/l).
The averaged migration data from the most commonly used baby bottles in the survey was taken into account (2.7 μg/l).
The maximum amount of release from a used bottle in boiling was taken into account (14 μg/l).
The results presented in Table 5 indicated that infants in both the age groups did not have any serious health risk because of BPA intake through formula feeding. Moreover, BPA intake in the worst-case scenario did not exceed the defined t-TDI for BPA.
4. Discussion
Results of risk assessment of a toxic chemical could be different from population to population with respect to genetic, race, common habits, traditions and regulations. It has been more than a decade that safety authorities in developed countries have issued a body of acts against BPA usage in baby feeding bottles. However, in the absence of any BPA legislation in some countries, this is rational to investigate the risk of exposure to BPA from baby feeding bottles according to the formula feeding and formula preparation pattern in the society.
The results of present study, revealed that the associated risk is negligible because the concentration of released BPA from different examined bottles was much lower than the standard level set by the European committee. In 1991, the European Committee defined a significant migration limit of 3 mg/kg for BPA in foods or food stimulants. In 2004, this value was decreased to 0.6 mg/kg [12]. A review of literature showed high variation among the results of various studies with respect to the amount of BPA migrated from polymeric baby bottles into their contents.
Some studies show release of BPA from baby feeding bottles was as low as what was detected in our study; Kawamura et al. [48] examined four PC baby bottles by using a standard migration test and reported BPA concentration of approximately 0.5 μg/l of food simulants. Further, Sun et al. [41] used chemiluminescence techniques and showed that the amount of BPA released in hot water after the first migration test could be 0.59⿿0.75 μg/l [41]. Brede et al. [7] used new baby bottles and showed that the average amount of BPA released in the first migration test was 0.23 μg/l of food simulants. Another study showed that new polymer bottles released BPA at a concentration of 0.11⿿1.18 μg/l of water [23]. In contrast, there is a body of evidence that the level of leached BPA from baby bottles was much higher than that found in our study; In a recent study in India, examining three new baby bottles from different brands, liberated BPA in pure water averaged as 19 μg/l. Using 70 °C water, the migration assay in the latter study was less vigorous than our study. However, the duration of migration assay was longer (1 h) than our experiment Shrinithivihahshini et al. [49].
Cao and Corriveau [9], who performed a six-day migration test at 70 °C, reported that the concentration of BPA released from new bottles was 228⿿521 μg/l and that from used bottles was only 0.63⿿2.47 μg/l.
Some studies examined the migration of BPA from tested bottles by employing several washing cycles. These studies reported conflicting results. Kawamura et al. [48] used two baby bottles and five washing cycles and observed that BPA leaching decreased from 26.9 to1.5 μg/l and from 0.5 to undetectable level in the first and second tested bottle, respectively, indicating that the amount of migrated BPA decreased after continuous washing.
Another study by Sun et al. [41] showed five-fold reduction in the concentration of migrated BPA after the first and second washing cycle of polymeric baby bottles.
The above studies are one of the few instances where the amount of BPA leached from baby bottles decreased after repeated experiments. Other studies involving migration tests showed that the amount of BPA leached from baby bottles increased with each experiment. Brede et al. [7] showed that the average amount of BPA released from 12 PC baby bottles significantly increased after 51 washing cycles but reduced again after further washing (169 washing cycles).
Similar observation was reported using dishwasher, detergents and subsequent sterilization. The amount of BPA released showed noticeable increase after the initial washing of baby bottles but showed a significant decrease after up to 20 washing cycles [31].
BPA release from new baby bottles is attributed to free or non-polymerised molecules on the inner layer of PC bottles that are gradually released by repeated use of these bottles [34]. Use of high temperature water as well as physical damage to the polymer structure during sanitation practices may enhance the leaching process [9], [5], [15]. Due to the results of a recent study conducted by Santillana et al., baby bottles exposed to infant formula released increased amount of BPA under detergent effect. In the present study the effects of detergents was not investigated, however, since the used bottles in our work were in use for at least two months, considering the procedure of washing mentioned by the participants and the low levels of migrated BPA from used baby bottles, being exposed to formula didn't impose specific effect on the release of BPA. It is interesting that despite the sound design and experimental process in the latter study, dispersion of the results obtained from samples was regarded as a limiting factor for evaluation of the effect of aging on BPA migration.
National Toxicology estimated that formula-fed babies aged below 6 months and 6⿿12 months are exposed to 1⿿24 and 1.65⿿13 μg of BPA per kg bw/d, respectively, through their daily diet, indicating that the related risk was considerable according to the current TDI for BPA (4 μg/kgbw).
In 2002, the European Scientific Committee on Food proposed that the daily exposure limit for babies aged 0⿿4 months and 6⿿12 months was as much as1.6 and 0.8 μg/kgbw/d, respectively, for the migration of approximately 10 μg of BPA per kg formula from baby bottles [16].
According to World Health Organization, daily exposure to BPA for babies fed breast milk, those fed formula by using non-polymeric bottles and those fed formula using polymeric bottles was 0.2, 2.3 and 11 μg/kgbw/d, respectively [39]. Studies performed by EFSA [14], by considering that 4 and 50 μg of BPA migrated per litre of formula estimated that daily exposure of babies to BPA could be as much as 10⿿11 μg/kgbw/d. According to primary BPA risk assessments in Canada, the probable daily intake (PDI) for adults and infants was 0.18and 1.35 μg/kg bw/d, respectively. However, recent probabilistic assessment showed that the mentioned value for adults declined to 0.005 μg/kg bw/d, which was 3-times lower than the evaluation in 2008.
An independent study also showed a PDI (probably daily intake) of 0.083 μg/kgbw/d for 1-month-old infants and of 0.164 μg/kgbw/d for infants aged four to seven months [1] which is in accordance to results obtained in this study when the mean amount of BPA migrated from all tested bottles was considered as contaminant concentration.
Lack of internal standard in the BPA assay and absence of different migration test conditions can be considered as limitation of the present work. However, very low detection limit in the analytical protocol, appropriate chromatography method and probabilistic exposure estimation, which provide reliable information from the surveyed population, could be regarded as the strong points of the present study.
5. Conclusion
In the present study, exposure assessment was performed to identify the risks associated with BPA intake through formula consumption among Iranian infants using both distributions based and point estimation approaches. The results showed that BPA released from these bottles was lower than that observed in similar studies and the corresponding risk was very low. In particular, when distribution based approach which is more realistic came in to consideration.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2015.09.002.
Contributor Information
Zohreh Abdi Moghadam, Email: Z.abdimoghadam@gmail.com.
Maryam Mirlohi, Email: m_mirlohi@hlth.mui.ac.ir, mm_mirlohi@hotmail.com.
Hamidreza Pourzamani, Email: a.malekpour@chem.ui.ac.ir.
Akbar Malekpour, Email: pourzamani@hlth.mui.ac.ir.
Zohreh Amininoor, Email: amininoor1390@gmail.com.
Mohammad Reza Merasi, Email: mrmaracy@yahoo.co.uk.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.R. Addy, BPA risk assessment needs reassessing (Reports) [Online], 2012.
- 2.Ahmadkhaniha R., Mansouri M., Yunesian M., Omidfar K., Jeddi M.Z., Larijani B., Mesdaghinia A., Rastkari N. Association of urinary bisphenol a concentration with type-2 diabetes mellitus. J. Environ. Health Sci. Eng. 2014;12:64. doi: 10.1186/2052-336X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballesteros-Gómez A., Rubio S., Pérez-bendito D. Analytical methods for the determination of bisphenol A in food. J. Chromatogr. A. 2009;1216:449–469. doi: 10.1016/j.chroma.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Jonathan N., Hugo E.R., Brandebourg T.D. Effects of bisphenol A on adipokine release from human adipose tissue: implications for the metabolic syndrome. Mol. Cell. Endocrinol. 2009;304:49–54. doi: 10.1016/j.mce.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biedermann-Brem S., Grob K., Fjeldal P. Release of bisphenol A from polycarbonate baby bottles: mechanisms of formation and investigation of worst case scenarios. Eur. Food Res. Technol. 2008;227:1053–1060. [Google Scholar]
- 6.Boas M., Feldt-Rasmussen U., Main K.M. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2012;355:240–248. doi: 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Brede C., FjeldaL P., Skjevrak I., Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit. Contam. 2003;20:684–689. doi: 10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- 8.Calafat A.M., Kuklenyik Z., Reidy J.A., Caudill S.P., Ekong J., Needham L.L. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao X.-L., Corriveau J. Migration of bisphenol A from polycarbonate baby and water bottles into water under severe conditions. J. Agric. Food Chem. 2008;56:6378–6381. doi: 10.1021/jf800870b. [DOI] [PubMed] [Google Scholar]
- 10.Cao X.-L., Corriveau J., Popovic S. Levels of bisphenol A in canned soft drink products in Canadian markets. J. Agric. Food Chem. 2009;57:1307–1311. doi: 10.1021/jf803213g. [DOI] [PubMed] [Google Scholar]
- 11.Crain D.A., Eriksen M., Iguchi T., Jobling S., Laufer H., Leblanc G.A., Guillette L.J. An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod. Toxicol. 2007;24:225–239. doi: 10.1016/j.reprotox.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 12.De Coensel N., David F., Sandra P. Study on the migration of bisphenol-A from baby bottles by stir bar sorptive extraction-thermal desorption-capillary GC⿿MS. J. Sep. Sci. 2009;32:3829–3836. doi: 10.1002/jssc.200900349. [DOI] [PubMed] [Google Scholar]
- 13.Edginton A.N., RItter L. Predicting plasma concentrations of bisphenol A in children younger than 2 years of age after typical feeding schedules, using a physiologically based toxicokinetic model. Environ. Health Perspect. 2009;117:645–652. doi: 10.1289/ehp.0800073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EFSA. Bisphenol A [Online]. agency of the European Union. Available: http://www.efsa.europa.eu/en/topics/topic/bisphenol.htm [accessed 21.01.15], 2015.
- 15.Ehlert K., Beumer C., Groot M. Migration of bisphenol A into water from polycarbonate baby bottles during microwave heating. Food Addit. Contam. 2008;25:904–910. doi: 10.1080/02652030701867867. [DOI] [PubMed] [Google Scholar]
- 16.European Commission S. Scientific Committee on Food; Brusssels, Belgium: 2002. Opin Ion of the Scientific Committee on Food on Bisphenol A. [Google Scholar]
- 17.Geens T., Goeyens L., Covaci A. Are potential sources for human exposure to bisphenol-A overlooked? Int. J. Hyg. Environ. Health. 2011;214:339–347. doi: 10.1016/j.ijheh.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 18.González-castro M., Olea-Serrano M., Rivas-Velasco A., Medina-Rivero E., Ordoñez-Acevedo L.G., De León-Rodríguez A. Phthalates and bisphenols migration in Mexican food cans and plastic food containers. Bull. Environ. Contam. Toxicol. 2011;86:627–631. doi: 10.1007/s00128-011-0266-3. [DOI] [PubMed] [Google Scholar]
- 19.Hadjmohammadi M.R., Saeidi I. Determination of bisphenol A in Iranian packaged milk by solid-phase extraction and HPLC. Monatsh. Chem.-Chem. Mon. 2010;141:501–506. [Google Scholar]
- 20.Kang J.-H., Katayama Y., Kondo F. Biodegradation or metabolism of bisphenol A: from microorganisms to mammals. Toxicology. 2006;217:81–90. doi: 10.1016/j.tox.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Kang J.-H., Kondo F., Katayama Y. Human exposure to bisphenol A. Toxicology. 2006;226:79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan A.V., Stathis P., Permuth S.F., Tokes L., Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinol.-Phila. 1993;132:2279. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- 23.Kubwabo C., Kosarac I., Stewart B., Gauthier B., Lalonde K., Lalonde P. Migration of bisphenol A from plastic baby bottles, baby bottle liners and reusable polycarbonate drinking bottles. Food Addit. Contam. 2009;26:928–937. doi: 10.1080/02652030802706725. [DOI] [PubMed] [Google Scholar]
- 24.Kuo H.-W., Ding W.-H. Trace determination of bisphenol A and phytoestrogens in infant formula powders by gas chromatography⿿mass spectrometry. J. Chromatogr. A. 2004;1027:67–74. doi: 10.1016/j.chroma.2003.08.084. [DOI] [PubMed] [Google Scholar]
- 25.Kuroda N., Kinoshita Y., Sun Y., Wada M., Kishikawa N., Nakashima K., Makino T., Nakazawa H. Measurement of bisphenol A levels in human blood serum and ascitic fluid by HPLC using a fluorescent labeling reagent. J. Pharm. Biomed. Anal. 2003;30:1743–1749. doi: 10.1016/s0731-7085(02)00516-2. [DOI] [PubMed] [Google Scholar]
- 26.Le H.H., Carlson E.M., Chua J.P., Belcher S.M. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol. Lett. 2008;176:149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X., Ying G.-G., Su H.-C., Yang X.-B., Wang L. Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ. Int. 2010;36:557–562. doi: 10.1016/j.envint.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Li X., Ying G.-G., Zhao J.-L., Chen Z.-F., Lai H.-J., Su H.-C. 4-Nonylphenol, bisphenol-A and triclosan levels in human urine of children and students in China, and the effects of drinking these bottled materials on the levels. Environ. Int. 2011;52:81–86. doi: 10.1016/j.envint.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 29.Maia J., Cruz J., Sendón R., Bustos J., Sanchez J., Paseiro P. Effect of detergents in the release of bisphenol A from polycarbonate baby bottles. Food Res. Int. 2009;42:1410–1414. [Google Scholar]
- 30.Maia J., Cruz J.M., Sendón R., Bustos J., Cirugeda M.E., Sanchez J.J., Paseiro P. Effect of amines in the release of bisphenol A from polycarbonate baby bottles. Food Res. Int. 2010;43:1283–1288. [Google Scholar]
- 31.Maragou N.C., Makri A., Lampi E.N., Thomaidis N.S., Koupparis M.A. Migration of bisphenol A from polycarbonate baby bottles under real use conditions. Food Addit. Contam. 2008;25:373–383. doi: 10.1080/02652030701509998. [DOI] [PubMed] [Google Scholar]
- 32.Moghadam Z., Pourzamani H., Malekpour A., Mirlohi M., Amininoor Z. Preliminary estimation of infantile exposure to BPA based on the standard quality of baby bottles distributed in Isfahan urban society. J. Int. Environ. Health Eng. 2013;2:23. [Google Scholar]
- 33.Muncke J. Endocrine disrupting chemicals and other substances of concern in food contact materials: an updated review of exposure, effect and risk assessment. J. Steroid Biochem. Mol. Biol. 2011;127:118–127. doi: 10.1016/j.jsbmb.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Nam S.H., Seo Y.M., Kim M.G. Bisphenol A migration from polycarbonate baby bottle with repeated use. Chemosphere. 2010;79:949–952. doi: 10.1016/j.chemosphere.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 35.Onn Wong K., Woon Leo L., Leng Seah H. Dietary exposure assessment of infants to bisphenol A from the use of polycarbonate baby milk bottles. Food Addit. Contam. Part A. 2005;22:280–288. doi: 10.1080/02652030500077502. [DOI] [PubMed] [Google Scholar]
- 36.Ozaki A., Yamaguchi Y., Okamoto A., Kawai N. Determination of alkylphenols, bisphenol A, benzophenone and phthalates in containers of baby food, and migration into food simulants]. Shokuhin eiseigaku zasshi. J. Food Hyg. Soc. Jpn. 2002;43:260. doi: 10.3358/shokueishi.43.260. [DOI] [PubMed] [Google Scholar]
- 37.Rastkari N., Ahmadkhaniha R., Yunesian M., Baleh L., Mesdaghinia A. Sensitive determination of bisphenol A and bisphenol F in canned food using a solid-phase microextraction fibre coated with single-walled carbon nanotubes before GC/MS. Food Addit. Contam. 2010;27:1460–1468. doi: 10.1080/19440049.2010.495730. [DOI] [PubMed] [Google Scholar]
- 38.Richter C.A., Birnbaum L.S., Farabollini F., Newbold R.R., Rubin B.S., Talsness C.E., Vandenbergh J.G., Walser-Kuntz D.R., Vom Saal F.S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.R. Reuss, G.A. Leblanc, Background Paper on Bisphenol A Exposure Assessment[Online], 2010.
- 40.Rykowska I., Szymanski A., Wasiak W.A. Determination of bisphenol-A in drinking water using new SPE sorbents with chemically bonded ketoimine groups. Pol. J. Food Nutr. Sci. 2005;14:237. [Google Scholar]
- 41.Sun Y., Wada M., Al-Dirbashi O., Kuroda N., Nakazawa H., Nakashima K. High-performance liquid chromatography with peroxyoxalate chemiluminescence detection of bisphenol A migrated from polycarbonate baby bottles using 4-(4,5-diphenyl-1H-imidazol-2-yl) benzoyl chloride as a label. J. Chromatogr. B: Biomed. Sci. Appl. 2000;749:49–56. doi: 10.1016/s0378-4347(00)00387-x. [DOI] [PubMed] [Google Scholar]
- 42.Szymaſski A., Rykowska I., Wasiak W. Determination of bisphenol A in water and milk by micellar liquid chromatography. Acta Chromatogr. 2006;17:161–172. [Google Scholar]
- 43.Taylor J.A., Vom Saal F.S., Welshons W.V., Drury B., Rottinghaus G., Hunt P.A., Toutain P.-L., Laffont C.M., Vandevoort C.A. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure. Environ. Health Perspect. 2011;119:422. doi: 10.1289/ehp.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vom Saal F.S., Akingbemi B.T., Belcher S.M., Birnbaum L.S., Crain D.A., Eriksen M., Farabollini F., Guillette L.J., Jr, Hauser R., Heindel J.J. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. (Elmsford NY) 2007;24:131. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vom Saal F.S., Myers J.P. Bisphenol A and risk of metabolic disorders. JAMA J. Am. Med. Assoc. 2008;300:1353–1355. doi: 10.1001/jama.300.11.1353. [DOI] [PubMed] [Google Scholar]
- 47.Yi B., Kim C., Yang M. Biological monitoring of bisphenol A with HLPC/FLD and LC/MS/MS assays. J. Chromatogr. B. 2010;878:2606–2610. doi: 10.1016/j.jchromb.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Kawamura Y., Koyama Y., Takeda Y., Yamada T. Migration of bisphenol A from poly-carbonate products. J. Food Hyg. Soc. Jpn. 1998 [Google Scholar]
- 49.Shrinithivihahshini N.D., Mahamuni D., Praveen N. Bisphenol A migration study in baby feeding bottles of selected brands available in the Indian market. Curr. Sci. 2014;106:1081–1084. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.