Abstract
Objective
To investigate the toxicological effects of cleistanthin A and cleistanthin B using sub-chronic toxicity testing in rodents.
Method
Cleistanthins A and B were isolated from the leaves of Cleistanthus collinus. Both the compounds were administered orally for 90 days at the concentration of 12.5, 25 and 50 mg/kg, and the effects on blood pressure, biochemical parameters and histology were assessed. The dose for sub-chronic toxicology was determined by fixed dose method according to OECD guidelines.
Result
Sub-chronic toxicity study of cleistanthins A and B spanning over 90 days at the dose levels of 12.5, 25 and 50 mg/kg (once daily, per oral) revealed a significant dose dependant toxic effect in lungs. The compounds did not have any effect on the growth of the rats. The food and water intake of the animals were also not affected by both cleistanthins A and B. Both the compounds did not have any significant effect on liver and renal markers. The histopathological analysis of both cleistanthins A and B showed dose dependent morphological changes in the brain, heart, lung, liver and kidney. When compared to cleistanthin A, cleistanthin B had more toxic effect in Wistar rats. Both the compounds have produced a dose dependent increase of corpora amylacea in brain and induced acute tubular necrosis in kidneys. In addition, cleistanthin B caused spotty necrosis of liver in higher doses.
Conclusion
The present study concludes that both cleistanthin A and cleistanthin B exert severe toxic effects on lungs, brain, liver, heart and kidneys. They do not cause any significant pathological change in the reproductive system; neither do they induce neurodegenerative changes in brain. When compared to cleistanthin A, cleistanthin B is more toxic in rats.
Keywords: Cleistanthin A, Cleistanthin B, Cleistanthus collinus, Toxicity
1. Introduction
Cleistanthin A and cleistanthin B are toxic constituents present in the leaves of Cleistanthus collinus. The plant is most commonly used as a suicidal poison in the Southeast Asian countries [1]. The mortality rate of C. collinus leaf poisoning is around 20–60%. In the 1970s a series of phytoconstituents was isolated from the leaves of C. collinus and it includes eargic acid, diphyllin, collinusin, cleistanthin A and cleistanthin B [2]. Later, it was reported that the diphyllin glycoside includes cleistanthins A and B and they are responsible for the C. collinus poisoning [3] which causes severe toxic effects on the cardiac and the respiratory systems in patients. The poisonous effect of C. collinus was observed after 48–72 h of administration. Globally plant poisoning is not common, but it is contributing a significant percentage to mortality in rural India. Young women in rural Indian subcontinent use C. collinus poisoning as a method of deliberate self-harm [4]. According to literature, the C. collinus plant contains highly toxic lignan glycosides such as cleistanthins A and B which were reported to inhibit a specific thio-dependent enzyme [5]. Apart from cleistanthin A and cleistanthin B, the C. collinus leaves have more than 25 compounds [6]. The major constituents of the genus Cleistanthus are arylnaphthalide lignan glycosides which are insoluble in water. Arylnaphthalide lignan includes diphyllin, collinusin, cleistanthin, taiwanin and other related compounds. Arylnaphthalide lignin is commonly present in Cleistanthus patulus, Haplophyllum bucharicum, and Phyllanthus toxodiifolius and some of these plants are used for medicinal purpose [7]. The mechanism of toxic effects of the plant leaves or its phytoconstituents such as cleistanthins A and B remains unknown. Further these compounds have been reported to exert anticancer activity in vitro and in vivo and may have the potential to become lead compounds [8], [9], [10]. Hence the present study was planned to determine the toxicological profile of cleistanthins A and B and a sub-chronic toxicity study of both the compounds was planned for the duration of 90 days.
2. Method
2.1. Animals
The study was approved by the Institute Animal Ethics Committee, and all experiments were carried out as per the guidelines of the Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA), India in the Department of Pharmacology, JIPMER, Pondicherry.
Healthy, adult, inbred strain of Wistar rats of both sexes (120 ± 10 g), Wistar female rats (150 ± 10 g) and Swiss albino female mice (25 ± 3 g) were obtained from the central animal house at JIPMER, Pondicherry. The Wistar rats were used for the chronic toxicological studies and Swiss albino mice were used for the single oral dose toxicity studies. The rodents were housed at 25 ± 2 °C and 55–65% humidity in a 12:12 ± 1 h light-and-dark cycle. The animals were fed with standard rat pellets (VRK Nutritional Solution, Sangli, Maharastra) and water ad libitum.
2.2. Isolation of cleistanthin A and cleistanthin B
The leaves of C. collinus (Roxb.) (Family: Euphorbiaceae) were collected in Sedrapet village of Puducherry, Union tertiary of Pondicherry. The plant was identified and certified (BSI/SC/5/23/08–09/Tech.241) by the Botanical Survey of India, Southern Circle, Coimbatore. Cleistanthins A and B were isolated from the leaves using chromatographic techniques, and the structure of each compound was confirmed spectroscopically. Air-dried leaves of the plant were powdered and defatted with n-hexane and subsequently extracted with acetone. The acetone extract was dissolved in benzene and passed through a neutral alumina column. The fractions were collected with benzene, benzene:ethyl acetate (4:1), benzene:ethyl acetate (1:1) and methanol. The fractions were subjected to thin layer chromatography and spectroscopic analysis. Cleistanthins A and B were obtained from the benzene:ethyl acetate (1:1) and methanol fractions, respectively [11].
2.2.1. Single oral dose toxicity
The single oral dose toxicity was performed to determine the dose for toxicity testing. The fixed dose method was adopted for the study. The mice (25 ± 3 g) were then randomly divided into different groups; each group consisted of three mice. Both cleistanthins A and B were administered at the dose levels of 3, 30, 300, and 600, 1200 mg/kg by gavage. The study started with administration of the compounds (3 mg/kg) and the animals were observed for 24 h continually for mortality. The animals which were alive were observed for next 14 days. After completion of the observation period, the next set of animals was administered with next dose level and observed [12].
2.3. Repeated dose toxicological evaluation of cleistanthin A and cleistanthin B
Repeated dose toxicological evaluation of cleistanthins A and B was performed to determine the maximum tolerable dose for toxicity studies. Inbred strain of albino Wistar rats of female gender weighing 150 ± 10 g used for the experiment. Six animals per group were used for the repeated dose toxicity testing which was carried out for 30 days with doses of 50, 100, 200 and 400 mg/kg to determine the maximum tolerable dose. The study was initiated with a control group and a test group receiving a high dose i.e., 400 mg/kg of cleistanthin A or B. Since the mortality was high, the evaluation dose was slashed to 200 and 100 mg/kg. Cleistanthins A and B (100 and 200 mg/kg respectively) induced mortality after 3 weeks and hence the repeated dose toxicity testing was carried out with 50 mg/kg dose level. During the study period, the organs of animals which died due to cleistanthins A and B were collected and preserved in 10% v/v neutral formalin for histopathological evaluation.
2.4. Dose optimization
Based on the dose determination study results, the <1/10 of the toxic dose was selected for sub-chronic toxicity testing. As per OECD guidelines, the maximum tolerable dose (MTD) was optimized and used for the sub-chronic toxicological evaluation of cleistanthins A and B [13], [14]. The repeated dose toxicological study was started with doses of 50, 100, 200 and 400 mg/kg, and observed for physiological and behavioural changes and mortality in rats. At the end of the first month, 100, 200 and 400 mg/kg cleistanthins A and B administered groups showed a high rate of mortality and behavioural alterations. The maximum tolerable dose was determined to be 50 mg/kg. Based on MTD, three doses viz. 12.5, 25 and 50 mg/kg were selected for the sub-chronic toxicity testing.
2.5. Sub-chronic toxicological evaluation of cleistanthin A and cleistanthin B
Inbred strain of albino Wistar rats of both sexes weighing 120 ± 10 g was used for the experiment. The animals were divided into seven groups and each group comprised of six male and six female Wistar rats.
| Group-I: | Control |
| Group-II: | Cleistanthin A 12.5 mg/kg |
| Group-III: | Cleistanthin A 25 mg/kg |
| Group-IV: | Cleistanthin A 50 mg/kg |
| Group-V: | Cleistanthin B 12.5 mg/kg |
| Group-VI: | Cleistanthin B 25 mg/kg |
| Group-VII: | Cleistanthin B 50 mg/kg |
Animals were selected and allowed to adapt for the laboratory condition for a week and 0.5 ml of blood was collected from all animals for biochemical evaluation. The animals which had normal biochemical profiles were included in the study and divided randomly into experimental groups. The experimental animals were observed for normal biological and physical functions for a week. During that time the animal blood pressure was recorded using a non-invasive procedure (Non-invasive blood pressure (NIBP) system, IITC Inc., USA).
The compounds were administered once daily as oral suspension in the morning (between 7.00 am and 9.00 am) by gavage. The doses of the compounds were adjusted to the body weight every week after the measurement of weekly body weight. During the experimental period, animals were fed with standard palatable diet and water ad libitum. Weekly body weight variations were calculated for all the experimental animals. The variations in blood pressure were measured bimonthly and blood was collected every 30 days intervals and subjected to biochemical evaluation. Before termination of the study, the last blood sample was collected from all groups of animals for biochemical estimation and comet assay. Finally the experiential animals were euthanized by cervical dislocation and the organs were collected and relative weight was measured. A part of the brain, lung, heart, stomach, kidney and bone marrow were preserved in 10% v/v neutral formalin for histopathological examination [15].
2.6. Biochemical estimation
One ml of blood sample was collected in sodium EDTA (1% sodium EDTA in normal saline) containing sample collection tube. The blood sample was collected through the retro orbital sinus. Collected blood sample was centrifuged at 3000 rpm for 20 min. The plasma was separated and immediately processed for biochemical estimations.
The plasma sample was subjected to analysis of biochemical parameters such as random blood glucose, urea, creatinine, aspartate aminotransferase (AST/SGOT), alanine amino transferase (ALT/SGPT), alkaline phosphatase (ALP), total protein, albumin and creatinine kinase–MB (CK-MB) using commercial kits and an auto analyser (Olympus AU 400, Japan).
2.7. Measurement of non-invasive blood pressure (NIBP)
The NIBP was performed with a tail-cuff apparatus (3 measurements per rat) with the use of non-invasive blood pressure monitoring device through BP-MONWIN software (NIBP system, IITC, USA). The blood pressure was recorded between 10–12 am and 3–5 pm of the day. The animals were acclimatized to stay comfortably in a rat restrainer. After restraining the animal the tail was connected to a photosensitive detector which in turn was connected to a pressure unit and a pressure cuff. After recording the blood pressure, the animal was relieved from the restrainer. After 10/15 min, the experimental procedure was repeated for constancy [16], [17].
2.8. Blood sample collection for comet assay
One ml of the blood was collected in a heparinized tube through the retro orbital sinus for analysis of percentage of DNA damage using comet assay [18].
2.9. Relative organ weight analysis
At the end of the study, experimental animals from all the groups were sacrificed by cervical dislocation procedure. The abdomen was cut open and organs such as brain, heart, lung, stomach, liver, kidney, testes in male rats and uterus in female rats were dissected. The removed organs were washed with normal saline, dried with filter paper and weighed [19].
2.10. Comet assay
The lymphocytes from the blood sample were isolated using lymphocyte separation media (Histopaque 1077; Sigma, USA) and diluted with phosphate buffer saline (PBS). The diluted lymphocytes were mixed with low melting point agarose and the sandwich was prepared using normal agarose on glass microscopic slide and coverslip. The lymphocytes-agarose sandwich was preserved at 10 °C for 15 min to complete solidification. The coverslip of lymphocytes-agarose sandwich preparation was removed and placed in horizontal electrophoresis unit and allowed for DNA unwinding for 30 min after that the electrophoresis was run for 30 min at 10 V current. Then the slide was washed thrice with Tris buffer. The washed slide was allowed to overnight drying at room temperature.
Next day the slides were fixed with freshly prepared fixating solution (trichloroacetic acid, zing sulphate and glycerol in water) for 10 min. After 10 min the slide was removed from fixating solution and washed with distilled water (three times) and allowed for drying. The dried slide was stained using silver, sodium carbonate in water – solution A; ammonium nitrate, silver nitrate and silicotungstic acid in water – solution B; Solutions A and B in the ratio of 1:2 were used as silver staining solutions and then the slide was transferred to a tray containing stopping solution (glacial acetic acid). Finally the slide was washed again with distilled water and allowed to dry completely in an inclined position at room temperature. The dried slides were examined under bright-field light microscope and captured using CCD camera [18].
2.11. Histopathological analysis
Part of the brain, heart, lung, liver and kidney were excised quickly and fixed in 10% v/v buffered neutral formalin. The paraffin blocks were prepared and the 5–10 μm thick sections were prepared, stained with haematoxylin and eosin and mounted in neutral DPX medium. The histological slides were examined under light microscope [20].
2.12. Statistics
All the data presented are mean ± SEM. One-way analysis of variance (ANOVA) followed by Bonferroni test for blood pressure, and two-way repeated measures ANOVA followed by Bonferroni test for biochemical parameters were used to find out the statistically significant difference between groups. P values <0.05 was considered significant.
3. Results and discussion
The single oral dose administration of cleistanthins A and B up to 800 mg/kg did not lead to mortality in mice. Both cleistanthin A and cleistanthin B exhibited mortality at 1200 and 1000 mg/kg respectively. From the results of the repeated dose toxicity study, the maximum tolerable dose (MTD) for cleistanthins A and B was calculated to be 50 mg/kg.
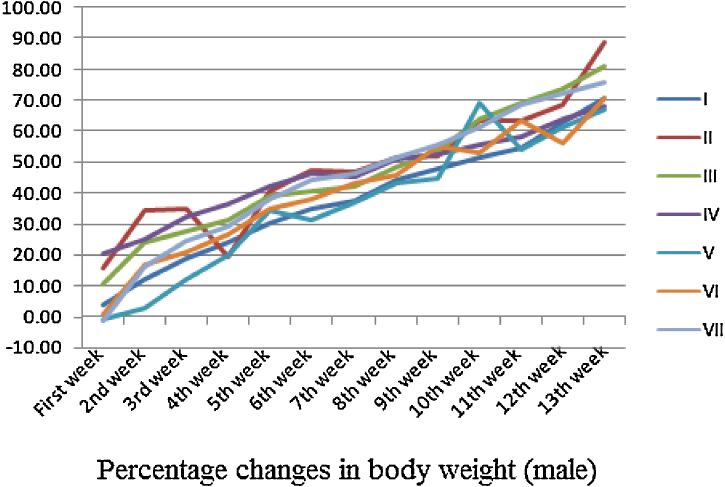
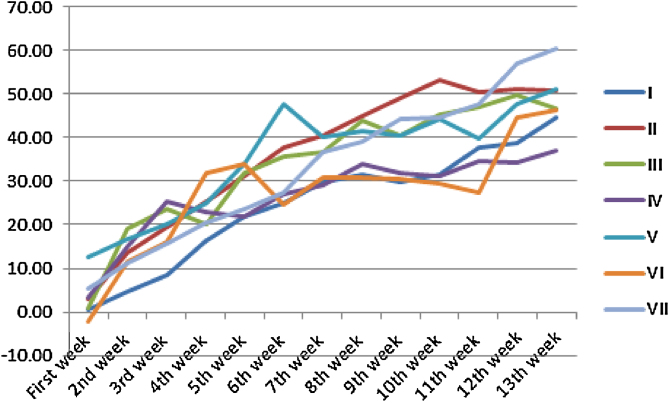
Fixed single oral dose toxicity determination of cleistanthin A and cleistanthin B in mice showed Straub tail phenomenon. But such an effect was not observed in sub-chronic toxicological study on rats. Both single and chronic administration of cleistanthin A and cleistanthin B in rodents do not have any effect on growth (Fig. 1, Fig. 2). The food intake and the water intake of the animals were also not affected by cleistanthins A and B. Single oral dose toxicity studies were carried out using mice because of their high basal metabolic rate (BMR) and hence their tolerance to higher dose (per kg body weight). This reduced the mortality and helped us find out the safe dose with the increasing doses of cleistanthins A and B. The sub-chronic toxicity studies were conducted using rats considering their longer lifespan.
Fig. 1.
Effect of cleistanthins A and B on growth of the male Wistar rats.
Fig. 2.
Effect of cleistanthins A and B on growth of the female Wistar rats.
Sub-chronic toxicity study of cleistanthin A and cleistanthin B spanning over 90 days at the dose levels of 12.5, 25 and 50 mg/kg (single dose, oral toxicity testing) revealed a significant dose dependant toxic effect in lungs. The effect (percentage change and area under curve [AUC]) of cleistanthins A and B on diastolic blood pressure and mean blood pressure is presented in Table 1, Table 2. Both cleistanthins A and B did not show any significant change in systolic, diastolic and mean blood pressures.
Table 1.
Effect of cleistanthins A and B on blood pressure and heart rate of male Wistar rat.
| Group | Systolic AUC | Diastolic AUC | Mean AUC | Heart rate AUC | Systolic % changes (mmHg) | Diastolic % changes (mmHg) | Mean % changes (mmHg) |
|---|---|---|---|---|---|---|---|
| Control | 4563.75 ± 227.93 | 3408.75 ± 234.30 | 3772.50 ± 201.38 | 18,626.25 ± 1831.30 | −16.89 ± 7.21 | 19.06 ± 4.59 | 5.57 ± 4.37 |
| Cleistanthin A 12.5 mg/kg | 4905.00 ± 201.97 | 3977.50 ± 156.97 | 4233.75 ± 191.22 | 17,142.50 ± 1544.47 | −1.83 ± 9.41 | 35.14 ± 3.80 | 22.94 ± 5.53 |
| Cleistanthin A 25 mg/kg | 4245.00 ± 713.78 | 2865.00 ± 453.04 | 3310.00 ± 533.18 | 15,325.00 ± 2784.91 | 6.26 ± 6.92 (n = 5) | 25.32 ± 4.17 (n = 5) | 18.28 ± 5.18 (n = 5) |
| Cleistanthin A 50 mg/kg | 4591.25 ± 269.26 | 3227.50 ± 228.86 | 3742.50 ± 173.36 | 16,855.00 ± 888.99 | 2.14± 8.83 (n = 5) | 21.77 ± 7.47 (n = 5) | 16.63 ± 6.33 (n = 5) |
| Cleistanthin B 12.5 mg/kg | 4416.25 ± 292.71 | 2921.25 ± 310.56 | 3362.50 ± 328.66 | 9417.50 ± 1746.90 | 8.99± 3.58 (n = 5) | 27.24 ± 7.16 (n = 5) | 20.39 ± 5.15 (n = 5) |
| Cleistanthin B 25 mg/kg | 4683.75 ± 567.70 | 3291.25 ± 456.68 | 3938.75 ± 401.75 | 12,877.50 ± 2214.42 | 4.06 ± 7.60 (n = 5) | 23.27 ± 5.40 (n = 5) | 16.17 ± 5.62 (n = 5) |
| Cleistanthin B 50 mg/kg | 5240.00 ± 375.65 | 3020.00 ± 364.65 | 4055.00 ± 272.62 | 19,711.25 ± 1582.22 | 5.37 ± 4.10 (n = 5) | 24.11 ± 5.77 (n = 5) | 17.59 ± 5.42 (n = 5) |
The values are mean ± SEM; n = 6 in each group. The % change was calculated using pre-study values and 90th day values. One-way ANOVA followed by Bonferroni test. No group showed significant differences.
Table 2.
Effect of cleistanthins A and B on blood pressure and heart rate of female Wistar rat.
| Group | Systolic AUC | Diastolic AUC | Mean AUC | Heart rate AUC | Systolic % changes (mmHg) | Diastolic % changes (mmHg) | Mean % changes (mmHg) |
|---|---|---|---|---|---|---|---|
| Control | 5207.50 ± 255.17 | 3143.75 ± 247.51 | 3768.75 ± 200.88 | 12,216.25 ± 1873.08 | 6.04 ± 11.90 | 16.30 ± 20.07 | 11.94 ± 14.79 |
| Cleistanthin A 12.5 mg/kg | 5318.75 ± 136.02 | 3907.50 ± 152.72 | 4330.00 ± 115.72 | 17,661.25 ± 673.31 | −4.36 ± 6.37 (n = 5) | 20.49 ± 6.43 (n = 5) | 11.44 ± 6.30 (n = 5) |
| Cleistanthin A 25 mg/kg | 5005.00 ± 170.03 | 3170.00 ± 151.35 | 3788.75 ± 158.60 | 9413.75 ± 2432.35 | 8.19 ± 3.51 | 17.22 ± 3.78 | 14.09 ± 3.19 |
| Cleistanthin A 50 mg/kg | 4662.50 ± 115.33 | 2995.00 ± 201.39 | 3658.75 ± 89.42 | 13,215.00 ± 2565.74 | 3.06 ± 3.05 | 24.16 ± 5.38 | 15.44 ± 3.73 |
| Cleistanthin B 12.5 mg/kg | 4396.25 ± 541.48 | 2632.50 ± 345.94 | 3308.75 ± 480.62 | 15,048.75 ± 2297.50 | 2.96 ± 8.88 | 15.19 ± 6.21 | 10.73 ± 6.60 |
| Cleistanthin B 25 mg/kg | 4502.50 ± 195.56 | 2572.50 ± 334.55 | 3615.00 ± 144.94 | 16,326.25 ± 3155.61 | −1.60 ± 7.56 (n = 5) | 13.38 ± 4.91 (n = 5) | 10.41 ± 5.95 (n = 5) |
| Cleistanthin B 50 mg/kg | 5280.00 ± 553.22 | 3055.00 ± 648.48 | 4106.25 ± 479.33 | 17,698.75 ± 2494.93 | 9.88 ± 10.3 (n = 5) | 22.77 ± 12.15 (n = 5) | 17.80 ± 11.67 (n = 5) |
The values are mean ± SEM; n = 6 in each group. The % change was calculated using pre-study values and 90th day values. One-way ANOVA followed by Bonferroni test. No group showed significant differences.
Both cleistanthins A and B significantly altered the biochemical parameters (Table 3, Table 4, Table 5, Table 6) and the mortality observed at the end of the study, in cleistanthins A and B groups is given in Table 7, Table 8). In both the groups, mortality is more with the highest dose used (400 mg/kg). Histopathology of the lung also showed severe interstitial pneumonitis and crystalline deposition in the cleistanthin A (50 mg/kg) administered group (Table 9) and extensive destruction of lung parenchyma, crystalline deposition, severe interstitial pneumonitis, necrosis and pulmonary haemorrhage in the cleistanthin B (50 mg/kg) administered group (Table 10). The results of DNA comet assay which are presented in Table 11 clearly indicate the toxic effects of the compounds on the DNA.
Table 3.
Effect of cleistanthins A and B on biochemical parameters of male Wistar rats.
| Urea (mg/dL) |
Creatinine (mg/dL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre-study | 1st month | 2nd month | 3rd month | Pre-study | 1st month | 2nd month | 3rd month | |
| Control | 29.50 ± 1.45 | 20.67 ± 1.17** | 28.33 ± 0.76 | 21.17 ± 0.79** | 0.78 ± 0.02 | 0.72 ± 0.02 | 0.72 ± 0.02 | 0.63 ± 0.02*** |
| Cleistanthin A 12.5 mg/kg | 23.67 ± 2.38 | 23.17 ± 1.25 | 32.00 ± 0.87* | 21.00 ± 2.48 | 0.75 ± 0.03 | 0.70 ± 0.00 | 0.70 ± 0.00 | 0.63 ± 0.02** |
| Cleistanthin A 25 mg/kg | 29.67 ± 3.70 | 20.20 ± 1.20 (n = 5)* | 27.00 ± 0.58 (n = 5) | 22.40 ± 0.74 (n = 5)* | 0.70 ± 0.07 | 0.72 ± 0.02 (n = 5) | 0.70 ± 0.00 (n = 5) | 0.64 ± 0.02 (n = 5)* |
| Cleistanthin A 50 mg/kg | 27.60 ± 1.18 | 29.00 ± 0.90 | 30.50 ± 1.43 | 22.83 ± 0.48 | 0.66 ± 0.11 | 0.80 ± 0.00* | 0.72 ± 0.02* | 0.63 ± 0.02* |
| Cleistanthin B 12.5 mg/kg | 31.50 ± 3.02 | 22.00 ± 0.82 | 27.20 ± 1.02 | 24.60 ± 1.21 (n = 5) | 0.78 ± 0.02 | 0.70 ± 0.00 | 0.70 ± 0.00 | 0.62 ± 0.02 (n = 5)*** |
| Cleistanthin B 25 mg/kg | 31.00 ± 2.73 | 22.20 ± 1.27** | 31.00 ± 0.82 | 35.20 ± 1.72 (n = 5)* | 0.77 ± 0.02 | 0.70 ± 0.00 | 0.70 ± 0.00 | 0.68 ± 0.02 (n = 5)*** |
| Cleistanthin B 50 mg/kg | 31.00 ± 1.73 | 21.67 ± 1.78 | 33.17 ± 0.70* | 41.00 ± 1.89 (n = 5)* | 0.80 ± 0.00 | 0.70 ± 0.000 | 0.70 ± 0.00 | 0.620 ± 0.02 (n = 5)** |
| AST (IU/L) | ALT (IU/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-study | 1st month | 2nd month | 3rd month | Pre-study | 1st month | 2nd month | 3rd month | |
| Control | 122.17 ± 3.53 | 132.80 ± 2.60 | 122.33 ± 10.79 | 121.00 ± 2.96 | 55.67 ± 4.32 | 38.60 ± 3.25 | 35.50 ± 2.93 | 48.33 ± 1.50 |
| Cleistanthin A 12.5 mg/kg | 114.17 ± 3.30 | 124.00 ± 4.44 | 118.80 ± 2.22 | 147.33 ± 4.78* | 49.83 ± 3.29 | 40.67 ± 2.04 | 41.00 ± 1.08 | 50.00 ± 3.04 |
| Cleistanthin A 25 mg/kg | 112.00 ± 2.40 | 125.80 ± 7.31 (n = 5) | 115.20 ± 3.70 (n = 5) | 174.60 ± 6.40 (n = 5) * | 51.17 ± 5.70 | 34.60 ± 1.21 (n = 5) | 36.40 ± 1.75 (n = 5)* | 50.60 ± 3.16 (n = 5) |
| Cleistanthin A 50 mg/kg | 116.40 ± 5.09 | 189.83 ± 15.42* | 119.83 ± 4.63 | 158.17 ± 2.54* | 46.20 ± 3.13 | 37.80 ± 3.06 | 35.17 ± 2.46 | 43.00 ± 1.39 |
| Cleistanthin B 12.5 mg/kg | 118.00 ± 4.16 | 125.40 ± 8.13 | 99.20 ± 13.46 | 150.60 ± 3.58 (n = 5) | 64.67 ± 4.63 | 38.60 ± 2.91 | 26.60 ± 5.27 | 40.20 ± 1.06 (n = 5) |
| Cleistanthin B 25 mg/kg | 111.00 ± 3.51 | 134.40 ± 6.50 | 159.00 ± 3.18 | 189.80 ± 22.00 (n = 5) | 57.67 ± 3.29 | 34.40 ± 2.21 | 34.60 ± 1.75 | 47.20 ± 9.06 (n = 5) |
| Cleistanthin B 50 mg/kg | 113.17 ± 4.87 | 140.33 ± 5.02 | 169.67 ± 18.06 | 169.20 ± 5.88 (n = 5) | 50.67 ± 2.92 | 39.67 ± 2.32 | 36.50 ± 1.59 | 38.00 ± 1.66 (n = 5) |
The values are mean ± SEM; n = 6 in each group; *P < 0.05, **P < 0.01 compared to pre-study (fixed variable). Two-way repeated measures ANOVA followed by Bonferroni test.
Table 4.
Effect of cleistanthins A and B on biochemical parameters of male Wistar rats.
| Total protein (g/dL) |
Albumin (g/dL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre-study | 1st month | 2nd month | 3rd month | Pre-study | 1st month | 2nd month | 3rd month | |
| Control | 6.53 ± 0.10 | 6.72 ± 0.15* | 6.95 ± 0.08** | 7.15 ± 0.08** | 3.80 ± 0.07 | 3.86 ± 0.02 | 3.82 ± 0.05 | 3.82 ± 0.03 |
| Cleistanthin A 12.5 mg/kg | 6.58 ± 0.60 | 6.85 ± 0.11 | 7.08 ± 0.08 | 7.22 ± 0.16 | 3.40 ± 0.24 | 3.87 ± 0.09 | 3.82 ± 0.08 | 3.85 ± 0.04 |
| Cleistanthin A 25 mg/kg | 5.12 ± 0.19 | 6.64 ± 0.11 (n = 5) | 6.94 ± 0.10 (n = 5) | 6.88 ± 0.07 (n = 5) | 3.35 ± 0.08 | 3.86 ± 0.05 (n = 5) | 3.88 ± 0.05 (n = 5) | 3.82 ± 0.03 (n = 5) |
| Cleistanthin A 50 mg/kg | 7.08 ± 0.55 | 7.68 ± 0.08 | 7.12 ± 0.13 | 7.350 ± 0.11 | 3.58 ± 0.20 | 4.48 ± 0.06* | 3.68 ± 0.12 | 3.73 ± 0.10 |
| Cleistanthin B 12.5 mg/kg | 6.72 ± 0.18 | 6.72 ± 0.14 | 6.02 ± 0.97 | 7.180 ± 0.15 (n = 5) | 3.78 ± 0.14 | 3.88 ± 0.05 | 3.44 ± 0.49 | 3.92 ± 0.04 (n = 5) |
| Cleistanthin B 25 mg/kg | 6.50 ± 0.23 | 6.72 ± 0.20* | 7.28 ± 0.11* | 7.96 ± 0.19 (n = 5)* | 3.65 ± 0.07 | 3.84 ± 0.08* | 3.96 ± 0.05* | 4.12 ± 0.09* (n = 5) |
| Cleistanthin B 50 mg/kg | 6.82 ± 0.12 | 6.78 ± 0.10 | 7.37 ± 0.12 | 7.90 ± 0.05 (n = 5) | 3.90 ± 0.04 | 3.93 ± 0.07 | 3.93 ± 0.11 | 4.14 ± 0.11* (n = 5) |
| CKMB (IU/L) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Pre-study | 1st month | 2nd month | 3rd month | |||||
| Control | 30.67 ± 4.14 | 42.00 ± 5.09 | 20.33 ± 4.77 | 13.17 ± 1.25 | ||||
| Cleistanthin A 12.5 mg/kg | 29.17 ± 5.67 | 21.50 ± 2.70 | 12.60 ± 1.60 | 10.83 ± 1.14 | ||||
| Cleistanthin A 25 mg/kg | 30.67 ± 6.40 | 26.20 ± 3.92 (n = 5) | 17.40 ± 3.20 (n = 5) | 21.60 ± 5.47 (n = 5) | ||||
| Cleistanthin A 50 mg/kg | 31.20 ± 6.13 | 38.20 ± 9.74 | 13.67 ± 3.53 | 13.33 ± 2.08 | ||||
| Cleistanthin B 12.5 mg/kg | 29.00 ± 4.07 | 23.40 ± 2.13 | 13.80 ± 2.62 | 13.40 ± 1.34 (n = 5) | ||||
| Cleistanthin B 25 mg/kg | 31.00 ± 4.03 | 24.80 ± 2.19 | 18.00 ± 4.02 | 52.60 ± 13.72 (n = 5) | ||||
| Cleistanthin B 50 mg/kg | 29.83 ± 4.42 | 24.33 ± 3.60 | 12.50 ± 0.76 | 80.40 ± 23.88* (n = 5) | ||||
The values are mean ± SEM; n = 6 in each group; *P < 0.05, **P < 0.01, compared to pre-study (fixed variable). Two-way repeated measures ANOVA followed by Bonferroni test.
Table 5.
Effect of cleistanthins A and B on biochemical parameters of female Wistar rats.
| Urea (mg/dL) |
Creatinine (mg/dL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre-study | 1st month | 2nd month | 3rd month | Pre-study | 1st month | 2nd month | 3rd month | |
| Control | 26.33 ± 2.14 | 27.83 ± 2.41 | 33.33 ± 1.86 | 31.67 ± 1.20 | 0.60 ± 0.00 | 0.83 ± 0.03 | 0.77 ± 0.02 | 0.70 ± 0.00 |
| Cleistanthin A 12.5 mg/kg | 30.50 ± 1.18 | 24.67 ± 1.69 | 37.33 ± 1.73 | 39.50 ± 1.31 | 0.82 ± 0.02 | 0.82 ± 0.02 | 0.75 ± 0.02* | 0.73 ± 0.02* |
| Cleistanthin A 25 mg/kg | 25.17 ± 0.48 | 29.00 ± 0.90** | 31.83 ± 0.87** | 39.83 ± 4.11* | 0.80 ± 0.00 | 0.80 ± 0.00 | 0.74 ± 0.02* | 0.67 ± 0.03* |
| Cleistanthin A 50 mg/kg | 23.00 ± 2.46 | 28.67 ± 1.23* | 39.00 ± 2.03* | 39.00 ± 2.78* | 0.55 ± 0.10 | 0.80 ± 0.00 | 0.74 ± 0.02* | 0.65 ± 0.03* |
| Cleistanthin B 12.5 mg/kg | 26.00 ± 1.63 | 26.17 ± 1.74 | 29.00 ± 1.29 | 31.40 ± 1.40 (n = 5) | 0.63 ± 0.02 | 0.78 ± 0.02*** | 0.80 ± 0.03*** | 0.78 ± 0.02*** (n = 5) |
| Cleistanthin B 25 mg/kg | 26.33 ± 3.16 | 28.33 ± 2.01 | 40.00 ± 10.02 | 31.33 ± 1.61 | 0.62 ± 0.02 | 0.80 ± 0.00 | 0.93 ± 0.13 | 0.78 ± 0.02 |
| Cleistanthin B 50 mg/kg | 28.00 ± 2.40 | 20.17 ± 1.49 | 30.25 ± 1.35 (n = 5) | 34.67 ± 1.84 (n = 3) | 0.60 ± 0.00 | 0.82 ± 0.02* | 0.82 ± 0.02* (n = 5) | 0.80 ± 0.00 (n = 3) |
| AST (IU/L) |
ALT (IU/L) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre-study | 1st month | 2nd month | 3rd month | Pre-study | 1st month | 2nd month | 3rd month | |
| Control | 116.83 ± 4.19 | 120.50 ± 6.23 | 128.00 ± 6.29 | 110.33 ± 4.48 | 49.17 ± 8.29 | 37.83 ± 1.85 | 26.67 ± 1.45 | 33.83 ± 1.94 |
| Cleistanthin A 12.5 mg/kg | 114.00 ± 3.47 | 167.17 ± 15.48* | 158.67 ± 13.76* | 152.83 ± 1.54* | 55.50 ± 3.60 | 49.25 ± 7.48* | 36.33 ± 7.45* | 34.17 ± 1.74* |
| Cleistanthin A 25 mg/kg | 112.33 ± 3.81 | 189.83 ± 15.42** | 144.60 ± 3.50* | 161.00 ± 5.82* | 50.67 ± 2.87 | 37.80 ± 3.06 | 35.20 ± 3.36 | 39.67 ± 4.26 |
| Cleistanthin A 50 mg/kg | 117.83 ± 2.65 | 168.33 ± 11.24* | 137.20 ± 7.06 | 157.67 ± 5.67* | 49.33 ± 4.15 | 40.33 ± 1.78* | 35.00 ± 2.58* | 37.83 ± 3.21* |
| Cleistanthin B 12.5 mg/kg | 123.20 ± 10.78 | 125.50 ± 6.52 | 135.00 ± 4.39 | 116.40 ± 4.81 (n = 5) | 49.20 ± 8.83 | 43.17 ± 4.06 | 27.33 ± 0.96 | 35.20 ± 1.34 (n = 5 |
| Cleistanthin B 25 mg/kg | 110.50 ± 3.22 | 246.17 ± 72.76** | 147.00 ± 3.06 | 122.50 ± 4.09 | 39.33 ± 3.51 | 163.83 ± 82.82 | 29.33 ± 2.68 | 36.33 ± 1.02 |
| Cleistanthin B 50 mg/kg | 113.00 ± 3.44 | 127.17 ± 6.88 | 171.75 ± 11.40 (n = 5)* | 122.33 ± 6.61 (n = 3) | 40.60 ± 3.04 | 38.50 ± 2.26 | 39.00 ± 2.06 (n = 5) | 35.00 ± 2.04 (n = 3) |
The values are mean ± SEM; n = 6 in each group; *P < 0.05, **P < 0.01, ***P < 0.001 compared to pre-study values (fixed variable). Two-way repeated measures ANOVA followed by Bonferroni test.
Table 6.
Effect of cleistanthins A and B on biochemical parameters of female Wistar rats.
| Total protein (g/dL) |
Albumin (g/dL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre-study | 1st month | 2nd month | 3rd month | Pre-study | 1st month | 2nd month | 3rd month | |
| Control | 5.60 ± 0.13 | 7.78 ± 0.11 | 7.37 ± 0.08 | 7.02 ± 0.08 | 3.57 ± 0.08 | 4.18 ± 0.08* | 4.08 ± 0.15** | 3.67 ± 0.21* |
| Cleistanthin A 12.5 mg/kg | 7.17 ± 0.22 | 8.16 ± 0.18* | 8.07 ± 0.20* | 8.45 ± 0.18 | 4.12 ± 0.07 | 4.42 ± 0.09* | 4.27 ± 0.17 | 4.45 ± 0.07 |
| Cleistanthin A 25 mg/kg | 6.97 ± 0.12 | 7.68 ± 0.08 | 7.80 ± 0.21 | 8.17 ± 0.11 | 4.20 ± 0.07 | 4.48 ± 0.07 | 4.20 ± 0.25** | 4.48 ± 0.10* |
| Cleistanthin A 50 mg/kg | 7.35 ± 0.320 | 7.97 ± 0.18* | 7.98 ± 0.06 | 8.00 ± 0.12 | 3.75 ± 0.13 | 4.57 ± 0.11* | 4.54 ± 0.11* (n = 5) | 4.47 ± 0.13* (n = 5) |
| Cleistanthin B 12.5 mg/kg | 6.02 ± 0.04 | 7.32 ± 0.12 | 7.50 ± 0.20 | 7.46 ± 0.18 (n = 5) | 3.68 ± 0.05 | 4.05 ± 0.08** | 4.18 ± 0.09** | 3.98 ± 0.11** (n = 5) |
| Cleistanthin B 25 mg/kg | 6.17 ± 0.14 | 7.18 ± 0.20 | 7.68 ± 0.29 | 7.73 ± 0.19 | 3.78 ± 0.10 | 4.07 ± 0.10*** | 4.18 ± 0.07*** | 4.00 ± 0.08*** |
| Cleistanthin B 50 mg/kg | 6.04 ± 0.05 | 6.97 ± 0.12 | 7.55 ± 0.12 (n = 5) | 7.77 ± 0.12 (n = 3) | 3.60 ± 0.06 | 3.80 ± 0.08 | 4.32 ± 0.10 (n = 5) | 4.03 ± 0.09 (n = 3) |
| CKMB (IU/L) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Pre-study | 1st month | 2nd month | 3rd month | |||||
| Control | 34.00 ± 5.09 | 12.17 ± 1.17 | 15.50 ± 3.48 | 23.33 ± 4.97 | ||||
| Cleistanthin A 12.5 mg/kg | 28.67 ± 3.88 | 37.75 ± 4.81 | 12.67 ± 0.99 | 33.83 ± 4.75 | ||||
| Cleistanthin A 25 mg/kg | 27.50 ± 3.47 | 36.60 ± 4.08 | 13.40 ± 1.93 | 45.50 ± 10.81 | ||||
| Cleistanthin A 50 mg/kg | 31.33 ± 4.99 | 38.00 ± 5.01 | 14.00 ± 0.82 | 65.17 ± 23.30 | ||||
| Cleistanthin B 12.5 mg/kg | 29.50 ± 3.91 | 17.67 ± 2.17 | 12.83 ± 3.049 | 139.60 ± 31.19* (n = 5) | ||||
| Cleistanthin B 25 mg/kg | 31.17 ± 4.39 | 31.50 ± 9.88 | 10.67 ± 2.94 | 74.17 ± 11.97 | ||||
| Cleistanthin B 50 mg/kg | 27.60 ± 6.03 | 16.17 ± 3.06 | 8.50 ± 1.27 (n = 5) | 76.67 ± 23.14 (n = 3) | ||||
The values are mean ± SEM; n = 6 in each group; *P < 0.05, **P < 0.01, ***P < 0.001 compared to pre-study values (fixed variable). Two-way repeated measures ANOVA followed by Bonferroni test.
Table 7.
Effect of cleistanthin A on mortality of rat.
| Drug and dose | Number of animals died |
Description | |
|---|---|---|---|
| M (n = 6) | F (n = 6) | ||
| Control (0.5% CMC) | 0 | 0 | |
| Cleistanthin A 12.5 mg/kg | 0 | 0 | |
| Cleistanthin A 25 mg/kg | 1 | 0 | Male rate died on 14th day |
| Cleistanthin A 50 mg/kg | 0 | 0 | |
| Cleistanthin A 100 mg/kg | 2 | 2 | Male rats died on 28th and 30th day and female rats died on 25th and 30th day |
| Cleistanthin A 200 mg/kg | 2 | 2 | Male rats died on 10th and 12th day and female rats died on 7th and 14th day |
| Cleistanthin A 400 mg/kg | 2 | 3 | Male rats died on 4th and 6th day and female rats died on 4th, 5th and 6th day |
Table 8.
Effect of cleistanthin B on mortality of rat.
| Drug and dose | Number of animals died |
Description | |
|---|---|---|---|
| M (n = 6) | F (n = 6) | ||
| Control (0.5% CMC) | 0 | 0 | |
| Cleistanthin B 12.5 mg/kg | 1 | 1 | Male rats died on 70th day and female animal died on 65th day |
| Cleistanthin B 25 mg/kg | 1 | 0 | Male rats died on 82nd day |
| Cleistanthin B 50 mg/kg | 1 | 3 | Male rats died on 80th and female rats died on 52nd, 67th and 72nd day of drug administration |
| Cleistanthin B 100 mg/kg | 2 | 3 | Male rats died on 18th and 25th day of and female rats died on 17th, 23rd and 31th day |
| Cleistanthin B 200 mg/kg | 3 | 3 | Male rats died on 5rd and 9th (2 Nos.) day and female rats died on 4th (2 Nos.) and 7th day |
| Cleistanthin B 400 mg/kg | 5 | 4 | Male rats died on 3rd, 4th (2 Nos.) and 5th (2 Nos.) day and female rats died on 2nd (2 Nos.) and 3rd (2 Nos.) day |
Table 9.
Histopathological summary report of cleistanthin A.
| Treatment | Organ | Male | Female |
|---|---|---|---|
| Control | Normal | Normal | |
| Cleistanthin A 12.5 mg/kg | Brain | Normal | ↑ Corpora amylacea |
| Lung | Interstitial pneumonitis | Severe interstitial pneumonitis | |
| Heart | Normal | Normal | |
| Liver | Normal | Normal | |
| Stomach | Normal | Normal | |
| Kidney | Normal | Acute tubular necrosis | |
| Bone marrow | Normal | Normal | |
| Cleistanthin A 25 mg/kg | Brain | ↑ Corpora amylacea | ↑ Corpora amylacea |
| Lung | Inflammation in lung; Interstitial pneumonitis (↑↑) and collapse of alveolar air spaces | Severe interstitial pneumonitis | |
| Heart | Normal | Normal | |
| Liver | Normal | Normal | |
| Stomach | Normal | Normal | |
| Kidney | Normal | Acute tubular necrosis | |
| Bone marrow | Normal | Normal | |
| Cleistanthin A 50 mg/kg | Brain | ↑ Corpora amylacea | ↑ Corpora amylacea |
| Lung | Severe interstitial pneumonitis, crystalline deposition and foreign body giant cell | Severe interstitial pneumonitis, crystalline deposition and foreign body giant cell reaction | |
| Heart | Normal | Normal | |
| Liver | Normal | Normal | |
| Stomach | Normal | Normal | |
| Kidney | Normal | Acute tubular necrosis | |
| Bone marrow | Normal | Normal |
Table 10.
Histopathological summary report of cleistanthin B.
| Treatment | Organ | Male | Female |
|---|---|---|---|
| Control | Normal | Normal | |
| Cleistanthin B 12.5 mg/kg | Brain | ↑ Corpora amylacea | Normal |
| Lung | Mild interstitial pneumonitis | Interstitial pneumonitis and severe focal necrosis | |
| Heart | Normal | Normal | |
| Liver | Normal | Spotty necrosis | |
| Stomach | Normal | Normal | |
| Kidney | Normal | Acute tubular necrosis | |
| Bone marrow | Normal | Normal | |
| Cleistanthin B 25 mg/kg | Brain | ↑ Corpora amylacea | ↑ Corpora amylacea |
| Lung | Severe interstitial pneumonitis with dense infiltration of neutrophils, eosinophils and foamy macrophages with collapse of alveolar space | Lung-severe interstitial pneumonitis; collection of macrophages, pulmonary oedema and extensive destruction | |
| Heart | Normal | Normal | |
| Liver | Normal | Normal | |
| Stomach | Normal | Normal | |
| Kidney | Normal | Acute tubular necrosis | |
| Bone marrow | Normal | Normal | |
| Cleistanthin B 50 mg/kg | Brain | ↑ Corpora amylacea | ↑ Corpora amylacea |
| Lung | Extensive destruction of lung parenchyma, severe interstitial pneumonitis | Crystalline deposition, severe interstitial pneumonitis, extensive necrosis, pulmonary haemorrhage | |
| Heart | Myocardial infarction | Normal | |
| Liver | Spotty necrosis | Spotty necrosis | |
| Stomach | Normal | Normal | |
| Kidney | Acute tubular necrosis | Acute tubular necrosis | |
| Bone marrow | Normal | Normal |
Table 11.
Effect of cleistanthins A and B on DNA toxicity.
| Group | Number of DNA analysed | Comet length (μm) | Head diameter (μm) | %DNA in head | Tail length (μm) | %DNA in tail |
|---|---|---|---|---|---|---|
| Control | 152 | 23.37 ± 0.40 | 23.18 ± 0.36 | 97.69 ± 0.24 | 1.14 ± 0.18 | 2.31 ± 0.24 |
| Cle A 25 mg/kg | 152 | 32.10 ± 1.28** | 24.12 ± 0.41 | 92.07 ± 0.76*** | 8.35 ± 1.02* | 7.93 ± 0.76*** |
| Cle A 50 mg/kg | 180 | 63.55 ± 2.54*** | 28.18 ± 0.41*** | 79.82 ± 0.89*** | 35.46 ± 2.27*** | 20.18 ± 0.89*** |
| Cle A 25 mg/kg | 150 | 37.94 ± 0.68*** | 26.14 ± 0.42*** | 87.89 ± 0.56*** | 11.80 ± 0.60*** | 12.11 ± 0.56*** |
| Cle B 50 mg/kg | 158 | 76.34 ± 2.21*** | 32.83 ± 0.51*** | 78.89 ± 0.80*** | 43.50 ± 2.00*** | 21.11 ± 0.80*** |
All the values are mean ± SEM; n = 3 animals; *P < 0.05, **P < 0.01, ***P < 0.001 compared to control. One-way ANOVA followed by Bonferroni test.
The overall blood pressure throughout the course has not changed significantly as evident from the AUC of systolic, diastolic and mean blood pressures. This is true for both the sexes. The change in blood pressure between pre-study and the 90th day also did not change. Though in male rats the %change appears to be more in cleistanthin groups the difference could not achieve statistical significance probably due to the small sample size. Heart rate varied so much within the groups and hence the statistical significance was not determined (only the AUC for heart rate is presented).
We expected a gradual fall in blood pressure when cliestanthins were administered for a long time in our study based on the results from our earlier study but this did not happen [21]. Though the blood pressure seemed to have fallen, the change is not statistically significant probably due to the small sample size. Further our earlier study was invasive with drug being administered intravenously. The dose used in the acute study was much higher and it produced a discernible reduction in blood pressure. Since the dose used in the current study of sub-chronic toxicity is low, the blood pressure did not fall much.
In male animals cleistanthin A showed a significant increase in the absolute organ weight of kidney and testis at the dose level of 12.5 mg/kg. This effect was not shared by cleistanthin B. But cleistanthin B showed an increase in the absolute organ weight of lung, kidney, liver and testis when the dose was raised to 25 mg/kg in male animals when compared to control. While cleistanthin A at 50 mg/kg increased the relative organ weights of heart, kidney and testis (P < 0.05), cleistanthin B at 25 mg/kg raised the relative organ weight of heart, lung, liver, kidney and testis but only in male animals (P < 0.01). Effect of cleistanthins A and B on absolute and relative organ weight is shown in Table 12, Table 13, Table 14, Table 15.
Table 12.
Effect of cleistanthins A and B on absolute organ weight of male Wistar rats (g).
| Group | Brain | Heart | Lung | Stomach | Liver | Kidney (L) | Kidney (R) | Mean kidney wt. | Testis (L) | Testis (R) | Mean testis wt. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1.85 ± 0.14 | 0.69 ± 0.03 | 1.58 ± 0.09 | 2.00 ± 0.11 | 7.48 ± 0.58 | 0.88 ± 0.05 | 0.80 ± 0.04 | 0.84 ± 0.05 | 2.30 ± 0.19 | 2.01 ± 0.22 | 2.15 ± 0.20 |
| Cleistanthin A 12.5 mg/kg | 1.96 ± 0.09 | 0.80 ± 0.07 | 1.45 ± 0.17 | 2.23 ± 0.14 | 8.74 ± 0.99 | 1.03 ± 0.07 | 0.97 ± 0.06** | 1.00 ± 0.07* | 2.76 ± 0.20 | 2.59 ± 0.25 | 2.67 ± 0.23 |
| Cleistanthin A 25 mg/kg | 1.94 ± 0.07 | 0.77 ± 0.01 | 1.57 ± 0.09 | 2.14 ± 0.14 | 8.53 ± 0.70 | 0.92 ± 0.03 | 0.91 ± 0.04 | 0.92 ± 0.03 | 2.55 ± 0.21 | 2.64 ± 0.25 | 2.60 ± 0.23 |
| Cleistanthin A 50 mg/kg | 1.87 ± 0.07 | 0.83 ± 0.06 | 1.65 ± 0.11 | 2.05 ± 0.16 | 8.02 ± 0.92 | 0.99 ± 0.07 | 0.94 ± 0.10* | 0.97 ± 0.08 | 2.63 ± 0.08 | 2.43 ± 0.11 | 2.53 ± 0.09 |
| Cleistanthin B 12.5 mg/kg | 1.87 ± 0.13 | 0.85 ± 0.05 | 2.00 ± 0.18*** | 1.92 ± 0.17 | 8.67 ± 0.61 | 1.12 ± 0.11*** | 1.06 ± 0.10*** | 1.09 ± 0.10*** | 2.97 ± 0.31 | 2.88 ± 0.30 | 2.92 ± 0.30 |
| Cleistanthin B 25 mg/kg | 1.92 ± 0.05 | 0.83 ± 0.05 | 1.64 ± 0.13 | 1.94 ± 0.10 | 8.97 ± 0.33* | 1.13 ± 0.09*** | 1.04 ± 0.09*** | 1.08 ± 0.09*** | 2.53 ± 0.18 | 2.43 ± 0.19 | 2.48 ± 0.18 |
| Cleistanthin B 50 mg/kg | 2.10 ± 0.10* | 0.78 ± 0.07 | 1.83 ± 0.10* | 1.88 ± 0.08 | 9.28 ± 0.77** | 1.17 ± 0.11*** | 1.00 ± 0.06*** | 1.09 ± 0.08*** | 2.60 ± 0.06 | 2.57 ± 0.13 | 2.58 ± 0.09 |
The values are mean ± SEM; n = 6 in each group; *P < 0.05, **P < 0.01, ***P < 0.001, compared to control. One-way ANOVA followed by Bonferroni test.
Table 13.
Effect of cleistanthins A and B on relative organ weight of male Wistar rats (g).
| Group | Brain | Heart | Lung | Stomach | Liver | Kidney (L) | Kidney (R) | Avg. kidney wt. | Testis (L) | Testis (R) | Mean. Testis wt. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.85 ± 0.04 | 0.32 ± 0.02 | 0.74 ± 0.05 | 0.92 ± 0.02 | 3.46 ± 0.25 | 0.41 ± 0.03 | 0.37 ± 0.02 | 0.39 ± 0.02 | 1.07 ± 0.10 | 0.93 ± 0.10 | 1.00 ± 0.10 |
| Cleistanthin A 12.5 mg/kg | 0.85 ± 0.07 | 0.34 ± 0.02 | 0.62 ± 0.06* | 0.96 ± 0.06 | 3.68 ± 0.27 | 0.44 ± 0.03 | 0.41 ± 0.02 | 0.42 ± 0.02 | 1.18 ± 0.09 | 1.12 ± 0.12* | 1.15 ± 0.10 |
| Cleistanthin A 25 mg/kg | 0.84 ± 0.040 | 0.33 ± 0.01 | 0.67 ± 0.01 | 0.91 ± 0.03 | 3.64 ± 0.22 | 0.40 ± 0.01 | 0.39 ± 0.02 | 0.39 ± 0.01 | 1.09 ± 0.06 | 1.12 ± 0.06* | 1.10 ± 0.06 |
| Cleistanthin A 50 mg/kg | 0.89 ± 0.06 | 0.40 ± 0.03*** | 0.78 ± 0.05 | 0.98 ± 0.11 | 3.75 ± 0.37 | 0.47 ± 0.04 | 0.44 ± 0.04* | 0.46 ± 0.04 | 1.24 ± 0.06 | 1.15 ± 0.07* | 1.20 ± 0.06* |
| Cleistanthin B 12.5 mg/kg | 0.92 ± 0.07 | 0.42 ± 0.02*** | 0.98 ± 0.09*** | 0.94 ± 0.08 | 4.25 ± 0.34** | 0.55 ± 0.06*** | 0.52 ± 0.05*** | 0.53 ± 0.05*** | 1.47 ± 0.18*** | 1.42 ± 0.16*** | 1.44 ± 0.17*** |
| Cleistanthin B 25 mg/kg | 0.90 ± 0.04 | 0.39 ± 0.03*** | 0.77 ± 0.08 | 0.91 ± 0.06 | 4.20 ± 0.21** | 0.53 ± 0.05*** | 0.49 ± 0.05*** | 0.509 ± 0.05*** | 1.18 ± 0.09 | 1.14 ± 0.09* | 1.16 ± 0.09 |
| Cleistanthin B 50 mg/kg | 1.07 ± 0.04*** | 0.38 ± 0.01* | 0.94 ± 0.05*** | 0.96 ± 0.02 | 4.71 ± 0.45*** | 0.60 ± 0.06*** | 0.51 ± 0.03*** | 0.55 ± 0.04*** | 1.36 ± 0.07 | 1.27 ± 0.05*** | 1.31 ± 0.06*** |
The values are mean ± SEM; n = 6 in each group; *P < 0.05, **P < 0.01, ***P < 0.001, compared to control. One-way ANOVA followed by Bonferroni test.
Table 14.
Effect of cleistanthins A and B on absolute organ weight of female Wistar rats (g).
| Group | Brain | Heart | Lung | Stomach | Liver | Kidney (L) | Kidney (R) | Mean kidney wt. | Uterus |
|---|---|---|---|---|---|---|---|---|---|
| Control | 1.88 ± 0.06 | 0.59 ± 0.04 | 1.63 ± 0.06 | 1.43 ± 0.13 | 6.06 ± 0.32 | 0.70 ± 0.03 | 0.66 ± 0.03 | 0.68 ± 0.03 | 3.62 ± 0.35 |
| Cleistanthin A 12.5 mg/kg | 1.90 ± 0.24 | 0.65 ± 0.06 | 1.70 ± 0.16 | 1.87 ± 0.25*** | 6.45 ± 0.56 | 1.10 ± 0.17*** | 0.92 ± 0.14*** | 1.01 ± 0.15*** | 2.96 ± 0.30* |
| Cleistanthin A 25 mg/kg | 1.66 ± 0.14 | 0.59 ± 0.04 | 1.63 ± 0.14 | 1.56 ± 0.06 | 6.42 ± 0.27 | 0.80 ± 0.03 | 0.75 ± 0.02 | 0.78 ± 0.03 | 3.47 ± 0.38 |
| Cleistanthin A 50 mg/kg | 1.98 ± 0.06 | 0.63 ± 0.05 | 1.59 ± 0.11 | 1.48 ± 0.131 | 6.13 ± 0.32 | 0.85 ± 0.04 | 0.74 ± 0.03 | 0.79 ± 0.03 | 2.86 ± 0.30** |
| Cleistanthin B 12.5 mg/kg | 2.38 ± 0.05*** | 0.72 ± 0.05** | 1.89 ± 0.03** | 1.86 ± 0.07*** | 6.70 ± 0.63 | 0.93 ± 0.04** | 0.90 ± 0.05*** | 0.92 ± 0.05*** | 3.24 ± 0.29 |
| Cleistanthin B 25 mg/kg | 2.31 ± 0.12*** | 0.62 ± 0.03 | 1.89 ± 0.08** | 1.76 ± 0.10** | 7.37 ± 0.06*** | 1.11 ± 0.18*** | 0.90 ± 0.04*** | 1.00 ± 0.11*** | 3.78 ± 0.30 |
| Cleistanthin B 50 mg/kg | 2.20 ± 0.08** | 0.69 ± 0.09 | 1.85 ± 0.02** | 1.99 ± 0.08*** | 6.25 ± 0.14 | 0.97 ± 0.05*** | 0.91 ± 0.04*** | 0.94 ± 0.05*** | 3.55 ± 0.45 |
The values are mean ± SEM; n = 6 in each group; *P < 0.05, **P < 0.01, ***P < 0.001, compared to control. One-way ANOVA followed by Bonferroni test.
Table 15.
Effect of cleistanthins A and B on relative organ weight of female Wistar rats (g).
| Group | Brain | Heart | Lung | Stomach | Liver | Kidney (L) | Kidney (R) | Avg. kidney wt. | Uterus |
|---|---|---|---|---|---|---|---|---|---|
| Control | 1.25 ± 0.03 | 0.39 ± 0.03 | 1.09 ± 0.04 | 0.95 ± 0.08 | 4.04 ± 0.20 | 0.47 ± 0.01 | 0.44 ± 0.01 | 0.45 ± 0.01 | 2.42 ± 0.23 |
| Cleistanthin A 12.5 mg/kg | 1.17 ± 0.13 | 0.40 ± 0.02 | 1.05 ± 0.08 | 1.14 ± 0.13* | 4.01 ± 0.27 | 0.70 ± 0.14*** | 0.58 ± 0.11*** | 0.64 ± 0.12*** | 1.85 ± 0.19*** |
| Cleistanthin A 25 mg/kg | 1.13 ± 0.12 | 0.39 ± 0.02 | 1.09 ± 0.07 | 1.05 ± 0.04 | 4.31 ± 0.15 | 0.54 ± 0.01 | 0.50 ± 0.01 | 0.52 ± 0.01 | 2.31 ± 0.20 |
| Cleistanthin A 50 mg/kg | 1.36 ± 0.10 | 0.43 ± 0.04 | 1.10 ± 0.12 | 0.83 ± 0.18 | 4.15 ± 0.22 | 0.58 ± 0.04 | 0.50 ± 0.03 | 0.54 ± 0.04 | 1.96 ± 0.27** |
| Cleistanthin B 12.5 mg/kg | 1.54 ± 0.03** | 0.48 ± 0.03** | 1.20 ± 0.02 | 1.18 ± 0.05** | 4.22 ± 0.41 | 0.59 ± 0.03 | 0.58 ± 0.03*** | 0.58 ± 0.03** | 1.90 ± 0.14** |
| Cleistanthin B 25 mg/kg | 1.45 ± 0.08* | 0.39 ± 0.01 | 1.20 ± 0.08 | 1.11 ± 0.08 | 4.65 ± 0.18** | 0.69 ± 0.11*** | 0.56 ± 0.02** | 0.63 ± 0.07*** | 2.38 ± 0.20 |
| Cleistanthin B 50 mg/kg | 1.31 ± 0.09 | 0.41 ± 0.06 | 1.11 ± 0.07 | 1.18 ± 0.06** | 3.73 ± 0.21 | 0.57 ± 0.03 | 0.54 ± 0.03* | 0.56 ± 0.03 | 2.05 ± 0.09 |
The values are mean ± SEM; n = 6 in each group; *P < 0.05, **P < 0.01, ***P < 0.001, compared to control. One-way ANOVA followed by Bonferroni test.
Cleistanthin B from 12.5 mg/kg onwards showed a significant increase in the absolute organ weight of heart, lung, liver, brain, stomach, kidney and uterus in female rats. When compared to cleistanthin A, cleistanthin B significantly increased the absolute and relative organ weight of the heart, lung and liver in female rats. We have no explanation as to why the weight of the organs changes other than that it could be due to some defence mechanism of the body to tackle the continuous presence of a poisonous substance. Such attribution draws some support from the histopathological data presented here. While the histopathologically affected organs such as heart, lung, liver and kidney showed significant weight changes, those organs such as stomach and testes did not show any change either in histopathology or weight.
The baseline (pre-study) values of biochemical parameters are within the normal range in all the experimental animals. Cleistanthins A and B significantly altered the fasting blood glucose, urea, creatinine, ALT, ALP, total protein, albumin and CKMB level in both the genders during the course of the experiment (Table 3, Table 4, Table 5, Table 6). But most of the parameters returned close to their original levels at the end of the study. There is no clear cut dose dependent effect exerted by either cleistanthin A or cleistanthin B. But creatinine levels remain raised in both the sexes even at the end of the study (Table 3, Table 5). The continuous presence of cleistanthins made the biochemical parameters to rise but it appears the body gets adapted to cleistanthins by about 3 months. This explains the full or partial return from raised levels of parameters. In case of creatinine, it is already reported that the kidney functions are affected by cleistanthin and hence it is no surprise that the levels of creatinine remain higher at the end of the study. Parasuraman et al. studied the effect of cleistanthins A and B on pH, urine volume and urinary ions, and they concluded that both cleistanthins A and B did not alter the pH of the urine at acute dosing, increased the urine excretion level at 5–24 h and increased the potassium ion excretion at 0–5 h and 5–24 h. The diuretic effect of both cleistanthins A and B is not dose dependent [22].
It is important to note even though cleistanthins A and B caused significant alterations in biochemical parameters such as glucose, creatinine, AST, ALT, ALP, albumin, urea and total protein, the values still remained in the normal range. The significant changes are due to the effect of the investigational compounds on morphology and function of liver and kidney.
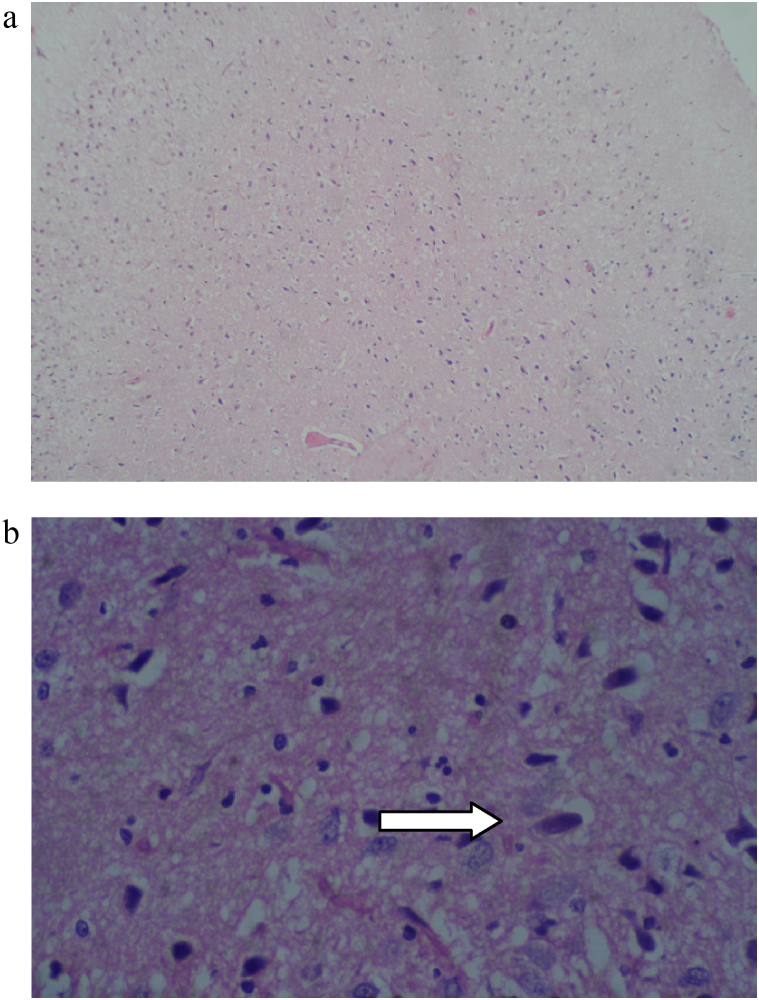
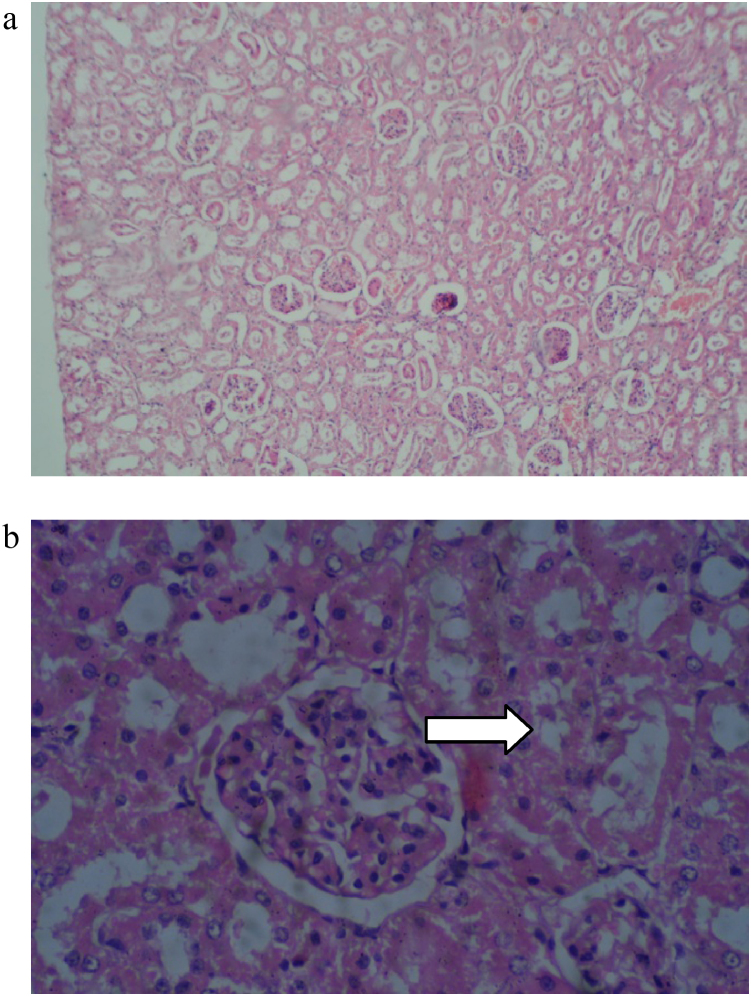
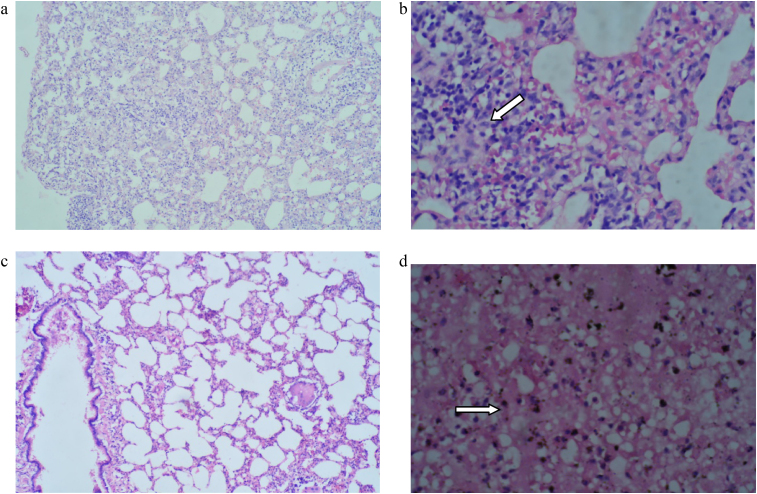
Histopathological findings are presented in Table 9, Table 10. The histopathological analysis showed dose dependent morphological changes in the lung, kidney and heart. Control group animals did not show any changes in the brain, lung, heart, liver, stomach, kidney and bone marrow. Both the compounds produced a dose dependent increase of corpora amylacea in brain (Fig. 3a and b) and induced acute tubular necrosis in kidneys (Fig. 4a and b). In addition, cleistanthin B caused spotty necrosis of liver at higher doses (Fig. 5). Cleistanthin B at a dose of 50 mg/kg and above caused infarction in cardiac muscle (Fig. 6). Comparatively cleistanthin B had more toxic effect in Wistar rats. Cleistanthins A and B caused a dose dependent toxic effect in lungs which includes mild to severe interstitial pneumonitis with dense infiltration by neutrophils, eosinophils and foamy macrophages, pulmonary edema and destruction of lung parenchyma. Cleistanthin B alone caused crystalline deposition and pulmonary haemorrhage in lungs at the doses of 50 mg/kg and above (Fig. 7a–d). Both cleistanthins A and B caused spotty necrosis and ground glass hepatocytes in liver at a dose higher than 50 mg/kg (Fig. 8). Histological analysis of heart showed the presence of myocardial infarction in animals treated with a high dose of cleistanthin B.
Fig. 3.
(a) Section from brain of cleistanthin A (25 mg/kg) treated animal shows increased corpora amylacea in cerebral cortex, H&E, 100×. (b) Section from brain of cleistanthin B (12.5 mg/kg) treated animal shows increased corpora amylacea (arrow) in cerebral cortex, H&E, 400×.
Fig. 4.
(a) Section from kidney of cleistanthin A (25 mg/kg) treated animals shows acute tubular necrosis, H&E, 100×. (b) Section from kidney of cleistanthin A (12.5 mg/kg) treated animal shows acute tubular necrosis (arrow), H&E, 400×.
Fig. 5.
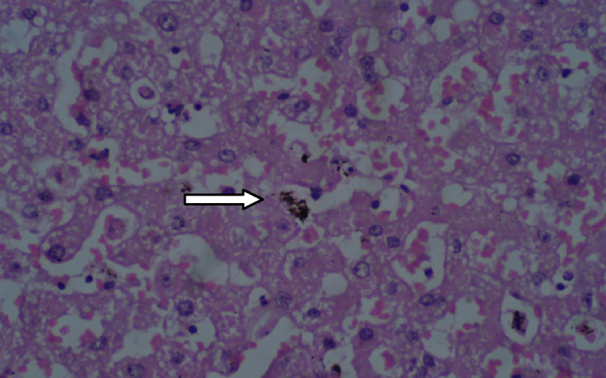
Section from liver of cleistanthin B (50 mg/kg) treated animal shows ground glass hepatocytes. Kupfer cell hyperplasia with increased hemosiderin deposition (arrow), H&E, 400×.
Fig. 6.
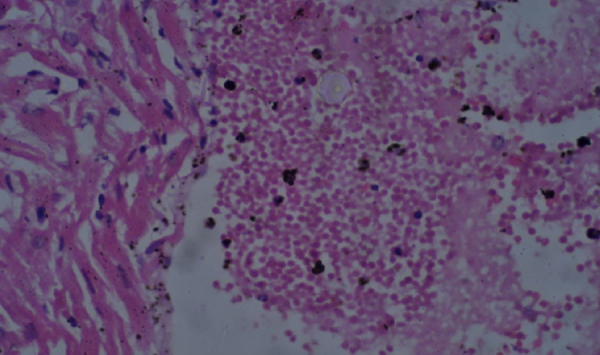
Section from heart of cleistanthin B (50 mg/kg) treated animal shows myocardial infarction, H&E, 400×.
Fig. 7.
(a) Section from lung of cleistanthin A (25 mg/kg) treated animal shows dense interstitial infiltration by lymphocytes and monocytes, H&E, 100×. (b) Section from lung of cleistanthin A (50 mg/kg) treated animal shows acute interstitial pneumonitis with dense infiltration of neutrophils, lymphocytes and plasma cells. There is evidence of focal retractile crystalline deposition surrounded by foreign body giant cell reaction (arrow), H&E, 400×. (c) Section from lung of cleistanthin B (12.5 mg/kg) treated animal shows increased hemosiderin deposition, H&E, 50×. (d) Section from lung parenchyma of cleistanthin B (50 mg/kg) treated animal shows extensive necrosis with collapsed air space and marked interstitial inflammation, H&E, 400×.
Fig. 8.
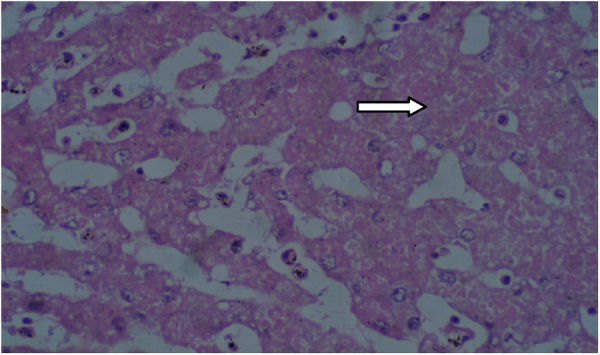
Section from liver parenchyma of cleistanthin B (50 mg/kg) treated animals shows spotty hepatic necrosis, H&E, 400×.
The histological finding of the bone marrow was normal indicating that the investigational compounds do not affect the rapidly dividing cells. But it is interesting to note that these compounds cause DNA damage in mature cells like lymphocytes. There were reports that these compounds showed anticancer effect in vitro and it is worth exploring the anticancer effects of the compounds further since they do not affect the rapidly dividing normal cells [8], [10].
Cleistanthin A and cleistanthin B caused significant DNA damage (Table 11). Both the compounds showed a significant increase in comet length and head diameter of the DNA. The percentage of DNA in head portion was reduced significantly in cleistanthins A and B treated animals. The visual observation images are presented in Fig. 9. Since cleistanthins A and B are capable of causing significant DNA damage, the anti-tumour activity of these compounds deserves exploration.
Fig. 9.
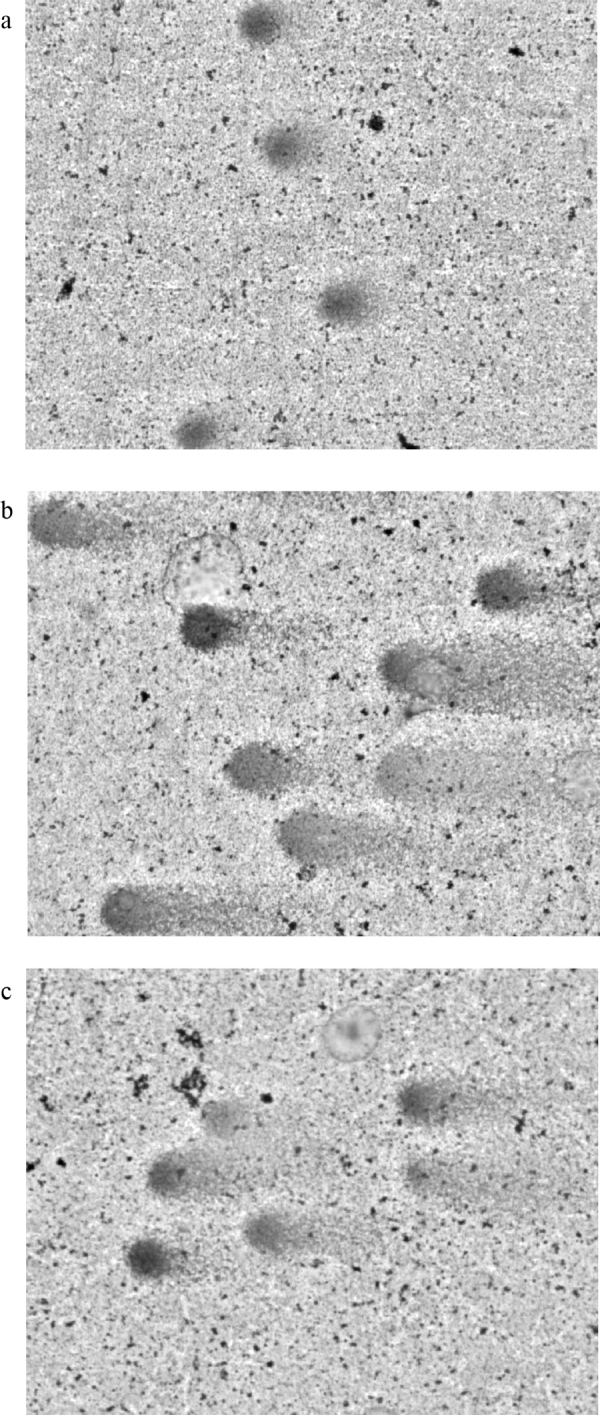
Effects of cleistanthins A and B on genotoxicity (comet assay). (a) Visual scoring of DNA damage from control animal, study sample: albino Wistar rat mononuclear cells, stain: silver nitrate, magnification: ×400. (b) Visual scoring of DNA damage from cleistanthin A 50 mg/kg treated animal, study sample: albino Wistar rat mononuclear cells, stain: silver nitrate, magnification: ×400. (c) Visual scoring of DNA damage from cleistanthin B 50 mg/kg treated animal, study sample: albino Wistar rat mononuclear cells, stain: silver nitrate, magnification: ×400.
Histological findings showed alterations in normal architecture of the tissue. It is suggested that cleistanthins A and B exert a dose dependent toxic effect in liver and heart. Biochemical analysis for cardiac marker such as CK-MB showed a significant increase within 2 months of treatment. This finding was supported by histological analysis of heart tissue.
The computer aided predictions for toxicity of cleistanthin A and cleistanthin B did not show any significant/major toxic effect [23]. The calculated NOEL (no observable effect level) dose [NOEL = LD50 (mg/kg) × 70 (kg a person)/Empirical constant (2000)] for cleistanthins A and B was 42 and 35 mg/day respectively [24].
In humans the plant was reported to induce death after 3–5 days after the consumption of leaf extract or paste made of leaves. The cause of death in humans was either cardiac arrest or respiratory arrest. In the present study the respiratory depression is evident in low doses and the compounds also cause cardiac toxicity. This explains the cause of death reported in humans. The higher doses of the compounds cause death in rodents around 5 days which is conformity with timeline of mortality occurring in humans poisoned with C. collinus.
4. Conclusion
Both cleistanthins A and B exert severe toxic effects on lungs, brain, liver, heart and kidneys. Also, they induce genotoxicity in blood lymphocytes but have no effect on bone marrow. We conclude that the investigational products exert toxicity on multiple organs but spare the rapidly dividing cells like stem cells and progenitor cells and this property would be much desirable, if these compounds are successfully developed into anticancer agents as they have been reported to exert anticancer activity. Further the toxicity profile presented here might be of help, if investigations for the treatment of poisoning are undertaken and also if the compounds themselves are investigated as lead compounds for therapeutic purposes in future.
Transparency document
Acknowledgements
The authors are thankful to Ramachandra Rao K., Department of Anatomy, JIPMER; Ananthanarayanan P.H., Department of Biochemistry, JIPMER; and Surendra Kumar V., Department of Pathology, JIPMER for providing the laboratory facilities to carry out the biochemical and histological analyses.
Footnotes
Available online 19 August 2014
Contributor Information
Subramani Parasuraman, Email: parasuphd@gmail.com.
Ramasamy Raveendran, Email: dr.ravee@gmail.com.
References
- 1.Eswarappa S., Chakraborty A.R., Palatty B.U., Vasnaik M. Cleistanthus collinus poisoning: case reports and review of the literature. J. Toxicol. Clin. Toxicol. 2003;41:369–372. doi: 10.1081/clt-120022005. [DOI] [PubMed] [Google Scholar]
- 2.Govindachari T.R., Sathe S.S., Viswanathan N., Pai B.R., Srinivasan M. Chemical constituents of Cleistanthus collinus (Roxb.) Tetrahedron. 1969;25:2815–2821. [Google Scholar]
- 3.Annapoorani K.S., Periakali P., Ilangovan S., Damodaran C., Sekharan P.C. Spectrofluorometric determination of the toxic constituents of Cleistanthus collinus. J. Anal. Toxicol. 1984;8:182–186. doi: 10.1093/jat/8.4.182. [DOI] [PubMed] [Google Scholar]
- 4.Chrispal A. Cleistanthus collinus poisoning. J. Emerg. Trauma Shock. 2012;5:160–166. doi: 10.4103/0974-2700.96486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ragupathi G., Prabhasankar P., Sekharan P.C., Annapoorani K.S., Damodaran C. Dipstick ELISA kit for the detection of Cleistanthus collinus toxins. Hindustan Antibiot. Bull. 1992;34:6–12. [PubMed] [Google Scholar]
- 6.Parasuraman S., Raveendran R., Madhavrao C. GC–MS analysis of leaf extracts of Cleistanthus collinus Roxb. (Euphorbiaceae) Int. J. Pharm. Sci. 2009;1:284–286. [Google Scholar]
- 7.Pinho P.M.M., Kijjoa A. Chemical constituents of the plants of the genus Cleistanthus and their biological activity. Phytochem. Rev. 2007;6:175–182. [Google Scholar]
- 8.Prabhakaran C., Kumar P., Panneerselvam N., Rajesh S., Shanmugam G. Cytotoxic and genotoxic effects of cleistanthin B in normal and tumour cells. Mutagenesis. 1996;11:553–557. doi: 10.1093/mutage/11.6.553. [DOI] [PubMed] [Google Scholar]
- 9.Pradheepkumar C.P., Shanmugam G. Anticancer potential of cleistanthin A isolated from the topical plant Cleistanthus collinus. Oncol. Res. 1999;11:225–232. [PubMed] [Google Scholar]
- 10.Pradheepkumar C.P., Panneerselvam N., Shanmugam G. Cleistanthin A causes DNA strand breaks and induces apoptosis in cultured cells. Mutat. Res. 2000;464:185–193. doi: 10.1016/s1383-5718(99)00179-5. [DOI] [PubMed] [Google Scholar]
- 11.Parasuraman S., Raveendran R. Effect of cleistanthin A and B on adrenergic and cholinergic receptors. Pharmacogn. Mag. 2011;7:243–247. doi: 10.4103/0973-1296.84239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stallard N., Whitehead A. Reducing animal numbers in the fixed-dose procedure. Hum. Exp. Toxicol. 1995;14:315–323. doi: 10.1177/096032719501400401. [DOI] [PubMed] [Google Scholar]
- 13.2010. OECD Guidance Document for the Performance of Chronic Toxicity and Carcinogenicity Studies, Supporting TG 451, 452 and 453 [Internet] Available from: http://www.oecd.org/dataoecd/19/33/41829966.pdf (accessed 24.01.10) [Google Scholar]
- 14.Parasuraman S. Toxicological screening. J. Pharmacol. Pharmacother. 2011;2:74–79. doi: 10.4103/0976-500X.81895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muralidhara S., Ramanathan R., Mehta S.M., Lash L.H., Acosta D., Bruckner J.V. Acute, subacute, and subchronic oral toxicity studies of 1,1-dichloroethane in rats: application to risk evaluation. Toxicol. Sci. 2001;64:135–145. doi: 10.1093/toxsci/64.1.135. [DOI] [PubMed] [Google Scholar]
- 16.Somova L.O., Nadar A., Rammanan P., Shode F.O. Cardiovascular antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine. 2003;10:115–121. doi: 10.1078/094471103321659807. [DOI] [PubMed] [Google Scholar]
- 17.Hayek T., Pavlotzky E., Hamoud S., Coleman R., Keidar S., Aviram M. Tissue angiotensin-converting-enzyme (ACE) deficiency leads to a reduction in oxidative stress and in atherosclerosis: studies in ACE-knockout mice type 2. Arterioscler. Thromb. Vasc. Biol. 2003;23:2090–2096. doi: 10.1161/01.ATV.0000098653.74209.C6. [DOI] [PubMed] [Google Scholar]
- 18.Nandhakumar S., Parasuraman S., Shanmugam M.M., Rao K.R., Chand P., Bhat B.V. Evaluation of DNA damage using single-cell gel electrophoresis (comet assay) J. Pharmacol. Pharmacother. 2011;2:107–111. doi: 10.4103/0976-500X.81903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey S.A., Zidell R.H., Perry R.W. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint. Toxicol. Pathol. 2004;32:448–466. doi: 10.1080/01926230490465874. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee K. vol. 1. Tata MC-Graw Hill, Publication Company; New Delhi: 1988. (Medical Laboratory Technology). [Google Scholar]
- 21.Parasuraman S., Raveendran R., Selvaraj R. Effects of cleistanthins A and B on blood pressure and electrocardiogram in Wistar rats. Z. Naturforsch. C. 2011;66c:581–587. doi: 10.1515/znc-2011-11-1207. [DOI] [PubMed] [Google Scholar]
- 22.Parasuraman S., Raveendran R. Diuretic effects of cleistanthin A and cleistanthin B from the leaves of Cleistanthus collinus in Wistar rats. J. Young Pharm. 2012;4:73–77. doi: 10.4103/0975-1483.96616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parasuraman S., Raveendran R. Computer-aided prediction of biological activity spectra, pharmacological and toxicological property of cleistanthin A and B. Int. J. Res. Pharm. Sci. 2010;1:333–337. [Google Scholar]
- 24.Williams P. second ed. Wiley; New York: 2000. Principles of Toxicology: Environmental and Industrial Applications. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.