Abstract
Ricin, a highly toxic plant-derived toxin, is considered a potential weapon in biological warfare due to its high availability and ease of preparation. Pulmonary exposure to ricin results in the generation of an acute edematous inflammation followed by respiratory insufficiency and death. Passive immunization with polyclonal anti-ricin antibodies conferred protection against pulmonary ricinosis, however, at clinically-relevant time points for treatment, survival rates were limited. In this study, intranasal instillation of a lethal dose of ricin to mice, served as a lung challenge model for the evaluation and comparison of different therapeutic modalities against pulmonary ricinosis. We show that treatment with doxycycline resulted in a significant reduction of pro-inflammatory cytokines, markers of oxidative stress and capillary permeability in the lungs of the mice. Moreover, survival rates of mice intoxicated with ricin and treated 24 h later with anti-ricin antibody were significantly improved by co-administration of doxycycline. In contrast, co-administration of the steroid drug dexamethasone with anti-ricin antibodies did not increase survival rates when administered at late hours after intoxication, however dexamethasone did exert a positive effect on survival when applied in conjunction with the doxycycline treatment. These studies strongly suggest that combined therapy, comprised of neutralizing anti-ricin antibodies and an appropriate anti-inflammatory agent, can promote high-level protection against pulmonary ricinosis at clinically-relevant time points post-exposure.
Abbreviations: BALF, bronchoalveolar lavage fluid; ChE, cholinesterase; MMP-9, matrix-metalloproteinase-9; PBS, phosphate-buffered saline; sPLA2, secretory phospholipase A2; RCA, ricinus communis agglutinin; VEGF, vascular endothelial growth factor; XO, xanthine oxidase
Keywords: Ricin, Pulmonary, Antibodies, Doxycycline, Combined therapy
1. Introduction
Ricin, a toxin derived from the plant Ricinus communis, is an N-glycosidase that irreversibly inactivates the 28S rRNA of the mammalian 60S ribosome subunit, subsequently arresting cell protein synthesis [1]. The toxicity of ricin depends on the route of exposure, inhalatory exposure being considered most dangerous, the estimated dose causing death to 50% of the population (LD50) being within the range of several microgram/kilogram [2]. Pathological studies of pulmonary ricin intoxication have demonstrated that injury is mostly confined to the lungs [3]. The damage inflicted to the lungs is manifested by perivascular, interstitial and alveolar edema, influx of neutrophils to the lungs and the mounting of an acute inflammatory response. Flooding of the lungs leads to respiratory insufficiency and death [3].
Prophylactic anti-ricin vaccines have been developed and are now in human phase I studies [4], however, post-exposure medical countermeasures are needed for treatment of victims after pulmonary exposure to lethal doses of the toxin. Previous studies have examined the possibility to protect animal models against pulmonary ricinosis by passive immunization with polyclonal anti-ricin antibodies. Protection levels declined with the passage of time after intoxication, and when the anti-ricin antibodies were administered 24 h after exposure, survival rates were within the 30–50% range [5], [6]. At this late time point, the pathophysiological state of some of the intoxicated mice may have deteriorated so that the loss of function of the lungs is irreversible. Conversely, it may be that higher survival rates can be attained even at this late time point if the raging pulmonary inflammation is assuaged through additional medical intervention.
A growing body of studies supports the notion that antibiotic tetracyclines, restrain inflammatory responses of various etiologies. Doxycycline, a tetracycline derivative, has been shown to inhibit staphylococcal exotoxin-induced production of cytokines and chemokines by peripheral blood mononuclear cells [7] and to attenuate polymorphonuclear cell recruitment in models of lung injury secondary to LPS, bleomycin or Streptococcus pneumoniae pneumonia [8], [9], [10]. Recently, it was reported that doxycycline exhibits anti-inflammatory activity in CF bronchial epithelial cells by inhibiting ERK 1/2, P38 and JNK dependent cell signaling (Bensman et al., 2012). Interestingly, signaling pathways involving ERK, P38 and JNK were shown in the past to be stimulated by ricin [11].
In the present study, we examined the possibility to improve survival of mice exposed intranasally to a lethal dose of ricin, by co-administration of doxycycline together with polyclonal anti-ricin antibodies. Survival rates of mice subjected to this combination treatment were compared to those attained by co-administration of the highly potent anti-inflammatory steroid, dexamethasone, with the anti-ricin antibodies. We demonstrate that co-administration of doxycycline together with anti-ricin antibodies significantly improved the ability to protect mice even when treatment with the drug commenced at late hours (24 h post-intoxication), while dexamethasone confers slightly improved survival only when administered early after exposure. Nevertheless, dexamethasone did exert some positive effect on survival when administered late after intoxication in conjunction with both doxycycline and anti-ricin antibodies.
2. Methods
2.1. Ricin preparation
Crude ricin was prepared from seeds of endemic R. communis, essentially as described before [12]. Briefly, seeds were homogenized in a Waring blender in 5% acetic acid/phosphate buffer (Na2HPO4, pH 7.4) the homogenate was centrifuged and the clarified supernatant containing the toxin was subjected to ammonium sulphate precipitation (60% saturation). The precipitate was dissolved in phosphate-buffered saline (PBS) and dialyzed extensively against the same buffer. The toxin preparation appeared on a Coomassie blue stained non-reducing 10% polyacrylamide gel as 2 major bands of molecular weight approximately 65 kDa (= ricin toxin, ∼80%) and 120 kDa (=R. communis agglutinin (RCA), ∼20%). Protein concentration was determined as 2.86 mg/ml by 280 nm absorption (Nanodrop). Pure toxin was prepared as described previously [12], [13]. Briefly, the crude ricin preparation was loaded onto a gel-filtration column (Superdex 200HR 16/60 Hiload 16/600 superdex 200 pg on an AKTA explorer, GE Healthcare Bio-Science AB; Uppsala; Sweden) and washed out with PBS to yield two well-separated protein peaks corresponding to RCA and ricin. The purity of the ricin fraction was estimated by SDS-PAGE analysis to be >98%.
2.2. Anti-ricin antibodies
Rabbits were immunized with pure ricin toxin with Freund's adjuvant in a stepwise manner, injections 1, 2 and 3 containing 4, 16 and 16 μg toxin/rabbit respectively and subsequent injections containing 100 μg toxin/rabbit, with 4-week intervals between injections. Blood samples were collected (1 week after injection) to ascertain anti-ricin antibody titer build-up. Immunization was continued until steady high anti-ricin titers were observed.
Anti-ricin antibody titers were determined by ELISA. Microtiter plates (Nunc) were coated with pure ricin (2.5 ng/ml in carbonate buffer pH 9.6, overnight incubation at room temperature), washed 3 times in wash buffer (0.8%NaCl + 0.05% Tween-20) and then incubated with blocking buffer (PBS + 0.05% Tween 20 + 2% BSA) for 1 h at 37 °C. Rabbit antisera samples were added in 2-fold serial dilutions and incubated at 37 °C for 1 h. Plates were then washed 3 times with wash buffer and incubated at 37 °C for 1 h with AP-conjugated goat anti-rabbit immunoglobulin (Sigma, 1:500 in blocking buffer). After washing as above, the microtiter plates were developed with substrate (p-NPP, Sigma) and optical densities were measured at 405 nm using an ELISA reader (Molecular Devices).
Concentrated anti-ricin IgG preparations were generated from pooled hyperimmune antisera by precipitation of the proteins with ammonium sulfate (40% saturation, overnight with constant stirring). Following centrifugation (5000 rpm, 50 min, 4 °C), the pellet was dissolved in purified water, and subjected to dialysis (overnight, 30 mM phosphate buffer pH = 7.4). The dialyzed proteins were applied on an anion-exchange column (Express-Ion, exchanger D; Whatman) and eluted with 60 mM phosphate buffer containing 1 M NaCl. The sample was precipitated by the addition of ammonium sulfate (40%, 3 h), and following centrifugation (5000 rpm, 60 min, 4 °C) the pellet was dissolved in 300 mM glycine buffer (pH = 7.4) and dialyzed (300 mM glycine buffer pH = 7.4 overnight). The concentrated anti-ricin antibody preparations were stored at 4 °C until used in in vivo experiments.
2.3. Animal studies
Animal experiments were performed in accordance with the Israeli law and were approved by the Ethics Committee for Animal Experiments at the Israel Institute for Biological Research. Treatment of animals was in accordance with regulations outlined in the USDA Animal Welfare Act and the conditions specified in the Guide for Care and Use of Laboratory Animals (National Institute of Health, 1996).
All animals in this study were female CD-1 mice (Charles River Laboratories Ltd., UK) weighing 27–32 g. Prior to exposure, animals were habituated to the experimental animal unit for 5 days. All mice were housed in filter-top cages in an environmentally controlled room and maintained at 21 ± 2 °C and 55 ± 10% humidity. Lighting was set to mimic a 12/12 h dawn to dusk cycle. Animals had access to food and water ad libitum.
For intoxication, mice were anesthetized by an intraperitoneal injection of ketamine (1.9 mg/mouse) and xylazine (0.19 mg/mouse). Crude ricin (50 μl; 7 μg/kg diluted in PBS) was applied intranasally (2× 25 μl) and mortality was monitored over 14 days. Preceding these studies, we determined that 3.5 μg crude ricin/kg body weight is approximately equivalent to one mouse (intranasal) LD50 (95% confidence intervals of 2.3–4.5 μg/kg body weight).
Treatments were performed on mice anesthetized as above. For antibody treatment, a volume of 50 μl of anti-ricin antibody preparation was delivered intranasally (2× 25 μl), intramuscularly or intravenously at various time points following intoxication. Doxycycline-hyclate (Sigma) was dissolved in PBS and administered (200 μl) intraperitoneally at 24, 48, 72 and 96 h post-exposure, at doses of 100, 100, 50 and 25 mg/kg body weight, respectively. Dexamethasone 21-phosphate disodium salt (Sigma) was administered intranasally at a dose of 4 mg/kg body weight.
2.4. Bronchoalveolar lavage fluid (BALF) preparation and analysis
BALF, collected by instillation of 1 ml PBS at room temperature, was centrifuged at 1500 rpm at 4 °C for 10 min. Supernatants were collected and stored at −20 °C until further use. The levels of IL-6, IL-1β, TNF-α, and vascular endothelial growth factor (VEGF) were determined by ELISA using commercial kits purchased from R&D Systems. Levels of secretory phospholipase A2 (sPLA2) and (xanthine oxidase) XO in BALF were determined by activity assay kits purchased from Assay Designs and Molecular Probes, respectively.
Cholinesterase (ChE) enzymatic activity was measured according to Ellman [14]. Assays were performed in the presence of 0.5 mM acetylthiocholine, 50 mM sodium phosphate buffer pH 8.0, 0.1 mg/ml BSA and 0.3 mM 5,5′-dithiobis-(2-nitrobenzoic acid). The assay was carried out at 27 °C and monitored by a Thermomax microplate reader (Molecular Devices).
Matrix-metalloproteinase (MMP) activity in BALF samples was determined by zymography. BALF samples were electrophoresed on 10% SDS-PAGE gels co-polymerized with gelatin (10% Ready Gel® zymogram gel, Biorad). SDS was expelled by 30 min incubation with 2.5% Triton X-100, and gels were incubated with developing solution (50 mM Tris, 0.2 M NaCl, 5 mM CaCl2, and 0.02% (w/v) Brij® 35 (Sigma) (pH 7.6)) over night. Gels were then stained for 30 min with 0.5% Coomassie G250 (Bio-Rad, Israel) in methanol:acetic acid:water (30:10:60) and destained in tap water for 24 h. Relative densities of MMP-9 were analyzed with Bio-Rad Quantity One software (Bio-Rad, Hercules, CA, USA). Results were compared to a positive standard and expressed as mean ± SEM.
2.5. Statistical analysis
Animal survival experimental data was obtained by pooling results of three or more independent experiments, each with 8–10 mice per experiment group. Comparison of the Kaplan–Meier survival curves of mice in each individual experiment using the log-rank (Mantel–Cox) test showed that there are no statistically significant differences among groups of animals receiving the same treatment in independent experiments. No discrepancies in treatment effects from experiment to experiment were noted. In other experiments, individual groups were compared using unpaired t test analysis. To estimate p values, all statistical analyses were interpreted in a two-tailed manner. Values of p < 0.05 were considered to be statistically significant. All data is presented as means ± S.E.M.
3. Results
Pulmonary exposure to ricin results in the generation of a severe localized edematous inflammation which eventually leads to respiratory insufficiency and death [2]. The lack of antidotes against pulmonary ricinosis, prompted us to examine the prospect to alleviate intoxication by post-exposure combined therapy, comprising anti-ricin antibodies and a drug. In the present study, intranasal instillation served as a lung challenge model for the evaluation and comparison of different therapeutic modalities.
3.1. Treatment with anti-ricin antibodies
To determine the therapeutic window for protection against pulmonary exposure to ricin by antibodies, a lethal dose of ricin (7 μg/kg body weight) was instilled intranasally to groups of mice and at various time points prior or following intoxication, mice were intranasally administered a fixed volume (50 μl) of anti-ricin antiserum prepared at our laboratory from hyperimmune rabbits. Mice were monitored for survival for 14 days following exposure.
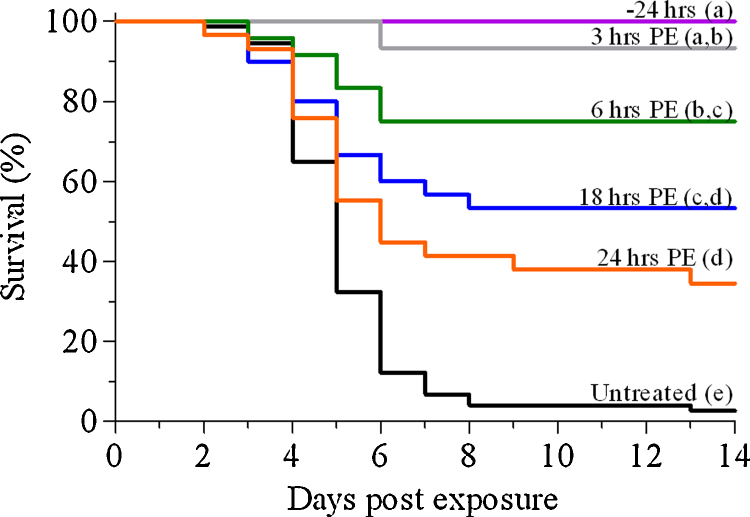
Administration of anti-ricin antibodies by the intranasal route 24 h before exposure, or a short period of time (3 h) after exposure, conferred protection to 100 and >90% of the mice, respectively. Treatment with antibodies at later time points resulted in descending levels of protection; 75, 53 and 34% of the mice survived when antibodies were administered 6, 18 and 24 h after intoxication, respectively (Fig. 1). In contrast, intramuscular administration of the same anti-ricin antiserum preparation at 3 h after intoxication resulted in low-level protection only (20%), while none of the mice survived when anti-ricin antibodies were delivered intramuscularly 18–24 h after intoxication. In fact, treatment via the i.m. route resulted in poor survival (33%) even when antibody administration preceded intoxication. These findings indicate that effective protection depends on the introduction to the lungs of sufficient amounts of anti-ricin antibodies within the period of time relevant for protection, a requirement met by the topical administration to the lungs by intranasal application. It is interesting to note, that intravenous administration of the anti-ricin antibodies at 6 or 24 h post-exposure conferred protection similar to that observed for intranasal administration of the antibodies (data not shown).
Fig. 1.
Therapeutic window for post-exposure treatment of ricin-intoxicated mice with anti-ricin antibodies. Mice were intoxicated intranasally with ricin (7 μg/kg body weight) and treated with anti-ricin antibodies at the indicated time points. Animals were observed for a 2-week period after ricin challenge. Curves with different letters are significantly different (p < 0.05). Number of animals per experimental group: 24 h, 6 h PE: n = 25; 3 h PE: n = 15; 18 h PE: n = 30; 24 h PE: n = 29; untreated: n = 74.
In these protection experiments, we used antiserum collected from hyperimmune rabbits vaccinated against ricin. To determine whether the antibody dose in these experiments limits survival levels, mice intranasally exposed to a lethal dose of ricin were treated 24 h post-intoxication side-by-side either with the antiserum or with IgG-enriched preparations derived from this antiserum, the latter exhibiting higher anti-ricin titers in ELISA. Protection levels following treatment with concentrated IgG preparations exhibiting anti-ricin titers that are 8- or 16-fold higher (5 × 106 and 107) than that of the antiserum (6.4 × 105) were not significantly higher than the protection level conferred by the antiserum (42% and 45% protection, respectively, as opposed to 39% protection with antiserum). The rather similar survival rate values exhibited following treatment with different dosages of antibody indicate that the upper limit of protection by treatment with antibodies has been reached and that survival values cannot be further improved in a significant manner, merely by increasing the dose of antibodies.
3.2. Effect of dexamethasone on the survival of mice
Pulmonary exposure to ricin causes a severe localized inflammation which is accompanied by a massive recruitment of neutrophils to the lungs (Lindauer et al., 2009(. High levels of protein were found in the BALF sampled from the inflamed lungs ([6] and our results, not shown), indicating that the lung–blood barrier has been disrupted. Since steroids are known both to suppress neutrophil migration and to mitigate capillary permeability [15], we examined the effect of dexamethasone, a potent steroid drug, on the survival rates of mice that were intranasally exposed to a lethal dose of ricin.
Administration of dexamethasone in conjunction with the 24 h PE anti-ricin antibody treatment, exerted a significant positive effect on survival of mice intranasally-exposed to a lethal dose of ricin only when the steroid was first administered prior to intoxication (8 h before intoxication) (Table 1). When dexamethasone treatment commenced at an early time point after intoxication (6 h PE), the increase in protection was considerably lower, while administration of the steroid at 24 h PE, concomitant with the antibody treatment, did not lead to any noticeable improvement in survival.
Table 1.
Survival of ricin-intoxicated mice following combined treatment with anti-ricin antibodies and dexamethasone. Survival of mice lethally challenged with ricin (7μg/kg), and treated with anti-ricin antibodies (intranasal, 2× 25μl) at 24 h PE and/or dexamethasone (4 mg/kg body weight) at the indicated time points.
| Time of treatment (h PE) |
% Survival | |
|---|---|---|
| Anti-ricin antibodies | Dexamethasone | |
| – | 0, 24 | 3a |
| 24 | – | 34b |
| 24 | −8, 24 | 82c |
| 24 | 6, 24 | 44b |
| 24 | 24 | 30b |
Represent groups displaying significant statistic difference (a, b, c).
Steroids operate mostly at the transcriptional level, exerting their anti-inflammatory effects by respectively promoting and repressing the expression of genes encoding for anti- and pro-inflammatory elements [16]. If transcription of pro-inflammatory factors occurs rapidly following ricin exposure, the effectiveness of steroid compounds such as dexamethasone are expected to be compromised when applied late after exposure. Conversely, compounds that interact directly with pro-inflammatory proteins may be more efficient in their ability to subdue inflammation at clinically-relevant time points. In an effort to tailor a treatment against pulmonary ricinosis, which includes a drug that is effective even when administered late after intoxication, we scrutinized the progressing lung pathophysiology following intranasal exposure of mice to ricin, for mediators of inflammation which can serve as targets for post-exposure drug therapy.
3.3. Pro-inflammatory markers in the lung
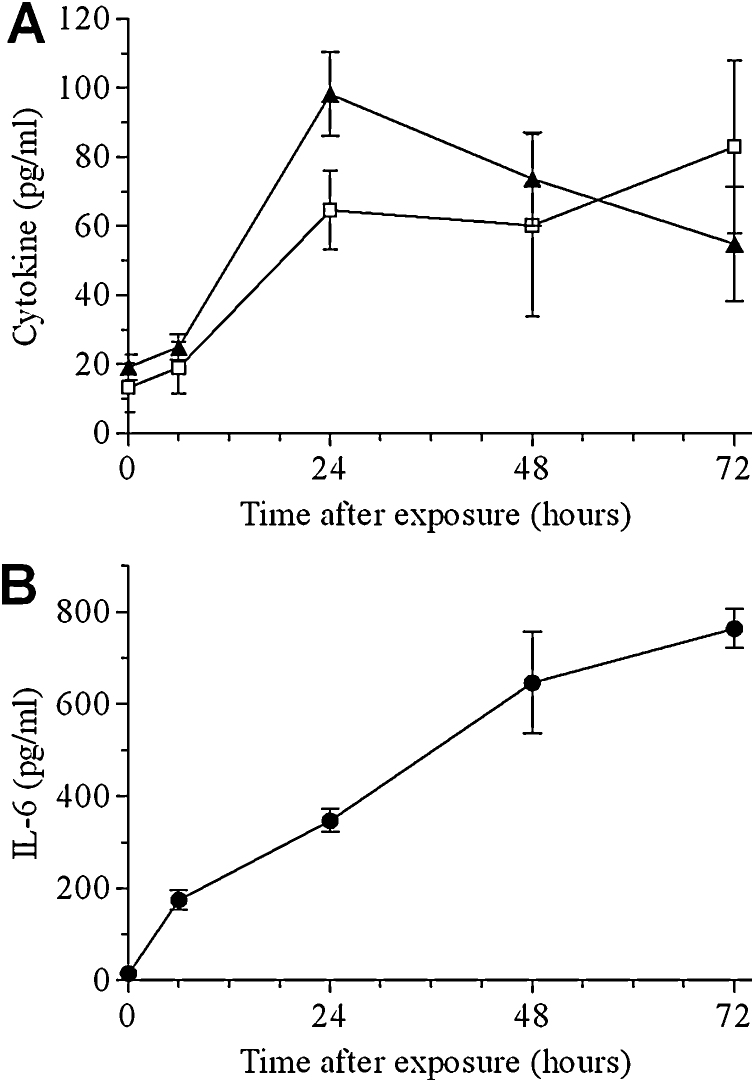
Elevated levels of the pro-inflammatory cytokines TNF-α, IL-1β and IL-6 were measured in BALF samples collected from mice at various time points following intranasal ricin intoxication. Peak levels of TNF-α were reached at 24 h PE, after which there was a moderate decrease during the next 48 h (Fig. 2A). IL-1β levels also rose during the 24 h following intoxication, yet unlike TNF-α, remained elevated during the next 48 h (Fig. 2A). Cytokine levels of IL-6 in BALF rose rapidly, displaying a significant 12-fold increase as soon as 6 h PE (Fig. 2B). IL-6 levels continued to rise, displaying a 24-, 45- and >50-fold increase above control, at 24, 48 and 72 h PE, respectively.
Fig. 2.
Pro-inflammatory cytokines in the BALF of ricin intoxicated mice. Mice were intranasally exposed to 7 μg/kg ricin and BALF samples collected at the indicated time points were monitored for: A. IL-1β (squares), TNF-α (triangles), B. IL-6. Data are mean ± S.E.M. N = 5.
Vascular endothelial growth factor (VEGF) promotes vascular permeability and interstitial edema [17] and therefore we determined VEGF levels in the BALF samples collected from these ricin-intoxicated mice. Indeed, 24 h post-ricin intoxication, VEGF increased significantly, thereafter, remaining unchanged for the following 48 h (Fig. 3A). In line with this finding, significantly increased levels of the serum-resident enzyme cholinesterase, were found in the BALF of intoxicated mice, albeit at a later stage (Fig. 3B), attesting to the fact that the blood–lung barrier has been severely impaired.
Fig. 3.
Markers of vascular permeability in the BALF of ricin-intoxicated mice. Mice were intranasally exposed to 7 μg/kg ricin and BALF samples collected at the indicated time points were monitored for: (A) VEGF, (B) ChE. Data are mean ± S.E.M. N = 5.
Next, we sought to examine in the BALF of ricin-intoxicated mice, markers associated with oxidative stress, lipolysis and proteolysis. Xanthine oxidase (XO), an enzyme associated with oxidative damage, increased only slightly (2-fold over control levels) during the first 48 h after intoxication, however, during the next 24 h levels of this enzyme accelerated, displaying an overall 20-fold increase (Fig. 4A). Expression of XO results in localized formation of reactive oxygen species that correlate with the degree of lung pathologies [18] and contributes to lung injury [19]. Levels of the lipolytic enzyme secretory phospholipase A2 (sPLA2), a potent mediator of inflammation responsible for hydrolysis of surfactant phospholipids [20], rose significantly after ricin-intoxication displaying a 44-fold increase during the 72 h period following intoxication (Fig. 4B). Levels of the gelatinolytic enzyme matrix-metalloproteinase-9 (MMP-9) ascended rapidly after ricin-intoxication, displaying a peak level equivalent to >100-fold increase already at 24 h PE. (Fig. 4C). MMP-9 plays an important role in lung injuries [21] and correlate with alveolar-capillary permeability [22].
Fig. 4.
Oxidative, lipolytic and proteolytic pro-inflammatory parameters in the BALF of ricin-intoxicated mice. Mice were intranasally exposed to 7 μg/kg ricin and BALF samples collected at the indicated time points were monitored for: (A) xanthine oxidase (XO), (B) secretory phospholipase A2 (sPLA2), (C) matrix-metalloproteinase-9 (MMP-9). Data are mean ± S.E.M. N = 5.
3.4. Doxycycline treatment reduces signs of ricin-induced pulmonary inflammation
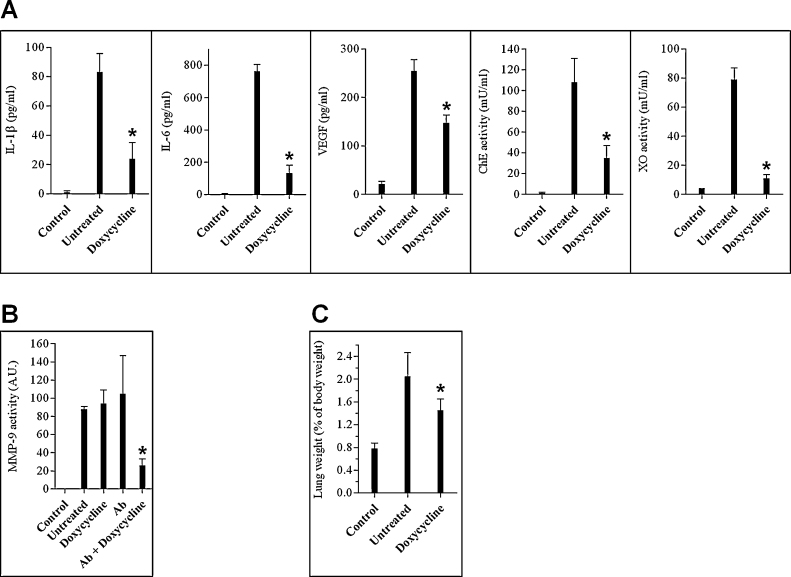
Doxycycline, a compound well-known for its antimicrobial activity, has been also shown to possess diverse anti-inflammatory capabilities. These include down regulation of proinflammatory cytokines [7], anti-oxidant activity [23], inhibition of matrix-metalloproteinases [9] and sPLA2 [24], and mitigation of vascular hyperpermeability [25]. Since, as detailed above, these inflammation-related characteristics were observed following pulmonary exposure to ricin, we decided to examine the effect of doxycycline treatment in itself on these inflammation-related elements in the lungs of ricin-intoxicated mice. To this end, mice intranasally exposed to a lethal dose of ricin were administered doxycycline only (100 mg/kg body weight, immediately after intoxication and then 100, 50 and 25 mg/kg body weight at 24, 48 and 69 h post-intoxication, respectively), lungs were lavaged at 72 h PE and BALF was subjected to analysis. Doxycycline exerted an anti-inflammatory effect on the majority of the inflammation-related parameters examined (Fig. 5A). Levels of the proinflammatory cytokines IL-1β and IL-6 were considerably lower in mice treated with doxycycline. Likewise, XO was substantially reduced in the doxycycline-administered mice, as compared to ricin-intoxicated mice that were not treated. Capillary permeability was also attenuated in these mice, as judged by the reduced levels of VEGF and ChE (Fig. 5A), the latter being a serum-resident protein whose incongruous presence in the lumen of the lungs is indicative of lung–blood barrier disruption. In contrast, administration of doxycycline did not affect BALF levels of MMP-9 (Fig. 5B), which remained as high as in intoxicated mice that were not treated with doxycycline. A positive effect of doxycycline on MMP-9 levels was however exhibited when mice were administered both doxycycline and antibodies shortly after intoxication, while mice that received antibodies only did not exhibit reduction in this marker (Fig. 5B).
Fig. 5.
Pathological and pro-inflammatory changes in the BALF of ricin intoxicated mice following treatment with α-ricin antibodies or with α-ricin antibodies and doxycycline. Mice were intranasally exposed to 7 μg/kg ricin and subjected to different treatment modes. (A) Ricin-intoxicated mice were treated with doxycycline immediately after intoxication and BALF collected at 72 h was monitored for IL-1β, IL-6, VEGF, ChE and XO. (B) Ricin-intoxicated mice were treated with doxycycline and/or anti-ricin antibodies and BALF collected at 72 h was monitored for MMP-9. (C) Ricin-intoxicated mice were treated with doxycycline and lungs were harvested at 72 h PE and weighed. Wet lung weight is presented as percent of total body weight. Error bars represent the standard error of the mean of the samples. *p < 0.05 in comparison to non-treated mice. N = 5.
Overall, treatment with doxycycline led to reduced pulmonary edema, as witnessed by the weight reduction of mice lungs following doxycycline administration (Fig. 5C). Nevertheless, treatment with doxycycline in itself conferred no more than marginal protection to the ricin intoxicated mice; approximately 90% of the mice died without displaying an increase in mean time to death values. We therefore examined the effect of doxycycline administration on mice survival, in conjunction with the antibody-based treatment.
3.5. Effect of doxycycline on the survival of mice
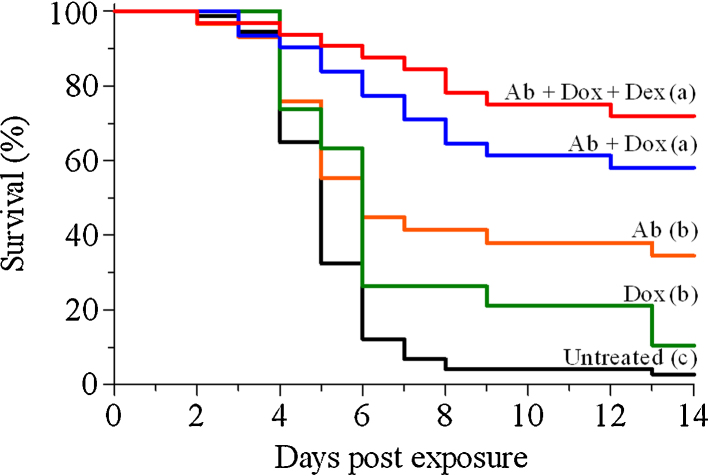
Mice were intranasally exposed to a lethal dose of ricin and 24 h later were subjected to combined anti-ricin antibodies and doxycycline treatment. Indeed, adding doxycycline to the antibody treatment improved survival rates, which reached 58%, nearly 2-fold higher compared to mice treated with antibodies only (34% survival) (Fig. 6). As mentioned above, adding dexamethasone failed to improve protection when administered at late hours with anti-ricin antibodies, nevertheless, when dexamethasone was applied as an adjunct to the doxycycline/anti-ricin antibody treatment, survival rates seem to be further elevated (74% protection, Fig. 6). Taken together, these findings demonstrate that anti-inflammatory drugs exert a positive effect on mice survival, when applied as add-ons to the post-exposure antibody-based therapy against pulmonary ricinosis.
Fig. 6.
Kaplan–Meier survival curves of mice intoxicated with ricin and subjected to post-exposure treatment. Mice were intoxicated intranasally with ricin (7 μg/kg) and subjected to various treatments as indicated. Animals were observed for a 2-week period after ricin challenge. The various treatments were commenced at 24 h post exposure and included α-ricin antibodies (Ab), doxycycline (dox), or dexamethasone (dex), or their combinations, as indicated within the figure (n = 29–31). Curves corresponding to the “untreated” and “Ab” groups are those displayed in Fig. 1.
4. Discussion
In the present study, we demonstrate that the survival rates of mice treated with anti-ricin antibodies at relatively late hours (24 h) post-pulmonary intoxication can be considerably improved by co-administration of doxycycline. The improved survival displayed in mice treated with doxycycline stems from the anti-inflammatory properties of this compound; doxycycline promotes significant reduction of pro-inflammatory cytokines and markers of oxidative stress and capillary permeability in the lungs of the intoxicated mice. Various studies have shown that doxycycline exerts its anti-inflammatory effects not only at the transcriptional level by down-regulation of pro-inflammatory cytokines [7], [16], but also by interacting with pro-inflammatory mediators, or compromising their ability to operate. Thus, doxycycline has been shown to: (a) act as an anti-oxidant agent by reacting with free radicals [23], (b) serve as a chelator of zinc/calcium ions, thereby inhibiting MMPs [26], (c) prevent vascular hyperpermeability by inducing expression of VE-cadherin on endothelial cells [25], and (d) inhibit sPLA2 via direct interaction with this enzyme [27].
Although administration of doxycycline had a marked beneficial effect on inflammation-related markers, doxycycline in itself provided no more than marginal (10%) protection to mice that were intranasally exposed to a lethal dose of ricin. It seems that ricin by virtue of being highly inflammogenic, persistently stimulates acute inflammatory responses with which doxycycline alone cannot cope. Anti-ricin antibody treatment is therefore required to halt any further pro-inflammatory firing, whilst doxycycline exerts its positive effect by dampening inflammation that has already developed. Indeed, though treatment with doxycycline alone reduced inflammation-related factors to a considerable extent, values remained higher than in control mice (see Fig. 5). Alternatively, doxycycline treatment in itself may not suffice for protection, since anti-ricin antibodies are required for curbing inflammation-independent ricin-induced cytotoxicity caused by protein synthesis arrest. The inter-relationship between protein synthesis arrest and the clinical manifestation of pulmonary ricinosis, namely, the onset of a severe edematous inflammation accompanied by massive recruitment of neutrophils and cytokine storming, has yet not been resolved. It may well be that large scale disruption of ribosomes (= quantitative protein synthesis arrest) is not required for establishment of full-blown pulmonary ricinosis. A “minimal” role for protein synthesis arrest in pulmonary ricinosis, would maintain that low-key ricin-induced cessation of protein synthesis is sufficient as a trigger for launching full-scale inflammation. Previous studies suggested that termination of protein synthesis, or perhaps the actual damage to ribosomes by ricin, initiates a MAPKinase-driven proinflammatory signaling cascade termed “ribotoxic stress response”, whereby cytokines such as TNF-α and IL1-β are upregulated [11], [28]. In another study, it was suggested that termination of cellular protein synthesis by ricin initiates inflammation by allowing turnover of labile suppressor proteins that under normal conditions prevent the conversion of inactive pro-IL1-β into biologically active proinflammatory IL1-β [29]. A recent study demonstrated that sessile alveolar macrophages and pulmonary epithelial cells, mutually suppress cytokine release [30]. It may well be that ricin-inflicted injury to alveolar macrophages, an early event following pulmonary intoxication [31], prompts the onset of pro-inflammatory responses by de-repressing cytokine synthesis by the epithelial cells. Conversely, in a model which assumes a “maximal” role for protein synthesis arrest in pulmonary ricinosis, ricin-mediated intoxication leads to large-scale ribosome damage, which in turn, gives rise to a protein synthesis arrest-related disease which is life-threatening in itself, regardless of inflammation. The scope and magnitude of the ricin-induced disease that develops in the absence of inflammation, has not been determined. Regardless of the exact model, our study amply demonstrates that inflammation plays a decisive role in pulmonary ricin toxicity and that alleviation of inflammation contributes significantly to survival.
Unlike the combined doxycycline/anti-ricin antibody treatment, dexamethasone improved survival rates only if the steroid was applied before or shortly after intoxication, but not when it was administered simultaneously with the antibody treatment, 24 h post-exposure. Nevertheless, dexamethasone did exert some positive effect when co-administered at 24 h after ricin intoxication together with both the anti-ricin antibodies and doxycycline. Survival rates following this tripartite treatment were higher than when only doxycycline was added to the antibody treatment (74% survival after dexamethasone/doxycycline/Ab treatment, 58% survival after doxycycline/Ab treatment, p = 0.07). In what way does the dexamethasone improve survival in this unique therapeutic set-up, in terms of the underlying anti-inflammatory reactions, remains to be deciphered.
In summary, we show that compounds displaying multifaceted anti-inflammatory traits, such as doxycycline, can bestow improved protection following pulmonary ricin-intoxication even when administered at clinically-relevant time points. Judicious choice of other anti-inflammatory agents for treatment, based on in-depth knowledge of pulmonary ricinosis pathology should lead to further improvement in medical treatment, as well as providing valuable tools for unraveling the intricate cascades and pathways underlying ricin intoxication.
Transparency document
Acknowledgments
We thank Dr. Arik Makovitzki and Mr. Avi Agami for the production of the concentrated anti-ricin IgG preparations.
Footnotes
Available online 1 August 2014
References
- 1.Olsnes S., Kozlov J.V. Ricin. Toxicon. 2001;39:1723–1728. doi: 10.1016/s0041-0101(01)00158-1. [DOI] [PubMed] [Google Scholar]
- 2.Audi J., Belson M., Patel M., Schier J., Osterloh J. Ricin poisoning: a comprehensive review. JAMA. 2005;294:2342–2351. doi: 10.1001/jama.294.18.2342. [DOI] [PubMed] [Google Scholar]
- 3.Wilhelmsen C.L., Pitt M.L.M. Lesions of acute inhaled lethal ricin intoxication in rhesus monkeys. Vet. Pathol. 1996;33:296–302. doi: 10.1177/030098589603300306. [DOI] [PubMed] [Google Scholar]
- 4.O’Hara J.M., Brey R.N., Mantis N.J. Comparative efficacy of two leading candidate ricin toxin a subunit vaccines in mice. Clin. Vaccine Immunol. 2013;20:789–794. doi: 10.1128/CVI.00098-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holley J.L., Poole S.J.C., Cooper I.A.M., Griffiths G.D., Simpson A.J. Defence against the Effects of Chemical Hazards: Toxicology, Diagnosis and Medical Countermeasures. Meeting Proceedings RTO-MP-HFM-149, Paper 12; Neuilly-sur-Seine, France: 2007. The production and evaluation of ricin antitoxins; pp. 12-1–12-8. Available from http://www.rto.nato.int. [Google Scholar]
- 6.Pratt T.S., Pincus S.H., Hale M.L., Moriera A.L., Roy C.J., Tchou-Wong K.M. Oropharyngeal aspiration of ricin as a lung challenge model for evaluation of the therapeutic index of antibodies against ricin A-chain for post-exposure treatment. Exp. Lung Res. 2007;33:459–481. doi: 10.1080/01902140701731805. [DOI] [PubMed] [Google Scholar]
- 7.Krakauer T., Buckley M. Doxycycline is anti-Inflammatory and inhibits staphylococcal exotoxin-induced cytokines and chemokines. Antimicrob. Agents Chemother. 2003;47:3630–3633. doi: 10.1128/AAC.47.11.3630-3633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita M., Ye Q., Ouchi H., Harada E., Inoshima I., Kuwano K., Nakanishi Y. Doxycycline attenuated pulmonary fibrosis induced by bleomycin in mice. Antimicrob. Agents Chemother. 2006;50:739–743. doi: 10.1128/AAC.50.2.739-743.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita M., Harada E., Ikegame S., Ye Q., Ouchi H., Inoshima I., Nakanishi Y. Doxycycline attenuated lung injury by its biological effect apart from its antimicrobial function. Pulm. Pharmacol. Ther. 2007;20:669–675. doi: 10.1016/j.pupt.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Moon A., Gil S., Gill S.E., Chen P., Matute-Bello G. Doxycycline impairs neutrophil migration to the airspaces of the lung in mice exposed to intratracheal lipopolysaccharide. J. Inflamm. 2012;9:31–35. doi: 10.1186/1476-9255-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korcheva V., Wong J., Corless C., Iordanov M., Magun B. Administration of ricin induces a severe inflammatory response via nonredundant stimulation of ERK, JNK and P38 MAPK and provides a mouse model of hemolytic uremic syndrome. Am. J. Pathol. 2005;166:323–339. doi: 10.1016/S0002-9440(10)62256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin J.Y., Liu S.Y. Studies on the antitumor lectins isolated from the seeds of Ricinus Communis (Castor bean) Toxicon. 1986;24:757–765. doi: 10.1016/0041-0101(86)90100-5. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths G.D., Lindsay C.D., Upshall D.G. Examination of the toxicity of several protein toxins of plant origin using bovine pulmonary endothelial cells. Toxicology. 1994;90:11–27. doi: 10.1016/0300-483x(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 14.Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 15.Ohta N., Shimaoka M., Imanaka H., Nishimura M., Taenaka N., Kiyono H., Yoshiya I. Glucocorticoid suppresses neutrophil activation in ventilator-induced lung injury. Crit. Care Med. 2001;29:1012–1016. doi: 10.1097/00003246-200105000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Singh N., Rieder M.J., Tucker M.J. Mechanisms of glucocortico-mediated anti-inflammatory and immunosuppressive action. Paediatr. Perinat. Drug Ther. 2004;6:107–115. [Google Scholar]
- 17.Kaner R.J., Ladetto J.V., Singh R., Fukuda N., Matthay M.A., Crystal R.G. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am. J. Cell. Mol. Biol. 2000;22:657–664. doi: 10.1165/ajrcmb.22.6.3779. [DOI] [PubMed] [Google Scholar]
- 18.Komaki Y., Sugiura H., Koarai A., Tomaki M., Ogawa H., Akita T., Hattori T., Ichinose M. Cytokine-mediated xanthine oxidase upregulation in chronic obstructive pulmonary disease's airways. Pulm. Pharmacol. Ther. 2005;18:297–302. doi: 10.1016/j.pupt.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Wright R.M., Ginger L.A., Kosila N., Elkins N.D., Essary B., McManaman J.L., Repine J.E. Mononuclear phagocyte xanthine oxireductase contributes to cytokine-induced acute lung injury. Am. J. Respir. Cell Mol. Biol. 2004;30:479–490. doi: 10.1165/rcmb.2003-0309OC. [DOI] [PubMed] [Google Scholar]
- 20.Touqui L., Arbibe L. A role for phospholipase A2 in ARDS pathogenesis. Mol. Med. Today. 1999;5:244–249. doi: 10.1016/s1357-4310(99)01470-7. [DOI] [PubMed] [Google Scholar]
- 21.Carney D.E., McMann U.G., Schiller H.J., Gatto L.A., Steinberg J., Picone A.L., Nieman G.F. Metalloproteinase inhibition prevents acute respiratory distress syndrome. J. Surg. Res. 2001;99:245–252. doi: 10.1006/jsre.2001.6180. [DOI] [PubMed] [Google Scholar]
- 22.Soccal P.M., Gasche Y., Pache J.C., Schneuwly O., Slosman D.O., Morel D.R., Spiliopoulos A., Suter P.M., Nicod L.P. Matrix metalloproteinases correlate with alveolar-capillary permeability alteration in lung ischemia-reperfusion injury. Transplantation. 2000;70:998–1005. doi: 10.1097/00007890-200010150-00002. [DOI] [PubMed] [Google Scholar]
- 23.Akamatsu H., Asada M., Komura J., Asada Y., Niwa Y. Effect of doxycycline on the generation of reactive oxygen species: a possible mechanism of action of acne therapy with doxycycline. Acta. Derm. Venereol. 1992;72:178–179. [PubMed] [Google Scholar]
- 24.Pruzanski W., Greenwald R.A., Street I.P., Laliberte F., Stefanski E., Vadas P. Inhibition of enzymatic activity of phospholipase A2 by minocycline and doxycycline. Biochem. Pharmacol. 1992;44:1165–1170. doi: 10.1016/0006-2952(92)90381-r. [DOI] [PubMed] [Google Scholar]
- 25.Fainaru O., Adini I., Benny O., Bazinet L., Pravda E., D’Amato R., Folkman J. Doxycycline induces membrane expression of VE-cadherin on endothelial cells and prevents vascular hyperpermeability. FASEB J. 2008;22:3728–3735. doi: 10.1096/fj.08-110494. [DOI] [PubMed] [Google Scholar]
- 26.Golub L.M., Lee H.M., Ryan M.E., Giannobile W.V., Payne J., Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv. Dent. Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 27.Dalm D., Palm G.J., Aleksandrov A., Simonson T., Hinrichs W. Nonantibiotic properties of tetracyclines: structural basis for inhibition of phospholipase A2. J. Mol. Biol. 2010;398:83–96. doi: 10.1016/j.jmb.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 28.Korcheva V., Wong J., Lindauer M., Jacoby D.B., Iordanov M., Magun B. Role of apoptotic signaling pathways in regulation of inflammatory responses to ricin in primary murine macrophages. Mol. Immunol. 2007;44:2761–2771. doi: 10.1016/j.molimm.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindauer M., Wong J., Magun B. Ricin toxin activates the NALP3 inflammasome. Toxins. 2010;2:1500–1514. doi: 10.3390/toxins2061500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westphalen K., Gusarova G.A., Islam M.N., Subramanian M., Cohen T.S., Prince A.S., Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–506. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown R.F.R., White D.E. Ultrastructure of rat lung following inhalation of ricin aerosol. Int. J. Exp. Pathol. 1997;78:267–276. doi: 10.1046/j.1365-2613.1997.300363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.