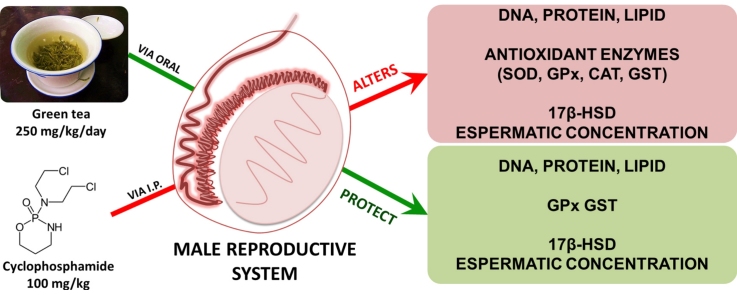
Graphical abstract
Keywords: Cyclophosphamide, Green tea, Oxidative stress, Antioxidant, Male reproductive system
Abstract
Green tea presents catechins as its major components and it has a potential antioxidant activity. Cyclophosmamide (CP) is an antineoplastic and immunosuppressive agent, known to reduce fertility. In the present study, we evaluated the effect of green tea infusion on cyclophosphamide-induced damage in male mice reproductive system. Mice received green tea infusion (250 mg/kg) or vehicle by gavage for 14 days. Saline or CP were injected intraperitoneally at a single dose (100 mg/kg) at the 14th day. Animals were euthanized 24 h after CP administration and testes and epididymis were removed for biochemical analysis and sperm evaluation. Catechins concentration in green tea infusion was evaluated by HPLC. CP increased lipid peroxidation, DNA damage and superoxide dismutase activity whereas sperm concentration, glutathione peroxidase (GPx), glutathione S-transferase (GST) and 17β-hydroxysteroid (17β-HSD) dehydrogenase activities were reduced in both tissues tested. Catalase activity and protein carbonyl levels were changed only in testes, after CP administration. Green tea pre-treatment reduced significantly lipid peroxidation, protein carbonylation, DNA damage and restored GPx and GST activity in testes. In epididymis, therapy significantly increased sperm concentration and restored GPx and 17β-HSD activity. Green tea improves CP-induced damage on reproductive system, probably due to their high catechins content.
1. Introduction
Cyclophosphamide (CP) is an antineoplastic agent and immunosuppressive. It is a drug used in the treatment of various types of tumors, organ transplant rejection, as well as in treatment of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis [1]. CP is a pro drug which undergoes a metabolic activation by hepatic microsomal cytochrome P450, primarily being oxidized to 4-hydroxycyclophosphamide. This metabolite enters cells and spontaneously decomposes to phosphoramide-mustard and acrolein [2], [3]. The therapeutic activity of CP involves alkylation of tumor cell and its cytotoxic effect is due to phosphoramide-mustard metabolite. On the other hand, acrolein is reported to cause toxic effects on normal cells, it activates reactive oxygen species (ROS) and nitric oxide production, leading to peroxynitrite formation which ultimately damages the lipids, proteins and DNA inside the cell [4].
CP therapy leads to gonadal toxicity as a side effect of the drug and the possible resultant infertility can have great physical and emotional impact on both men and women. Patients treated with CP exhibited an increased incidence of oligospermia and azoospermia [5]. In other study, CP treatment at its therapeutic dose in rat resulted in inhibition of gonadal steroidogenesis [6].
Recently, it was shown that a single dose of CP (100 mg/kg, i.p.) induced oxidative stress in liver of Swiss mice, evidenced by increased levels of malondialdehyde (MDA) and reduced antioxidant defenses [1]. In order to overcome the toxic side effect of anti-cancer drugs, some antioxidant agents are considered useful to alleviate oxidative stress. Several studies show the beneficial effects of antioxidants on cyclophosphamide induced damage [1], [7], [8]. Green tea is obtained from the Camellia sinensis plant, and has antioxidant activity and free radical-scavenging ability mainly related to polyphenol components [9]. The high concentration of polyphenols in green tea is responsible for the cancer preventive effects. Green tea consumption is inversely associated with the cancer risk including stomach cancer, ovarian cancer and breast cancer [10], [11], [12].
Studies have suggested that green tea polyphenols can act as direct antioxidants by scavenging ROS, or indirectly, by up-regulating phase II antioxidant enzymes [13]. Regarding its therapeutic safety, it was showed that green tea extract does not cause toxicity in mice up to a dose of 2500 mg/kg body weight/day administered by gavage for 28 days [14]. Recently, it was shown that green tea infusion at a dose of 250 mg/kg restores the δ-aminolevulinate dehydratase (δ-ALA-D) enzyme activity inhibited by cadmium exposure in mice ovary [15]. This enzyme participates in heme biosynthesis pathway, and more recently is considered a marker of oxidative stress.
In this study, we investigated if green tea infusion could be effective in protecting male reproductive system from CP-induced toxicity.
2. Materials and methods
2.1. Chemicals
Cyclophosphamide, glutathione reductase, b-nicotinamide adenine dinucleotide phosphate reduced tetrasodium salt (NADPH), 5,50-dithio-bis (2-nitrobenzoic acid) (DTNB), reduced glutathione (GSH) and glutathione disulfide (GSSG) were purchased from Sigma–Aldrich (St. Louis, MO, USA). 1-Chloro-2,4-dinitrobenzene (CDNB) was purchased from Aldrich Chemical Co. (USA).
2.2. Infusion preparation
Green tea (Madrugada Alimentos Ltda, Venâncio Aires, RS, Brazil) was purchased from a local supermarket. The infusion was prepared immediately before each administration using double distilled-deionized water (95–100 °C). After 10 min the infusion was filtered through filter paper and administered in mice (250 mg/kg per day).
2.3. HPLC analysis of catechins
The chromatographic assay was conducted using a reversed phase technique. The analyses of tea infusions and standards were performed in a gradient elution mode with a 1.0 mL/min flow, using a mobile phase of either 5% (v/v) acetonitrile (solvent A) or 50% (v/v) acetonitrile (solvent B) containing 0.05% (v/v) phosphoric acid (85%) [16]. A calibration curve, with concentrations ranging from 25 to 250 μg/mL, was built from a standard solution containing a mixture of three catechins: (−)-epicatechin (EC), (−)-epicatechin gallate (ECG) and (−)-epigallocatechin gallate (EGCG). Tea infusion was properly diluted to fit the calibration curve. The presence of these compounds in tea solutions were identified by comparison to those authentic standards, evaluating the chromatographic profile and UV absorption. All measurements and analysis were carried out in triplicate.
2.4. Animals and treatments
Male adult Swiss albino mice (25–30 g) were used for this experiment. The animals were kept in appropriate animal cabinet with forced air ventilation, in a 12 h light/dark cycle, at a controlled room temperature of 22 °C, with food (Puro Trato, RS, Brazil) and water ad libitum. The animals were used according to the guidelines of the Committee on Care and Use of Experimental Animal Resources (Federal University of Santa Maria, Santa Maria, Brazil) and all efforts were made to reduce the number of animals used and their suffering. This study was approved by the Ethics Committee on the Use of Animals of Federal University of Pampa (Protocol no 016/2013).
Animals were separated in three groups: control, CP, CP + green tea. Infusion of green tea was given daily, for fourteen days, at the dose of 250 mg/kg b.w. via oral. Green tea dose was chosen based in a previous study demonstrating that aqueous extract of green tea did not demonstrate adverse effect at up to 2500 mg/kg/day for 28 days [14]. Additionally, we recently reported that green tea infusion (250 mg/kg) was effective to protect ovarian tissue against cadmium toxicity [15]. Saline or CP (100 mg/kg b.w.) were administrated intraperitoneally only once, 1 h after the last administration of green tea (14th day). The dose of CP was selected based on earlier reports [1], [8], [17]. Animals were euthanized with pentobarbital (100 mg/kg b.w.) 24 h after CP administration and testes and epididymis were removed and homogenized in 50 mM Tris–HCl, pH 7.4 (1/10, w/v). A fraction of homogenized was used for comet assay and the remainder was centrifuged at 2400 × g for 15 min. The supernatant (S1) obtained was used for TBARS, carbonyl protein, antioxidant enzymes activity and 17-βHSD activity analysis.
2.5. Non-enzymatic assay
2.5.1. Lipid peroxidation (TBARS)
Lipid peroxidation was determined by formation of the thiobarbituric acid reactive species (TBARS) as described by Ohkawa et al. [18], in which the malondialdehyde (MDA), one of the end products of fatty acids peroxidation, reacts with thiobarbituric acid (TBA) to form a colored complex. MDA values are determined with the absorbance coefficient of MDA–TBA complex at 532 nm = 1.56 × 105 cm/mmol [18].
2.5.2. Carbonyl groups determination
The formation of carbonyl groups, a parameter of oxidative damage to proteins, was measured based on the reaction of these groups with dinitrophenylhidrazine (DNPH), as previously described by Levine et al. [19]. Samples were incubated at laboratory temperature in the dark for 30 min, stirring at 15-min intervals. After centrifugation, the samples were washed three times with 1 mL of ethanol–ethyl acetate (1:1; v/v) to remove the residual DNPH reagent. The final precipitates were dissolved in buffer SDS 2% and placed in a water bath at 37 °C for 10 min. The reaction product absorbation was measured in a spectrophotometer at 370 nm. Results were expressed as nmol carbonyl/mg protein.
2.5.3. Single cell gel electrophoresis (comet assay)
The alkaline comet assay was performed as described by Singh et al. [20] in accordance with general guidelines for use of the comet assay [21], [22], [23]. Homogenized testes and epididymis were suspended in agarose and spread into a glass microscope slide pre-coated with agarose. Agarose was allowed to set at 4 °C for 5 min. Slides were incubated in ice-cold lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, pH 10.0, and 1% triton X-100 with 10% DMSO) to remove cell proteins, leaving DNA as ‘nucleoids’. After the lysis procedure, slides were placed on a horizontal electrophoresis unit, covered with a fresh solution (300 mM NaOH and 1 mM EDTA, pH > 13) for 20 min at 4 °C to allow DNA unwinding and the expression of alkali-labilesites. Electrophoresis was performed for 20 min (25 V; 300 mA; 0.9 V/cm). Slides were then neutralized, washed in bidistilled water and stained using a silver staining protocol [24], [25]. After drying at room temperature overnight, gels were analyzed using an optical microscope. One hundred cells (50 cells from each of the two replicate slides) were selected and analyzed. Cells were visually scored according to tail length and receive scores from 0 (no migration) to 4 (maximal migration) according to tail intensity. Therefore, the damage index (DI) for cells ranged from 0 (all cells with no migration) to 400 (all cells with maximal migration). The slides were analyzed under blind conditions at least by two different individual. Median values of the scores were presented.
2.5.4. Epididymal sperm characteristics
The criteria used in the evaluation of sperm viability were motility, vigor, concentration and membrane integrity. An estimation of the percentage of progressively motile cells was performed at 100× magnification. These evaluations were performed by the same observer. For measuring their concentration, the semen samples were diluted (1:10) in 90 μL of formaldehyde solution. The counting was performed in a hematimetric Neubauer chamber under 400× magnification. Spermatozoa in 10 of the 25 squares were counted, with each square measuring 0.04 mm2 (volume = 0.004 mm3, with a height of 0.1 mm). The results were converted to a concentration in sperm per mL by multiplying the number of sperm counted in five squares by 0.5 × 106.
The functional integrity of the sperm membrane was determined using the hypoosmotic-swelling test (HOS) as described by Lomeo and Giamberso [26]. The assay was performed by mixing 10 μL of semen with 50 μL of hypoosmotic solution (100 mosm) and incubating this mixture at 37 °C for 45 min. A total of 100 cells were evaluated in at least five different fields under 400× magnification. Spermatozoa with changes were denoted as swelled or HOS positive (HOS+) [26].
2.6. Antioxidant enzymes
2.6.1. Superoxide dismutase (SOD) activity
The activity of SOD was determined as described by Misra and Fridovich [27]. This method is based on the ability of SOD to inhibit the auto-oxidation of adrenaline to adrenochrome. The color reaction is measured at 480 nm. One unit of enzyme (1 IU) is defined as the amount of enzyme required to inhibit the rate of auto-oxidation of adrenaline to 50% at 26 °C [27].
2.6.2. Catalase (CAT) activity
The CAT activity was determined spectrophotometrically according to the method of Aebi [28], which involves monitoring the consumption of H2O2 in the presence of the sample (S1) (20 μL) at 240 nm. Enzyme activity is expressed in units (1 U decomposes 1 μmol H2O2/min at pH 7 and 25 °C) [28].
2.6.3. Glutathione peroxidase (GPx) activity
GPx activity was analyzed spectrophotometrically by the method of Paglia and Valentine [29]. GPx analysis was made by adding GSH, GR, NADPH and a peroxide to begin the reaction, monitored at 340 nm as NADPH is converted to NADP+ [29].
2.6.4. Glutathione S-transferase (GST) activity
GST activity was analyzed spectrophotometrically at 340 nm, as described by Habig et al. [30]. The reaction mixture contained an aliquot of the homogenized tissue (S1), buffer sodium phosphate 0.1 M pH 7, GSH (100 mM) and 1-chloro-2,4-dinitrobenzene (CDNB) (100 mM), which was used as a substrate. Enzyme activity is expressed as nmol of CDNB conjugated/min/mg protein [30].
2.7. 17β-Hydroxysteroid dehydrogenase (17β-HSD) activity
17β-HSD activity was assayed according Jarabak et al. [31]. The supernatant fluid (200 μL) was mixed with 950 μL of 440 μM sodium pyrophosphate buffer (pH 8.9), 250 μL of bovine serum albumin (25 mg crystalline BSA) and 20 μL of 0.3 mM 17β-estradiol. The enzymatic activity was expressed as nmol NADH/min/mg protein [31].
2.8. Protein determination
Protein concentration was measured by the method of Bradford [32], using bovine serum albumin as the standard.
2.9. Statistical analysis
The data were expressed as mean ± SD (n = 6). Statistical analysis was performed using analysis of variance (ANOVA) one-way and differences between the means of experimental and control groups were analyzed statistically by Duncan's test (Statistica Software, 1999). A difference was considered significant at P < 0.05.
3. Results
3.1. HPLC analysis of catechins
Catechins are the major flavonoids present in green tea. Concentration of epigallocatechin gallate (EGCG), epicatechin (EC) and epicatechin gallate (ECG) were identified in green tea infusion. EGCG was present in higher concentration in our infusion (1340.2 μg/mL), followed by EC (500.95 μg/mL) and ECG (302.84 μg/mL).
3.2. Non-enzymatic assay
3.2.1. Lipid peroxidation (TBARS)
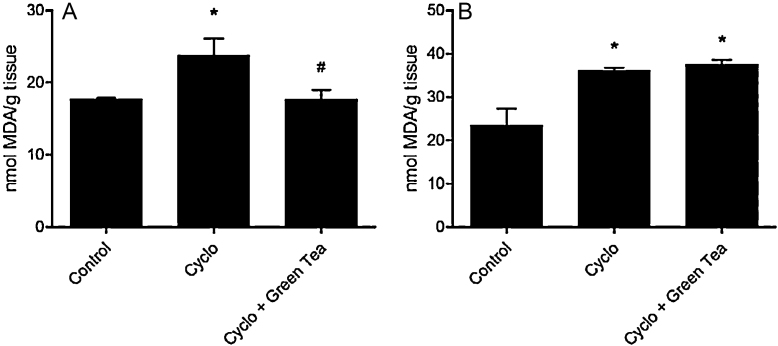
CP significantly increased MDA levels in testes (34.5% or 1.3 folds) and epididymis (53.3% or 1.5 folds) when compared to the control group. Green tea treatment was effective to prevent this damage in testes, but not in epididymis (Fig. 1).
Fig. 1.
Effect of cyclophosphamide (100 mg/kg) and green tea pre-treatment (250 mg/kg) on MDA level in testes (A) and epididymis (B). All the values are expressed as mean ± SD (n = 6) *P < 0.01, compared with control group. #P < 0.001, compared with cyclo group.
3.2.2. Carbonyl groups determination
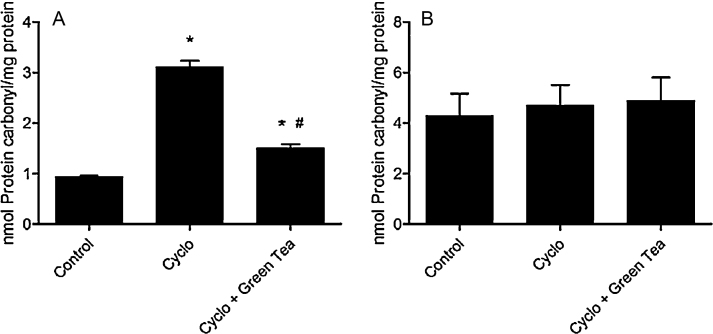
The protein carbonyl levels in testes were increased in the CP group (234.4% or 3.3 folds) when compared to the control group. Green tea was effective in reducing protein carbonyl levels enhanced by CP exposure, but not at the control level. No alteration was observed in protein carbonyl levels in epididymis (Fig. 2).
Fig. 2.
Effect of cyclophosphamide (100 mg/kg) and green tea pre-treatment (250 mg/kg) on protein carbonyl level in testes (A) and epididymis (B). All the values are expressed as mean ± SD (n = 6). *P < 0.001, compared with control group. #P < 0.001, compared with cyclo group.
3.2.3. Single cell gel electrophoresis (comet assay)
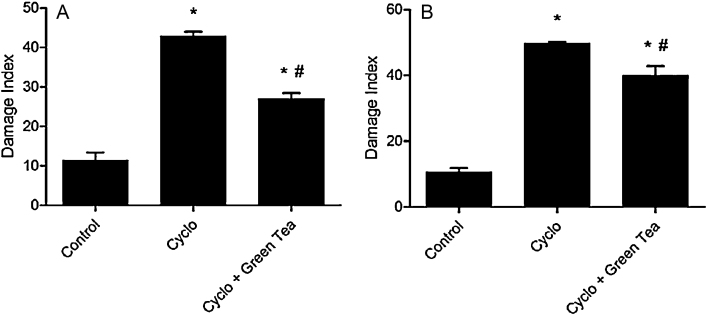
Comet assay demonstrates the damage caused on DNA strand. Higher DNA strand migration through the electrophoresis gel represents a pronounced DNA fragmentation. The control group presented an average damage index (DI) of 11.33 ± 2.08 for testes and 10.66 ± 1.15 for epididymis. The CP group presented an average damage index (DI) of 43.0 ± 1.0 for testes and 49.66 ± 0.58 for epididymis, showing an increase of 279.5% or 3.8 folds and 365.8% or 4.6 folds, respectively. Pre-treatment with green tea significantly reduced damage index in relation to CP group in testes and epididymis (Fig. 3).
Fig. 3.
Effect of cyclophosphamide (100 mg/kg) and green tea pre-treatment (250 mg/kg) on damage index DNA in testes (A) and epididymis (B). All the values are expressed as mean ± SD (n = 6). *P < 0.01, compared with control group. #P < 0.01, compared with cyclo group.
3.2.4. Sperm viability
The sperm motility, vigor and integrity of the CP group were reduced compared with the control group, but was not significantly different. The sperm concentration was decreased in CP group to 50% and pre-treatment with green tea improved this parameter (Table 1).
Table 1.
Effect of cyclophosphamide and green tea infusion on epididymal sperm characteristics.
| Control | Cyclo | Cyclo + green tea | |
|---|---|---|---|
| Motility (%) | 42.5 ± 9.57 | 31.66 ± 2.88 | 35.71 ± 9.16 |
| Vigor | 2.6 ± 0.81 | 2.3 ± 1.3 | 2.12 ± 0.25 |
| Concentration | 12.71 ± 2.67 | 6.35 ± 2.61* | 8.88 ± 3.34# |
| Integrity | 46.66 ± 5.50 | 39.33 ± 2.51 | 43.0 ± 9.84 |
All the values are expressed as mean ± SD (n = 6).
P < 0.01, compared with control group.
P < 0.05, compared with cyclo group.
3.3. Antioxidant enzymes
3.3.1. Superoxide dismutase (SOD) activity
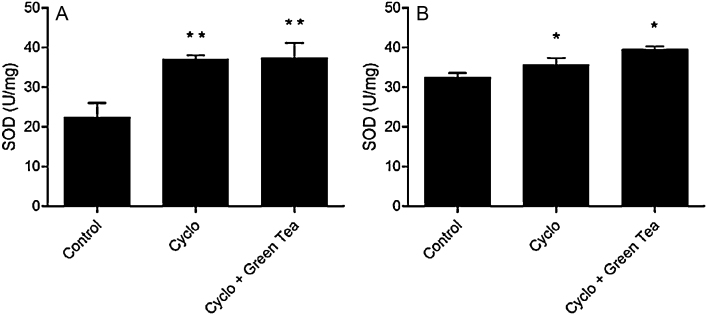
Cyclophosphamide treatment increased significantly the SOD activity in testes and epididymis (65.6% or 1.6 folds and 10% or 1.0 folds, respectively), and green tea infusion was not effective to improve this parameter (Fig. 4).
Fig. 4.
Effect of cyclophosphamide (100 mg/kg) and green tea pre-treatment (250 mg/kg) on SOD activity in testes (A) and epididymis (B). All the values are expressed as mean ± SD (n = 6). *P < 0.05, compared with control group. **P < 0.01, compared with control group.
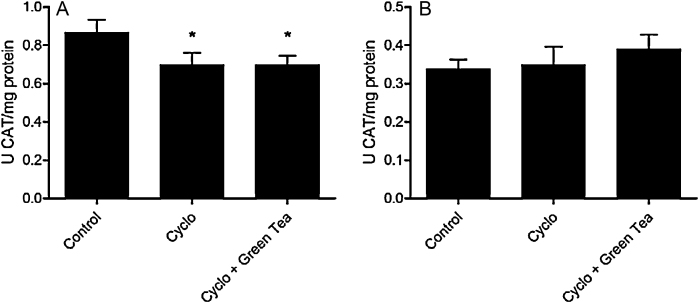
3.3.2. Catalase (CAT) activity
Treatment with CP reduced CAT activity in testes (19.5%) and green tea therapy was not effective in restoring this parameter. No alteration was observed on CAT activity in epididymis (Fig. 5).
Fig. 5.
Effect of cyclophosphamide (100 mg/kg) and green tea pre-treatment (250 mg/kg) on CAT activity in testes (A) and epididymis (B). All the values are expressed as mean ± SD (n = 6). *P < 0.05, compared with control group.
3.3.3. Glutathione peroxidase (GPx) activity
Treatment with CP reduced GPx activity in testes and epididymis (45.6% and 36.2%, respectively), and treatment with green tea was effective to restore GPx activity in both tissues (Fig. 6).
Fig. 6.
Effect of cyclophosphamide (100 mg/kg) and green tea pre-treatment (250 mg/kg) on GPx activity in testes (A) and epididymis (B). All the values are expressed as mean ± SD (n = 6). *P < 0.01, compared with control group. #P < 0.01, compared with cyclo group.
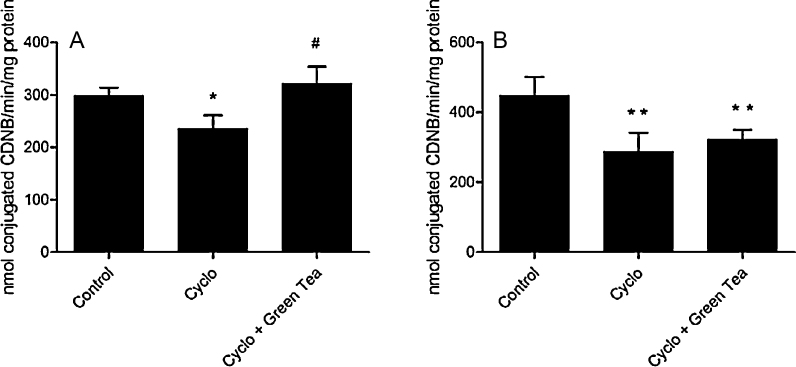
3.3.4. Glutathione S-transferase (GST) activity
GST activity was decreased in testes and epididymis of mice after CP administration (21.2% and 13.8%, respectively). Green tea therapy was able to protect the enzyme activity in testes, but not in epididymis (Fig. 7).
Fig. 7.
Effect of cyclophosphamide (100 mg/kg) and green tea pre-treatment (250 mg/kg) on GST activity in testes (A) and epididymis (B). All the values are expressed as mean ± SD (n = 6). *P < 0.05, compared with control group. **P < 0.01, compared with control group. #P < 0.01, compared with cyclo group.
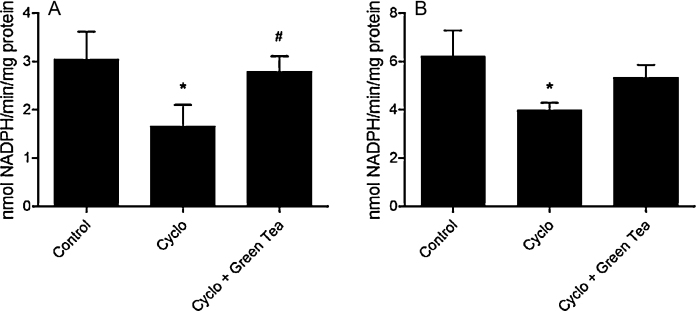
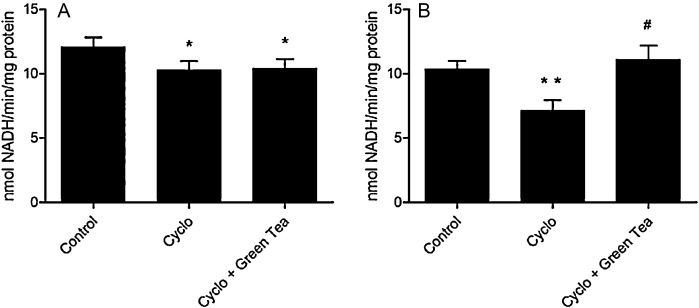
3.4. 17β-Hydroxysteroid dehydrogenase (17β-HSD)
CP reduced the 17β-HSD activity in testes (15%), and its effect was even more pronounced on the epididymis (31.2%). Green tea therapy was effective in restoring this parameter only in epididymis (Fig. 8).
Fig. 8.
Effect of cyclophosphamide (100 mg/kg) and green tea pre-treatment (250 mg/kg) on 17β-HSD activity in testes (A) and epididymis (B). All the values are expressed as mean ± SD (n = 6). *P < 0.05, compared with control group. **P < 0.01, compared with control group.
4. Discussion
The use of cyclophosphamide as chemotherapy drug and immunosuppressive therapy is limited, because of its toxicity to normal tissue, in which infertility is one of the major concerns in the younger patients. CP is an alkylating agent and its cytotoxic effect occurs by inhibiting cell division through DNA damage [1]. CP metabolization generates active alkylating compounds such as 4-hydroxycyclophosphamide, aldophosphamide mustard and acrolein. Acrolein, which is highly toxic in nature, generates oxidative stress by increasing reactive species production and by inducing both normal cells apoptosis and necrotic cell death [33]. This metabolite can interact with the cellular macromolecules such as proteins, membrane lipids and DNA.
Based on our results we can infer that: (a) the acute CP-exposure cause toxic effects on male reproductive system, which are related to oxidative stress. In fact, CP-exposure enhances lipid peroxidation, protein carbonylation and DNA damage. Epididymis may be less sensitive to CP effects in relation to carbonyl protein when compared with testes. Green tea therapy may have a better effect in preventing these parameters on testicular tissue, since it was not able to prevent lipid peroxidation on epididymis; (b) It is probable that the oxidative effects produced by CP-exposure are related with an over production and/or an accumulation of hydrogen peroxide (H2O2). It is fomented by enhanced SOD activity as well as a reduction on GPx and CAT activities. SOD is responsible to anion superoxide (O2−) detoxification, producing H2O2. Thus, an increase on SOD activity could be producing a higher H2O2 concentration. CAT and GPx are involved on H2O2 detoxification. Considering that these enzymes presented reduced activities, it could be corroborating to H2O2 accumulation; (c) reduced semen concentration could be related to ROS over production which causes an increase on lipid peroxidation and DNA damage. On the other hand, it could be related to hormonal disruption which alter the semen production; 17β-HSD is a enzyme that plays a key role in sex steroid biology and a reduction in its activity implies in decreased production of hormones such as testosterone; (d) finally, the beneficial effect of green tea infusion could be attributed to antioxidant properties of their constituents, specially to high catechins content, evidenced in preparation.
Corroborating to our study, other studies showed that oxidative stress is involved in CP-induced testicular damage. Selvakumar et al. [34] found that CP increased MDA and hydrogen peroxide levels as well as changed SOD, GPx and GR activities in the mitochondrial fraction of testes. Recently, Maremanda et al. [35] demonstrated that CP administration significantly increased the oxidative stress, sperm DNA damage and reduced sperm count and motility.
17β-HSD is an enzyme that catalyzes the interconversion of androstenedione to testosterone and a reduction of its activity can decrease spermatid count per testis, sperm count per epididymis, daily sperm production/gram testis, sperm motility, and significantly increased abnormal sperm rates [36]. Proteins can also be modified by the free radicals action, including amino acids modification, denaturation and formation of carbonyl compounds [37], [38]. CP binds covalently to DNA and induces DNA damage in the form of strand breaks, DNA–DNA cross-links and DNA–protein cross-links [39]. Considering that this damage may lead to the secondary tumors in humans, compounds that have the ability to decrease DNA damage, such organo-selenium compound and Rubus imperialis extract have been studied [40], [41].
Natural antioxidants have been studied to decrease the damage caused by anticancer drugs. Kenji Sato et al. [42] suggest that green tea extracts exert protective effects against doxorubicin-induced spermatogenic disorders in mice. In another study, quercetin and methanolic extract of Viscum album attenuates cardiotoxicity, urotoxicity and genotoxicity induced by CP [43]. Moreover, vitamin C, vitamin E and glutathione have been demonstrated to help treat male infertility [44], [45].
Tea is among the most highly consumed beverages worldwide, especially green tea, which contains phenolic compounds including the catechins: epigallocatechin gallate (EGCG), epicatechin (EC) and epicatechin gallate (ECG). The antioxidant activity of green tea polyphenols and, more recently, the pro-oxidant effects of these compounds, resulting in indirect antioxidant effects, have also been suggested as potential mechanisms for cancer prevention [46], [47]. In the present study, we demonstrated that the reproductive system toxicity in male mice was attenuated by green tea infusion. This is important, since the green tea infusion used seems tea consumption in humans. It is daring to attribute the benefic effect observed with green tea infusion to a specific constituent as catechins.
Taking into account that decreased fertility rate remains one of the challenging tasks to be addressed in younger patients treated with CP, new therapeutic approach to manage its toxicity is necessary. Oxidative stress appears to be directly related to testicular steroidogenesis and normal function of the male reproductive system. In this way, natural antioxidants have utmost importance in maintaining the integrity of sperm and fertility. This study showed damage caused by CP on male reproductive system, even after a single administration, involving oxidative stress, which could impair the fertility of these animals. The pre-treatment (14 days) with green tea infusion was effective in partially prevent the CP-induced damage, and its effect is probably due to high concentrations of catechins and antioxidant activity. However, more studies are needed to understand the mechanism of green tea in relation to its beneficial effect and possible interaction with anticancer drugs.
Conflict of interest
The authors declare that they have no conflict of interest.
Transparency document
Acknowledgments
The financial support by CNPq and FAPERGS is gratefully acknowledged. FAPERGS and CAPES are also acknowledged for financial support (M.Sc. Fellowship) to M.M.Z and A.P.I.
References
- 1.Tripathi D.N., Jena G.B. Intervention of astaxanthin against cyclophosphamide-induced oxidative stress and DNA damage: a study in mice. Chem. Biol. Interact. 2009;180(3):398–406. doi: 10.1016/j.cbi.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Ren S., Yang J.S., Kalhorn T.F., Slattery J.T. Oxidation of cyclophosphamide to 4-hydroxycyclophosphamide and deschloroethylcyclophosphamide in human liver microsomes. Cancer Res. 1997;57(19):4229–4235. [PubMed] [Google Scholar]
- 3.Kehrer J.P., Biswal S.S. The molecular effects of acrolein. Toxicol. Sci. 2000;57(1):6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 4.Korkmaz A., Topal T., Oter S. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biol. Toxicol. 2007;23(5):303–312. doi: 10.1007/s10565-006-0078-0. [DOI] [PubMed] [Google Scholar]
- 5.Kenney L.B., Laufer M.R., Grant F.D., Grier H., Diller L. High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer. 2001;91(3):613–621. doi: 10.1002/1097-0142(20010201)91:3<613::aid-cncr1042>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh D., Das U.B., Ghosh S., Mallick M., Debnath J. Testicular gametogenic and steroidogenic activities in cyclophosphamide treated rat: a correlative study with testicular oxidative stress. Drug Chem. Toxicol. 2002;25(3):281–292. doi: 10.1081/dct-120005891. [DOI] [PubMed] [Google Scholar]
- 7.Patra K., Bose S., Sarkar S., Rakshit J., Jana S., Mukherjee A., Roy A., Mandal D.P., Bhattacharjee S. Amelioration of cyclophosphamide induced myelosuppression and oxidative stress by cinnamic acid. Chem. Biol. Interact. 2012;195(3):231–239. doi: 10.1016/j.cbi.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Wei X., Su F., Su X., Hu T., Hu S. Stereospecific antioxidant effects of ginsenoside Rg3 on oxidative stress induced by cyclophosphamide in mice. Fitoterapia. 2012;83(4):636–642. doi: 10.1016/j.fitote.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Frei B., Higdon J.V. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J. Nutr. 2003;133(10) doi: 10.1093/jn/133.10.3275S. 3275S-384S. [DOI] [PubMed] [Google Scholar]
- 10.Yuan J.M., Koh W.P., Sun C.L., Lee H.P., Yu M.C. Green tea intake, ACE gene polymorphism and breast cancer risk among Chinese women in Singapore. Carcinogenesis. 2005;26:1389–1394. doi: 10.1093/carcin/bgi080. [DOI] [PubMed] [Google Scholar]
- 11.Yu G.P., Hsieh C.C., Wang L.Y., Yu S.Z., Li X.L., Jin T.H. Green-tea consumption and risk of stomach-cancer – a population-based case-control study in Shanghai, China. Cancer Causes Control. 1995;6(6):532–538. doi: 10.1007/BF00054162. [DOI] [PubMed] [Google Scholar]
- 12.Nagle C.M., Olsen C.M., Bain C.J., Whiteman D.C., Green A.C., Webb P.M. Tea consumption and risk of ovarian cancer. Cancer Causes Control. 2010;21(9):1485–1491. doi: 10.1007/s10552-010-9577-7. [DOI] [PubMed] [Google Scholar]
- 13.Forester S.C., Lambert J.D. Antioxidant effects of green tea. Mol. Nutr. Food Res. 2011;55(6):844–854. doi: 10.1002/mnfr.201000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu Y.W., Tsai C.F., Chen W.K., Huang C.F., Yen C.C. A subacute toxicity evaluation of green tea (Camellia sinensis) extract in mice. Food Chem. Toxicol. 2011;49(10):2624–2630. doi: 10.1016/j.fct.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Soares M.B., Izaguirry A.P., Vargas L.M., Mendez A.S., Spiazzi C.C., Santos F.W. Catechins are not major components responsible for the beneficial effect of Camellia sinensis on the ovarian d-ALA-D activity inhibited by cadmium. Food Chem. Toxicol. 2013;55:463–469. doi: 10.1016/j.fct.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 16.Goto T., Yoshida Y., Kiso M., Nagashima H., Mejia E.G. Simultaneous analysis of individual catechins and caffeine in green tea. J. Chromatogr. Anal. 1996;749(1–2):295–299. [Google Scholar]
- 17.Elangovan N., Chiou T.J., Tzeng W.F., Chu S.T. Cyclophosphamide treatment causes impairment of sperm and its fertilizing ability in mice. Toxicology. 2006;222(1–2):60–70. doi: 10.1016/j.tox.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawa H., Ohishi N., Yagi K. Assay for lipide peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Levine R.L., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.G., Ahn B.W., Shaltiel S., Stadtman E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 20.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell. Res. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 21.Tice R.R., Agurell E., Anerson D., Burlinson B., Hartmann A., Kobayashi H., Miyamae Y., Rojas E., Ryu J.C., Sasaki Y.F. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000;35(3):206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann A., Agurell E., Beevers C., Brendler-Schwaab S., Burlinson B., Clay P., Collins A., Smith A., Speit G., Thybaud V., Tice R.R. Recommendations for conducting the in vivo alkaline Comet assay. Mutagenesis. 2003;18:45–51. doi: 10.1093/mutage/18.1.45. [DOI] [PubMed] [Google Scholar]
- 23.Bajpayee M., Pandey A., Parmar D., Dhawan A. Current status of short-term tests for evaluation of genotoxicity, mutagenicity, and carcinogenicity of environmental chemicals and NCEs. Toxicol. Mech. Methods. 2005;15(3):155–180. doi: 10.1080/15376520590945667. [DOI] [PubMed] [Google Scholar]
- 24.Maluf S., Erdtmann B. Follow-up study of the genetic damage in lymphocytes of pharmacists and nurses handling antineoplastic drugs evaluated by cytokinesis-block micronuclei analysis and single cell gel electrophoresis assay. Mutat. Res. 2000;471(1–2):21–27. doi: 10.1016/s1383-5718(00)00107-8. [DOI] [PubMed] [Google Scholar]
- 25.Nadin S., Vargas-Roig L., Ciocca D. A silver staining method for single cell gel assay. J. Histochem. Cytochem. 2001;49(9):1183–1186. doi: 10.1177/002215540104900912. [DOI] [PubMed] [Google Scholar]
- 26.Lomeo A.M., Giamberso A.M. Water test: a simple method to asses sperm-membrane integrity. Int. J. Androl. 1991;14(4):278–282. doi: 10.1111/j.1365-2605.1991.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 27.Misra H.P., Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide-dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 28.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 29.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70(1):158–169. [PubMed] [Google Scholar]
- 30.Habig W.H., Pabst M.J., Jokoby W.B. Glutathione S-transferases, the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 31.Jarabak J., Adams J.A., Williams-Ashman H.G., Talalay P. Purification of 1 17-hydroxysteroid dehydrogenase of human placenta and studies on its transhydrogenase function. J. Biol. Chem. 1962;237:345–357. [PubMed] [Google Scholar]
- 32.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Kern J.C., Kehrer J.P. Acrolein-induced cell death: a caspase-influenced decision between apoptosis and oncosis/necrosis. Chem. Biol. Interact. 2002;139(1):79–95. doi: 10.1016/s0009-2797(01)00295-2. [DOI] [PubMed] [Google Scholar]
- 34.Selvakumar E., Prahalathan C., Mythili Y., Varalakshmi P. Beneficial effects of dl-alpha-lipoic acid on cyclophosphamide-induced oxidative stress in mitochondrial fractions of rat testis. Chem. Biol. Interact. 2005;152(1):59–66. doi: 10.1016/j.cbi.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Maremanda K.P., Khan S., Jena G. Zinc protects cyclophosphamide-induced testicular damage in rat: involvement of metallothionein, tesmin and Nrf2. Biochem. Biophys. Res. Commun. 2014;445(3):591–596. doi: 10.1016/j.bbrc.2014.02.055. [DOI] [PubMed] [Google Scholar]
- 36.Abarikwu S.O., Otuechere C.A., Ekor M., Monwuba K., Osobu D. Rutin ameliorates cyclophosphamide-induced reproductive toxicity in male rats. Toxicol. Int. 2012;19(2):207–214. doi: 10.4103/0971-6580.97224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J., Xu L., Eu J.P., Stamler J.S., Meissner G. Classes of thiols that influence the activity of skeletal muscle calcium release channel. J. Biol. Chem. 2001;276:15625–15630. doi: 10.1074/jbc.M100083200. [DOI] [PubMed] [Google Scholar]
- 38.Song D.U., Jung Y.D., Chay K.O., Chung M.A., Lee K.H., Yang S.Y., Shin B.A., Ahn B.W. Effect of drinking green tea on age-associated accumulation Maillard-type fluorescence and carbonyl groups in aortic and skin collagen. Arch. Biochem. Biophys. 2002;397(2):424–429. doi: 10.1006/abbi.2001.2695. [DOI] [PubMed] [Google Scholar]
- 39.Colvin O.M. An overview of cyclophosphamide development and clinical applications. Curr. Pharm. Des. 1999;5(8):555–560. [PubMed] [Google Scholar]
- 40.Roy S.S., Chakraborty P., Bhattacharva S. Intervention in cyclophosphamide induced oxidative stress and DNA damage by a flavonyl-thiazolidinedione based organoselenocyanate and evaluation of its efficacy during adjuvant therapy in tumor bearing mice. Eur. J. Med. Chem. 2014;73:195–209. doi: 10.1016/j.ejmech.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Alves A.B., Santos R.S., Calil S.D., Niero R., Lopes J.D., Perazzo F.F., Rosa P.C., Andrade S.F., Cechinel-Filho V., Maistro E.L. Genotoxic assessment of Rubus imperialis (Rosaceae) extract in vivo and its potential chemoprevention against cyclophosphamide-induced DNA damage. J. Ethnopharmacol. 2014;153(3):694–700. doi: 10.1016/j.jep.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 42.Sato K., Sueoka K., Tanigaki R., Tajima H., Nakabayashi A., Yoshimura Y., Hosoi Y. Green tea extracts attenuate doxorubicin-induced spermatogenic disorders in conjunction with higher telomerase activity in mice. J. Assist. Reprod. Genet. 2010;27(8):501–508. doi: 10.1007/s10815-010-9438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Şekeroğlu V., Aydın B., Şekeroğlu Z.A. Viscum album L. extract and quercetin reduce cyclophosphamide-induced cardiotoxicity, urotoxicity and genotoxicity in mice. Asian Pac. J. Cancer Prev. 2011;12(11):2925–2931. [PubMed] [Google Scholar]
- 44.Tavares D.C., Cecchi A.O., Antunes L.M., Takahashi C.S. Protective effects of the amino acid glutamine and of ascorbic acid against chromosomal damage induced by doxorubicin in mammalian cells. Teratog. Carcinog. Mutagen. 1998;18(4):153–161. [PubMed] [Google Scholar]
- 45.Agarwal A., Sharma R.K., Nallella K.P., Thomas A.J., Jr., Alvarez J.G., Sikka S.C. Reactive oxygen species as an independent marker of male factor infertility. Fertil. Steril. 2006;86(4):878–885. doi: 10.1016/j.fertnstert.2006.02.111. [DOI] [PubMed] [Google Scholar]
- 46.Hou Z., Sang S., You H., Lee M.J., Hong J., Chin K.V., Yang C.S. Mechanism of action of (−)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65(17):8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- 47.Butt M.S., Sultan M.T. Green tea: nature's defense against malignancies. Crit. Rev. Food Sci. Nutri. 2009;49(5):463–473. doi: 10.1080/10408390802145310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.