Highlights
-
•
Lower doses of cyanide did not alter the biochemical compositions in rat sperms.
-
•
Higher doses of cyanide had induced significant decrease in biochemical compositions in rat sperms.
-
•
Cyanide induced alterations in secondary structure of proteins present in rat sperms.
-
•
FT-IR spectroscopy is a powerful tool used to investigate oxidative damage in sperms.
Keywords: Cyanide, Rat sperm, Sperm lipids, Sperm proteins, FT-IR
Abstract
In the recent years, great attention had been focused on cyanide toxicity because of its widespread use in industries and considered to be a ubiquitous pollutant in the environment. Therefore, the current study aimed to evaluate the toxic effect of cyanide on rat sperms at molecular level by using FT-IR technique. For this purpose, rats were randomly divided into four groups and treated with 0.0, 0.64, 1.2 and 3.2 mg kg−1 body weight (BW) for the period of 90 days. The group treated with lower dose (0.64 mg kg−1 BW) showed an insignificant change in all the peaks, except the peaks assigned to olefinic C—H, CH2 asymmetric and CH2 symmetric stretching vibration in the lipids. While, the groups treated with higher doses (1.2 and 3.2 mg kg−1 BW) showed the significant decrease in the area under the peaks corresponds to different bio-molecules. In addition, spectral second derivative analysis showed the significant alteration in α-helix, turns, β-sheet, aggregated β-sheet and random coil structures in the proteins. In conclusion, the selected higher dosage of cyanide had caused significant decrease in the biochemical composition of rat sperms along with structural changes in the proteins. The FT-IR technique is an excellent tool used for the analysis of oxidative damage in the sperms.
1. Introduction
During recent years, great attention had been focused on cyanide toxicity because of its widespread use in the industries and considered to be a ubiquitous pollutant in the environment. Human exposure to this substance may occur through mining, industrial usage, smoke from fire, propulsion motors and tobacco [1], [2], [3], [4]. Exposure to other chemicals like organonitriles, cyanogens and cyanide containing pesticides would also result in cyanide toxicity [5], [6], [7]. In addition to this, ingestion of cyanogenic glycoside-containing plant products is a major source of cyanide toxicity in human [8], [9]. Furthermore, studies have reported that the occupational cyanide exposure occurs through intake of water, ingestion of cassava-based food, inhalation of cyanide contaminated air and dermal absorption of hydrocyanic acid discharged into the environment during the courses of industrial process [10].
Cyanide could be immediately absorbed into the blood and forms reversible complex with methemoglobin and haemoglobin; and distributed throughout the body followed by any route of exposure [11], [12]. Since these complexes are reversible, cyanide could be dissociated from them under the organ system and inhibits the several cellular enzymes, including cytochrome c oxidase resulting in cellular hypoxia and cytotoxic anoxia, which is potentially fatal [13]. Studies have reported that, cyanide also inhibit several other metalloenzymes including superoxide dismutase (SOD), xanthine oxidase, catalase (CAT), peroxidases (Px), nitrogenase, nitrate reductase and subsequently induce oxidative stress in functionally different tissues by enhanced generation of reactive oxygen species (ROS) [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]. However, sperms are most sensitive to oxidative stress due to the lack of their own antioxidant mechanism [24]. Shiva et al. [25] demonstrated that, the elevated level of ROS in male reproductive organs will induce oxidative damage in the sperm bio-molecules. Consequently, it will certainly lead to an altered sperm function and cause male infertility.
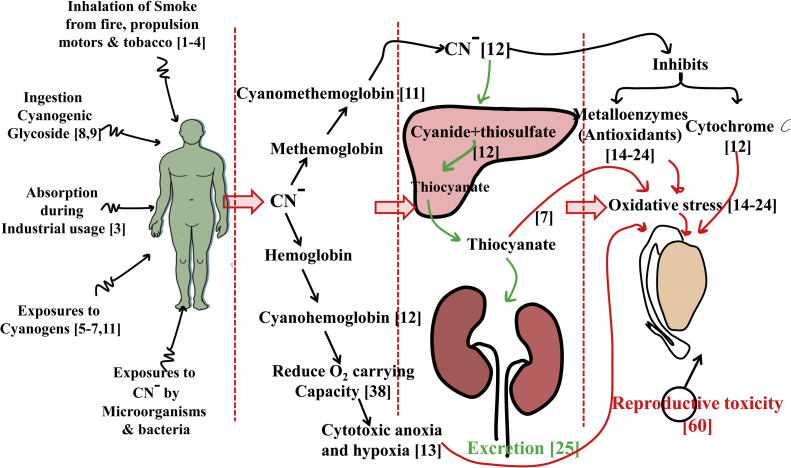
In fact, the body is having a well established cyanide detoxification mechanism, involves the enzyme rhodanese. The rhodanese can bring the catalytic reaction between cyanide and thiosulfate and produce thiocyanate (SCN−) [13]. However, the SCN− is less toxic than the cyanide and it will be excreted within few days [26]. Some studies have stated that, excretion of SCN− is based on the kidney performance and it would get accumulated in the body of the patients suffering from renal disorders and induced oxidative stress in functionally different tissues [25], [27], [28]. In addition to this, Rocha-e-Silva et al. [13] reported that, detoxification of cyanide was nutritionally cost effective and it requires protein based diet. The lower doses of cyanide will get detoxified by providing the diet with sufficient proteins [29]. Consequently, studies have reported that, cyanide toxicity was occurring more in malnourished population, who have been suffering from protein deficiency [11]. In addition to this, male infertility was also occurring more in the same group of population [30]. Fig. 1 represents the different sources of cyanide exposures, its distribution and detoxification in the body and possible routes of male reproductive toxicity.
Fig. 1.
The schematic representation of different sources of cyanide exposures, its distribution and detoxification in the body and possible routes of male reproductive toxicity. The numbers correspond to the references.
On the other perspective, several studies reported that infertility in human has increased massively from last two decades [21], [31], [32]. In particular, 50% of infertility was accounted to males [33], [34]. The main reason for increased male infertility was attributed to increased sperm abnormalities along with declining sperm numbers and motility. Furthermore, studies have reported that, increasing use of chemicals and their pollution, change in lifestyle and diet, and exposure to occupational and environmental factors are known to cause such deformities in the sperms [34], [35], [36], [37]. Moreover, studies pertaining to the molecular investigation of cyanide toxicity on rat sperm are very rare.
Nearly two decades back in 1993, National Toxicology Program (NTP) had evaluated the cyanide toxicity on male reproductive system of F344/N rat strain and found that, the dose equivalent to 4.5 mg kg−1 BW will cause the mild (insignificant) effect on male reproductive system [38]. In contrast, the review of literature revealed that, the dose of 2 mg kg−1 BW will induce oxidative stress in functionally different tissues and further same dose was reported to induce hepatotoxicity, renal toxicity and neurotoxicity in rats, quails and goats [9], [13], [39], [40], [41], [42], [43], [44], [45], [46]. Therefore, in the current study, we made an attempt to evaluate the cyanide toxicity on rat sperm biomolecules with different sublethal doses (0.64, 1.2 and 3.2 mg kg−1 BW), those are lower than the dose reported to induce mild reproductive toxicity in male rats.
To date, different techniques have been employed to diagnose infertility in males, among them the use of vibration spectroscopy is a new technique in the field of reproductive medicine [47]. This technique has been utilized well in biophysical and biochemical research for both quantitative and qualitative analysis of biomolecules such as proteins, lipids, carbohydrates and nucleic acids [48]. In addition to this, FT-IR spectrum provides sensitive ‘fingerprint’ for the oxidative damage to the different biomolecules present in the sperm [47]. However, the spectra can be obtained by molecular vibration of functional groups of different biomolecules; it provides the information about the structure, symmetry and bonding pattern of the molecules. Therefore, in the current study, we made an attempt to use this molecular fingerprinting approach (FT-IR) to investigate the subchronic cyanide induced oxidative damage in the rat sperms, which to the best of our knowledge this technique has not been previously employed for the same.
2. Material and methods
2.1. Chemicals
Sodium cyanide of 95% purity was procured from Loba Chemie Pvt., Ltd., Mumbai, India. All other chemicals used in the current study were of analytical grade and commercially available.
2.2. Animals
Healthy adult (90 days old) Wistar male albino rats weighing about 160–170 gm were utilized for the current study. They were maintained in plastic cages at the animal care facility in the Department of Zoology, Karnatak University, Dharwad, with a room temperature of 23 ± 2 °C, relative humidity of 65 ± 5%, and exposed to a 12 h of light/dark cycle. Before the initiation of the experiment animals were acclimatized for one week and handled in accordance with the CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) guidelines for the care and use of laboratory animals.
2.3. Experimental design
After the period of acclimation, animals were randomly divided into four groups of six animals each and treated as follows.
-
•
Group I : Control animals (received the vehicle only).
-
•
Group II : 0.64 mg kg−1 BW cyanide (1/10th of LD50).
-
•
Group III : 1.2 mg kg−1 BW cyanide (1/5th of LD50).
-
•
Group IV : 3.2 mg kg−1 BW cyanide (1/2th of LD50).
The selected LD50 (6.44 mg kg−1 BW) value of NaCN was based on the available literature [49]. The treatment was given orally with the dose volume of 1 ml 100 gm−1 BW in the morning (between 09:00 and 10:00 am) to non-fasted rats for 90 days. However, the first two selected doses (0.64 and 1.2 mg kg−1 BW) were considered to be environmentally relevant [50], [51]. Furthermore, it has been reported that, the dose of 0.5–3.5 mg kg−1 BW is lethal to human beings [52], [53], [54]. Hence, according to OECD (Organization for Economic Cooperation and Development) guidelines for chemical testing on animals, we have selected one more dose (3.2 mg kg−1 BW) with a higher sublethal concentration to evaluate the toxicity on rat sperms.
2.4. Sperm collection
The epididymis was collected as quickly as possible after the dissection and placed in a clean Petri plate. Then the cauda epididymis was separated from the whole epididymis and cut into several pieces and immersed in 3 ml pre-warmed phosphate buffer saline (PBS) solution and incubated for 10 min at 37 °C to allow sperms to release from the epididymal lumen. The obtained sperm suspension was pipetted several times and used for FT-IR spectral analysis.
2.5. Sample preparation and FT-IR spectral analysis
For FT-IR spectral analysis of rat sperms, the sperm suspension was normalized based on their number by transferring the required volume of sperm suspension to different centrifugation tubes and made final volume of 1 ml with PBS. Then the tubes were subjected to centrifugation at 1000× g for 10 min at 4 °C. The obtained sperm pellets were immersed in liquid nitrogen and then they were dried in a lyophilizer (VIRTIS 6 KBEL 85) for 12 h to remove the water content in the samples. In order to obtain fine sperm powders, completely dried sperm pellets were grounded with IR grad potassium bromide (KBr) in an agate mortar and pestle. Then the powdered sperm samples from control and cyanide treated groups were again dried under a halogen lamp to remove any trace of water before preparing the pellet. Finally, KBr pellets were prepared in triplet by applying the high pressure of 5 tonnes for 5 min. The prepared KBr pellets were with a diameter of 12 mm and thickness of 1 mm. The FT-IR spectra was recorded at room temperature (25 ± 1 °C) in the region ∼4000–400 cm−1 on a Nicolet-6700 FT-IR spectrometer equipped with KBr beam splitter and an air cold DTGS (Dueterated Triglycine Sulfate) detector. Every pellet was scanned under identical condition and obtained the spectra then it was further analyzed using the ORIGIN.8 software.
2.6. Statistical analysis
The data were analyzed using SPSS (Statistical Package for the Social Sciences) 16.0 for Windows and expressed as mean ± SE (standard error). The significance (P < 0.05) between control and cyanide treated groups was determined by using one-way ANOVA (Analysis of Variance) followed by Tukey’s post-doc or student’s t -test.
3. Results
3.1. FT-IR spectral analysis
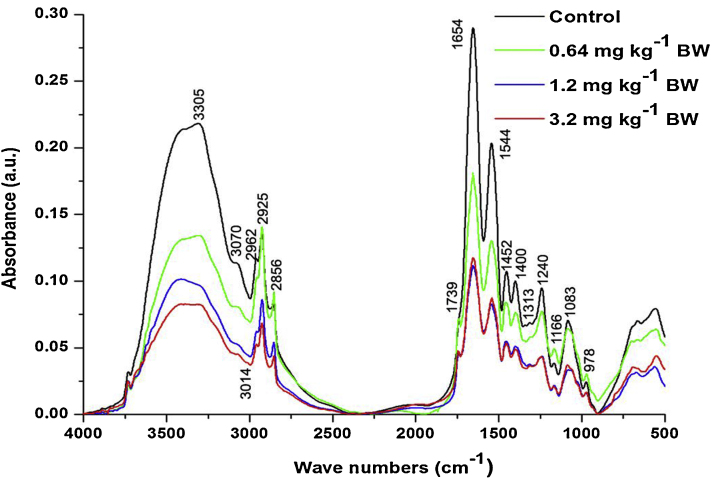
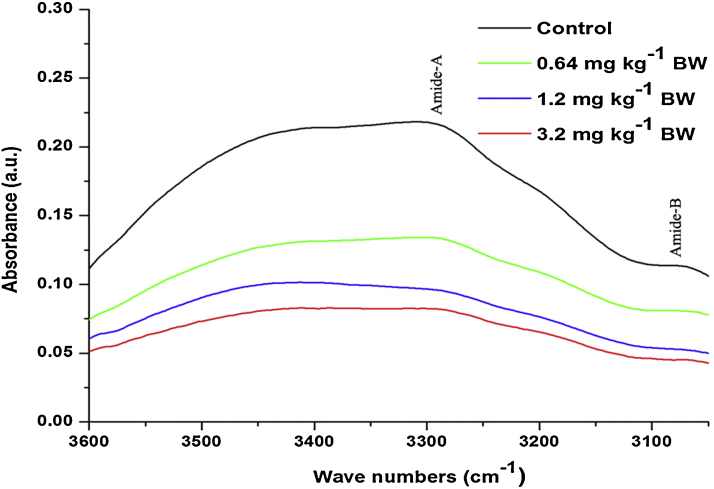
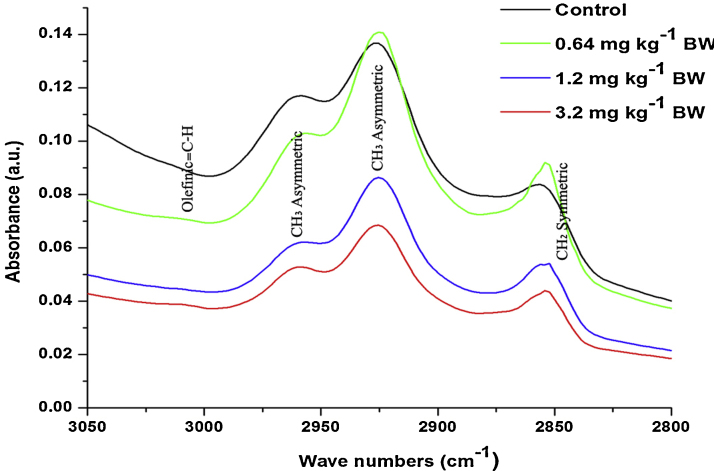
The control and different doses of cyanide treated on rat sperm FT-IR spectra (∼3600–400 cm−1) is shown in Fig. 2. The peak position and detailed band assignments were done according to available literature [55], [56], [57] (Table 1). In order to get detailed spectral analysis the spectra was divided into three distinct frequency ranges, namely ∼3600–3050 cm−1(Fig. 3), ∼3050–2800 cm−1 (Fig. 4) and ∼1800–800 cm−1 (Fig. 5). Furthermore, the protein and lipid secondary derivatives were analysed in ∼1700–1600 cm−1 (Fig. 6) and ∼3050–2800 cm−1 (Fig. 7) respectively.
Fig. 2.
The representative FT-IR spectra of the control and different dose cyanide treated rat sperms in the ∼4000–500 cm−1 range.
Table 1.
Subchronic (90 days) cyanide induced changes peak position with their general band assignment based on the literature [55], [56], [57]].
| Wave number (cm−1) |
Vibrational peak assignments | |||
|---|---|---|---|---|
| Control | 0.64 mg kg−1 BW | 1.2 mg kg−1 BW | 3.2 mg kg−1 BW | |
| 3305.40 | 3305.39 | 3003.46 | 3297.89 | Amide-A: N—H stretching of proteins |
| 3070.11 | 3070.11 | 3070.10 | 3068.19 | Amide-B: N—H stretching of proteins |
| 3014.19 | 3016.12 | 3012.26 | 3008.40 | Olefinic C—H stretching: unsaturated lipids |
| 2962.19 | 2958.26 | 2958.26 | 2958.26 | CH3 asymmetric stretching: mainly lipids |
| 2925.48 | 2925.48 | 2925.48 | 2925.48 | CH2 asymmetric stretching: mainly lipids |
| 2856.05 | 2856.13 | 2852.20 | 2854.13 | CH2 symmetric stretching: lipids |
| 1739.47 | 1741.40 | 1739.47 | 1745.26 | Ester C O stretching: lipids |
| 1654.62 | 1654.62 | 1654.62 | 1652.69 | Amide–I (protein C O stretching) |
| 1544.72 | 1544.70 | 1544.70 | 1540.84 | Amide–II (protein N—H bend, C—N stretch) |
| 1452.13 | 1455.99 | 1454.06 | 1455.99 | CH2 bending; lipids & proteins |
| 1400.06 | 1400.06 | 1398.13 | 1396.21 | COO— symmetric stretching: fatty acids |
| 1313.28 | 1313.28 | 1311.35 | 1309.42 | CH3 CH2 stretching: collagen |
| 1240.00 | 1240.00 | 1238.07 | 1238.07 | PO2– asymmetric stretching: nucleic acids and phospholipids |
| 1166.72 | 1166.72 | 1164.79 | 1168.65 | CO—O—C stretching: glycogen |
| 1083.79 | 1083.79 | 1083.79 | 1079.94 | PO2– asymmetric stretching: nucleic acids |
Fig. 3.
The average FT-IR spectra of the control and different dose cyanide treated albino rat sperms in the ∼3600–3100 cm−1 region.
Fig. 4.
The average FT-IR spectra of the control and different dose cyanide treated albino rat sperms in the ∼3050–2800 cm−1 region.
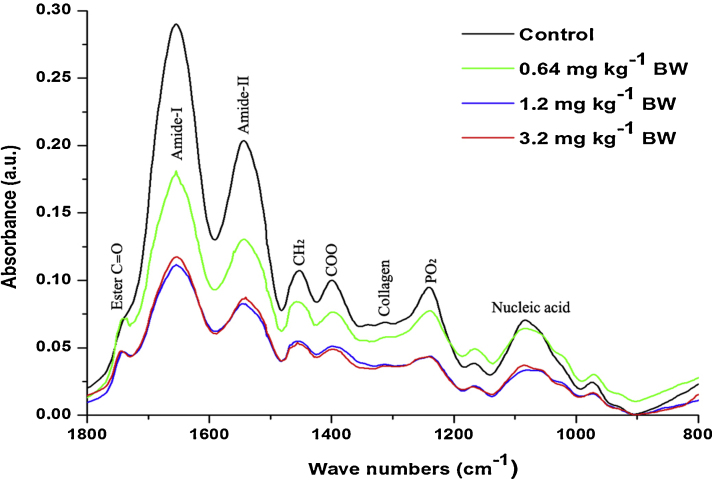
Fig. 5.
The average FT-IR spectra of the control and different dose cyanide treated albino rat sperms in the ∼1800–800 cm−1 region.
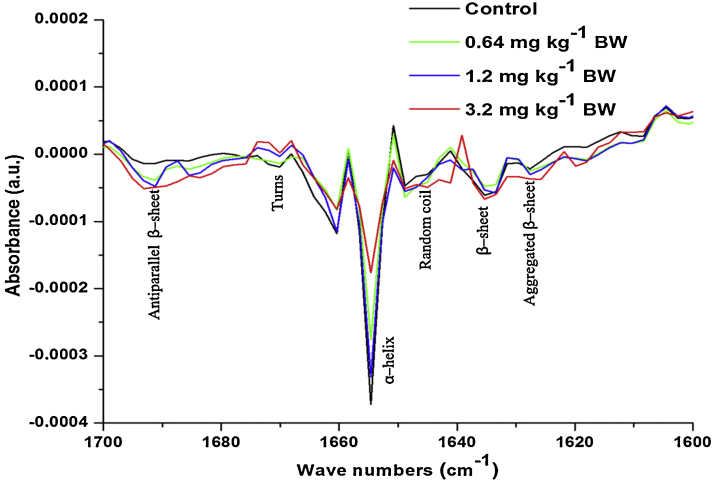
Fig. 6.
Second derivative of FT-IR spectra of control and different dose cyanide treated rat sperms amide-I band (∼1700–1600 cm−1) region.
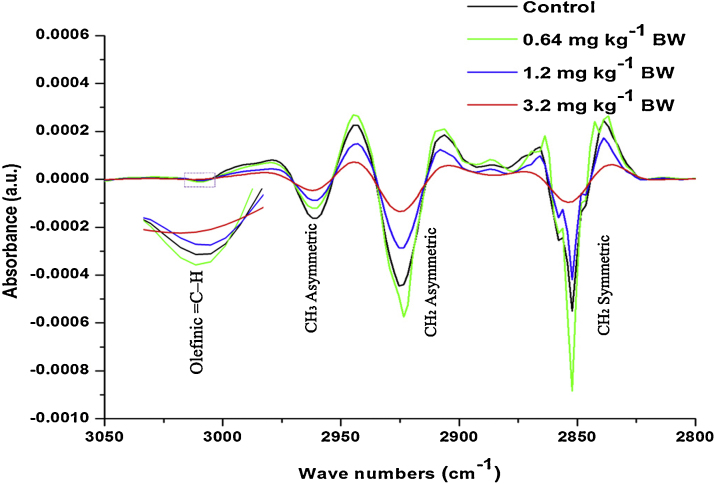
Fig. 7.
Second derivative of FT-IR spectra of the control and different dose cyanide treated rat sperms lipid (∼3050–2800 cm−1) region.
Fig. 3 shows the average FT–IR spectra of rat sperms from control and cyanide treated groups in the range of ∼3600–3050 cm−1. The absorption in this range was dominated by amid-A band at ∼3305 cm−1 of protein arising from N—H and O—H stretching modes of proteins and intermolecular H banding. The weak band observed at ∼3070 cm−1 represents N—H stretching of amide-B. The area under these bands mainly revealed about the protein content in the samples. In the current study, the group treated with 0.64 mg kg−1 BW showed insignificant (P > 0.05) changes in the area under these bands compared to control (Table 2). While, the groups treated with 1.2 and 3.2 mg kg−1 BW showed the significant (P < 0.05) decrease by −12% and −28% in the area value of these bands respectively compared to control. In addition to these bands, one more band at ∼1544 cm−1 in the third spectral range (∼1800–800 cm−1) (Fig. 5) was assigned to the amid-II protein in the sperms (Table 3). Results showed that, the group treated with 1.2 mg kg−1 BW showed significant decrease at P < 0.05 and the group treated with 3.2 mg kg−1 BW showed significant decrease at P < 0.01 in the area value of this band. The evaluated ratio value of amid-A band to amid-I band and amid-II band to amid-I band indicates the dose dependent decrease in the composition of protein in the rat sperm after sub-chronic cyanide treatment compare to control (Table 3).
Table 2.
Subchronic (90 days) cyanide induced changes in the band areas assigned to different biomolecules present in rat sperms.
| Wave number (cm−1) | Experimental groups |
|||
|---|---|---|---|---|
| Control | 0.64 mg kg−1 BW | 1.2 mg kg−1 BW | 3.2 mg kg−1 BW | |
| 3305 | 235.52 ± 24.11 | 228.88 ± 35.23 | 192.53 ± 23.32** | 172.62 ± 19.11*** |
| 3070 | 0.49 ± 0.01 | 0.46 ± 0.03 | 0.41 ± 0.01** | 0.36 ± 0.02*** |
| 3014 | 0.14 ± 0.03 | 0.10 ± 0.01 | 0.06 ± 0.08** | 0.04 ± 0.06** |
| 2962 | 0.15 ± .05 | 0.18 ± 0.03 | 0.11 ± 0.03** | 0.07 ± 0.06*** |
| 2925 | 2.53 ± 0.21 | 2.48 ± 0.42 | 2.36 ± 0.45* | 1.56 ± 0.48** |
| 2856 | 0.92 ± 0.08 | 0.70 ± 0.04* | 0.62 ± 0.06** | 0.56 ± 0.04*** |
| 1739 | 0.42 ± 0.01 | 0.62 ± 0.03 | 0.53 ± 0.07** | 0.48 ± 0.06*** |
| 1654 | 28.73 ± 0.42 | 26.43 ± 0.52 | 23.23 ± 0.42* | 20.41 ± 0.72** |
| 1544 | 8.32 ± 0.26 | 7.12 ± 0.36 | 5.12 ± 0.31** | 4.42 ± 0.36*** |
| 1452 | 0.28 ± 0.07 | 0.25 ± 0.04 | 0.20 ± 0.02** | 0.18 ± 0.05*** |
| 1313 | 2.42 ± 0.05 | 2.38 ± 0.06 | 1.95 ± 0.03** | 1.35 ± 0.07*** |
| 1240 | 2.53 ± 0.05 | 2.43 ± 0.07 | 2.12 ± 0.04** | 1.43 ± 0.06*** |
| 1166 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00* | 0.01 ± 0.00** |
Values are mean ± SE for six rats in each group.
P < 0.05.
P < 0.01.
P < 0.001 for cyanide treated groups vs. control group.
Table 3.
Subchronic (90 days) cyanide induced changes in FT-IR absorption band area ratio for selected bands in rat sperm spectra.
| Band area ratio | Experimental groups |
|||
|---|---|---|---|---|
| Control | 0.64 mg kg−1 BW | 1.2 mg kg−1 BW | 3.2 mg kg−1 BW | |
| I1544/I3305 | 0.038 ± 0.004 | 0.032 ± 0.005 | 0.026 ± 0.003** | 0.025 ± 0.002*** |
| I1544/I1654 | 0.28 ± 0.004 | 0.26 ± 0.005 | 0.22 ± 0.008* | 0.21 ± 0.006** |
| I2962/I2856 | 0.16 ± 0.007 | 0.21 ± 0.008 | 0.15 ± 0.003 | 0.12 ± 0.001** |
Values are mean ± SE for six rats in each group.
P < 0.05.
P < 0.01.
P < 0.001 for cyanide treated groups vs. control group.
In order to investigate the changes in lipid content of rat sperms, the spectral range from ∼3050 to ∼2800 cm−1 was considered (Fig. 4). The assignment of the bands arises in this region given in Table 1. The groups treated with 1.2 and 3.2 mg kg−1 BW showed significant decrease by −34% and −56% respectively in the area value compared to control group (Table 2).
The average FT-IR spectra of control and cyanide treated groups rat sperm in the ∼1800–800 cm−1 region is shown Fig. 5. The detailed band assessment was given in Table 4. The band at ∼1739 cm−1 was assigned to C O stretching vibration in ester groups of triglycerides. The cyanide treated groups showed the dose-dependent increase in the area under this peak. One more band observed at ∼1313 cm−1 was assigned to CH3·CH2 stretching in collagen. The group treated with 3.2 mg kg−1 BW showed the significant (P < 0.01) decrease in the area under the band compared to control, followed by the group treated with 1.2 mg kg−1 BW (Table 2). While the group treated with lower dose (0.64 mg kg−1 BW) showed insignificant changes in the area value of these bands compared to control.
Table 4.
Subchronic (90 days) cyanide induced changes in protein (∼1700–1600 cm−1) and lipid (∼3050–2800 cm−1) secondary structures with their general band assignment based on the literature [58], [59]].
| Wave number in cm−1 | Experimental groups |
Peak assignments | |||
|---|---|---|---|---|---|
| Control | 0.64 mg kg−1 BW | 1.2 mg kg−1 BW | 3.2 mg kg−1 BW | ||
| Protein | |||||
| 1691.26 | −0.000013 | −0.000038* | −0.000047** | −0.000051*** | Antiparallel β-sheet |
| 1670.05 | −0.000018 | −0.000013 | −0.000003** | −0.000002*** | Turns |
| 1654.62 | −0.000372 | −0.000331 | −0.000275* | −0.000176** | α-helix |
| 1644.98 | −0.000030 | −0.000041* | −0.000043* | −0.000049** | Random coil |
| 1633.41 | −0.000055 | −0.000051 | −0.000054 | −0.000059* | β-sheet |
| 1627.62 | −0.000021 | −0.000024 | −0.000029* | −0.000037** | Aggregated β-sheet |
| Lipid | |||||
| 3014.01 | −0.000007 | −0.00001 | −0.000004** | −0.00000006*** | Olefinic C—H stretching: unsaturated lipids |
| 2962.12 | −0.00016 | −0.00012 | −0.00008** | −0.00004*** | CH3 asymmetric stretching |
| 2925.48 | −0.00044 | −0.00057 | −0.00028* | −0.00013** | CH2 asymmetric stretching |
| 2856.20 | −0.00052 | −0.00081 | −0.00043* | −0.00009** | CH2 symmetric stretching |
Values are mean ± SE (±0.0001 to 0.000001) for six rats in each group.
*P < 0.05.
**P < 0.01.
***P < 0.001 for cyanide treated groups vs. control group.
Further, in order to investigate the changes in the secondary structure of proteins and lipids, the second derivative of IR spectra in the region from ∼1700 to ∼1600 cm−1 for protein (Fig. 6) and ∼3050 to ∼2800 cm−1 for lipids (Fig. 7) were investigated. The changes in the peak position and detailed peak assessments were given in Table 4. Overall intensity of all the observed peaks in the protein and lipid secondary derivative showed significant changes compared to control. The ratio between α-helix and β-sheet and α-helix and random coils indicated that, the groups treated with 1.2 and 3.2 mg kg−1 BW showed significant increase in the aggregated β-sheet compared to control. In addition to this, the random coils in the proteins were also increased in 1.2 and 3.2 mg kg−1 BW compared to control. While the group treated with 0.64 mg kg−1 BW showed insignificant changes in the α-helix and β-sheet and α-helix and random coils compared to control.
Fig. 7 shows the alteration in the lipid composition of control and different doses of cyanide treated on rat sperms. The selected spectral region (∼3050 to ∼2800 cm−1) for the analysis of alteration in lipid composition showed the presence of different bands. The band at ∼3014 cm−1, ∼2925 cm−1 and ∼2856 cm−1 were assigned to olefinic C—H stretching in unsaturated lipids, CH2 asymmetric and CH2 symmetric in the lipids. The group treated with lower dose (0.64 mg kg−1 BW) showed the insignificant increase in intensity compared to the control. While the groups treated with 1.2 and 3.2 mg kg−1 BW showed the significant decrease in the intensity compared to the control. However, the band at ∼2962 cm−1 was assigned to CH3 asymmetric stretching vibrational groups in the lipids. Dose-dependent decrease was observed in the intensity of this band compared to control (Table 4).
4. Discussion
The current study was undertaken to investigate the oxidative damage in rat sperms induced by subchronic cyanide intoxication. The results obtained from body weight gain, reproductive organ weight, hormone levels, sperm count, sperm motility and sperm abnormality were in line with our previous work [60]. Further, in our previous work we have also revealed that, higher doses of cyanide can induce changes in histoarchitecture of testis, epididymis and prostate and it has been presumed due to the induced oxidative stress. Several studies have demonstrated that, cyanide can induce oxidative stress in functionally different tissues [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]. As a consequence, the induced oxidative stress in the male reproductive organs will cause harmful effect on sperms due to the lack of their own antioxidant mechanism. Hence, in the current study FT-IR has been particularly utilized to investigate the oxidative damage in the rat sperms.
In the current study, the care has been taken during sample preparation for FT-IR spectral analysis. All samples were carefully evaluated for the number of sperms in order to the normalization sample based on sperm number with final volume of 1 ml in PBS buffer by following the method (explained in Section 2.5). Then the samples were centrifuged and the obtained sperm pellets were immersed in the liquid nitrogen and subjected to lyophilization. After lyophilization, KBr pellets with even thickness were prepared by applying equal pressure. Eventually, the FT-IR spectra were collected in triplet and observed that they were identical. Therefore, it is possible to directly relate the change in intensity and more accurately an area under the bands to the concentration of the corresponding biomolecules in the sperms.
The results of the FT-IR spectral analysis indicated the dose diffident changes in the absorption and an area under the peaks aroused by the different biomolecules present in the rat sperms (Fig. 2). The spectral range from ∼3600 cm−1 to ∼3050 cm−1 (Fig. 3) consists mainly of two bands at ∼3305 cm−1 and ∼3070 cm−1. The band at ∼3305 cm−1 assigned to the amide-A, mainly due to the N—H stretching modes in proteins with a negligible contribution of O—H stretching of intermolecular hydrogen bonding since the unbound water was removed from the samples [61], [62]. The second band at ∼3070 cm−1 corresponds to N—H stretching of amide-B proteins. In the current study, the groups treated with 1.2 and 3.2 mg kg−1 BW showed significant decrease in the area under both these bands (Table 4). The decreased area under these bands was the indication of reduced protein contained in the sperms. Studies have demonstrated that, cyanide induced the protein catabolism in functionally different tissues and caused a decrease in the protein content. In addition to this, the groups treated with 1.2 and 3.2 mg kg−1 BW showed shifting of peak position of the band assigned to amide-A from ∼3305 cm−1 in control to ∼3003 cm−1 and ∼3297 cm−1 respectively (Table 1). Such change in the peak position implied the changes in the protein secondary structure configuration and it had been presumed due to the ROS attack on the proteins present in the sperms.
The second spectral region (∼3050 cm−1 to ∼2800 cm−1) was mainly considered for the evaluation of alteration in the lipid content, which showed the presence of several peaks aroused from different functional groups in the lipids. This spectral region showed the presence of three prominent bands at ∼2962 cm−1, ∼2925 cm−1 and ∼2856 cm−1 were assigned to CH3 asymmetric and CH2 asymmetric and CH2 symmetric stretching modes in lipids respectively (Table 1). In the current study, the groups treated with 1.2 and 3.2 mg kg−1 BW showed significant decrease in the area under these bands. The decreased area under these bands represents the decreased lipid content in the sperms. In addition to this, the higher dose treated groups showed shifting in frequency of these peaks to lower values. Studies have considered the shifting in frequencies of a CH2 symmetric stretching band to lower values by 2.3 cm−1 as a marker for decreased membrane fluidity [63]. However, membrane lipids play an important role in maintaining the normal shape of sperms, which is required for the competitive forward motility in female genital track to reach the site of fertilization. In addition to this, studies have demonstrated that the changes in the sperms lipid bilayers will cause the increased abnormalities in sperms, which will certainly lead to increased male infertility [34], [64]. Further, lipids in plasma membrane will also play an important role in the regulation of membrane functions [65]. The plasma membrane in the acrosomal region of sperms carries the receptors to recognize the ova in the female genital track. Results from the current study indicates that the alteration of lipid content in the sperms from groups treated with 1.2 and 3.2 mg kg−1 BW may lead to fail in recognition of ova and which may cause infertility.
However, the analysis of second derivatives of spectra revealed that the very small peaks are not prominent or absent in the first derivative spectra. In the current study, the second derivative of the spectral region from ∼3050 cm−1 to ∼2800 cm−1 showed the presence of band assigned to olefinic functional group in unsaturated lipids, mainly due to C—H stretching modes at ∼3014 cm−1. Studies have mainly used this band to investigate the lipid peroxidation in the tissues [66]. In the current study, the groups treated with 1.2 and 3.2 mg kg−1 BW showed the significant decrease in the intensity of the olefinic unsaturated lipid band. Studies have demonstrated that, the sperm plasma membrane contains higher amount of polyunsaturated lipids, which is more susceptible to ROS [24]. As a result of induced oxidative stress in the epididymis, the elevated level of ROS will attack on polyunsaturated lipids and caused lipid peroxidation in the sperm plasma membrane. Studies have reported that lipid peroxidation in the sperm plasma membrane will lead to the elevated number of abnormal sperm, which will certainly lead to increased male infertility [33]. While, the group treated with lower dose (0.64 mg kg−1 BW) showed the increased area and intensity of the bands observed at ∼3014 cm−1, ∼2925 cm−1 and ∼2856 cm−1 assigned to olefinic C—H, CH2 symmetric and asymmetric stretching modes in the lipids (Fig. 4, Fig. 7). The increased area under these bands was the indication of increased lipid content in the sperms. Such increase in the lipid content was the indication of body response to mild toxic effect of xenobiotics [67].
The third spectral region (∼1800 to 800 cm−1) contains two sharp bands with several other bands arising from different functional groups of proteins, lipids and nucleic acids (Fig. 5). The first sharp band observed at ∼1654 cm−1 is assigned to amide-I, mainly due to C—O stretching of ά-helix proteins and second band observed at ∼1544 cm−1 assigned to amid-II, mainly due to N—H bending and C—N stretching vibration modes in proteins (Table 4). In the current study, the groups treated with 1.2 and 3.2 mg kg−1 BW showed the significant decrease in the area under these bands (Table 3). The reduced area under these bands confirms the decreased protein content in the rat sperm. This decreased protein content maybe due to cyanide induced increased protein oxidation in the sperms. In addition to this, bands at ∼1452 cm−1 and ∼1400 cm−1 aroused mainly from asymmetric and symmetric CH3 bending modes of methyl groups of proteins with some contribution of the vibrations of fatty acids respectively. The groups treated with 1.2 and 3.2 mg kg−1 BW indicated the significant decreased area under the bands. Such a decrease in the area under these bands had given further confirmation of decreased protein content in sperms.
Furthermore, in order to evaluate the changes in protein to lipid ratio the area value of amid-I proteins to CH2 symmetric stretching was used to calculate the ratio between proteins to lipids (Table 3). The results indicated that, the groups treated with 1.2 and 3.2 mg kg−1 BW showed decrease in both protein and lipid content compared to control. Such decrease in the lipids and protein content was a result of ROS induced oxidation in proteins and lipids present in the sperms. While, the group treated with lower dose (0.64 mg kg−1 BW) showed the insignificant changes in the area under the bands assigned to proteins and lipids. Studies have demonstrated that lower doses of cyanide may detoxify without causing any harmful effect on the body [25]. Consequently, in the current study, the results indicated that, the selected lower dose of (0.64 mg kg−1 BW) showed no significant changes in the sperm biochemical composition.
In addition to quantitative analysis of proteins present in sperms, the qualitative and structural analysis was down with the second derivative of the spectra in the region (∼1700 cm−1 to ∼1600 cm−1) assigned to amide-I. In the current study, the groups treated with 1.2 and 3.2 mg kg−1 BW showed significant decrease in the intensity of the peak assigned to ά-helical structures and turn in the proteins which is accompanied by an increase in the intensity of the peaks assigned to β-sheets, aggregated β-sheets and random coil structures. The decreased intensity of the peak assigned to ά-helical and turns and subsequent increase in the intensity of the peaks assigned to β-sheets, aggregated β-sheets and random coil structures indicated the denaturation of proteins [68]. Palaniappa and Pramod, [69] have demonstrated that, the decrease in ά-helical structure is responsible for the increase in the β-sheets secondary structure in proteins. Further, these results also indicated decreased protein content of rat sperms. However, both membrane proteins and lipids played an important role in the maintenance of cell membrane dynamics [70] and which is most important in the sperms to achieve fertilization.
5. Conclusion
In conclusion, our study demonstrated that exposure to higher doses (1.2 and 3.2 mg kg−1 BW) of cyanide caused significant alteration in the biochemical constituents of the rat sperms. Further, the results clearly indicated a significant change in the composition and structure of proteins. A significant decrease in ά-helical structures and turns in the proteins was accompanied by a subsequent increase in the intensity of the peaks assigned to β-sheets, aggregated β-sheets and random coil structures were observed in the high-dose cyanide intoxicated rat sperms. On the whole, the results showed the sperms are most vulnerable to oxidative stress induced by cyanide intoxication. In addition to this, the current study revealed that the FT-IR is an excellent tool for the investigation of oxidative damage in the sperms.
References
- 1.Miller G.C., Pritsos C.A. Cyanide. Soc. Ind. Econ. Aspects Proc. Symp. Annu. Meet. 2001:7. [Google Scholar]
- 2.McAllister J.L., Roby R.J., Levine B., Purser D. The effect of sodium fluoride on the stability of cyanide in postmortem blood samples from fire victims. Forensic Sci. Int. 2011;209:29–33. doi: 10.1016/j.forsciint.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez R., Ord́o∼nez A., Loredo J., Younger P.L. Wetland-based passive treatment systems for gold ore processing effluents containing residual cyanide, metals and nitrogen species. Environ. Sci. 2013;15:2115–2124. doi: 10.1039/c3em00410d. [DOI] [PubMed] [Google Scholar]
- 4.Gwon S.-Y., Rao B.A., Kim H.-S., Son Y.-A., Kim S.-H. Novel styrylbenzothiazolium dye-based sensor for mercury, cyanide and hydroxide ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;44:226–234. doi: 10.1016/j.saa.2015.02.094. [DOI] [PubMed] [Google Scholar]
- 5.Dubey S.K., Holmes D.S. Biological cyanide destruction mediated by microorganisms. World J. Microbiol. Biotechnol. 1995;11:257–265. doi: 10.1007/BF00367095. [DOI] [PubMed] [Google Scholar]
- 6.Fetoui H., Makni M., Garoui E.M., Zeghal N. Toxic effects of lambda-cyhalothrin, a synthetic pyrethroid pesticide, on the rat kidney: involvement of oxidative stress and protective role of ascorbic acid. Exp. Toxicol. Pathol. 2010;62(6):593–599. doi: 10.1016/j.etp.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Potter J., Smith R.L., Api A.M. Urinary thiocyanate levels as a biomarker for the generation of inorganic cyanide from benzyl cyanide in the rat. Food Chem. Toxicol. 2001;39:41–146. doi: 10.1016/s0278-6915(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 8.Liu C.-Y., Tseng W.-L. Colorimetric assay for cyanide and cyanogenic glycoside using polysorbate 40-stabilized gold nanoparticles. Chem. Commun. (Cambridge) 2011;47(9):2550–2552. doi: 10.1039/c0cc04591h. [DOI] [PubMed] [Google Scholar]
- 9.Mathangi D.C., Namasivayam A. Protective effect of diltiazem on cyanide-induced neurotoxicity in Wistar strain rats. Food Chem. Toxicol. 2004;42:605–608. doi: 10.1016/j.fct.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Okafor P.N., Okorowkwo C.O., Maduagwu E.N. Occupational and dietary exposures of humans to cyanide poisoning from large-scale cassava processing and ingestion of cassava foods. Food Chem. Toxicol. 2002;40:1001–1005. doi: 10.1016/s0278-6915(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 11.EPA, Toxicological review of hydrogen cyanide and cyanide salts, EPA/635/R-08/016F, (2010) 1-153.
- 12.Rao P., Singh P., Yadav S.K., Gujar N.L., Bhattacharya R. Acute toxicity of some synthetic cyanogens in rats: time-dependent cyanide generation and cytochrome oxidase inhibition in soft tissues after sub-lethal oral intoxication. Food Chem. Toxicol. 2013;59:595–609. doi: 10.1016/j.fct.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 13.Rocha-e-Silva R.C., Cordeiro L.A.V., Soto-Blanco B. Cyanide toxicity and interference with diet selection in quail (Coturnix coturnix) Comp. Biochem. Physiol. Part C. 2010;151:294–297. doi: 10.1016/j.cbpc.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Solomonson L.P. In: Cyanide in Biology. Vennesland B., Conn E.E., Knowles C.J., Westley J., Wissing F., editors. Academic Press; New York: 1981. Cyanide as a metabolic inhibitor; pp. 11–28. [Google Scholar]
- 15.Ardelt B.K., Borowitz J.L., Isom G.E. Brain lipid peroxidation and antioxidant protectant mechanisms following acute cyanide intoxication. Toxicology. 1989;56:147–154. doi: 10.1016/0300-483x(89)90129-7. [DOI] [PubMed] [Google Scholar]
- 16.Ezeanyika L.U.S., Obidoa O., Shoyinka V.O. Comparative effects of scopoletin and cyanide on ratbrain, 1: histopathology. Plant Foods Hum. Nutr. 1999;53:351–358. doi: 10.1023/a:1008096801895. [DOI] [PubMed] [Google Scholar]
- 17.Kamendulis L.M., Zhang H., Wang Y., Klaunig J.E. Morphological transformation and oxidative stress induced by cyanide in Syrian Hamster Embryo (SHE) cells. Toxicol. Sci. 2002;68:437–443. doi: 10.1093/toxsci/68.2.437. [DOI] [PubMed] [Google Scholar]
- 18.Okolie N.P., Iroanya C.U. Some histological and biochemical evidence for mitigation of cyanide-induced tissue lesions by antioxidant vitamin administration in rabbits. Food Chem. Toxicol. 2003;41:463–469. doi: 10.1016/s0278-6915(02)00224-7. [DOI] [PubMed] [Google Scholar]
- 19.Okolie N.P., Asonye C.C. Mitigation of cataractogenic potential of cyanide by antioxidant vitamin administration. J. Med. Biomed. Res. 2004;3(1):48–52. [Google Scholar]
- 20.David M., Vadingadu M., Ramesh H., Marigoudar S.R. Impact of sodium cyanide on catalase activity in the freshwater exotic carp Cyprinus carpio (Linnaeus) Pestic. Biochem. Physiol. 2008;92:15–18. [Google Scholar]
- 21.Jo T.G., Na Y.J., Lee J.J., Lee M.M., Lee S.Y., Kim C. A multifunctional colorimetric chemosensor for cyanide and copper(II) ions. Sens. Actuators B Chem. 2015;211:498–506. [Google Scholar]
- 22.Wang K., Liu Z., Guan R., Cao D., Chen H., Shan Y., Wu Q., Xu Y. Coumarin benzothiazole derivatives as chemosensors for cyanide anions. Spectrochim. Acta Part A Mol. Biomol .Spectrosc. 2015;144:235–242. doi: 10.1016/j.saa.2015.02.072. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y., Dai X., Zhao B.-X. A coumarin-indole based colorimetric and ‘turn on’ fluorescent probe for cyanide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;138:164–168. doi: 10.1016/j.saa.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal A., Saleh R.A. Role of oxidants in male infertility: rationale, significance, and treatment. Urol. Clin. N. Am. 2002;29:817–827. doi: 10.1016/s0094-0143(02)00081-2. [DOI] [PubMed] [Google Scholar]
- 25.Shiva M., Gautam A.K., Verma Y., Shivgotra V., Doshi H., Kumar S. Association between sperm quality, oxidative stress, and seminal antioxidant activity. Clin. Biochem. 2011;44:319–324. doi: 10.1016/j.clinbiochem.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Zottola M.A. A partial exploration of the potential energy surfaces of SCN and HSCN: implications for the enzyme-mediated detoxification of cyanide. J. Mol. Graphics Modell. 2009;28:183–186. doi: 10.1016/j.jmgm.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Hasuike Y., Nakanishi T., Moriguchi R., Otaki Y., Nanami M., Hama Y., Naka M., Miyagawa K., Izumi M., Takamitsu Y. Accumulation of cyanide and thiocyanate in haemodialysis patients. Nephrol. Dial. Transplant. 2004:1474–1479. doi: 10.1093/ndt/gfh076. [DOI] [PubMed] [Google Scholar]
- 28.Lockwood A., Patka J., Rabinovich M., Wyatt K., Abraham P. Sodium nitroprusside-associated cyanide toxicity in adult patients-fact or fiction—a critical review of the evidence and clinical relevance. Open Access J. Clin. Trials. 2010;2:133–148. [Google Scholar]
- 29.Curtis A.J., Grayless C.C., Fall R. Simultaneous determination of cyanide and carbonyls in cyanogenic plants by gas chromatography-electron capture/photoionization detection. Analyst. 2002;127(11):1446–1449. doi: 10.1039/b205378k. [DOI] [PubMed] [Google Scholar]
- 30.Rinaldi J.C., Justulin L.A., Jr, Lacorte L.M., Sarobo C., Boer P.A., Scarano W.R., Felisbino S.L. Implications of intrauterine protein malnutrition on prostate growth, maturation and aging. Life Sci. 2013;92:763–774. doi: 10.1016/j.lfs.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Highland H.N., Kalaria B., George L.B. A study on the trend in semen parameters of males from Ahmedabad City (Gujarat), over a two-decade period. EthnoMed. 2010;4(3):199–202. [Google Scholar]
- 32.Vela G., Luna M., Sandler B., Copperman A.B. Advances and controversies in assisted reproductive technology. Mt. Sinai J. Med. 2009;76:506–520. doi: 10.1002/msj.20147. [DOI] [PubMed] [Google Scholar]
- 33.Niederberger C., Joyce G.F., Wise M., Meacham R.B. In: Urologic Diseases in America. Litwin M.S., Saigal C.S., editors. US Government Printing Office; Washington, DC: 2007. Male infertility. [Google Scholar]
- 34.Simon L., Proutski I., Stevenson M., Jennings D., McManus J., Lutton D., Lewis S.E.M. Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod. Biomed. Online. 2013;26:68–78. doi: 10.1016/j.rbmo.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Cox C. Chlorpyrifos, part 1: toxicology. J. Pestic. Ref. 1994;14:15–20. [Google Scholar]
- 36.Sinclair S. Male infertility: nutritional and environmental considerations. Altern. Med. Rev. 2000;5:28–38. [PubMed] [Google Scholar]
- 37.Barratt C.L.R., Aitken R.J., Björndahl L., Carrell D.T., De Boer P., Kvist U., Lewis S.E.M., Perreault S.D., Perry M.J., Ramos L., Robaire B., Ward S., Zini A. Sperm DNA: organization, protection and vulnerability: from basic science to clinical applications—a position report. Hum. Reprod. 2010;25:824–838. doi: 10.1093/humrep/dep465. [DOI] [PubMed] [Google Scholar]
- 38.Research Triangle Park, NC, National Institutes of Health, National Toxicology Program Toxicology Report Series No. 37. NIH Publication; 1993. NTP studies, sodium cyanide administered in drinking water to F344/N rats and B6C3F1 mice; pp. 94–3386. [Google Scholar]
- 39.Mathangi D.C., Namasivayam A. Effecet of chronic cyanide intoxication on memory in albino rats. Food Chem. Toxicol. 2000;38:51–55. doi: 10.1016/s0278-6915(99)00118-0. [DOI] [PubMed] [Google Scholar]
- 40.Mathangi D.C., Namasivayam A. Effect of chronic sublethal cyanide administration on brain neurotransmitters and behaviour in rats. J. Occup. Health. 2000;42:88–90. [Google Scholar]
- 41.Mathangi D.C., Namasivayam A. Neurochemical and behavioural correlates in cassava-induced neurotoxicity in rats. Neurotox. Res. 2000;2:29–35. doi: 10.1007/BF03033325. [DOI] [PubMed] [Google Scholar]
- 42.Odunuga O.O., Adenuga G.A. Sodium nitrite alone protects the brain microsomal Ca2-ATpase against potassium cyanide-induced neurotoxicity in rats. Biosci. Rep. 1997;17(6):543–546. doi: 10.1023/a:1027312324077. [DOI] [PubMed] [Google Scholar]
- 43.Soto-Blanco B., Górniak S.L. Prenatal toxicity of cyanide in goats—a model for teratological studies in ruminants. Theriogenology. 2004;62:1012–1026. doi: 10.1016/j.theriogenology.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 44.Soto-Blanco B., Pereira F.T.V., Flávia de Carvalho A., Miglino M.A., Górniak S.L. Fetal and maternal lesions of cyanide dosing to pregnant goats. Small Rumin. Res. 2009;87:76–80. [Google Scholar]
- 45.Soto-blanco B., Górniak S.L. Toxic effects of prolonged administration of leaves of cassava (Manihot esculenta Crantz) to goats. Exp. Toxicol. Pathol. 2010;62:361–366. doi: 10.1016/j.etp.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Mathangi D.C., Shyamala R., Vijayashree R., et al. Effect of alpha-ketoglutarate on neurobehavioral, neurochemical and oxidative changes caused by sub-chronic cyanide poisoning in rats. Neurochem. Res. 2011;36:540–548. doi: 10.1007/s11064-010-0376-z. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez V., Redmann K., Wistuba J., Frank Wubbeling M., Burger H., Oldenhof W.F., Wolkers S., Kliesch S., Schlatt Mallidis C. Oxidative DNA damage in human sperm can be detected by Raman microspectroscopy. Fertil. Steril. 2012;98:1124–1129.e3. doi: 10.1016/j.fertnstert.2012.07.1059. [DOI] [PubMed] [Google Scholar]
- 48.Shivanoor S.M., David M. Protective role of turmeric against deltamethrin induced renal oxidative damage in rats. Biomed. Preventive Nutr. 2014;4:543–553. [Google Scholar]
- 49.Egekeze J.O., Oehme F.W. Cyanides and their toxicity: a literature review. Vet. Q. 1980;2:104–114. doi: 10.1080/01652176.1980.9693766. [DOI] [PubMed] [Google Scholar]
- 50.Clark D.J., Hothem R. Mammal mortility at Arizona: California and Navada gold mines using cyanide extraction. Calif. Fish Game. 1991;77:61–69. [Google Scholar]
- 51.Brasel J.M., Cooper R.C., Pritsos C.A. Effect of environmental relevant dose of cyanide on flight time in pigeons, Culumba livia. Bull. Environ. Contam. Toxicol. 2006;76:202–209. doi: 10.1007/s00128-006-0908-z. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization; Geneva: 1996. Guidelines for Drinking-water Quality. [Google Scholar]
- 53.Tomasulo M., Sortino S., White A.J.P., Raymo F.M. Chromogenic oxazines for cyanide detection. J. Org. Chem. 2006;71:744–753. doi: 10.1021/jo052096r. [DOI] [PubMed] [Google Scholar]
- 54.Y. Ding T., Li W., Zhu Y. Xie. Highly selective colorimetric sensing of cyanide based on formation of dipyrrin adducts. Org. Biomol. Chem. 2012;10:4201–4207. doi: 10.1039/c2ob25297j. [DOI] [PubMed] [Google Scholar]
- 55.Sánchez V., Redmann K., Wistuba J., Wübbeling F., Burger M., Oldenhof H., et al. Oxidative DNA damage in human sperm can be detected by Raman microspectroscopy. Fertil. Steril. 2012;98:1124–1129. doi: 10.1016/j.fertnstert.2012.07.1059. e1–3. [DOI] [PubMed] [Google Scholar]
- 56.Chu H.-L., Liu T.-Y., Lin S.-Y. Effect of cyanide concentrations on the secondary structures of protein in the crude homogenates of the fish gill tissue. Aquat. Toxicol. 2001;55:171–176. doi: 10.1016/s0166-445x(01)00177-1. [DOI] [PubMed] [Google Scholar]
- 57.Roque A., Ponte I., Suau P. Secondary structure of protamine in sperm nuclei: an infrared spectroscopy study. BMC Struct. Biol. 2011:1–8. doi: 10.1186/1472-6807-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Byler D.M., Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986;25:469–487. doi: 10.1002/bip.360250307. [DOI] [PubMed] [Google Scholar]
- 59.Iconomidou V.A., Chryssikos D.G., Gionis V., Pavlidis M.A., Paipetis A., Hamodrakas S.J. Secondary structure of chorion proteins of the teleostean fish dentex dentex by ATR FT-IR and FT-Raman Spectroscopy. J. Struc. Biol. 2000;122:112–122. doi: 10.1006/jsbi.2000.4307. [DOI] [PubMed] [Google Scholar]
- 60.Shivanoor S.M., David M. Subchronic cyanide toxicity on male reproductive system of albino rat. Toxicol. Res. 2014;4:57–64. [Google Scholar]
- 61.Jung C. Insight into protein structure and protein–ligand recognition by Fourier transform infrared spectroscopy. J. Mol. Recognit. 2000;13:325–351. doi: 10.1002/1099-1352(200011/12)13:6<325::AID-JMR507>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 62.Cakmak G., Togan I., Severcan F. 17 Beta-estradiol induced compositional, structural and functional changes in rainbow trout liver, revealed by FT-IR spectroscopy: a comparative study with nonylphenol. Aquat. Toxicol. 2006;77:53–63. doi: 10.1016/j.aquatox.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 63.Liu K.Z., Schultz C.P., Mohammad R.M., Johnston J.B., Mantsch H.H. Infrared spectroscopic study of bryostatin 1-induced membrane alterations in a B-CLL cell line. Leukemia. 1999;13:1273. doi: 10.1038/sj.leu.2401463. [DOI] [PubMed] [Google Scholar]
- 64.Donnelly E.T., Lewis S.E., Thompson W., Chakravarthy U. Sperm nitric oxide and motility: the effects of nitric oxide synthase stimulation and inhibition. Mol. Hum. Reprod. 1997;3:755–762. doi: 10.1093/molehr/3.9.755. [DOI] [PubMed] [Google Scholar]
- 65.Oldenhof H., Friedel K., Sieme H., Glasmacher B., Wolkers W.F. Membrane permeability parameters for freezing of stallion sperm as determined by Fourier transform infrared spectroscopy. Cryobiology. 2010;61:115–122. doi: 10.1016/j.cryobiol.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Severcana F., Gorgulub G., Gorgulua S.T., Gurayb T. Rapid monitoring of diabetes-induced lipid peroxidation by Fourier transform infrared spectroscopy: evidence from rat liver microsomal membranes. Anal. Biochem. 2005;339:36–40. doi: 10.1016/j.ab.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Palaniappan P.L.R.M., Vijayasundaram V. The FT-IR study of the brain tissue of Labeo rohita due to arsenic intoxication. Microchem. J. 2009;91:118–124. [Google Scholar]
- 68.Toyran N., Zorlu F., Donmez G., Ode K., Severcan F. Chronic hypoperfusion alters the content and structure of proteins and lipids of rat brain homogenates: a fourier transform infrared spectroscopy study. Eur. Biophys. J. 2004;33:549–554. doi: 10.1007/s00249-004-0396-1. [DOI] [PubMed] [Google Scholar]
- 69.Palaniappan P.L.R.M., Pramod K.S. The effect of titanium dioxide on the biochemical constituents of the brain of Zebrafish (Danio rerio): an FT-IR study. Spectrochim. Acta Part A. 2011;79:206–212. doi: 10.1016/j.saa.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 70.Szalontai B., Kota Z., Nonaka H., Murata N. Structural consequences of genetically engineered saturation of the fatty acids of phosphatidylglycerol in tobacco thylakoid membranes. An FTIR study. Biochemistry. 2003;42:4292–4299. doi: 10.1021/bi026894c. [DOI] [PubMed] [Google Scholar]