Highlights
-
•
Insulin formulations are cytotoxic in vitro.
-
•
Toxicity is caused by the excipients phenol and m-cresol.
-
•
Phenolic excipients activate stress kinases and attenuate AKT phosphorylation.
-
•
Phenolic excipients induce pro-inflammatory responses and MCP-1 release.
-
•
The toxic effects of excipients might explain inflammation of infusion sites in vivo.
Abbreviations: APC, allophycocyanin; CCL2, chemokine ligand 2; CD, cluster of differentiation; CSII, continuous subcutaneous insulin infusion; DMSO, dimethyl sulfoxide; ERK, extracellular signal-regulated kinase; IgG, immunoglobulin G; IL, interleukin; JNK, Jun N-terminal kinase; MAP kinase, mitogen-activated protein kinase; MCP-1, monocyte chemotactic protein-1; Mip-1α, macrophage inflammatory protein-1alpha; PE, phycoerythrin; TNFα, tumor necrosis factor alpha; XTT, 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
Chemical compounds studied in this article: Phenol (PubChem CID: 996), m-Cresol (PubChem CID: 342), Insulin (PubChem CID: 70678557), NovoRapid (PubChem CID: 16132418), Apidra (PubChem CID: 72941761), Humalog (PubChem CID: 16132438)
Keywords: Insulin, Phenolic excipients, Adverse effects, MCP-1, Inflammation
Abstract
Skin reactions at the infusion site are a common side effect of continuous subcutaneous insulin infusion therapy. We hypothesized that local skin complications are caused by components of commercial insulin formulations that contain phenol or m-cresol as excipients. The toxic potential of insulin solutions and the mechanisms leading to skin reactions were explored in cultured cells.
The toxicity of insulin formulations (Apidra, Humalog, NovoRapid, Insuman), excipient-free insulin, phenol and m-cresol was investigated in L929 cells, human adipocytes and monocytic THP-1 cells. The cells were incubated with the test compounds dose- and time-dependently. Cell viability, kinase signaling pathways, monocyte activation and the release of pro-inflammatory cytokines were analyzed.
Insulin formulations were cytotoxic in all cell-types and the pure excipients phenol and m-cresol were toxic to the same extent. P38 and JNK signaling pathways were activated by phenolic compounds, whereas AKT phosphorylation was attenuated. THP-1 cells incubated with sub-toxic levels of the test compounds showed increased expression of the activation markers CD54, CD11b and CD14 and secreted the chemokine MCP-1 indicating a pro-inflammatory response.
Insulin solutions displayed cytotoxic and pro-inflammatory potential caused by phenol or m-cresol. We speculate that during insulin pump therapy phenol and m-cresol might induce cell death and inflammatory reactions at the infusion site in vivo. Inflammation is perpetuated by release of MCP-1 by activated monocytic cells leading to enhanced recruitment of inflammatory cells. To minimize acute skin complications caused by phenol/m-cresol accumulation, a frequent change of infusion sets and rotation of the infusion site is recommended.
1. Introduction
Patients suffering from insulin-dependent diabetes mellitus have two main options for insulin administration: multiple daily injections or continuous subcutaneous insulin infusion (CSII) using insulin pumps. It is well documented that many patients benefit from CSII therapy by achieving a better glycemic control [1]. Although CSII is a safe and efficient therapy, it may be hampered by metabolic and non-metabolic complications. Among these are technical problems with the infusion sets (occlusion, kinked tubes, leakage), lipohypertrophy, and local skin reactions at the infusion site (inflammation, infection, scarring) [2], [3]. Inflammation at the infusion site has high prevalence, especially among pediatric patients (25–42%) [4], [5], [6]. The occurrence of these adverse events increases with longer indwelling time of the insulin catheter, which is reflected by the limited application time of 3–4 days found in clinical studies or surveys [7], [8], [9], [10] and the fact that most manufacturer recommend to change the infusion set every 2–3 days. Infusion sets have been optimized to reduce skin reactions by means of material selection and design. Using modern infusion sets the occurrence of true infections at the infusion site is very low compared to what was seen in clinical trials in the 1980ies [11], [12]. However, skin irritation and inflammation is a persistent problem for patients on CSII therapy and are a major reason for premature catheter replacement and even for giving up on CSII [13], [14]. However, the underlying mechanism for acute skin complications has not been resolved.
Preparations of insulin or rapid-acting insulin analogs contain phenolic excipients that serve two purposes: maintaining sterility of the solution and stabilization of the insulin molecule in the hexameric form to avoid aggregation [15], [16]. All insulin formulations for pump use contain either phenol, m-cresol or a mixture of both in considerable amounts (29–32 mM or 2.7–3.2 mg/ml). These compounds are problematic since they are known to be toxic and irritant, and are suspected sensitizers and carcinogens [17], [18]. There is limited knowledge on the toxicity of insulin solutions when administered to subcutaneous tissue. One clinical study reported that skin irritation at the infusion site are more common in patients using insulin preparations with m-cresol as compared to insulin containing methyl p-hydroxybenzoate as preserving agent [19]. However, this study was conducted with a small number of patients and it is not clear if the results can be transferred to current insulin formulations.
The aim of the study was to investigate the toxic potential of current pump insulins in vitro using cultured cells. We hypothesized that m-cresol or phenol in relevant concentrations could lead to cell death and trigger pro-inflammatory responses. The murine fibroblastic cell line L929 was selected as a model, because it is a well-established tool for cytotoxicity testing generating highly reproducible results. Although not of human origin, L929 cells are commonly used for risk assessment of medical devices like infusion catheters. This cell line was preferred for cytotoxicity testing of insulin formulations to obtain comparable results. Besides fibroblasts, adipose tissue mainly consists of adipocytes. Therefore, we have chosen human adipocytes to confirm our results in a second model that is closer to human physiology. In addition, we tested whether phenol or m-cresol would stimulate the secretion of pro-inflammatory cytokines by immune cells. For this purpose, human monocytic THP-1 cells were preferred, as cell activation by pro-inflammatory stimuli as well as cytokine secretion can be monitored in this cell line.
2. Materials and methods
2.1. Chemicals
Phenol (CAS No.108-95-2) and m-cresol (CAS No. 108-39-4) were obtained from Sigma–Aldrich. Jun N-terminal kinase (JNK) inhibitor SU3327 (10–20 μM in dimethyl sulfoxide (DMSO)) and p38 Inhibitor SB202190 (1–2 μM in DMSO) were from Tocris Bioscience.
The following formulations of insulin or insulin analogs were used, each at 100 I.U./ml: Apidra (3.15 mg/ml m-cresol, Sanofi-Aventis), Insuman Infusat (2.7 mg/ml phenol, Sanofi-Aventis), Humalog (3.15 mg/ml m-cresol, Eli Lilly), NovoRapid (1.72 mg/ml m-cresol and 1.5 mg/ml phenol, Novo Nordisk). Recombinant human insulin (Roche Diagnostics GmbH) was dissolved in phosphate buffer at 3 mg/ml (equivalent to 100 U/ml).
2.2. Cell culture and viability assay
The murine fibroblast cell line L929 and the human monocytic cell line THP-1 were cultured in RPMI1640 (Sigma–Aldrich) supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin at 37 °C and 5% CO2.
Cell viability assay was performed using the Cell Proliferation kit II (XTT) (Roche Diagnostics GmbH). Briefly, the tetrazolium salt 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) is cleaved by viable cells to form an orange formazan dye that can be quantified photometrically at 492 nm. Before the assay, 1 × 104 L929 cells were cultured in 96-well plates for 24 h. The culture medium was replaced by medium containing the desired concentration of insulin solutions, phenol or m-cresol. The cells were incubated for 24 h or otherwise as indicated. The XTT reagent was added and absorption was measured after 2 h using the Synergy4 plate reader (BioTek Instruments). Data analysis was performed using the Gen5 Data Analysis Software (BioTek Instruments). The threshold for toxicity was set to <70% cell viability.
2.3. Cytokine Immunoassay
5 × 105 THP-1 cells were incubated with insulin solutions or phenol or m-cresol (0.2 mg/ml) for 24 h. The release of the cytokines interleukin (IL)-1β, IL-6, IL-8, IL-12, tumor necrosis factor alpha (TNFα), macrophage inflammatory protein-1 alpha (Mip-1α) and monocyte chemotactic protein-1 (MCP-1) was determined in cell-free supernatants obtained by centrifugation. Immunodetection was performed using cytometric bead assays (CBA, BD Bioscience) and a BD FACSarray flow cytometer (BD Bioscience) as described by the manufacturer. Data analysis was performed using FCAP array software v1.0.1 (Soft flow Hungary Ltd.).
2.4. CD54, CD11b and CD14 immunostaining
Monocyte activation was determined by cluster of differentiation (CD)54, CD11b and CD14 expression. THP-1 cells were incubated with insulin solutions or phenol or m-cresol (0.2 mg/ml) for 24 h. Cells were washed and blocked in 10% human serum and then were incubated with phycoerythrin (PE)-labeled CD54, PE-labeled CD11b or allophycocyanin (APC)-labeled CD14 antibodies (BD Bioscience). Isotype controls were incubated with PE-labeled immunoglobulin (Ig) G1κ and APC-labeled IgG2a antibodies (BD Bioscience). After washing cells were analyzed by flow cytometry (BD FACSArray, BD Bioscience).
2.5. Phospho-kinase array
The Proteome Profiler Phospho-Kinase Array kit (R + D Systems) was used. Cell lysis and assay procedure were performed as described by the manufacturer. A total of 600 μg of protein was used in each array. Image acquisition was performed using a ChemiDoc XRS Imager (Biorad Laboratories) and Quantity One analysis software (Biorad Laboratories).
2.6. Western blot analysis
30–50 μg protein were subjected to SDS gel electrophoresis. The proteins were then transferred to PVDF Western Blotting Membrane (Roche Diagnostics GmbH), blocking was performed with 1% Western Blocking Reagent (Roche Diagnostics GmbH) for 1 h. The membrane was then incubated with the respective primary and secondary antibodies for 1 h. After washing, Lumi-LightPlus Western Blotting Substrate (Roche Diagnostics GmbH) was added and signals were analyzed with a ChemiDoc XRS Imager (Biorad Laboratories) and Quantity One software (Biorad Laboratories). Antibodies used: JNK, phospho-JNK (T183/Y185), p38α, phospho-P38α (T180/Y182) from R + D Systems; protein kinase B (AKT), phospho-AKT (S473), anti-rabbit-horseradish peroxidase conjugate from Cell Signaling Technology; anti-β-actin-peroxidase conjugate from Sigma–Aldrich.
2.7. Statistics
Standard deviation is indicated by error bars. Unpaired, two-tailed t-tests were performed and significance was assumed for p values less than 0.05.
3. Results
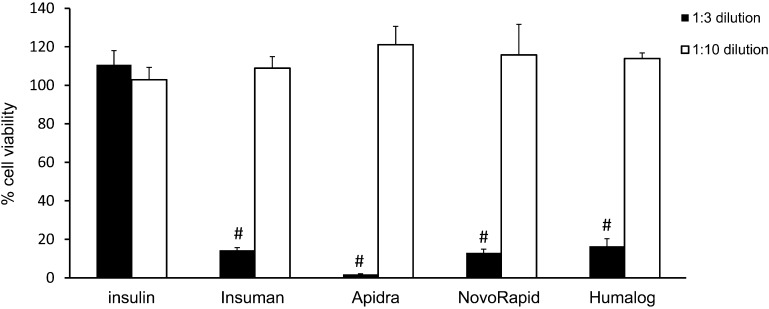
In order to test the cytotoxic potential of various insulin formulations, L929 cells were incubated with Humalog, Apidra, NovoRapid, Insuman, all diluted 1:3 with culture media, or excipient-free human insulin at a concentration of 1 mg/ml for 24 h. The XTT test revealed that all commercial insulin solutions caused a significant drop in cell viability below the toxicity threshold of 70%. In contrast, insulin dissolved in medium without any excipient did not impair cell viability (Fig. 1). Therefore it can be excluded that cell-death was caused by the supra-physiological insulin concentrations used in these experiments. A 1:10 dilution of the insulin formulations did not cause toxicity in L929 cells.
Fig. 1.
Insulin formulations are cytotoxic in L929 cells. L929 cells were exposed to different insulin solutions for 24 h. Cell viability was assessed relatively to culture medium control (= 100%) by XTT assay. Insulin formulations were diluted 1:3 (filled bars) or 1:10 (open bars) in culture medium. Insulin concentration was 1 or 0.3 mg/ml, respectively. Error bars indicate S.D., n = 6, # p < 0.001.
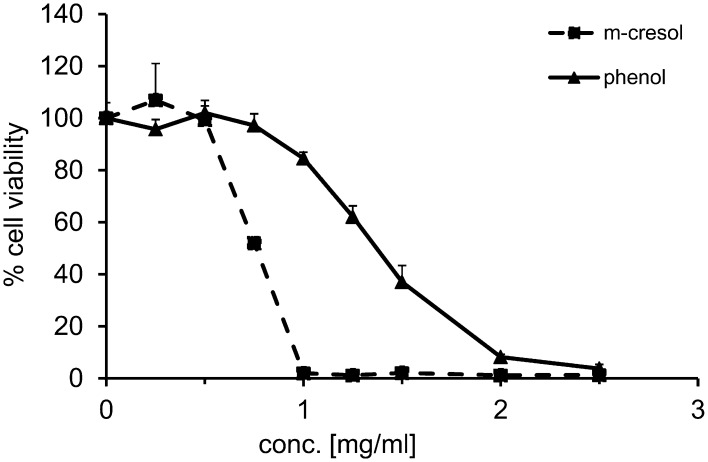
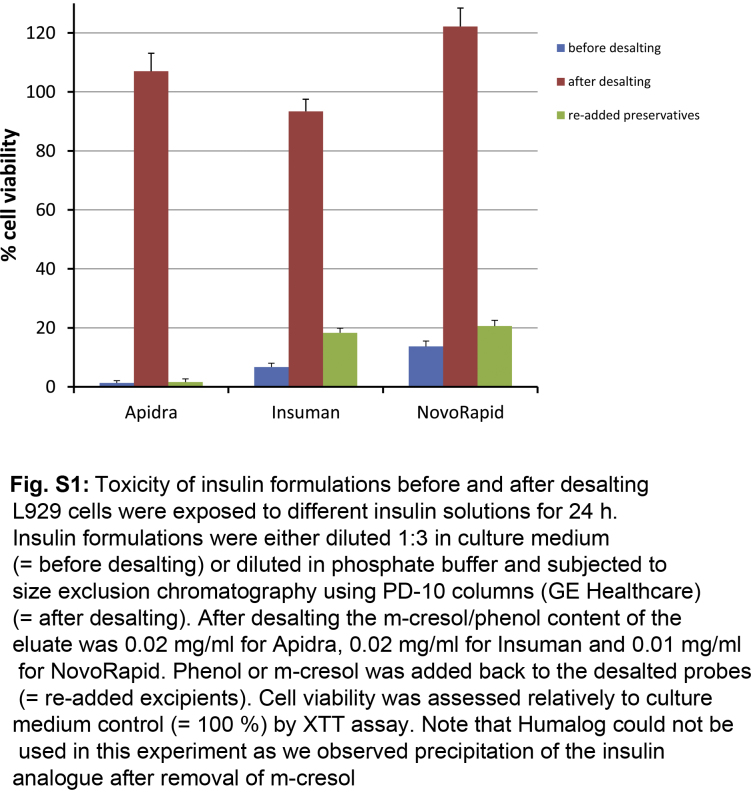
After this initial observation, we hypothesized that the excipients phenol and m-cresol caused the toxic effect of insulin solutions. To test this, all low molecular weight components were removed from different insulin formulations using desalting columns. The resulting eluate contained insulin or insulin analogs in phosphate buffer with only trace amounts of phenol or m-cresol. The XTT test revealed no cytotoxicity of these eluate. Upon re-addition of phenol or m-cresol, the eluate caused cell death to the same degree as the insulin formulations (Fig. S1). This indicated that the compounds causing cytotoxicity of insulin formulations are phenol and m-cresol. In addition, L929 cells were incubated with either pure phenol or m-cresol in a dose dependent manner (0–2.5 mg/ml) for 24 h, which revealed the threshold of toxicity at 0.6 mg/ml for m-cresol. For phenol the toxic threshold was 1.2 mg/ml indicating that it is less toxic to the cells than m-cresol (Fig. 2).
Fig. 2.
Concentration-dependent phenol and m-cresol toxicity in L929 cells. L929 cells were exposed to phenol or m-cresol in a dose dependent manner (0.0–2.5 mg/ml) for 24 h. XTT test was performed to analyze cell viability in relation to culture medium control (= 100%). Phenol: triangles and full line; m-cresol: squares and dashed line.
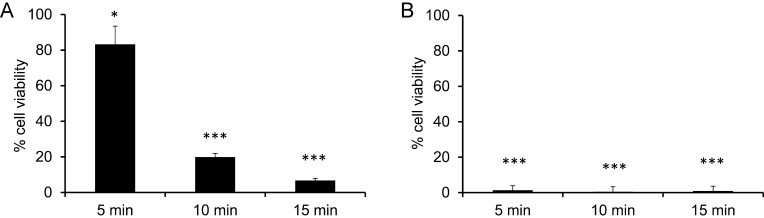
When patients infuse insulin into subcutaneous tissue, the contact time of insulin solutions with cells is limited, since small volumes are injected into the body that will be absorbed rapidly. To further analyze the toxic potential, L929 cells were incubated with undiluted insulin formulations (2.7–3.2 mg/ml phenol or m-cresol) for short time periods of 5, 10 or 15 min. Insulin solutions were then replaced by fresh cell culture medium and the cells were incubated for 24 h before cell viability was assessed. An incubation of only 5 min with undiluted insulin solutions was sufficient to induce cell death (Table 1). Cells treated with Insuman for 5 min showed 61.2% cell viability which declined to 34.4% after 15 min treatment. NovoRapid treated cells showed 49.2% viability or 10.8% viability after 5 min or 15 min treatment, respectively. Induction of cell death was somewhat stronger in Humalog and Apidra treated cells (less than 1% viable cells after 5 min treatment), both of which contain m-cresol as preservative. Incubation with excipient-free insulin only marginally affected cell viability. Using pure phenol or m-cresol for short term incubations the same degree of toxicity was observed as with insulin solutions. Phenol and m-cresol were used at a concentration of 3 mg/ml, which is equivalent to the concentration in undiluted insulin preparations. Cells treated with 3 mg/ml of m-cresol for 5 min were not viable. Incubation with phenol for 5 min resulted in 83.3% viability which declined to 19.9% after 10 min and 6.7% after 15 min incubation (Fig. 3).
Table 1.
Cell viability after short-term exposure with insulin formulations.
| Cell viability in % (SD) |
|||
|---|---|---|---|
| 5 min | 10 min | 15 min | |
| Apidra | 0.4 (0.22)# | 0.0 (0.17)# | 0.2 (0.16)# |
| Humalog | 0.5 (0.62)# | 0.2 (0.14)# | 0.0 (0.25)# |
| Insuman | 61.2 (5.72)# | 36.2 (3.42)# | 34.4 (2.68)# |
| NovoRapid | 49.2 (4.60)# | 20.9 (2.35)# | 10.8 (0.80)# |
| Insulin | 88.6 (2.21) | 92.5 (2.31) | 94.0 (2.26) |
L929 cells were incubated with undiluted insulin formulations for the indicated time periods. The solutions were then replaced by fresh culture medium and cell viability was assessed after 24 h. n = 6, # p < 0.001 (versus insulin).
Fig. 3.
Cell viability after short-term exposure of L929 cells with phenol or m-cresol. L929 cells were treated with 3 mg/ml phenol (a) or m-cresol (b) for 5, 10 or 15 min. The supernatant was replaced by fresh culture medium and XTT test was performed after 24 h. Cell viability is given in relation to culture medium control (= 100%). Error bars indicate S.D., n = 6, * p < 0.05, *** p < 0.001.
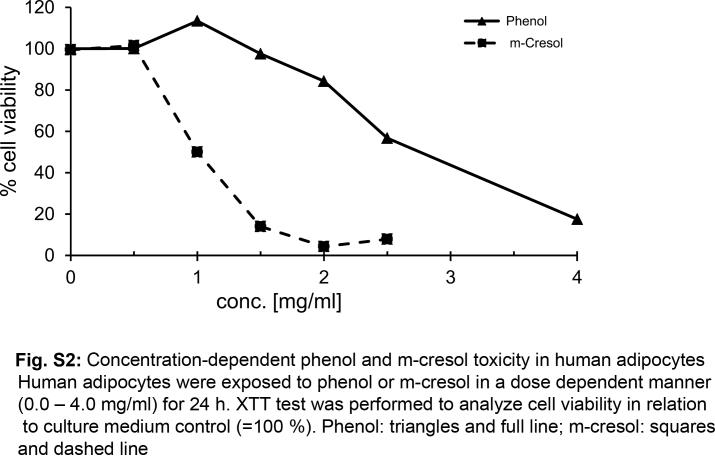
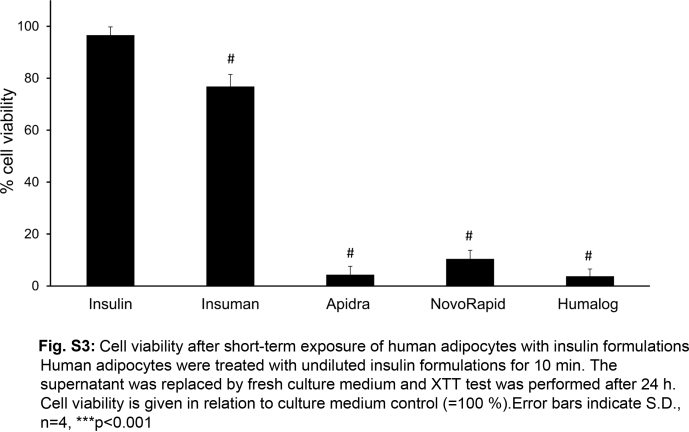
For cytotoxicity studies, rapidly dividing cell lines with high metabolic activity (like L929 cells) are the preferred model. However, we wanted to confirm the toxic effects of insulin formulations in a model that more closely resembles the situation in subcutaneous tissue. Therefore, human adipocytes were chosen as a second cell model. Human white pre-adipocytes from subcutaneous tissue were differentiated toward mature adipocytes. These highly differentiated cells showed no proliferation. Adipocytes were incubated with various concentrations (0.0–4.0 mg/ml) of either phenol of m-cresol for 24 h. The threshold of toxicity was 0.9 mg/ml for m-cresol and 2.3 mg/ml in case of phenol (Fig. S2). This data confirmed the results obtained with L929 cells that phenol is less toxic than m-cresol. Adipocytes tolerated higher concentrations of the preservatives compared to fibroblasts, which could be explained by their lower metabolic activity. To analyze the effects of short-term exposure with insulin formulations, adipocytes were incubated with undiluted insulin solutions for 10 min which were then replaced by fresh medium for 24 h before cell viability was assessed. The insulin formulations caused high levels of cell death, except for Insuman that was at the toxicity threshold (76% cell viability) and caused higher toxicity only when added to the cells > 15 min (Fig. S3). Excipient-free insulin solutions had no impact on cell viability. Taken together, these data confirm the toxicity of insulin formulations after very short contact-time in a second cell model.
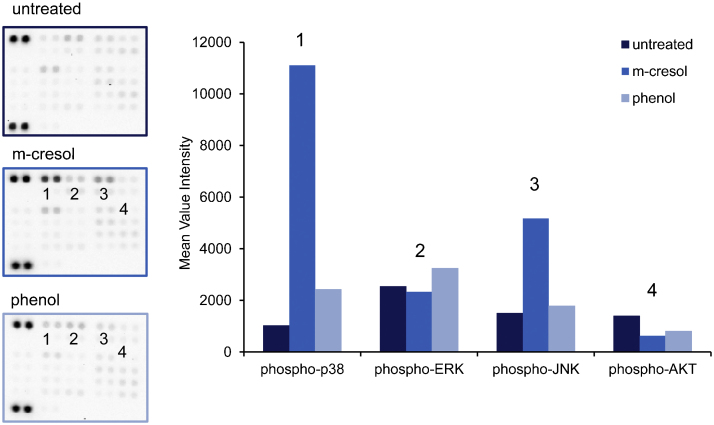
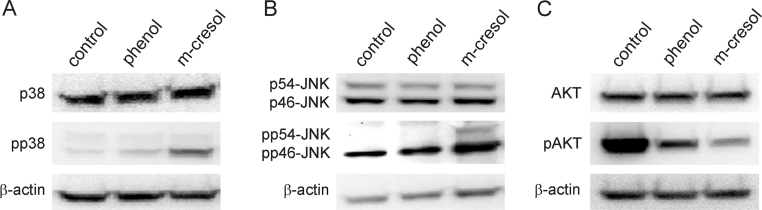
To identify molecular signaling pathways affected by m-cresol or phenol treatment, L929 cell-lysates were applied to a phospho-kinase array. This tool allowed us to detect the relative phosphorylation levels of 43 different kinases (Fig. 4). Compared to untreated cells, phosphorylation of the mitogen-activated protein (MAP)-kinases p38 and JNK was significantly induced by m-cresol, whereas extracellular signal-regulated kinase (ERK) phosphorylation was not altered. In phenol-treated cells there was a moderate up-regulation of p38 (2.4-fold), whereas there was no effect on ERK or JNK. The phosphorylation of AKT was reduced by approx. 50% in phenol or m-cresol-treated cells. The phosphorylation of other kinases was not significantly altered (Fig. 4, left panel).
Fig. 4.
Phospho-kinase array analysis of phenol or m-cresol treated L929 cells. L929 cells were treated with 1 mg/ml phenol or m-cresol for 30 min. Cell lysates were prepared and incubated with a phospho-kinase array. Untreated cells served as controls. Left: images of the arrays (untreated, phenol- or m-cresol-treated cells). Right: densitometric analysis of the arrays. The mean intensity of two array spots is depicted to illustrate the relative induction of kinase phosphorylation in phenol or m-cresol treated cells versus untreated cells.
These observations were confirmed by Western-blotting. Phosphorylation of p38 was strongly induced by treatment with m-cresol for 30 min. There was only a slight induction of phospho-p38 in phenol-treated cells. The amount of total p38 was unaffected (Fig. 5a). JNK-phosphorylation was induced by treating cells with m-cresol for 30 min. The phosphorylation was much weaker in phenol-treated cells. Total JNK-levels remained unaltered (Fig. 5b). Phosphorylation of AKT was reduced after 30 min treatment with phenol or m-cresol (Fig. 5c).
Fig. 5.
Western blot analysis of phenol or m-cresol treated L929 cells. L929 cells were incubated with 1 mg/ml phenol or m-cresol for 30 min. Cell lysates were subjected to Western blot analysis to determine levels of (a) p38 and phospho-p38 (pp38); (b) JNK and phospho-JNK (pp54-JNK or pp46-JNK); (c) AKT and phospho-AKT (pAKT). β-Actin served as loading control.
We hypothesized that, in addition to the cytotoxic effect, phenol/m-cresol might activate immune cells, thereby promoting an inflammatory response. For this purpose the monocytic cell line THP-1 was chosen as a model. The toxic threshold for THP-1 cells was determined first (>0.3 mg/ml for m-cresol; >0.45 mg/ml for phenol). In the following experiments, THP-1 cells were incubated with sub-toxic levels of phenol/m-cresol (0.2 mg/ml) to exclude release of pro-inflammatory mediators from disintegrated cells. This is equivalent to the concentration of phenol/m-cresol in a 1:15 dilution of commercial insulin preparations.
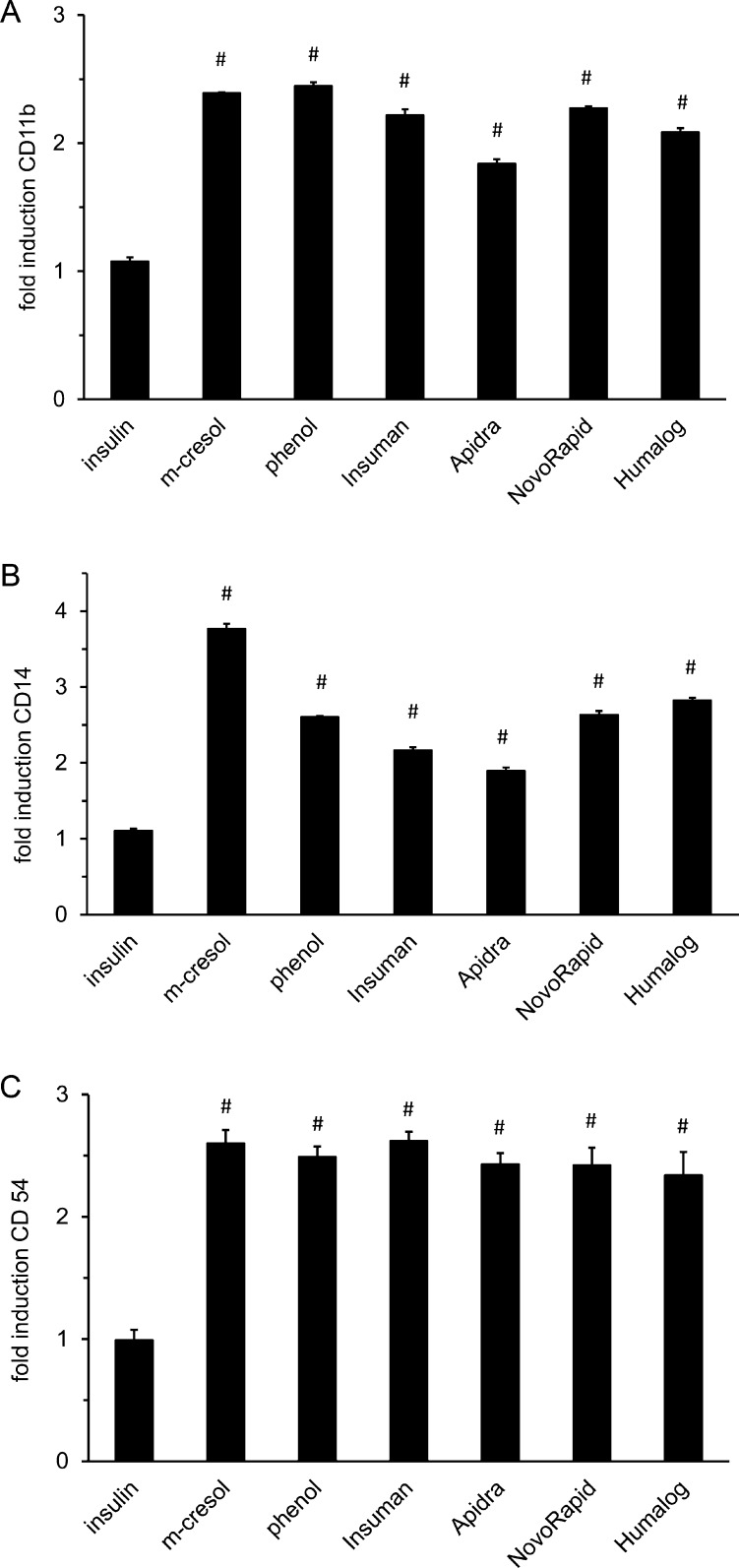
As markers of inflammatory cell activation we explored the expression of the cell surface proteins CD54, CD11b and CD14. THP-1 cells were subjected to sub-toxic doses of phenol, m-cresol or insulin solutions for 24 h. Insulin solutions caused a 2–3-fold induction of CD11b, CD14 and CD54 surface expression, while insulin without preservatives had no effect (Fig. 6a–c). CD11b and CD54 expression were induced 2–3-fold by phenol and m-cresol. CD14 was induced almost 4-fold by m-cresol and 2.5-fold by phenol (Fig. 6b). The elevated expression of CD54, CD11b and CD14 indicated that THP-1 cells were activated by m-cresol or phenol.
Fig. 6.
Expression of inflammatory cell-surface proteins in THP-1 cells. THP-1 cells were incubated with different insulin solutions, phenol or m-cresol for 24 h. Cell surface expression of (a) CD11b; (b) CD14; (c) CD54 was determined by incubation with fluorophor-conjugated antibodies and subsequent flow cytometric analysis. Data is presented as relative fluorescence intensity compared to untreated control cells (set to 1). Error bars indicate S.D., n = 3, # p < 0.001.
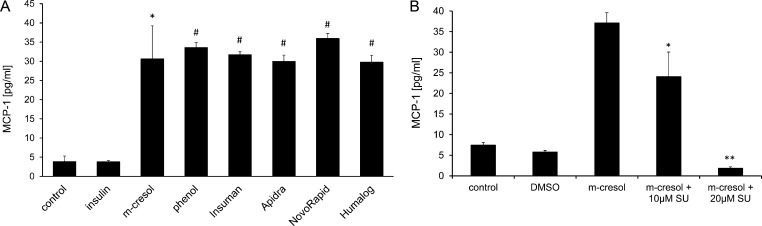
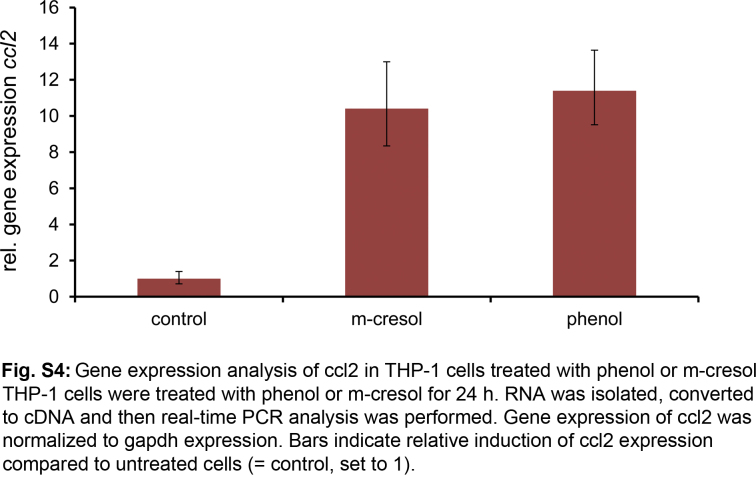
Next we assessed if THP-1 activation resulted in release of pro-inflammatory cytokines. The cells were incubated with 0.2 mg/ml of phenol or m-cresol for 24 h and thereafter the secretion of various pro-inflammatory cytokines into the supernatant was determined. We did not observe significantly more IL-1β, IL-6, IL-8, IL-12, TNFα or Mip-1α in treated cells as compared to untreated cells (Table S1). In contrast, MCP-1 secretion increased from <5 pg/ml in untreated cells up to 30–40 pg/ml in phenol and m-cresol treated THP-1 cells (Fig. 7a). Likewise, incubating THP-1 cells with sub-toxic levels of Apidra, Humalog, NovoRapid or Insuman resulted in significant release of MCP-1 into the supernatant, whereas insulin solution lacking excipients had no effect (Fig. 7a). To analyze whether MCP-1 induction occurred on the gene transcription level, RNA was isolated from m-cresol- or phenol-treated THP-1 cells and cDNA was generated by reverse transcription. Real-time PCR amplification was used to determine expression of the chemokine ligand 2 (ccl2) gene which codes for the MCP-1 protein. A 10.4-fold or 11.4-fold induction of ccl2 gene expression was observed after treatment with m-cresol or phenol, respectively (Fig. S4).
Fig. 7.
MCP-1 release of THP-1 cells treated with insulin solutions, m-cresol or phenol. (a) THP-1 cells were incubated with insulin solutions, phenol or m-cresol for 24 h. The supernatant was collected and MCP-1 release was determined using an immunoassay (cytometric bead assay). Error bars indicate S.D., n = 3, * p < 0.05, *** p < 0.001. (b) THP-1 cells were incubated with m-cresol or m-cresol + JNK inhibitor SU3327 (SU) for 24 h. Release of MCP-1 into the supernatant was analyzed by immunoassay (cytometric bead assay). Untreated or vehicle (DMSO) treated cells served as controls. Error bars indicate S.D., n = 3, * p < 0.05, ** p < 0.01.
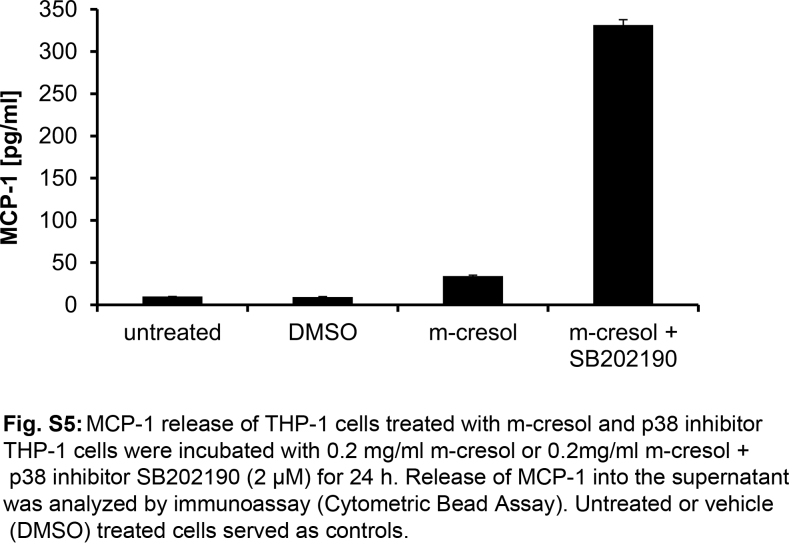
As it is known that MCP-1 expression is regulated by the MAP kinases p38 and JNK in monocytes and other cell-types [20], we tested whether specific inhibitors of these kinases would affect MCP-1 release after m-cresol treatment in THP-1 cells. The cells were incubated with 0.2 mg/ml m-cresol for 24 h in the presence of the JNK inhibitor SU3327 or the p38-inhibitor SB202190. At a concentration of 10 μM, the JNK inhibitor SU3327 partially inhibited MCP-1 release from m-cresol treated cells. At a concentration of 20 μM, MCP-1 release was fully inhibited (Fig. 7b). In contrast, the p38 Inhibitor SB202190 rather increased m-cresol-induced MCP-1 release (Fig. S5). This data indicated that m-cresol induced MCP-1 expression in THP-1 cells via the JNK signaling pathway.
4. Discussion
We have shown that commercial insulin solutions have a cytotoxic potential in vitro. The toxic effect was assessed using the fibroblastic cell line L929, primary human adipocytes and monocytic THP-1 cells. Insulin solution without excipients did not cause cell death, whereas pure phenol or m-cresol displayed the same level of toxicity as the commercial insulin formulations indicating that they are the main cause for toxicity. Importantly, cytotoxicity was detected even after very short exposure-time of only 5 min in L929 cells and 10 min in human adipocytes. This emphasizes the relevance of our findings to the real life application of phenol/m-cresol containing drugs that are injected into the subcutaneous tissue in small volumes forming a transient depot before they are absorbed. Using cell cultures has limitations since there are important differences between the in vitro situation and the subcutaneous tissue in vivo. In the culture dish, there is only one cell-type present in a single layer. The toxic compounds tested have direct access to the cells and the concentration will be uniform throughout the dish. In contrast, the subcutaneous tissue is a complex three-dimensional structure consisting of various cell types (fibroblasts, adipocytes, immune cells and others), extracellular matrix as well as lymphatics and blood vessels. The toxic compound injected will initially form a depot. By diffusion and convection the compound spreads in the tissue and will eventually be absorbed via the lymphatics and the blood stream. This results in an anisotropic distribution of the toxic compound and formation of concentration gradients.
Phenol and/or m-cresol are essential components of insulin solutions and the concentrations applied by insulin injections are considered to be save [15], [18], [17]. By CSII the tissue is repetitively subjected to high local phenol/m-cresol concentrations. There is only little information available how this will affect the subcutaneous tissue locally. In cultured rat liver slices the toxic effect of m-cresol was apparent at concentrations above 5 mM (= 0.54 mg/ml) [21], but further detailed information on cytotoxicity or pro-inflammatory effects of m-cresol is lacking. The cytotoxicity of phenol was analyzed in several cell types among them L929 cells, human fibroblasts and keratinocytes [22], [23], [24], [25]. However, short-term exposure of cells with phenol or m-cresol was not investigated. The cytotoxic effects of phenol may be due to oxidation of phenolic compounds to free radical intermediates known as phenoxyl radicals [26]. It was shown that free radicals formed from phenol are capable of inducing oxidative stress and causing an inflammatory response in the skin [27]. Furthermore, phenol induced arachidonic acid, TNFα and IL-8 release in cultured keratinocytes [23], [24], [28] and IL-1β in cultured epidermal cells [27].
We have shown that the phosphorylation of MAP kinases p38 and JNK is strongly induced by m-cresol in L929 cells while induction by phenol is much weaker. Both kinases have essential roles in cellular stress-signaling [29]. P38 activation leads to upregulation and activation of a variety of pro-inflammatory mediators [30]. Sustained JNK activation triggers apoptosis by expression of pro-apoptotic genes and by modulating the function of pro- and anti-apoptotic proteins in mitochondria [31]. Additionally, phenol or m-cresol treated cells showed a strong reduction of AKT phosphorylation. AKT is one of the most critical pathways regulating cell survival. AKT provides pro-survival signals and blocks apoptotic stimuli by modulating several downstream targets [32]. The strong down-regulation of AKT phosphorylation in phenol or m-cresol treated cells is in line with the induction of cell death by these compounds. Collectively, these data show that specific signaling pathways are modulated by m-cresol or phenol treatment. These pathways provide a molecular mechanism explaining the patho-physiological responses to these excipients.
In THP-1 cells, we observed upregulation of inflammatory cell surface proteins CD11b (an integrin and receptor for complement C3), CD14 (receptor for bacterial lipopolysaccharide) and the cell adhesion molecule CD54. In line with the enhanced cell activation, we assumed that phenol/m-cresol could enhance inflammatory responses by triggering the release of cytokines. The pro-inflammatory cytokine MCP-1 was selectively secreted after phenol/m-cresol treatment, while several other well-known cytokines were not secreted. So far it has not been reported that MCP-1 is induced by phenol or m-cresol. MCP-1 is the main factor mediating macrophage recruitment to the site of injury after wounding [33]. The ccl2 gene (which codes for the MCP-1 protein) is regulated by AP-1 transcription factors that are targets of p38 and JNK signaling [20]. Accordingly, JNK or p38 signaling was reported to mediate MCP-1 release in THP-1 cells and other cell types [30], [34], [35], [36].
Using the JNK-inhibitor SU3327 we clearly showed the m-cresol induced MCP-1 release in THP-1 cells is mediated via the JNK pathway. The fact that sub-toxic doses of phenol/m-cresol activated THP-1 cells and increased MCP-1 secretion significantly contributes to the understanding of local tissue reactions at the infusion sites of subcutaneously delivered drugs. The reason for the high prevalence of skin irritation at the infusion site remained unknown. We propose a model in which the insertion trauma by the infusion needle is a first trigger for inflammatory cell recruitment. Indeed, it has been shown by insertion of microdialysis catheters into subcutaneous adipose tissue that within a few hours cytokines are released from the wounded tissue [37], [38], [39]. As shown here, even short exposure of phenol/m-cresol induces cell death which further stimulates inflammatory and repair processes at the infusion site. Additionally, phenol/m-cresol induce monocyte activation and MCP-1 release to sustain the inflammatory response. These processes will likely lead to the clinical signs of skin irritation/inflammation and are therefore, at least in part, responsible for the occurrence of skin complications in CSII therapy. Clearly, this model has to be tested in a clinical trial. Health care professionals and patients using CSII should be aware that it is important to change the infusion set at least every 2–3 days and rotate the infusion site. We hypothesize that this limits tissue inflammation as the toxic stimulus by the excipients is removed before more monocytes are recruited and a sustained inflammation at the infusion site is triggered.
Conflict of interest
AHL, BS and CW are employed by Roche Diagnostics GmbH. DK is employed by Metecon GmbH.
Transparency document
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.11.019.
Appendix A. Supplementary data
References
- 1.Jeitler K., Horvath K., Berghold A., Gratzer T.W., Neeser K., Pieber T.R., Siebenhofer A. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia. 2008;51:941–951. doi: 10.1007/s00125-008-0974-3. [DOI] [PubMed] [Google Scholar]
- 2.Bode B.W. Comparison of pharmacokinetic properties, physicochemical stability, and pump compatibility of 3 rapid-acting insulin analogues-aspart, lispro, and glulisine. Endocr. Pract. 2011;17:271–280. doi: 10.4158/EP10260.RA. [DOI] [PubMed] [Google Scholar]
- 3.Ponder S.W., Skyler J.S., Kruger D.F., Matheson D., Brown B.W. Unexplained hyperglycemia in continuous subcutaneous insulin infusion: evaluation and treatment. Diabetes Educ. 2008;34:327–333. doi: 10.1177/0145721708315682. [DOI] [PubMed] [Google Scholar]
- 4.Conwell L.S., Pope E., Artiles A.M., Mohanta A., Daneman A., Daneman D. Dermatological complications of continuous subcutaneous insulin infusion in children and adolescents. J. Pediatrics. 2008;152:622–628. doi: 10.1016/j.jpeds.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Jarosz-Chobot P., Nowakowska M., Polanska J. Seeking the factors predisposing to local skin inflammatory state development in children with type 1 diabetes (T1DM) treated with continuous subcutaneous insulin infusion (CSII) Exp. Clin. Endocrinol. Diabetes. 2007;115:179–181. doi: 10.1055/s-2007-970593. [DOI] [PubMed] [Google Scholar]
- 6.Schober E., Rami B. Dermatological side effects and complications of continuous subcutaneous insulin infusion in preschool-age and school-age children. Pediatric Diabetes. 2009;10:198–201. doi: 10.1111/j.1399-5448.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- 7.Johansson U.B., Adamson U., Lins P.E., Wredling R. Patient management of long-term continuous subcutaneous insulin infusion. J. Adv. Nurs. 2005;51:112–118. doi: 10.1111/j.1365-2648.2005.03475.x. [DOI] [PubMed] [Google Scholar]
- 8.Patel P.J., Benasi K., Ferrari G., Evans M.G., Shanmugham S., Wilson D.M., Buckingham B.A. Randomized trial of infusion set function: steel versus teflon. Diabetes Technol. Thera. 2014;16:15–19. doi: 10.1089/dia.2013.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickup J.C., Yemane N., Brackenridge A., Pender S. Nonmetabolic complications of continuous subcutaneous insulin infusion: a patient survey. Diabetes Technol. Thera. 2014;16:145–149. doi: 10.1089/dia.2013.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Bon A.C., Bode B.W., Sert-Langeron C., DeVries J.H., Charpentier G. Insulin glulisine compared to insulin aspart and to insulin lispro administered by continuous subcutaneous insulin infusion in patients with type 1 diabetes: a randomized controlled trial. Diabetes Technol. Thera. 2011;13:607–614. doi: 10.1089/dia.2010.0224. [DOI] [PubMed] [Google Scholar]
- 11.Guinn T.S., Bailey G.J., Mecklenburg R.S. Factors related to discontinuation of continuous subcutaneous insulin-infusion therapy. Diabetes Care. 1988;11:46–51. doi: 10.2337/diacare.11.1.46. [DOI] [PubMed] [Google Scholar]
- 12.Mecklenburg R.S., Benson E.A., Benson J.W., Jr., Fredlund P.N., Guinn T., Metz R.J., Nielsen R.L., Sanner C.A. Acute complications associated with insulin infusion pump therapy. Report of experience with 161 patients. JAMA. 1984;252:3265–3269. [PubMed] [Google Scholar]
- 13.Heinemann L., Krinelke L. Insulin infusion set: the Achilles heel of continuous subcutaneous insulin infusion. J. Diabetes Sci. Technol. 2012;6:954–964. doi: 10.1177/193229681200600429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renard E., Guerci B., Leguerrier A.M., Boizel R. Lower rate of initial failures and reduced occurrence of adverse events with a new catheter model for continuous subcutaneous insulin infusion: prospective, two-period, observational, multicenter study. Diabetes Technol. Thera. 2010;12:769–773. doi: 10.1089/dia.2010.0073. [DOI] [PubMed] [Google Scholar]
- 15.Dunn M.F. Zinc-ligand interactions modulate assembly and stability of the insulin hexamer – a review. Biometals. 2005;18:295–303. doi: 10.1007/s10534-005-3685-y. [DOI] [PubMed] [Google Scholar]
- 16.Groenning M., Frokjaer S., Vestergaard B. Formation mechanism of insulin fibrils and structural aspects of the insulin fibrillation process. Curr. Protein Pept. Sci. 2009;10:509–528. doi: 10.2174/138920309789352038. [DOI] [PubMed] [Google Scholar]
- 17.Cosmetic Ingredient Review Expert Panel Final report on the safety assessment of sodium p-chloro-m-cresol, p-chloro-m-cresol, chlorothymol, mixed cresols, m-cresol, o-cresol, p-cresol, isopropyl cresols, thymol, o-cymen-5-ol, and carvacrol. Int. J. Toxicol. 2006;25(Suppl. 1):29–127. doi: 10.1080/10915810600716653. [DOI] [PubMed] [Google Scholar]
- 18.Environmental Protection Agency . U.S. Environmental Protection Agency; Washington, DC: 2002. Toxicological Review of Phenol. EPA/635/R-02/006. [Google Scholar]
- 19.van Faassen I., Razenberg P.P., Simoons-Smit A.M., van der Veen E.A. Carriage of Staphylococcus aureus and inflamed infusion sites with insulin-pump therapy. Diabetes Care. 1989;12:153–155. doi: 10.2337/diacare.12.2.153. [DOI] [PubMed] [Google Scholar]
- 20.Panee J. Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine. 2012;60:1–12. doi: 10.1016/j.cyto.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson D.C., Perera K., Fisher R., Brendel K. Cresol isomers: comparison of toxic potency in rat liver slices. Toxicol. Appl. Pharmacol. 1994;125:51–58. doi: 10.1006/taap.1994.1048. [DOI] [PubMed] [Google Scholar]
- 22.Jover R., Ponsoda X., Castell J.V., Gomez-Lechon M.J. Acute cytotoxicity of ten chemicals in human and rat cultured hepatocytes and in cell lines: correlation between in vitro data and human lethal concentrations. Toxicol. In Vitro. 1994;8:47–54. doi: 10.1016/0887-2333(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 23.Muller-Decker K., Furstenberger G., Marks F. Keratinocyte-derived proinflammatory key mediators and cell viability as in vitro parameters of irritancy: a possible alternative to the Draize skin irritation test. Toxicol. Appl. Pharmacol. 1994;127:99–108. doi: 10.1006/taap.1994.1144. [DOI] [PubMed] [Google Scholar]
- 24.Newby C.S., Barr R.M., Greaves M.W., Mallet A.I. Cytokine release and cytotoxicity in human keratinocytes and fibroblasts induced by phenols and sodium dodecyl sulfate. J. Investig. Dermatol. 2000;115:292–298. doi: 10.1046/j.1523-1747.2000.00056.x. [DOI] [PubMed] [Google Scholar]
- 25.Pace D.M., Elliott A. Effects of acetone and phenol on established cell lines cultivated in vitro. Cancer Res. 1962;22:107–112. [PubMed] [Google Scholar]
- 26.Shvedova A.A., Kommineni C., Jeffries B.A., Castranova V., Tyurina Y.Y., Tyurin V.A., Serbinova E.A., Fabisiak J.P., Kagan V.E. Redox cycling of phenol induces oxidative stress in human epidermal keratinocytes. J. Investig. Dermatol. 2000;114:354–364. doi: 10.1046/j.1523-1747.2000.00865.x. [DOI] [PubMed] [Google Scholar]
- 27.Murray A.R., Kisin E., Castranova V., Kommineni C., Gunther M.R., Shvedova A.A. Phenol-induced in vivo oxidative stress in skin: evidence for enhanced free radical generation, thiol oxidation, and antioxidant depletion. Chem. Res. Toxicol. 2007;20:1769–1777. doi: 10.1021/tx700201z. [DOI] [PubMed] [Google Scholar]
- 28.Wilmer J.L., Burleson F.G., Kayama F., Kanno J., Luster M.I. Cytokine induction in human epidermal keratinocytes exposed to contact irritants and its relation to chemical-induced inflammation in mouse skin. J. Investig. Dermatol. 1994;102:915–922. doi: 10.1111/1523-1747.ep12383512. [DOI] [PubMed] [Google Scholar]
- 29.Kyriakis J.M., Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 30.Schieven G.L. The p38alpha kinase plays a central role in inflammation. Curr. Top. Med. Chem. 2009;9:1038–1048. doi: 10.2174/156802609789630974. [DOI] [PubMed] [Google Scholar]
- 31.Dhanasekaran D.N., Reddy E.P. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song G., Ouyang G., Bao S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell. Mol. Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillitzer R., Goebeler M. Chemokines in cutaneous wound healing. J. Leukocyte Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- 34.Giri R.K., Selvaraj S.K., Kalra V.K. Amyloid peptide-induced cytokine and chemokine expression in THP-1 monocytes is blocked by small inhibitory RNA duplexes for early growth response-1 messenger RNA. J. Immunol. 2003;170:5281–5294. doi: 10.4049/jimmunol.170.10.5281. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto K., Ichiyama T., Hasegawa M., Hasegawa S., Matsubara T., Furukawa S. Cysteinyl leukotrienes induce monocyte chemoattractant protein-1 in human monocyte/macrophages via mitogen-activated protein kinase and nuclear factor-kappaB pathways. Int. Arch. Aller. Immunol. 2009;149:275–282. doi: 10.1159/000199724. [DOI] [PubMed] [Google Scholar]
- 36.Shanmugam N., Reddy M.A., Guha M., Natarajan R. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes. 2003;52:1256–1264. doi: 10.2337/diabetes.52.5.1256. [DOI] [PubMed] [Google Scholar]
- 37.Clausen T.S., Kaastrup P., Stallknecht B. Proinflammatory tissue response and recovery of adipokines during 4 days of subcutaneous large-pore microdialysis. J. Pharmacol. Toxicol. Methods. 2009;60:281–287. doi: 10.1016/j.vascn.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Murdolo G., Herder C., Wang Z., Rose B., Schmelz M., Jansson P.A. In situ profiling of adipokines in subcutaneous microdialysates from lean and obese individuals. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1095–E1105. doi: 10.1152/ajpendo.90483.2008. [DOI] [PubMed] [Google Scholar]
- 39.Pachler C., Ikeoka D., Plank J., Weinhandl H., Suppan M., Mader J.K., Bodenlenz M., Regittnig W., Mangge H., Pieber T.R., Ellmerer M. Subcutaneous adipose tissue exerts proinflammatory cytokines after minimal trauma in humans. Am. J. Physiol. Endocrinol. Metab. 2007;293:E690–E696. doi: 10.1152/ajpendo.00034.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.