Abstract
The effect of Roundup® on adrenal gland steroidogenesis and signaling pathway associated with steroid production was investigated. Doses of 10, 50, 100 and 250 mg/kg bw/d Roundup® were administered for two weeks to adult male rats. The 10 mg/kg bw/d dose which reduced circulatory corticosterone levels, but did not change food consumption and body weight, was selected for further study. The expression of cholesterol receptor (low density lipoprotein receptor), de novo cholesterol synthesis enzyme (3-hydroxy-3-methylglutaryl-coenzyme A synthase), hormone-sensitive lipase, steroidogenic acute regulatory protein (StAR) mRNA and phosphorylated form was decreased. Adrenocorticotropic hormone receptor (ACTH), melanocortin-2 receptor, expression was not changed but circulatory ACTH levels and adrenal cortex protein kinase A (PKA) activity were reduced. Surprisingly, exogenous ACTH treatment rescued steroidogenesis in Roundup®-treated animals. Apoptosis was evident at 250 mg/kg bw/d, but not at 10 mg/kg bw/d dose. These results suggest that Roundup® may be inhibitory to hypothalamic–pituitary axis leading to reduction in cyclic adenosine monophosphate (cAMP)/PKA pathway, StAR phosphorylation and corticosterone synthesis in the adrenal tissue.
Abbreviations: EDC, endocrine disrupting chemical; LD50, lethal dose, 50%; Ldlr, low density lipoprotein receptor; Sr-b1, scavenger receptor class B member 1; Hmgcs, 3-hydroxy-3-methylglutaryl-coenzyme A synthase; Hmgcr, 3-hydroxy-3-methylglutaryl-CoA reductase; Hsl, hormone-sensitive lipase; StAR, steroidogenic acute regulatory protein; Creb, cAMP response element-binding protein; ACTH, adrenocorticotropic hormone; Mc2r, melanocortin-2 receptor; PKA, protein kinase A; cAMP, cyclic adenosine monophosphate; L:D cycle, light–dark cycle; RIA, radioimmunoassay; ELISA, enzyme-linked immunosorbent assay; EIA, enzyme Immunoassay; qPCR, quantitative real-time PCR; DPX, distrene, plasticiser, xylene; DAPI, 4′,6-diamidino-2-phenylindole; RIPA buffer, radioimmunoprecipitation assay buffer; SDS PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; EDTA, ethylenediaminetetraacetate; EGTA, ethylene glycol tetraacetate; β ME, beta mercaptoethanol; PBS, phosphate buffer saline; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; TdT, terminal deoxynucleotidyl transferase; SD, standard deviation
Keywords: Endocrine disruptor, Glyphosate, Steroidogenesis, StAR, Adrenal gland, Rat
1. Introduction
Agricultural advancements have increased production and correspondingly increased the usage and release of herbicides into the environment. Among the herbicides, the glyphosate-based herbicide Roundup® is most extensively used world over [14]. Roundup® is non-selective and broad spectrum herbicide utilized in agricultural fields, gardens, play grounds, road sides etc. [10]. The half life of Roundup® is ∼47 days in soil and up to 90 days in water with low microbial metabolism and disintegration [18], [37], [39]. In rats, Roundup® whole body pharmacokinetics is biphasic for single 10 mg/kg bw dose with half-life of the alpha phase is 6 h and 79–106 h for beta phase [43]. Roundup® and its metabolite, aminomethyl-phosphonic acid, have been detected in water and crops [11], [25], [26], [35]. Therefore, there is increased probability of Roundup® exposure to animals and human, and it becomes of interest to study its toxic effects, if any.
Glyphosate or its formulation Roundup® acts via specific inhibition of plant enzyme 5-enolpyruvylshikimate-3-phosphate synthase which is essential for synthesis of aromatic amino acids [19], [34], [36] and thus, considered non toxic to animals. However in recent past, several studies have suggested glyphosate toxic effects such as carcinogen [28], [38], [40], teratogen [12], [13] and as an endocrine disruptor (ED). The endocrine disrupting chemicals (EDC) represent a broad class of exogenous substances that adversely affect the endocrine system by interfering with hormone biosynthesis, metabolism or action [23]. As an ED, glyphosate and its formulation, Roundup® was reported to decrease testosterone hormone levels in adult rats [9]. The prenatal exposure of glyphosate disrupted the masculinization process and caused endocrine dysfunction in reproductive parameters of male offspring [32]. Moreover, various formulations of glyphosate including Roundup® were reported to disrupt aromatase activity, enzyme required for estrogen synthesis, in human liver HepG2 cells [15]. The human placental JEG3 cells treated with Roundup® altered aromatase mRNA levels and enzymatic activity by interacting with the active site of the purified enzyme [31]. A study involving MA-10 Leydig tumor cell line reported down regulation of StAR mRNA levels, a key regulatory steroidogenic gene, and dibutyryl cAMP-stimulated progesterone production upon treatment [42]. It has been observed that the commercial formulation had more adverse effects than the active ingredient i.e., glyphosate [3]. The EDC effects of Roundup® in male reproductive system have been described; however, studies detailing EDC effect on the adrenal gland steroidogenesis have not been reported in animals.
In the present study, experiments have been conducted to examine effects of Roundup® on adrenal steroidogenesis at systemic as well as at the tissue level. Different doses of Roundup® were orally administered to adult male rats for 14 days daily although, only the lowest dose required for disrupting the major adrenal gland steroid hormone i.e., corticosterone level was selected for further detailed study. The EDC effect of Roundup® was examined on important regulatory genes for steroidogenesis; StAR and p450scc, the steroid precursor, cholesterol levels, and cholesterol homeostasis genes and the signaling involved. An ACTH challenge experiment was also performed to evaluate Roundup® action to be via hypothalamic pituitary axis or directly upon the gland.
2. Material and methods
2.1. Chemicals and antibodies
Glyphosate formulation, Roundup® was procured from Monsanto India Ltd., Mumbai, India. Porcine ACTH, TRIzol®, custom made primers, oligo(dT) and dNTPs were obtained from Sigma–Aldrich Co. (Bangalore, India). The kits purchased for various hormone assays were as follows: rat corticosterone ELISA from Neogen (Lansing, MI), rat corticosterone EIA from Cayman (Ann Arbor, MI), testosterone RIA from Immunotech (Marseille, France) and rat ACTH Ultra-sensitive lumELISA kit from Calbiotech Inc (Spring Valley, CA). Amplex® red cholesterol assay kit and SYBR Green PCR Master Mix were purchased from Molecular Probes, Life Technologies (Carlsbad, CA). Reverse transcriptase (RevertAid) was from Thermo scientific (Waltham, MA). DNase 1 (RNase free) was from New England Biolabs Inc. (Ipswich, MA). PVDF membrane (Immobilon pSQ) was procured from Millipore (Billerica, MA). Protein molecular weight markers (PageRuler™ Prestained Protein Ladder) and Western blotting detection reagents (SuperSignal™ West Femto Maximum Sensitivity Substrate) were from Thermo Fisher Scientific Inc. (Waltham, MA). Antibody against pCREB (Ser133) (#9198), CREB (#9197), cleaved Caspase 3 (#9915), goat anti-rabbit IgG, HRP-linked Antibody (#7074) were from Cell Signaling Technology (Danvers, MA) and β-actin (#A3854) from Sigma–Aldrich Co. (Bangalore, India). Antibodies for pStAR and StAR were kind gifts from Professor Steven King (Baylor College of Medicine, Houston, TX) and Professor DM Stocco (Texas Tech University Health Sciences Center, Lubbock, TX). HDL and LDL/VLDL quantitation kit was procured from Sigma–Aldrich Co. (Bangalore, India). All other chemicals, unless otherwise noted were purchased from Sigma–Aldrich Co. (Bangalore, India) or sourced locally.
2.2. Animal experiments
All procedures in animals were approved by the Institutional Animal Ethical Committee, Indian Institute of Science, Bangalore, India. In this study, 2–2.5 months old male Harlan Wistar rats were used. One rat per cage was housed in the room with L:D cycle of 12 h each under a controlled temperature of 24–26 °C. Rats were allowed free access to standard chow diet and drinking water ad libitum. For this study, rats were randomly divided into five groups with 5 or more animals per group. Rats were administered with 24.4, 121.9, 244 and 609.8 μl of Roundup® (41% w/w) which corresponds to 10, 50, 100 and 250 mg/kg bw/d of glyphosate, respectively. The highest dose tested was well below LD50 of 4900 mg/kg bw. Roundup® was dissolved in deionized water to make the total volume of 300 μl except for 250 mg/kg bw/d dose, in which Roundup® was administered directly. The vehicle (0 mg/kg bw/d) group received 300 μl of deionized water only. The treatments were oral, once daily (0800–0900 h) for 14 days. Body weight and food consumption were monitored twice weekly. Food consumption was calculated by subtracting food pellet weight remaining in the cage mesh from the total food pellet weight provided to the each rat cage mesh. A total 10 rats for control, n = 10 rats for 10 mg/kg, n = 5 rats for 50 mg/kg, n = 10 rats for 100 mg/kg and n = 10 rats for 250 mg/kg bw/d dose group were utilized in the study. On day 15 of treatment, animals were weighed and anesthetized with 50 mg/kg bw/d pentobarbitone sodium (Sigma Chemical Co., St. Louis, MO) and blood was collected by cardiac puncture. Plasma was separated by centrifugation at 2000 × g, 4 °C for 15 min and stored at −20 °C until analyzed. The anaesthetized animals were killed by cervical dislocation, adrenal glands were dissected out, weighed, transferred to neutral buffered formaldehyde (NBF) solution or snap frozen in liquid nitrogen and stored in −70 °C freezer until analysis. To examine the effect of exogenous adrenocorticotropic hormone (ACTH) on adrenal gland steroidogenesis, a standardized dose of 5 IU of Porcine ACTH was injected i.v., based on the protocol reported by others [21], [30], [41] to vehicle (n = 4 rats) as well as Roundup® 10 mg/kg bw/d (n = 3 rats) treated animals. Blood sample and adrenal glands were collected after 60 min of ACTH treatment.
2.3. Hormone assays
Two different kits have been utilized for determining plasma corticosterone levels. The steroids extraction from plasma was carried out by using diethyl ether or methylene chloride (Merck, Billerica, MA) as per requirement of the kits. The corticosterone levels obtained from different sources gave similar concentration of corticosterone. The inter- and intra-coefficient of variations of assay were <15%. Plasma concentrations of testosterone were measured by testosterone RIA kit according to the manufacturer's protocol. ACTH in plasma was measured using luminescence based ELISA kit for rats.
2.4. Cholesterol assay
Adrenal gland tissue lysate was prepared by homogenizing 0.5 mg tissue in unit ml of 10% SDS containing phosphate buffered saline (PBS) (Sigma–Aldrich Co., Bangalore, India). Tissue debris was removed by centrifugation. The tissue lysate and plasma were analyzed for total cholesterol and esterified cholesterol by using the Amplex® red cholesterol assay kit as per the manufacturer’s instructions. Briefly, plasma or tissue sample were mixed with equal volume of Amplex® red working reagent with and without cholesterol esterase. The reaction mixture was then incubated for 30 min at 37 °C in the dark. The fluorescence values were read at an excitation wavelength of 545 nm and an emission wavelength of 590 nm (Tecan Infinite F200 Microplate Reader, Männedorf, Switzerland). A series of cholesterol standards were prepared that were provided in the kit and ran alongside the plasma and tissue lysate samples. Plasma HDL (high-density lipoprotein) and LDL (low-density lipoprotein) were analyzed by commercially available HDL and LDL/VLDL quantitation kit according to the manufacturer’s instruction.
2.5. qPCR analysis
mRNA expression of key regulatory receptors, enzymes and carrier proteins involved in the cholesterol homeostasis and steroidogenesis were determined by qPCR analysis as previously described from the laboratory Priyanka et al. [46] using ABI 7500 Real-Time PCR instrument. Briefly, total RNA was isolated from adrenal glands by TRIzol® method, treated with DNaseI before performing reverse transcription for cDNA preparation with oligo(dT). Real time PCR was performed with each reaction carrying 10 ng of cDNA. Primers have been preferably designed from exon junction sequences, except for gene. The details of primers employed along with the sequence source are provided in Table 1. Expression level of individual gene was normalized to Rpl19 expression which was used as calibrator (internal control) for each cDNA sample. PCR for each sample was set up in duplicates and the average Ct value was used in the Δ∆Ct equation.
Table 1.
List of primers used for qPCR analysis.
| Gene | NCBI gene ID | Forward primer (5′3′) | Reverse primer (3′5′) |
|---|---|---|---|
| Rpl19 | 81767 | CGTCCTCCGCTGTGGTAAA | AGTACCCTTCCTCTTCCCTATGC |
| Srb1 | 25073 | TGGGATGAACGACTCGAGT | AGTACCATTGATCATGTTGCAC |
| Ldlr | 300438 | GAGTCCCCTGAGACATGCAT | GGGAGCAGTCTAGTTCATCCG |
| Hmgcr | 25675 | GGGTCAAGATGATCATGTCT | ATTCTCTTGGACACATCTTCAG |
| Hmgcs | 29637 | ACGATACGCTTTGGTAGTTG | AAGCCCTCGGTCAAAAAT |
| Hsl | 25330 | CCTGCAACAGAGACACTGC | CTCTGAGTTGCCCTTAAAGCTC |
| P450SCC | 29680 | ACCCAACTCGTTGGTTGGA | CACGTTGATGAGGAAGATGGT |
| StAR | 25557 | GGCCCCGAGACTTCGTAA | TGGCAGCCACCCCTTGA |
| Mc2r | 282839 | GTCCCCCGTGTACTTTTTCATC | GGACGAACATGCAGTCAATGAT |
2.6. Hematoxylin and eosin (H&E) staining
The neutral buffered formaldehyde (NBF) fixed adrenal gland was sectioned (5 μm thick) and stained as reported elsewhere [45]. The sections were mounted in DPX (Sigma–Aldrich Co., Bangalore, India) and visualized under light microscope (Olympus IX81 inverted microscope, Tokyo, Japan).
2.7. Oil Red O staining
The 5 μm thick cryosections of frozen adrenal glands were prepared and fixed in NBF, washed with PBS (Sigma–Aldrich Co., Bangalore, India) and stained with Oil Red O (ORO) (Sigma–Aldrich Co., Bangalore, India) in 60% isopropanol (Sigma–Aldrich Co., Bangalore, India) for lipid detection. For nuclear staining, sections were equilibrated in McIlvaine’s Citric acid; Na2HPO4 buffer and stained with DAPI and the sections were visualized under florescent microscope (Olympus IX81 inverted microscope, Tokyo, Japan).
2.8. Immunoblot analysis
The adrenal gland tissue was homogenized using RIPA buffer with protease inhibitors as per the procedure reported earlier [44] and the lysates were stored at −70 °C until further use. Total protein estimation was performed by Bradford method (Bio Rad lab. Inc, Berkeley, CA). Tissue lysates (30 μg protein) were resolved on 12% SDS PAGE and transferred onto PVDF membrane using a wet transfer unit (Bio Rad Laboratories, Berkeley, CA). Non specific sites on the membrane were blocked using 10% milk in TBST (20 mM Tris–HCl, pH 7.6, 150 mM NaCl, 0.1% Tween 20) by incubating it for 1 h at room temperature. The membrane was incubated overnight at 4 °C with primary antibody at 1:1000 dilution specific for pCREB (Ser133), CREB, Cleaved Caspase-3, pStAR, StAR and β-actin. The membrane was washed with TBST and incubated with secondary antibody (horseradish peroxidase labeled anti rabbit IgG) at 1:3000 dilutions. The bands were visualized using Western blot imaging system (Flourchem FC2, Cell Biosciences Inc., Santa Clara, CA) and the band intensity was quantitated by Gene tool software (Syngene, Cambridge, UK).
2.9. PKA assay
PKA assays were performed as per the previously published procedure [29] using SignaTECT® cAMP dependent protein kinase (PKA) assay kit (Promega, Madison, Wisconsin). The activity of PKA was determined by measuring the incorporation of 32P from [γ32P] ATP via adrenal gland PKA to biotinylated kemptide, a highly specific peptide substrate. Briefly, the medullary region of adrenal gland was removed carefully from the decapitated gland under dissecting microscope in ice cold PBS solution. The adrenal medulla region specific gene, PNMT expression was found to be undetectable in the dissected cortical fraction (data not shown). The cortical fraction was homogenized in cold extraction buffer containing 25 mM Tris–HCl, 0.5 mM EDTA, 0.5 mM EGTA, 10 mM β ME, 1 μg/ml Aprotinin and 1 μg/ml Leupeptin and centrifuged at 4 °C, 14,000 × g for 5 min. After protein estimation, 10 μg of protein was used to perform the assay by incubating with [γ32P] ATP at 30 °C for 5 min. The reaction was terminated by adding stopping buffer provided with the kit and 10 μl of reaction mixture was spotted onto SAM biotin capture membrane. The individual membrane square was dried and then transferred to liquid scintillation vials for counting in β counter (Tri Carb B2910TR liquid scintillation analyzer, Waltham, MA). A control reaction without the substrate was also performed for determining the background activity which was subtracted from the total activity of samples.
2.10. TUNEL assay
Assay was performed using TACS® TdT DAB in situ apoptosis detection kit (Trevigen Inc., Gaithersburg, MD) according to manufacturer’s protocol. Briefly, NBF fixed adrenal gland from vehicle, 10 and 250 mg/kg bw/d treated animals was sectioned into 5 μm thickness. Sections were cleared in xylene and rehydrated using ascending grades of alcohol solutions and PBS. Sections were treated with Proteinase K followed by TdT labeling reaction mix and buffer incubation. The reaction was stopped by TdT Stop Buffer provided with the kit. The sections were washed thoroughly in PBS and treated with HRP conjugated streptavidin in a humid chamber. After PBS washing, the sections were exposed to DAB provided in the kit, till development of brown color, followed by haematoxylin staining for nuclei and the sections were observed under light microscope. The positive and negative controls for the technique were included by addition of nuclease during incubation and removal TdT enzyme from the labeling mix, respectively.
2.11. Statistical analysis
Data were expressed as mean ± SEM. A t-test was used to calculate p value between two groups. Multiple comparisons were made between vehicle and Roundup® treatment groups using Bonferroni’s test after one way ANOVA. Prism version 5 (GraphPad, California) was utilized for statistical analysis. A p value <0.05 was considered statistically significant throughout.
3. Results
3.1. Effect of Roundup® on body weight and food consumption
No overt signs of toxicity were observed during oral administration of Roundup® up to dose of 250 mg/kg bw/d for 14 days. However, food consumption and body weight were significantly lower beginning with 50 mg/kg bw/d dose (Table 2A and B). This might be due to toxic effects of Roundup® other than EDC effect, hence doses higher than 50 mg/kg bw/d were not included for further analysis.
Table 2.
(A) Average food consumption (g) per animal per day and (B) body weight (g) during Roundup® treatment.
| (A) | |||||
|---|---|---|---|---|---|
| Days of treatment | Control (n = 4) |
10 mg/kg bw/d (n = 4) |
50 mg/kg bw/d (n = 3) |
100 mg/kg bw/d (n = 3) |
250 mg/kg bw/d (n = 3) |
| 0–3 | 16.75 ± 1.2 | 16.3 ± 1.5 | 16.7 ± 0.4 | 16.1 ± 1.3 | 14.3 ± 2.1 |
| 4–7 | 21.6 ± 1.8 | 19.9 ± 1.6 | 18.55 ± 0.12 | 15.2 ± 1.04* | 13.03 ± 0.8*** |
| 8–11 | 22.4 ± 1.9 | 20.2 ± 1.8 | 19.15 ± 0.6 | 13.5 ± 1.08*** | 11.8 ± 0.4*** |
| 12–15 | 23.19 ± 1.9 | 20.3 ± 1.7 | 18.4 ± 0.8 | 12.8 ± 1.1*** | 11.7 ± 0.4*** |
| (B) | |||||
|---|---|---|---|---|---|
| Days of treatment | Control (n = 6) |
10 mg/kg bw/d (n = 6) |
50 mg/kg bw/d (n = 3) |
100 mg/kg bw/d (n = 5) |
250 mg/kg bw/d (n = 5) |
| 0 | 233 ± 15.6 | 225.2 ± 15.1 | 192 ± 5.9 | 188.4 ± 7.1 | 187.6 ± 4.1 |
| 3 | 244.8 ± 14.4 | 235.2 ± 16.6 | 196.6 ± 4.4 | 178.4 ± 7.2** | 173 ± 6.02** |
| 7 | 253.5 ± 15.3 | 243.2 ± 17.2 | 201.3 ± 4.7 | 176.8 ± 8.3*** | 176.2 ± 11.6*** |
| 11 | 262.3 ± 17.04 | 248.2 ± 18.2 | 205.2 ± 7.2 | 178.8 ± 4.7*** | 160.4 ± 9.5*** |
| 15 | 270.8 ± 18.2 | 254.4 ± 20.5 | 207.5 ± 8.9* | 170.4 ± 6.8*** | 176.6 ± 5.9*** |
Values are mean ± SEM. Vehicle group received milliQ water for 14 days and other groups administered with different doses of Roundup® for 14 days. Statistical significance from vehicle group was determined by two way ANOVA followed by Bonferroni test.
p < 0.05.
p < 0.01.
p < 0.001.
3.2. Roundup® effect on circulatory levels of steroid
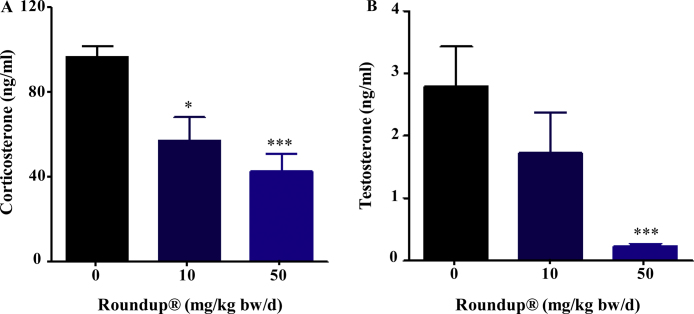
Plasma testosterone and corticosterone, the representative steroid hormone from major endocrine sources i.e., testis and adrenal gland of male rat, were assayed (Fig. 1A and B). Both hormone concentrations were lowered after Roundup® treatment in a dose dependent manner. The lowest dose 10 mg/kg bw/d itself could decrease corticosterone levels significantly (p < 0.05), therefore this dose was selected for detailed EDC studies.
Fig. 1.
Circulatory hormone levels in vehicle and Roundup®-treated animals. Male rats were treated orally with vehicle, 10 and 50 mg/kg bw/d Roundup® for 14 days. Circulatory corticosterone (A) and testosterone (B) levels were measured at the end of 14 days treatment. Data are presented as mean ± SEM (n = 5 per group). ‘t’ test was performed to compare each treatment group to the vehicle group. *, ***, significantly different from vehicle group by p < 0.05, 0.0001 viz.
3.3. Roundup® effect on expression of genes associated with steroidogenesis
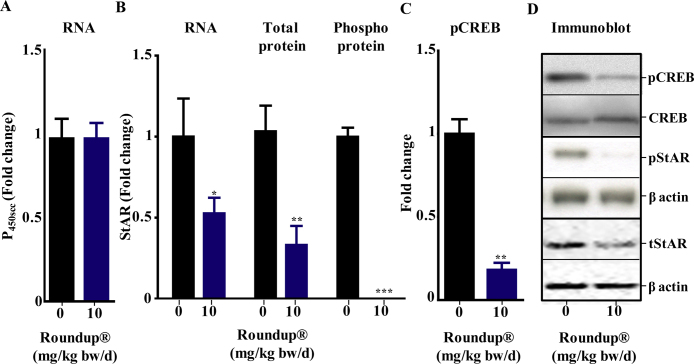
The key regulatory steps in steroidogenesis are transport of cholesterol from outer to inner mitochondrial membrane by StAR protein and cholesterol side chain cleavage step by P450scc enzyme. The mRNA expression of P450scc was unchanged, while StAR mRNA and total protein were down regulated in Roundup® (10 mg/kg bw/d) treated animals compared to vehicle group (Fig. 2A and B). Phosphorylated form of CREB, the transcriptional regulator of StAR expression, was down regulated in 10 mg/kg bw/d Roundup®-treated rats and phosphorylated StAR protein was also found to be significantly lower (p < 0.05) in the 10 mg/kg bw/d treatment group (Fig. 2C and D).
Fig. 2.
Expression of steroidogenic genes in vehicle and Roundup®-treated rats. Male rats were treated orally with vehicle or 10 mg/kg bw/d Roundup® for 14 days. Total RNA from adrenal gland was isolated and qPCR analysis was performed to quantitate the fold change of P450scc (A) and StAR gene expression (B). Rpl19 was used as internal control. The mRNA expression value in vehicle treated animals was set as 1 fold and the mRNA expression value of the treated group was expressed in relation to the vehicle group. Immunoblot analysis of total and phosphorylated form of StAR and CREB proteins (C and D) was performed using the adrenal gland protein lysate from vehicle and Roundup®-treated rats. Immunoblot densitometry arbitrary values for vehicle group was set as fold 1 and the values in treated groups were represented compare in comparison to the vehicle group. Blots are representative of three or more experiments. Values are presented as mean ± SEM (n = 5 per group). ‘t’ test was performed to compare each treatment group with vehicle group. *, **, ***, significantly different from vehicle group by p < 0.05, 0.01, 0.001.
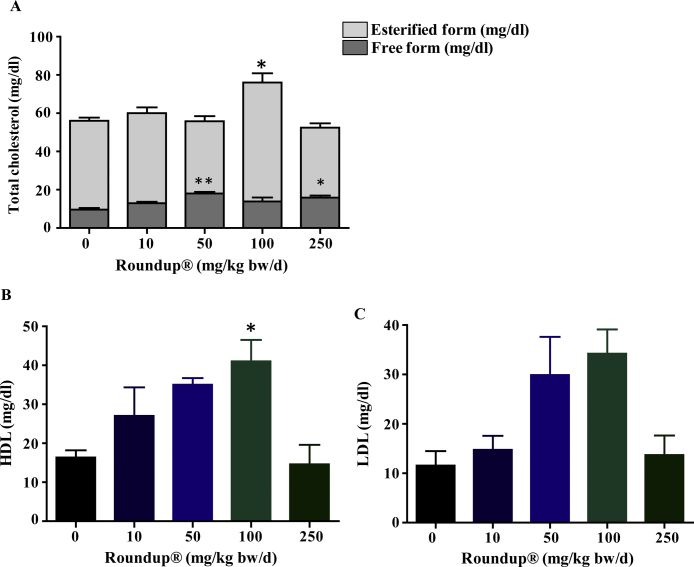
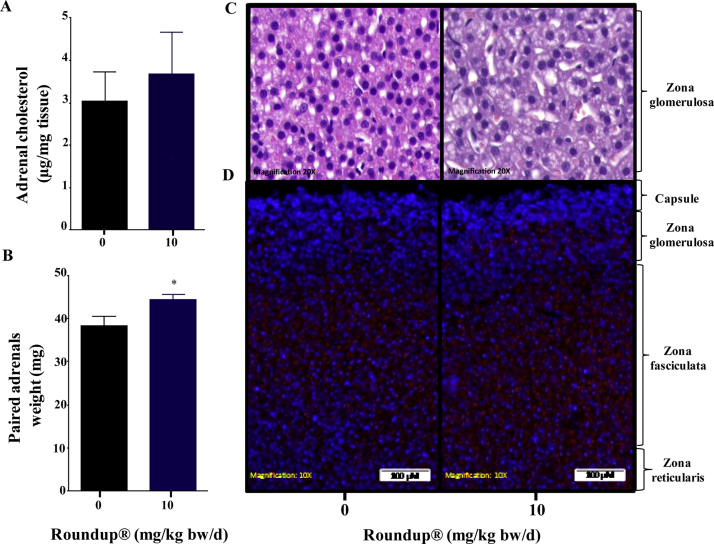
3.4. Roundup® effect upon circulatory and adrenal gland lipid & cholesterol content
Together with steroidogenesis process, the homeostasis of steroid precursor cholesterol was also examined. The level of cholesterol in the circulation as well as in the adrenal gland was measured in vehicle and Roundup®-treated animals. Circulatory levels of total, free and esterified cholesterol were unchanged except for 100 mg/kg bw/d dose (Fig. 3A). A dose-dependent increase in HDL and LDL levels were observed (Fig. 3B and C). Cholesterol was found to be moderately higher in the adrenal gland (Fig. 4A). The weight of adrenal glands (paired weight) was observed to be significantly higher (p < 0.05) (Fig. 4B) in Roundup® -treated animals. H&E and ORO staining indicated moderately higher number of lipid droplets present in the adrenal gland of 10 mg/kg bw/d Roundup®-treated rats (Fig. 4C and D).
Fig. 3.
Plasma cholesterol and lipoprotein levels in vehicle and Roundup®-treated animals. Plasma from control and treatment group were collected and subjected to total, free, esterified cholesterol analysis and HDL, LDL measurement. Values are presented as mean ± SEM (n = 3–4 per group). ‘t’ test was performed to compare each treatment group with vehicle group. *,**, p < 0.05, p < 0.01 viz.
Fig. 4.
Total cholesterol levels in adrenal gland lysate (A) of vehicle and Roundup®-treated group. The adrenal glands were weighed (B), sectioned and stained for H&E (C) and ORO (D) post Roundup® treatment. Sections are representative of two or more runs. Values are presented as mean ± SEM (n = 3–5 per group). ‘t’ test was performed to compare each treatment group with vehicle group. *, p < 0.05.
3.5. Effect of Roundup® treatment on genes involved in cholesterol intake and de novo synthesis
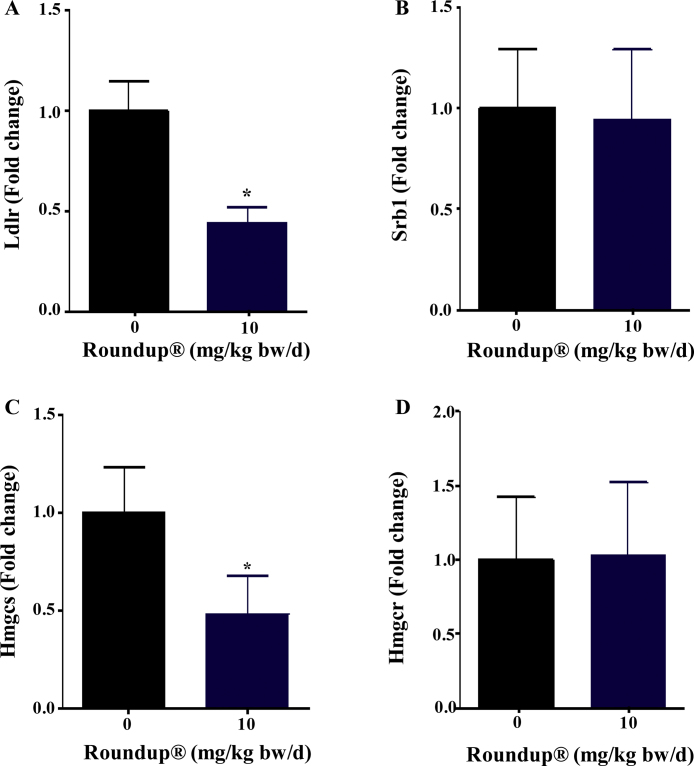
The RNA expression of receptors for cholesterol uptake i.e., low-density lipoprotein receptor (Ldlr) found to be significantly lower (p < 0.05) however, expression of high-density lipoproteins receptor (Srb1) was unaltered in the adrenal gland of 10 mg/kg bw/d Roundup®-treated group compared to vehicle group (Fig. 4A and B). Gene expression for enzymes involved in cholesterol de novo synthesis were found to be unchanged (Hmgcr) or significantly lower (p < 0.05) (Hmgcs) in the adrenal gland of 10 mg/kg bw/d Roundup®-treated animals (Fig. 4C and D). Although expression of genes associated with cholesterol mobilization in the gland was lower, there was a moderately higher accumulation of cholesterol in adrenal cells which could be due to decreased utilization of cholesterol in Roundup®-treated animals.
3.6. Effect of Roundup® treatment on esterified cholesterol (CE) and ester hydrolase
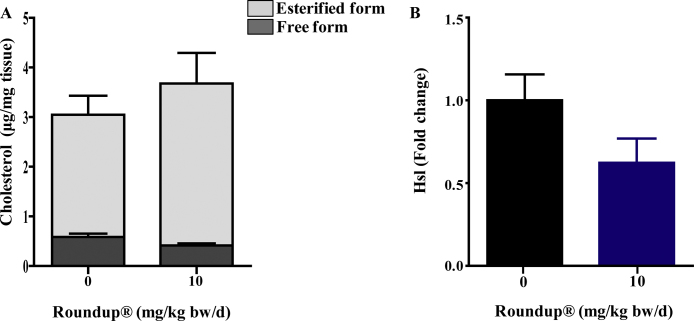
Stored form of cholesterol i.e., CE was estimated by calculating free from total cholesterol. CE tended to be moderately higher in the adrenal gland of Roundup®-treated animals (Fig. 5A). Cholesterol ester hydrolase or hormone sensitive lipase (Hsl), catalyzes the hydrolysis of CE into their free form, was found to be lower (Fig. 5B) even though changes in both, CE and Hsl, were not statistically significant (p > 0.05).
Fig. 5.
Expression of genes associated with cholesterol import and de novo synthesis in the adrenal gland of vehicle and Roundup®-treated animals. qPCR analysis was performed to quantitate the expression of genes involved in cholesterol transport (Srb1, Ldlr) (A and B) and Cholesterol de novo synthesis (Hmgcr, Hmgcs) (C and D). Rpl19 was used as internal control. mRNA expression in the vehicle treated group was set as 1 fold and the expression in treatment group was calculated in relation to the vehicle group. Data presented as mean ± SEM (n = 5 rats per group). ‘t’ test was performed to compare treatment group from vehicle group. *, significantly different from the vehicle group (p < 0.05).
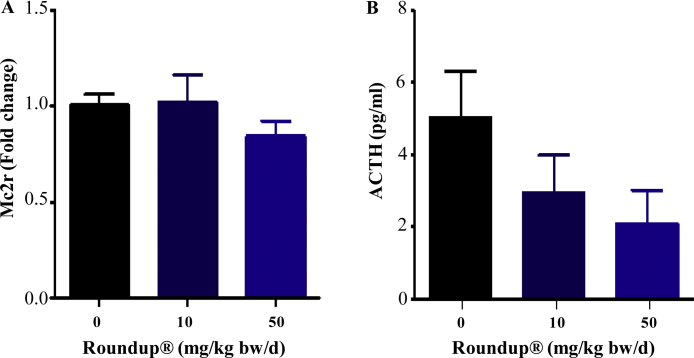
3.7. Roundup® effect on adrenal gland ACTH receptor expression and circulatory ACTH levels
The ACTH receptor, Mc2r, expression in the adrenal gland was found to be unchanged in 10 and 50 mg/kg bw/d Roundup®-treated rats (Fig. 6A), however the circulatory levels of ACTH were lower in Roundup®-treated rats (Fig. 6B). The results suggest that Roundup® treatment might have decreased the synthesis or release of ACTH from the pituitary gland. Lower ACTH levels might explain observed down regulation in StAR and Hsl expression, expression of both genes regulated by ACTH in the adrenal gland post Roundup® treatment.
Fig. 6.
Esterified cholesterol levels (A) of adrenal gland from vehicle and 10 mg/kg bw/d Roundup®-treated animals. Adrenal glands were isolated post treatment and lysate was prepared. Esterified cholesterol levels were obtained by subtracting free cholesterol levels from total cholesterol values. qPCR was performed to detect Hsl gene expression in the adrenal gland of vehicle and 10 mg/kg bw/d Roundup®-treated animals (B). Rpl19 was used as internal control. qPCR value for vehicle was set as 1 fold and Roundup®-treated group values were plotted in relation to the vehicle treated group. Values are presented in mean ± SEM (n = 6 rats per group). ‘t’ test was performed to compare the two groups.
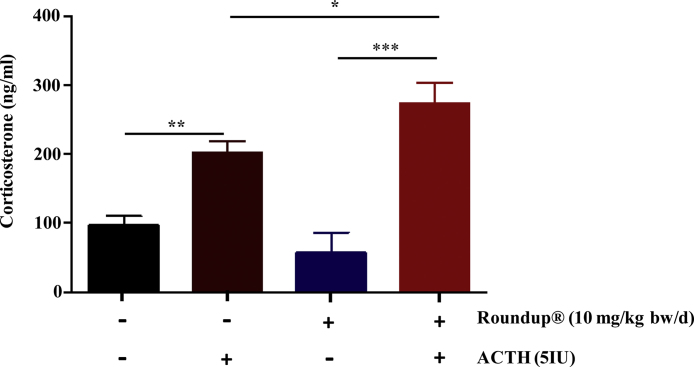
3.8. Effect of exogenous ACTH upon circulatory corticosterone levels in Roundup®-treated animals
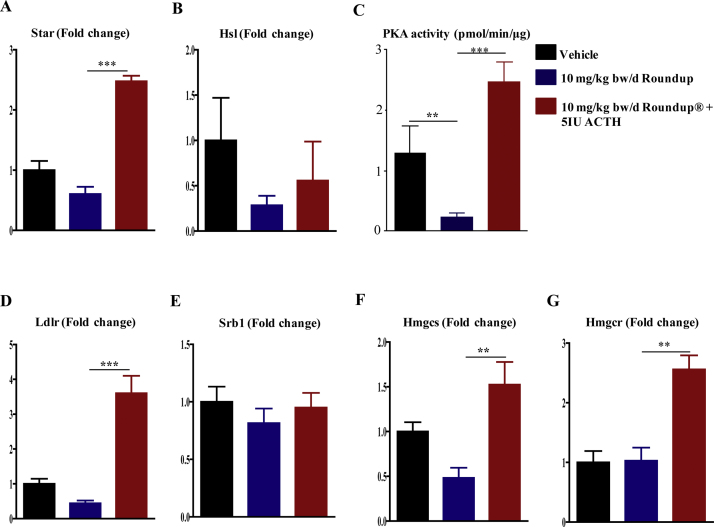
To examine ACTH responsiveness in the adrenal glands of Roundup®-treated animals, a 5 IU dose [based on previous literature as well as pilot study carried out for the present work (data not presented)] of porcine ACTH administered i.v. to vehicle and Roundup®-treated animals. Administration of exogenous ACTH increased corticosterone levels significantly (p < 0.05) at 60 min in Roundup®-treated rats compared to vehicle treated rats (Fig. 7). Exogenous ACTH treatment also increased StAR, Hsl expression (Fig. 8A and B) and the expression of cholesterol homeostasis genes (Srb1, Ldlr, Hmgcr, Hmgcs, Hsl) in the adrenal gland of Roundup®-treated rats (Fig. 8D–G). The results indicate that responsiveness of the adrenal gland to ACTH was intact in the Roundup®-treated rats.
Fig. 7.
Dose dependent adrenal ACTH receptor expression (A) and circulatory ACTH levels (B) present in vehicle and Roundup®-treated animals. Blood plasma was collected and ACTH levels were measure utilizing luminescence based assay kit. ACTH receptor, Mc2r, expression was examined by qPCR analysis. Rpl19 was used as internal control. Vehicle group values were set as one fold and values in the treated groups were expressed in relation to the vehicle group. One way ANOVA was performed to compare the vehicle group with the treatment groups.
Fig. 8.
Circulatory corticosterone levels in ACTH administered and/or vehicle and Roundup®-treated groups. Animals were treated with vehicle and Roundup® (10 mg/kg bw/d). 5 IU of porcine ACTH was administered intravenously. Post 1 h of ACTH treatment, corticosterone levels were measured in the plasma. Data presented as mean ± SEM (n = 3–4 rats per group). ‘t’ test was performed to compare vehicle and treatment groups. *, ** & ***, significantly different from the vehicle group by p < 0.05. p < 0.01, p < 0.001 viz.
3.9. Effect of Roundup® on adrenal gland PKA activity
The cAMP/PKA pathway activated by ACTH in the adrenal gland was examined by quantifying PKA activity. Equal amount of gland cortical region total protein from vehicle and 10 mg/kg bw/d Roundup® and/or ACTH treated rats, was utilized for the assay. The PKA activity was significantly lower (p < 0.01) in Roundup®-treated rats, while exogenous ACTH treatment increased the activity (p < 0.001) (Fig. 8C). The results suggested that Roundup® decreased endogenous ACTH levels and in turn cAMP/PKA pathway in the adrenal gland tissue Fig. 9, Fig. 10.
Fig. 9.
Expression of StAR and genes associated with cholesterol homeostasis post vehicle, Roundup® and/or ACTH treatments. qPCR analysis was performed for StAR (A), Hsl (B), cholesterol homeostasis related genes, Srb1, Ldlr, Hmgcr, Hmgcs (D–G). The cortical region lysate was utilized to quantitate PKA activity (C). Values are presented as mean ± SEM (n = 3–4 rats per group). ‘t’ test was performed to calculate significance between treatment and vehicle groups. *, ** & ***, significantly different from the vehicle group by p < 0.05, p < 0.01, p < 0.001 viz.
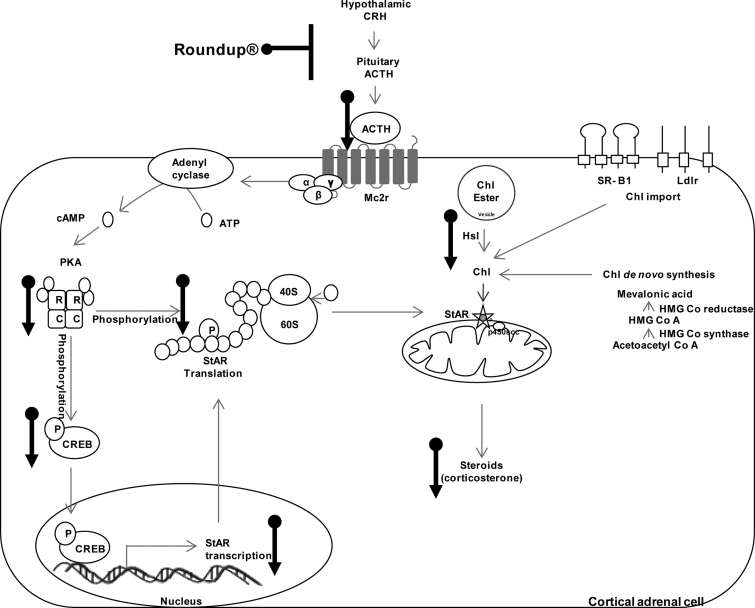
Fig. 10.
Schematic representation of EDC mechanism of glyphosate formulation on adrenal gland steroidogenesis under in vivo condition. Roundup® appears to act at HPA axis to down regulate endogenous ACTH levels which in turn down regulates cAMP/PKA pathway. Lowered activity of PKA leads to down regulation in CREB and StAR phosphorylation, leading to down regulation of StAR function and steroidogenesis.
3.10. Analysis of Roundup® effect on cell toxicity
Immunoblot analysis of cleaved caspase 3 (as marker of apoptosis) was performed on the adrenal gland tissue lysate of vehicle and Roundup®-treated animals (Supplementary Fig. 1A and B). It was observed that cleaved caspase-3 level was up regulated in 250 mg/kg bw/d dose treated rats while the levels were unchanged in the 10 mg/kg bw/d treated group. TUNEL assay for in vivo apoptosis was performed to determine DNA fragmentation (Supplementary Fig. 1C). The incidence of apoptotic cells in the adrenal gland cortical region was higher in 250 mg/kg bw/d treated rats compared to vehicle and 10 mg/kg bw/d dose of Roundup®-treated rats.
4. Discussion
4.1. The effect of Roundup® is demonstrable at the low dose of 10 mg/kg bw/d, while toxic effects are evident at the high dose
The present study was conducted to examine EDC effect of Roundup® on adrenal gland steroidogenesis and to determine its mechanism of action. For this purpose, after determining the effect of ranges of doses on different parameters such as food consumption, body weight etc., the lowest dose was selected which was devoid of obvious toxic effects other than the EDC effect. A dose of 50 mg/kg bw/d Roundup® manifested significant loss in body weight but not in food consumption, while doses higher than 50 mg/kg bw/d caused decrease in food consumption as well as body weight of rats in the second week of the treatment. In previous studies, it was observed that Roundup® exposure did not change body weight of male Spraque–Dawley rats at a dose of 560 mg/ kg bw Roundup® for 91 days [4], while female Spraque–Dawley pregnant rats exposed orally to Roundup® with 500 mg/kg bw/d and higher doses for 10 days of pregnancy, reduced food consumption as well as body weight [12]. The discrepancy of the effect of Roundup® observed in the present as well as in other studies may be related to the differences in strains of rats, age of rats and duration of treatment employed. In order to circumvent possible effects of Roundup® on causing stress and other toxicity related effects, the doses of Roundup® higher than 50 mg/kg were not used for studying the endocrine disrupting effect.
Further, circulatory corticosterone levels in the doses 10 and 50 mg/kg bw/d were observed to be lower compared to the vehicle treated group. To verify that the decrease in corticosterone is due to possible EDC effect of Roundup®, another steroid hormone, testosterone level was also determined. The Roundup® has previously been reported to inhibit testosterone levels and the results in present study are in agreement with others [9], [5], [32]. The result observed suggest suitability of the dose 10 mg/kb bw to assess the EDC effect of the Roundup®.
In cancer cell line studies, Roundup® has been reported to have apoptotic effect [7], [27]. We examined one of the makers of apoptosis and performed TUNEL assay in adrenal glands and observed evidence for increased apoptosis at higher dose of 250 mg/kg bw/d compared to vehicle but not in 10 mg/kg bw/d treated rats. Therefore, decreased corticosterone level that was seen at low dose may not be attributed to toxicity or cell death induced by Roundup® treatment.
4.2. Roundup® negatively regulates StAR in the adrenal gland via cAMP/PKA pathway
The analysis of expression of genes associated with steroidogenesis such as StAR and P450scc, revealed no change in P450scc expression, but decrease in StAR expression, both at mRNA and protein levels. Moreover, phosphorylated StAR expression showed greater degree of down regulation compared to total protein and RNA levels. The difference between total StAR and phosphorylated StAR expression levels post Roundup® treatment, suggests gene regulation at two levels; one at transcription and another at phosphorylation process. Phosphorylation of StAR at serine 194/195 enhances the cholesterol transport capacity to at least 40–50% [2]. Significant down regulation in phosphorylated StAR levels observed in the present study suggest decreased pStAR could be responsible for down regulation of steroidogenesis in the adrenal gland of Roundup®-treated rats. Interestingly, in the present study higher lipid droplet accumulation was observed in Roundup®-treated rats and this is one of the characteristic of the StAR gene knockout mice [17]. The finding that phosphorylated CREB was lower, may be correlated to StAR expression down regulation, since CREB phosphorylation is involved in StAR transcription [24]. However to what extent that CREB down regulation contributed to decrease in StAR mRNA levels, remains to be explored. It should be pointed out that in the adrenal gland, ACTH upon binding to its cognate receptor Mc2r, activates cAMP/PKA pathway leading phosphorylation of CREB and StAR proteins [2]. In the adrenal gland of Roundup®-treated rats, the lowered PKA activity was reversed post ACTH treatment confirming cAMP/PKA pathway to be disrupted in the adrenal gland of Roundup®-treated animals.
4.3. Roundup® alters cholesterol homeostasis moderately
Roundup®-treated animals did not show altered total cholesterol levels in circulation at the lowest dose i.e., 10 mg/kg bw/d, but it was observed to be moderately higher in the adrenal gland. With this observation, it can be hypothesized that the cholesterol homeostasis within gland may be altered by increased cholesterol intake and/or de novo synthesis. Interestingly, there was down regulation of genes associated with cholesterol intake (Ldlr, Srb1) and de novo synthesis (Hmgcs, Hmgcr). Also, analysis revealed higher levels of esterified or stored form of cholesterol in the adrenal gland of Roundup®-treated rats. The data taken together suggest that increased levels of stored cholesterol might be due to lowered utilization and/or lowered hydrolysis of esterified form. Hsl expression was not significantly altered in the present study. Hsl or lipe gene is involved in cholesterol ester hydrolysis [22] and reported to be regulated by cAMP/PKA pathway [20]. Therefore, Roundup® appears to act via cAMP/PKA pathway and regulate StAR phosphorylation negatively leading to decrease in cholesterol utilization and increase in cholesterol stored in adrenal glands.
Interestingly, increase in the weight of adrenal gland was observed in Roundup®-treated animals, but its significance is not clear. The study examining diethylstilbestrol effects on the adrenal gland steroidogenesis has suggested steroid metabolic changes to be the contributing factor for increased weight [16].
4.4. Roundup® acts via HPA axis
Since Roundup® treatment at a dose of 10 mg/kg bw/d decreased corticosterone levels, it became of interest to examine the responsiveness of adrenal gland to exogenous ACTH treatment. The findings that the adrenal gland was responsive to ACTH treatment in Roundup® treated animals suggest that Roundup® acts at a site higher than the adrenal gland and this indirectly suggests that ACTH synthesis and/or release may be affected. Since the adrenal gland responsiveness to external ACTH was found to be similar or higher compared to vehicle treated animals suggest that the process of steroidogenesis in the adrenal gland appears to be intact post herbicide treatment. Therefore, it can be inferred that the stimulation of adrenal gland i.e., ACTH synthesis and release appears to be impaired rather than defects in the steroidogenesis machinery of the adrenal gland. A significantly higher increase in corticosterone levels in response to supraphysiological dose of ACTH was observed in Roundup®-treated rats compared to vehicle treated rats is perhaps due to higher stored cholesterol content in the adrenal gland of Roundup®-treated animals. The mechanism of action may vary with different experimental system e.g., [33] observed higher testosterone, LH and FSH concentrations in second generation or pups of Roundup®-treated Wistar rat dams in contrast to lowered testosterone observed in the present study as well as other studies where adult rats or different cell lines have been studied. Nonetheless, the results are in agreement with a pilot study of glyphosate exposure to Jundiá fish [6]. In sum, the data suggest Roundup® appears to act at the hypothalamo-pituitary level under in vivo conditions.
4.5. Implications of the study
The recent information regarding Roundup® and its metabolites detection in food, water and in human urine [1] signifies the relevance of toxicological studies as one presented. The findings that Roundup® treatment down regulates endogenous ACTH, is similar to the condition known as adrenal insufficiency in humans. This condition manifests as fatigue, anorexia, sweating, anxiety, shaking, nausea, heart palpitations and weight loss. Chronic adrenal insufficiency could be fatal, if untreated. A progressive increase in its prevalence has been observed in humans [8], while a very few studies relating to xenobiotic exposure and adrenal insufficiency development have been reported. The present study describes one of the possible mechanisms of adrenal insufficiency due to Roundup® and suggests more systematic studies, to investigate the area further.
Acknowledgements
We are grateful to Professor Steven King (Baylor College of Medicine, Houston, TX) and Professor DM Stocco (Texas Tech University Health Sciences Center, Lubbock, TX) for providing phospho StAR and total StAR antibodies. AP was supported by a fellowship from the Council of Scientific and Industrial Research, New Delhi, India. This work was supported by grant from the Department of Science and Technology FIST, Government of India.
Contributor Information
Aparamita Pandey, Email: aparamita@mrdg.iisc.ernet.in.
Medhamurthy Rudraiah, Email: rmm@mrdg.iisc.ernet.in.
References
- 1.Acquavella J.F., Alexander B.H., Mandel J.S., Gustin C., Baker B., Chapman P., Bleeke M. Glyphosate biomonitoring for farmers and their families: results from the Farm Family Exposure Study. Environm. Health Perspect. 2004;112:321–326. doi: 10.1289/ehp.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakane F., King S.R., Du Y., Kallen C.B., Walsh L.P., Watari H., Stocco D.M., Strauss J.F., 3rd. Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J. Biol. Chem. 1997;272:32656–32662. doi: 10.1074/jbc.272.51.32656. [DOI] [PubMed] [Google Scholar]
- 3.Benachour N., Seralini G.E. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem. Res. Toxicol. 2009;22:97–105. doi: 10.1021/tx800218n. [DOI] [PubMed] [Google Scholar]
- 4.Caglar S., Kolankaya D. The effect of sub-acute and sub-chronic exposure of rats to the glyphosate-based herbicide Roundup. Environ. Toxicol. Pharmacol. 2008;25:57–62. doi: 10.1016/j.etap.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Cavalli V.L.L.O., Cattani D., Rieg C.E.H., Pierozan P., Zanatta L., Parisotto E.B., Filho D.W., Silva F.R.M.B., Pessoa-Pureur R., Zamoner A. Roundup disrupts male reproductive functions by triggering calcium-mediated cell death in rat testis and Sertoli cells. Free Radic. Biol. Med. 2013;65:335–346. doi: 10.1016/j.freeradbiomed.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 6.Cericato L., Neto J.G., Kreutz L.C., Quevedo R.M., da Rosa J.G., Koakoski G., Centenaro L., Pottker E., Marqueze A., Barcellos L.J. Responsiveness of the interrenal tissue of Jundia (Rhamdia quelen) to an in vivo ACTH test following acute exposure to sublethal concentrations of agrichemicals. Comp. Biochem. Physiol. Toxicol. Pharmacol.: CBP. 2009;149:363–367. doi: 10.1016/j.cbpc.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Chaufan G., Coalova I., Rios de Molina Mdel C. Glyphosate commercial formulation causes cytotoxicity, oxidative effects, and apoptosis on human cells: differences with its active ingredient. Int. J. Toxicol. 2014;33:29–38. doi: 10.1177/1091581813517906. [DOI] [PubMed] [Google Scholar]
- 8.Charmandari E., Nicolaides N.C., Chrousos G.P. Adrenal insufficiency. Lancet. 2014;383(9935):2152–2167. doi: 10.1016/S0140-6736(13)61684-0. [DOI] [PubMed] [Google Scholar]
- 9.Clair E., Mesnage R., Travert C., Seralini G.E. A glyphosate-based herbicide induces necrosis and apoptosis in mature rat testicular cells in vitro, and testosterone decrease at lower levels. Toxicol. In Vitro. 2012;26:269–279. doi: 10.1016/j.tiv.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Cox C. Glyphosate herbicide fact sheet. J. Pestic. Reform (Winter) 2004;24:10–15. [Google Scholar]
- 11.Cox C., Surgan M. Unidentified inert ingredients in pesticides: implications for human and environmental health. Environ. Health Perspect. 2006;114:1803–1806. doi: 10.1289/ehp.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dallegrave E., Mantese F.D., Coelho R.S., Pereira J.D., Dalsenter P.R., Langeloh A. The teratogenic potential of the herbicide glyphosate-Roundup in Wistar rats. Toxicol. Lett. 2003;142:45–52. doi: 10.1016/s0378-4274(02)00483-6. [DOI] [PubMed] [Google Scholar]
- 13.Dallegrave E., Mantese F.D., Oliveira R.T., Andrade A.J., Dalsenter P.R., Langeloh A. Pre- and postnatal toxicity of the commercial glyphosate formulation in Wistar rats. Arch. Toxicol. 2007;81:665–673. doi: 10.1007/s00204-006-0170-5. [DOI] [PubMed] [Google Scholar]
- 14.EPA (Environmental Protection Agency), 2013. Pesticide Industry Sales and Usage EPA home page. Available: <http://www.epa.gov/opp00001/pestsales/07pestsales/usage2007_2.htm/>. Updated on July 2013.
- 15.Gasnier C., Dumont C., Benachour N., Clair E., Chagnon M.C., Seralini G.E. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology. 2009;262:184–191. doi: 10.1016/j.tox.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Haeno S., Maeda N., Yagi T., Tahata S., Sato M., Sakaguchi K., Miyasho T., Ueda H., Yokota H. Diethylstilbestrol decreased adrenal cholesterol and corticosterone in rats. J. Endocrinol. 2014;221:261–272. doi: 10.1530/JOE-13-0460. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa T., Zhao L., Caron K.M., Majdic G., Suzuki T., Shizawa S., Sasano H., Parker K.L. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol. Endocrinol. 2000;14:1462–1471. doi: 10.1210/mend.14.9.0515. [DOI] [PubMed] [Google Scholar]
- 18.A.M. Henderson, J.A. Gervais, B. Luukinen, K. Buhl, D. Stone, Glyphosate Technical Fact Sheet. National Pesticide Information Center). Oregon State University Extension Services. Available: <http://npic.orst.edu/factsheets/glyphotech.html/> Updated on September 2010.
- 19.Hollander-Czytko H., Amrhein N. 5-enolpyruvylshikimate 3-phosphate synthase, the target enzyme of the herbicide glyphosate, is synthesized as a precursor in a higher plant. Plant physiology. 1987;83:229–231. doi: 10.1104/pp.83.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollysz M., Derebecka-Holysz N., Trzeciak W.H. Transcription of LIPE gene encoding hormone-sensitive lipase/cholesteryl esterase is regulated by SF-1 in human adrenocortical cells: involvement of protein kinase A signal transduction pathway. J. Mol. Endocrinol. 2011;46:29–36. doi: 10.1677/JME-10-0035. [DOI] [PubMed] [Google Scholar]
- 21.Kitay J.I. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- 22.Kraemer F.B., Shen W.J. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J. Lipid Res. 2002;43:1585–1594. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- 23.le Maire A., Bourguet W., Balaguer P. A structural view of nuclear hormone receptor: endocrine disruptor interactions. Cell. Mol. Life Sci.: CMLS. 2010;67:1219–1237. doi: 10.1007/s00018-009-0249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manna P.R., Dyson M.T., Eubank D.W., Clark B.J., Lalli E., Sassone-Corsi P., Zeleznik A.J., Stocco D.M. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol. Endocrinol. 2002;16:184–199. doi: 10.1210/mend.16.1.0759. [DOI] [PubMed] [Google Scholar]
- 25.Marek L.J., Koskinen W.C. Simplified analysis of glyphosate and aminomethylphosphonic acid in water, vegetation and soil by liquid chromatography–tandem mass spectrometry. Pest Manag. Sci. 2014;70:1158–1164. doi: 10.1002/ps.3684. [DOI] [PubMed] [Google Scholar]
- 26.Mercurio P., Flores F., Mueller J.F., Carter S., Negri A.P. Glyphosate persistence in seawater. Mar. Pollut. Bull. 2014;85:385–390. doi: 10.1016/j.marpolbul.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Mesnage R., Bernay B., Seralini G.E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology. 2013;313:122–128. doi: 10.1016/j.tox.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Mink P.J., Mandel J.S., Sceurman B.K., Lundin J.I. Epidemiologic studies of glyphosate and cancer: a review. Regul. Toxicol. Pharmacol.: RTP. 2012;63:440–452. doi: 10.1016/j.yrtph.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Priyanka S., Medhamurthy R. Characterization of cAMP/PKA/CREB signaling cascade in the bonnet monkey corpus luteum: expressions of inhibin-alpha and StAR during different functional status. Mol. Human Reprod. 2007;13:381–390. doi: 10.1093/molehr/gam015. [DOI] [PubMed] [Google Scholar]
- 30.Rich E.L., Romero L.M. Exposure to chronic stress downregulates corticosterone responses to acute stressors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R1628–R1636. doi: 10.1152/ajpregu.00484.2004. [DOI] [PubMed] [Google Scholar]
- 31.Richard S., Moslemi S., Sipahutar H., Benachour N., Seralini G.E. Differential effects of glyphosate and Roundup on human placental cells and aromatase. Environ. Health Perspect. 2005;113:716–720. doi: 10.1289/ehp.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romano R.M., Romano M.A., Bernardi M.M., Furtado P.V., Oliveira C.A. Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Arch. Toxicol. 2010;84:309–317. doi: 10.1007/s00204-009-0494-z. [DOI] [PubMed] [Google Scholar]
- 33.Romano M.A., Romano R.M., Santos L.D., Wisniewski P., Campos D.A., de Souza P.B., Viau P., Bernardi M.M., Nune M.T., de Oliveira C.A. Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Arch. Toxicol. 2012;86:663–673. doi: 10.1007/s00204-011-0788-9. [DOI] [PubMed] [Google Scholar]
- 34.Rubin J.L., Gaines C.G., Jensen R.A. Glyphosate Inhibition of 5-enolpyruvylshikimate 3-phosphate synthase from suspension-cultured cells of Nicotiana silvestris. Plant Physiol. 1984;75:839–845. doi: 10.1104/pp.75.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruiz-Toledo J., Castro R., Rivero-Perez N., Bello-Mendoza R., Sanchez D. Occurrence of glyphosate in water bodies derived from intensive agriculture in a tropical region of southern Mexico. Bull. Environ. Contam. Toxicol. 2014;93:289–293. doi: 10.1007/s00128-014-1328-0. [DOI] [PubMed] [Google Scholar]
- 36.Schonbrunn E., Eschenburg S., Shuttleworth W.A., Schloss J.V., Amrhein N., Evans J.N., Kabsch W. Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1376–1380. doi: 10.1073/pnas.98.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuette J. Environmental Monitoring & Pest Management. Department of Pesticide Regulation; Sacramento, CA 95824-5624: 1998. Environmental fate of glyphosate. Available: < http://www.cdpr.ca.gov/docs/emon/pubs/fatememo/glyphos.pdf/> Updated on November 1998. [Google Scholar]
- 38.Seralini G.E., Clair E., Mesnage R., Gress S., Defarge N., Malatesta M., Hennequin D., de Vendomois J.S. Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food Chem. Toxicol. 2012;50:4221–4231. doi: 10.1016/j.fct.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Tu M., Hurd C., Robison R., Randall J.M. The Nature Conservancy; 2001. Glyphosate Weed Control Methods Handbook; pp. 7e1–7e10. Available: < http://www.invasive.org/gist/products/handbook/14Glyphosate.pdf/>. Updates on November 2001. [Google Scholar]
- 40.Thongprakaisang S., Thiantanawat A., Rangkadilok N., Suriyo T., Satayavivad J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. 2013;59:129–136. doi: 10.1016/j.fct.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 41.van Oers J.W., Hinson J.P., Binnekade R., Tilders F.J. Physiological role of corticotropin-releasing factor in the control of adrenocorticotropin-mediated corticosterone release from the rat adrenal gland. Endocrinology. 1992;130:282–288. doi: 10.1210/endo.130.1.1309333. [DOI] [PubMed] [Google Scholar]
- 42.Walsh L.P., McCormick C., Martin C., Stocco D.M. Roundup inhibits steroidogenesis by disrupting steroidogenic acute regulatory (StAR) protein expression. Environ. Health Perspect. 2000;108:769–776. doi: 10.1289/ehp.00108769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willium G.M., Kroes R., Munro I.C. Safety evaluation and risk assessment of the herbicide roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol. 2000;31:117–165. doi: 10.1006/rtph.1999.1371. [DOI] [PubMed] [Google Scholar]
- 44.Yadav V.K., Sudhagar R.R., Medhamurthy R. Apoptosis during spontaneous and prostaglandin F(2alpha)-induced luteal regression in the buffalo cow (Bubalus bubalis): involvement of mitogen-activated protein kinases. Biol. Reprod. 2002;67:752–759. doi: 10.1095/biolreprod.102.004077. [DOI] [PubMed] [Google Scholar]
- 45.Zhao H.L., Sui Y., Guan J., He L., Zhu X., Fan R.R., Xu G., Kong A.P., Ho C.S., Lai F.M., Rowlands D.K., Chan J.C., Tong P.C. Fat redistribution and adipocyte transformation in uninephrectomized rats. Kidney Int. 2008;74:467–477. doi: 10.1038/ki.2008.195. [DOI] [PubMed] [Google Scholar]
- 46.Priyanka S., Jayaram P., Sridaran R., Medhamurthy R. Genome-wide gene expression analysis reveals a dynamic interplay between luteotropic and luteolytic factors in regulation of corpus luteum function in the bonnet monkey (Macaca radiata) Endocrinology. 2009;150:1473–1484. doi: 10.1210/en.2008-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]