Abstract
Soft drinks are consumed daily in Nigeria due to its affordability, characteristic taste, and thirst quenching potential. However, the high demand may compromise the quality of production with possible contamination of heavy metals which have shown to cause intoxication and death in humans. This study evaluated some constituents of twenty-six soft drinks in Nigeria and investigated the presence of some heavy metal contaminants. The soft drinks were screened for the presence of sugar, carbon dioxide, phosphate and alcohol as well as the pH and acidity determined. The level of cadmium, mercury and lead were determined using atomic absorption spectrophotometer. The study showed the presence of sugar, carbon dioxide, phosphate, and alcohol in the soft drinks. The soft drinks were acidic in nature, pH ranging from 3 to 5 with a mean of 3.6 and the acid concentration was relatively low between 3 and 12 g/L with a mean of 8.1 g/L. Lead was present in all the samples ranging from 0.17 to 3.39 mg/L with a mean of 0.8, mercury was present in 22 samples ranging from 0.29 to 11.32 mg/L with a mean of 2.08 mg/L while cadmium was present only in one sample (0.149 mg/L). When compared to EPA, WHO and NIS standards, the levels of the heavy metal contaminants were above the tolerated limits for good quality drinking water in most samples. These results suggest that soft drinks in Nigeria may be contaminated with heavy metals which constitute a major public health problem. Thus, quality control is recommended during the production process especially at the stages of sterilization and purification.
Keywords: Soft drink, Heavy metal, Intoxication, Public health, Nigeria, Contamination
1. Introduction
In Nigeria today, soft drink is one of the most consumed beverages. The consumption of non-alcoholic beverages in Nigeria was rated at 159.85 g/person/day in 2007 [1]. Soft drinks exist in various forms and brands and are marketed by different brewery industries across the country [2], [3]. These drinks are readily consumed on daily bases especially when undergoing tedious activities like hard work and sport [4]. Also, with the relatively affordable prices, they are highly consumed during leisure and relaxation outings and serve the general public in celebrations such as traditional marriages, weddings, funerals, etc. [5].
The high consumption rate of soft drink is attributed to the characteristic taste and flavour as well as their thirst quenching potential [6]. These characteristics are defined by the constituents present such as sugar which is responsible for its sweetness, carbonated water which is water compressed with carbon dioxide to make it an ultimate thirst quencher and flavouring agents to add flavour to the drinks [7]. In addition to taste satisfaction, soft drinks contain other constituents such as vitamins, phosphates, acids, antioxidants, etc. which are of nutritional and health benefits to the body [8].
However, due to the high level of consumption and demand of soft drinks, quality control within the process of production especially during sterilization and purification may be compromised and the quality of soft drinks may be challenging. As such, soft drinks have been shown to contain traces of alcohols as a result of microbial contamination [9]. Also, the presence of some heavy metals such as cadmium, lead, mercury arsenic, zinc, etc. in soft drinks which may be due to environmental pollution from surface and underground water, food and fruits utilized during production have been reported [10].
Heavy metals are metals that have shown to be harmful and toxic to the human body [11], [12] and constitute a major public health concern [13], [14]. These metals have the potential of causing acute and chronic toxicity by various modes of action in both children and adults [15], [16]. Some heavy metals act as catalyst in oxidative reactions of biological macromolecules, therefore their intoxication may lead to oxidative tissue damage [17]. Others have genotoxic/carcinogenic potential causing chromosomal aberrations and mutation as well as cancer [18]. One of the major mechanisms by which heavy metals exert toxic effect is through impairment of cellular respiration by inhibition of various mitochondrial enzymes, and the uncoupling of oxidative phosphorylation [19], [20], [21]. Some of the heavy metals of health importance include: cadmium, lead, mercury, etc.
Cadmium is a heavy metal whose long term accumulation may lead to cancer since it is a carcinogenic element [18]. Also, over a long period of intake, cadmium may accumulate in the kidney and liver because of its long biological half life and may lead to kidney damage [22]. Lead is known to affect humans and animals of all ages but the effects of lead are most serious in young children [23]. The most common childhood presentation of lead poisoning is central neurotoxicity [24]. Other symptoms of childhood lead toxicity include; anaemia, peripheral motor neuropathy, gastrointestinal complaints such as anorexia, vomiting, and abdominal pain, and growth delay [25]. Mercury is also another critical health hazard. Its intoxication can occur in infants and adults and has shown to interfere with numerous cellular processes including protein and nucleic acid synthesis, oxidative stress, calcium homeostasis, and protein phosphorylation [26].
Thus, this study was aimed to evaluate some constituents of soft drinks in Nigeria which may be characteristic of their taste and consumption and also assessed some heavy metal for possible contamination.
2. Materials and methods
Twenty-six soft drinks were purchased from local grocery stores in the commercial city of Enugu, Enugu State of Nigeria and were qualitatively analyzed for the presence of sugar, carbon dioxide, alcohol and phosphate while the acidity, pH, and heavy metals concentration were quantified. The presence of sugar, carbon dioxide, phosphates and acidity were determined according to the procedures of AOAC [27].
2.1. Test for sugar
Benedict solution was used to test for the presence of sugar. In this procedure, 3 ml of the sample of different brands were taken into a test tube and 2 ml of Benedict reagent was added. The test tube was heated in a water bath for 5 min and the formation of reddish colour confirmed the presence of sugar in soft drinks.
2.2. Test for reducing sugar
The presence of reducing sugar was tested using Fehling solution. In this test, 3 ml of the sample was taken in a test tube and 2 ml of a mixture of Fehling's A and Fehling's B solutions in equal amount was added. The test tube was heated in a water bath for 10 min and the appearance of brown precipitation confirmed the presence of reducing sugar.
2.3. Test for phosphates
3 ml of sample for each brand of soft drinks was taken into separate test tubes. 2 ml of ammonium molybdate followed by 2 ml of concentrated nitric acid (HNO3) was added. The solution was heated in a water bath for 10 min and appearance of canary-yellow precipitate confirmed the presence of phosphate ions in soft drinks.
2.4. Test for alcohol
3 ml of sample for each brand of soft drink was transferred into a separate test tube. 1 ml of iodine was added, followed by 1 ml of potassium iodide and 1 ml of sodium hydroxide (NAOH) solution. The test tubes were boiled at 100 °C in a water bath for 30 min. Appearance of yellow coloured precipitate confirmed the presence of alcohol in soft drinks.
2.5. Test for carbon dioxide
As soon as the bottles were opened, 3 ml of the sample for each brand of soft drinks was added to 2 ml of lime water (calcium hydroxide). The change of lime water from colourless to milky confirmed the presence of dissolved carbon dioxide in the soft drinks.
2.6. Quantification of acid concentration
The acidity of the soft drinks was done by the acid titration method. 1 ml of 0.1 M sodium hydroxide was added into a 25 ml capacity burette. 10 ml of the prepared sample was added followed by 2 drops of 1% phenolphthalein. Small amount of the sample was further titrated until the end point was attained marked by a colour changed from colourless to pink and the acid concentration was determined.
2.7. Determination of pH
This was done by the dipstick method. A pH paper was dipped into 2 ml of the soft drinks contained in a test tube. The change in colour of the pH paper was noticed and compared with the standard pH scale of the kit.
2.8. Quantification of heavy metals
2.8.1. Sample preparation
Prior to analysis, the samples were digested according to the method of Wallace [28]. In this method, 10 ml of 69% concentrated nitric acid was added to 25 ml of the sample and the mixture was evaporated on a hot plate in a fume cupboard until the brown fumes disappears leaving white fumes. 50 ml of distilled water was then added and the solution was concentrated by evaporation on a hot plate to 25 ml. Subsequently, additional 25 ml of distilled water was added to make up the volume to 50 ml which was then filtered and ready for atomic absorption spectrophotometer (AAS) analysis using the Varian AA240 model.
2.8.2. Preparation of metal ion and ASS analysis
The calibration plot method described in the British Pharmacopoeia [29] was adopted for the preparation of metal ion and AAS analysis. A stock standard solution, 1000 ppm, of the metal ion was prepared by dividing the molar mass of the compound containing the element by the molar mass of the element. The weight obtained was equivalent to 1.0 g of the metal ion. This weight (which is equivalent to 1.0 g of the metal) was dissolved in 1000 ml to give 1000 ppm. A working solution of 100 ppm was prepared from the stock solution and serial dilutions were made from the working solution. The absorbance of these solutions was obtained using AAS at 228.8, 283.3 and 253.7 nm for cadmium, lead and mercury respectively. The calibration graph was plotted and the regression equation was used to determine the heavy metal concentration. Deionised water was used as control.
2.8.3. Analysis for heavy metal contamination in soft drink
As defined by the United States Environmental protection agency, the standard for the determination of heavy metal contamination in soft drink is based on two units of measurements; the maximum contaminant level goal (MCLG) and maximum contaminant level (MCL). MCLG is the level of a contaminant in drinking water below which there is no known or expected risk to health and hence allow for a margin of safety and are non-enforceable public health goals. On the other hand, MCL is the highest level of a contaminant that is allowed in drinking water. The MCLG and MCL are measured in milligrams per litre (mg/L) which is equivalent to parts per million [34].
2.9. Statistical analysis
Data obtained were subjected to statistical analysis by mean comparison using analysis of variance (ANOVA) test and values of p ≤ 0.05 were considered to be significantly different.
3. Results
Qualitative analysis showed the presence of sugar, phosphate, alcohol, and carbon dioxide in the soft drinks. Sugar was abundantly present in all the 26 samples except for reducing sugar which was absent in 6 samples but present abundantly in 20 samples. Phosphate was present abundantly in 11 samples as well as alcohol in 18 samples. Carbon dioxide was present in 20 samples and absent in 6 (results are summarized in Table 1). All the soft drinks were acidic with low pH ranging from 3 to 5 with a mean of 3.6 and the acid concentration was relatively low between 3 and 12 g/L with a mean of 8.1 g/L (see Table 2). Heavy metal analysis showed the presence of cadmium, lead and mercury. Cadmium was detectable only in bottled coke (0.149 mg/L), while mercury was present in 22 samples and lead detected in all the samples. Lead ranged from 0.17 to 3.39 mg/L with a mean of 0.8 while mercury ranged from 0. 29 to 11.32 mg/L with a mean of 2.08 mg/L (see Table 3).
Table 1.
The presence of sugar, phosphate, alcohol and carbon dioxide in soft drinks.
| Samples | Benedict test | Fehling's solution test | Test for phosphate | Test for alcohol | Test for (Co2) |
|---|---|---|---|---|---|
| Bottled coke | +++ | +++ | − | +++ | +++ |
| Bottled fanta | +++ | +++ | − | +++ | + |
| Bottled sprite | +++ | +++ | +++ | − | +++ |
| Bottled Maltina | +++ | +++ | +++ | − | ++ |
| Bottled Amstel | +++ | +++ | − | − | + |
| Bottled Pepsi | +++ | +++ | − | +++ | +++ |
| Bottled Miranda | +++ | +++ | +++ | +++ | +++ |
| Bottled 7up | +++ | − | − | − | +++ |
| Can coke | +++ | +++ | +++ | +++ | +++ |
| Can zero coke | +++ | +++ | − | +++ | +++ |
| Can fanta | +++ | +++ | − | +++ | +++ |
| Can sprite | +++ | +++ | +++ | +++ | +++ |
| Can Amstel | +++ | − | − | − | + |
| Can Maltina | +++ | − | − | − | + |
| Plastic fanta | +++ | +++ | − | +++ | +++ |
| Plastic coke | +++ | +++ | +++ | +++ | +++ |
| Plastic sprite | +++ | +++ | − | +++ | +++ |
| Plastic Pepsi | +++ | +++ | +++ | +++ | +++ |
| Plastic Miranda | +++ | +++ | − | +++ | +++ |
| Plastic 7up | +++ | − | − | − | + |
| Chivita premium pineapple and coconut juice | +++ | +++ | +++ | − | − |
| Don Simon pineapple juice | +++ | +++ | +++ | +++ | − |
| Chi active 5, citrus fruit juice | +++ | +++ | +++ | +++ | − |
| Fresh pineapple and coconut juice | +++ | − | +++ | +++ | − |
| Fresh pineapple juice | +++ | +++ | − | +++ | − |
| Fresh citrus juice | +++ | − | +++ | − | − |
+ Present; ++ moderately present; +++ abundantly present; − absent.
Table 2.
pH and acid concentration.
| Samples | pH | Acid level (g/L) |
|---|---|---|
| Bottled coke | 2 | 3.26 |
| Bottled fanta | 3 | 12.67 |
| Bottled sprite | 4 | 9.15 |
| Bottled Maltina | 5 | 12.10 |
| Bottled Amstel | 4 | 12.67 |
| Bottled Pepsi | 3 | 5.95 |
| Bottled Miranda | 3 | 12.86 |
| Bottled 7up | 4 | 12.29 |
| Can coke | 3 | 9 |
| Can zero coke | 3 | 11.71 |
| Can fanta | 3 | 11.71 |
| Can sprite | 4 | 11.52 |
| Can Amstel | 5 | 9.6 |
| Can Maltina | 4 | 10.35 |
| Plastic fanta | 3 | 11.52 |
| Plastic coke | 3 | 11.14 |
| Plastic sprite | 4 | 12.86 |
| Plastic Pepsi | 3 | 13.06 |
| Plastic Miranda | 3 | 9.9 |
| Plastic 7up | 4 | 9.75 |
| Chivita premium pineapple and coconut juice | 4 | 11.90 |
| Don Simon pineapple juice | 4 | 12.86 |
| Chi active 5, citrus fruit juice | 4 | 12.48 |
| Fresh pineapple and coconut juice | 5 | 12.67 |
| Fresh pineapple juice | 4 | 12.67 |
| Fresh citrus juice | 3 | 8.064 |
| Mean | 3.62 | 10.91 |
| S.D. | 0.75 | 2.36 |
Table 3.
Cadmium, lead and mercury level.
| Samples | Cadmium (mg/L) | Lead (mg/L) | Mercury (mg/L) |
|---|---|---|---|
| Bottled coke | 0.149 | 2.379 | 6.139 |
| Bottled fanta | ND | 0.731 | 7.184 |
| Bottled sprite | ND | 0.471 | 6.796 |
| Bottled Maltina | ND | 0.270 | 11.325 |
| Bottled Amstel malt | ND | 0.256 | 7.671 |
| Bottled Pepsi | ND | 0.241 | 3.315 |
| Bottled Miranda | ND | 0.382 | 2.495 |
| Bottled 7up | ND | 0.388 | 2.184 |
| Can coke | ND | 0.671 | 1.196 |
| Can zero coke | ND | 0.476 | 1.735 |
| Can fanta | ND | 0.331 | 0.086 |
| Can sprite | ND | 0.171 | 0.069 |
| Can Amstel malt | ND | 1.305 | 0.099 |
| Can Maltina | ND | 0.260 | 0.447 |
| Plastic fanta | ND | 0.659 | 0.503 |
| Plastic coke | ND | 1.540 | 0.677 |
| Plastic sprite | ND | 0.676 | 0.078 |
| Plastic Pepsi | ND | 0.791 | ND |
| Plastic Miranda | ND | 3.392 | ND |
| Plastic 7up | ND | 0.317 | ND |
| Chivita premium pineapple and coconut juice | ND | 1.305 | 0.341 |
| Don Simon pineapple juice | ND | 0.983 | 0.289 |
| Chi active 5, citrus fruit juice | ND | 1.413 | ND |
| Fresh pineapple and coconut juice | ND | 0.691 | 0.167 |
| Fresh pineapple juice | ND | 0.302 | 0.936 |
| Fresh citrus juice | ND | 0.563 | 0.353 |
| MCLG | 0.005 | 0.00 | 0.002 |
| MCL | 0.005 | 0.015 | 0.002 |
| Mean | 0.0057 | 0.81 | 2.08 |
| S.D. | 0.029 | 0.74 | 3.08 |
| P value | 0.000 | ||
ND signifies non-detectable levels of heavy metals.
4. Discussion
The most liable reasons for soft drink consumption are usually its sweetness due to the presence of sugar as well as the thirst quenching nature of carbonated water used [30]. From this study, all the soft drinks showed the presence of sugar; both reducing and non-reducing sugars which are responsible for its sweetness. Sucrose which is a non-reducing sugar was absent in a few samples as confirmed by a negative result for Fehling test. Carbon dioxide which gives soft drink the frizzy effect as an ultimate taste quencher was present in most of the drinks except for the fresh juices which are not usually carbonated.
Phosphorous is an important element for the body. It forms a major constituent of the DNA, cell membrane layer and channels and is also vital for teeth and bone formation [4]. Phosphorous naturally exist as phosphates which are acidic in nature and can be obtained from dietary sources. Phosphate was present in most of the soft drinks and thus could be beneficial especially in children for the development of teeth and bones.
Although low acid concentration could be of importance in killing gastrointestinal bacteria in the body, low pH could cause teeth erosion [31], [32]. The effect of low pH has been shown in so many studies to be responsible for tooth decay especially when the acidity (acid concentration per litre) of the soft drink is high [33]. In this study, most of the soft drinks had a relatively low pH with an average of about 3.6 g/L. However, the acidity was low thus minimizes the risk of causing tooth erosion and hence, makes soft drinks relatively save for consumption.
Industrial processes require good sterilization procedures and quality control to ensure the safety of soft drinks. Poor sterilization during production may lead to microbial contamination which may ferment sugar to alcohol [9]. Results from this study showed the presence of alcohol in soft drinks. Though the level of alcohol was not quantified, previous studies have shown alcohol level in soft drinks to be present in very minute amounts less than 0.05% which is very in significant to cause related alcohol intoxication problems such as drunkenness [9].
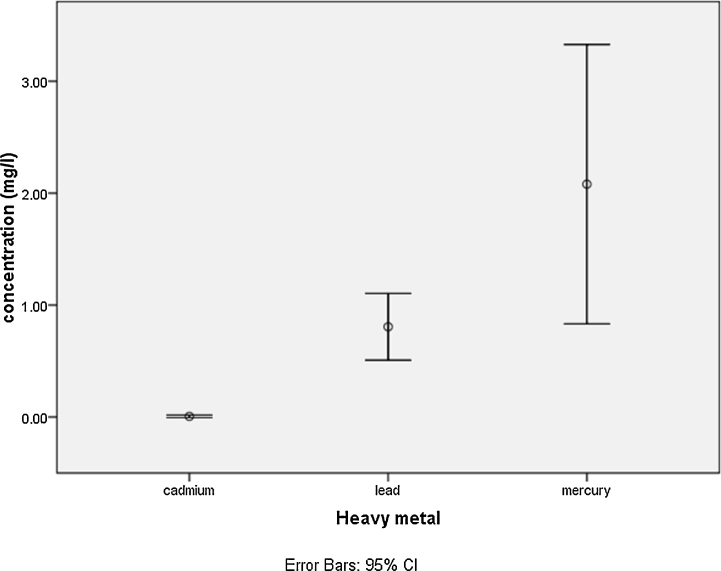
Water, if not purified during the production process of soft drink may be another source of contamination by heavy metals which constitute a major threat to public health. As such, certain standards and guidelines on the tolerable levels of heavy metal contaminants in water have been defined by World health Organisation, and United States Environmental Protection Agency [34], [35]. Also, the Nigerian Industrial Standard for drinking water has also been defined to control the quality of drinking water in Nigeria [36]. In this study, cadmium, lead and mercury were present in soft drinks and the quantities varied significantly (p ≤ 0.05) among the various soft drinks (Table 3 and Fig. 1). Previous studies in Nigeria have also shown the presence of heavy metals in soft drinks [37], [38]. In a study conducted in 2012 by Adepoju-Bello and collaborators in Lagos showed the presence of lead, cadmium, nickel and silver in some soft drinks and the level was above the WHO tolerable limits.
Fig. 1.
Mean level of cadmium, lead and mercury.
Cadmium was non-detectable in most samples except for one sample (bottled coke) with a concentration of 0.149 mg/L (Table 3). This value was far above the MCL and MCLG of NIS (0.003 mg/L) as well as those of WHO and EPA (0.005 mg/L). Previous studies in Nigeria have also confirmed the presence of cadmium in soft drinks and have shown the level to be above the tolerated limit [37]. Chromium contamination in soft drinks may not only be a Nigerian issue as it has also been shown to be present abroad. A study in Spain also showed the presence of chromium in soft drinks [39] thus may be a global problem.
Another heavy metal that was investigated in this study was lead. Lead was shown to be detectable in all the soft drinks and the concentrations were also found to be beyond the accepted MCLG (0.00) and MCL (0.015) limits. Lead contamination in Nigeria has not only been observed in soft drinks but also in water sediments and fish. This was observed in a study carried out in Bayelsa State where lead as well as other heavy metals were identified in tilapia fish and water sediments [40]. However, in a study conducted by Adepoju-Bello et al. [37] in Nigeria showed lead levels to be below MCL limit.
Mercury was also detected in most samples and the values were far above the recommended acceptable MCL limit of NIS (0.001 mg/L) and EPA (0.002 mg/L) as well as their MCLG limit of 0.002 mg/L. Mercury poisoning of fish has been a major problem in Nigeria as a result of contamination of rivers and fishing farm lands [10].
5. Conclusion
The presence of sugar, carbon dioxide, phosphate and acidity in soft drinks in Nigeria gives it the characteristic taste which justifies its frequent consumption. However, this high consumption gives room for the risk of heavy metal contamination and intoxication as cadmium, lead and mercury were found to be present in most of the soft drinks and the values were above the accepted limits for consumption. As such, soft drink consumption may constitute a major public health concern for heavy metal contamination in Nigeria and thus, there is need for regulatory bodies to monitor and control the quality of the soft drinks in order to ensure safe consumption and minimize the possible underlying risk. Quality control should be ensured during production and the quality of sugar and water used for soft drink production be evaluated for the presence of heavy metals at the level of purification and sterilization to reduce or prevent subsequent health effects of intoxication.
Conflict of interest
The authors declare that there is no conflict of interest. The research was conducted with no financial conflict or others factors which is considered to be declared as conflict.
Transparency document
Acknowledgements
This study would not have been possible without the financial and moral support of the management of Godfrey Okoye University Nigeria. Special thanks go to the Vice Chancellor, Rev. Fr. Prof. Christian Anieke for financial assistance and to the staff and final year students of Chemical Sciences Department for their technical assistance in carrying out this project.
References
- 1.FAO (United Nations Food and Agriculture Organisation) 2011. FAOSTAT data.http://faostat.fao.org (accessed February 2011) [Google Scholar]
- 2.Asiegbu I.F. Salesforce competence development and marketing performance of industrial and domestic products firms in Nigeria. Far East J. Psychol. Bus. 2011;2(3) [Google Scholar]
- 3.Ambler T., Styles C. Brand development versus new product development: toward a process model of extension decisions. J. Prod. Brand Manage. 1997;6(4):222–234. [Google Scholar]
- 4.EFSA (European Food Safety Authority) 2006. The setting of nutrient profiles for foods bearing nutrition and health claims pursuant to Article 4 of the Regulation (EC) No 1924/2006: Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies.http://www.efsa.europa.eu/en/efsajournal/doc/644.pdf (accessed February 2011) [Google Scholar]
- 5.Dharmasena K.A. Texas A&M University; 2010. The Non-Alcoholic Beverage Market in the United States: Demand Interrelationships, Dynamics, Nutrition Issues and Probability Forecast Evaluation. (PhD thesis) [Google Scholar]
- 6.Phillip B.B., Shittu A.M., Ashaolu O.F. Demand for non-alcoholic beverages among urban households in SouthWest, Nigeria. Afr. J. Food Agric. Nutr. Dev. 2013;13(3):7853–7869. [Google Scholar]
- 7.Kirk R.S. 9th ed. Longman; 1991. Pearson's Composition and Analysis of Foods. [Google Scholar]
- 8.Pofahl G.M., Capps O., Jr., Clauson A. Paper presented at the American Agricultural economics Association annual meeting, Providence, Rhode Island, 24–27 July; 2005. Demand for non alcoholic beverages: evidence from the AC-Nelson Home scan panel. [Google Scholar]
- 9.Juvonen R., Virkajärvi V., Priha O., Laitila A. Microbiological spoilage and safety risks in non-beer beverages produced in a brewery environment. Espoo. 2011:p107. VTT Tiedotteita – Research Notes 2599. [Google Scholar]
- 10.Galadima A., Garba Z.N. Heavy metals pollution in Nigeria: causes and consequences. Elixir Pollut. 2012;45:7917–7922. [Google Scholar]
- 11.Life Extention. Heavy Metal Toxicity, http://www.lef.org/ (updated 06.12.03).
- 12.Duffus J.H. “Heavy metal” – a meaningless term? Pure Appl. Chem. 2002;74:793–807. [Google Scholar]
- 13.Bingol M., Yentur G., Buket E.R., Oktem A.B. Determination of some heavy metal levels in soft drinks from Turkey using ICP-OES method. Czech J. Food Sci. 2010;28:213–216. [Google Scholar]
- 14.Cabrera C., Lorenzo M.L., Lopaz M.C. Lead and cadmium contamination in dairy product and its repercussion on total dietary intake. J. Agric. Food Chem. 1995;43:1605–1609. [Google Scholar]
- 15.Ibrahim D., Froberg B., Wolf A., Rusyniak D.E. Heavy metal poisoning: clinical presentations and pathophysiology. Clin. Lab. Med. 2006;26:67–97. doi: 10.1016/j.cll.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Berry W.L., Wallace A. 1981. Toxicity – The Concept and Relationship to the Dose–Response Curve. [Google Scholar]
- 17.Shaw B.P., Sahu S.K., Mishra R.K. In: Heavy Metal Stress in Plants. 2nd ed. Prasad M.N.V., editor. Springer; Berlin: 2004. pp. 84–126. [Google Scholar]
- 18.Rubio C., Hardisson A., Reguera J.I., Revert C., Lafuente M.A., Gonzalez-Iglesias T. Cadmium dietary intake in the Canary Islands, Spain. Environ. Res. 2006;100:123–129. doi: 10.1016/j.envres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Sharma R.J., Agrawal M. Biological effects of heavy metals: an overview. J. Exp. Bot. 2005;26:2. [PubMed] [Google Scholar]
- 20.Lösch R. Plant mitochondrial respiration under the influence of heavy metals. In: Prasad Manton D.J., Cai F., Yuan Y., Walker G.D., Cochrane N.J., Reynolds C., Brearley-Messer L.J., MNV, editors. Heavy Metal Stress in Plants. 3rd ed. Springer; Berlin: 2004. pp. 182–200. [Google Scholar]
- 21.Van Assche F., Clijsters H. Effects of heavy metals on enzyme activity in plants. Plant Cell. 1990 [Google Scholar]
- 22.Godt J., Scheidig F., Grosse-Siestrup C., Esche V., Brandenburg P., Reich A., Groneberg D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006;1:22. doi: 10.1186/1745-6673-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staudinger K.C., Roth V.S. Occupational lead poisoning. Am. Fam. Physician. 1998;57(Suppl.):301–313. [PubMed] [Google Scholar]
- 24.Lidsky T.I., Schneider J.S. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126:5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- 25.Lockitch G. Perspectives on lead toxicity. Clin. Biochem. 1993;26:371–381. doi: 10.1016/0009-9120(93)90113-k. [DOI] [PubMed] [Google Scholar]
- 26.Chang L.W., Verity M.A. Mercury neurotoxicity: effects and mechanisms. In: Chang L.W., Dyer R.S., editors. Handbook of Neurotoxicology. Marcel Dekker; New York: 1995. pp. 31–59. [Google Scholar]
- 27.AOAC . 18th ed. AOAC International; Gaithersburg, MD, USA: 2005. Official Methods of Analysis of AOAC International. [Google Scholar]
- 28.Wallace H.A. 4th ed. Taylor and Francis Publishing Inc.; Philadelphia: 2001. Principles and Methods of Toxicology; pp. 50–55. [Google Scholar]
- 29.British Pharmacopoeia . 2005. Atomic Spectrophotometry: Emission and Absorption, Appendix IID, CD. [Google Scholar]
- 30.Banumathy, Hemameena Customer satisfaction and customer preferences towards Soft drinks. Total Qual. Manage. Bus. Excell. 2006;19(7):843–853. [Google Scholar]
- 31.Bamise C.T., Kolawol K.A., oloyede E.O. The determinants and control of soft drinks-incited dental erosion. Rev. Clín. Pesq. Odontol. 2009;5(2):141–154. [Google Scholar]
- 32.Panich M., Poolthong S. The effect of casein phosphopeptide–amorphous calcium phosphate and a cola soft drink on in vitro enamel hardness. J. Am. Dental Assoc. 2009;140(4):455–460. doi: 10.14219/jada.archive.2009.0195. [DOI] [PubMed] [Google Scholar]
- 33.Larsen M.J., Nyvad B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Carr. Res. 1999;33:81–87. doi: 10.1159/000016499. [DOI] [PubMed] [Google Scholar]
- 34.EPA (United States Environmental Protection Agency) Office of Water U.S. Environmental Protection Agency; Washington, DC: 2011. 2011 Edition of the Drinking Water Standards and Health Advisories. pp. 8, 719–726, 731–732. [Google Scholar]
- 35.WHO . 4th ed. 2011. WHO Guidelines for Drinking Water Quality; pp. 72–475. Geneva. [Google Scholar]
- 36.NIS (Nigerian Industrial Standard) 2007. Nigerian Standard for Drinking Water Quality. Approved by Standard Organisation of Nigeria, Lagos Nigeria. [Google Scholar]
- 37.Adepoju-Bello A.A., Oguntibeju O.O., Onuegbu M.T., Ayoola G.A.A., Coker H.A.B. Analysis of selected metallic impurities in soft drinks marketed in Lagos. Niger. Afr. J. Biotechnol. 2012;11(March (20)):4676–4680. [Google Scholar]
- 38.Onianwa P.C., Adetola I.G., Iwegbue C.M.A., Ojo M.E., Tella O.O. Trace heavy metals composition of some Nigerian beverages and food drinks. Food Chem. 1999;66:275–279. [Google Scholar]
- 39.Garcia E.M., Cabrera C., Sanchez J., Lorenzo M., Lopez M.C. Chromium levels in public water, fruit juices and soft drinks: influence on dietary intake. Sci. Total Environ. 1999;241:143–150. doi: 10.1016/s0048-9697(99)00340-x. [DOI] [PubMed] [Google Scholar]
- 40.Ebenezer A., Eremasi Y. Determination of heavy metals in water sediments and tilapia zilli from Kolo-creek, ogbia local government area, Bayelsa State, Nigeria. Sci. Afr. 2012;11(1):44–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.