Abstract
The aim of this study was to estimate the protective effect of N-acetyl-l-cysteine (NAC) against cisplatin-induced cardiotoxicity under conditions of ischemic-reperfusion injury.
Wistar albino rats were randomly divided into three groups (n = 8): control, cisplatin (5 mg/kg/w, i.p., 5 weeks) and cisplatin + NAC group (cisplatin – 5 mg/kg/w, i.p. and NAC – 500 mg/kg/w, i.p., 5 weeks). Isolated hearts were perfused according to the modified Langendorff technique at constant pressure (70 cmH2O). Following cardiodynamic parameters were measured: maximum rate of left ventricular pressure development, minimum rate of left ventricular pressure development, left ventricular systolic pressure (SLVP), left ventricular diastolic pressure and heart rate. The ischemic vasodilation episodes were induced by the complete interruption of coronary inflow for 30, 60 and 120 s. The samples of the coronary venous effluent (CVE) were continuously collected during the reperfusion period for determination of coronary flow (CF) rate and oxidative stress markers (H2O2, O2−, NO2− and thiobarbituric acid reactive substances – TBARS).
Cisplatin reduced CF, heart rate and overflow (total, maximal and duration of overflow) during reperfusion, and increased SLVP (under basal conditions and after global ischemias). Cisplatin increased levels of H2O2 (under basal conditions), O2− and TBARS (under basal conditions and after ischemia), but decreased NO2− levels (during reperfusion) in CVE, and decreased superoxide dismutase and reduced glutathione in serum. NAC attenuated cisplatin-induced changes of cardiodynamic parameters (except CF under basal conditions) and oxidative stress parameters.
Those results suggest that NAC, by decreasing oxidative stress, may be useful in cardioprotection during cisplatin therapy.
Keywords: Isolated rat heart, Cisplatin, N-acetylcysteine, Ischemia-reperfusion
1. Introduction
Since the accidental discovery four decades ago, cisplatin (cis-dichlorodiammine-platinum(II), CDDP) remained one of the most commonly used chemotherapeutic drugs. The effect of this antineoplastic agent has been demonstrated against various types of tumors, such as solid tumors and hematological malignancies, including testicular, ovarian, bladder, head and neck, esophageal, stomach and lung cancer, as well as lymphoma and osteosarcoma [7]. Mechanism of cisplatin action is achieved by interaction with DNA via formation of covalent adduct [56]. However, despite of its beneficial antitumor activity, cisplatin showed numerous adverse effects and toxicities affecting gastrointestinal, renal, neurological and hematological system, even when administered at standard doses [62]. Although statistically classified as rare, cardiotoxicity is one of the most serious side effects of cisplatin therapy described in numerous studies.
This cardiotoxic manifestations of cisplatin include electrocardiographic changes, arrhythmias, cardiomyopathy, congestive heart failure [70] and tromboembolic events [45]. Ischemia syndrome, palpitation and myocardial infarction can occur during treatment with cisplatin, or even as long-term consequences [57]. Treatment with cisplatin can be also associated with occasional complete atrioventricular block, hypotension [51] and recurrent sinus bradycardia [4].
It has been reported that cisplatin is able to generate reactive oxygen species (ROS), such as hydroxyl radical and superoxide anion radical [67]. This very reactive oxygen species can cause tissue damage through reaction with biological macromolecules (lipids, proteins and nucleic acids) leading to the formation of oxidized substances [31]. Nephrotoxicity induced by cisplatin, as one of the most common side effect of cisplatin, is associated with an increase of lipid peroxidation in the kidney tissue [6]. Also, some studies showed that cisplatin treatement may induce decrease of antioxidants, such as superoxide dismutase (SOD) and reduced glutathione (GSH) in plasma [1] and this may lead to failure of the antioxidative defense mechanisms against free radical-mediated damage [66].
Cisplatin therapy is usually associated with oxidative stress that has important role in cardiovascular toxicity after cisplatin treatment [22]. Not surprisingly, there are numerous data suggesting that administration of various antioxidants may be effective in ameliorating of cisplatin-induced toxicity in various tissues [34], [47]. N-Acetyl-l-cysteine (NAC) is a cysteine analog with free radical scavenging activity, and is widely used as a chemoprotective agent [39]. Numerous studies reported beneficial effects of NAC on cisplatin-induced toxicity in various tissues and species, by means of blocking apoptosis through the caspase-signaling pathway [69], increase of intracellular GSH [58], scavenging ROS and via phospho-extracellular regulated kinase1/2 (ERK1/2) activation [72].
Aside of that, there are numerous reports concerning increase in oxygen free radicals during the reperfusion of ischemic cardiac tissue [19], [32]. Oxidative damage induced by increased ROS has been confirmed by increased levels of lipid peroxidation [25].
However, the interplay between two mechanisms that induce oxidative damage in the heart (of cisplatin and ischemic-reperfusion origin) has not been evaluated yet. This study was conducted in order to estimate the desirable protective effect of NAC (which has been proven to have antioxidant potentials) against cisplatin-induced cardiotoxicity under conditions of ischemic-reperfusion injury.
2. Material and methods
2.1. Animals and treatment
Male Wistar albino rats (3 months old, 250–300 g body weight) were used in this study. Animals (obtained from Military Medical Academy, Serbia) were maintained under well-controlled environmental conditions of temperature (23 ± 1 °C) and light (12/12 h light/dark cycle), and had free access to food and water. Twenty four rats were randomly divided into three groups (each group containing 8 rats): control, cisplatin and cisplatin + NAC group. Cisplatin group were injected (5 mg/kg/w, i.p.) for 5 weeks, while control group were injected by saline (i.p.), also for 5 weeks. Group cisplatin + NAC received cisplatin (5 mg/kg/w, i.p., Merck, France) and NAC (500 mg/kg/w, i.p., Sigma–Aldrich, Germany). Applied doses of cisplatin [60] and NAC [33] are comparable to patient treatment regimens. All experiments were performed according to EU Directive for welfare of laboratory animals (86/609/EEC) and principles of Good Laboratory Practice (GLP) approved by Ethical Committee of the Faculty of Medical Sciences, University of Kragujevac, Serbia.
2.2. Determination of coronary flow rate and cardiodynamic parameters
After short-term narcosis induced by intraperitoneal application of ketamine (10 mg/kg) and xylazine (5 mg/kg), rats were sacrified by decapitation, and after prompt thoracotomy, hearts were rapidly excised. According to the modified Langendorff technique at constant pressure (70 cmH2O), hearts were perfused (Experimetria Ltd., Hungary) via aortic cannula with Krebs–Henseleit solution (mM/l): NaCl 118, KCI 4.7, CaCl2·2H2O 2.5, MgSO4·7H2O 1.7, NaHCO3 25, KH2PO4 1.2, glucose 11, and pyruvate 2. Solution was gassed with 95% O2 plus 5% CO2, with pH maintained at 7.4 and temperature of 37 °C. Cardiodynamic parameters were measured by sensor (transducer BS4 73-0184, Experimetria Ltd., Hungary) inserted in the left ventricle. The following parameters were registered: maximum rate of left ventricular pressure development (dp/dt max), minimum rate of left ventricular pressure development (dp/dt min), left ventricular systolic pressure (SLVP), left ventricular diastolic pressure (DLVP) and heart rate (HR).
Following the equilibration period of 20 min at the 70 cmH2O, basal (preischemic) values of coronary flow (also used for biochemical assays) and cardiodynamic parameters were determined. The ischemic vasodilation episodes (short-term global ischemia) were induced by the interruption of coronary inflow (global ischemia) for 30, 60 and 120 s, respectively. The intervals between the interruptions of the coronary inflow were approximately 15–20 min. The following parameters for the coronary flow during the reperfusion were calculated: maximal (peak) overflow during reperfusion (maximal value of coronary flow rate during reperfusion comparing to basal coronary flow rate, given as percentage of increase), total overflow during reperfusion (value of total coronary flow rate during reperfusion comparing to basal coronary flow rate for the same time period, given as percentage of increase), and total duration of overflow (total duration of increased coronary flow above the basal flow rate during reperfusion, given in minutes). Values of cardiodynamic parameters were determined at the end of each minute through the reperfusion period.
2.3. Biochemical assays
The samples of the coronary venous effluent (CVE) were collected under basal conditions – before global ischemia, as well as during the reperfusion periods and prepared for further analysis – the determination of biochemical markers of myocardial injury (creatine kinase – CK and lactate dehydrogenase – LDH) and oxidative stress markers (hydrogen peroxide H2O2; superoxide anion radical – O2−; nitrite – NO2− and index of lipid peroxidation, thiobarbituric acid reactive substances – TBARS) in CVE. For determination of the levels for mentioned markers under basal conditions we collected the samples of CVE when it was considered as stable (with no change during 5 min). Levels of biochemical markers of myocardial injury and oxidative stress markers after global ischemia of different durations in CVE were estimated in samples that were collected continuously during the whole reperfusion period (until the values of CF reached basal values). Samples of adequate volumes of CVE were used for further analysis.
The samples for determination of biochemical markers of myocardial injury (CK and LDH), as well as for determination of antioxidants in blood (SOD and GSH) were collected immediately after decapitation. Freshly separated sera for estimation of CK and LDH levels in blood was obtained after centrifugation at 3000 rpm for 10 min and preserved at −20 °C until the determination of CK and LDH levels. Isolated erythrocytes were washed three times with three volumes of ice-cold 0.9 mM/l NaCl, and then lysed with dH2O (1:3, v/v) at 0 °C and preserved also at −20 °C until the determination of SOD and GSH levels in blood.
2.3.1. Determination of CK and LDH
Levels of CK and LDH in CVE and serum were determined spectrophotometrically using Roche Cobas system according to manufacturer’s protocol. The values are presented as U/L.
2.3.2. Determination of H2O2
The measurement of H2O2 is based on the oxidation of Phenol Red by H2O2 in a reaction catalyzed by horseradish peroxidase – HRPO [55]. A volume of 200 μl of perfusate was precipitated with 800 μl of fresh Phenol Red solution, along with 10 μl of 1:20HRPO (made ex tempore). An adequate volume of Krebs–Henseleit solution was used for a blank probe (instead of coronary venous effluent).
2.3.3. Determination of O2−
The level of the O2− was measured by Nitro Blue Tetrazolium reaction in TRIS-buffer with coronary venous effluent. Krebs–Henseleit solution was used as a blank probe [10].
2.3.4. Determination of NO2−
Nitrite was determined and used as an index of nitrite oxide production by the spectrophotometric method using Gries’s reagent. A total of 0.5 ml of perfusate was precipitated with 200 μl of 30% sulphosalicylic acid, vortexed for 30 min, and centrifuged at 3000 × g. Equal volumes of the supernatant and Gries’s reagent, containing 1% sulphanilamide in 5% phosphoric acid/0.1% naphthalene ethylenediamine–dihydrochloride, were added, incubated for 10 min in the dark. The nitrite levels were calculated using sodium nitrite as the standard [29].
2.3.5. Determination of TBARS
For estimation of the degree of lipid peroxidation we measured TBARS using 1% thiobarbituric acid in 0.05 NaOH incubated with the coronary effluent at 100 °C for 15 min. As a blank probe Krebs–Henseleit solution was used [49].
2.3.6. Determination of SOD
SOD activity was determined by the epinephrine method [44] in which 100 μl lysate and 1 ml carbonate buffer were mixed, and then 100 μl epinephrine was added. The activity of SOD in red blood cells (RBCs) is presented in units per gram of hemoglobin × 103 (U/g Hb × 103).
2.3.7. Determination of GSH
Level of GSH was determined according to Beutler [12] based on GSH oxidation via 5.5 dithiobis-6.2-nitrobenzoic acid (DTNB). GSH extract was obtained by combining 0.1 ml 0.1% EDTA, 50 μl erythrocyte lysate and 750 μl precipitation solution. After vortexing and extraction on cold ice for 15 min, centrifugation at 4000 rpm was performed for 10 min. 300 μl, 750 μl Na2HPO4 and 100 μl DTNB was pipetted into test tubes. As a blank probe, distilled water was used. Concentration and the amount of GSH were determined on the basis of a standard curve for each assay. For standard curve construction, standard stock-solution of GSH at a concentration 1.5 mM/l was used. The concentration is expressed as nanomoles per millilitre of erythrocytes.
All markers of oxidative stress (H2O2, O2−, NO2− and TBARS) as well as the levels of antioxidants (SOD and GSH) were measured spectrophotometrically (Shimadzu UV 1800, Japan) at 610, 530, 543 and 530 nm, respectively, for markers of oxidative stress, and 470 and 412 nm, for SOD and GSH, respectively.
2.4. Statistical analysis
All values were expressed as mean ± S.E.M. Statistical analysis was performed by IBM SPSS Statistics version 20. Variables were checked for normal distributions of the data with the Shapiro–Wilks test. Comparison of means was done by the Student’s t-test (One way ANOVA) and the Kruskal–Wallis test. Data were considered statistically significant at p value ˂0.05.
3. Results
3.1. Changes of coronary flow
Basal rates of coronary flow (before ischemia) observed in cisplatin group were significantly lower than in control group. Cisplatin plus NAC group increased basal coronary flow comparing to cisplatin group, but it was still lower comparing to control group (Table 1).
Table 1.
Parameters of the coronary flow under basal conditions and during reperfusion after global ischemia of different durations.
| Parameter | Group (treatment) | Determination of coronary flow | ||
|---|---|---|---|---|
| Basal conditions (before ischemia) | ||||
| Coronary flow (ml/min) | Control | 9.76 ± 0.70c, * | ||
| Cisplatin | 6.76 ± 0.60a, * | |||
| Cisplatin + NAC | 7.75 ± 0.26 | |||
| During reperfusion | ||||
| 30 s ischemia | 1 min ischemia | 2 min ischemia | ||
| Total overflow after ischemia of different duration (% of increase comparing to basal CF values) |
Control | 22.35 ± 5.15 | 39.19 ± 10.34 | 72.43 ± 17.25 |
| Cisplatin | 7.38 ± 4.88a, * | 12.00 ± 5.02a, * | 8.61 ± 6.70a, * | |
| Cisplatin + NAC | 34.22 ± 2.86b, ** | 68.83 ± 6.34b, ** | 103.33 ± 13.07b, ** | |
| Maximal overflow after ischemia of different duration (% of increase comparing to basal CF values) |
Control | 17.30 ± 1.31c, * | 24.59 ± 4.65 | 26.25 ± 4.48c, * |
| Cisplatin | 5.64 ± 2.56a, * | 9.03 ± 2.27a, * | 5.41 ± 3.63a, * | |
| Cisplatin + NAC | 22.63 ± 1.69b, ** | 36.76 ± 0.87b, ** | 42.55 ± 1.43b, ** | |
| Duration of overflow after ischemia of different duration (in minutes) |
Control | 2.00 ± 0.32 | 2.60 ± 0.60c, * | 4.00 ± 0.63 |
| Cisplatin | 0.80 ± 0.37a, * | 1.80 ± 0.90 | 1.00 ± 0.71a, * | |
| Cisplatin + NAC | 2.50 ± 0.29b, * | 4.50 ± 0.29b, ** | 4.75 ± 0.25b, * | |
Values are expressed as mean ± S.E.M. for eight animals.
Control vs. cisplatin.
Cisplatin vs. cisplatin + NAC.
Control vs. cisplatin + NAC.
p < 0.05.
p < 0.01.
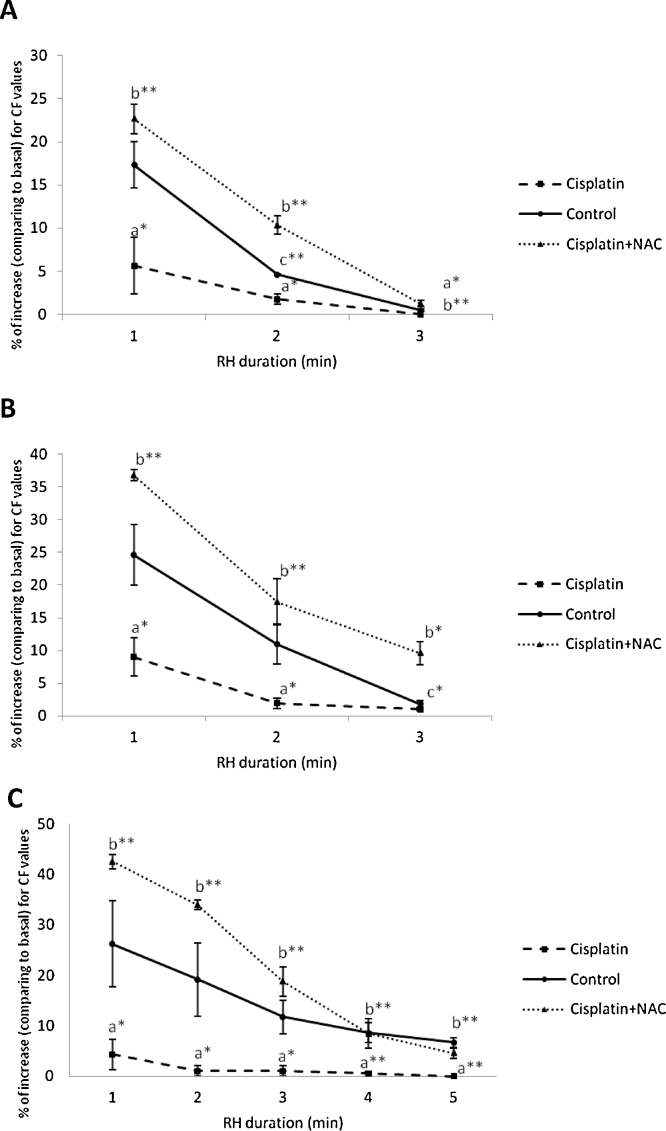
The total overflow after global ischemia gradually increased in control group following increase in duration of ischemia. In cisplatin group, total overflow remained the same values, significantly lower comparing to control, with no change after the elongation of global ischemia. NAC administration along with cisplatin significantly increased total overflow during reperfusion for all three durations of global ischemia comparing to cisplatin group (Table 1).
Maximal (peak) flow during reperfusion progressively enhanced following the increase in duration of global ischemia. Peak flow in cisplatin group was almost constant, at significantly lower levels, for all three durations of ischemia comparing to control group. Cisplatin plus NAC group showed significant increase in maximal flow following global ischemia of all three different durations comparing to the cisplatin group (Table 1). This increase in maximal flow during reperfusion was even significantly higher comparing to control group (for global ischemia of 30 s and 2 min).
The total duration of overflow observed in control group showed progressive increase depending on the duration of global ischemia. Total duration of overflow during reperfusion was significantly lower comparing to control group, maintaining the same values regardless of the ischemia duration. Simultaneous administration of NAC with cisplatin significantly increased total duration of overflow during reperfusion comparing to the cisplatin group for all three different durations of global ischemia. This effect of NAC on duration of overflow was above the values in the control group after global ischemia of 1 min (Table 1).
Overflow following global ischemia observed in the cisplatin group was significantly lower comparing to control by means of all evaluated parameters (i.e., total and maximal overflow, duration of overflow) throughout the whole reperfusion period (Fig. 1). Decrease of coronary overflow in cisplatin group was manifested after global ischemia of all three durations. NAC administration along with cisplatin significantly increased coronary overflow after global ischemia comparing to cisplatin group, and even comparing to control group (for peak overflow and for the total duration of overflow).
Fig. 1.
Overflow after global ischemia of 30 (A), 60 (B) and 120 (C) seconds.
Values are expressed as mean ± S.E.M. for eight animals (*p < 0.05, **p < 0.01). a – control vs. cisplatin; b – cisplatin vs. cisplatin + NAC; c – control vs. cisplatin + NAC
3.2. Changes of cardiodynamic parameters
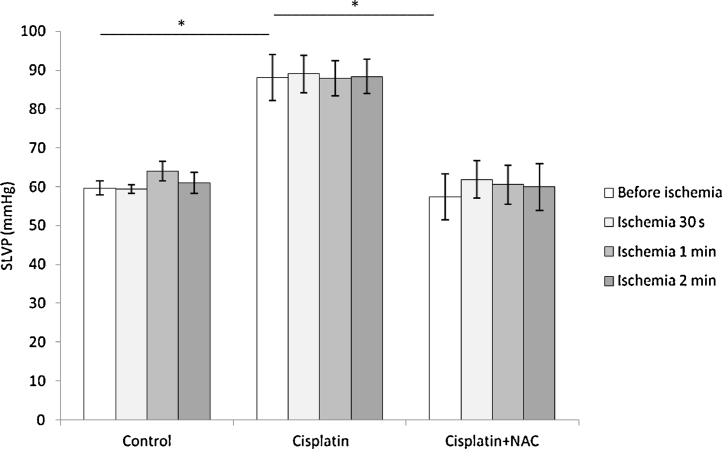
SLVP before ischemia was significantly higher in cisplatin group comparing to control group. Simultaneous administration of NAC with cisplatin significantly reduced basal SLVP (before ischemia) comparing to cisplatin group, backing SLVP almost to control values (Fig. 2). Values of SLVP did not significantly change during reperfusion period in all three groups and for all three durations of global ischemia, comparing to their basal values, maintaining the differences observed for basal values in the groups. There was no significant difference in basal DLVP between control, cisplatin and cisplatin with NAC group (5.08 ± 0.84, 4.92 ± 0.20 and 4.68 ± 0.65 mmHg, respectively). Also, DLVP did not change following global ischemia of different durations in all three groups preserving the values similar to basal.
Fig. 2.
Changes of SLVP before and after global ischemia of different durations.
Values are expressed as mean ± S.E.M. for eight animals (*p < 0.05).
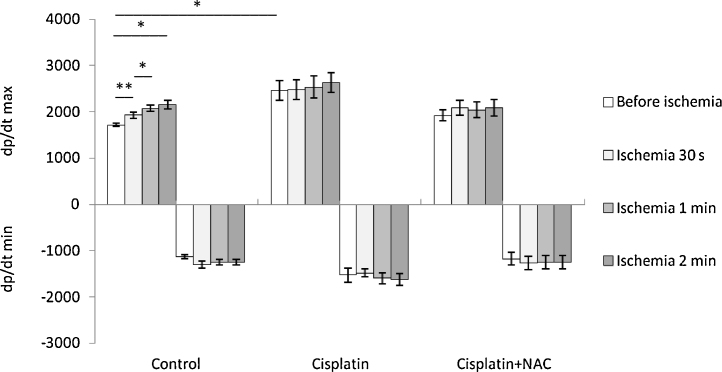
Left ventricle contractility (dp/dt max) before global ischemia was significantly higher in cisplatin group comparing to control group, while NAC supplementation attenuated this cisplatin effect on basal dp/dt max (Fig. 3, top). Contractility significantly increased during reperfusion in control group comparing to the basal values following the enhancing of ischemia duration. This was not observed in cisplatin and cisplatin plus NAC groups (Fig. 3, top). There was no significant difference in left ventricle relaxation (dp/dt min) between control, cisplatin and cisplatin plus NAC group (Fig. 3, bellow). Also, there was no change in relaxation rate during reperfusion comparing to basal (preishemic) values in all three groups for global ischemia of different durations.
Fig. 3.
Changes of dp/dt max and dp/dt min before and after global ischemia of different durations.
Values are expressed as mean ± S.E.M. for eight animals (*p < 0.05, **p < 0.01).
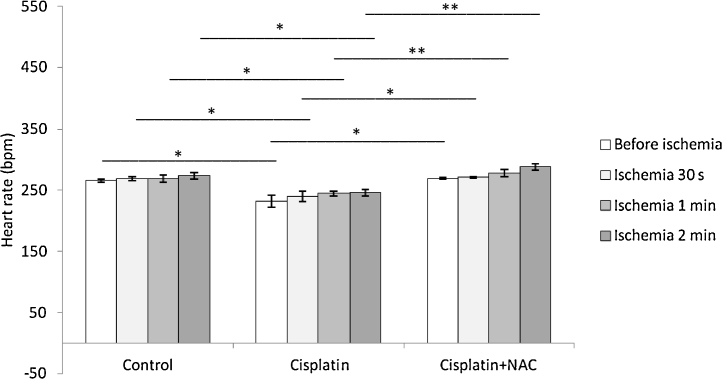
Heart rate in cisplatin group was significantly lower comparing to control group. Simultaneous administration of NAC with cisplatin significantly increased HR comparing to cisplatin group, returning HR to the values similar to control (Fig. 4). Heart rate did not change following global ischemia of different durations in all experimental groups preserving the values similar to basal.
Fig. 4.
Changes of heart rate before and after global ischemia of different durations.
Values are expressed as mean ± S.E.M. for eight animals (*p < 0.05, **p < 0.01).
3.3. Changes of markers of myocardial injury
The levels of CK in CVE under basal conditions in cisplatin group were significantly higher comparing to control (Table 2). NAC application reduced CK levels in CVE under basal conditions, but they were still above the control values. Also, CK levels in CVE following global ischemia of different durations were significantly increased in cisplatin group for all durations of ischemia comparing to control group. NAC supplementation along with cisplatin therapy abolished the increase in CK observed in cisplatin group. There were no significant changes in CK levels during reperfusion after global ischemia of any duration in cisplatin and cisplatin + NAC groups comparing to control values. Serum levels of CK in cisplatin group were also significantly higher comparing to control group. NAC supplementation reduced CK levels comparing to cisplatin group (not significantly), although the CK levels remained significantly increased comparing to control group (Table 2).
Table 2.
Levels of creatin kinase (CK) and lactate dehydrogenase (LDH) in serum and coronary venous effluent (CVE) under basal conditions and during reperfusion after global ischemia of different durations.
| Group (treatment) | Basal conditions (U/L in CVE) | 30 s ischemia (U/L in CVE) | 1 min ischemia (U/L in CVE) | 2 min ischemia (U/L in CVE) | Serum (U/L) |
|---|---|---|---|---|---|
| CK | |||||
| Control | 7.00 ± 0.96c, * | 6.33 ± 0.67 | 6.66 ± 0.41 | 7.00 ± 0.71 | 198 ± 21.55c, ** |
| Cisplatin | 12.20 ± 2.10a, * | 13.20 ± 2.92a, * | 11.70 ± 2.25a, * | 11.33 ± 2.27a, * | 374.4 ± 9.72a, ** |
| Cisplatin + NAC | 10.94 ± 1.39 | 9.33 ± 2.53 | 9.33 ± 2.48 | 10.00 ± 2.63 | 337 ± 25.70 |
| LDH | |||||
| Control | 2.33 ± 0.41c, * | 2.00 ± 0.27 | 3.33 ± 0.31 | 2.00 ± 0.27 | 223.00 ± 15.92c, * |
| Cisplatin | 4.00 ± 0.53a, * | 4.50 ± 1.01a, * | 4.17 ± 0.23a, * | 3.83 ± 0.80a, * | 491.00 ± 60.68a, ** |
| Cisplatin + NAC | 3.97 ± 0.64 | 3.00 ± 0.53 | 3.67 ± 0.82 | 3.33 ± 1.11 | 330.50 ± 17.12b, * |
Values are expressed as mean ± S.E.M. for eight animals.
Control vs. cisplatin.
Cisplatin vs. cisplatin + NAC.
Control vs. cisplatin + NAC.
p < 0.05.
p < 0.01.
LDH levels in CVE in cisplatin group were significantly higher when compared to control under basal conditions (Table 2). NAC administration along with cisplatin did not decrease LDH levels observed in cisplatin group, remaining significantly higher levels comparing to control. Cisplatin induced increase in LDH levels comparing to control group was also observed after global ischemia of all durations, while NAC supplementation failed to decrease high levels of LDH following cisplatin application comparing to control group. LDH concentrations during reperfusion following global ischemia of all durations showed no change comparing to preischemic conditions in al groups. LDH concentrations in serum after 5 weeks of cisplatin therapy were significantly higher comparing to control group. NAC administration along with chronic cisplatin therapy significantly decreased LDH levels, but they remained significantly above the control values (Table 2).
3.4. Changes of oxidative stress parameters
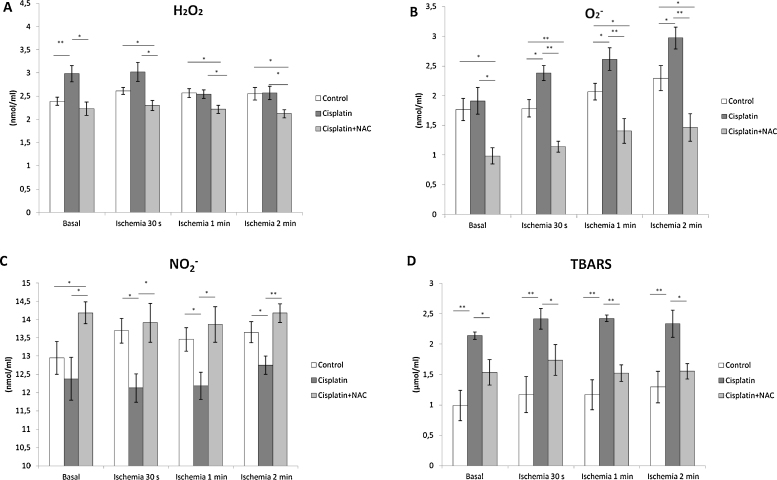
The levels of H2O2 under basal conditions (preischemic values) were significantly higher in cisplatin group comparing to control group (Fig. 5A). However, there was no significant difference in H2O2 concentration after global ischemia (of any duration) in cisplatin group comparing to control. Simultaneous administration of NAC along with cisplatin significantly reduced basal concentrations of H2O2 comparing to cisplatin group, as well as after global ischemia of all durations. Even with no difference under basal conditions, decrease in H2O2 in cisplatin plus NAC group was also significant following global ischemia (of all durations) comparing to control group. Levels of H2O2 did not significantly change following global ischemia of different durations (comparing to basal values) in all three groups preserving the values similar to differences observed under basal conditions.
Fig. 5.
Changes in oxidative stress markers (A – H2O2, B – O2−, C – NO2−, D – TBARS) in coronary venous effluent before and after global ischemia of different durations.
Values are expressed as mean ± S.E.M. for eight animals (*p < 0.05, **p < 0.01).
In contrary, O2− release in cisplatin group was not significantly higher comparing to preischemic values in control group. Concentration of O2− significantly increased after global ischemia of 30 s and maintained significantly higher levels with increasing duration of global ischemia comparing to control group (Fig. 5B). Simultaneous administration of NAC along with cisplatin significantly decreased concentration of O2− under basal conditions, as well as after global ischemia of all durations, comparing to cisplatin group. Also, decrease in O2− release in cisplatin plus NAC group was significant comparing to control group. Released O2− did not significantly change following global ischemia of different durations (comparing to basal values) in control and cisplatin plus NAC group, maintaining the values similar to observed under basal conditions. However, even with no significant change between two consecutive durations of global ischemia, O2− concentrations in cisplatin group gradually increased comparing to basal values (basal vs. 60 s and 120 s ischemia – 1.91 ± 0.22 vs. 2.61 ± 0.19 and 2.97 ± 0.18; p ˂ 0.05 and p ˂ 0.01, respectively).
NO2− release in cisplatin group was not significantly lower comparing to control group before ischemia. However, significant suppression of NO2− release was observed in cisplatin group during reperfusion after all three durations of global ischemia. NAC supplementation increased NO2− concentration comparing to cisplatin group under basal conditions, as well as after global ischemia (all three durations). Administration of NAC with cisplatin resulted in increased levels of NO2− in preischemic period with no change during reperfusion period comparing to control group after all three durations of global ischemia (Fig. 5C).
Preishemic TBARS concentration was significantly increased in cisplatin group comparing to control group (Fig. 5D). Higher levels of TBARS in cisplatin group comparing to control were also observed during reperfusion following global ischemia (all three durations). Simultaneous administration of NAC along with cisplatin significantly reduced TBARS concentration comparing to cisplatin group under basal conditions, as well as after all three durations of global ischemia. Still, TBARS levels in cisplatin plus NAC group remained above the control values (not significant) under both pre and after ischemic conditions (all three durations of global ischemia).
Blood levels of SOD and GSH observed in cisplatin group were significantly lower comparing to control group (Table 3). Simultaneous administration of NAC with cisplatin significantly increased SOD and GSH when compare to cisplain group. SOD activity following NAC application along with cisplatin therapy remained significantly lower comparing to control group (Table 3).
Table 3.
Serum levels of superoxide dismutase (SOD) and reduced glutathione (GSH).
| Group (treatment) | SOD (U/g Hgb × 103) | GSH (nM/ml erythrocytes) |
|---|---|---|
| Control | 6.00 ± 0.58c, * | 1131 ± 31.45 |
| Cisplatin | 2.40 ± 0.33a, ** | 661.67 ± 35.16a, ** |
| Cisplatin + NAC | 4.00 ± 0.58b, * | 1014.17 ± 65.45b, ** |
Values are expressed as mean ± S.E.M. for eight animals.
Control vs. cisplatin.
Cisplatin vs. cisplatin + NAC.
Control vs. cisplatin + NAC.
p < 0.05.
p < 0.01.
4. Discussion
Numerous cardiotoxic effects are reported to be accompanied with chronic cisplatin therapy, such as various types of arrhythmia, ischemic heart disease and heart failure [23]. Many of cardiac (and other) adverse effects of cisplatin alone, and in combination with some other chemotherapeutic agents, are connected with cisplatin-induced oxidative damage [17]. The aim of this study was to evaluate the effects of chronic cisplatin treatment on coronary flow and cardiodynamic parameters of the isolated rat heart after global ischemia. The protocol for cisplatin application was chosen in order to mimic cyclic administration of cisplatin in various chemotherapeutical protocols in humans [16]. Also, we evaluated the effects of NAC, as an antioxidant agent, on cisplatin-induced changes in rat heart under ischemic conditions. Applied NAC dose has been reported to have cardioprotective effects (by reducing oxidative damage) in rat heart [30].
Five weeks of cisplatin treatment (5 mg/kg/week) resulted in decreased basal coronary flow (app. 30%). Although, reduction of coronary flow was reported after acute cisplatin application in isolated perfused rat heart [43], the effect of chronic cisplatin therapy on overall coronary flow was not previously observed. Due to lack of data obtained on animal models concerning this cardiotoxic effect of cisplatin, we can only compare our results with some case reports for the patients undergoing cisplatin therapy. Observed reduction of coronary flow in our study is in line with some manifestations of ischemic heart disease accompanied with cisplatin therapy, such as coronary syndrome [52], unstable angina [36], and acute myocardial infarction [27]. Causal connection between cisplatin treatment and decrease in coronary flow could be found in the vasospasm [35] and alterations of endothelial cells integrity and increase of systemic procoagulant factors [15]. NAC supplementation along with cisplatin therapy increased basal coronary flow comparing to cisplatin alone (10–15%, not significant), but it was still below the control values (Table 1). Observed increase of coronary flow after NAC treatment leads to the conclusion that at least part of adverse effect of cisplatin on coronary flow may be attributed to increased oxidative damage. It seems that cisplatin treatment leads to both decrease of coronary flow (under basal conditions) and coronary reactivity. Those adverse effects of cisplatin were attenuated with NAC. However, NAC did not produce significant change (increase) of CF under basal conditions while that beneficial effect of NAC on CF was more pronounced during reperfusion. Such difference could be explained by the fact that ischemic events resulted in increased production of ROS, and that enabled better insight in beneficial effect of NAC in coronary circulation. The possible mechanism of NAC antioxidative effect [8] could be found in increased GSH levels caused by NAC (Table 3). This is in accordance with literature data that report beneficial effect of different antioxidants by means of the reduction of the oxidative stress induced by various chemotherapeutic agents. Cisplatin-induced oxidative damage was reduced with ginger extract [9], dl-alpha-lipoic acid [34], garlic extract [47] and ellagic acid [71].
All parameters defined in order to quantify coronary flow during reperfusion following global ischemia of different durations had been evidently changed after chronic treatment with cisplatin. Total overflow and peak (maximal) overflow, as well as the duration of overflow, after cisplatin treatment were 3–10 fold lower comparing to control. Also, there was no progressive increase of those parameters following the increase of the global ischemia duration that was observed in control group (Table 1). Adverse cisplatin effect on coronary flow during reperfusion was even more pronounced than the effect on the coronary flow under basal conditions suggesting devastating influence of chronic cisplatin treatment on the coronary vessels reactivity. This cardiotoxic effect of cisplatin was clearly observed through the whole reperfusion (Fig. 1). However, application of NAC along with cisplatin completely restored reactivity of coronary vessels. Even more, parameters of coronary flow during reperfusion following global ischemia of different durations after NAC treatment were above the values obtained in control group through the whole reperfusion period (Fig. 1). This obvious cardioprotective effect of NAC on cisplatin-induced decrease of coronary reactivity after ischemia is in line with previously reported beneficial effects of NAC in myocardial ischemia [37], [50], [61].
Chronic cisplatin treatment increased SLVP and left ventricle contractility (30% and 20%, respectively) under basal conditions, i.e., before global ischemia, with no change in DLVP and left ventricle relaxation (Fig. 2, Fig. 3). Except the left ventricle contractility in control group (gradual increase along the elongation of global ischemia), none of those left ventricular function parameters changed during reperfusion period following global ischemia (for all three durations of ischemia) suggesting that postischemic events, under this experimental protocol, did not affect left ventricular function in all experimental groups. Previous studies reported cisplatin-induced reduction of left ventricle function in mice [40] and in humans [3]. Those results are not in line with the results of our study. The opposite effects of chronic cisplatin treatment on left ventricular function parameters may be explained by species differences, as well as by different protocols (and doses) for cisplatin therapy. Our previous results show similar effects of acute cisplatin treatment (slight increase) on left ventricle [43]. Cisplatin-induced increase of SLVP and left ventricle contractility may be causally connected to increased systolic arterial pressure after long-term cisplatin therapy accompanied with renal failure [42] and increased peripheral vascular resistance due to vasospasm [35]. The both mentioned possible reasons for induced increase of left ventricle function are closely related to increased oxidative damage after cisplatin therapy [38], [63]. Therefore, it is not surprising that NAC supplementation along with cisplatin therapy reduced cisplatin-induced increase of SLVP (app. 25%) and left ventricle contractility (app. 20%, not significant). However, it still remains unclear whether this cardioprotective effect of NAC was predominantly achieved via direct action on cardiac muscle or due to decreased afterload.
Negative chronotropic effect of cisplatin was clearly observed under basal (preischemic) conditions, as well during the reperfusion period for all durations of global ischemia. Possible explanation for this cardiotoxic effect of cisplatin may be found in evidence for the deposition of cisplatin in the region of sinoatrial node [59]. It has been reported that cisplatin-induced bradicardia may be related to recurrent AV block [51] Also, negative chronotropic effect of cisplatin was observed after acute cisplatin application in isolated perfused rat heart [43]. However, NAC application in combination with cisplatin completely reversed cisplatin-induced negative chronotropic effect (Fig. 4). Cardioprotective effect of NAC on HR suggests that cisplatin-induced oxidative damage may be involved in accumulation of cisplatin in sinoatrial node and/or in the disturbances of AV conduction. Furthermore, it should be underlined that negative chronotropic effect of cisplatin, simultaneously accompanied with the marked reduction of coronary flow, may be the cause of compensatory mechanism within the heart that can rely only on positive inotropic response (Fig. 2, Fig. 3) in order to maintain required (critical) coronary flow.
It has been previously reported that chronic cisplatin treatment increased the levels of biochemical markers of myocardial injury in humans [18]. Results of this study concerning the increased levels CK and LDH after cisplatin therapy in serum are in line previous observations for cisplatin induced elevation of CK and LDH in rats [64]. Elevated levels of those markers of myocardial injury remained above the control values after NAC application along with cisplatin treatment (Table 2). However, NAC lowered CK (not significantly) and LDH (significantly) levels comparing to cisplatin therapy group alone. Beneficial effect of various antioxidants, such as dl-alpha-lipoic acid and silymarin [22] and ginger extract [9], on CK and LDH levels in blood following cisplatin therapy have been confirmed, suggesting that cisplatin-induced oxidative damage may lead to disruption of cell membranes resulting in release of intracellular proteins, such as CK, LDH and troponin T [18].
Although, there is no data concerning the effects of cisplatin on myocardial injury after global (and any other) ischemia, it was hard to believe that such pronounced disturbance of oxidative balance and coronary homeostasis would not affect the reactivity of coronary vessels during reperfusion. Indeed, CK and LDH levels in CVE were significantly higher after chronic cisplatin treatment under basal conditions (values before global ischemia), and remained higher during reperfusion following global ischemia of all durations. NAC supplementation along with cisplatin therapy failed to reduce CK and LDH levels under basal conditions, but it resulted in abolishing of cisplatin-induced increase of CK and LDH levels during reperfusion following ischemia of all durations (Table 2). Differences in CK and LDH levels observed between the effects of NAC under basal (preischemic) conditions and after ischemia could be explained by significant increase of CF during reperfusion (Fig. 1) leading to better washout and dilution of released of intracellular proteins. Beneficial effects of NAC on ischemic injury of the human and rabbit hearts have been previously reported [54]. Also, the other antioxidants (lavender flower and tribulosin) showed cardioprotective effects by means of reduced levels of myocardial injury markers in CVE during ischemia-reperfusion injury [65], [73], respectively). Furthermore, it is obvious that levels of CK and LDH did not change following global ischemia of any durations comparing to control values. This confirms that applied durations of ischemia did not caused further lesions of myocardium, but those short-term episodes of global ischemia were sufficient for testing the changes of coronary vessels reactivity between the groups.
Recent studies have demonstrated that cisplatin application interrupts redox homeostasis by stimulation of ROS overproduction [13] and reduction of antioxidative enzymes activity [22], which strongly encourage the using of free radical scavengers and antioxidants, such as NAC and others, to counteract cisplatin-induced toxicities [20], [53]. However, the vast majority of these findings come from experimental and clinical investigations on kidneys (probably due to evident nephrotoxity of cisplatin), while data related to effects of cisplatin on the cardiac muscle are still inconsistent and insufficient.
Polyunsaturated fatty acids of membrane lipids are highly susceptible to peroxidation enhanced by cisplatin activity [34]. In present study we used TBARS levels, as marker of lipid peroxidation, and found increased basal TBARS concentration in cisplatin group comparing to control, which is in accordance with previous results on similar experimental protocols [2]. The increase in TBARS levels after cisplatin treatment was also observed following all three durations of ischemia. Previous studies have shown that reperfusion-induced increasing of free radicals was paralleled with elevated rates of lipid peroxidation [14]. However, elongation of ischemia did not produce any difference in TBARS levels in both control and cisplatin group, suggesting that the most of differences in lipid peroxidation for this experimental protocol was previously achieved (during chronic cisplatin treatment) with no significant changes regarding acute ischemia/reperfusion episodes. Decrease in TBARS concentrations following NAC treatment, comparing to cisplatin group, was clearly observed under basal conditions, as well as after global ischemia of all three durations (Fig. 5D). This beneficial effect of NAC may be achieved by abolishing of cisplatin-induced oxidative damage, due to diminishing nuclear factor (NF)-κB signaling pathway [68], which has been noted to cause cell apoptosis.
NO, another ROS whose mechanism of action is widely investigated, can inhibit lipid peroxidation, and also act as an antioxidant agent regarding the cell protection from oxidative stress [21]. Thus NO influences the regulation of many biological responses such as induction and activation of genes, inhibition of thrombocyte aggregation, cytostasis, apoptosis, neurotransmission, stimulation of immune response, and relaxation of vascular smooth muscles [41]. When NO and О2− are excessively produced, they can interact and generate a highly toxic peroxynitrite (ONOO−) and thus reduces NO concentration [26]. In this investigation, NO concentrations were estimated by means of NO2− levels in coronary venous effluent. Even cisplatin had no effect on NO2− production under basal conditions, ischemia-induced response in NO2− release during reperfusion showed significant decrease after cisplatin therapy (Fig. 5C). Cisplatin-induced suppression of NO2− production was observed after all three durations of ischemia comparing to control. Possible explanation for decreased NO2− release after ischemia could be found on the fact that during reperfusion excessive amount of O2− can be produced and thus reduce the function of nitric oxide synthases [24], shortening the half-life of NO and decreasing its bioavailability. However, administration of NAC along with cisplatin resulted in significant increase in NO2− levels both under basal conditions and during reperfusion periods following ischemia of all durations. Furthermore, NAC increased NO2− production even above the levels achieved in control group under basal conditions. This certain cardioprotective effect of NAC could be also attributed to increased nitric oxide synthases activity [24].
Although with no effect on O2− release under basal conditions, cisplatin-induced increase in O2− levels after global ischemia of 30 s (maintaining higher levels following increase in duration of global ischemia) comparing to control group. This is in line with the findings observed in human population [5]. Nevertheless, NAC administration attenuates this effect of cisplatin by reducing O2− levels. Similar results have been reported on cell cultures of the infracted heart [5], confirming antioxidative potentials of this agent. Moreover, suppression of O2− production after NAC supplementation was significant even comparing to control group (Fig. 5B).
In contrary, cisplatin induced rise in basal H2O2 levels, but after global ischemia (of any duration) H2O2 lowered, resuming almost the same values comparing to control group. Observed increase in basal production of H2O2 after cisplatin administration is in accordance with literature data, confirming that cisplatin-induced oxidative stress may be considered as the major mechanism of cell apoptosis [11]. The lack of further post-ischemic increase in H2O2 levels in cisplatin group may be attributed to limited cell capacity to generate this ROS during observed period of reperfusion above already high levels achieved under basal conditions. Reduction of both basal and post-ischemic H2O2 concentrations after NAC administration confirmed its ability to reduce H2O2 production (Fig. 5A). This result is in line with recent report for muscle cell cultures [46].
Furthermore, we noticed interesting dynamic for some parameters of antioxidative defense system, by means of alterations of SOD and GSH levels (Table 3). Chronic cisplatin treatment performed in this study significantly decreased blood levels of GSH and SOD (for 40% and 60%, respectively) comparing to control group. Our results are in accordance with previously described effects of cisplatin (and its analogs) on isolated human breast cancer cells [28]. Results of that study showed that cisplatin treatment reduced the activity of antioxidant enzymes such as glutathione peroxidase and glutathione reductase and also decreased levels of small-molecule antioxidants such as GSH, vitamins E and A. Moreover, decreased levels of GSH, as well as the inhibition of the catalase and SOD activity in rats treated with cisplatin were recently confirmed [1]. NAC supplementation along with cisplatin treatment in our protocol resulted in increased intracellular GSH and SOD levels comparing to cisplatin group. However, SOD levels remained significantly lower comparing to control group. Those results are in line with the data concerning beneficial effects of antioxidants (quercetin) on ciplatin-induced nephrotoxicity in rats [1]. Furthermore, GSH, as well as superoxide dismutase and dimethylthiourea, has been shown to reduce the degree of renal failure and tubular cell damage when administered simultaneously with cisplatin in rats [48], [74]. Possible mechanism which is included in positive effects of NAC on antioxidative enzymes on molecular level is still unknown. Yet, it has been proposed that the beneficial effects of NAC, as a cell-permeable precursor of GSH, may be related to either increase of intracellular GSH, or the ability to sustain high intracellular GSH levels [58]. Moreover, it has been reported that NAC exerts its protective effect in part directly by scavenging ROS and in part via phospho-extracellular regulated kinase1/2 (ERK1/2) activation [72], suggesting that cysteinyl thiol (of NAC) provided its antioxidant properties.
In conclusion, we can summarize that chronic cisplatin treatment produced numerous adverse effects in isolated rat heart (reduction of coronary flow, heart rate, etc.). Those carditoxic effects of cisplatin even worsened after the episodes of short-term ischemia. Also, findings of present study regarding oxidative stress markers suggest that cisplatin-induced cardiotoxity may be accomplished via induction of oxidative stress and that cardiac effect of cisplatin under ischemia/reperfusion conditions depend on specific type of ROS. NAC has been showed to possess a wide range of cardioprotective efficiency, scavenging all estimated pro-oxidative markers (and enhancing antioxidant capacity of cardiomyocytes), in both pre- and post-ishemic periods, leading to restoration of cardiodynamic parameters. This data could have potential clinical importance pointing NAC as a possible adjuvant during cisplatin therapeutic algorithms.
Acknowledgements
This work was supported by Faculty of Medical Sciences (JP 01/13), University of Kragujevac, Serbia. We wish to thank Telekom Srbija a.d. who had provided much help for our work.
Contributor Information
Gvozden Rosic, Email: grosic@medf.kg.ac.rs.
Ivan Srejovic, Email: ivan_srejovic@hotmail.com.
Vladimir Zivkovic, Email: vladimirziv@gmail.com.
Dragica Selakovic, Email: dragica984@gmail.com.
Jovana Joksimovic, Email: jovana_joksimovic@yahoo.com.
Vladimir Jakovljevic, Email: drvladakgbg@yahoo.com.
References
- 1.Almaghrabi O.A. Molecular and biochemical investigations on the effect of quercetin on oxidative stress induced by cisplatin in rat kidney. Saudi J. Biol. Sci. 2015;22(2):227–231. doi: 10.1016/j.sjbs.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Majed A.A., Sayed-Ahmed M.M., Al-Yahya A.A., Aleisa A.M., Al-Rejaie S.S., Al-Shabanah O.A. Propionyl-l-carnitine prevents the progression of cisplatin-induced cardiomyopathy in a carnitine-depleted rat model. Pharmacol. Res. 2006;53(3):278–286. doi: 10.1016/j.phrs.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Altena R., Hummel Y.M., Nuver J., Smit A.J., Lefrandt J.D., de Boer R.A., Voors A.A., van den Berg M.P., de Vries E.G., Boezen H.M., Gietema J.A. Longitudinal changes in cardiac function after cisplatin-based chemotherapy for testicular cancer. Ann. Oncol. 2011;22(10):2286–2293. doi: 10.1093/annonc/mdr408. [DOI] [PubMed] [Google Scholar]
- 4.Altundağ O., Celik I., Kars A. Recurrent asymptomatic bradycardia episodes after cisplatin infusion. Ann. Pharmacother. 2001;35:641–642. doi: 10.1345/aph.10180. [DOI] [PubMed] [Google Scholar]
- 5.Andre L., Fauconnier J., Reboul C., Feillet-Coudray C., Meschin P., Farah C., Fouret G., Richard S., Lacampagne A., Cazorla O. Subendocardial increase in reactive oxygen species production affects regional contractile function in ischemic heart failure. Antioxid. Redox Signal. 2013;18(9):1009–1020. doi: 10.1089/ars.2012.4534. [DOI] [PubMed] [Google Scholar]
- 6.Antunes L.M., Darin J.D., Bianchi M.D. Protective effects of vitamin c against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: a dose-dependent study. Pharmacol. Res. 2000;41(4):405–411. doi: 10.1006/phrs.1999.0600. [DOI] [PubMed] [Google Scholar]
- 7.Arany I., Safirstein R.L. Cisplatin nephrotoxicity. Semin. Nephrol. 2003;23(5):460–464. doi: 10.1016/s0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 8.Atkuri K.R., Mantovani J.J., Herzenberg L.A., Herzenberg L.A. N-Acetylcysteine – a safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007;7(4):355–359. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attyah A.M., Ismail S.H. Protective effect of ginger extract against cisplatin-induced hepatotoxicity and cardiotoxicity in rats. Iraqi J. Pharm. Sci. 2012;21(1):27–33. [Google Scholar]
- 10.Auclair C., Voisin E. Nitroblue tetrazolium reduction. In: Greenwald R.A., editor. Handbook of Methods for Oxygen Radical Research. CRC Press; Boca Raton: 1985. pp. 123–132. [Google Scholar]
- 11.Berndtsson M., Hagg M., Panaretakis T., Havelka A.M., Shoshan M.C., Linder S. Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Int. J. Cancer. 2006;120:175–180. doi: 10.1002/ijc.22132. [DOI] [PubMed] [Google Scholar]
- 12.Beutler E. Reduced glutathione (GSH) In: Beutler E., editor. Red Cell Metabolism, a Manual of Biochemical Methods. Grune and Stratton; New York, NY, USA: 1975. pp. 112–114. [Google Scholar]
- 13.Chirino Y.I., Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp. Toxicol. Pathol. 2009;61:223–242. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Cordis G.A., Maulik N., Das D.K. Detection of oxidative stress in heart by estimating the dinitrophenylhydrazine derivative of malonaldehyde. J. Mol. Cell. Cardiol. 1995;27(8):1645–1653. doi: 10.1016/s0022-2828(95)90656-8. [DOI] [PubMed] [Google Scholar]
- 15.Czaykowski P.M., Moore M.J., Tannock I.F. High risk of vascular events in patients with urothelial transitional cell carcinoma treated with cisplatin based chemotherapy. J. Urol. 1998;160(6 Pt 1):2021–2024. doi: 10.1097/00005392-199812010-00022. [DOI] [PubMed] [Google Scholar]
- 16.Daugaard G., Abildgaard U., Holstein-Rathlou N.H., Bruunshuus I., Bucher D., Leyssac P.P. Renal tubular function in patients treated with high-dose cisplatin. Clin. Pharmacol. Ther. 1988;44(2):164–172. doi: 10.1038/clpt.1988.132. [DOI] [PubMed] [Google Scholar]
- 17.Demkow U., Białas-Chromiec B., Stelmaszczyk-Emmel A., Radzikowska E., Wiatr E., Radwan-Rohrenschef P., Szturmowicz M. The cardiac markers and oxidative stress parameters in advanced non-small cell lung cancer patients receiving cisplatin-based chemotherapy. eJIFCC. 2011;22(1) [PMC free article] [PubMed] [Google Scholar]
- 18.Demkow U., Stelmaszczyk-Emmel A. Cardiotoxicity of cisplatin-based chemotherapy in advanced non-small cell lung cancer patients. Respir. Physiol. Neurobiol. 2013;18(1):64–67. doi: 10.1016/j.resp.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Dhalla N.S., Elmoselhi A.B., Hata T., Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc. Res. 2000;47(3):446–456. doi: 10.1016/s0008-6363(00)00078-x. [DOI] [PubMed] [Google Scholar]
- 20.Dickey D.T., Wu Y.J., Muldoon L.L., Neuwelt E.A. Protection against cisplatin induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular and in vivo levels. J. Pharmacol. Exp. Ther. 2005;314:1052–1058. doi: 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- 21.Eiserich J.P., Patel R.P., O’Donnell V.B. Pathophysiology of nitric oxide and related species: free radical reactions and modification of biomolecules. Mol. Aspects Med. 1998;19(4–5):221–357. doi: 10.1016/s0098-2997(99)00002-3. [DOI] [PubMed] [Google Scholar]
- 22.El-Awady el-S.E., Moustafa Y.M., Abo-Elmatty D.M., Radwan A. Cisplatin-induced cardiotoxicity: mechanisms and cardioprotective strategies. Eur. J. Pharmacol. 2011;650(1):335–341. doi: 10.1016/j.ejphar.2010.09.085. [DOI] [PubMed] [Google Scholar]
- 23.Ewer M.S., Yeh E.T.H. Second ed. People’s Medical Publishing House; USA: 2013. Cancer and the Heart. [Google Scholar]
- 24.Fan N.C., Tsai C.M., Hsu C.N., Huang L.T., Tain Y.L. N-Acetylcysteine prevents hypertension via regulation of the ADMA-DDAH pathway in young spontaneously hypertensive rats. Biomed. Res. Int. 2013;2013:696317. doi: 10.1155/2013/696317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.J.C. Fantinelli, I.A. Pérez Núñez, L.F. González Arbeláez, S.M. Mosca. Lipid Peroxidation and Reperfusion Injury in Hypertrophied Hearts, Lipid Peroxidation, In: Angel Catala (Ed.), ISBN: 978-953-51-0716-3, InTech, doi: 10.5772/45913. Available from: http://www.intechopen.com/books/lipid-peroxidation/lipid-peroxidation-and-reperfusion-injury-in-hypertrophied-heartsPubMed PMID: 8498563 (2012).
- 26.Ferdinandy P., Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br. J. Pharmacol. 2003;138(4):532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukunaga T., Soejima H., Sugamura K., Kojima S., Sugiyama S., Sakamoto T., Yoshimura M., Tanoue T., Kageshita T., Ono T., Ogawa H. Acute myocardial infarction induced by cisplatin based combination chemotherapy for malignant melanoma: a case report. J. Cardiol. 2006;47(4):191–195. [PubMed] [Google Scholar]
- 28.Gęgotek A., Cyuńczyk M., Łuczaj W., Bielawska A., Bielawski K., Skrzydlewska E. The redox status of human breast cancer cell lines (MCF-7 and MDA-MB231) treated with novel dinuclear berenil-platinum(II) complexes. Pharmazie. 2014;69(12):923–928. [PubMed] [Google Scholar]
- 29.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 30.Haleagrahara N., Julian V., Chakravarthi S. N-Acetylcysteine offers cardioprotection by decreasing cardiac lipid hydroperoxides and 8-isoprostane level in isoproterenol-induced cardiotoxicity in rats. Cardiovasc. Toxicol. 2011;11(4):373–381. doi: 10.1007/s12012-011-9132-0. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141(2):312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry T.D., Archer S.L., Nelson D., Weir E.K., From A.H. Postischemic oxygen radical production varies with duration of ischemia. Am. J. Physiol. 1993;264(5 Pt 2):H1478–H1484. doi: 10.1152/ajpheart.1993.264.5.H1478. [DOI] [PubMed] [Google Scholar]
- 33.Hurd R.W., Wilder B.J., Helveston W.R., Uthman B.M. Treatment of four siblings with progressive myoclonus epilepsy of the Unverricht–Lundborg type with N-acetylcysteine. Neurology. 1996;47(5):1264–1268. doi: 10.1212/wnl.47.5.1264. [DOI] [PubMed] [Google Scholar]
- 34.Hussein A., Ahmed A.A., Shouman S.A., Sharawy S. Ameliorating effect of dl-α-lipoic acid against cisplatin-induced nephrotoxicity and cardiotoxicity in experimental animals. Drug Discov. Ther. 2012;6(3):147–156. [PubMed] [Google Scholar]
- 35.Içli F., Karaoğuz H., Dinçol D., Demirkazik A., Günel N., Karaoğuz R., Uner A. Severe vascular toxicity associated with cisplatin-based chemotherapy. Cancer. 1993;72(2):587–593. doi: 10.1002/1097-0142(19930715)72:2<587::aid-cncr2820720242>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 36.Khan S., Chen C.L., Brady M.S., Parameswaran R., Moore R., Hassoun H., Carvajal R.D. Unstable angina associated with cisplatin and carboplatin in a patient with advanced melanoma. J. Clin. Oncol. 2012;30(18):e163–e164. doi: 10.1200/JCO.2011.38.7852. [DOI] [PubMed] [Google Scholar]
- 37.Khanna G., Diwan V., Singh M., Singh N., Jaggi A.S. Reduction of ischemic, pharmacological and remote preconditioning effects by an antioxidant N-acetylcysteine pretreatment in isolated rat heart. Yakugaku Zasshi. 2008;128(3):469–477. doi: 10.1248/yakushi.128.469. [DOI] [PubMed] [Google Scholar]
- 38.Laursen J.B., Somers M., Kurz S., McCann L., Warnholtz A., Freeman B.A., Tarpey M., Fukai T., Harrison D.G. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103(9):1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 39.Links M., Lewis C. Chemoprotectants: a review of their clinical pharmacology and therapeutic efficacy. Drugs. 1999;57(3):293–308. doi: 10.2165/00003495-199957030-00003. [DOI] [PubMed] [Google Scholar]
- 40.Ma H., Jones K.R., Guo R., Xu P., Shen Y., Ren J. Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: role of endoplasmic reticulum stress. Clin. Exp. Pharmacol. Physiol. 2010;37(4):460–465. doi: 10.1111/j.1440-1681.2009.05323.x. [DOI] [PubMed] [Google Scholar]
- 41.Mayer B., Hemmens B. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem. Sci. 1997;22(12):477–481. doi: 10.1016/s0968-0004(97)01147-x. [DOI] [PubMed] [Google Scholar]
- 42.Meinardi M.T., Gietema J.A., van der Graaf W.T., van Veldhuisen D.J., Runne M.A., Sluiter W.J., de Vries E.G., Willemse P.B., Mulder N.H., van den Berg M.P., Koops H.S., Sleijfer D.T. Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. J. Clin. Oncol. 2000;18(8):1725–1732. doi: 10.1200/JCO.2000.18.8.1725. [DOI] [PubMed] [Google Scholar]
- 43.Misic M.M., Jakovljevic V.L., Bugarcic Z.D., Zivkovic V.I., Srejovic I.M., Barudzic N.S., Djuric D.M., Novokmet S.S. Platinum complexes-induced cardiotoxicity of isolated, perfused rat heart: comparison of Pt(II) and Pt(IV) analogues versus cisplatin. Cardiovasc. Toxicol. 2014 doi: 10.1007/s12012-014-9293-8. [Epub ahead of print] PubMed PMID: 25404470. [DOI] [PubMed] [Google Scholar]
- 44.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 45.Moore R.A., Adel N., Riedel E., Bhutani M., Feldman D.R., Tabbara N.E., Soff G., Parameswaran R., Hassoun H. High incidence of thromboembolic events in patients mtreated with cisplatin-based chemotherapy: a large retrospective analysis. J. Clin. Oncol. 2011;29(25):3466–3473. doi: 10.1200/JCO.2011.35.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moraes L.H., Bollineli R.C., Mizobuti D.S., Silveira Ldos R., Marques M.J., Minatel E. Effect of N-acetylcysteine plus deferoxamine on oxidative stress and inflammation in dystrophic muscle cells. Redox Rep. 2015;20(3):109–115. doi: 10.1179/1351000214Y.0000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nasr A.Y. Protective effect of aged garlic extract against the oxidative stress induced by cisplatin on blood cells parameters and hepatic antioxidant enzymes in rats. Toxicol. Rep. 2014;1:682. doi: 10.1016/j.toxrep.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noori S., Mahboob T. Antioxidant effect of carnosine pretreatment on cisplatin-induced renal oxidative stress in rats. Indian J. Clin. Biochem. 2010;25(1):86–91. doi: 10.1007/s12291-010-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira D.M., Gomes E.S., Mussivand T., Fiorelli A.I., Gomes O.M. Effects of n-acetylcysteine on ischemic preconditioning: study in isolated rat hearts. Rev. Bras. Cir. Cardiovasc. 2009;24(1):23–30. doi: 10.1590/s0102-76382009000100006. [DOI] [PubMed] [Google Scholar]
- 51.Ozcan T., Cirit A., Kiykim A. Recurrent complete atrioventricular block during cisplatin infusion: a case report. J. Clinic. Exp. Cardiol. 2011;2(151) [Google Scholar]
- 52.Paiva C.E., Michelin O.C., Okoshi K. Acute coronary syndrome during chemotherapy: report of three cases. Rev. Bras. Cancerol. 2009;55(1):55–58. [Google Scholar]
- 53.Panchuk R., Skorokhyd N., Chumak V., Lehka L., Omelyanchik S., Gurinovich V., Moiseenok A., Heffeter P., Berger W., Stoika R. Specific antioxidant compounds differentially modulate cytotoxic activity of doxorubicin and cisplatin: in vitro and in vivo study. Croat. Med. J. 2014;55(3):206–217. doi: 10.3325/cmj.2014.55.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng Y.W., Buller C.L., Charpie J.R. Impact of N-acetylcysteine on neonatal cardiomyocyte ischemia-reperfusion injury. Pediatr. Res. 2011;70(1):61–66. doi: 10.1203/PDR.0b013e31821b1a92. [DOI] [PubMed] [Google Scholar]
- 55.Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods. 1980;38(1–2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- 56.Pil P.M., Lippard S.J. Specific binding of chromosomal protein HMG1 to DNA damaged by the anticancer drug cisplatin. Science. 1992;256(5054):234–237. doi: 10.1126/science.1566071. [DOI] [PubMed] [Google Scholar]
- 57.Ryberg M. Recent advances in cardiotoxicity of anticancer therapies. Am. Soc. Clin. Oncol. 2012;32:555–559. doi: 10.14694/EdBook_AM.2012.32.40. [DOI] [PubMed] [Google Scholar]
- 58.Saito C., Zwingmann C., Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51(1):246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schlaeffer F., Tovi F., Leiberman A. Cisplatin-induced bradycardia. Drug Intell. Clin. Pharm. 1983;17(12):899–901. doi: 10.1177/106002808301701207. [DOI] [PubMed] [Google Scholar]
- 60.Ta L.E., Low P.A., Windebank A.J. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol. Pain. 2009;26:5–9. doi: 10.1186/1744-8069-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang L.D., Sun J.Z., Wu K., Sun C.P., Tang Z.M. Beneficial effects of N-acetylcysteine and cysteine in stunned myocardium in perfused rat heart. Br. J. Pharmacol. 1991;102(3):601–606. doi: 10.1111/j.1476-5381.1991.tb12219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsang R.Y., Al-Fayea T., Au H.J. Cisplatin overdose toxicities and management. Drug Saf. 2009;32(12):1109–1122. doi: 10.2165/11316640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 63.Vijayan F.P., Rani V.K., Vineesh V.R., Sudha K.S., Michael M.M., Padikkala J. Protective effect of Cyclea peltata Lam on cisplatin-induced nephrotoxicity and oxidative damage. J. Basic Clin. Physiol. Pharmacol. 2007;18(2):101–114. doi: 10.1515/jbcpp.2007.18.2.101. [DOI] [PubMed] [Google Scholar]
- 64.Wang J., He D., Zhang Q., Han Y., Jin S., Qi F. Resveratrol protects against cisplatin-induced cardiotoxicity by alleviating oxidative damage. Cancer Biother. Radiopharm. 2009;24(6):675–680. doi: 10.1089/cbr.2009.0679. [DOI] [PubMed] [Google Scholar]
- 65.Wang D., Guo X., Zhou M., Han J., Han B., Sun X. Cardioprotective effect of the aqueous extract of lavender flower against myocardial ischemia/reperfusion injury. J. Chem. 2014;2014 Article ID 368376, 6 p. [Google Scholar]
- 66.Weijl N.I., Hopman G.D., Wipkink-Bakker A., Lentjes E.G., Berger H.M., Cleton F.J., Osanto S. Cisplatin combination chemotherapy induces a fall in plasma antioxidants of cancer patients. Ann. Oncol. 1998;9(12):1331–1337. doi: 10.1023/a:1008407014084. [DOI] [PubMed] [Google Scholar]
- 67.Wozniak K., Czechowska A., Blasiak J. Cisplatin-evoked DNA fragmentation in normal and cancer cells and its modulation by free radical scavengers and the tyrosine kinase inhibitor STI571. Chem. Biol. Interact. 2004;147(3):309–318. doi: 10.1016/j.cbi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 68.Wu X.Y., Luo A.Y., Zhou Y.R., Ren J.H. N-Acetylcysteine reduces oxidative stress, nuclear factor-(B activity and cardiomyocyte apoptosis in heart failure. Mol. Med. Rep. 2014;10(2):615–624. doi: 10.3892/mmr.2014.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Y.J., Muldoon L.L., Neuwelt E.A. The chemoprotective agent N-acetylcysteine blocks cisplatin-induced apoptosis through caspase signaling pathway. J. Pharmacol. Exp. Ther. 2005;312(2):424–431. doi: 10.1124/jpet.104.075119. [DOI] [PubMed] [Google Scholar]
- 70.Yousef M.I., Saad A.A., El-Shennawy L.K. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem. Toxicol. 2009;47(6):1176–1183. doi: 10.1016/j.fct.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 71.Yüce A., Ateşşahin A., Ceribaşi A.O., Aksakal M. Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic. Clin. Pharmacol. Toxicol. 2007;101(5):345–349. doi: 10.1111/j.1742-7843.2007.00129.x. [DOI] [PubMed] [Google Scholar]
- 72.Zhang F., Lau S.S., Monks T.J. The cytoprotective effect of N-acetyl-l-cysteine against ROS-induced cytotoxicity is independent of its ability to enhance glutathione synthesis. Toxicol. Sci. 2011;120(1):87–97. doi: 10.1093/toxsci/kfq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang S., Li H., Yang S.J. Tribulosin protects rat hearts from ischemia/reperfusion injury. Acta Pharmacol. Sin. 2010;31(6):671–678. doi: 10.1038/aps.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zunino F., Pratesi G., Micheloni A., Cavalletti E., Sala F., Tofanetti O. Protective effect of reduced glutathione against cisplatin-induced renal and systemic toxicity and its influence on the therapeutic activity of the antitumor drug. Chem. Biol. Interact. 1989;70(1–2):89–101. doi: 10.1016/0009-2797(89)90065-3. [DOI] [PubMed] [Google Scholar]