Abstract
Campomanesia adamantium (Myrtaceae) is an antioxidant compounds-rich Brazilian fruit popularly known as gabiroba. In view of this, it was evaluated the hepatoprotective effects of pulp (GPE) or peel/seed (GPSE) hydroalcoholic extracts of gabiroba on injured liver-derived HepG2 cells by CCl4 (4 mM). The results showed the presence of total phenolic in GPSE was (60%) higher when compared to GPE, associated with interesting antioxidant activity using DPPH• assay. Additionally, HPLC chromatograms and thin layer chromatography of GPE and GPSE showed the presence of flavonoids. Pretreatment of HepG2 cells with GPE or GPSE (both at 800–1000 μg/mL) significantly (p < 0.0001) protected against cytotoxicity induced by CCl4. Additionally, the cells treated with both extracts (both at 1000 μg/mL) showed normal morphology (general and nuclear) contrasting with apoptotic characteristics in the cells only exposed to CCl4. In these experiments, GPSE also was more effective than GPE. In addition, CCl4 induced a marked increase in AST (p < 0.05) and ALT (p < 0.0001) levels, while GPE or GPSE significantly (p < 0.0001) reduced these levels, reaching values found in the control group. In conclusion, the results suggest that gabiroba fruits exert hepatoprotective effects on HepG2 cells against the CCl4-induced toxicity, probably, at least in part, associated with the presence of antioxidant compounds, especially flavonoids.
Keywords: Campomanesia adamantium (Myrtaceae), Carbon tetrachloride, Flavonoids, Hepatoprotection, HepG2 cells
1. Introduction
Brazilian biodiversity has underexploited native fruit plants which have promising economic role to local population and industries [1] such as Campomanesia adamantium (Myrtaceae) from the Cerrado biome. This plant has nutritive fruits popularly known as gabiroba, which grow on small shrubs and present peculiar features such as bright colors ranging from green to yellow, with a strong citric scent and sour taste (Fig. 1).
Fig. 1.
Photomicrographic representation of Campomanesia adamantium (Myrtaceae) fruits, popularly known as gabiroba, from Cerrado, a Brazilian biome.
Scientific findings have shown that gabiroba fruits present antimicrobial activity [2], [3], antiproliferative and apoptotic activities in PC-3 human prostate carcinoma cells [4]. Moreover, in vivo studies demonstrated that these fruits cause no toxicity and have anti-inflammatory, antihyperalgesic and antidepressive activities [5]. In addition, these fruits have important nutritional elements such as iron, potassium and calcium and high levels of antioxidant compounds, particularly vitamin C and phenolic compounds (flavonoids and chalcones) [2], [3], [6]. Therefore, these fruits have been considered as a potential candidate against pathological processes in which reactive oxygen species (ROS) are a key role, such as several liver diseases [7], [8], [9].
The liver is especially susceptible to ROS due to its central role in the metabolism of nutrients and detoxification of xenobiotics which can lead to an increase in ROS production, thereby reducing cell defense capacity and establishing oxidative stress. Cell damage arising from this process may result in inflammation and fibrosis [10], [11], [12], [13].
Nowadays, research of phenolic compounds in dietary sources with hepatoprotective effect is increasing [7], [10], [14], [15]. For such purposes, the use of an established cell line for in vitro evaluation is crucial to identify potential foods. The HepG2 human hepatoma cell line presents most biochemical requirements and routes for the detoxification and metabolism of the xenobiotics of intact hepatocytes [8], representing an interesting in vitro experimental model.
Carbon tetrachloride (CCl4) is widely used for induce oxidative stress and liver injury, being extensively used in the evaluation of therapeutic potential of drugs and dietary antioxidants using HepG2 cells [16], [17], [18], [19], [20]. This xenobiotic is metabolized by cytochrome P450 2E1 in hepatocytes, generating trichloromethyl radical (CCl3•). This radical can bind to cellular molecules (nucleic acid, protein and lipid) or react with oxygen to form a highly reactive species, trichloromethylperoxy radical (CCl3OO•), impairing critical cellular events such as lipid peroxidation and DNA damage [21], [22], [23].
Given the above, the present study evaluated the phenolic content, antioxidant activity, and the presence of flavonoids as well as the in vitro hepatoprotective effect of pulp (GPE) and peel/seed (GPSE) hydroalcoholic extracts of gabiroba fruits against CCl4-induced toxicity in HepG2 cells.
2. Materials and methods
2.1. Chemicals
Folin–Ciocalteu, quercetin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), Dulbecco's modified eagle medium (DMEM), penicillin, l-glutamine, sodium bicarbonate and carbon tetrachloride (CCl4) were purchased from Sigma–Aldrich® (MO, USA). May-Grünwald–Giemsa stainings was acquired from Merck (Darmstadt, HE, Germany). Fetal bovine serum (FBS), Hoechst 33342 dye and 3-(4,5-dimetylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Invitrogen (Gibco®, NY, USA), whereas ethanol, methanol, acetonitrile, acetic acid, diphenylboryloxiethylamine and dimethyl sulfoxide (DMSO) were obtained from Vetec (RJ, Brazil).
2.2. Harvesting and processing of gabiroba fruits
Gabiroba fruits were collected in two regions of Cerrado biome located in the State of Goiás, Brazil (16°19′37″ S; 48°57′10″ O and 16°58′5″ S; 49°13′54″ O). A voucher specimen was deposited in the Federal University of Goiás Herbarium (UFG No. 47620). The fruits were stored at −40 °C before processing. Samples were pulped in a fruit and vegetable depulper (Itametal®, BA, Brazil) and the resulting materials, pulp and residue (comprising peel and seeds), were lyophilized in a Liotop L101 lyophilizer (Liobras®, SP, Brazil) and ground to a fine powder, yielding 17.3% dry pulp and 40.6% dry peel and seeds.
2.3. Preparation of pulp (GPE) and peel/seed extracts (GPSE) of gabiroba fruits
For obtaining of hydroalcoholic extracts, 70 g of dry matter were mixed with 300 mL ethanol:water (7:3, v/v) under magnetic agitation for 1 h. The mixture was filtered in Whatman No. 1 filter paper. The filtration residue was extracted twice more with 300 mL ethanol:water (7:3, v/v) and all the filtrates collected were combined and concentrated under reduced pressure at 37 °C in a rotator evaporator. The pulp (GPE) and peel/seed (GPSE) extracts were maintained at −20 °C for further use.
2.4. Determination of total phenolic content of GPE and GPSE
Total phenolic content was determined by using the Folin–Ciocalteu method, according to Swain and Hillis [24], with modifications. Aliquots of both extracts were diluted in distilled water to form a stock solution (0.5 mg/mL) and were allowed to react with 10% Folin–Ciocalteu reagent and 7.5% sodium carbonate solution in glass tubes. The reaction mixture was maintained in a water bath at 60 °C for 5 min. Color development was measured at 772 nm and the amount of phenolic compounds was expressed as the gallic acid equivalent (GAE) mg/100 g of fresh material.
2.5. Determination of the antioxidant potential of GPE and GPSE
The antioxidant activity of fruits extracts was determined by the DPPH• (2,2-diphenyl-1-picrylhydrazil) free radical-scavenging method, as described by Brand-Williams et al. [25], with modifications. Briefly, 50 μL of GPE (0, 50, 75, 125, 150 or 200) or GPSE (0, 20, 25, 30, 40 or 50 μg/mL) ethanolic solution were evaluated against 150 μL DPPH (0.3 mM) in ethanol. Quercetin was used as a standard for comparison. After 30 min, absorbance was measured using a microplate reader (Thermo Scientific Multiskan® Spectrum, MA, USA) at 517 nm. The antioxidant activity of the samples was performed in triplicate and a nonlinear fit curve was obtained to determine EC50 (sample concentration necessary to reduce free radicals’ initial concentration by 50%). It is assumed that the lower the EC50 value, the higher the antioxidant potential of the extract.
2.6. Chromatographic profiles by HPLC and qualitative investigation of flavonoids through thin layer chromatography of GPE and GPSE
Chromatographic profiles of GPE and GPSE were determined by high performance liquid chromatography in a Waters e2695 chromatograph (Milford, MA, USA), using a Zorbax XDB C18 column (Santa Clara, CA, USA) (25 cm × 4.6 mm × 5 μm). The mobile phase was eluted by linear gradient composed of spectroscopic grade methanol (A) and acetonitrile (B) and 2% ultrapure water acidified with 2% acetic acid (C). During 10 min, the proportion was 15% (A), 17% (B) and 68% (C). At 12 min, it was 26% (A), 30% (B) and 44% (C) and remained like that for 16 min. Between 16 and 17 min, the mobile phase returned to its initial proportion and remained thus for 5 min. The injection volume was of 10 μL and flow rate was of 1 mL/min. Waters 2998 Photodiode array detector (Milford, MA, USA) recorded absorbances from 200 to 430 nm and data were collected using Empower software. All chromatographic analyses were performed at 25 °C.
Thin layer chromatography was performed to confirm the presence of flavonoids in the samples as following: 10 μL of a 0.3 mg/mL solution of both extracts in methanol was applied to an aluminum plate coated with a thin layer of silica gel (Silicycle®, Quebec, Canada). The mobile phase consisted of toluene: ethyl acetate: formic acid in the proportion of 25:20:5 (v/v/v) and, after the elution, the plate was left at room temperature for 15 min. The revealer, diphenylboryloxiethylamine diluted in methanol, was applied and the plates were observed under UV light at 365 nm. The occurrence of yellow spots indicated the presence of flavonoids.
2.7. Evaluation of hepatoprotective activity of GPE and GPSE in HepG2 cells exposed to CCl4
2.7.1. Cell culture
HepG2 cell line was purchased from the Rio de Janeiro Cell Bank at the Paul Ehrlich Technical Scientific Association (RJ, Brazil). HepG2 cells were cultured in DMEM medium supplemented with 10% FBS, HEPES (4.5 mM), penicillin (100 U/mL), l-glutamine (2 mM) and sodium bicarbonate (0.17 M). Cells were maintained under standard culture conditions at 37 °C and 5% CO2 in a humid environment and trypsinized for experiments whenever the cellular confluence was 70%.
2.7.2. Cytotoxicity assay
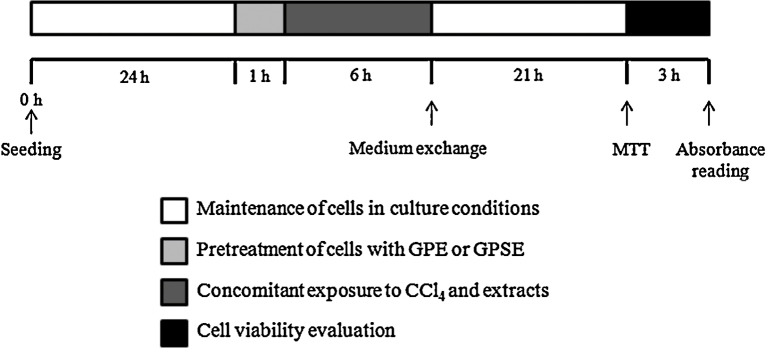
The assessment of the hepatoprotective effect of GPE and GPSE was performed using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) reduction assay, adapted from Mosmann [26]. The experimental design was done as described by Deb et al. [27], with modifications (Fig. 2). Cells (1 × 105 cells/well) were seeded in 96-well plates and remained for 24 h in culture conditions in order to adhere to them. The experimental design involved pre-treatment of HepG2 cells with GPE or GPSE (both at 800, 850, 900, 950 or 1000 μg/mL, diluted in DMEM) for 1 h followed by exposure with CCl4 (4 mM, diluted in 0.5% DMSO) for 6 h. Control extracts groups were carried out without CCl4 exposure. At the exposure time, the medium was exchanged and after 21 h, sufficient time for the cells to restore metabolic functions after the insult, 10 μL of MTT (5 mg/mL) were added to each well and the plates were incubated for 3 h in culture conditions. Supernatants were removed and 100 μL of DMSO were added to each well to solubilize the formazan crystals. The absorbance was measured on a microplate reader at 570 nm.
Fig. 2.
Experimental design of the evaluation of pulp (GPE) and peel/seed extracts (GPSE) of gabiroba fruits on CCl4-induced cytotoxicity. HepG2 cells were pre-treated with GPE or GPSE (both at 800, 850, 900, 950 or 1000 μg/mL) for 1 h and then exposed to CCl4 (4 mM) for 6 h. Posteriorly, the medium was exchanged and after 21 h, sufficient time for the cells to restore metabolic functions after the insult, the cell viability was evaluated by the MTT assay.
2.7.3. Cell morphology evaluation
Cell morphology was evaluated using May-Grünwald–Giemsa dye [28]. Round sterile coverslips (HDA Instruments® Co., Jiangsu, China) were placed in the wells of a 6-well plate. Cells (1 × 106 cells/well) were plated and incubated for 24 h in culture conditions. Then, the assay for hepatoprotective activity was carried out. However, only the concentration of 1000 μg/mL of GPE or GPSE was applied, because it exerted the best effect on cell viability. At the end of the experiment, the coverslips were withdrawn from each well, washed with PBS and left at room temperature until completely dry. Coverslips were placed over glass slides (Exacta®, SP, Brazil) using resin to attach them. Cells were stained for 3 min, washed and left to dry at room temperature. The slides were observed under a light microscope (AxioScope A1 Carl ZEISS®, Gottingen, Germany) using 100× magnification and photographed using an AxioCam MRc Carl Zeiss camera and AxioVs40V 4.7.2.0 Carl Zeis software.
2.7.4. Nuclei morphology evaluation
Apoptotic nuclear changes were assessed using the DNA binding dye Hoechst 33342 as described by Mota et al. [29]. HepG2 cells (1 × 106 cells/well) were cultured over coverslips in 6 well plates and pre-treated with GPE or GPSE (both at 1000 μg/mL) followed by exposure to CCl4. Then, cells were fixed with methanol for 5 min, washed with ice-cold PBS T20 twice and stained with 100 μL of Hoechst 33342 (10 μg/mL, diluted in PBS) for each slide, for 20 min. The slides were observed under a fluorescence microscope (Leica DMI 4000B®, Solms, Germany) using 40× magnification and photographed using a Leica DCF 340 FX® camera.
2.7.5. Activities of ALT and AST in cell supernatants
An evaluation of the activities of the liver-specific enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) is recommended for the assessment of hepatocellular function [30]. For this purpose, HepG2 cells (1 × 106 cells/well) were seeded in 24 well plates, incubated for 24 h in culture conditions and subsequently pre-treated with GPE or GPSE (both at 1000 μg/mL) followed by exposure to CCl4. After supernatants were collected and centrifuged at 5000 rpm for 5 min using a microtube centrifuge (Espresso centrifuge, Thermo Electron Corporation®, Waltham, MA, USA). Analyses of the enzymatic activities of AST and ALT were performed according to the manufacturer's instructions on their respective analysis kit (LabTest®, SP, Brazil).
2.8. Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.01 software for windows (San Diego, CA, USA). The data were expressed as the mean ± standard deviation of three independent experiments in triplicates. The inter group variation was measured by one-way Analysis of Variance (ANOVA) followed by Bonferroni's test for cytotoxicity and aminotransferases assays and Tukey test for antioxidant activity. The Student's t test was applied to compare the results of total phenolic content. Statistical significance was established as p < 0.05.
3. Results
3.1. Total phenolic content and antioxidant activity of GPE and GPSE
Results of total phenolic content and antioxidant activity analysis are shown in Table 1. The total phenolic content of both extracts was measured by Folin–Ciocalteu, and the values found of phenolic compounds for GPSE (515.9 mg AGE/100 g fw) were 60% higher than those found in GPE (324.8 mg AGE/100 g fw) (p < 0.001). This data correlates with that of antioxidant activity using DPPH• free radical scavenging assay. The EC50 obtained for GPSE (34 mg/L) was 74% lower than GPE (130.4 mg/L) (p < 0.05), showing that GPSE is more potent showing a higher antioxidant activity.
Table 1.
Total phenolic contents measured by Folin–Ciocalteu method and antioxidant activity using DPPH• free radical scavenging assay of both pulp (GPE) and peel/seed extracts (GPSE) of gabiroba fruits.
| Total phenolic content (mg AGE/100 g fw) | Antioxidant activity (EC50 mg/L) | |
|---|---|---|
| GPE | 324.8 ± 8.5 | 130.4 ± 3.5b |
| GPSE | 515.9 ± 4.4a | 34 ± 0.9b,c |
Data are expressed as means ± SD of three independent experiments in triplicate.
p < 0.001. Student's t test, p < 0.05.
p < 0.0001 vs. quercetin (3.5 ± 0.1 mg/L).
p < 0.0001 vs. GPE. One way ANOVA and Tukey test, p < 0.05.
3.2. Chromatographic profiles of GPE and GPSE by HPLC
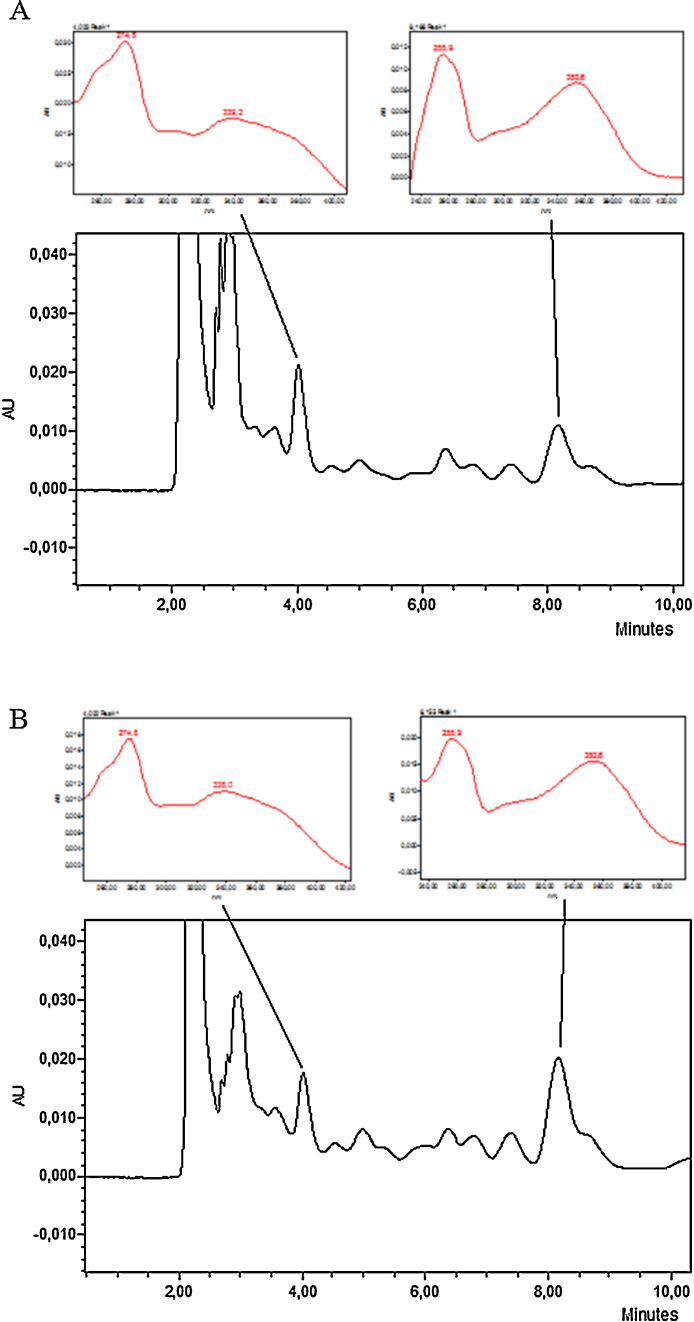
The analysis of chromatogram spectrums of GPE and GPSE showed compounds with characteristics of flavonoids, i.e. a common basic C6—C3—C6 skeleton organized into two aromatic rings: ring A occurs between two maxima of absorption bands (240–285 nm) identified as band II, and ring B occurs between 300 and 400 nm, identified as band I [31]. Two components were verified as probably being flavonoids (i.e. flavonols) in the GPE profile, the first showing band II at 274.8 nm and band I at 339.2 nm, and the second structure showing band II at 255.9 nm and band I at 353.6 nm (Fig. 3A). Similar results were observed in the GPSE chromatogram in which the first structure presented band II at 274.8 nm and band I at 338 nm, and the second presented band II absorption at 255.9 nm and band I at 356.3 nm (Fig. 3B). Along with these data, the thin layer chromatography analysis confirmed the presence of flavonoids in both extracts.
Fig. 3.
Chromatographic profiles of pulp (GPE) (A) and peel/seed extracts (GPSE) (B) of gabiroba fruits by HPLC, traced from 200 to 430 nm. UV–vis absorption spectra are highlighted and bands of absorption are characteristic of flavonoids.
3.3. Hepatoprotective activity of GPE and GPSE in HepG2 cells exposed to CCl4
3.3.1. Effects of GPE and GPSE on CCl4-induced cytotoxicity
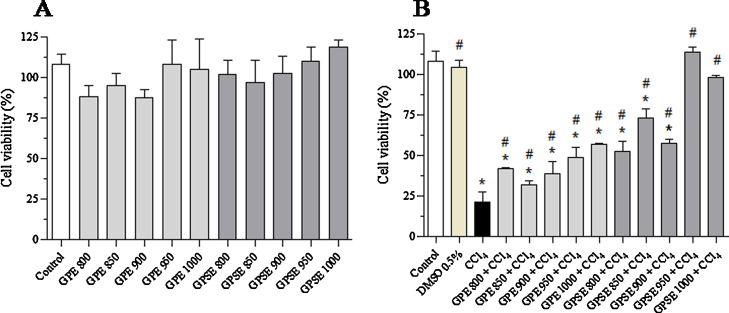
Cells only exposed to both extracts of gabiroba fruits (800–1000 μg/mL) or CCl4 vehicle (DMSO 0.5%) showed no changes in cell viability (Fig. 4A). On the other hand, HepG2 cells exposed to CCl4 (4 mM) presented a significant reduction of 79% (p < 0.0001) in cell viability, when compared to the control group. In turn, pre-treatment of HepG2 cells with all concentrations of GPE or GPSE followed by exposure to CCl4 significantly (p < 0.0001) protected against cytotoxicity. In this experiment, GPSE was more effective than GPE (Fig. 4B). The two higher concentrations of 950 and 1000 μg/mL of both extracts showed an increase of viability of 126.4 and 163.5% for GPE and 423 and 326.8% for GPSE, respectively, when compared to CCl4 group. In view of these results, 1000 μg/mL of GPE and GPSE were chosen for subsequent assays.
Fig. 4.
Effect of pulp (GPE) and peel/seed extracts (GPSE) of gabiroba fruits with or without exposure to CCl4 on cell viability of HepG2 cells. The viability of cells (1 × 105 cells/well) was assessed using MTT assay: (A) Viability of cells treated with GPE or GPSE (both at 800, 850, 900, 950 or 1000 μg/mL); (B) Viability of cells pretreated for 1 h with GPE or GPSE (both at 800, 850, 900, 950 or 1000 μg/mL) followed by exposure to CCl4 (4 mM) for 6 h. Experiments are expressed as mean ± SD of three independent experiments in triplicate (*p < 0.0001 vs. control and #p < 0.001 vs. CCl4. One way ANOVA and Bonferroni test, p < 0.05).
3.3.2. Effects of GPE and GPSE on the morphology of HepG2 cells exposed to CCl4
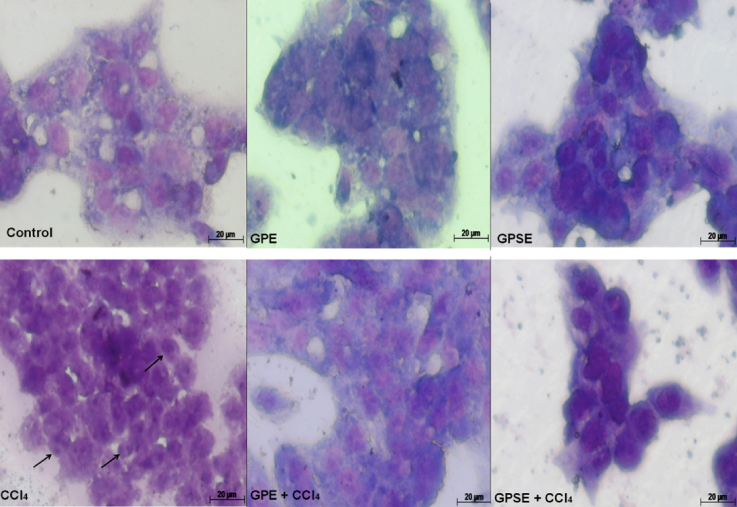
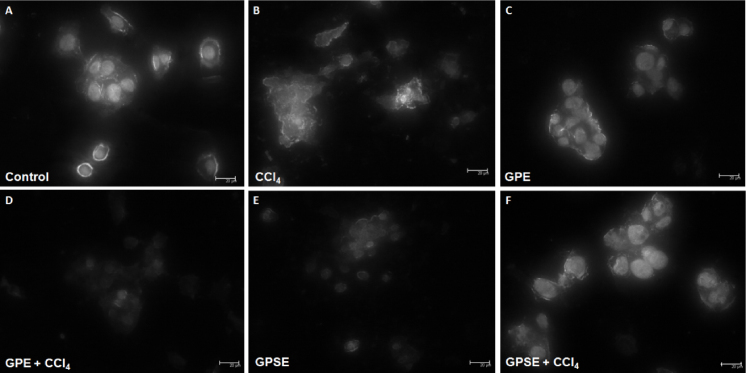
As shown in Fig. 5, HepG2 cells pretreated with GPE or GPSE (both at 1000 μg/mL) showed a general morphology similar to control group using May-Grünwald–Giemsa staining. In contrast, cells exposed to CCl4 presented characteristics of apoptosis such as cell shrinkage, blebbed surfaces, prominent cytoplasmic aggregation, disintegrated nuclear membrane and DNA fragmentation. On the other hand, pre-treatment with GPE or GPSE protected against CCl4-induced cell morphology damage. In parallel, as shown in Fig. 6, an evaluation of nuclei morphology by Hoechst 33342 staining showed that GPE, GPSE and control groups had normal-shaped nuclei with uniform staining. However, the cells exposed to CCl4 resulted in unorganized nuclei morphology, condensed nuclear fragments and chromatin aggregation, typical events of cells undergoing apoptosis. On the other hand, the pre-treatment of cells with GPE or GPSE followed by exposure to CCl4 protected against nuclei damage, which reflects chemoprotective activity of gabiroba fruits.
Fig. 5.
Photomicrography of representative morphological changes of HepG2 cells using May-Grünwald–Giemsa staining. The cells (1 × 106 cells/well) were pretreated with or without the pulp (GPE) or peel/seed extracts (GPSE) of gabiroba fruits (both at 1000 μg/mL) for 1 h followed by exposure to CCl4 (4 mM) for 6 h. Posteriorly, the medium was exchanged and after 24 h, the cells were stained and morphological changes were analyzed. The images were taken using a 100× objective. Arrows in CCl4 group indicate DNA fragmentation.
Fig. 6.
Photomicrography of representative of nuclear morphological changes of HepG2 cells using Hoechst 33258 dye. The cells (1 × 106 cells/well) were pretreated with or without the pulp (GPE) or peel/seed extracts (GPSE) of gabiroba fruits (both at 1000 μg/mL) for 1 h followed by exposure to CCl4 (4 mM) for 6 h. Posteriorly, the medium was exchanged and after 24 h the cells were stained and nuclear morphological changes were analyzed. The images were taken using a 40× objective.
3.3.3. Effects of GPE and GPSE on AST and ALT activities of HepG2 cells exposed to CCl4
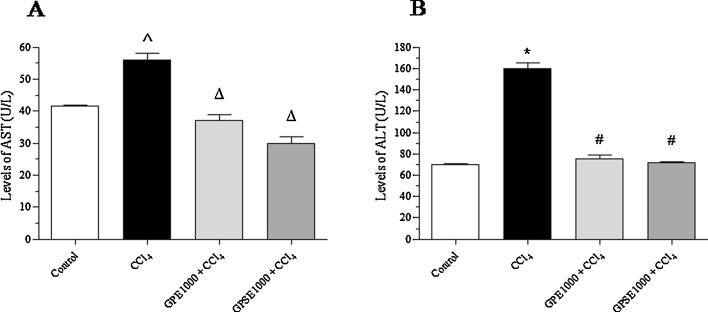
In HepG2 cells exposed to CCl4, both AST and ALT levels presented a marked increase of 35% (p < 0.05) and 124% (p < 0.0001), respectively, in relation to the control group. By contrast, the pre-treatment of HepG2 cells with GPE (p < 0.001) or GPSE (p < 0.0001) significantly reduced AST and ALT activities, reaching normal values, when compared to control (Fig. 7). These results indicate that the gabiroba fruits extracts succeeded in protecting cell membranes from the disruption induced by the toxic effects of CCl4.
Fig. 7.
Effect of pulp (GPE) and peel/seed extracts (GPSE) of gabiroba fruits after exposure to CCl4 on aminotransferases activity in HepG2 cells. Cells were pretreated for 1 h with GPE or GPSE (both at 1000 μg/mL) followed by exposure to CCl4 (4 mM) for 6 h. After supernatants were collected and analyses of the enzymatic activities of AST (A) and ALT (B) were performed. Each bar presents mean ± SD of three independent experiments. (*p < 0.0001 and ^p < 0.05 vs. control and #p < 0.0001 and Δp < 0.001 vs. CCl4. One way ANOVA and Bonferroni test, p < 0.05).
4. Discussion
The liver is a multifunctional organ and plays a central role in the detoxification and metabolic homeostasis. When overloaded, its cellular redox state is susceptible to imbalances which lead to the progression of a variety of hepatic diseases [32]. Since dietary components can modulate antioxidant status of human body, adequate food intake is important for the maintenance of liver health [13], [33], [34].
In this study, gabiroba fruits, especially the GPSE, protected HepG2 cells against CCl4-induced damage. CCl4 is metabolized by citochrome P450 in highly reactive free radicals such as CCl3OO• and CCl3• that promoted marked cellular changes [35], [36]. Thus, pretreatment of HepG2 cells with GPE or GPSE followed by exposure to this xenobiotic protected cells against the cytotoxicity, indicating that the extracts counteracted the toxicity of products generated by metabolism of CCl4. Weber et al. [21] show that CCl3OO• is more reactive, has a shorter half-life than CCl3• and responsible for the chain reaction that leads to cell death. Thus, gabiroba extracts seem to act in the initial steps of metabolism of CCl4.
The oxidative stress triggered by CCl4 leads to apoptotic cell death [49] as noted herein in the morphological analysis of HepG2 cells exposed to this xenobiotic. However cells pretreated with GPE or GPSE followed by exposure to CCl4 showed morphology similar to that of the control group, confirming that the gabiroba fruits extracts inhibited molecular derangements that would culminate in structural alterations and cell death. This result was corroborated by the analysis of ALT and AST liver enzymes, which overflow to extracellular medium due to membrane permeability alterations after cellular injury [37], [38]. As expected, exposure to CCl4 induced a marked release of ALT and AST in HepG2 cells, while the pretreatment with GPE or GPSE promoted normalization of liver enzyme levels, indicating the protection of the cell membrane.
Several studies have reported the protective effects of food components. Malta et al. [39] found Campomanesia cambessedes, a fruit of the same genus that gabiroba, protects DNA of hydrogen peroxide-induced genotoxicity in rat hepatocytes. Ávila et al. [40] showed that pomegranate, a phenolic compounds-rich fruit, protects mice against hexavalent chromium-induced genotoxicity. Li et al. [41] showed pretreatment of HepG2 cells with tea polyphenols have protective action against CCl4-induced damage equivalent to those exhibited by GPE and GPSE, while pretreatment of cells with gallic acid had an effect similar to that observed by GPSE. Similarly, Bhavsar et al. [42] evaluated the protective activity of ethyl acetate fraction of Citrus limon which exerted activity comparable to that presented by GPSE.
The hepatoprotective effect observed by gabiroba fruits extracts is possibly, at least in part, attributed to the presence of phenolic compounds, especially flavonoids, as observed here. Corroborating these results, Souza et al. [5] found that hydroethanolic extract obtained from gabiroba fruit barks presents flavonoids such as quercetin and myricetrin, which are also found in the leaves of C. adamantium [43]. The intake of flavonoids-rich foods has been associated with the prevention of many degenerative diseases, such as liver diseases [9], [48]. Some studies have showed antioxidant activity and phenolic contents of whole Campomanesia pubescens fruits ethanolic extract are similar to those obtained here by GPE or GPSE [44], [45]. However the second stands out in greater amount of polyphenols and antioxidant activity, which could be related with the best results found here against CCl4-induced damage. In this sense its worth to mention, according to Ignat et al. [9], fruit waste, mainly peel and seeds, presents higher amounts of phenolic compounds than their edible parts.
In addition, the expression of cytoprotective enzymes via the nuclear factor erythroid-2 related factor 2 (Nrf2) pathway is activated in response to oxidative stress [32]. Polyphenols-rich foods can overexpress highly protective gene via Nrf2 protecting the cells against the oxidative damage and promoting cell survival [46], [47], [48]. Thus, in view of the large amount of polyphenols found in gabiroba fruits, it is suggested that, at least in part, that the protective effect found in GPE and GPSE could be also related to modulation of the Nrf2 pathway.
In conclusion, the results showed that gabiroba fruits had a positive effect in HepG2 cells injured by CCl4, which could be associated with the antioxidant activity exerted by phenolic compounds of these fruits. This reinforces the importance of dietary antioxidants for the nutritional support of pathological conditions associated to oxidative stress, such as liver diseases [50]. Therefore, further studies should be conducted on gabiroba fruits to evaluate its liver protective activity using animal models and clinical trials. The gabiroba's liver protective activity seen in this research leads one to believe that it should be considered in further studies aiming to include it in the composition of healthy diets for people with liver disturbances and also for healthy people. It should also be considered in the formulation of nutraceuticals and dietary supplements intended for disease risk reduction.
Conflict of interest
The authors declare that there are no conflicts of interest in this study.
Transparency document
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG), Fundação de Apoio à Pesquisa – UFG (FUNAPE); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Financiadora de Estudos e Projetos (FINEP); and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Contributor Information
Maria Margareth Veloso Naves, Email: mmvnaves@gmail.com.
Marize Campos Valadares, Email: marizecv@ufg.br.
References
- 1.Almeida M.M.B., Sousa P.H.M., Arriaga A.M.C., Prado G.M., Magalhães C.E.C., Maia G.A., Lemos T.L.G. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res. Int. 2011;44:2155–2159. [Google Scholar]
- 2.Pavan F.R., Leite C.Q.F., Coelho R.G., Coutinho I.D., Honda N.K., Cardoso C.A.L., Vilegas W., Leite S.R.A., Sato D.N. Evaluation of anti-Mycobacterium tuberculosis activity of Campomanesia adamantium (Myrtaceae) Quim. Nova. 2009;32:1222–1226. [Google Scholar]
- 3.Cardoso C.A., Salmazzo G.R., Honda N.K., Prates C.B., Vieira M.C., Coelho R.G. Antimicrobial activity of the extracts and fractions of hexanic fruits of Campomanesia species (Myrtaceae) J. Med. Food. 2010;13:1273–1276. doi: 10.1089/jmf.2009.0047. [DOI] [PubMed] [Google Scholar]
- 4.Pascoal A.C., Ehrenfried C.A., Lopez B.G., Araujo T.M., Pascoal V.D., Gillioli R., Anhê G.F., Ruiz A.L., Carvalho J.E., Stefanello M.E., Salvador M.J. Antiproliferative activity and induction of apoptosis in PC-3 cells by the chalcone cardamonin from Campomanesia adamantium (Myrtaceae) in a bioactivity-guided study. Molecules. 2014;19:1843–1855. doi: 10.3390/molecules19021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Souza J.C., Piccinelli A.C., Aquino D.F.S., Souza V.V., Schmitz W.O., Traesel G.K., Cardoso C.A.L., Kassuya C.A.L., Arena A.C. Toxicological analysis and antihyperalgesic, antidepressant, and anti-inflammatory effects of Campomanesia adamantium fruit barks. Nutr. Neurosci. 2014 doi: 10.1179/1476830514Y.0000000145. [DOI] [PubMed] [Google Scholar]
- 6.Vallilo M.I., Lamardo L.C.A., Gaberlotti M.L., Oliveira E.D., Moreno P.R.H. Chemical composition of Campomanesia adamantium (Cambessédes) O. Berg. fruits. Cienc. Tecnol. Aliment. 2006;26:805–810. [Google Scholar]
- 7.Alía M., Ramos S., Mateos R., Granado-Serrano A.B., Bravo L., Goya L. Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol. Appl. Pharmacol. 2006;212:110–118. doi: 10.1016/j.taap.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Goya L., Martín M.A., Ramos S., Mateos R., Bravo L. A cell culture model for the assessment of the chemopreventive potential of dietary compounds. Curr. Nutr. Food Sci. 2009;5:56–64. [Google Scholar]
- 9.Ignat I., Volf I., Popa V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Lima C.F., Fernandes-Ferreira M., Pereira-Wilson C. Phenolic compounds protect HepG2 cells from oxidative damage: relevance of glutathione levels. Life Sci. 2006;79:2056–2068. doi: 10.1016/j.lfs.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 11.Loguercio C., Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic. Biol. Med. 2003;34:1–10. doi: 10.1016/s0891-5849(02)01167-x. [DOI] [PubMed] [Google Scholar]
- 12.Stagos D., Amoutzias G.D., Matakos A., Spyrou A., Tsatsakis A.M., Kouretas D. Chemoprevention of liver cancer by plant polyphenols. Food Chem. Toxicol. 2012;50:2155–2170. doi: 10.1016/j.fct.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Vitaglione P., Morisco F., Caporaso N., Fogliano V. Dietary antioxidant compounds and liver health. Crit. Rev. Food Sci. Nutr. 2004;44:575–586. doi: 10.1080/10408690490911701. [DOI] [PubMed] [Google Scholar]
- 14.Goya L., Mateos R., Bravo L. Effect of the olive oil phenol hydroxytyrosol on human hepatoma HepG2 cells. Eur. J. Nutr. 2007;46:70–78. doi: 10.1007/s00394-006-0633-8. [DOI] [PubMed] [Google Scholar]
- 15.Martin M.A.A., Ramos S., Mateos R., Granado Serrano A.B.N., Izquierdo-Pulido M.A., Bravo L., Goya L. Protection of human HepG2 cells against oxidative stress by cocoa phenolic extract. J. Agric. Food Chem. 2008;56:7765–7772. doi: 10.1021/jf801744r. [DOI] [PubMed] [Google Scholar]
- 16.Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003;189:113–127. doi: 10.1016/s0300-483x(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 17.Noh J.R., Gang G.T., Kim Y.H., Yang K.J., Hwang J.H., Lee H.S., Oh W.K., Song K.S., Lee C.H. Antioxidant effects of the chestnut (Castanea crenata) inner shell extract in t-BHP-treated HepG2 cells, and CCl4- and high-fat diet-treated mice. Food Chem. Toxicol. 2010;48:3177–3183. doi: 10.1016/j.fct.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Pareek A., Godavarthi A., Issarani R., Nagori B.P. Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. J. Ethnopharmacol. 2013;150:973–981. doi: 10.1016/j.jep.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 19.Ozerkan D., Ozsoy N., Yilmaz E. Vitamin D and melatonin protect the cell's viability and ameliorate the CCl4 induced cytotoxicity in HepG2 and Hep3B hepatoma cell lines. Cytotechnology. 2014;66:543–551. doi: 10.1007/s10616-014-9738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simeonova R., Kondeva-Burdina M., Vitcheva V., Mitcheva M. Some in vitro/in vivo chemically induced experimental models of liver oxidative stress in rats. Biomed. Res. Int. 2014;2014:1–6. doi: 10.1155/2014/706302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber L.W., Boll M., Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 22.Huang C.-C., Tung Y.-T., Cheng K.-C., Wu J.-H. Phytocompounds from Vitis kelungensis stem prevent carbon tetrachloride-induced acute liver injury in mice. Food Chem. 2011;125:726–731. [Google Scholar]
- 23.Wang S.-H., Kao M.-Y., Wu S.-C., Lo D.-Y., Wu J.-Y., Chang J.-C., Chiou R.Y.Y. Oral administration of Trapa taiwanensis Nakai fruit skin extracts conferring hepatoprotection from CCl4-caused injury. J. Agric. Food Chem. 2011;59:3686–3692. doi: 10.1021/jf1048386. [DOI] [PubMed] [Google Scholar]
- 24.Swain T., Hillis W.E. The phenolic constituents of Prunus domestica: the quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959;10:63–68. [Google Scholar]
- 25.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT – Food Sci. Technol. 1995;28:25–30. [Google Scholar]
- 26.Mosmann T. Rapid Colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Deb D.D., Parimala G., Devi S.S., Chakrabarti T. Role of Carum copticum seeds in modulating chromium-induced toxicity on human bronchial epithelial cells and human peripheral blood lymphocytes. Exp. Toxicol. Pathol. 2012;64:889–897. doi: 10.1016/j.etp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeld G. Pancromic staining for clinical hematology and cytology: a novel combination of May-Grunwald and Giemsa components in one rapid usage staining. Mem. Inst. Butantan. 1947;20:329–335. [Google Scholar]
- 29.Mota M.F., Benfica P.L., Batista A.C., Martins F.S., Paula J.R., Valadares M.C. Investigation of Ehrlich ascites tumor cell death mechanisms induced by Synadenium umbellatum Pax. J. Ethnopharmacol. 2012;139:319–329. doi: 10.1016/j.jep.2011.04.055. [DOI] [PubMed] [Google Scholar]
- 30.Boone L., Meyer D., Cusick P., Ennulat D., Provencher-Bolliger A., Everds N., Meador V., Elliott G., Honor D., Bounous D., Jordan H. Selection and interpretation of clinical pathology indicators of hepatic injury in preclinical studies. Vet. Clin. Pathol. 2005;34:182–188. doi: 10.1111/j.1939-165x.2005.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 31.Zuanazzi J.A.S. Flavonóides. In: Gosmann G., Schenkel E.P., Simoes C.M.O., editors. Farmacognosia da planta ao medicamento. UFSC; Santa Catarina: 2011. pp. 489–517. [Google Scholar]
- 32.Shin S.M., Yang J.H., Ki S.H. Role of the Nrf2-ARE pathway in liver diseases. Oxid. Med. Cell. Longev. 2013;2013:1–9. doi: 10.1155/2013/763257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morisco F., Vitaglione P., Amoruso D., Russo B., Fogliano V., Caporaso N. Foods and liver health. Mol. Aspects Med. 2008;29:144–150. doi: 10.1016/j.mam.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Tell G., Vascotto C., Tiribelli C. Alterations in the redox state and liver damage: hints from the EASL Basic School of Hepatology. J. Hepatol. 2012;58:365–374. doi: 10.1016/j.jhep.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Manibusan M.K., Odin M., Eastmond D.A. Postulated carbon tetrachloride mode of action: a review. J. Environ. Sci. Health C. 2007;25:185–209. doi: 10.1080/10590500701569398. [DOI] [PubMed] [Google Scholar]
- 36.Porubsky P.R., Meneely K.M., Scott E.E. Structures into the binding of human cytochrome P-450 2E1: inhibitors and both small molecular weight and fatty acid substrates. J. Biol. Chem. 2008;283:33698–33707. doi: 10.1074/jbc.M805999200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha H.L., Shin H.J., Feitelson M.A., Yu D.Y. Oxidative stress and antioxidants in hepatic pathogenesis. World J. Gastroenterol. 2010;16:6035–6043. doi: 10.3748/wjg.v16.i48.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramaiah S.K. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem. Toxicol. 2007;45:1551–1557. doi: 10.1016/j.fct.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Malta L.G., Ghiraldini F.G., Reis R., Oliveira M.V., Silva L.B., Pastore G.M. In vivo analysis of antigenotoxic and antimutagenic properties of two Brazilian Cerrado fruits and the identification of phenolic phytochemicals. Food Res. Int. 2012;49:604–611. [Google Scholar]
- 40.Ávila R.I., Guerra M.T., Borges K.A.S., Vieira M.S., Júnior Oliveira L.M., Furtado H., Mota M.F., Arruda A.F., Valadares M.C. Punica granatum L. protects mice against hexavalent chromium-induced genotoxicity. Braz. J. Pharm. Sci. 2013;49:689–697. [Google Scholar]
- 41.Li T., Zhang X., Zhao X. Powerful protective effects of gallic acid and tea polyphenols on human hepatocytes injury induced by hydrogen peroxide or carbon tetrachloride in vitro. J. Med. Plants Res. 2010;4:247–253. [Google Scholar]
- 42.Bhavsar S.K., Joshi P., Shah M.B., Santani D.D. Investigation into hepatoprotective activity of Citrus limon. Pharm. Biol. 2007;45:303–311. [Google Scholar]
- 43.Ferreira L.C., Grabe-Guimarães A., Paula C.A., Michel M.C.P., Guimarães R.G., Rezende S.A., Souza Filho J.d., Saúde-Guimarães D.A. Anti-inflammatory and antinociceptive activities of Campomanesia adamantium. J. Ethnopharmacol. 2013;145:100–108. doi: 10.1016/j.jep.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 44.Chang R., Morais S.A.L., Nascimento E.A., Cunha L.C.S., Rocha E.O., Aquino F.J.T., Souza M.G.M., Cunha W.R., Martins C.H.G. Essential oil composition and antioxidant and antimicrobial properties of Campomanesia pubescens O. Berg, native of Brazilian Cerrado. Acta Farm. Bonaer. 2011;30:1843–1848. [Google Scholar]
- 45.Haminiuk C.W.I., Plata-Oviedo M.S.V., Guedes A.R., Stafussa A.P., Bona E., Carpes S.T. Chemical, antioxidant and antibacterial study of Brazilian fruits. Int. J. Food Sci. Technol. 2011;46:1529–1537. [Google Scholar]
- 46.Niture S.K., Kaspar J.W., Shen J., Jaiswal A.K. Nrf2 signaling and cell survival. Toxicol. Appl. Pharmacol. 2010;244:37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scapagnini G., Vasto S., Abraham N.G., Caruso C., Zella D., Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 2011;44:192–201. doi: 10.1007/s12035-011-8181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez-Panchon M.S., Villano D., Troncoso A.M., Garcia-Parrilla M.C. Antioxidant activity of phenolic compounds: from in vitro results to in vivo evidence. Crit. Rev. Food Sci. Nutr. 2008;48:649–671. doi: 10.1080/10408390701761845. [DOI] [PubMed] [Google Scholar]
- 49.Guo X., Liang B., Wang X., Fan F., Jin J., Lan R., Yang J., Wang X., Jin L., Cao Q. Glycyrrhizic acid attenuates CCl4-induced hepatocyte apoptosis in rats via a p53 mediated pathway. World J. Gastroenterol. 2013;19:3781–3791. doi: 10.3748/wjg.v19.i24.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobo-Velazquez D., Cisneros-Zevallos L. Correlations of antioxidant activity against phenolic content revisited: a new approach in data analysis for food and medicinal plants. J. Food Sci. 2009;74:107–113. doi: 10.1111/j.1750-3841.2009.01352.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.