Abstract
This study was aimed to compare the relative activities of the purified pomegranate peels polyphenols (PPPs) with some other plant polyphenols including punicalagin, ellagic acid, gallic acid, phlorizin, and epigallocatechin gallate (EGCG) on the lipid metabolism regulation, and the cholesterol efflux mechanisms of PPPs and punicalagin were also investigated. In this paper, a convenient and accurate in vitro HL7702 steatosis hepatic cell model was applied to evaluate the lipid-lowering effects of the tested polyphenols. The results showed that PPPs possessed the strongest lipid-lowering effects. Prevention group (treated with polyphenols when establishing of steatosis model) was more effective than treatment group (treated with polyphenols after establishment of steatosis model). Punicalagin displayed the strongest lipid-lowering effects among all the tested components of pomegranate peel polyphenols. Moreover, PPPs and punicalagin (10, 20, 40 μg/mL) significantly increased the mRNA expression of LXRα (Liver X receptor alpha) and its target genes-ABCA1 (ATP-binding cassette transporter A1) in a dose-dependent manner in HL7702 steatosis hepatic cells. The high mRNA expression of LXRα and ABCA1, next to lovastatin, was observed in cells treated with 40 μg/mL of PPPs. These in vitro findings suggested that PPPs might have great potential in the clinic treatment of hyperlipemia.
Chemical compounds studied in this article: Gallic acid (PubChem CID: 370), Punicalagin (PubChem CID: 44584733), Ellagic acid (PubChem CID: 5281855), Epigallocatechin gallate (PubChem CID: 65064), Phlorizin (PubChem CID: 6072)
Keywords: Pomegranate peel polyphenols (PPPs), Plant polyphenols, Hepatic cell, Lipid-lowering effects, Molecular mechanisms
1. Introduction
Pomegranate (Punica granatum L.), a seeded or granular apple, is derived from the name Pomum (apple) granatus (grainy) [1], [2]. It is one of the most investigated fruits in recent years [3]. Numerous studies show that pomegranate fruits possess significant anti-diabetic, anti-inflammatory, anti-oxidant, anti-tumor, and anti-obesity activities in vivo and in vitro [4], [5], [6]. Consumption of pomegranate is of great benefits to patients with metabolic syndrome, such as coronary heart disease, diabetes, and hyperlipidemia [7], [8]. It has reported that consumption of pomegranate juice is able to decrease oxidative stress in serum and the macrophage uptake of oxidized low-density lipoprotein (ox-LDL) of diabetic patients [9].
Pomegranate fruits consist of carbohydrates, minerals, crude fibers, vitamin C, and considerable varieties of phenolic compounds, including anthocyanins (3-glucosides and 3, 5-diglucosides of delphinidin, cyanidin, and pelargonidin), ellagic acid (EA), gallic acid (GA), punicalin, punicalagin, pedunculagin, and different flavanols [5], [10]. There are abundant phenolic compounds, punicalagin, EA and GA in the pomegranate peels, and they have known as natural antioxidants [11]. Pomegranate shows potent anti-atherosclerotic activity which could be attributed to the abundant contents of polyhenols. Punicalagin is the most abundant polyphenols, and it presents in two anomers: punicalagin A and B, [6], [11], [12], [13], [14]. Studies have shown that punicalagin has antioxidant, antifungal and antibacterial properties [2]. Ellagic acid has also been demonstrated to reduce white fat deposits and triglycerides accumulation in the body during regular intake of high-fat diets [15].
In addition, scientific researches have suggested that green tea and plant sterols possess the lipid-lowering effects, and lower the risk of heart diseases [16], [17], [18]. Epigallocatechin gallate (EGCG), the main component of tea polyphenols, has attracted much attention in recent years. EGCG exhibits significant lipid-lowering activity in mice [19], [20]. However, there are many questions remaining to be answered, such as, which components are the main bioactive ingredients of PPPs? How they play this role? Moreover, are the lipid-lowering effects of the bioactive monomers of PPPs stronger than other common plant polyphenols? Further studies are required to compare the lipid-lowering and hepatoprotective effects between PPPs and its main components (including punicalagin, EA, GA), EGCG, and phlorizin.
In this study, the lipid-lowering effects of the six kinds of polyphenols were investigated in vitro simultaneously. In order to better understand the lipid-lowering effects of pomegranate peel polyphenols cholesterol, efflux mechanisms were also investigated. LXRα and its target gene-ABCA1 were known to play an important role in the cholesterol efflux pathway [21], [22], [23]. However, the effects and putative mechanisms of pomegranate peel polyphenols on these two genes remains poorly understood. The present study investigated the cholesterol efflux by evaluating LXRα and its target gene-ABCA1, and provided reference to the effective treatment and prevention of nonalcoholic fatty liver diseases and to the decrease in the risk rates of hyperlipidemia and cardiovascular diseases.
2. Materials and methods
2.1. Materials and chemicals
HL7702 human hepatic cells were purchased from China Center for Type Culture Collection (CCTCC). Punicalagin, ellagic acid (EA), gallic acid (GA), phlorizin, epigallocatechin gallate (EGCG), and lovastatin were obtained from Sigma–Aldrich (St. Louis, MO, USA), and the purities of all the standards were not less than 98%. Fetal calf serum was purchased from Hangzhou Sijiqing Company. Oil red O dye, insulin, penicillin–streptomycin solution, trypsin, dimethyl sulfoxide (DMSO), 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were provided by Sigma Chemical Company (Shanghai, China). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total triglycerides (TG), and total cholesterol (TC) testing cassette were purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China).
2.2. Preparation of purified pomegranate peel extracts
Ripe pomegranates were obtained from Lintong, Shaanxi province of China. The peel was separated and cut into pieces, then dried, milled into fine powders and kept in a dessicator overnight before extraction for polyphenols. The extraction was performed using ethanol ultrasonic-assisted extraction method. Briefly, 1 g of sample was weighed, extraction solution was then obtained with ultrasonic power of 120 W, ethanol concentration of 60% (v/v), temperature at 60 °C, solid–liquid ratio of 1–20, as well as ultrasonic extraction time of 30 min. Thereafter, the extraction solution was concentrated by rotary evaporator at 40 °C, followed by vacuum freeze-drying to obtain crude extracts of pomegranate peel polyphenols. The crude extracts were dissolved in water, extracted by equal volume of petroleum ether and the aqueous phase was collected. The aqueous phase was then extracted by equal volume of chloroform for three times, and aqueous phase was collected with pH adjusted to 6.5 by 1 mol L−1 NaOH. The aqueous phase was then extracted by two times volume of ethyl acetate for three times, and the ethyl acetate phase was collected. Thereafter, the pH of the aqueous phase was adjusted to 2.0 by 1 mol L−1 HCl, then extracted by two times volume of ethyl acetate for three times. Finally, the six times of extracted acetate phase were merged together, the solvent was removed using a vacuum rotary evaporator at 40 °C, followed by vacuum drying to obtain the purified polyphenols obtained from pomegranate peel extracts.
2.3. HPLC analysis of the purified polyphenols extracts
The identification of phenolic compounds in the pomegranate peel polyphenols purified extracts was performed by HPLC. The analysis was carried out using a ZORBAX SB-C18 chromatographic column (4.6 mm i.d. ×250 mm, 5 μm, Agilent, American) on a 1525 Waters HPLC system equipped with an UV detector (Waters, USA). A gradient elution was performed by varying the proportion of solvent A (water containing 1% glacial acetic acid) and solvent B (methanol). The g–radient program was as follows: 0–70 min from 5% to 44% methanol; 70–80 min with 44% methanol. The flow rate of the mobile phase was 1 mL/min; the UV detection wavelength was 280 nm, the sample injection volume was 20 μL, and the column temperature was 30 °C.
2.4. Culture of HL7702 hepatic cells
Cells were kept at 37 °C, 95% air, 5% CO2 in RPMI-1640 supplemented with 10% (v/v) FCS, 100 U/mL penicillin and 100 μg/mL streptomycin. Every 2 days, medium was refreshed, at 75–80% confluence; cells were split to a new 75 cm2 flask.
2.5. Building of steatosis hepatic cell model
Cells were seeded in 6-well tissue culture plates (Jetbiofil, Guangzhou) at a cell density of 2 × 105 in 1000 μL/well and then incubated for 12 h with 10% (v/v) FBS-PRMI 1640 medium till 20–30 confluence. Subsequently, cells were exposed to 10% FBS-RPMI (Fetal Bovine Serum-Roswell Park Memorial Institute) 1640 medium (M1 medium) and 50% FBS-RPMI 1640 medium (M2 medium), respectively. After 24, 48, 72 h, the accumulation of orange red droplets were determined using oil red O staining, a vital lipophilic dye used to label fat accumulation in the cytosol. Contents of AST, ALT, TG, and TC were tested by the testing cassette to evaluate the lipid accumulation level and damage degree of HL7702 hepatic cell.
2.6. MTT assay for optimum concentration of polyphenols
The stock solution of all the tested polyphenols, PPPs, punicalagin, EA, GA, phlorizin, EGCG, lovastatin (positive control), was achieved by dissolving the solid samples in dimethylsulfoxide (DMSO) at a concentration of 10 mg/mL and then further diluted appropriately with 1640 medium to concentration from 160 to 5 μg/mL.
This MTT assay was divided into four groups: normal group (control group), model group (steatosis cells), treatment group, and prevention group. Firstly, HL7702 human hepatic cells were seeded in the 96 well tissue culture plates at a cell density of 1000 in 100 μL/well. After 12 h incubation, normal group and treatment group were exposed to M1 medium, model group exposed to M2 medium, while prevention group exposed to M2 medium with polyphenols concentration gradient (5, 10, 20, 40, 80, 160 μg/mL respectively); 48 h later, normal group, model group, and treatment group were exposed to M1 medium, while prevention group exposed to M1 medium with the same polyphenols concentration gradient. Thereafter, MTT assay was conducted. The cells exhibiting blue formazan/endosomes or needle-like formazan/crystals were considered as reactive cells, whereas the ones without displaying formazan/endosomes or formazan/crystals were labeled as non-reactive cells. Cell viability was expressed as a percentage of absorbance values in testing group to that in control group, which was considered as 100%.
2.7. Evaluation of lipid-lowering effects of tested polyphenols
HL7702 cells were seeded in 6-well tissue culture plates at a cell density of 2 × 105 in 1000 μL/well and then incubated for 12 h with M1 medium till 20–30 confluence. Thereafter, normal group, model group, treatment group, and prevention group cells were treated with the optimum polyphenols concentration determined by MTT experiment above. Finally, oil red O staining, contents of AST, ALT, TC and TG testing experiment were conducted.
2.8. Oil red O staining
Cell monolayers were firstly washed twice with phosphate buffer saline (PBS, pH 7.0) and were fixed with 4% (m/v) paraformaldehyde for 5 min, then were washed with PBS and were fixed with propanediol for 5 min. Thereafter, monolayers were incubated for 15 min with 0.5% (m/v) at 60 °C, and then washed with 85% (v/v) propanediol for 5 min. Finally, the monolayers were washed with PBS; the accumulation of orange red droplets of different groups was determined by ordinary light of inverted fluorescence microscope (Leica DMIRB, Germany).
2.9. Measurement of TG, TC, ALT, and AST contents
After treatment, cell culture medium and the cells were collected, respectively. ALT and AST contents of the Cell culture mediums were tested according to the methods of ALT and AST enzyme kit (Nanjing, Jiancheng). TG and TC contents in the cells were measured using TG and TC enzyme kit (Nanjing, Jiancheng).
2.10. RNA extraction and q-PCR
Punicalagin and PPPs were selected as the tested polyphenols. Low, middle, high doses (10, 20, 40 μg/mL) were chosen as the gradient concentration. After culture, normal group, model group, treatment group cells were collected, and the total RNA was extracted using the total RNA kit (Omega, American) according to the manufacturer's protocol. The cDNA was synthesized from 1 μg of RNA using RevertAid™ First Strand cDNA Synthesis Kit (Fermentas). Primer sequences for PCR are as follows: LXRα, 5′-TCTGGAGACATCTCGGAGGTA-3′ (forward), and 5′-GGCTCACCAGTTTCATTAGCA-3′ (reverse); RXRα, 5′-CTCCTCAGGCAAGCACTATG-3′ (forward), and 5′-CAGCTCCGTCTTGTCCATCTG-3′ (reverse); ABCA1, 5′-GGGAGGCTCCCGGAGTT-3′ (forward), and 5′-GTATAAAAGAAGCCTCCGAGCATC-3′ (reverse); GAPDH, 5′-TCATTGACCTCAACTACATGG-3′ (forward), and 5′-AAATGAGCCCCAGCCTTCTCC-3′ (reverse). The real-time PCR (Step One Plus, Applied Biosystems, USA) conditions were: 30 s at 95 °C, followed by 40 cycles of 95 °C for 5 s, 58 °C for 30 s, and 58 °C for 30 s. GAPDH was used as the control in the comparative CT method.
2.11. Statistical analysis
Experiments were performed at least in triplicate. The data in this study are expressed as mean ± SD. One-way analysis of variance (ANOVA) was performed using SPSS software. A probability of p < 0.05 was considered as significant.
3. Results
3.1. HPLC analysis for polyphenols composition of PPPs
The major polyphenols were identified and quantified by HPLC as shown in Table 1. Components of polyphenols of the purified pomegranate peel extracts were identified in the following order: gallic acid (9.97 min), punicalagin including two peaks (Isomers, 15.22 and 22.49 min), catechin (26.65 min), chlorogenic acid (31.53 min), caffeic acid (35.75 min), epicatechin (41.36 min), rutin (68.60 min), and ellagic acid (70.86 min). In the present study, linear regression was used for the calculation, and the assay showed excellent linearity with the correlation coefficients (R2) in the range of 0.9987–0.9994. Punicalagin, with the level of 65.38 mg/100 mg, was identified to be the dominant component of PPPs, followed by catechin, ellagic acid, gallic acid, epicatechin, chlorogenic acid, rutin, and caffeic acid at the concentration of 12.66, 2.93, 2.53, 0.99, 0.37, 0.34, and 0.03 mg/100 mg, respectively.
Table 1.
Calibration curves and the contents of the identified polyphenols obtained from the pomegranate peel extracts by HPLC.
| Component | tR (min) | Linearity (μg/mL) | Regression equation (Y = aX + b) | R2 | Content (mg/100 mg) |
|---|---|---|---|---|---|
| Gallic acid | 9.97 ± 0.09 | 22–110 | Y = 5443X + 422.17 | 0.9991 | 2.53 |
| Punicalagin | 15.22 ± 0.09 22.49 ± 0.08 |
11–500 | Y = 2898X − 1121.61 | 0.9989 | 65.38 |
| Catechin | 26.65 ± 0.05 | 22–110 | Y = 2031X + 447.94 | 0.9992 | 12.66 |
| Chlorogenic acid | 31.53 ± 0.07 | 1.4–110 | Y = 4728X + 156.11 | 0.9993 | 0.37 |
| Caffeic acid | 35.75 ± 0.09 | 0.04–80 | Y = 8734X + 313.97 | 0.9987 | 0.03 |
| Epicatechin | 41.36 ± 0.04 | 2.2–110 | Y = 1684X + 145.86 | 0.9994 | 0.99 |
| Rutin | 68.60 ± 0.06 | 2.8–110 | Y = 1890X + 242.67 | 0.9990 | 0.34 |
| Ellagic acid | 70.86 ± s0.08 | 11–275 | Y = 2491X − 320.39 | 0.9992 | 2.93 |
3.2. Evaluation of the established steatosis hepatic model
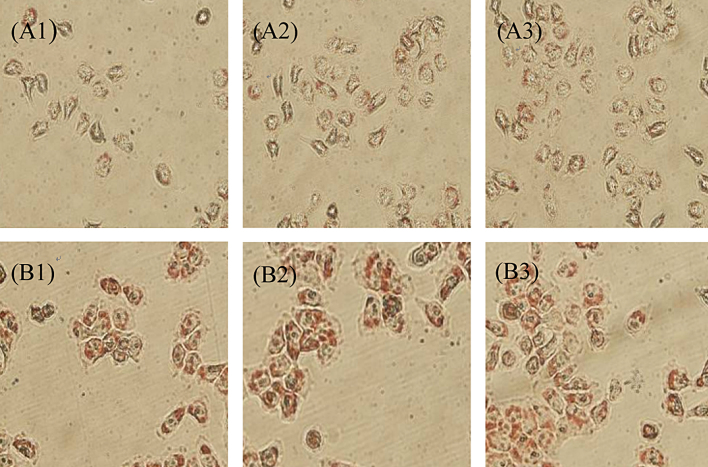
The oil red O staining results of normal group and model group were presented in Fig. 1. Cell edges in normal group were clear, the accumulation of orange red droplets of normal group was evidently less than model group, and it was not increased within the modeling time. Whereas, after modeling, morphological observations showed that the orange red droplets were accumulated.
Fig. 1.
Changes of the accumulation of orange red droplets with modeling time increasing, where A1, A2, and A3 represent the control group, 24 h, 48 h, and 72 h, respectively; B1, B2, and B3 represent the modeling time of model group, 24 h, 48 h, and 72 h, respectively.
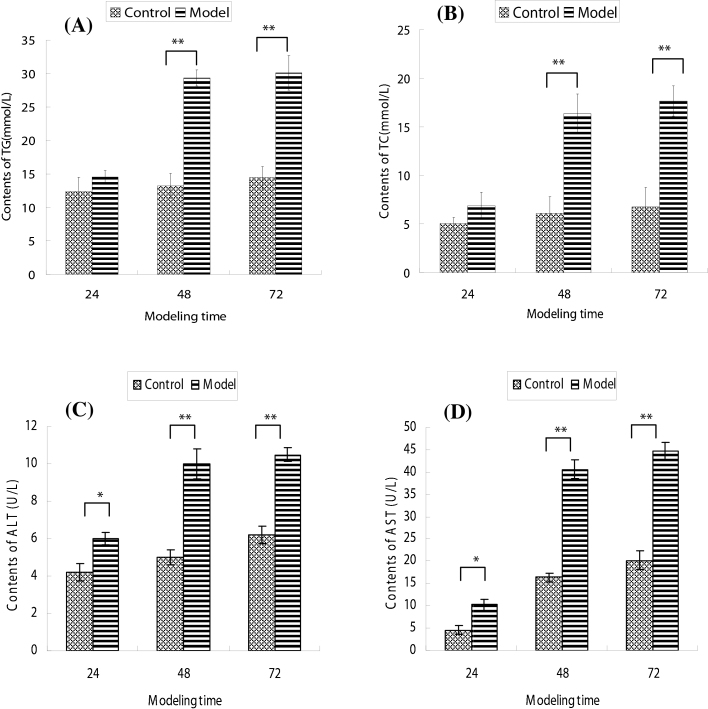
Additionally, contents of TG and TC increased over time as shown in Fig. 2A and B. Although there were no significant changes of TG and TC contents at 24 h after modeling, the significant change was shown at 48 and 72 h, as compared with normal group (p < 0.01).
Fig. 2.
A and B represent the changes of TG and TC contents with modeling time increasing, respectively; C and D represent the changes of ALT and AST contents with modeling time increasing, respectively. All values are expressed as means ± SD (n = 4). *p < 0.05 and **p < 0.01 as compared to normal group.
Moreover, AST and ALT contents were observed in Fig. 2C and D. Compared with normal group, AST and ALT contents in cultural supernatants were increased dramatically after modeling for 24 h (p < 0.05), 48 and 72 h (p < 0.01).
3.3. Optimum dose of the tested polyphenols
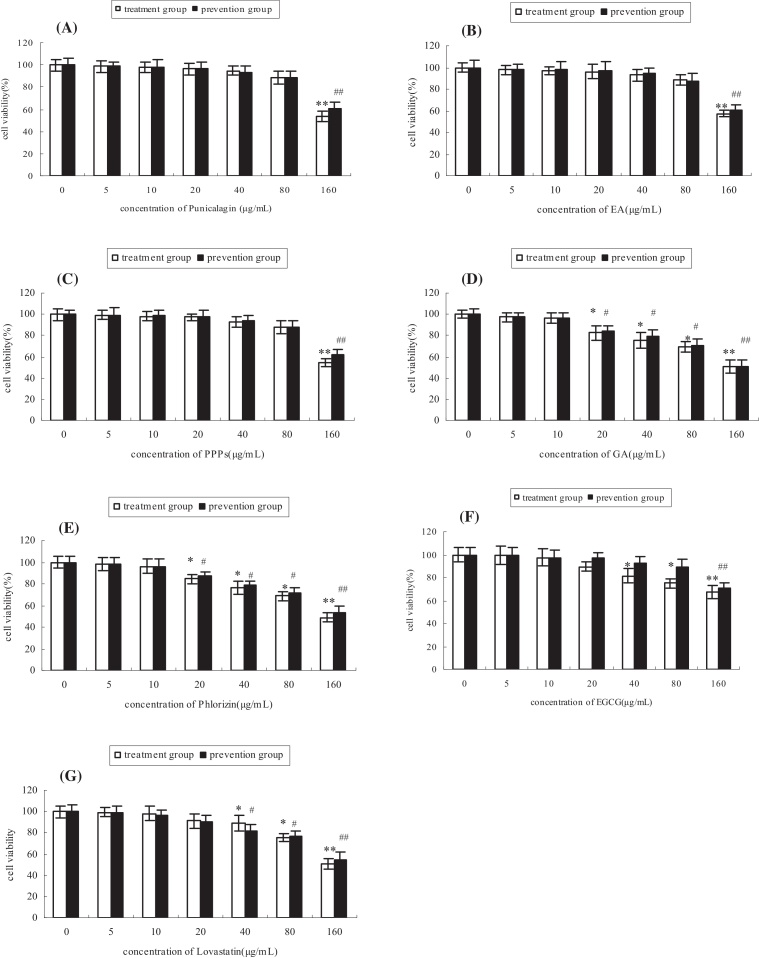
Cell survival rate of all the treatment and prevention groups decreased as the polyphenols concentration increased as presented in Fig. 3. When the concentration of the polyphenols ranged 0–10 μg/mL, no significant cell survival rates were observed in all the groups; 20 μg/mL polyphenols significantly (p < 0.05) decreased cell survival rate in GA and phlorizin groups (Fig. 3D and E); treatment with 40 μg/mL and 80 μg/mL polyphenols resulted in significant (p < 0.05) decreases in cell survival rate of GA, phlorizin, EGCG, lovastatin groups (Fig. 3D–F). However, there was no significant difference in punicalagin, EA and PPPs groups, suggesting that the toxicity of punicalagin, EA, and PPPs was lower to cells, as compared with other polyphenols. Cell survival rate of all the groups consistently remarkably (p < 0.01) decreased, when cells were exposed to 160 μg/mL polyphenols. Therefore, 10 μg/mL was selected as the appropriate concentration in the following experiments.
Fig. 3.
Determination of the appropriate concentration of the tested polyphenols by MTT. All values are expressed as means ± SD (n = 4), A, B, C, D, E, F, and G represent the change of cell viability of punicalagin, EA, PPPs, GA, phlorizin, EGCG and lovastatin groups with the concentration increasing of polyphenols, respectively. *p < 0.05 and **p < 0.01 as compared to normal group (polyphenols’ concentration of treatment group = 0 μg/mL). #p < 0.05 and ##p < 0.01 as compared to normal group (polyphenols’ concentration of prevention group = 0 μg/mL).
3.4. Comparison of the lipid-lowering effects
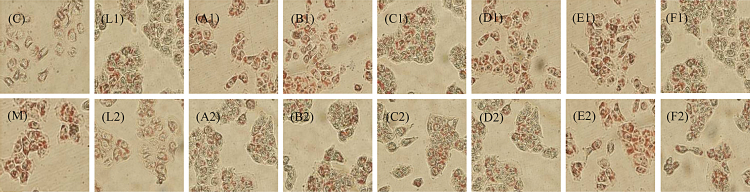
Treatment or prevention with 10 μg/mL polyphenols (punicalagin, EA, PPPs, GA, phlorizin, EGCG, and lovastatin) resulted in significant decreases in the accumulation of orange red droplets, indicating that all these tested polyphenols possessed lipid-lowering effects as presented in Fig. 4. The accumulation degree of orange red droplets of the prevention group declined more evident than the treatment group, suggesting that the lipid-lowering effects of the prevention group were stronger than the treatment group. Furthermore, orange red droplets accumulation of punicalagin, PPPs, EGCG, and lovastatin groups were less than other groups. These results indicated that punicalagin, PPPs, EGCG, and lovastatin were more effective as the lipid-lowering agents than other tested polyphenols.
Fig. 4.
Changes of the accumulation of orange red droplets of different groups, C and M represent normal group and model group respectively; L1, A1, B1, C1, D1, E1, and F1 represent lovastatin, punicalagin, EA, PPPs, GA, phlorizin, EGCG treatment group, respectively; L2, A2, B2, C2, D2, E2, and F2 represent lovastatin, punicalagin, EA, PPPs, GA, phlorizin, and EGCG prevention group, respectively.
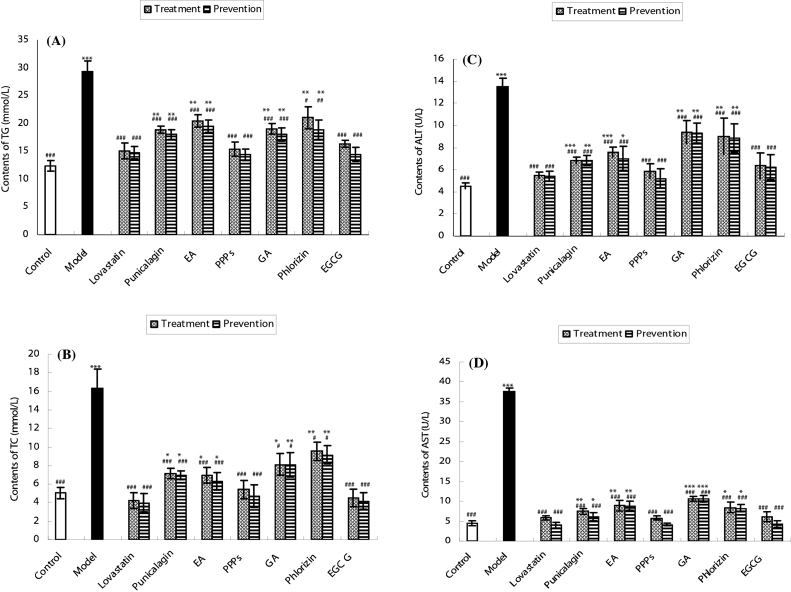
To further strengthen the above results, TG and TC contents were tested. As presented in Fig. 5A and B, the contents of TG and TC of model group were significantly (p < 0.001) higher than that of normal group. When compared with model group, TG and TC levels of punicalagin, EA, PPPs, phlorizin, EGCG, and lovastatin groups decreased significantly, indicating that all tested polyphenols possessed lipid-lowering effects, which was consistent with the results in Fig. 4.
Fig. 5.
A and B represent the changes of TG and TC contents exposed to different types of plant polyphenols, respectively; C and D represent the changes of ALT and AST contents exposed to different types of plant polyphenols, respectively. All values are expressed as means ± SD (n = 4). *p < 0.05, **p < 0.01, and ***p < 0.001 as compared to normal group. #p < 0.05, ##p < 0.01, ###p < 0.001 as compared to model group.
Additionally, there were no significant differences in TG and TC contents of PPPs and EGCG groups, as compared with normal group, which suggested that TG and TC contents in PPPs and EGCG groups returned to normal level. Moreover, when compared with positive control group, no significant changes of TG and TC level of PPPs and EGCG groups, indicating that lipid-lowering effects of PPPs and EGCG were approximately approaching to lovastatin.
3.5. Comparison of the hepatocytes damage alleviating effects
ALT and AST contents in the cultural supernatants of model group were significantly (p < 0.001) higher than that in normal group as shown in Fig. 5C and D. When compared with model group, AST and ALT contents in cultural supernatants of groups were all significantly (p < 0.001) decreased by polyphenols, suggesting that the hepatocytes damage was alleviated. ALT and AST levels in PPPs and EGCG treated cells were not significantly changed, as compared with normal group. This observation indicates that the AST and ALT contents of PPPs and EGCG groups returned to normal level, and the results further elucidate that PPPs and EGCG possessed the strongest liver damage alleviating effects among all the tested polyphenols. In addition, there were no significant differences in ALT and AST contents of PPPs and EGCG groups as compared with positive control group, illustrating that the hepatocytes damage alleviating effects of PPPs and EGCG were approximately approaching to lovastatin.
3.6. The cholesterol efflux mechanisms of pomegranate peel polypheols
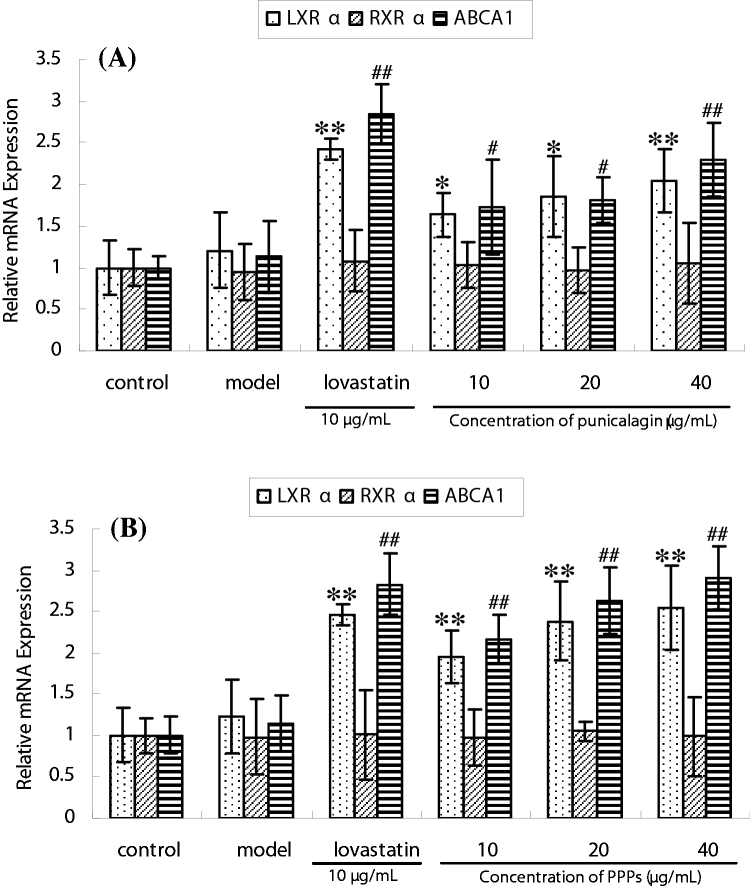
The cholesterol efflux mechanisms of PPPs and its main component, punicalagin, were also investigated in this study. The mRNA expressions of LXRα and ABCA1 were notably increased after incubating with PPPs and punicalagin, and a dose-dependent manner was shown in the cells treated with the polyphenols which ranged from low dose to high dose (10, 20, 40 μg/mL) as shown in the Fig. 6A and B. However, there were no significant changes of the mRNA expression of RXRα (Retinoid X receptor alpha) as the results shown in Fig. 6. These results indicated that LXRα and ABCA1 played an important role in the cholesterol efflux regulation of the cells. Furthermore, when the concentration of these two tested polyphenols reached to high dose (40 μg/mL), the mRNA expression of LXRα and ABCA1 regulated by PPPs showed significant enhancement (p < 0.01), next to positive group.
Fig. 6.
Expressions of LXRα, RXRα, and ABCA1 of each group of HL7702 hepatic cells treated with punicalagin and PPPs, respectively. *p < 0.05, **p < 0.01, as compared to normal group (LXRα). #p < 0.05, ##p < 0.01, as compared to normal group (ABCA1).
4. Discussion
The objective of this study was to compare the lipid-lowering effects of pomegranate peel polyphenols with several other plant polyphenols as well as its cholesterol efflux mechanisms. Lovastatin, which has been widely used for lipid-lowering purpose as a hepatoprotective drug, can reduce cell death in vitro and protect against the cardiotoxicity of anthracyclines in vivo [24], [25]. It therefore has been chosen as the positive control drug in the present study.
The results indicate that RPMI 1640 medium contained 50% FBS could remarkably increase the accumulation of lipid droplets, AST, ALT, TC, and TG contents in a time-dependent manner. Furthermore, at 48 h, the contents of AST, ALT, TC and TG were all increased significantly (p < 0.05). Therefore, 48 h was chosen as the appropriate modeling time.
To accurately compare the lipid-lowering effects of pomegranate peel polyphenols with other plant polyphenols, MTT experiment were performed to eliminate the interruptions of the toxicology that different kinds of polyphenols may have on the cells. The results revealed that 10 μg/mL could be the appropriate concentration, since cell viability among different groups was approximately the same.
As part of systematic effort to assess the lipid-lowering effects of the tested plant polyphenols, both lipid accumulation rate and cholesterol level were analyzed. All the tested polyphenols could significantly (p < 0.05) decrease lipid accumulation in both the treatment group and the prevention group. Furthermore, compared with model group, the lipid-lowering effects of the prevention group were stronger than that in the treatment group, as well as TG, TC AST and ALT levels. These data indicated that punicalagin, EA, PPPs, GA, phlorizin, and EGCG exhibited significant lipid-lowering and hepatoprotective effects. Moreover, the contents of TG, TC, AST, and ALT in the prevention group were closer to normal group when compared with the treatment group, suggesting that the prevention group was more effective than the treatment group. Significant difference of TG, TC, AST, and ALT contents of PPPs group were observed as compared with control group (p < 0.05). However, no significant differences in TG, TC, AST, and ALT contents of its main monomer components, punicalagin, EA, and GA, were noticed when compared with control group (p > 0.05). This observation suggested that PPPs (mixture) had stronger lipid-lowering and hepatoprotective effects than the single compositing monomer components. The stronger lipid-lowering effects of PPPs were again confirmed when compared with phlorizin group, which showed no significant differences in AST, ALT, TC, and TG contents as compared with the control group (p > 0.05). Additionally, EGCG exhibited effective lipid-lowering effects which were approaching to PPPs as compared with punicalagin, EA, GA, and phlorizin. This complies with the previous studies which reported that EGCG can effectively reduce the cholesterol contents [26], [27]. When compared with phlorizin, we found that AST, ALT, TC, and TG contents of punicalagin and EA groups were closer to normal group than phlorizin group. This observation suggests that punicalagin and EA possessed stronger lipid-lowering effects. Contents of AST and ALT in phlorizin group were closer to normal group than GA group, indicating that phlorizin was more effective than GA as the hepatic cell damage alleviating agent. Whereas, contents of TC and TG of GA group were closer to normal group than phlorizin group, indicating that GA exhibited stronger lipid-lowering effects than phlorizin. Moreover, the contents of AST, ALT, TC, and TG of punicalagin group were closer to normal group than EA group as compared with EA group, suggesting that punicalagin possessed stronger lipid-lowering effects than EA. Finally, when the lipid-lowering effects of PPPs were compared with EGCG, contents of AST and ALT of PPPs and EGCG prevention group were 4.13, 5.22 and 4.35, 6.25 U/L, respectively, which value was close to normal group (4.57, 4.53 U/L) and positive group (4.02, 5.44 U/L); TG and TC contents of PPPs and EGCG prevention group were 14.36, 4.16 and 14.39, 4.72 mmol/L respectively, which also had close value to normal group (12.38, 5.03 mmol/L) and positive control group (14.78, 3.99 mmol/L). The above results suggest that PPPs and EGCG possessed effective lipid-lowering effects approaching to positive drug that can lower the contents of AST, ALT, TC, and TG of hepatic steatosis cells to almost normal level. In addition, the results show that the lipid-lowering effects of the PPPs were slightly stronger than EGCG.
In order to explore the cholesterol efflux mechanisms of pomegranate peel plyphenols in the cells, PPPs and its most abundant polyphenols – punicalagin were chosen as the tested polyphenols. The correlation of LXRα, RXRα, and ABCA1 regulation with the cholesterol efflux was further investigated. The relative mRNA expression of both LXRα and its target genes ABCA1 showed a dose-dependent manner, suggesting that PPPs and punicalagin could regulate the cholesterol efflux through increasing mRNA expression of LXRα and ABCA1. Furthermore, when cells were treated with high dose of punicalagin and PPPs, the mRNA expression of LXRα and ABCA1 of the group treated with PPPs was closer to the positive control group. As shown in Fig. 6B, the relative mRNA expression of LXRα and ABCA1 in cells treated with PPPs were 2.5 and 2.91, respectively; while positive group, 2.46 and 2.83, respectively. The result suggested that PPPs was more effective in the regulation of the cholesterol efflux related genes-LXRα and ABCA1. This was consistent with the lipid-lowering effects result above (Fig. 7.).
Fig. 7.
Chemical structure of the studied polyphenols.
In conclusion, this study highlights the different lipid-lowering effects of a variety of polyphenols (punicalagin, EA, PPPs, GA, phlorizin, and EGCG) as well as the cholesterol efflux mechanisms of pomegranate peel polyphenols. We found that PPPs and EGCG were the most effective polyphenols among all the tested samples, which had similar lipid-lowering effects to lovastatin. Furthermore, the prevention group showed stronger lipid-lowering effects than the treatment group. In addition, the lipid-lowering effects of PPPs were stronger than its main monomer components (punicalagin, EA, and GA). Among the monomer components, punicalagin was the most effective monomer at the lipid-lowering aspects. Although the hepatocytes damage alleviating effects of phlorizin were stronger than GA, the cholesterol-lowering effects of GA were stronger. The effective lipid-lowering of PPPs might be attributed to the combined effects of several monomer components of polyphenols obtained from pomegranate peels. The cholesterol efflux mechanism of PPPs and its main component, punicalagin, may be related with the expression of LXRα and ABCA1. Moreover, LXRα and ABCA1 showed a dose-dependent manner with tested pomegranate peel polyphenols. 40 μg/mL PPPs could up-regulate LXRα and ABCA1 sufficiently, which was next to the positive group. The strong lipid-lowering effects of PPPs were consistent with the results above. These findings may not only lead to a better understanding of the preventive roles of plant polyphenols, but also suggest that PPPs may have more satisfactory effects in the clinic treatment of hyperlipemia than its monomer components and EGCG.
Conflict of interest
Dr. Zhao has nothing to disclose.
Transparency document
Acknowledgment
The Project was supported by the National Natural Science Foundation of China (Grant No. 31171677).
Footnotes
Available online 29 October 2014
References
- 1.Teixeira da Silva J.A., Rana T.S., Narzary D., Verma N., Meshram D.T., Ranade S.A. Pomegranate biology and biotechnology: a review. Sci. Hortic. 2013;160:85–107. [Google Scholar]
- 2.Ismail T., Sestili P., Akhtar S. Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012;143:397–405. doi: 10.1016/j.jep.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Çam M., İçyer N.C., Erdoğan F. Pomegranate peel phenolics: microencapsulation, storage stability and potential ingredient for functional food development. LWT-Food Sci. Technol. 2014;55:117–123. [Google Scholar]
- 4.Shaban N.Z., El-Kersh M.A.L., El-Rashidy F.H., Habashy N.H. Protective role of Punica granatum (pomegranate) peel and seed oil extracts on diethylnitrosamine and phenobarbital-induced hepatic injury in male rats. Food Chem. 2013;141:1587–1596. doi: 10.1016/j.foodchem.2013.04.134. [DOI] [PubMed] [Google Scholar]
- 5.Wu D., Ma X., Tian W. Pomegranate husk extract, punicalagin and ellagic acid inhibit fatty acid synthase and adipogenesis of 3T3-L1 adipocyte. J. Funct. Foods. 2013;5:633–641. [Google Scholar]
- 6.Al-Muammar M.N., Khan F. Obesity: the preventive role of the pomegranate (Punica granatum) Nutrition. 2012;28(6):595–604. doi: 10.1016/j.nut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Esmaillzadeh A., Tahbaz F., Gaieni I., Alavi-Majd H., Azadbakht L. Cholesterol-lowering effect of concentrated pomegranate juice consumption in type II diabetic patients with hyperlipidemia. Int. J. Vitam. Nutr. Res. 2006;76(3):147–151. doi: 10.1024/0300-9831.76.3.147. [DOI] [PubMed] [Google Scholar]
- 8.Hashemi M., Kelishadi R., Hashemipour M., Zakerameli A., Khavarian N., Ghatrehsamani S., Poursafa P. Acute and long-term effects of grape and pomegranate juice consumption on vascular reactivity in paediatric metabolic syndrome. Cardiol. Young. 2010;20(1):73–77. doi: 10.1017/S1047951109990850. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblat M., Hayek T., Aviram M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis. 2006;187(2):363–371. doi: 10.1016/j.atherosclerosis.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Fischer U.A., Carle R., Kammerer D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MSn. Food Chem. 2011;127:807–821. doi: 10.1016/j.foodchem.2010.12.156. [DOI] [PubMed] [Google Scholar]
- 11.Aqil F., Munagala R., Vadhanam M.V., Kausar H., Jeyabalan J., Schultz D.J., Gupta R.C. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res. Int. 2012;49:345–353. doi: 10.1016/j.foodres.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pande G., Akoh C.C. Antioxidant capacity and lipid characterization of six Georgia-grown pomegranate cultivars. J. Agric. Food Chem. 2009;57(20):9427–9436. doi: 10.1021/jf901880p. [DOI] [PubMed] [Google Scholar]
- 13.Park S.H., Kim J.L., Lee E.S., Han S.Y., Gong J.H., Kang M.K., Kang Y.H. Dietary ellagic acid attenuates oxidized LDL uptake and stimulates cholesterol efflux in murine macrophages. J. Nutr. 2011;141(11):1931–1937. doi: 10.3945/jn.111.144816. [DOI] [PubMed] [Google Scholar]
- 14.Negi P.S., Jayaprakasha G.K., Jena B.S. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003;80:393–397. [Google Scholar]
- 15.Lei F., Zhang X.N., Wang W., Xing D.M., Xie W.D., Su H., Du L.J. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int. J. Obesity (London) 2007;31:1023–1029. doi: 10.1038/sj.ijo.0803502. [DOI] [PubMed] [Google Scholar]
- 16.Bursill C.A., Roach P.D. Modulation of cholesterol metabolism by the green tea polyphenol (−)-epigallocatechin gallate in cultured human liver (HepG2) cells. J. Agric. Food Chem. 2006;54(5):1621–1626. doi: 10.1021/jf051736o. [DOI] [PubMed] [Google Scholar]
- 17.Huang J., Zhang Y., Zhou Y., Zhang Z., Xie Z., Zhang J., Wan X. Green tea polyphenols alleviate obesity in broiler chickens through the regulation of lipid-metabolism-related genes and transcription factor expression. J. Agric. Food Chem. 2013;61(36):8565–8572. doi: 10.1021/jf402004x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y.B., Wan X.C., Shang Y.Y., Hu J.W., Shao L., Chen W., Li D.X. Polyphenol content of plasma and litter after the oral administration of green tea and tea polyphenols in chickens. J. Agric. Food Chem. 2012;60(7):1619–1627. doi: 10.1021/jf2039789. [DOI] [PubMed] [Google Scholar]
- 19.Schmid B., Rippmann J.F., Tadayyon M., Hamilton B.S. Inhibition of fatty acid synthase prevents preadipocyte differentiation. Biochem. Biophys. Res. Commun. 2005;328:1073–1082. doi: 10.1016/j.bbrc.2005.01.067. [DOI] [PubMed] [Google Scholar]
- 20.Moon H.S., Chung C.S., Lee H.G., Kim T.G., Choi Y.J., Cho C.S. Inhibitory effect of (−)-epigallocatechin-3-gallate on lipid accumulation of 3T3-L1 cells. Obesity. 2007;15:2571–2582. doi: 10.1038/oby.2007.309. [DOI] [PubMed] [Google Scholar]
- 21.Chen J.H., Wang C.J., Wang C.P., Sheu J.Y., Lin C.L., Lin H.H. Hibiscus sabdariffa leaf polyphenolic extract inhibits LDL oxidation and foam cell formation involving up-regulation of LXRa/ABCA1 pathway. Food Chem. 2013;141:397–406. doi: 10.1016/j.foodchem.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Yang C.M., Lu Y.L., Chen H.Y., Lu M.L. Lycopene and the LXRα agonist T0901317 synergistically inhibit the proliferation of androgen-independent prostate cancer cells via the PPARγ-LXRα-ABCA1 pathway. J. Nutr. Biochem. 2012;23:1155–1162. doi: 10.1016/j.jnutbio.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Chawla A., Boisvert W.A., Lee C.H., Laffitte B.A., Barak Y., Joseph S.B., Liao D., Nagy L., Edwards P.A., Curtiss L.K., Evans R.M., Tontonoz P. A PPAR-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 24.Michalik M., Soczek E., Kosińska M., Rak M., Wójcik K.A., Lasota S., Pierzchalska M., Czyż J., Madeja Z. Lovastatin-induced decrease of intracellular cholesterol level attenuates fibroblast-to-myofibroblast transition in bronchial fibroblasts derived from asthmatic patients. Eur. J. Pharmacol. 2013;704:23–32. doi: 10.1016/j.ejphar.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 25.Henninger C., Huelsenbeck J., Huelsenbeck S., Grösch S., Schad A., Lackner K.J., Kaina B., Fritz G. The lipid lowering drug lovastatin protects against doxorubicin-induced hepatotoxicity. Toxicol. Appl. Pharmacol. 2012;261(1):66–73. doi: 10.1016/j.taap.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Raederstorff D.G., Schlachter M.F., Elste V., Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003;14(6):326–332. doi: 10.1016/s0955-2863(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 27.Shan D., Fang Y., Ye Y., Liu J. EGCG reducing the susceptibility to cholesterol gallstone formation through the regulation of inflammation. Biomed. Pharmacother. 2008;62(10):677–683. doi: 10.1016/j.biopha.2007.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.