Abstract
Imatinib mesylate, a selective tyrosine kinase inhibitor, is the first line treatment against chronic myelogenous leukemia and gastrointestinal stromal tumors. The aim of the present study is to investigate the effects of imatinib mesylate on the pregnant rats and their fetuses. Pregnant rats were divided into three groups; the first group served as a control group. The second and third groups were orally administered imatinib at doses of 36 mg/kg body weight or 54 mg/kg b.wt. on gestation days (SDs) 6 through 13 or SDs 13 through 19, respectively. All animals were sacrificed on the 20th day of gestation. Treatment with imatinib caused a reduction of maternal body weight gain, uterine and placental weights, increased rate of abortion and fetal resorptions. High dose of imatinib caused fetal congenital deformities represented in harelip, contraction of the fore limbs, and paralysis of the hind limbs, exencephaly, encephalocoele and distended abdominal wall, besides occurrence of wavy ribs and absence of other ribs in addition to skeletal growth retardation and lack of ossification of the most skeletal elements. The present work concluded that imatinib is teratogenic when given orally to pregnant rats at 54 mg/kg b.wt. and causes direct maternal or developmental toxicity.
Keywords: Imatinib, Pregnant rat, Fetuses, Congenital deformities, Skeleton
1. Introduction
Pregnancy and cancer are complex situations. The treatment of cancer during pregnancy is a difficult problem because of the potential effects of the therapy on the mother and fetus [1]. Cancer, known medically as a malignant neoplasm, is a broad group of various diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. Cancer may also spread to more distant parts of the body through the lymphatic system or bloodstream. Not all tumors are cancerous. Benign tumors do not grow uncontrollably, nor invade neighboring tissues, and do not spread throughout the body [2]. There are over 200 different known cancers that afflict humans, 90–95% of cases attributed to environmental factors and 5–10% are due to genetics [3]. Researchers have long searched for a more selective method of targeting cancer cells exclusively. The ideal drug would “zero in” on cancer cells and leave other body cells unharmed. Recently, the drug imatinib, which is referred to as a “guided missile” against cancer has captured the interest of oncologists [4]. It is the first anticancer drug to specifically inhibit a molecular abnormality unique to human cancer cells. Imatinib selectively inhibits BCR-ABL gene which has been identified being the cause of chronic myeloid leukemia and was approved by the United States of America FDA as a first line treatment for chronic myeloid leukemia [5].
Imatinib is rapidly absorbed when given by mouth, and is highly bioavailable: 98% of an oral dose reaches the bloodstream. Metabolism of imatinib occurs in the liver and is mediated by several isozymes of the cytochrome P450 system, including CYP3A4 and, to a lesser extent, CYP1A2, CYP2D6, CYP2C9, and CYP2C19. The main metabolite, N-demethylatedpiperazine derivative, is also active. The major route of elimination is in the bile and feces; only a small portion of the drug is excreted in the urine. Most of imatinib is eliminated as metabolites; only 25% is eliminated unchanged. The half-lives of imatinib and its main metabolite are 18 and 40 h, respectively. It blocks the activity of Abelson cytoplasmic tyrosine kinase (ABL), c-Kit and the platelet-derived growth factor receptor (PDGFR). As an inhibitor of PDGFR, imatinib mesylate appears to have utility in the treatment of a variety of dermatological diseases. Imatinib has been reported to be an effective treatment for FIP1L1-PDGFRalpha+ mast cell disease, hypereosinophilic syndrome, and dermatofibrosarcoma protuberans [6].
Although imatinib is an effective therapy for newly diagnosed CP-CML patients, 40–45% of patients discontinue treatment due to adverse events (20–25%) or imatinib resistance (20%) [7]. Importantly, 7–8% of patients transform to accelerated phase (AP) or blast crisis (BC), with most transformations occurring within the first 3 years of imatinib therapy [7], [8]. Treatment with imatinib is generally well tolerated, and the risk for severe adverse effects is low. Adverse effects most commonly include mild-to-moderate edema, nausea and vomiting, diarrhea, muscle cramps, and cutaneous reactions. Hepatic transaminase level elevations and myelosuppression occur less frequently and resolve with interruption of imatinib therapy [9].
The beneficial effects of treatment with imatinib in pregnant patients with chronic myelogenous leukemia should be balanced with the risk of teratogenicity and congenital abnormality in fetus. The risk of teratogenicity has been variable and in most cases it is low and is not well established. A more extensive surveillance is needed for better decision making in treating pregnant women with chronic myelogenous leukemia [10]. So, the aim of the present study is to investigate the possible side effects from the use of imatinib on the pregnant rats and their fetuses.
2. Material and methods
2.1. Animals
Total number of 40 adult female Sprague Dawley rats weighing 150–170 g and 20 male rats weighing 150 g of the same strain were obtained from the Farm of National Organization for Drug Control and Research. Animals had free access to tap water and to standard food diet ad libitum. All rats were allowed to adapt to the laboratory environment for 1 week before being used. The experimental procedures complied with the guidelines of the Committee on Care and Use of National Organization for Drug Control and Research Center, Cairo, Egypt. Each two adult virgin females in proestrus were caged overnight in an animal plastic cage with normal mature male. Vagina was examined daily for suggesting pregnancy by a vaginal smear technique according to the method of Matthews and Kenyon [11]. Females with positive vaginal smears were considered pregnant at zero day of gestation.
2.2. Chemical used
Imatinib mesylate film-coated tablets containing imatinib mesylate equivalent to 100 mg of imatinib free base was used (Novartis.Com, Switzerland). Imatinib mesylate is designated chemically as 4-[(4-Methyl-1-piperazinyl) methyl]-N-[4-methyl-3-[[4-(3-yridinyl)-2-pyrimidinyl]amino]-phenyl] benzamidemethanesulfonate and its structural chemical formula is
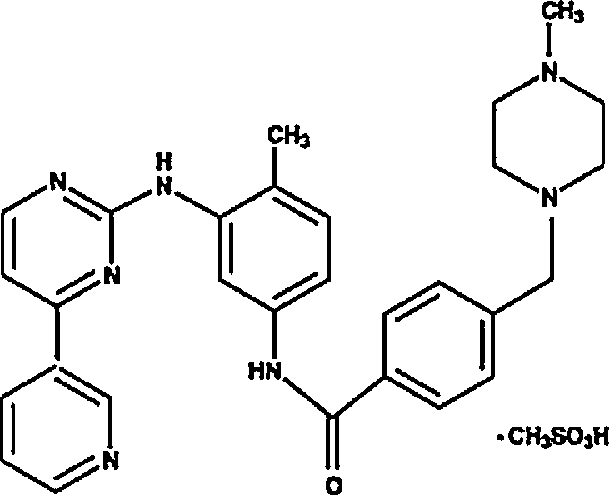
Imatinib mesylate is a white to yellowish tinged crystalline powder. Its molecular formula is C29H31N7O·CH4SO3 and it has low molecular weight which is 493.603 g/mol. Imatinib mesylate is freely soluble in water and aqueous buffers ≤pH 5.5.
2.3. Experimental design and procedures
All pregnant rats were divided into three main groups. The first main group (about 8 animals) served as control. The second main group was divided into two subgroups (8 rats each) and was orally administered low dose of imatinib mesylate free base pure active compound (36 mg/kg body weight dissolved in 1 ml distilled water) on gestation days (SDs) 6 through 13 and on SDs 13 through 19. The third main group of pregnant rats was divided into two subgroups (8 rats/each) and was orally administered high dose of imatinib (54 mg/kg b.wt.) dissolved in 1 ml distilled water from 6th day to 13th day of gestation and from 13th day to 19th day of gestation, respectively. The drug doses (36 mg/kg and 54 mg/kg) are related to the low and high human therapeutic doses [12], [13], [14], [15]. The drug doses (36 mg/kg and 54 mg/kg) were chosen in the present work as the dose 36 mg/kg body weight is considered the lowest-observed-adverse-effect level (LOAEL) of imatinib that can cause the least developmental toxic effects and mortality rate to the pregnant rats and their fetuses. Lower than 36 mg/kg showed no observed adverse effect level (NOAEL) of imatinib. The dose equal to 54 mg/kg b.wt. was considered as the maximum dose that can cause direct maternal and developmental toxicity. Higher than 54 mg/kg b.wt. showed complete mischarge and increased percentage of dams mortality rates. At the 20th day of gestation – one day before the date of expected delivery, because mothers usually cannibalize malformed or incompletely vital neonates [16] – pregnant females are sacrificed. The ovaries and uteri from each female were removed and examined for the number of corpora lutea, status of all the implantation sites (i.e., live and dead fetuses, early and late resorptions and total implantations sites). A resorption site was defined as an implantation site resembling a brown to greenish blood clot, with just placental tissue [early resorption] or with placental and embryonic tissue [late resorption] [17]. A non-viable fetus – still birth – which was described as a fetus that does not react to stimuli, has a pale color, stemming from a lack of blood flow, and is smaller in size compared to the viable fetuses. The number of alive and dead fetuses was recorded. Each live fetus was dried on a bloating paper, weighed and subsequently, the fetal weight and crown rump length were measured. Each fetus was examined carefully for any congenital anomalies. Fetuses were fixed in 96% ethanol solution for skeletal anomalies. Alterations were classified as malformations (rare structural changes) or anomalies (minor structural differences from normal detected relatively frequently). The percentage of pre-implantation loss and post-implantation loss was calculated according to the following equations:
Pre-implantation loss = (number of corpora lutea) − (number of implantation sites)/number of corpora lutea × 100.
Post-implantation loss = (number of implantation sites) − (number of viable fetuses)/number of implantation sites × 100.
2.4. Skeletal preparations of fetuses
For the skeletal studies, fetuses of rats were skinned and fixed in 96% ethanol for 5 days for dehydration. Staining was performed with alcian blue–alizarin red solution according to the method described by Taylor [18], which is prepared by dissolving (15 mg alcian blue stain in 80 ml 96% ethanol + 20 ml of glacial acetic acid). Then fetuses were immersed in a solution containing 0.01 g alizarin red stain dissolved in 100 ml of 1% aqueous KOH. Clearing process was done by placing the fetuses in 2% aqueous KOH solution for 7 days, until skeletons were clearly visible through the surrounding tissue. After staining, the specimens were transferred to ascending series of glycerol in 1% aqueous potassium hydroxide solution, after which, they were preserved in 100% glycerin. The stained preparations were carefully examined under the dissecting binocular microscope to study the various parts of the axial and appendicular skeleton for any abnormalities.
2.5. Statistical analysis
Statistical significance was evaluated by one-way ANOVA using SPSS Version 19 and the individual comparison was obtained by LSD method. Values were considered statistically significant when p < 0.05.
3. Results
3.1. Effect of imatinib on the pregnant rats
Maternal food and water consumption were slightly decreased (non-significantly) as compared to the control group and no obvious clinical observations were noticed in the treated groups.
3.1.1. Body weight
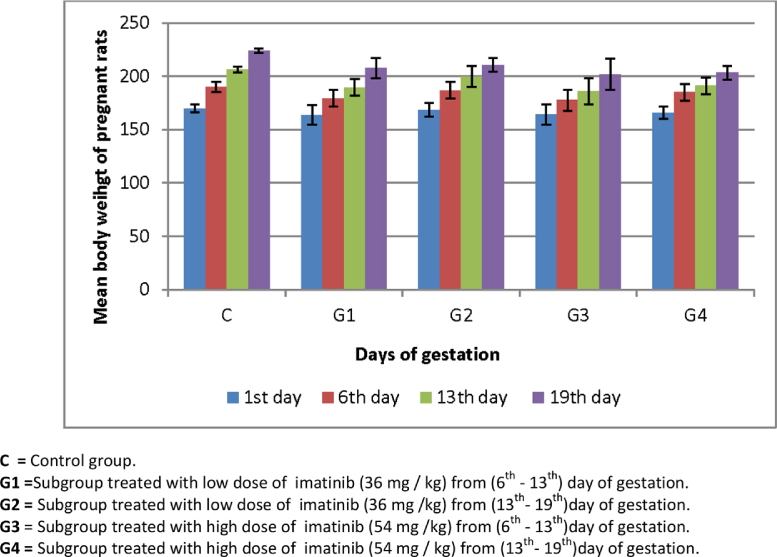
In the present study, the average maternal body weights for the control and experimental subgroups recorded on the 1st, 6th, 13th and 19th days of gestation were represented in Fig. 1. The data indicated that the pregnant females of both the control and all experimental groups showed a steady increase in the body weight (weight gain) during the first week of gestation before administration of imatinib. On the 13th day of gestation, the body weight of rats of the control group continued to increase approximately at the same rate, while those of the experimental subgroups showed a less rate of increase in the body weight gain. On the 19th day, pregnant females of the control group exhibited high percentage of body weight gain (31.79%). The rate of increase in weight of the treated subgroups decreased than the control one being 26.51% and 27% for animals that were treated with the low dose of imatinib from the 6th day to the 13th day and from the 13th day to the 19th day of pregnancy, respectively, while those which were treated with high dose of imatinib from the 6th day to the 13th day recorded (23.01%) and from the 13th day to the 19th day recorded (22.62%) from control group.
Fig. 1.
Average body weight of control and imatinib treated groups (mean ± S.E.). C = Control group. G1 = Subgroup treated with low dose of imatinib (36 mg/kg) from (6th–13th) day of gestation. G2 = Subgroup treated with low dose of imatinib (36 mg/kg) from (13th–19th) day of gestation. G3 = Subgroup treated with high dose of imatinib (54 mg/kg) from (6th–13th) day of gestation. G4 = Subgroup treated with high dose of imatinib (54 mg/kg) from (13th–19th) day of gestation.
3.1.2. Percentage of abortion
No abortions were recorded among mothers of control group. On the other hand, two cases of complete abortions were recorded in subgroup (25%) which were treated with the low dose of imatinib (36 mg/kg b.wt.) from the 6th to the 13th day of gestation, while no abortions were recorded in the subgroup treated with imatinib at the same dose from the 13th to the 19th day of gestation compared to the normal control group. Treatment with high dose (54 mg/kg body weight) of imatinib from the 6th to the 13th day of gestation recorded three cases of complete abortions (37.5%) and two cases (25%) of abortions were recorded in the subgroup treated with imatinib from the 13th to the 19th day of gestation as compared to control group. Abortions among rats of both treated groups occurred on the 13th or the 19th day of gestation. A sudden drop in maternal body weight and presence of drops of blood were considered as signs of abortion.
3.1.3. Percentage of resorption
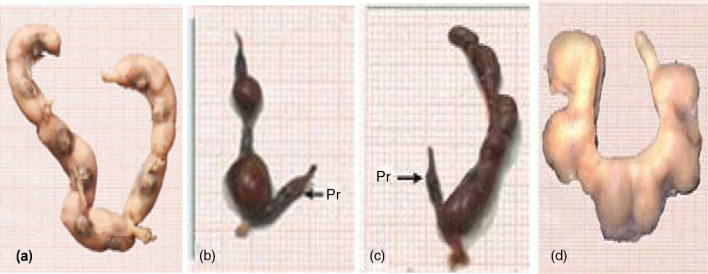
Uteri with partial and complete resorption sites were calculated and recorded in Table 1. There was no resorption among the control group (Fig. 2) or in the subgroup treated with imatinib at 36 mg/kg body weight from the 6th to the 13th day of gestation. On contrary, the percentage of uteri with partial resorption in rats administered the same dose of imatinib from the 13th to the 19th day of gestation recorded 62.5% compared to the normal values. Pregnant rats treated with imatinib (54 mg/kg b.wt.) exhibited about 62.5% of uteri with partial resorption (Fig. 2b and c), while the percentage of uteri with complete resorption recorded about 12.5% as shown in Fig. 2.
Table 1.
Average weights of uteri and placenta of pregnant rats at the 20th day of gestation.
| Groups | Total no. of pregnant rats | Total number of sacrificed uteri | Uteri |
Placenta |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Without resorption C% | With partial resorption C% | With complete resorption C% | Average weight of uteri | C% | Average weight of placenta | C% | |||
| Control | 8 | 8 | 8 (100) | – | – | 35.52 ± 0.70 | – | 1.53 ± 0.08 | – |
| Imatinib low dose (6th–13th) | 8 | 6 | 6 (75) | – | – | 24.41 ± 3.23 | 11.11 (−31.28) | 1.0 ± 0.15 a*e* |
−34.64 |
| Imatinib low dose (13th–19th) | 8 | 8 | 3 (37.5) | 5 (62.5) | – | 22.76 ± 4.87 a* |
12.76 (−35.92) | 0.87 ± 0.17 a** |
−43.13 |
| Imatinib high dose (6th–13th) | 8 | 5 | 4 (50) | – | 1 (12.5) | 16.06 ± 4.36 a** |
19.46 (−54.78) | 0.62 ± 0.13 a*** |
−59.47 |
| Imatinib high dose (13th–19th) | 8 | 6 | 1 (12.5) | 5 (62.5) | – | 15.28 ± 5.69 a*** |
20.24 (−56.98) | 0.53 ± 0.18 a***b* |
−65.35 |
Values are means (±SE) of 8 animals.
a = compared to the normal control.
b = compared to the low dose of imatinib (36 mg/kg) from 6th to 13th day of gestation.
c = compared to the low dose of imatinib (36 mg/kg) from 13th to 19th day of gestation.
d = compared to the high dose of imatinib (54 mg/kg) from 6th to 13th day of gestation.
e = compared to the high dose of imatinib (54 mg/kg) from 13th to 19th day of gestation.
C% = percentage of change from normal control.
Significant (p < 0.05).
Highly significant (p < 0.01).
Very highly significant (p < 0.001).
Fig. 2.
(a) Photograph of control uterus. (b–d) Uteri of pregnant rats treated with 54 mg/kg b.wt. of imatinib on the 20th day of gestation showing partial resorption (Pr) (b and c) and complete resorption (d).
3.1.4. Average weight of uteri and placenta
The effect of imatinib on the weight of uteri and placenta of pregnant rats for control and all treated groups were recorded in Table 1.
3.1.4.1. Average weight of uteri
The average uterine weight is generally less in all experimental groups than those of the control group (Table 1). The average weight of uteri of pregnant rats treated with imatinib (36 mg/kg) from the 6th to the 13th day of gestation showed no statistical significant difference (p > 0.05) recording (24.41 ± 3.23) as compared to normal control one (35.52 ± 0.70). On contrast, the average weight of uteri of pregnant rats treated with imatinib (36 mg/kg) from the 13th to the 19th day of gestation exhibited a significant decrease (p < 0.05) recording (22.76 ± 4.87) than that of the control group. The average weight of uteri of pregnant rats treated with 54 mg/kg of imatinib from the 6th to the 13th day of gestation and from the 13th to the 19th day of gestation recorded (16.06 ± 4.36) and (15.28 ± 5.69) respectively, exhibited a highly significant (p < 0.01, p < 0.001) reduction than those of the control group (35.52 ± 0.70).
3.1.4.2. Average weight of placenta
The average weight of placenta exhibited a significant decrease (p < 0.05) at 36 mg/kg imatinib from the 6th to the 13th recorded (1.0 ± 0.15) and from the 13th to the 19th day of gestation recorded (0.87 ± 0.17) compared to the control group (1.53 ± 0.08). While the average weight of placenta revealed a very highly significant decrease (p < 0.001) at 54 mg/kg imatinib from the 6th to the 13th day of gestation recorded (0.62 ± 0.13) and from the 13th to the 19th day of gestation recorded (0.53 ± 0.18) as compared to the control group. The data were given in Table 1.
3.2. Effect of imatinib on the fetuses
3.2.1. External morphological studies
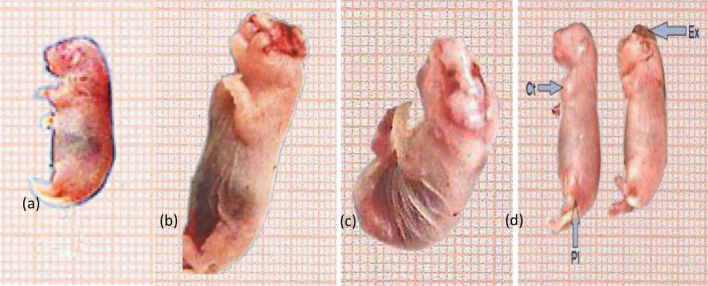
The mean number of fetal body weight and length were recorded in (Table 2). Growth retardation was indicated by the reduction of both fetal body weight and body length. The average fetal body weight and length in the two experimental subgroups, treated with imatinib at both doses, were less than that of the control group.Morphological examination of fetuses maternally treated with high dose of imatinib exhibited several abnormal external features as internal hemorrhage, congestion and growth retardation. Also, hematoma (subcutaneous hemorrhage or dark red patches under the skin) was found mainly in the head, in the back between shoulders and between fore and hind limbs. Harelip appeared as congenital cleft in the midline of the upper lip (Fig. 3b). Encephalocoele and protrusion of the brain tissue through a congenital fissure in the skull, abdominal distention and internal hemorrhage were also noticed (Fig. 3c and d), besides anomalies of limbs that were indicated by contraction of the fore limb and paralysis of the hind limb (Fig. 3d). In the fetuses maternally treated with the high dose of gleevec from the 13th to the 19th day of gestation, anomalies were also verified particularly in the fore and hind limbs that were rotated inward. In addition to, some fetuses exhibited edema and internal hemorrhage (Fig. 3b and c). No obvious abnormal changes in the fetuses internal organs were noticed in the treated groups. Table 4 illustrated the total number of fetuses with external anomalies.
Table 2.
Effect of imatinib on fetuses body weight and length.
| Parameters | Control | Imatinib low dose (6th–13th) | Imatinib low dose (13th–19th) | Imatinib high dose (6th–13th) | Imatinib high dose (13th–19th) |
|---|---|---|---|---|---|
| Mean body weight (g) (±SE) | 4.55 ± 0.09 | 3.47 ± 0.13 | 3.09 ± 0.12 | 2.56 ± 0.11 | 2.11 ± 0.16 |
| C% of change | – | −23.74 | −32.09 | −43.74 | −53.63 |
| p Value | – | a***c*d***e*** | a***b*d**e*** | a***b***c** | a***b***c*** |
| Mean body length (cm) (±SE) | 5.24 ± 0.06 | 4.56 ± 0.08 | 4.12 ± 0.10 | 3.81 ± 0.11 | 3.16 ± 0.15 |
| C% of change | – | −12.97 | −21.37 | −27.29 | −33.69 |
| p Value | – | a***c***d***e*** | a***b***d*e*** | a***b***c*e*** | a***b***c***d*** |
a = compared to the normal control.
b = compared to the low dose of imatinib (36 mg/kg) from 6th to 13th day of gestation.
c = compared to the low dose of imatinib (36 mg/kg) from 13th to 19th day of gestation.
d = compared to the high dose of imatinib (54 mg/kg) from 6th to 13th day of gestation.
e = compared to the high dose of imatinib (54 mg/kg) from 13th to 19th day of gestation.
C% = percentage of change from normal control.
Significant (p < 0.05).
Highly significant (p < 0.01).
Very highly significant (p < 0.001).
Fig. 3.
Photograph of control fetus (a). (b–d) Fetuses on the 20th day of gestation maternally treated with the high dose of gleevec (54 mg/kg b.wt.) showing: harelip with congenital cleft in the midline of the upper lip (b). Encephalocoele with protrusion of the brain tissue through a congenital fissure in the skull, abdominal distention and internal hemorrhage (c). Exencephaly (Ex) and the brain are completely exposed through a defect in the cranial vault, contraction (cr) of the fore limb and paralysis (pl) of the hind limb (d).
Table 4.
Total number of fetuses with external anomalies (%).
| No of fetuses with external anomalies (%) | Control | Imatinib low dose (6th–13th) | Imatinib low dose (13th–19th) | Imatinib high dose (6th–13th) | Imatinib high dose (13th–19th) |
|---|---|---|---|---|---|
| Harelip | 0 | 0 | 0 | 15 (38.46) | 6 (14.29) |
| Exencephaly | 0 | 0 | 0 | 10 (25.64) | 4 (9.52) |
| Encephalocoele | 0 | 0 | 0 | 4 (10.26) | 3 (7.14) |
| Paralysis of the hind limb | 0 | 2 (4.88) | 3 (4.41) | 2 (5.13) | 1 (2.38) |
| Contraction of the fore limb | 0 | 3 (7.32) | 3 (4.41) | 3 (7.69) | 1(2.38) |
| Congestion | 0 | 2 (4.88) | 4 (5.88) | 3 (7.69) | 2 (4.76) |
| Subcutaneous hemorrhage | 0 | 0 | 1(1.47) | 7 (17.95) | 5 (11.90) |
| Hematoma | 0 | 0 | 0 | 3 (7.69) | 0 |
3.2.2. Mortality rate
The total prenatal mortality rate was represented by fetal resorptions and stillbirths (dead fetuses). On the 20th day of gestation all dead and alive fetuses were calculated and recorded in Table 3. There were no mortality rates among the control and experimental subgroup maternally treated with low dose (36 mg/kg b.wt.) of imatinib from the 6th to the13th day of gestation, while mortality rate of fetuses maternally treated with the same dose from the 13th to the 19th of gestation was found to be 36.76% from the control group. The rate of dead fetuses maternally treated with high dose of imatinib (54 mg/kg b.wt.) from the 6th to the 13th and from the 13th to the 19th was 18.18% and 64.28% respectively as compared to the control group. Administration of imatinib at low dose from the 13th to the 19th of gestation increased the percentage of pre-implantation loss to 11.69% and post-implantation loss to 36.76% as compared with control group. Even more, high dose of imatinib elevated the percentage of pre-implantation loss and post-implantation loss to 29.09% and 30.77% respectively, in the group of rats given imatinib from the 6th to the 13th day of gestation and also in the group of rats given imatinib from the 13th to the 19th day of gestation to reach 17.65% and 64.29% respectively from normal control group (Table 3).
Table 3.
Effect of imatinib on fetal mortality rate at the 20th day of gestation.
| Groups | No. of sacrificed uteri | Developing fetuses |
Pre-implantation loss | Post-implantation loss | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number |
No. of living fetuses | Stillbirths % | Resorbed % | Total mortality % | |||||
| Total number | No. of fetuses per litter | No. % |
No. % |
No. % |
No. % |
||||
| Control | 8 | 57 | 7.12 | 57 100% |
– – |
– – |
– – |
0% | 0% |
| Imatinib low dose (6th–13th) | 6 | 41 | 5.12 | 41 100% |
– – |
– – |
– – |
0% | 0% |
| Imatinib low dose (13th–19th) | 8 | 68 | 8.5 | 43 63.23% |
1 1.47% |
24 35.29% |
25 36.76% |
11.69% | 36.76% |
| Imatinib high dose (6th–13th) | 5 | 39 | 4.12 | 27 81.81% |
– – |
6 18.18% |
6 18.18% |
29.09% | 30.77% |
| Imatinib high dose (13th–19th) | 6 | 42 | 5.25 | 15 35.71% |
11 26.19% |
16 38.09% |
27 64.28% |
17.65% | 64.29% |
3.2.3. Skeletal studies
3.2.3.1. Control cases
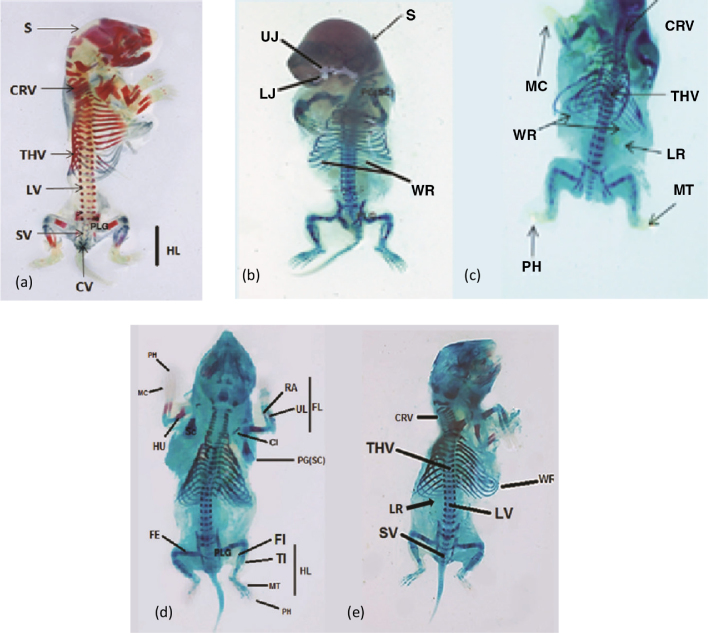
At the 20th day of gestation, the cleared cartilage and bone preparations of control rat fetuses have designated that the skeletal system shows numerous bony elements stained with alizarin and few cartilages stained with alcian blue. These cases, well illustrated in (Fig. 4a), were displaying the developed cartilage and bone in the different parts of skeleton of these control fetuses.
Fig. 4.
(a) Photograph of the skeletal system of 20 days old control fetus showing: well ossified skull (S), cervical (CRV), thoracic (THV), lumbar (LV), sacral (SV), caudal vertebrae (CV) and hind limbs (HL). (b) Photograph of fetus maternally treated with imatinib (36 mg/kg) from 6th to 13th day of gestation showing: retardation of ossification of skull (S), upper jaw (UJ), Lower jaw (LJ) and presence of wavy ribs (WR). (c) Skeleton of fetuses maternally treated with imatinib (36 mg/kg) from 13th to 19th of gestation showing incomplete ossification of cervical (CRV), thoracic vertebrae (THV), metacarpals (MC), metatarsals (MT) and phalanges (PH). Absence of the last rib (LR) (no. 13) and a fetus had wavy ribs (WR) (nos. 7, 8, and 9). (d) Skeleton of fetuses on the 20th day of gestation maternally treated with imatinib (54 mg/kg) from 6th to 13th day of gestation showing sever lack of ossification of scapulae (SC) and clavicles (Cl) bones of the pectoral girdle (PG). Lack of ossification of pelvic girdle (PLG) and humerus (HU), radius (RA), ulna (UL), metacarpals (MC) and phalanges (PH) of the fore limb (FL). Femur (FE), tibia (TI), fibula (FI), metatarsals (MT) and phalanges (PH) of the hind limb (HL) showed lower degree of ossification, shorter and thinner as compared to the control fetus (a). (e) Severe lack of ossification of cervical (CRV), thoracic vertebrae (THV), lumbar vertebrae (LV) and sacral vertebrae (SV). Severe rib anomalies: wavy ribs (WR) on rib numbers 7, 8, and 9 and absence of the last rib (LR) number 13.
3.2.3.2. Fetuses maternally treated mothers
Fetuses of mothers treated with imatinib during gestation have exhibited several skeletal alterations as compared with control ones. Bones of the skull of 20 days old fetuses maternally treated with low dose of imatinib (36 mg/kg b.wt.) from the 6th to the13th day of gestation, showed mild degree of lack of ossification of frontal, parietal, interparital, squamosal and occipital bones of the skull (Fig. 4b). Less ossification of the cervical, thoracic, lumber, sacral and caudal vertebrae of the vertebral column was also noticed. Twenty days old fetuses maternally treated with imatinib (36 mg/kg b.wt.) from the 13th to the19th day of gestation revealed, retardation of ossification of scapulae and clavicles of the pectoral girdle and also the bones of the fore limbs (humerus, radius, ulna, carpals, metacarpals and phalanges). Moreover, less ossification of the bones of the pelvic girdle (ilium, ischium and pubis) and the bones of the hind limbs (femur, tibia, fibula, tarsals, metatarsals and phalanges) were also seen. The fore and hind limbs not only showed lower degree of ossification but also became more shorter and thinner as compared with the corresponding bones of the control fetuses (Fig. 4a). In addition, some fetuses had wavy ribs; numbers 7, 8, and 9 and the last rib (number 13) were lost (Fig. 4c).
Fetuses of dams treated with (54 mg/kg b.wt.) imatinib from the 6th to the13th day and the 13th to the19th day of gestation showed severe lack of ossification of the dermal bones of the skull and bones of the upper and lower jaws. Also, the effect of imatinib on the pectoral and pelvic girdles of the fetuses maternally treated with high dose of imatinib was manifested by severe shortness and lack of ossification of all their bones and reduction of the ossification parts of both scapulae and clavicles as compared with those of the control. The long bones of the fore and hind limbs of these fetuses whose mothers were treated with high dose of imatinib were considerably shorter, thinner and less ossified, compared with those of the control but the phalangeal formula was still the same as that of the control 2:3:3:3:3 (Fig. 4d). In addition to the above mentioned lesions, some fetuses had wavy ribs in number; 7, 8, and 9 as shown in Fig. 4e in addition to severe lack of ossification and shortness of the vertebral column (cervical, thoracic, lumbar and sacral vertebrae). However, vertebral column showed non-ossification of their central and neural arches. Moreover, the caudal vertebrae were absolutely non-ossified in all treated groups.
4. Discussion
Although the chemotherapy drug imatinib mesylate is used to treat certain types of cancer (e.g., chronic myeloid leukemia, gastrointestinal stromal tumors, and myelodysplastic/myeloproliferative diseases) by stopping or slowing the growth of cancer cells (tumors) and causing cancer cells to die. This medication can increase the risk of fetal malformation when administered to the pregnant rats. Imatinib mesylate was found to be teratogenic in rats when administered during organogenesis period at dose 54 mg/kg b.wt. It caused reduction of maternal body weight, uterine and placental weights. It increased the percentages of pre- and post-implantation loss, percentage of abortion, resorptions and caused fetal growth retardation with respect to normal control group. Fetuses maternally treated with the imatinib mesylate suffered from subcutaneous hemorrhage, hematoma, contraction in the fore limbs and paralysis in the hind limbs, in addition to the presence of harelip, exencephaly, encephalocoele and distended abdominal wall. Moreover, Skeletal examination of 20-day old fetuses showed wavy ribs (numbers 7, 8, and 9), absence of rib number 13 and incomplete ossification of some skeletal elements such as skull bones, vertebral column, sternum, ribs, vertebrae, pectoral girdle, fore limb bones, pelvic girdle and hind limb bones.
From the results of the present work, it was found that the first noticed effect of imatinib on the pregnant rats was the reduction of body weight gain in the experimental groups than in the control group. The rate of reduction in body weight increased as the dose of imatinib was increased being 26.51% and 27% for animals that were treated with the low dose of imatinib from the 6th day to the 13th day and from the 13th day to the 19th day of pregnancy, respectively. While, those which were treated with high dose of imatinib from the 6th day to the 13th day recorded (23.01%) and from the 13th day to the 19th day recorded (22.62%) from control group. Similar results were recorded by Chabner et al. [19] and Togo et al. [20], as they stated that many anticancer drugs damage the epithelial cells of the digestive tract, resulting anorexia, nausea, vomiting, malabsorption and all of these effects can cause the reduction of the body weight. In accordance with the present results, El-Sayyad et al. [21] found a significant decrease in the body weight gain after treatment of rats with therapeutic doses of many anticancer drugs as cisplatin, doxorubicin (1 mg/kg) and 5-FU (20 mg/kg) for 20 days as compared to control group and the authors attributed that to hepatotoxicity which might have contributed to this loss as previously reported by King and Perry [22]. The second harmful action on mothers was represented by high percentage of abortions (37.5%) which was noticed in the treated group injected with the high dose of imatinib (54 mg/kg b.wt.) from the 6th to the 13th as compared with the other treated groups and (25%) were recorded in the subgroup treated with imatinib from 13th to 19th day of gestation as compared to control group. Our results are in agreement with many authors [23], [24] who found the same results in animal studies and in patients treated with different chemotherapy agents. The authors added that, increased risk of abortion, birth defects, genetic or neoplastic disease in the offspring of cancer survivors may be caused from germ cells injury as well as induction of transmissible genetic damage.
Placental transfer of imatinib to fetuses during pregnancy may be one of the reasons which led to the increased numbers of resorption and abortion in the treated groups. In confirmation with the present suggestion, many reports established the penetration of anticancer drugs through the placental barrier. Busulfan crosses the placenta and causes incidence of abortion in rhesus monkeys [25] and severe stunting of growth, fetal malformations and gonadal aplasia in the offspring of rats [26]. Inhibition of DNA synthesis has the potential to cause abortion, intrauterine growth retardation and congenital malformations after treatment with both hydroxyurea and busulfan [27].
In the present work, administration of imatinib to pregnant rats caused high percentage of fetal mortality including resorptions and stillbirths, thus leading to a decrease in maternal uterine weights in both treated groups compared with the control group. Comparing between the two treated groups (36 and 54 mg/kg b.wt. imatinib) it was clear that the decrease in the average uterine weight was proportional to the dose of imatinib. Similar results were recorded by Chung et al. [28] and Yeh et al. [29] in pregnant rats treated with different doses of anticancer drugs. Slott and Hales [30] and Higdon et al. [31] stated that, cisplatin and carboplatin are reported to be distributed in the uterine tissues in human, rats, and rabbits. Furthermore, there are persistent DNA adducts in the uterine tissue of rats [32]. Anticancer drug has also been shown to cause apoptosis in primary endometrial cell cultures [33]. It is possible that the administration of cisplatin causes uterine damage, and that damage to the uterus will either prevent implantation, leading to the increased pre-implantation loss, or will lead to damage that prevents the implanted fetuses from developing, leading to the increased post-implantation loss, or a combination of both. In accordance with the present results, Chung et al. [34] found an increase in the resorption rate in pregnant rats which were administered platinum compound and DA-125 from the 6th to the16th day of gestation. The authors suggested that the resorption may be as a result of insufficient hormone concentration, deficient placenta function or may be due to the direct effect of heptaplatin on the embryos.
In the present study, treatment with imatinib caused fetal growth retardation indicated by reduction of both body weight and length of the fetuses. Our results are in agreement with Bajt and Aggarwal [35], as they elucidated that, reduction in the number of fetuses and their body weights during organogenesis were linked to its efficacy in reducing the levels of pituitary hormones. Hormones, such as progesterone, prolactin and LH, are necessary for normal embryonic and fetal development. Their imbalance, due to the exposure of dams to chemotherapy, might result in a delay of embryonic and fetal development. The intrauterine development in mammals is a period of active cell proliferation, migration and differentiation and it is highly sensitive to chemical injuries. A number of effects, ranging from pre- and postnatal mortality and stunting of growth to severe organ malformation, and functional anomalies have been reported to result from chemical exposure to embryos [36]. Vandyke et al. [37] reported that the rapid acceleration of growth plate closure resulting from the inhibition of PDGF-β receptor signaling by imatinib placental hypoperfusion and consequently fetal hypoperfusion.
Imatinib induced various teratogenic pictures in fetuses maternally treated with imatinib (36 and 54 mg/kg b.wt.) from the 6th day to the 13th day and from the 13th day to the 19th day of pregnancy. These pictures were manifested by hemorrhage, hematoma, contraction of the fore limbs, paralysis in hind limbs, lack of ossification of the skeleton and reduced birth weight and length. Also, encephalocoele, exencephaly, distended abdominal wall with internal hemorrhage and harelip were noticed only in the fetuses maternally treated with the high dose of imatinib. Similar observations were recorded by Howle and Gale [38] as they found that, cis-platinum complexes have been reported to induce a severe and prolonged inhibition of DNA synthesis with minimal effects on RNA and protein synthesis. The complexes appear to produce a partial denaturation of DNA and an almost complete destruction of protein secondary structure [39]. Many chemotherapeutic agents are teratogenic, and they also inhibit DNA synthesis. The relationship between the inhibition of DNA synthesis and the production of malformations is not clear [40]. It may be interference with cell proliferation and induction of cell death that often follows inhibition of DNA synthesis that is responsible for the production of malformations. Ault et al. [41] and Pye et al. [42] found that pregnant women treated for chronic myeloid leukemia and exposed to imatinib by trimester were known to have resulted infants with abnormalities. Ognio et al. [43] found that the formation of reactive free radicals seems to be an important part of the embryonic cytotoxicity. The reductions in uterine blood flow may be associated with the embryo toxicity [44]. Nystrom et al. [45] claimed that, in rats, imatinib has been shown to be teratogenic. The theoretical mechanisms responsible for the teratogenicity of the drug include inhibition of platelet-derived growth factor beta (PDGFR-beta) that is important for the development of the microvascular system and for myelination of the peripheral nervous system.
5. Conclusions
Exposure to imatinib during pregnancy has adverse effects on pregnant rats and their fetuses. Imatinib developmentally toxic and was found to be teratogenic when given orally to pregnant rats at 54 mg/kg b.wt. The health risk was increased as the dose of imatinib increased. So, imatinib induced toxicity to pregnant rats and their fetuses in a dose dependent manner.
References
- 1.Buyukbayrak E.E., Ergen B., Karsidag Y.K., Kars B., Turan C., Argon D. Pregnancy complicated with chronic myelogeneous leukemia (cml) successfully treated with imatinib: a case report. Arch. Gynecol. Obstet. 2008;278:161–163. doi: 10.1007/s00404-007-0547-6. [DOI] [PubMed] [Google Scholar]
- 2.Nishanthi M., Prasanthi P., Teja K.M., Reddy K.M., Nishanthi P., Nagendramma M., Vijayakumar B. A cancer disease: a review. Asian J. Pharm. Res. 2013;3(1):47–52. [Google Scholar]
- 3.Anand P., Kunnumakkara A.B., Kunnumakara A.B., Sundaram C., Harikumar K.B., Tharakan S.T., Lai O.S., Sung B., Aggarwal B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008;25(9):2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman J.M., Melo J.V. Chronic myeloid leukemia advances in biology and new approaches treatment. N. Engl. J. Med. 2003;349(15):1451–1464. doi: 10.1056/NEJMra020777. [DOI] [PubMed] [Google Scholar]
- 5.Deininger M., Buchdunger E., Druker B. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. 2005;105:2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- 6.Scheinfeld N., Schienfeld N. A comprehensive review of imatinib mesylate (Gleevec) for dermatological diseases. J. Drugs Dermatol. 2006;5(2):117–122. [PubMed] [Google Scholar]
- 7.Deininger M., O’Brien S.G., Guilhot F., Goldman J.M., Hochhaus A., Hughes T.P. International randomized study of interferon vs sti571 (iris) 8-year follow-up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood. 2009;114(22):462. [Google Scholar]
- 8.Druker B.J., Guilhot F., O’Brien S.G., Gathmann I., Kantarjian H., Gattermann N. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 9.Guilhot F. Indications for imatinib mesylate therapy and clinical management. Oncologist. 2004;9(3):271–281. doi: 10.1634/theoncologist.9-3-271. [DOI] [PubMed] [Google Scholar]
- 10.Mashhadi M.A. Pregnancy outcome of two patients with chronic myelogenous leukemia treated with imatinib. Iran. J. Med. Sci. 2008;33(2):114–116. [Google Scholar]
- 11.Matthews M.K., Kenyon R. Four- versus five-day estrous cycles in rats: vaginal cycling and pregnancy. Physiol. Behav. 1984;33:65–67. doi: 10.1016/0031-9384(84)90014-3. [DOI] [PubMed] [Google Scholar]
- 12.Foringer J.R., Verani R.R., Tjia V.M., Finkel K.W., Samuels J.A., Guntupalli J.S. Acute renal failure secondary to imatinib mesylate treatment in prostate cancer. Ann. Pharmacother. 2005;39:2136–2138. doi: 10.1345/aph.1G131. [DOI] [PubMed] [Google Scholar]
- 13.Choudhary D.R., Mishra P., Kumar R., Mahapatra M., Choudhry V.P. Pregnancy on imatinib: fatal outcome with meningocele. Ann. Oncol. 2006;17(1):178–179. doi: 10.1093/annonc/mdj065. [DOI] [PubMed] [Google Scholar]
- 14.Ferrero D., Pogliani E.M., Rege-Cambrin G., Fava C., Mattioli G., Dellacasa C., Campa E., Perfetti P., Fumagalli M., Boccadoro M. Corticosteroids can reverse severe imatinib-induced hepatotoxicity. Haematologica. 2006;91(6):78–80. [PubMed] [Google Scholar]
- 15.Garderet L., Santacruz R., Barbu V., van den Akker J., Carbonne B., Gorin N.C. Two successful pregnancies in a chronic myeloid leukemia patient treated with imatinib. Hematol. J. 2007;92(1):9–10. doi: 10.3324/haematol.10935. [DOI] [PubMed] [Google Scholar]
- 16.Gleich J., Frohberg H. Methods in Prenatal Toxicology. Georgy Thieme Publishers; Stuttgart: 1977. General teratological techniques. [Google Scholar]
- 17.Kim J.C., Shin J.Y., Yang Y.S., Shin D.H., Moon C.J., Kim S.H. Evaluation of developmental toxicity of amitraz in Sprague-Dawley rats. Arch. Environ. Contam. Toxicol. 2007;52:137–144. doi: 10.1007/s00244-006-0021-7. [DOI] [PubMed] [Google Scholar]
- 18.Taylor P. Acad. Press Inc.; London: 1986. Practical Teratology. [Google Scholar]
- 19.Chabner B.A., Ryan D.P., Paz-Ares L., Garcia-Carbonero R., Calabresi P. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 10th ed. McGraw-Hill; New York: 2001. Anti-neoplastic agents; pp. 1389–1459. [Google Scholar]
- 20.Togo S., Akiyama H., Yamaguchi S., Ichikawa Y., Ike H., Shimada H. Clinical evaluation of granisetron as an inhibitor of nausea and vomiting induced by oral anticancer drugs. Oncol. Rep. 2002;9:277–282. [PubMed] [Google Scholar]
- 21.El-Sayyad H., Ismail M.F., Shalaby F.M., Abou-El-Magd R.F., Gaur R.L., Fernando A., Raj M.H.G., Ouhtit A. Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-fu) on the liver of male albino rats. Int. J. Biol. Sci. 2009;5(5):466–473. doi: 10.7150/ijbs.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King P.D., Perry M.C. Hepatotoxicity of chemotherapy. Oncologist. 2001;6(2):162–176. doi: 10.1634/theoncologist.6-2-162. [DOI] [PubMed] [Google Scholar]
- 23.Fassas A., Kartalis G., Klearchou N., Tsatalas K., Sinaco Z., Mantalenakis S. Chemotherapy for acute leukemia during pregnancy. Five case reports. Nouv. Rev. Fr. Hematol. 1984;26(1):19–24. [PubMed] [Google Scholar]
- 24.Pdydn E.F., Ataya K.M. Effect of cyclophosphamide on mouse oocytes in vitro fertilization and cleavage: recovery. Reprod. Toxicol. 1991;5(1):73–78. doi: 10.1016/0890-6238(91)90113-t. [DOI] [PubMed] [Google Scholar]
- 25.Baer M.R., Ozer H., Foon K.A. Interferon-α therapy during pregnancy in chronic myelogenous leukaemia and hairy cell leukaemia. Br. J. Haematol. 1992;81(2):167–169. doi: 10.1111/j.1365-2141.1992.tb08202.x. [DOI] [PubMed] [Google Scholar]
- 26.Bazarbashi M.S., Smith M.R., Karanes C., Zielinski I., Bishop C.R. Successful management of ph chromosome chronic myelogenous leukemia with leukapheresis during pregnancy. Am. J. Hematol. 1991;38:235–257. doi: 10.1002/ajh.2830380316. [DOI] [PubMed] [Google Scholar]
- 27.Celiloglu M., Altunyurt S., Undar B. Hydroxyurea treatment for chronic myeloid leukemia during pregnancy. Acta. Obstet. Gynecol. Scand. 2000;79:803–804. [PubMed] [Google Scholar]
- 28.Chung M.K., Kim C.Y., Kim J.C. Reproductive toxicity evaluation of a new camptothecin anticancer agent, ckd-602, in pregnant/lactating female rats and their offspring. Cancer Chemother. Pharmacol. 2007;59(3):383–395. doi: 10.1007/s00280-006-0290-x. [DOI] [PubMed] [Google Scholar]
- 29.Yeh J., Kim B.S., Peresie J. Reproductive toxic effects of cisplatin and its modulation by the antioxidant sodium 2-mercaptoethanesulfonate (mesna) in female rats. Rep. Biol. Insights. 2011;5:17–27. [Google Scholar]
- 30.Slott V.L., Hales B.F. Sodium 2-mercaptoethane sulfonate protection against cyclophosphamide-induced teratogenicity in rats. Toxicol. Appl. Pharmacol. 1986;82:80–86. doi: 10.1016/0041-008x(86)90440-0. [DOI] [PubMed] [Google Scholar]
- 31.Higdon R.E., Marchetti F., Mailhes J.B., Phillips G.L. The effects of cisplatin on murine metaphase II oocytes. Gynecol. Oncol. 1992;47:348–352. doi: 10.1016/0090-8258(92)90138-9. [DOI] [PubMed] [Google Scholar]
- 32.Mailhes J.B., Carabatsos M.J., Young D., London S.N., Bell M., Albertini D.F. Taxol-induced meiotic maturation delay, spindle defects, and aneuploidy in mouse oocytes and zygotes. Mutat. Res. 1999;423:79–90. doi: 10.1016/s0027-5107(98)00228-0. [DOI] [PubMed] [Google Scholar]
- 33.Drucker L., Stackievicz R., Radnay J., Shapira H., Cohen I., Yarkoni S. Tamoxifen enhances apoptotic effect of cisplatin on primary endometrial cell cultures. Anticancer Res. 2003;23:1549–1554. [PubMed] [Google Scholar]
- 34.Chung M.K., Kim J.C., Roh J.K. Embryotoxic effects of ski 2053 a new potential anticancer agent, in rats. Reprod. Toxicol. 1998;12(3):375–381. doi: 10.1016/s0890-6238(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 35.Bajt M.L., Aggarwal S.K. An analysis of factors responsible for responsible for resorption of embryos in cisplatin treated rats. Toxicol. Appl. Pharmacol. 1985;80:97–107. doi: 10.1016/0041-008x(85)90104-8. [DOI] [PubMed] [Google Scholar]
- 36.Garber J.E. Long-term follow-up of children exposed in utero to antineoplastic agents. Semin. Oncol. 1989;16:437–444. [PubMed] [Google Scholar]
- 37.Vandyke K., Dewar A.L., Fitter S., Menicanin D., To L.B., Hughes T.P. Imatinib mesylate causes growth plate closure in vivo. Leukemia. 2009;23:2155–2159. doi: 10.1038/leu.2009.150. [DOI] [PubMed] [Google Scholar]
- 38.Howle J.A., Gale G.R. CIS dichlorodiammineplatinum (II): persistent and selective inhibition of deoxyribonucleic acid synthesis in vivo. Biochem. Pharmacol. 1970;19:2757–2762. doi: 10.1016/0006-2952(70)90102-4. [DOI] [PubMed] [Google Scholar]
- 39.Herrmann M.S., Cardin A.D., Behnke W.D., Durig J.R. Nature and reactivity of platinum-pyrimidine blues with biomacromolecules. Biochem. Pharmacol. 1978;27:1571–1576. doi: 10.1016/0006-2952(78)90487-2. [DOI] [PubMed] [Google Scholar]
- 40.Ritter E.J. In: Wilson J.G., Fraser F.C., Ritter E.J., editors. vol. 2. Plenum Press; New York: 1977. pp. 99–116. (Handbook of Teratology). [Google Scholar]
- 41.Ault P., Kantarjian H., O’Brien S., Faderl S., Beran M., Rios M.B., Koller C., Giles F., Keating M., Talpaz M., Corte J. Pregnancy among patients with chronic myeloid leukemia treated with imatinib. J. Clin. Oncol. 2006;24(7):1204–1208. doi: 10.1200/JCO.2005.04.6557. [DOI] [PubMed] [Google Scholar]
- 42.Pye S.M., Cortes J., Ault P., Hatfield A., Kantarjian H., Pilot R., Rosti G., Apperley J.F. The effects of imatinib on pregnancy outcome. 2008;111:5505–5508. doi: 10.1182/blood-2007-10-114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ognio E., Lapide M., Mandys M.O.V., Peterka M., Viale B.P.M. Embryo-lethal and teratogenic effect of the new platinum compound DPR in pregnant mice. Arch. Toxicol. 2003;77:584–590. doi: 10.1007/s00204-003-0494-3. [DOI] [PubMed] [Google Scholar]
- 44.Millicovsky G., Desesso J.M., Kleiman L., Clark K. Effects of hydroxyurea on hemodynamics of pregnant rabbits: a maternally mediated mechanism of embryotoxicity. Am. J. Obstet. Gynecol. 1981;140:747–752. doi: 10.1016/0002-9378(81)90734-1. [DOI] [PubMed] [Google Scholar]
- 45.Nystrom H.C., Lindblom P., Wickman A., Andersson I., Norlin J., Faldt J. Platelet-derived growth factor B retention is essential for development of normal structure and function of conduit vessels and capillaries. Cardiovasc. Res. 2006;71:557–565. doi: 10.1016/j.cardiores.2006.05.019. [DOI] [PubMed] [Google Scholar]