Abstract
Background
The number of chemicals in household products has driven concern about potential adverse health through their use. Most research concentrates on product chemicals with reproductive and carcinogenic consequences, however some evidence exists that immune effects can lead to exacerbation of autoimmune illnesses such as lupus (SLE).
Objectives
This paper examines household and personal product exposure patterns in a pilot case/control study of female Australians. We also examined associations between common product exposure and SLE symptom exacerbation over a year period.
Methods
We enrolled 41 control and 80 SLE participants aged 18–80 years. Qualitative techniques of structured interview and thematic analysis retrospectively explored patterns of product use, and flare history data of SLE participants. Negative binomial regression models explored associations between self-reported flare (SRF) days and exposure to 34 common home product groups.
Results
Mean product counts did not differ between participant groups (mean 33.1: SD 11.8), or flare groups (flare mean 32.6:SD 12, no-flare 31.8:SD 6.6). Products used for personal hygiene and general house cleaning were most frequently used.Significant association with increased SRF day relative risk (IRR) was seen for bath oil use (IRR 1.008, CI 1.00–1.02). Paradoxical “protective” effects, (reduced SRF days) were found for cleansing beauty (IRR 0.999, CI 0.998–0.999), make-up (IRR 0.998, CI 0.997–0.999); adhesives (IRR 0.994, CI 0.991–0.997) and paint (IRR 0.99, CI 0.986–0.995).
Conclusions
Everyday product exposures can impact on symptom exacerbation in SLE. Some offering protection and others increased health risk. Identifying environmental associations offer the possibility of life-style interventions to reduce illness impact.
Keywords: Lupus, Flare, Personal products, Exposure
1. Introduction
The large number of new chemicals available in commercial and household products on the worldwide market has driven concern about potential adverse health impacts from these components [1], [2]. Most research to date has concentrated on animal models and on agents with potential for endocrine disruption, in particular those chemicals with reproductive and carcinogenic consequences, but many information gaps still exist,particularly in regards to immune- endocrine and neuro-endocrine systems.
Our lack of understanding regarding the impact of everyday exposures as well as the cumulative impact of exposure interactions presents a significant challenge in the assessment of health risk. Studies investigating the pathogenic potential of common environmental and chemical agents are limited, with most toxicological studies concentrating on single chemical components rather than end product mixtures [3]. The enclosed nature of modern indoor environments, (including homes, workplaces, activity centers, and also transport modes), and the time spent within these environments, increases an individual’s potential for environmental exposure. Estimates of time spent indoors within developed communities range from 85% to 95% [4]. This also increases the potential for chemicals to accumulate in tissues and organs creating a cumulative burden.
Impacts on health associated with exposure to a wide number of environmental agents have been reported, including immune effects leading to auto immune illnesses [3]. In addition, a collaborative investigation into autoimmunity and exposure to chemicals, by an international panel of experts, as part of the Interorganization Programme for the Sound Management of Chemicals (IPSMC), suggested that environmental exposures can also exacerbate pre-existing autoimmune illnesses [3].
SLE is characterized by multi-system involvement that can be mild through to life threatening and commonly exhibits periods of symptom quiescence and exacerbation (flare events). Environmental interactions along with intrinsic factors of genetics, hormones, and age play a role in the pathogenesis of the disease [5]. This interplay of endogenous and exogenous factors is thought to modulate endocrine and immune regulatory systems and to manifest in either suppression or heightening of immune system responses [3]. Stimuli such as UV sunlight, certain pharmaceutical compounds, hormones, infection and stress are the most researched and accepted symptom exacerbation effectors [5], [6], [7], however, in the vast majority of individuals the reasons for flares remains unclear.
Therefore, this paper will first explore and compare patterns of exposure to common household and personal products in a case/control study of female Australians, and will further examine product exposure in the SLE patient group in relation to frequency of self-reported symptom exacerbation “flare” events (SRF) over a one year period. The results of this pilot study will add to the limited knowledge available regarding common everyday products and the contribution these products may make to SLE symptom exacerbation.
2. Materials and methods
Validated qualitative research techniques using structured interview and thematic analysis [8] were used to explore data of everyday product use and also the flare history of SLE participants. Data was of a self-reported nature representing events over the preceding 12 month period. Institutional review and approval of study protocols and methods according to the Declaration of Helsinki 2008 revision [9] was undertaken and received.
2.1. Participants
Case (SLE) and control participants from Hunter/Central Coast regions of New South Wales, Australia were recruited for a study examining environmental determinants of lupus flares (EDOLF). Written study consent for participation in the study was received from all participants. The study cohort contains a mix of 41 control participants and also 80 SLE participants with a health audit confirmed diagnosis according to the American College of Rheumatology (ACR) Classification guidelines for SLE [10]. Participants were aged between 18 and 80 years and were all female. Participant recruitment and health audit methods have been previously outlined in detail [11].
2.2. Data collection
Participants were posted study specific questionnaires for completion prior to attending scheduled study appointments where questionnaires were collected and checked for completion. A primary study questionnaire captured participant demographic, lifestyle and relevant medical history including routine health management practices, use of medicines, sun protection products, and complementary medicines or therapies. Measurements of body mass index (BMI) were also taken and calculated according to Australian Government Health guideline categories [12]. A 0–100 point visual analogue scale (VAS) with end points of “not stressed at all” and “highly stressed” were used to record current stress levels. Additional study questions captured data related to previously reported SLE flare risk factors, in particular:
-
1.
Home environment characteristics including location of garages, recent renovation and fumigation activities, and use of either public or private water supply;
-
2.
lifestyle practices including indoors and outdoor activity types and times, hobbies and exercise regimes; and
-
3.
personal hygiene and beauty practices.
Participants also completed a Home Cleaning and Maintenance Product list (HCMPL) questionnaire which documented exposure and use of commercial products for personal hygiene and household cleaning.
The HCPML was to be completed in the month between receipt and the scheduled study appointment. Instructions were given to each participant to document all the products that are used within their home for cleaning and for personal care. Instructions clearly stated that products could be used by any member of the household including cleaners and carers. To aid the participant in the self-documentation task and to ensure a more comprehensive list of products, a prompt guide of 5 house areas (kitchen, bathroom/toilet, laundry, garage, garden shed) was included; however participants were not limited to these areas. The HCPML asked for descriptions and brand names of products recorded as well as how often the product was used over the past year (“daily”, “weekly”, “monthly”, “yearly” or “do not know”). The HCPML template is included as supplementary material.
2.2.1. Product exposure assessment
HCPML reported products were coded into 96 product subtypes and aggregated based on product type and description of use into the 34 product groups listed in Table 1. Home and personal product exposure for each participant was calculated as a day count by the collation of self-reported participant product activity information. The total product exposure day counts (EDC) were calculated from cumulative counts of each product's days of use within the HCPML with the addition of product group information contained within the primary questionnaire. Specifically, groups included: lifestyle and activity information; product group exposure due to hobbies; renovation or home fumigation activities; and fluoride exposure through drinking and cooking water source. Reported product use days were scored as ‘daily’ (365 days), ‘weekly’ (52), ‘monthly’ (12), ‘yearly’ (1), ‘when necessary’ or ‘as needed’ (1), ‘rarely’ (1), ‘never’ (0). In addition, if a participant specified a day number for a product group use this number was used within the calculation. Crosschecking of product groups listed in HCPML and questionnaire information occurred to ensure duplicate counts were not recorded and calculated within total EDC. Allocation of EDC for reported activities were based on available literature and personal communications with expert practitioner hobby groups for common exposures. Published out gassing estimates were also used when available for activities such as fumigation, renovation or new carpeting/furnishing as well as some hobbies involving solvents/adhesives, if not available then an estimate of 6 weeks or 42 EDC’s were used. Reported activity with associated product group and exposure EDC score allocation details are provided as a supplement to this paper. Calculated product EDC were standardized across the cohort by portioning the total day count in relation to individual participant nominated hours spent within an indoors environment within averaged weekday and weekend day hours.
Table 1.
Household cleaning and personal product groups.
| Product groups | |
|---|---|
| Adhesives | Fertilizers |
| Air fresheners/deodorisers | Solid fuels |
| Cleaners general | Fixatives |
| Bleach | Herbicides, insecticides, pesticides |
| Cleaners – furniture, homewares | Laundry cleaners |
| Protector waxes/polish | Make-up |
| Cleaners carpet | Paint |
| Cleansing beauty | Cleaning acids |
| Ammonia | Powder beauty |
| Degreasers | Solvents |
| Deodorant | Bath oil |
| Hair removers | Powdered salt |
| Dishwasher | Hydrocarbons |
| Disinfectants/antiseptics | Beauty colorants |
| Caustic cleaners | Perfumes |
| Dry cleaning | Flame retardants |
| Equipment oils & petrol | Fluoride (potable public water) |
2.2.2. Self-reported flare (SRF) assessment
SLE participants took part in a structured interview to record their personal perspectives and accounts of SRF activity over the 12 months prior to interview. To limit self-report error, a participant flare definition describing an autoimmune illness with symptom fluctuations was chosen and used in the interview.
An exacerbation is defined as: The appearance of a new clinical sign/symptom or the clinical worsening of a previous sign/symptom that had been stable for at least the previous 30 days and which persisted for a minimum of 24 h [13].
Participant reported flare counts and average length of flare events were used to establish a total count of SRF days for the study year. Detailed information of structured interview is specifically outlined in previously published material [14].
2.3. Statistics
Simple descriptive statistics summarize participant group demographic information. Association tests between participant groups were performed on product EDC via one-way Anova for continuous variables, and also Fisher’s exact test of independence for dichotomous variables.
Regression models explored the association between self-reported flare events and exposure to the 34 home product groups. General linear modeling based upon a negative binomial robust link function using dichotomous and continuous count data was used due to over dispersion of the dependent SRF variable. Initial models included all variables of interest with a backward stepwise approach adopted for covariates of age; diagnosis years; educational level; socio economic status (SES); body mass index (BMI) and use of vitamin D supplementation, hormones and immune therapy medications (ITM). Significant p values (<0.05) were noted for diagnosis years and ITM use, so they were retained in multivariate models with associations expressed as incidence rate ratios (IRR) with 95% confidence intervals.
Following principles adopted in exposure assessment, product EDC and SRF data were further explored by dichotomising participant exposure/non-exposure based on exposure prevalence cut-points. This approach is used when exposure monitoring data is absent or insufficient due to variability in exposure patterns across differing environments, time periods and work practices [15], [16], [17], [18], [19].
All analysis was performed using STATA v11.0 (StataCorp LP, College Station, Texas, USA).
3. Results
An audit of 159 patient health records was undertaken with 83 including documentary evidence of 4 or more SLE criteria according to the ACR classification guidelines. Three of these SLE participants self-reported “constant” flaring during interview and were not considered to be representative of a relapsing and remitting flare state, therefore their data were not included in the final analysis. Forty one control participants completed all comparison study components. Demographic data of relevance for both groups are shown in Table 2 along with analysis of difference between groups. Mean age of participants was 48 years for SLE participants and 50 for control participants. Length of time post SLE diagnosis averaged 7.7 years (SD 6.2). The study participants were all female and primarily Caucasian (97.5%) with similar levels of BMI, education and SES with 91 % reporting SES of either “above Australian average” or “Australian average”. The proportion of current smokers did not significantly differ (control 2.4%: SLE 7.5%).
Table 2.
Characteristics of participant groups.
| Characteristic | Control (N = 41) |
SLE (N = 80) |
Difference between groups |
|---|---|---|---|
| Mean ± SD | Mean ± SD | p-Value | |
| Age (years) | 49.8 ± 12.4 | 47.7 ± 13.5 | 1.0 |
| Diagnosis (years) | 7.7 ± 6.2 | ||
| Indoor hr portion | 0.81 ± 0.1 | 0.84 ± 0.13 | 0.97 |
| Body Mass Index score | 25.9 ± 4.8 | 27.4 ± 5.6 | 1.0 |
| Stress level (VAS) | 25.4 ± 22 | 50.1 ± 27.4 | 1.0 |
| Socio Economic Status (SES) | 1.2 ± 0.5 | 1.1 ± 0.55 | 0.1 |
| SRF days (year) | 29.2 ± 8.9 | ||
| SRF number (year) | 6.8 ± 2.1 | ||
| n (%) | n (%) | p-Value | |
| Educational background | 0.43 | ||
| Socio economic status | 0.48 | ||
| Above average | 10(24.4) | 15(18.8) | |
| Average | 29(70.7) | 56(70.0) | |
| Below average | 2(4.9) | 9(11.3) | |
| Current smoker | 1(2.4) | 6(7.5) | 0.42 |
| Regular sun | 28(68.3) | 39(48.8) | 0.05 |
| Use sunscreen | 32(78) | 65(81.3) | 0.81 |
Ultraviolet light (UV) exposure can lead to photosensitive responses as well as potentially triggering flare events. Therefore details of time spent in the sun with and without sunscreen were considered to be relevant. Regular time out in the sun differed between SLE and control groups, but was of borderline significance (p = 0.05);the reported use of sunscreen did not differ,reflecting general adherence to sun protective measures.
Patterns of usage: participant groups.
Characteristics of exposure and product counts for each participant group were collated from questionnaires. One-way Anova analysis results showed similarity between participant groups in respect to product use patterns of either exposure days or product counts. Total participant product counts ranged from a minimum of 13 products to a maximum of 81 different products used within the aggregated 34 product groups. Mean number of total products for the cohort was 33.1 with a standard deviation of 11.8. No significant differences between the groups were found for either total overall product counts or for the majority of different product groups. Significant differences were found for the number of carpet cleaners (p = 0.03) and dish washing products (p = 0.01) used with control participants reporting high use.
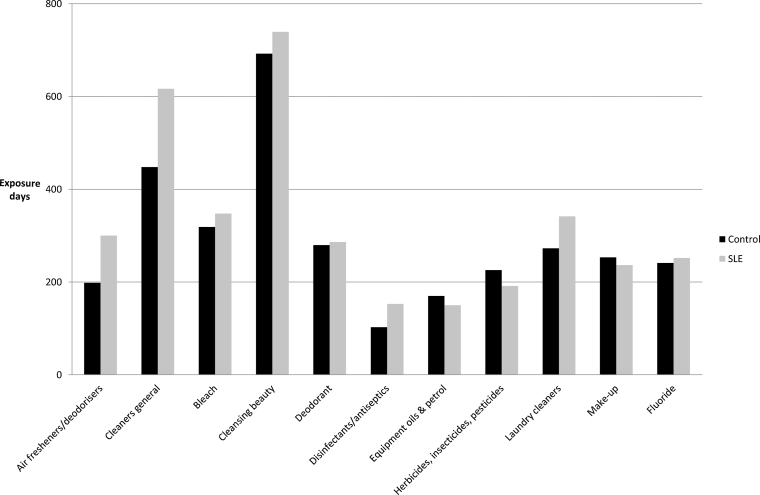
Along with EDC and product counts, similarities were found for the top 10 most frequently used product groups in each participant group. Some differences were evident in the overall order (Fig. 1); however, it was found that personal hygiene and household cleaning products were the most frequently used along with fluoride, due to a fluoridated potable public water supply. All but one participant reported use of tap water on a daily basis.However, a high percentage of both participant groups (control 14:34%, SLE 31:39%) reported limiting public water use for personal washing and laundry purposes only, and drinking and cooking water being drawn from either a filtered or bottled water supply.
Fig. 1.
Top product groups mean product group exposure days: Comparison of SLE participants and control group exposure days.
Patterns of usage: SLE participant flare groups.
SLE participant product group exposure sub analysis based on SRF status was undertaken. Means, standard deviations (SD) and p value differences for the most frequently used product groups and significant product groups are presented in Table 3. In summary, SLE participants reporting SRF events were found to use between 13 and 81 total products with non-flaring SLE participants reporting use of between 23 and 43 different products. Whilst count ranges did appear to indicate that flaring SLE participants used more products, Anova analysis showed that mean product counts did not differ between flare groups(flare mean 32.6:SD 12, no-flare 31.8:SD 6.6). Significant difference between the flare groups was only found for the use of beauty colorants (hair dye: p = 0.02) and powdered salts (p = 0.03); this could potentially be a false signal due to the number of comparisons being made or an indication that SLE patients avoided these exposures. Reported hair dye use either in the home or in a salon was higher in SLE participants that did not report any flares (mean 1.0:SD 0.4). The product group “powdered salts” included commercial products used with the care of swimming pools, cleaning salts of soda ash, bicarbonate of soda, and sugar soap (CaCO3), moisture absorbers, and therapeutic salts used for bathing and relaxation.
Table 3.
Summary of product exposure days and use by SLE flare groups.
| Product Group | SLE No flare (n = 12) |
SLE Flare (n = 68) |
Difference between groups |
|---|---|---|---|
| Mean ± SD | Mean ± SD | p-Value | |
| Adhesives | 8.9 ± 14.8 | 17.0 ± 47.8 | 0.87 |
| Air fresheners/deodorisers | 185 ± 201 | 124 ± 332 | 0.97 |
| General cleaning | 696 ± 320 | 684 ± 269 | 0.14 |
| Bleach | 331 ± 152 | 344 ± 148 | 0.60 |
| Cleaners furniture/homewares | 0.71 ± 0.71 | 1.30 ± 5.32 | 0.73 |
| Cleaners carpet | 1.43 ± 3.17 | 1.56 ± 7.74 | 0.54 |
| Cleansing beauty | 846 ± 786 | 675 ± 329 | 0.24 |
| Deodorant | 235 ± 118 | 293 ± 173 | 0.92 |
| Dishwashing | 64.3 ± 210 | 112 ± 157 | 0.77 |
| Disinfectant/antiseptic | 178 ± 210 | 150 ± 201 | 0.34 |
| Caustic cleaners | 126 ± 197 | 120 ± 163 | 0.46 |
| Dry cleaning | 71 ± 91.3 | 61 ± 107 | 0.37 |
| Equipment oils & petrol | 154 ± 239 | 142 ± 216 | 0.43 |
| Fertilisers | 74.4 ± 120 | 74.2 ± 129 | 0.50 |
| Solid fuels | 0 | 3.86 ± 31.9 | 0.84 |
| Herbicides, insecticides, pesticides | 145 ± 144 | 187 ± 208 | 0.80 |
| Laundry cleaners | 321 ± 287 | 338 ± 267 | 0.57 |
| Make-up | 264 ± 167 | 237 ± 177 | 0.31 |
| Paint | 53.8 ± 112 | 25.2 ± 47.4 | 0.20 |
| Bath oil | 0 | 1.97 ± 12.5 | 0.90 |
| Powdered salt | 27.3 ± 20.9 | 14.5 ± 21.4 | 0.03 |
| Beauty colorants | 10.1 ± 4.23 | 6.95 ± 6.02 | 0.02 |
| Perfumes | 172 ± 136 | 131 ± 147 | 0.17 |
| Fluoride | 208 ± 67.3 | 258 ± 90 | 0.98 |
| All products | 4292 ± 1402 | 4287 ± 1369 | 0.50 |
The most frequently used product groups were general cleaning, cleansing beauty inclusive of shampoos and wash gels, bleach, laundry, deodorant and make-up. It should be noted that the product group of make-up included both foundation make-up and sunscreen products only as other make-up of lipsticks, blushes or eye-shadows were not recorded by any participants.
Product groups displayed within Table 3 represent products found to have the highest exposure for the study year with the addition of product groups which indicate a difference in use between the control and SLE participant groups.
3.1. Associations between product exposure and SRF
Negative binomial regression was used to model SRF days (outcome) against independent EDC of all indoor hour adjusted product group variables with results summarized within Table 4. Covariate factors of age, diagnosis years, education level, SES, BMI, smoking, stress, therapeutic supplements of vitamin D and ITM use in the study year were tested for significance with univariate modeling. Significant effects were found for diagnosis years (p = 0.028) and ITM use (p = 0.009) which were retained in all subsequent multivariate models.
Table 4.
Negative binomial regression for flare days (Fc) and independent product group continuous variables (EDC) and dichotomised exposure or non-exposure with applied cut-points.
| Product groups | Continuous EDC |
Dichotomised no cut-point |
Dichotomised 5% cut-point |
Dichotomised 10% cut-point |
||||
|---|---|---|---|---|---|---|---|---|
| IRR | p < 0.05 | IRR | p < 0.05 | IRR | p < 0.05 | IRR | p < 0.05 | |
| Adhesives | 0.994 | 0.000 | 0.671 | 0.155 | 0.481 | 0.013 | 0.458 | 0.003 |
| Air fresheners/deodorisers | 0.999 | 0.757 | 0.823 | 0.620 | 1.032 | 0.915 | 1.092 | 0.764 |
| Cleaners general | 1.001 | 0.807 | 3.608 | 0.000 | 2.324 | 0.069 | ||
| Bleach | 1.000 | 0.772 | 2.974 | 0.000 | 2.974 | 0.000 | ||
| Cleaners furniture home wares | 1.000 | 0.413 | 2.974 | 0.000 | 0.939 | 0.890 | ||
| Cleansing beauty | 0.999 | 0.024 | 0.736 | 0.635 | 1.062 | 0.888 | ||
| Dishwashing | 1.002 | 0.068 | 1.594 | 0.092 | 1.650 | 0.079 | 1.750 | 0.052 |
| Disinfectants/antiseptics | 0.999 | 0.057 | 1.448 | 0.093 | 0.865 | 0.628 | 0.936 | 0.827 |
| Laundry cleaners | 0.999 | 0.205 | 1.408 | 0.572 | 1.075 | 0.849 | 1.002 | 0.995 |
| Make-up | 0.998 | 0.002 | 0.946 | 0.891 | 0.857 | 0.653 | 1.034 | 0.915 |
| Paint | 0.991 | 0.000 | 0.807 | 0.517 | 0.742 | 0.270 | 0.680 | 0.161 |
| Bath oil | 1.008 | 0.048 | 1.498 | 0.483 | 1.498 | 0.483 | 1.498 | 0.483 |
| Powdered salt | 1.005 | 0.504 | 1.641 | 0.059 | 1.214 | 0.520 | 1.214 | 0.520 |
| Beauty colourant | 0.969 | 0.126 | 0.691 | 0.373 | 0.585 | 0.107 | 0.510 | 0.024 |
| Fluoride | 1.001 | 0.559 | 2.689 | 0.000 | 2.689 | 0.000 | 2.689 | 0.000 |
Significant association consistent with increased SRF day risk represented by relative risk (IRR) was seen for bath oil use only (IRR 1.008, CI1.00–1.02). Paradoxical “protective” effects, (reduced flare days) were found for cleansing beauty (IRR 0.999, CI0.998–0.999), make-up use (IRR 0.998, CI0.997–0.999); adhesives (IRR 0.994, CI 0.991–0.997) and paint (IRR 0.99, CI 0.986–0.995).
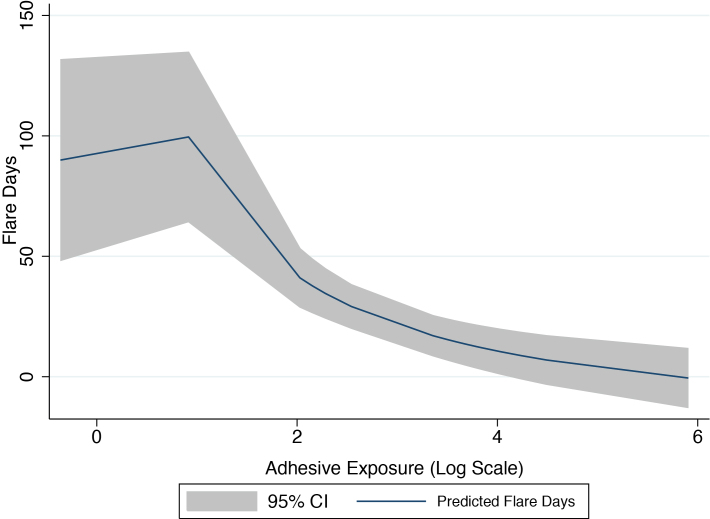
The surprising risk reduction results for some product groups were explored by plotting continuous group exposure data. These plots displayed features suggestive of a non-linear non-monotonic response as exemplified by graphing SRF days against adhesive exposure (Fig. 2). The figure represents an initial increase in SRF days with low levels of adhesive product exposure days and decreasing days with increasing exposure days. Alternatively, these may represent either false positives due to multiple comparisons or avoidance of these products by those with more severe SLE.
Fig. 2.
Non-linear non-monotonic exposure response – adhesive product group.
Based on established assessment methods of semi-quantified and quantified exposure estimates, robust negative binomial regression models were repeated using dichotomised product group data. As expert probability of exposure information was not obtained for product groups, assignment of exposure and non-exposure status was based on: any reported use in the study year; and also 5% and 10% cut-points of the maximum EDC score (max) of each product group (max <5% (10%) non-exposed; max ≥5% (10%) exposed). Results for dichotomised data showed a significant relationship (p < 0.001) with increased SRF risk associated with fluoride exposure. Relative risk (IRR 2.7) was maintained in all models regardless of applied cut-points. Sixty one percent of SLE participants reported not using fluoridated water for drinking and or cooking, limiting their exposure on a daily basis to skin and aerosol routes. This reported practice was accommodated in the data by scoring fluoride exposure EDC at half a year score (183 EDC). Therefore, whilst this finding is of interest, the almost ubiquitous use of fluoridated water daily; along with the varying rates of exposure based on purpose of use, warrants more specific prospective exposure measurement with reference to exposure routes and levels.
Significant results for regression modeling with 5% cut-point product group exposures indicate an increased likelihood of SRF days associated with exposure to general cleaners (IRR 3.61, CI 2.66–4.9), bleach and specialized cleaners of furniture and home wares (IRR 2.97, CI 2.04–4.34), and solid fuels (IRR 2.97, CI 0.85–1.7). Solid fuels were in the form of fire and BBQ starter products infused with flammable liquid; however positive exposure was related to only a few participants and should be interpreted with caution.Retention of significance and a risk reduction of 2.97 were also found for exposure to bleach at the 10% max cut-point.The 10% max cut-point also revealed significant results for beauty colorants (hair dye). Across the study year, hair dye was used by 62 (77.5%) of SLE participants and 50 (62.5%) participants reporting new event exposures either monthly or more frequently. Unexpectedly, a 50% reduction in risk was found with its use (IRR 0.51, CI 0.28–0.91).
4. Discussion
4.1. Exposure patterns of household and personal products
A major goal of this paper was to explore the types of products and patterns of exposure associated with routine household and personal activities over a one year study period. The patterns were examined across a small group of female Australians without a medical history of SLE and also in a larger group with SLE. The study then used exposure data to investigate associations between product group exposure and SRF in the SLE patient group. This was done to better inform our knowledge of flare triggers and products of concern that could potentially trigger flare events in SLE.
The process was resource-intensive but did provide useful information as a baseline snapshot of the Australian female population and their use of household and personal consumer products. In particular, the results largely showed that the use of products did not differ between those with SLE and those without. Personal hygiene and house/clothing cleaning were the most frequently used products with extension to products used for more aesthetic reasons such as deodorizers and air fresheners. These usage patterns are consistent with findings from other United States studies such as the “Study of use of products and exposure-related behaviors” (SUPERB) [20], [21] and the “Household exposure” study of the Silent Spring Institute [22] indicating limited population differences.
Our cohort used large numbers of different products (13–81: mean 33.1). Numerous products were used for similar purposes, particularly in regards to household general cleaners and for insect, pest and garden weed eradication. The majority of these products were used daily for personal care and hygienic cleaning of households. A large number of the products contained antibacterial ingredients and SLE patients nominated more products with marketed claims aimed at reducing overall infection risks and used these products more frequently than similar products without antibacterial claims. Therefore, it is plausible that this practice may have been adopted as a strategy to self-manage one potential trigger for ill health.
Personal hygiene and household products are complex mixtures of chemicals, colorings and perfumes of both “natural” and synthetic origins combined to produce products with targeted properties of use. Formulations and concentrations of commercial product ingredients change frequently,reducing the capacity to gauge exact cause and effects of exposure, with only generalized associations possible. However, some of the product groups commonly found in this study have been previously associated with SLE and other autoimmune health effects. For example, Triclos an, a common antimicrobial addition to paints and household cleaning products, has been found to be associated with presentation of common SLE and thyroid illness symptoms of fatigue, headache and skin irritation [23]. Fragrances, which can contain up to 300 different chemicals [24], have been associated with headaches, contact dermatitis, and mucosal dryness, a shared symptom between SLE and Sjögren’s [25]. Phthalate plasticizing compounds in cosmetics, cleansers, insect repellents, fragrant perfume additives, and food storage containers, are known respiratory irritants causing inflammation of the lungs and asthma exacerbation [26] and been directly linked to SLE development [5]. In addition, there is increasing evidence that inflammatory responses, as demonstrated by allergic contact dermatitis, have been linked to chemical components of parabens, formaldehyde and methylisothiazolinone added to cosmetic and cleaning products as preservatives [27], [28], [29].
Studies exploring health effects of available products are further hindered by the lack of consistencies and regulation in labeling of products and the inadequacies of Material safety data sheets (MSDS) [26]. Many products do not contain completed ingredient lists and may not be required to publicly supply MSDS for products.This study did attempt to retrieve information regarding product chemical ingredients based upon the product name supplied in the HCPML; however, MSDS and complete ingredients and concentration information were not readily obtained.
4.2. SRF days and association with product group use
The development and activity of hair dye use has been discussed in a number of studies [6], [30], [31] with conflicting health impact results. Analysis of difference between the flare and non- flaring SLE subgroups in this study showed that the participants that reported flares did not readily dye their hair and as such no association with health effects was found. It is probable that SLE patients, particularly those experiencing active phases of their SLE symptomology, adhere to precautions in choosing products they use, opting for perceived safer options and limiting exposure to products previously raised as having potential adverse impacts. Flare group differences were also observed with use of cleaning salts, specifically, flaring SLE participants reported higher use of therapeutic bath salts and simple cleaning salts of bicarbonate of soda in preference to products with a more complex chemistry. It is also of interest that an overall observed trend toward the use of “natural”, “greener”, products marketed as being less toxic, (“no nasties”, “paraben free”, “petroleum free”, and “environmentally friendly”) was found within the HCPML brand names supplied by all participants. However, the reason for purchase and use of such products was not explored and warrants further investigation. It of interest that both Dodson et al. [26] and Steinemann et al. [32] did not find significant differences in chemical ingredient analysis of ‘healthier choice products’, with many containing chemicals components of fragrance, preservatives, and parabens despite market labelling to the contrary.
Significant effects were found in univariate analysis in relation to the length of diagnosis time and also for use of ITM during the study year. As reported in Squance et al. [33] participants’ use of ITM was common (82.5%) with multiple ITM’s being prescribed, consistent withselection bias for those with more severe SLE requiring ITM for disease management.
Regression models found mixed protective and increased SRF day risk associations on continuous product EDC data. An increased SRF day risk (IRR 1.008) was indicated in participants that routinely used bath mineral oils. The HCPML named brand of bath oil ingredients were crosschecked and all contained hydrocarbon petrochemicals, fragrances, as well as parabens which have been associated with adverse SLE health impacts. In addition, mineral oils commonly contain pristane which has been linked to renal disease and autoanti body production inducing a lupus-like syndrome in mice [34], [35]. Overall 37 (46.3%) of SLE participants had a renal disorder in the form of persistent proteinuria or cellular cast presentation documented within their health record, and 73 (91.3%) had a positive result for auto antibody presence as part of their symptom spectrum. However, the retrospective study nature did not have the capacity to match individual participant SRF to times of product use, and so correlation to renal dysfunction events or changes in auto antibodies could not be drawn.
As indicated by relative risk values, inverse associations between products and SRF days were found in this study for personal cleansing products (IRR 0.999) and also for participant exposure to make up group (foundation make-up and sunscreen, FMSS) (IRR 0.998). A calculated reduction of SRF days by 0.15% was found for each day of FMSS product use and was not found to be contingent on ITM use; however, no assessment of SLE or SRF severity was made [33]. It is suggested that this protective effect could be directly related to the reduction of UV exposure, a known flare trigger. The study by Vila et al. [36] found significantly lower renal involvement, thrombocytopenia, hospitalisations, and a reduction in ITM use in patients that adopted regular UV protection strategies inclusive of sunscreen use. UV protective chemicals and mineral based pigments are a common manufacturing addition in many household and personal products [37], [38], [39]. Thisresults in almost ubiquitous population exposure as a result of daily activities. This is highlighted by raised urinary levels of the UV protective chemical, benzophenone-3, in a large general population study (NHANES) in the US with 96.8% of samples containing the chemical [40]. Despite this finding, it is important to understand that presence of a chemical in human tissue as well as urine or blood does not necessarily mean that adverse health effects will or have occurred.
In this study, a number of paradoxical protective effects were found for estimates of exposure to adhesives (IRR 0.994, CI 0.991–0.997) as well as paint (IRR 0.99, CI 0.986–0.995). These product groups contain shared chemical components of solvents and epoxy resins, aromatic amines and hydrocarbon structures which have been linked with increased risk in the development of SLE, lupus-like illnesses as well as other autoimmune illnesses [6], [31], [41]. It is thought that the aromatic amine, hydrazine and hydrocarbon structures contained in over 70 pharmaceutical drugs (e.g., clonidine, ibuprofen, penicillamine, tetracyclines) as well as commercial product mixtures of paints, dyes and adhesives can be a catalyst to the development and exacerbation of symptoms of drug induced lupus [6]. In light of the evidence supporting increased risk, our findings were surprising and are difficult to explain.This study’s inventory of products used self-reported measures of frequency of use allowing generalized semi-quantification of exposure. This, along with the retrospective nature without firm measures of timing, duration of use and consistency of exposure, may have contributed to either under or over estimation of exposure. It is also probable that over-estimation of exposure days in relation to out gassing and allocation of exposure for less toxic hobby adhesive and paint products may have caused misclassification bias.It is also possible that dose response associations for adhesives and paint display non-linear non-monotonic characteristics. These types of relationships are common in toxicology [42] , with reports that associations can display a phenomenon where low dose exposure may demonstrate paradoxically beneficial or protective effects, and higher doses displaying increased risk [43], [44]. This phenomenon is often described as “hormesis” and still remains a much-discussed concept.
This study’s paradoxical results and the appearance of non-linear non-monotonic dose-response relationships in plotted product group and SRF out come data prompted the use of multivariate models using dichotomous data. This method is applied in clinical research and exposure risk assessments when: exposures are small; incomplete concentrations of chemical components and safe exposure limits are available; or when differing environments are to be evaluated [15], [45], [46]. Whilst dichotomising continuous data is considered suboptimal with the tendency to lose statistical power of association and an increased risk of false positive results [47], it can offer insight into simple risk interpretation of data eliminating the need for linearity assumptions [48]. Dichotomised data for these variables did not support a protective effect indicating that these results represent type 1 error, i.e., false positives.
On the other hand, dichotomised outcomes did yield considerable increased SRF relative risk values for some product groups: general cleaners (IRR = 3.6), bleach (IRR = 2.9), specialist furniture and home appliance cleaners (IRR=2.9). All of these product groups are complex mixtures with solvent surfactants, fragrances, preservatives including parabens and formaldehyde, phthalates, and antibacterial additives which have reported adverse health impacts relevant to SLE patients.
The dichotomised model analysis also identified fluoride, present in fluoridated potable public water supplies, as a potential product which may increase the risk of SRF. However, interpretation of this result should be treated with caution due to almost universal exposure in participants and applied cut-point levels being too narrow.Thirty nine percent of SLE participants did report using fluoridated water for washing purposes only; however, despite choosing to limit purpose of use and therefore overall exposure, daily exposure would have still occurred in all but one participant.
Fluoride is a common additive in many oral hygiene products for dental caries prevention [49] which is particularly important in SLE patients with dryness symptoms or co-existing Sjögren’s syndrome. Reported adverse health effects of high levels of fluoride exposure have included skeletal fluorosis (brittle bones), joint pain and limitations to joint movements [4], [50]. Drinking fluoridated water wasalso found to be nephrotoxic in animals with chronic kidney disease and impaired renal excretion [51] , and reported to exacerbate SLE by disrupting the synthesis of collagen leading to breakdown in the skin, muscle, ligaments, bone, lungs and kidneys [52]. In view of these reported health effects and the high representation of joint/muscular pains and renal involvement in SLE patients [14], [53], fluoride exposure remains of investigative interest.
4.3. Study strengths and weaknesses
Whilst a case/control approach is suitable for studying retrospective multiple exposures particularly in rare illness [54], the study had some limitations. The basis of data collection was from the participants lived experience and provides retrospective self-reported data of a small relatively homogeneous female Australian population. This would be subject to recall bias. In addition, SRF was not able to be confirmed by review of clinical notes as the study was undertaken outside of clinical appointments with participants being recruited from multiple centers. Therefore SRF was not crosschecked against standard disease activity measures. To reduce bias, a single researcher used a standardized method incorporating a strict structured interview technique [14] with a clearly defined description of a flare event [13]. This technique may have resulted in an overestimation of SRF however it would be expected that any bias would be toward the null. The results should be viewed as ofa pilot nature with the need for: (i) a comprehensive prospective study protocol documenting specific product use over time; and (ii) assessment of disease activity with validated tools.
5. Conclusion
There is growing evidence that a wide array of these chemical components can produce SLE-like diseases;however, the interaction of environmental exposures and adverse health consequences for SLE, particularly their association with exacerbation of pre-existing SLE symptoms ‘flares’, is poorly understood.
We found that patterns of use did not significantly differ between case/control groups, with the exception that SLE participants had a tendency to opt for products that were marketed as ‘less toxic’ than others. Regression analyses indicated that there may be a protective element to use of personal cleaning and make-up products and an increased risk of flares with exposure to bath oils. The results also showed paradoxical effects of reduced risk associated with exposure to adhesives and paint which could not be readily explained but could be due to inflated type 1 error due to multiple comparisons. There was also some support for an increased risk of flares with general cleaners, bleach, and specialist furniture and home appliance cleaners but these remain to be independently verified. The reported environmental associations found in this study offer the possibility of life-style interventions to reduce autoimmune symptom exacerbation.
Financial interests declaration
The authors declare that they have no financial interests, assets or competing interests that might be perceived to influence the results and/or discussion reported in this article.
Acknowledgements
This study forms part of the Environmental Determinants of Lupus Flare (EDOLF) PhD study, University of Newcastle. The study was funded via resources provided by the Autoimmune Resource Research Center (Not-for-profit charity www.autoimmune.org.au) and the Val Badham Research Scholarship for Immunology, University of Newcastle Foundation.
References
- 1.E. Ballestar, Epigenetic Contributions in Autoimmune Disease. Boston, MA: Landes Bioscience and Springer Science + Business Media, LLC; 2011. Available from: <http://dx.doi.org/10.1007/978-1-4419-8216-2>.
- 2.Miller F.W. Environmental agents and autoimmune diseases. Adv. Exp. Med. Biol. 2011;711:61–81. doi: 10.1007/978-1-4419-8216-2_6. [DOI] [PubMed] [Google Scholar]
- 3.International Programme on Chemical Safety (IPCS) WHOWDoPotHE, Inter-Organization Programme for the Sound Management of Chemicals. Principles and Methods for Assessing Autoimmunity Associated with Exposure to Chemicals (Environmental Health Criteria 236 Series). GenevaHerndon: World Health OrganizationStylus Publishing; 2006. Available from: <http://whqlibdoc.who.int/publications/2006/9241572361_eng.pdf >.
- 4.Commonwealth of Australia SaerB, Department of Health and Aging. State of knowledge report: air toxics and indoor air quality in Australia/Environment Australia. Australia. Environment A, Australia. Environment Australia. Air Toxics S, editors. Canberra: Environment Australia. 2001.
- 5.Zandman-Goddard G., Solomon M., Rosman Z., Peeva E., Shoenfeld Y. Environment and lupus-related diseases. Lupus [Review] 2012;21(March (3)):241–250. doi: 10.1177/0961203311426568. [DOI] [PubMed] [Google Scholar]
- 6.Hess E.V. Environmental chemicals and autoimmune disease: cause and effect. Toxicol. [Review] 2002;181–182(December 27):65–70. doi: 10.1016/s0300-483x(02)00256-1. [DOI] [PubMed] [Google Scholar]
- 7.Grootscholten C., Ligtenberg G., Derksen R.H., Schreurs K.M., de Glas-Vos J.W., Hagen E.C. Health-related quality of life in patients with systemic lupus erythematosus: development and validation of a lupus specific symptom checklist. Qual. Life Res. [Research Support, Non-U.S. Gov’t Validation Studies] 2003;12(September (6)):635–644. doi: 10.1023/a:1025176407776. [DOI] [PubMed] [Google Scholar]
- 8.Israel B.A., Eng Eugenia Schulz Amy J., Parker Edith A. 1st ed. John Wiley & Sons; San Francisco, CA: 2005. Methods for Community-based Participatory Research for Health. [Google Scholar]
- 9.Association W.M. World Medical Association Declaration of Helsinki. www.wma.net/en/30publications/10policies/b3/17c.pdf; 2008 [cited 2009 25 November]; [Declaration of Helsinki 2008 revision].
- 10.Rheumatology ACo. 1997 Update of the 1982 American College of Rheumatology Revised Criteria for Classification of Systemic Lupus Erythematosus. [webpage]: http://www.rheumatology.org/practice/clinical/classification/sle/1997_update_of_the_1982_American_College_of_Rheumatology_Revised_Criteria_for_Classification_of_sle.pdf; 2005 [cited 2005 26 Oct 2005]; [Classification criteria for diagnosis of Systemic Lupus Erythematosus].
- 11.Squance M.L., Guest M., Reeves G., Attia J., Bridgman H. Exploring lifetime occupational exposure and SLE flare: a patient- focussed pilot study. Lupus Sci. Med. 2014;1(May 1):1–9. doi: 10.1136/lupus-2014-000023. e000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centre for Epidemiology and Research PHD, NSW Department of Health. New South Wales Population Health Survey 2010. In: Centre for Epidemiology and Research PHD, NSW Department of Health, editor: NSW Government, Australia. 2011.
- 13.Poser C.M., Paty D.W., Scheinberg L., McDonald W.I., Davis F.A., Ebers G.C. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann. Neurol. [Research Support, Non-U.S. Gov’t] 1983;13(March 3):227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 14.Squance M.L., Reeves G.E.M., Bridgman H. The lived experience of lupus flares: features, triggers, and management in an Australian female cohort. Int. J. Chronic Dis. 2014;2014:12. doi: 10.1155/2014/816729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.‘T Mannetje A.M., Mclean D.J., Eng A.J., Kromhout H., Kauppinen T., Fevotte J. Developing a general population job-exposure matrix in the absence of sufficient exposure monitoring data. Ann. Occup. Hyg. 2011;55(October 1 (8)):879–885. doi: 10.1093/annhyg/mer045. [DOI] [PubMed] [Google Scholar]
- 16.Kauppinen T., Heikkila P., Plato N., Woldbaek T., Lenvik K., Hansen J. Construction of job-exposure matrices for the Nordic Occupational Cancer Study (NOCCA) Acta Oncologica. 2009;48(5):791–800. doi: 10.1080/02841860902718747. [DOI] [PubMed] [Google Scholar]
- 17.Kauppinen T., Toikkanen J., Pukkala E. From cross-tabulations to multipurpose exposure information systems: a new job-exposure matrix. Am. J. Ind. Med. 1998;33(April (4)):409–417. doi: 10.1002/(sici)1097-0274(199804)33:4<409::aid-ajim12>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Teschke K., Olshan A.F., Daniels J.L., De Roos A.J., Parks C.G., Schulz M. Occupational exposure assessment in case-control studies: opportunities for improvement. Occup. Environ. Med. [Review] 2002;59(September (9)):575–593. doi: 10.1136/oem.59.9.575. discussion 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Tongeren M., Kincl L., Richardson L., Benke G., Figuerola J., Kauppinen T. Assessing occupational exposure to chemicals in an international epidemiological study of brain tumours. Ann. Occup. Hyg. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] 2013;57(June (5)):610–626. doi: 10.1093/annhyg/mes100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett D.H., Wu X.M., Teague C.H., Lee K., Cassady D.L., Ritz B. Passive sampling methods to determine household and personal care product use. J. Expo. Sci. Environ. Epidemiol. [Research Support, U.S.;1; Gov’t, Non- P.H.S.] 2012;22(March–April (2)):148–160. doi: 10.1038/jes.2011.40. [DOI] [PubMed] [Google Scholar]
- 21.I. Hertz-Picciotto, D. Cassady, K. Lee, D.H. Bennett, B. Ritz, R. Vogt, Study of Use of Products and Exposure-Related Behaviors (SUPERB): study design, methods, and demographic characteristics of cohorts. Environmental health: a global access science source. [Research Support, U.S. Gov’t, Non-P.H.S.]. 9 (2010) 54. [DOI] [PMC free article] [PubMed]
- 22.Dunagan S.C., Dodson R.E., Rudel R.A., Brody J.G. Toxics use reduction in the home: lessons learned from household exposure studies. J. Clean. Prod. 2011;19(March 1 (5)):438–444. doi: 10.1016/j.jclepro.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul K.B., Hedge J.M., DeVito M.J., Crofton K.M. Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in young long-evans rats. Toxicol. Sci. 2010;113(February 1 (2)):367–379. doi: 10.1093/toxsci/kfp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bickers D.R., Calow P., Greim H.A., Hanifin J.M., Rogers A.E., Saurat J.H. The safety assessment of fragrance materials. Regul. Toxicol. Pharmacol. [Review] 2003;37(April (2)):218–273. doi: 10.1016/s0273-2300(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 25.Nossent J.C., Swaak A.J. Systemic lupus erythematosus VII: frequency and impact of secondary Sjogren’s syndrome. Lupus. 1998;7(4):231–234. doi: 10.1191/096120398678920046. [DOI] [PubMed] [Google Scholar]
- 26.Dodson R.E., Nishioka M., Standley L.J., Perovich L.J., Brody J.G., Rudel R.A. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ. Health Perspect. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] 2012;120(July (7)):935–943. doi: 10.1289/ehp.1104052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundov M.D., Moesby L., Zachariae C., Johansen J.D. Contamination versus preservation of cosmetics: a review on legislation, usage, infections, and contact allergy. Contact Dermatitis. 2009;60(February (2)):70–78. doi: 10.1111/j.1600-0536.2008.01501.x. [DOI] [PubMed] [Google Scholar]
- 28.McFadden J.P., White I.R., Basketter D., Puangpet P., Kimber I. The cosmetic allergy conundrum: inference of an immunoregulatory response to cosmetic allergens. Contact Dermatitis. [Review] 2013;69(September (3)):129–137. doi: 10.1111/cod.12100. [DOI] [PubMed] [Google Scholar]
- 29.Travassos A.R., Claes L., Boey L., Drieghe J., Goossens A. Non-fragrance allergens in specific cosmetic products. Contact Dermatitis. 2011;65(November (5)):276–285. doi: 10.1111/j.1600-0536.2011.01968.x. [DOI] [PubMed] [Google Scholar]
- 30.Cooper G.S., Dooley M.A., Treadwell E.L., St Clair E.W., Gilkeson G.S. Smoking and use of hair treatments in relation to risk of developing systemic lupus erythematosus. J. Rheumatol. [Research Support, U.S. Gov’t, P.H.S.] 2001;28(December (12)):2653–2656. [PubMed] [Google Scholar]
- 31.Dooley M.A., Hogan S.L. Environmental epidemiology and risk factors for autoimmune disease. Curr Opin. Rheumatol. [Review] 2003;15(March (2)):99–103. doi: 10.1097/00002281-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Steinemann A.C., MacGregor I.C., Gordon S.M., Gallagher L.G., Davis A.L., Ribeiro D.S. Fragranced consumer products: chemicals emitted, ingredients unlisted. Environ. Impact Assess. Rev. 2011;31(3):328–333. [Google Scholar]
- 33.Squance M.L., Reeves G.E.M., Attia J. Patient reported frequency of lupus flare: associations with foundation makeup and sunscreen use journal of cosmetics. Dermatol. Sci. Appl. 2014;04(05):344–354. [Google Scholar]
- 34.Lee P.Y., Kumagai Y., Xu Y., Li Y., Barker T., Liu C. IL-1alpha modulates neutrophil recruitment in chronic inflammation induced by hydrocarbon oil. J. Immunol. [Research Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov’t] 2011;186(February 1 (3)):1747–1754. doi: 10.4049/jimmunol.1001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satoh M., Hamilton K.J., Ajmani A.K., Dong X., Wang J., Kanwar Y.S. Autoantibodies to ribosomal P antigens with immune complex glomerulonephritis in SJL mice treated with pristane. J. Immunol. [Comparative Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] 1996;157(October 1 (7)):3200–3206. [PubMed] [Google Scholar]
- 36.Vila L.M., Mayor A.M., Valentin A.H., Rodriguez S.I., Reyes M.L., Acosta E. Association of sunlight exposure and photoprotection measures with clinical outcome in systemic lupus erythematosus. P. R. Health Sci. J. [Comparative Study] 1999;18(June (2)):89–94. [PubMed] [Google Scholar]
- 37.Household Products Database [database on the Internet]. <http://householdproducts.nlm.nih.gov/index.htm> 2013 [cited 08 February 2013].
- 38.Nohynek G.J., Lademann J., Ribaud C., Roberts M.S. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit. Rev. Toxicol. [Research Support, Non-U.S. Gov’t Review] 2007;37(March (3)):251–277. doi: 10.1080/10408440601177780. [DOI] [PubMed] [Google Scholar]
- 39.Obermoser G., Zelger B. Triple need for photoprotection in lupus erythematosus. Lupus. 2008;17(June (6)):525–527. doi: 10.1177/0961203308089440. [DOI] [PubMed] [Google Scholar]
- 40.Calafat A.M., Wong L.Y., Ye X., Reidy J.A., Needham L.L. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: national health and nutrition examination survey 2003–2004. Environ. Health Perspect. 2008;116(July (7)):893–897. doi: 10.1289/ehp.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gourley M., Miller F.W. Mechanisms of disease: Environmental factors in the pathogenesis of rheumatic disease. Nat. Clin. Prac. Rheumatol. [Research Support, N.I.H., Intramural Review] 2007;3(March (3)):172–180. doi: 10.1038/ncprheum0435. [DOI] [PubMed] [Google Scholar]
- 42.Conolly R.B., Lutz W.K. Nonmonotonic dose-response relationships: mechanistic basis, kinetic modeling, and implications for risk assessment. Toxicol. Sci. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] 2004;77(January (1)):151–157. doi: 10.1093/toxsci/kfh007. [DOI] [PubMed] [Google Scholar]
- 43.Tuomisto J. Does mechanistic understanding help in risk assessment?the example of dioxins. Toxicol Appl. Pharmacol. [Research Support, Non-U.S. Gov’t Review] 2005;207(September 1 (2 Suppl.)):2–10. doi: 10.1016/j.taap.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 44.Tuomisto J.T., Pekkanen J. Assessing environmental health risks or net health benefits? Scand. J. Public Health [Research Support, Non-U.S. Gov’t Review] 2005;33(3):162–163. doi: 10.1080/14034940510006085. [DOI] [PubMed] [Google Scholar]
- 45.Commonwealth of Australia SaerB, Department of Health and Aging. Environmental health risk assessment: Guidelines for assessing human health risks from environmental hazards. Canberra ACT: Commonwealth of Australia, Dept. of Health and Ageing. 2012.
- 46.Kauppinen T., Uuksulainen S., Saalo A., Makinen I. Trends of occupational exposure to chemical agents in Finland in 1950–2020. Ann .Occup. Hyg. [Research Support, Non-U.S. Gov’t] 2013;57(June (5)):593–609. doi: 10.1093/annhyg/mes090. [DOI] [PubMed] [Google Scholar]
- 47.Altman D.G., Royston P. The cost of dichotomising continuous variables. BMJ [Review] 2006;332(May (6) (7549)):1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baneshi M.R., Talei A.R. Dichotomisation of continuous data: review of methods, advantages and disadvantages. Iranian J. Cancer Prevent. 2011;4(1):26–32. [Google Scholar]
- 49.Lehmann R., Wapniarz M., Hofmann B., Pieper B., Haubitz I., Allolio B. Drinking water fluoridation: bone mineral density and hip fracture incidence. Bone [Research Support, Non-U.S. Gov’t] 1998;22(March (3)):273–278. doi: 10.1016/s8756-3282(97)00273-1. [DOI] [PubMed] [Google Scholar]
- 50.Department of Health and Aged Care. Environmental Health Section., Unit. QDoPWBDBER. Indoor air quality: a report on health impacts and management options. Canberra: Environmental Health Section, Dept. of Health and Aged Care. 2000.
- 51.Martin-Pardillos A., Sosa C., Millan A., Sorribas V. Effect of water fluoridation on the development of medial vascular calcification in uremic rats. Toxicology [Research Support, Non-U.S. Gov’t] 2014;318(April (6)):40–50. doi: 10.1016/j.tox.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Thomas P. Gill & Macmillan; Dublin: 2001. Cleaning Yourself to Death: How Safe is Your Home? [Google Scholar]
- 53.Dooley M., Houssiau F., Aranow C., D'Cruz D., Askanase A., Roth D. Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus. 2013;22(January 1 (1)):63–72. doi: 10.1177/0961203312465781. [DOI] [PubMed] [Google Scholar]
- 54.Gregg M.B. 2nd ed. Oxford University Press; Oxford; New York: 2002. Field Epidemiology. [Google Scholar]