Abstract
Furin is a proprotein convertase enzyme. In the liver, it cleaves prohepcidin to form active hepcidin-25, which regulates systemic iron homeostasis. Hepcidin deficiency is a component of several iron overload disorders, including β-thalassemia. Several studies have identified factors that repress hepcidin gene transcription in iron overload. However, the effect of iron overload on furin, a post-translational regulator of hepcidin, has never been evaluated. The present study aimed to investigate the changes in furin and related factors in parenteral iron-overloaded mice, including those with β-thalassemia. Wild-type (WT) and β-thalassemia intermedia (th3/+) C57BL/6 mice were intraperitoneally injected with 9 doses of iron dextran (1 g iron/kg body weight) over 2 weeks. In the iron overload condition, our data demonstrated a significant Furin mRNA reduction in WT and th3/+ mice. In addition, the liver furin protein level in iron-overloaded WT mice was significantly reduced by 70% compared to control WT mice. However, the liver furin protein in iron-overloaded th3/+ mice did not show a significant reduction compared to control th3/+ mice. The hepcidin gene (hepcidin antimicrobial peptide gene, Hamp1) expression was increased in iron-overloaded WT and th3/+ mice. Surprisingly, the liver hepcidin protein level and total serum hepcidin were not increased in both WT and th3/+ mice with iron overload, regardless of the increase in Hamp1 mRNA. In conclusion, we demonstrate furin downregulation in conjunction with Hamp1 mRNA-unrelated pattern of hepcidin protein expression in iron-overloaded mice, particularly the WT mice, suggesting that, not only the amount of hepcidin but also the furin-mediated physiological activity may be decreased in severe iron overload condition.
Keywords: Furin, Hepcidin, Iron overload, β-Thalassemic mice
1. Introduction
Furin belongs to the family of enzymes called “proprotein convertases” which localize as transmembrane proteins in the trans-Golgi network, endosomes, and at the cell surface along the protein-sorting pathway. Furin converts various proproteins to mature proteins [1], [2]. It plays a role in post-translational modification of hepcidin, a liver peptide hormone that regulates systemic iron homeostasis. Increased hepcidin synthesis limits intestinal iron absorption, whereas hepcidin deficiency promotes iron absorption. Hepcidin is initially synthesized as a pro-hormone. Therefore, it must undergo a maturation process via furin cleavage to become an active hormone containing 25 amino acids [3], [4], [5].
Iron overload is an important clinical manifestation in β-thalassemia that is caused by blood transfusion and increased iron absorption secondary to ineffective erythropoiesis. The liver iron content (LIC) greater than 7.0–15.0 mg/g liver dry weight is considered to represent an increased risk of fatal complications in β-thalassemia patients [6], [7]. Despite iron overload, the levels of serum and urine hepcidin are extremely low, which contribute to the progressive iron absorption in these patients [8], [9].
Whereas most studies of iron overload focused on the changes in transcriptional regulation of hepcidin, the changes in factors influencing post-translation processes, especially furin, are still unknown. In the liver, furin is regulated by transferrin receptor 2 (TfR2), an iron-sensing molecule located on the hepatocyte cell surface. TfR2 (with HFE) activates furin expression through mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/Erk) signaling [10]. Moreover, a previous study has shown a significant decrease in TfR2 in parenteral iron-loaded C57BL/6 mice [11], which led us to ask whether this iron overload could also affect furin expression.
Several mouse models have been established to study the pathophysiology and to identify novel therapeutics in β-thalassemia [12]. Furthermore, some mouse models of iron overload (both β-thalassemia and others) mimic the iron burden patients after administration of exogenous iron to the mice [13], [14]. For example, iron dextran was injected in th1/th1 mouse model of β-thalassemia intermedia to generate a severe form of iron overload, and changes of various parameters including iron accumulation in target organs (i.e. serum, bone marrow, liver, spleen, and heart), red blood cell production in medullary site (bone marrow) and extramedullary sites (liver and spleen), and liver hepcidin gene expression were subsequently examined [15].
We investigated the effect of parenteral iron overload on the changes in furin and related parameters in both WT and th3/+ mice in a C57BL/6 background. Our model represents severe iron-overloaded condition without (WT) and with (th3/+) the underlying ineffective erythropoiesis [16]. We hypothesized that iron overload may affect liver furin expression, and subsequently disrupt the production of physiologically active hepcidin.
2. Materials and methods
2.1. Animals
Seven week old WT and th3/+ mice (both male and female) on a C57BL/6 background were obtained from the Thalassemia Research Center, Institute of Molecular Biosciences, Mahidol University, Thailand. The phenotype of th3/+ mice is comparable to β-thalassemia intermedia in humans, including mild anemia (hemoglobin level ∼8 g/dl), low red blood cell counts with the increase in reticulocytes, and enlarged spleen size [17]. The mice were acclimatized under conventional sterile conditions at 20 ± 2 °C with regular chow and water in a 12 h light/dark cycle and 60 ± 10% humidity for 1 week prior to the experiments. All experimental protocols were approved by the Animal Ethics Committee of the Faculty of Science, Mahidol University (Protocol No. 29 and MUSC56-004-266).
2.2. Experimental design
WT and th3/+ mice were divided into 2 groups (5 mice each, containing both male and female): one control (0.9% NaCl treatment) and one iron overload group. To induce iron overload, the mice were intraperitoneally (i.p.) injected with iron dextran (Sigma, St. Louis, MO) at a dose of 1 g iron/kg body weight for a total of 9 doses over 15 days as shown in Fig. 1. Ten days after the completion of the animal treatments, the mice were weighed and sacrificed. Blood samples were collected via cardiac puncture, and the sera were separated. Livers and spleens were subsequently harvested and weighed. Each liver sample was dissected into 3 parts: (1) stored at −80 °C for the measurement of iron content and western blot analysis, (2) immersed in RNAlater® (Sigma) and stored at −20 °C for real-time RT-PCR, and (3) fixed in 10% neutral buffered formalin for immunofluorescence.
Fig. 1.
Schedule of animal treatment.
2.3. Determination of liver iron content
The non-heme iron content was analyzed by the ferrozine method [18], [19]. In brief, the liver sample was weighed and homogenized in 1 ml of 0.1 M PBS buffer, pH 7.4. Iron was extracted by adding an equal volume of liver tissue homogenate (250 μl) and protein precipitants (125 μl of 1 N HCl and 125 μl of 10% trichloroacetic acid, Sigma). After mixing, the solution was incubated in a water bath at 95 °C for 45 min. Next, the solution was cooled at room temperature for 5 min, followed by centrifugation at 3500 rpm for 15 min. The supernatant was collected, and the precipitate was resuspended with protein precipitants to extract the remaining iron. An additional supernatant was then collected. To analyze the iron content, the supernatant was diluted with 1 N HCl to a total volume of 500 μl and further mixed with 1 ml of chromogen solution (0.5 mM ferrozine, 50 mM sodium ascorbate, and 1.05 M sodium acetate, Sigma). After 20 min incubation at room temperature, the absorbance was measured at 562 nm using the Cintra 10e UV visible spectrophotometer (GBC; Melbourne, VIC, Australia).
2.4. Measurements of serum erythropoietin and hepcidin
Serum erythropoietin (sEPO) and hepcidin were measured using the EPO Mouse ELISA kit (ab119593, Abcam, Cambridge, MA) and Mouse Hepcidin Antimicrobial Peptide (HAMP) ELISA kit (E03H0051, Blue Gene, Shanghai, China), respectively, according to the manufacturer's instructions.
2.5. Quantification of Hamp1, Tfr2, and Furin mRNA expression
Total liver RNA was isolated using the Ultraclean™ Tissue & Cells RNA isolation kit (MO BIO Laboratories, Carlsbad, CA) according to the manufacturer's instructions. The mRNA expression of Hamp1, Tfr2, and Furin was examined by real-time RT-PCR using the SYBR® Green One-Step qRT-PCR kit (11736-051, Invitrogen, Carlsbad, CA). The oligonucleotide primers (Invitrogen) used were 5′-AGAGCTGCAGCCTTTGCAC-3′ (forward) and 5′-ACACTGGGAATTGTTACAGCATT-3′ (reverse) for Hamp1 [20]; 5′-CAGCCTCGGTACACACAGAT-3′ (forward) and 5′-AGCTACACCTACGCCACAGA-3′ (reverse) for Furin [21]; 5′-CCTCTATGAACAAGTGGCACTCA-3′ (forward) and 5′-CCCGATCATCCTCCATGAAG-3′ (reverse) for Tfr2; 5′-TCCTGGCCTCACTGTCCAC-3′ (forward) and 5′-GTCCGCCTAGAAGCACTTGC-3′(reverse) for β-actin [22]. In a single 25 μl PCR reaction, 1 μl of total RNA was added. The target sequences were amplified in duplicate by an ABI PRISM 7500 thermal cycler (Applied Biosystems; Foster City, CA) using the following program: 50 °C for 3 min; 95 °C for 5 min; 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 40 °C for 1 min; and a final melting curve analysis. Relative quantification was performed using the Pfaffl method [23], and β-actin was chosen as the normalizer.
2.6. Liver furin protein detection and quantification
Liver tissue samples were homogenized in RIPA buffer (with protease inhibitors) and centrifuged for 20 min at 10,000 × g at 4 °C. The supernatant was then collected. Next, the concentration of protein was measured by Lowry method [24]. Western blot analyses were performed as previously described [25]. Briefly, proteins (20 μg/lane) were loaded into 10% SDS-polyacrylamide gel. After electrophoresis and electrotransfer, the blots were blocked by 5% skim milk in phosphate-buffered saline (PBS) pH 7.4 with 0.1% (v/v) Tween 20 (PBST) for 1 h and washed 3 times with PBST. Membranes were then incubated at 4 °C overnight with rabbit anti-furin polyclonal antibody (1:200) (sc-20801, Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit anti-β-actin polyclonal antibody (1:3000) (4967S, Cell Signaling Technology). Membranes were washed 3 times with PBST followed by a 1 h incubation at room temperature with enhanced chemiluminescence (ECL), donkey anti-rabbit IgG, HRP-linked secondary antibody (dilution 1:10,000) (NA934, GE Healthcare). After washing 3 times with PBST, the protein bands were visualized using Clarity™ western ECL substrate (170-5060, Bio-Rad, Hercules, CA) according to the protocol. β-actin was used as a loading control.
2.7. Liver hepcidin protein detection and quantification
Hepcidin protein in liver tissue was detected by immunofluorescence. The slides of formalin-fixed, paraffin-embedded liver tissue sections (5 μm thick) were heated at 80 °C for 30 min and deparaffinized by a 3-step immersion into Unyhol Plus® solution (Bio-Optica, Milano, Italy) (once for 10 min and twice for 5 min). Next, the tissues were rehydrated in a graded ethanol series (15 min in 100%, 15 min in 95%, and 5 min in 80%), followed by immersion in 0.25% NH3 in 70% ethanol for 1 h and 50% ethanol for 10 min [26]. Then, the slides were rinsed with distilled water and washed twice with 1× PBS (5 min each). For antigen retrieval, the slides were immersed in Tris–EDTA buffer (10 mM Tris base, 1 mM EDTA, and 0.05% Tween 20, pH 9.0), preheated in a water bath at 95 °C, and incubated for 40 min. After cooling at room temperature for 20 min, the tissue slides were treated with 0.1% Triton-X for 10 min and washed 3 times with 1× PBS (5 min each). The tissue sections were blocked with 5% normal goat serum in 3% BSA-containing 1× PBS for 1 h at room temperature. After removing the blocking agent, the samples were incubated with 20 μg/ml rabbit polyclonal anti-mouse hepcidin-25 antibody (ab81010, Abcam) (diluted in 1× PBS containing 3% BSA) at 4 °C overnight [27]. Later, the slides were washed 3 times with 1× PBS (5 min each) before incubating the tissue sections with Chromeo™ 488-labeled goat polyclonal anti-rabbit IgG antibody (ab60314, Abcam) (1:1000, diluted with 2% normal goat serum in 3% BSA-containing 1× PBS) for 1 h at room temperature in the dark. The slides were then washed 3 times with 1× PBS (5 min each), immersed into 0.1% Sudan black B in 70% ethanol for 30 min, rinsed and washed again with 1× PBS for 10 min [26]. Finally, Prolong® Gold antifade mounting medium (Invitrogen) was added, and a coverslip was placed on the slides. The fluorescent images were captured with a Fluoview FV10i-DOC laser scanning confocal microscope (Olympus; Shinjuku, Tokyo, Japan) with a 60 × 1.35 NA (numerical aperture) objective lens. Fluorescence intensities were analyzed by ImageJ Ver.1.47i software (National Institutes of Health, USA). The summation of fluorescence from 10 non-overlapping fields was defined as the total fluorescence intensity of the sample. Using the mean total fluorescence intensity in the WT control as a calibrator, the relative quantification was calculated as the total fluorescence intensity of the sample divided by the fluorescence intensity of the calibrator.
2.8. Statistical analysis
The data are presented as the mean ± SEM. The mean differences were compared using a Mann–Whitney U test. The correlation of parameters was examined by Spearman's correlation analysis. A P-value <0.05 was considered statistically significant.
3. Results
3.1. Characteristics of the animals
At the end of the experiment, no significant difference in body weight was measured among the groups of mice (Table 1). With the same liver mass as the WT mice, the th3/+ mice had a markedly (approximately 6-fold) higher spleen weight. Moreover, the th3/+ mice demonstrated significantly (approximately 7-fold) higher LICs compared to the WT mice.
Table 1.
Characteristics of the animals.
| Parameter | WT |
th3/+ |
||
|---|---|---|---|---|
| Control | Iron overload | Control | Iron overload | |
| Body weight (g) | 23.8 ± 0.9 | 21.6 ± 1.1 | 23.1 ± 0.8 | 21.9 ± 1.1 |
| Liver weight (g) | 1.0 ± 0.3a | 2.7 ± 0.2a | 1.1 ± 0.1b | 2.6 ± 0.2b |
| Spleen weight (mg) | 73.8 ± 7.1c,d | 145.3 ± 29.3c | 441.8 ± 37.7d | 528.5 ± 28.3 |
| LIC (mg/g) | 0.03 ± 0.00e,f | 14.5 ± 1.2f | 0.21 ± 0.03e,g | 14.6 ± 1.9g |
| Serum EPO (pg/ml) | <46.9* | 292.7 ± 77.9 | 231.0 ± 54.7 | 278.9 ± 48.2 |
| Total serum hepcidin (ng/ml) | 2.9 ± 1.1 | 1.1 ± 0.2 | 3.9 ± 0.9 | 1.5 ± 0.2 |
The data are the mean ± SEM (n = 4–5 per group). Comparisons for significant differences are indicated by the same letter; a, e, f, gP < 0.01, b,c,dP < 0.05.
Under the range of detection.
After iron loading, the LIC significantly increased to 15 mg/g liver wet weight in both WT and th3/+ mice. In addition, the liver weight increased by over 2-fold from the baseline in both WT and th3/+ mice. Furthermore, a similar increase in spleen weight was observed in iron-overloaded WT and th3/+ mice (70 and 85 mg increase, respectively).
The baseline sEPO in WT mice was under the range of detection. However, a concentration of 292.7 ± 77.9 pg/ml was detected in iron-overloaded mice (Table 1). In contrast, the baseline sEPO level was observed at 231.0 ± 54.7 pg/ml in th3/+ mice, and it was not changed in iron overload conditions (278.9 ± 48.2 pg/ml).
3.2. Furin expression in liver
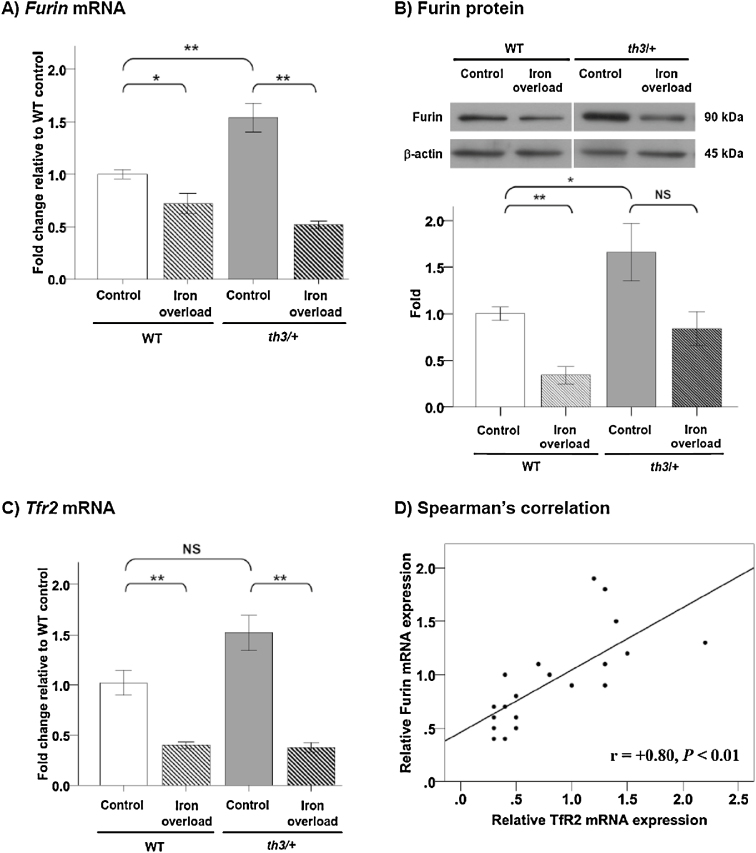
In th3/+ mice, Furin mRNA and furin protein expressions were increased by 50 and 70% compared to WT mice, respectively (Fig. 2A and 2B, respectively). Remarkably, Furin mRNA was significantly decreased by 30% in iron-overloaded WT mice and 67% in iron-overloaded th3/+ mice. Whereas furin protein was significantly decreased by 70% in iron-overloaded WT mice, it did not decrease significantly with iron overload in th3/+ mice (Fig. 2B).
Fig. 2.
Expression of furin and related factors in the liver. The data are the mean ± SEM (n = 5 per group). (A) Relative Furin mRNA expression by real-time RT-PCR. (B) Upper: furin protein expression by western blot analysis. Lower: quantitative furin protein expression. (C) Relative Tfr2 mRNA expression by real-time RT-PCR. (D) Spearman's correlation analysis of Tfr2 and Furin mRNA expression (all samples were included; n = 20). *P < 0.05, **P < 0.01, NS = not significant.
3.3. Tfr2 mRNA expression in liver
Compared to WT mice, Tfr2 mRNA expression in th3/+ mice was not significantly higher (P = 0.056) (Fig. 2C). In iron-overloaded mice, Tfr2 mRNA was significantly decreased in both WT and th3/+ mice (60 and 73% reduction compared to control, respectively). Furthermore, the result of Spearman's correlation analysis showed a strong, positive correlation between Tfr2 and Furin mRNA expression (r = +0.80, P < 0.01) as shown in Fig. 2D.
3.4. Hepcidin expression
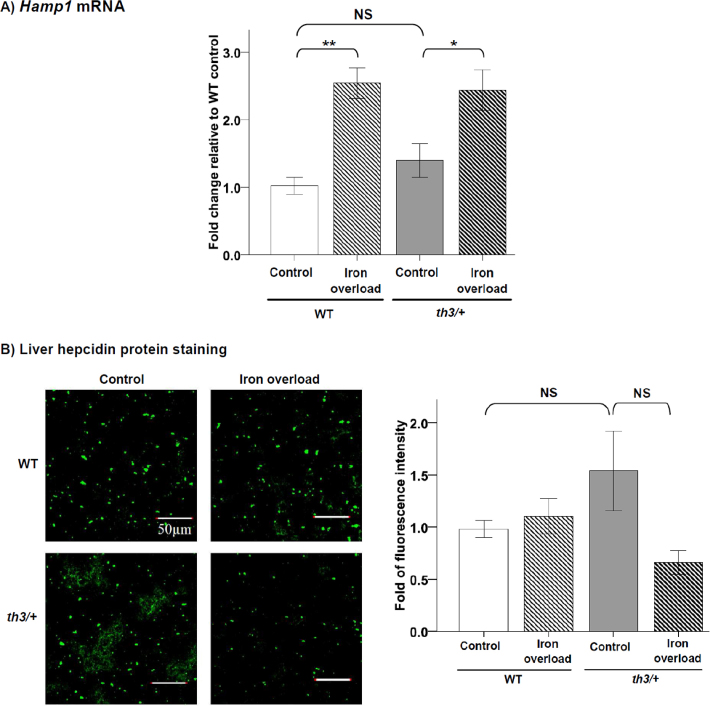
The results showed that Hamp1 mRNA (Fig. 3A), liver hepcidin protein (Fig. 3B), and total serum hepcidin (Table 1) in th3/+ mice did not significantly differ from those in WT mice. In iron overload condition, Hamp1 mRNA was significantly increased by 150% in WT mice, whereas the expression was increased by 70% in th3/+ mice (Fig. 3A). Surprisingly, the hepcidin protein level in both liver and serum did not increase in iron-overloaded WT and th3/+ mice, regardless of the increased level of Hamp1 mRNA (Fig. 3B and Table 1, respectively).
Fig. 3.
Hepcidin expression in the liver. The data are the mean ± SEM (n = 5 per group). (A) Relative Hamp1 mRNA expression by real-time RT-PCR. (B) Left: fluorescence images of hepcidin protein detection by immunofluorescence staining. Right: relative quantification of hepcidin protein expression. *P < 0.05, **P < 0.01, NS = not significant.
4. Discussion
According to the β-globin deficiency in β-thalassemia, erythroid precursors undergo apoptosis. Therefore, mature red blood cells cannot be effectively synthesized, resulting in a condition known as ineffective erythropoiesis (IE) [28], [29], [30]. In th3/+ mice, we observed the consequences of IE, including splenomegaly, elevated sEPO, and liver iron accumulation. The hematological parameters of this th3/+ mouse model have been described previously [17]. As in the previous studies [17], [31], our results demonstrated comparable body and liver weights between the WT and th3/+ mice.
In the presence of high sEPO and liver iron accumulation, the th3/+ mice had 50% increase of Furin mRNA expression compared to WT mice. The liver furin protein level was also consistent with the mRNA levels (70% higher in th3/+ mice). Serum hepcidin in normal WT mice was 2.9 ± 1.1 ng/ml, which is equivalent to the level observed in pooled mouse sera using liquid chromatography tandem mass spectrometry [32]. In addition, the total serum hepcidin and liver hepcidin protein expression in th3/+ mice were consistent with the result of Hamp1 mRNA. With a 7-fold increase in LIC, the level of hepcidin appeared to be insufficient in th3/+ mice [31]. Hence, it is possible that the elevated sEPO secondary to IE may continually impede Hamp1 mRNA expression. Thus, the ongoing hepcidin insufficiency ultimately leads to a further increase in intestinal iron absorption, contributing to the liver iron accumulation in th3/+ mice [8], [31].
After parenteral iron loading, the LIC was measured as ∼15 mg/g liver wet weight in both WT and th3/+ mice. In addition, a significant hepatosplenomegaly was found in WT mice and was more predominant in th3/+ mice. Hepatosplenomegaly is a common characteristic in iron-overloaded animal models and in thalassemia patients [28], [30]. Although sEPO concentration in WT mice was under the range of detection of the selected technique, it was detected at the same elevated level after iron overload in both WT and th3/+ mice. In addition to our result, previous study has demonstrated the apparent increase of both medullary and extramedullary erythropoiesis, and reticulocyte hemoglobin content in non-anemic, iron-overloaded C57BL/6 mice [15], which possibly supports the notion of iron overload-mediated erythropoiesis. However, the precise mechanism of sEPO elevation needs further experimentation.
With abundant iron loading, a significant down-regulation of Furin mRNA was observed in both WT and th3/+ mice (∼ 30 and 70% reduction, respectively). This was confirmed by the prominent reduction of furin protein in iron-overloaded WT mice. Nonetheless, it did not decrease significantly in iron-overloaded th3/+ mice. We also observed a marked Tfr2 mRNA reduction in iron-overloaded WT and th3/+ mice. Moreover, the strong, positive correlation between Tfr2 and Furin mRNA expression may support a role for TfR2 in regulating furin expression, as previously illustrated [10]. However, the study of changes in TfR2 protein and its signaling pathway that regulate furin expression would further clarify the underlying mechanism of furin down-regulation in this iron overload condition.
In terms of hepcidin, Hamp1 mRNA was significantly increased in the iron overload condition. This result also proves the principle that iron acts as a positive regulator of hepcidin gene expression, which in our model overcomes negative regulators such as elevated sEPO [33]. Nevertheless, the hepcidin protein levels in both the liver and serum did not increase in iron-overloaded mice, relating to the elevated Hamp1 mRNA expression. In addition to that aberrant hepcidin protein expression, the reduction of furin may further attenuate hepcidin function by affecting the maturation process of hepcidin.
As shown in an in vitro study, the accumulated prohepcidin was secreted from a hepatocyte cell line when furin was inhibited [34]. In addition, the impaired degradation of ferroportin, a target of hepcidin, has been observed in macrophage J774 cells after incubating with prohepcidin plus a furin inhibitor or Furin-specific siRNA [3]. These two lines of evidence indicate that a substantial amount of prohepcidin, an immature hormone, might be secreted in conditions of furin deficiency and that the prohormone produced is less physiologically meaningful. Therefore, we postulate in our study that both quantity and furin-mediated biological activity of hepcidin may be decreased in severe iron overload condition, including β-thalassemia.
In fact, the reduction of furin may also affect the maturation process of other proteins. Insulin-like growth factor-1 (IGF-1), which is mainly secreted by the liver, is a furin substrate [35]. In β-thalassemic patients with iron overload, serum IGF-1 is significantly lower. Lower serum IGF-1 is linked to the pathogenesis of growth failure and osteoporosis [36], [37]. Our study demonstrates the possible contribution of furin downregulation to the hepcidin deficiency in iron overload. Potential changes in other substrates, including IGF-1, could provide the basis for future studies related to the iron overload.
5. Conclusion
We found furin downregulation along with Hamp1 mRNA-unrelated pattern of hepcidin protein expression in iron-overloaded mice, particularly WT mice. This result suggests that hepcidin insufficiency may involve the decrease in both quantity and furin-mediated biological activity of hepcidin in sever iron overload condition.
Transparency document
Acknowledgments
This study was supported by Thailand Research Funds and the Commission on Higher Education (RMU5480001); the Office of the Higher Education Commission and Mahidol University under The National Research Universities Initiative; the Research Chair Grant, the National Science and Technology Development Agency (NSTDA), Thailand; and the Central Instrument Facility (CIF), Research Division, Faculty of Science, Mahidol University. The authors thank Miss Atiitaya Inthana for her assistance and for handling the animals.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2015.01.004.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Seidah N.G., Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 2012;11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 2.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gagliardo B., Kubat N., Faye A., Jaouen M., Durel B., Deschemin J.C., Canonne-Hergaux F., Sari M.A., Vaulont S. Pro-hepcidin is unable to degrade the iron exporter ferroportin unless maturated by a furin-dependent process. J. Hepatol. 2009;50:394–401. doi: 10.1016/j.jhep.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Pietrangelo A. Hepcidin in human iron disorders: therapeutic implications. J. Hepatol. 2011;54:173–181. doi: 10.1016/j.jhep.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Scamuffa N., Basak A., Lalou C., Wargnier A., Marcinkiewicz J., Siegfried G., Chretien M., Calvo F., Seidah N.G., Khatib A.M. Regulation of prohepcidin processing and activity by the subtilisin-like proprotein convertases Furin, PC5, PACE4 and PC7. Gut. 2008;57:1573–1582. doi: 10.1136/gut.2007.141812. [DOI] [PubMed] [Google Scholar]
- 6.Olivieri N.F. The beta-thalassemias. N. Engl. J. Med. 1999;341:99–109. doi: 10.1056/NEJM199907083410207. [DOI] [PubMed] [Google Scholar]
- 7.Olivieri N.F., Brittenham G.M. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–761. [PubMed] [Google Scholar]
- 8.Nemeth E. Hepcidin in beta-thalassemia. Ann. N.Y. Acad. Sci. 2010;1202:31–35. doi: 10.1111/j.1749-6632.2010.05585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Origa R., Galanello R., Ganz T., Giagu N., Maccioni L., Faa G., Nemeth E. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92:583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 10.Poli M., Luscieti S., Gandini V., Maccarinelli F., Finazzi D., Silvestri L., Roetto A., Arosio P. Transferrin receptor 2 and HFE regulate furin expression via mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/Erk) signaling. Implications for transferrin-dependent hepcidin regulation. Haematologica. 2010;95:1832–1840. doi: 10.3324/haematol.2010.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bondi A., Valentino P., Daraio F., Porporato P., Gramaglia E., Carturan S., Gottardi E., Camaschella C., Roetto A. Hepatic expression of hemochromatosis genes in two mouse strains after phlebotomy and iron overload. Haematologica. 2005;90:1161–1167. [PubMed] [Google Scholar]
- 12.Jamsai D., Zaibak F., Khongnium W., Vadolas J., Voullaire L., Fowler K.J., Gazeas S., Fucharoen S., Williamson R., Ioannou P.A. A humanized mouse model for a common beta0-thalassemia mutation. Genomics. 2005;85:453–461. doi: 10.1016/j.ygeno.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Moon S.N., Han J.W., Hwang H.S., Kim M.J., Lee S.J., Lee J.Y., Oh C.K., Jeong D.C. Establishment of secondary iron overloaded mouse model: evaluation of cardiac function and analysis according to iron concentration. Pediatr. Cardiol. 2011;32:947–952. doi: 10.1007/s00246-011-0019-4. [DOI] [PubMed] [Google Scholar]
- 14.Reardon T.F., Allen D.G. Iron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performance. Exp. Physiol. 2009;94:720–730. doi: 10.1113/expphysiol.2008.046045. [DOI] [PubMed] [Google Scholar]
- 15.Ginzburg Y.Z., Rybicki A.C., Suzuka S.M., Hall C.B., Breuer W., Cabantchik Z.I., Bouhassira E.E., Fabry M.E., Nagel R.L. Exogenous iron increases hemoglobin in beta-thalassemic mice. Exp. Hematol. 2009;37:172–183. doi: 10.1016/j.exphem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Yatmark P., Morales N.P., Chaisri U., Wichiyo S., Hemstapat W., Srichairatanakool S., Saovaros S., Fucharoen S. Iron distribution and histopathological characterization of the liver and heart of β-thalassemic mice with parenteral iron overload: effects of deferoxamine and deferiprone. Exp. Toxicol. Pathol. 2014;66:333–343. doi: 10.1016/j.etp.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Jamsai D., Zaibak F., Vadolas J., Voullaire L., Fowler K.J., Gazeas S., Peters H., Fucharoen S., Williamson R., Ioannou P.A. A humanized BAC transgenic/knockout mouse model for HbE/beta-thalassemia. Genomics. 2006;88:309–315. doi: 10.1016/j.ygeno.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Rebouche C.J., Wilcox C.L., Widness J.A. Microanalysis of non-heme iron in animal tissues. J. Biochem. Biophys. Methods. 2004;58:239–251. doi: 10.1016/j.jbbm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Sarradin P.M., Le Bris N., Le Gall C., Rodier P. Fe analysis by the ferrozine method: adaptation to FIA towards in situ analysis in hydrothermal environment. Talanta. 2005;66:1131–1138. doi: 10.1016/j.talanta.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Frazer D.M., Wilkins S.J., Darshan D., Badrick A.C., McLaren G.D., Anderson G.J. Stimulated erythropoiesis with secondary iron loading leads to a decrease in hepcidin despite an increase in bone morphogenetic protein 6 expression. Br. J. Haematol. 2012;157:615–626. doi: 10.1111/j.1365-2141.2012.09104.x. [DOI] [PubMed] [Google Scholar]
- 21.Mastrogiannaki M., Matak P., Mathieu J.R., Delga S., Mayeux P., Vaulont S., Peyssonnaux C. Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis. Haematologica. 2012;97:827–834. doi: 10.3324/haematol.2011.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weizer-Stern O., Adamsky K., Amariglio N., Rachmilewitz E., Breda L., Rivella S., Rechavi G. mRNA expression of iron regulatory genes in beta-thalassemia intermedia and beta-thalassemia major mouse models. Am. J. Hematol. 2006;81:479–483. doi: 10.1002/ajh.20549. [DOI] [PubMed] [Google Scholar]
- 23.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waterborg J.H., Matthews H.R. The Lowry method for protein quantitation. Methods Mol. Biol. 1994;32:1–4. doi: 10.1385/0-89603-268-X:1. [DOI] [PubMed] [Google Scholar]
- 25.Sisto M., Lisi S., Lofrumento D.D., Ingravallo G., Mitolo V., D’Amore M. Expression of pro-inflammatory TACE-TNF-alpha-amphiregulin axis in Sjogren's syndrome salivary glands. Histochem. Cell Biol. 2010;134:345–353. doi: 10.1007/s00418-010-0735-5. [DOI] [PubMed] [Google Scholar]
- 26.Baschong W., Suetterlin R., Laeng R.H. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM) J. Histochem. Cytochem. 2001;49:1565–1572. doi: 10.1177/002215540104901210. [DOI] [PubMed] [Google Scholar]
- 27.Ohtake T., Saito H., Hosoki Y., Inoue M., Miyoshi S., Suzuki Y., Fujimoto Y., Kohgo Y. Hepcidin is down-regulated in alcohol loading. Alcohol. Clin. Exp. Res. 2007;31:S2–S8. doi: 10.1111/j.1530-0277.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 28.Ginzburg Y., Rivella S. beta-thalassemia: a model for elucidating the dynamic regulation of ineffective erythropoiesis and iron metabolism. Blood. 2011;118:4321–4330. doi: 10.1182/blood-2011-03-283614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melchiori L., Gardenghi S., Rivella S. beta-Thalassemia: hijaking ineffective erythropoiesis and iron overload. Adv. Hematol. 2010;2010:938640. doi: 10.1155/2010/938640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rund D., Rachmilewitz E. Beta-thalassemia. N. Engl. J. Med. 2005;353:1135–1146. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 31.Gardenghi S., Marongiu M.F., Ramos P., Guy E., Breda L., Chadburn A., Liu Y., Amariglio N., Rechavi G., Rachmilewitz E.A., Breuer W., Cabantchik Z.I., Wrighting D.M., Andrews N.C., de Sousa M., Giardina P.J., Grady R.W., Rivella S. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109:5027–5035. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy A.T., Witcher D.R., Luan P., Wroblewski V.J. Quantitation of hepcidin from human and mouse serum using liquid chromatography tandem mass spectrometry. Blood. 2007;110:1048–1054. doi: 10.1182/blood-2006-11-057471. [DOI] [PubMed] [Google Scholar]
- 33.Zhao N., Zhang A.S., Enns C.A. Iron regulation by hepcidin. J. Clin Investig. 2013;123:2337–2343. doi: 10.1172/JCI67225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valore E.V., Ganz T. Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin. Blood Cells Mol. Dis. 2008;40:132–138. doi: 10.1016/j.bcmd.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brisson B.K., Barton E.R. New modulators for IGF-I activity within IGF-I processing products. Front. Endocrinol. 2013;4:42. doi: 10.3389/fendo.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papanikolaou G., Pantopoulos K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005;202:199–211. doi: 10.1016/j.taap.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Vidergor G., Goldfarb A.W., Glaser B., Dresner-Pollak R. Growth hormone reserve in adult beta thalassemia patients. Endocrine. 2007;31:33–37. doi: 10.1007/s12020-007-0018-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.