Abstract
The chemical inhibition of acetyl-cholinesterase (AChE) is a potent strategy for addressing signal related neuropathology and natural products are potential sources of compounds with such properties. Essential oil extracts from leaf, seed, stem and rhizome of four medicinal plants [Aframomum melegueta K. Schum, Crassocephalum crepidioides (Benth S. More), Monodora myristica (Gaertn.), and Ocimum gratissimum (Linn)] were tested for acetyl-cholinesterase inhibitory activity (AChEI) using Ellman's colorimentric method and compared to a reference acetyl-cholinesterase inhibitor (galantamine). The seed (IC50 = 6.71 mg/l) and leaf (IC50 = 6.54 mg/l) extracts from O. gratissimum showed values that matched the capacity of the reference inhibitor (IC50 = 6.62 mg/l). The least potent extract was rhizome extracts of A. melegueta (IC50 = 28.97 mg/l) about four times that of the reference inhibitor. Principal component analysis (PCA) showed that the intrinsic properties (bioactive ingredient factor) of each extract (PC1 = 29.50%) was the most important factor defining the difference or similarity in potency to the reference acetyl-cholinesterase inhibitor while ‘dose response’ (PC2 = 11.38%) was the second most important factor. The outstanding AChEI property of O. gratissimum extracts could largely be attributed to the high monoterpene content while the weak potency of rhizome extracts of A. melegueta may be attributed to its predominant concentrations of sesquiterpenes. Since potency could be related to interaction between bioactive components, understanding the interaction between ratios of monoterpene and sesquiterpene in extracts could be important in determining their potency for AChEI.
Keywords: Essential oils, Hydro-distillation, Medicinal plants, Acetyl-cholinesterase inhibition
1. Introduction
Acetylcholine (ACh) is the principal neurotransmitter which functions in all autonomic ganglia and is the only neurochemical that triggers motor division of the somatic nervous system. While normal cholinergic activity is defined by the sequence of release, binding and enzymatic deactivation of ACh by acetyl-cholinesterase (AChE), abnormal cholinergic activity on the other hand is characterized by a deficit or short-fall in cholinergic transmissions at synapses and has been attributed to reduced production of acetylcholine (ACh) or its excess deactivation/hydrolysis by AChE [34], [47]. The etiologies of cognitive problems highlight problems with signal transduction across synapses [32], [45] thus regulating the activities of AChE has become an important research focus [41].
Commercially available synthetic acetyl-cholinesterase inhibitors (AChEIs) include donepezil (Aricept®), rivastigmine (Exelon®), galantamine (Razadyne®), and tacrine (Cognex®) influence the dynamics of ACh by inhibiting the activity of AChE thus increasing the availability and interaction time of Ach with cholinergic receptors of synaptic cells. Considering the drawbacks of synthetic AChEIs which include gastrointestinal disturbances, moderate effectiveness, high cost and short half-life [38], [46], [49] natural product based compounds have been increasingly explored for better effects [39], [40]. Desirable properties of botanical extracts or natural product based compounds include a comparatively better penetration of the blood–brain barrier better than the pharmaceutical options and better specificity for human type AChE (G4) [49]. Although the anticholinesterase activity of many plant compounds have been demonstrated, in vitro laboratory trials using essential oil extracts of tropical plants are very limited.
Plant-derived essential oils exhibit pharmacological properties traceable to the presence of various structurally diverse bioactive chemical components [1] and are increasingly harnessed for their anti-cholinesterase (AChE) properties [33], [35], [48]. Smail et al. [48] reported the acetyl-cholinesterase inhibiting potentials of commercial essential oils of Citrus aurantium L., Cupressus sempervirens L., and Eucalyptus globulus Labill. Foeniculum vulgare Mill and Thymus vulgaris from Morocco. Politeo et al. [44] also reported high acetyl-cholinesterase inhibition activity of essential oil from Pinus nigra Arnold ssp. dalmatica (Vis.) and documented α-pinene, β-pinene, germacrene-d and β-caryophyllene as predominant bioactive compounds. Considering the importance of sufficient knowledge base for accurate recommendations on the use of plant extracts, this study compares the anti-cholinesterase activity of essential oil extracts from four tropical plant species to galantamine (a commercially available synthetic AChEI).
2. Materials and methods
2.1. Plant materials and extraction of essential oils
The plant species of medical importance i.e. Aframomum melegueta (K. Schum), Crassocephalum crepidioides (Benth S. More), Monodora myristica (Gaertn), and Ocimum gratissimum (Linn) were harvested at different farm locations in Akure, Ondo State Nigeria. Identification was carried out at the forest research institute Ibadan, Nigeria. Different plant parts i.e. seed, leaf, stem and rhizome were separated washed, cut into small sizes and subjected separately to hydro-distillation using an all glass Clevenger apparatus for 3–4 h according to European pharmacopoeia 2008. Oils were collected into glass sample bottles and kept in the refrigerator without further treatment before GC/MS and acetyl-cholinesterase inhibition analyses.
2.2. Gas chromatography/mass spectrometry (GC/MS)
The essential oils were analyzed using Agilent (USA) 6890N GC Coupled with MS-5973-634071 Series. The capillary column type was DB-1 (fused-silica) [30.0 m (length) × 320.00 μm (diameter) × 1.00 μm (film thickness)]. The carrier gas was Helium at constant flow rate of 1.0 ml/min and average velocity of 37 cm/s; the pressure was 0.78 psi. The initial column temperature was set at 100 °C (held for 5 min) to the final temperature of 250 °C at the rate of 5 °C/min. The injector was the split type and was set at 50:1, and volume injected was 1.0 μl. The chromatograms were auto-integrated by Shem-Station and the constituents were identified by comparison of the GC–MS data with (NIST02) library spectra and data from literature [2].
2.3. Acetyl-cholinesterase inhibition assay
The acetyl-cholinesterase inhibition assay was determined by Ellman colorimetric method [36] as modified by Albano et al. [31]. In a total volume of 1 ml, 415 μl of Tris–HCl buffer 0.1 M (pH 8), 10 μl of solution of essential oils in methanol with different concentrations and 25 μl of enzyme (electric eel acetyl-cholinesterase, type-VI-S, EC 3.1.1.7, Sigma–Aldrich, St. Louis, USA) solution containing 0.5 U/ml were incubated for 15 min at room temperature. 75 μl of a solution of AChI (acetyl-thiocholine) (Sigma–Aldrich, Steinheim, Germany) 1.83 mM and 475 μl of DTNB (5,5-dithiobis-2-nitrobenzoic acid), 3 mM (Sigma–Aldrich, Steinheim, Germany) were added and the final mixture incubated for 30 min, at room temperature. Absorbance of the mixture was measured at 412 nm in a UV-Visible 752 spectrophotometer (Techmel and Techmel, USA). Galanthamine hydrobromide (Sigma–Aldrich, Steinheim, Germany) was used as positive control. The percentage inhibition of enzyme activity was calculated by comparison with the negative control:
where A0 was the absorbance of the negative control (enzyme + methanol) and A1 was the absorbance of the sample (enzyme + solution of EO). Tests were carried out in triplicate and data were analyzed using descriptive statistics.
2.4. Estimation of IC50 values
The IC50 values (concentration of test compounds that inhibits the hydrolysis of substrates by 50%) were determined by spectrophotometric measurement of the effect of increasing concentrations of test compounds (plant extracts and positive controls) on AChE activity. Determinations were carried out in triplicates. To calculate the IC50 values, each sample was assayed at 5 concentrations (20, 15, 10, 5 and 2.5 mg/ml). IC50 values were obtained from dose–effect curves by linear regression.
2.5. Inhibition factor
This variable is defined as the inhibitory strength of an extract relative to the reference inhibitor. The values give the number of times the essential oil extract is more potent or less potent than the reference inhibitor.
It is calculated as
where ICx is the concentration of test substance or extract that inhibited x% of AChE activity.
2.6. Data analysis
Dose relationship plots and dose response equation were generated using the percentage inhibition of each extract at each given concentration of each extract. The 10% (IC10), 50% (IC50) and 90% (IC90) inhibitory concentration values were calculated from the dose response equation. All dose response plots and calculations were achieved using Originlab version 8 (Originlab Corp., USA).
Multivariate statistic i.e. principal component analysis (PCA) was applied to acetyl-cholinesterase inhibition data of all extracts to elucidate intricate relationships and patterns of inhibition across essential oil extracts used for this study. Principal components (PCs) were extracted, percentage variance and principal component scores tabulated for each PC. A PCA biplot created using PC 1 and 2 to graphically explain similarities or differences in inhibition patterns of extracts based on the orientation of the variables within ordination space. PCA was carried out using STATISTICA® version 7 (Statsoft Inc., USA).
3. Results
3.1. Dose response relationships
The summary of acetyl-cholinesterase inhibition by essential oil extracts from the different parts of the four plants used in this study is given in Table 1. The acetyl-cholinesterase inhibition of each extract was indicated primarily by its IC50 and complemented by the IC10 and IC90 as lower and upper limits of their inhibition capacity.
Table 1.
Summary of acetyl-cholinesterase inhibition by essential oil extracts.
| Extract | Dose response equation | IC10 (mg/l) | IC50 (mg/l) | IC90 (mg/l) | Inhibition factor (IF) (IC50) | Inhibition factor (IF) (IC90) |
|---|---|---|---|---|---|---|
| Galatamine (reference) | Y = 4.22x + 13.21 | ND | 6.62 | 18.22 | 1 | 1 |
| O. gratissimum leaf | Y = 3.94x + 24.23 | ND | 6.54 | 16.7 | 1.01 | 1.09 |
| O. gratissimum seed | Y = 3.65x + 25.53 | ND | 6.71 | 17.68 | 0.99 | 1.03 |
| C. crepidiodes leaf | Y = 3.64x + 10.14 | ND | 10.96 | 21.95 | 0.6 | 0.83 |
| A. melegueta seed | Y = 2.53x + 20.31 | ND | 11.75 | 27.59 | 0.56 | 0.66 |
| C. crepidiodes stem | Y = 4.02x + 1.14 | 2.2 | 12.15 | 22.11 | 0.55 | 0.82 |
| M. myristica seed | Y = 2.16x + 17.81 | ND | 14.9 | 33.42 | 0.44 | 0.55 |
| M. myristica stem | Y = 1.96x + 19.29 | ND | 15.56 | 36.13 | 0.43 | 0.51 |
| A. melegueta stem | Y = 2.16x + 17.08 | ND | 15.27 | 33.82 | 0.43 | 0.54 |
| A. melegueta leaf | Y = 2.66x + 7.42 | 0.97 | 16 | 31 | 0.4 | 0.59 |
| A. melegueta rhizome | Y = 1.79x + 1.62 | 6.52 | 28.97 | 51.42 | 0.23 | 0.35 |
From the IC50, O. gratissimum seed and leaf extracts showed an AChEI capacity that matched that of the reference inhibitor galatamine (Table 1). The upper limit of AChEI by the essential oil extracts as indicated by the IC90 showed that O. gratissimum seed (17.68 mg/l) and leaf (16.70 mg/l) extracts gave lower values than the reference inhibitor (18.22 mg/l). This demonstrates a higher potency because the essential oil extracts of O. gratissimum were able to inhibit up to 90% of AChE activity at lower concentrations than that of the reference inhibitor.
Essential oil extracts of A. melegueta stem and rhizome showed the least potency as indicated by their high IC50 and IC90 values (Table 1). A notable feature in the pattern of AChEI capacity of these essential oil extracts is that the most potent extracts including the reference inhibitor did not have a detectable IC10, an indication that their potency may not be easily managed to achieve low level inhibitions when and where necessary. Only essential oil extract from C. crepidioides stem (2.20 mg/l), A. melegueta leaf (0.97 mg/l) and A. melegueta rhizome (6.52 mg/l) showed the ability to achieve a 10% inhibition of AChE (IC10). The lower IC10 value of A. melegueta leaf (0.97 mg/l) showed that it had the highest potency for low level inhibition.
The inhibition factor of each essential oil extracts showed that the potency of O. gratissimum extracts where the IC50 of seed (0.99) and the leaf extracts (1.01) were approximately equal to that of the reference inhibitor while they were slightly more potent than galantamine (reference) at 90% inhibition of AChE. Essential oil extracts of C. crepidioides leaf [0.83 (83%)] and stem [0.82 (82%)] was the next most potent extract achieving up to 80% (0.8) of the inhibition potential of the reference (galatamine) at 90% inhibition of AChE (IC90). Extracts from A. melegueta rhizome was the only extracts that showed less than 50% (0.5) capacity of the reference inhibitor at IC90.
3.2. Principal component analysis (PCA)
Principal component analysis showed the extraction of three principal components PC 1, PC 2 and PC 3 each accounting for 29.50, 11.38 and 6.72% of the variance in AChE inhibition across essential oil extracts respectively (Table 2). PC 1 which explains the highest variances represents the primary factor responsible for the difference in inhibitory capacity of each essential oil extract.
Table 2.
Principal component scores of variables.
| Variables | PC 1 (intrinsic factor) | PC 2 (dose response factor) | PC 3 (not used) |
|---|---|---|---|
| 2.5 mg/l | 0.92 | −0.35 | 0.02 |
| 5.0 mg/l | 0.93 | −0.32 | −0.15 |
| 10.0 mg/l | 0.95 | −0.20 | 0.14 |
| 15.0 mg/l | 0.89 | 0.39 | 0.03 |
| 20.0 mg/l | 0.72 | 0.67 | −0.04 |
| Control | 0.02 | 0.03 | 0.08 |
| Extract {MN_seed} | 0.09 | −0.29 | −0.16 |
| Extract {MN_stem} | 0.10 | −0.43 | −0.17 |
| Extract {OC_seed} | −0.45 | −0.14 | 0.48 |
| Extract {OC_leaf} | −0.49 | 0.00 | 0.09 |
| Extract {CR_stem} | 0.10 | 0.59 | 0.48 |
| Extract {AF_seed} | −0.06 | −0.27 | −0.04 |
| Extract {AF_leaf} | 0.24 | 0.06 | 0.01 |
| Extract {AF_stem} | 0.11 | −0.32 | 0.12 |
| Extract {AF_rhizome} | 0.68 | 0.09 | 0.02 |
| Extract {CR_leaf} | −0.05 | 0.33 | −0.10 |
| Extract {galantamine} | −0.27 | 0.37 | −0.74 |
| Variance (%) | 29.50 | 11.38 | 6.72 |
AF, A. melegueta; CR, C. crepidioides; OC, O. gratissimum and MN, M. myristica.
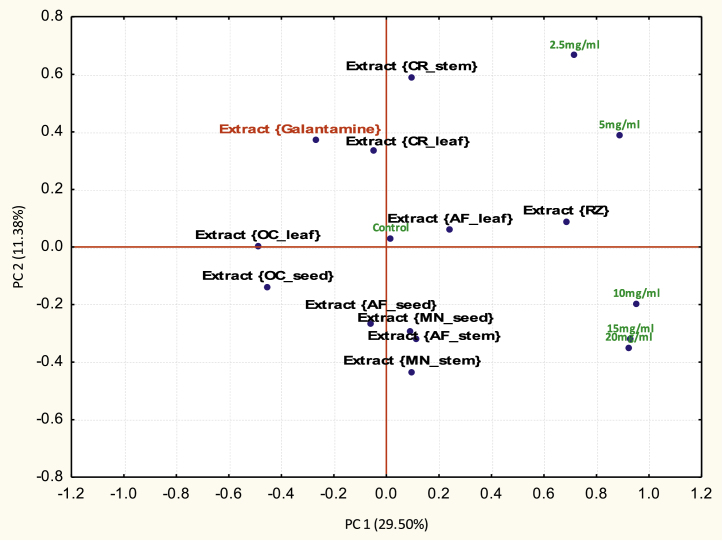
Viewing the principal component biplot from the PC 1 axis (Fig. 1), the orientation of OC_leaf and OC_seed alongside galatamine on the negative axis of PC 1 infers that they showed similar patterns of AChE inhibition. The orientation of OC_leaf and OC_seed farther ahead of galatamine on the negative axis implies that they achieved more AChE inhibition than galatamine (the reference inhibitor). The location of AF_rhizome on the positive axis of the PC 1 axis implies that it showed the least inhibition. The orientation of the exposure concentrations (2.5 mg/l, 5 mg/l, 10 mg/l, 15 mg/l and 20 mg/l) along the positive axis of PC 1 gives a first impression that the orientation of the extracts on the plots is irrespective of dose used for the study. Thus it may be implied that the orientation of extracts midway of the PC 1 axis alongside the control shows that the essential oil of such extracts were impotent if not applied in a dose-manner. Based on these inferences the PC 1 factor was indexed as “intrinsic property” implying that extracts that showed or did not show AChE inhibition could be traced to their intrinsic properties or bioactive ingredients.
Fig. 1.
Principal component biplot of essential oil extracts based on acetyl-cholinesterase inhibitory capacity observed in the in vitro tests (where AF, A. melegueta; CR, C. crepidioides; OC, O. gratissimum and MN, M. myristica).
From PC 2 axis the separation of lower concentrations (positive axis) from higher concentrations (negative axis) gives a first impression of a dose-response relationship in the distribution of extracts on the biplot. The cluster of extracts AF_seed, AF_stem, MN_stem and MN_seed on the negative axis of PC 2 alongside the higher concentrations implies that these essential oil extracts produce significant AChE inhibition only at higher concentrations. The orientation of CR_stem at the furthermost part of the PC 2 axis beyond galatamine and alongside 2.5 mg/l implies its potency at low concentrations unlike galatamine when applied in a dose approach.
Table 2 corroborates the depictions of the biplot. For PC 1 the concurrent positive correlations of the concentrations used for the study implies a non-dose relationship factor operational within that principal component. OC_leaf (−0.49) and OC_seed (−0.45) showed the highest negative principal component scores alongside galatamine (−0.27) implying their similarity in AChE inhibition trend. AF_rhizome had the highest positive principal component score implying that its inhibitory properties were negative correlated with that of galatamine (reference inhibitor).
The third principal component was not used for the study because it accounted for a very low percentage variance (6.73%) of the total inhibition trends of the essential oil extracts used in this study.
3.3. Predominant bioactive constituent and AChE inhibition patterns of essential oil extracts
The major compositions of the essential oils were determined and reported earlier [42], [43], [49], [50], [51], [52], [53]. The major chemical components of the leaf essential oil of A. melegueta were identified as myrtenyl acetate (29.06%), limonene (19.43%) and γ-elemene (8.84%). The stem essential oil contained caryophyllene oxide (19.70%), myrtenyl acetate (14.70%) and β-eudesmene (10.83%). The rhizome essential oils comprised of myrtenyl acetate (22.70%), pinocarvyl acetate (11.50%), cyperene (8.96%) and caryophyllene oxide (5.97%) while the seed volatile oil consisted of α-caryophyllene (48.78%) and β-caryophyllene (32.50%). The most abundant constituents of the leaf essential oil of C. crepidioides were α-caryophyllene (10.29%) and β-cubebene (13.77%) while the dominant constituents of the stem essential oil were thymol (43.93%) and 4-cyclohexybutyramide (20.94%). The essential oil of the leaves of O. gratissimum afforded γ-terpinene (52.86%), Z-tert-butyl-4-hydroxy anisole (13.93%) and caryophyllene (10.37%) while the seeds’ oil yielded α-pinene (48.19%) and caryophyllene (10.71%) as principal components. The GC/MS analyses of the essential oil of the seeds of M. myristica revealed germacrene-d-4-ol (25.48%), tricycle [5.2.1(1, 5)] dec-2-ene and stem-bark oil yielded γ-cadinene (31.31%) and α-elemene (17.98%) (6.70%) as dominant constituents (Table 3).
Table 3.
Predominant bioactive constituents of essential oil extracts.
| Plant species | Plant part extract | Predominant bioactive constituent | Percentage occurrence (%) | HYDROCARBON SUBCLASS |
|---|---|---|---|---|
| A. melegueta | Leaf | Myrtenyl acetate | 29.06 | Monoterpene ester |
| Limonene | 19.45 | Monoterpene olefin | ||
| γ-Elemene | 8.84 | Sesquiterpene | ||
| Stem | Caryophyllene oxide | 19.70 | Sesquiterpene | |
| Myrtenyl acetate | 14.70 | Monoterpene ester | ||
| β-Eudesmene | 10.83 | Monoterpene | ||
| Seed | α-Caryophyllene | 48.78 | Sesquiterpene | |
| β-Caryophyllene | 32.50 | Sesquiterpene | ||
| Rhizome | Myrtenyl acetate | 22.70 | Monoterpene ester | |
| Pinocarvyl acetate | 11.50 | Monoterpene ester | ||
| Cyperene | 8.96 | Sesquiterpene | ||
| Caryophyllene | 5.97 | Sesquiterpene | ||
| C. crepidioides | Leaf | α-Caryophyllene | 10.29 | Sesquiterpene |
| β-Cubebene | 13.77 | Sesquiterpene | ||
| Stem | Thymol | 43.93 | Monoterpene phenol | |
| 4-Cyclohexabutyramide | 20.94 | Monoterpene | ||
| O. gratissimum | Leaf | γ-Terpinene | 52.86 | Monoterpene |
| Z-tert-butyl-4-hydroxy anisol | 13.93 | Monoterpene | ||
| Caryophyllene | 10.37 | Sesquiterpene | ||
| Seed | α-Pinene | 48.19 | Monoterpene | |
| Caryophyllene | 10.71 | Sesquiterpene | ||
| M. myristica | Seed | Germocrene-d-4-ol | 25.48 | Sesquiterpene |
| Tricycle dec-2-ene | 6.71 | Sesquiterpene | ||
| Stem | γ-Cadinene | 31.31 | Sesquiterpene | |
| α-Elemene | 7.98 | Sesquiterpene | ||
Also given in Table 3 are the subclasses of the predominant bioactive constituents of the essential oil extracts. A very notable trend in the predominant bioactive components is that essential oils with the higher potency for inhibiting acetyl-cholinesterase activity had monoterpenes as their predominant component. The extract of O. gratissimum seed for instance showed a potency that exceeded the reference inhibitor (galatamine) (Table 1) and its predominant bioactive component was α-pinene (48.19%) (Table 3). Its leaf extract also matched the potency of the reference inhibitor (Table 1) and had γ-terpinene (52.86%) as the major bioactive component (Table 3). Similarly the next most potent essential oil extract which produced about 80% of the inhibition capacity of galatamine (Table 1) was the stem extract of C. crepidioides which had the monoterpene thymol (43.93%) as its major bioactive constituent.
Only essential oil extracts of C. crepidioides leaf showed a high potency and also had sesquiterpenes α-caryophyllene (10.29%) and β-cubebene (13.77%) as its predominant bioactive component. Extracts from M. myristica and A. melegueta showed moderate to weak AChE inhibition properties (Table 1) and predominant constituents were either monoterpene esters or sesquiterpenes (Table 3).
4. Discussion
Essential oils are mixtures of many bioactive compounds whose occurrence are strongly dependent on wide range of factors ranging from plant species used [3] the part of the plant used [4], the season the plant was harvested [5], population differences of the same species [6], or even between individuals of the same population [7], [8]. Although the bio-inhibitory effect of an essential oil could be explained in terms of the individual effects or joint effects of some main constituents there is still little knowledge on the nature of interaction between these compounds.
The essential oils of A. melegueta generally showed a weak to moderate potency for inhibiting AChE. The potency priority for extracts showed seed > leaf > stem > rhizome. Relating this trend to the profile of its predominant bioactive constituents the seed extract was the only one that had sesquiterpene in concentrations above 30%. The AChE inhibition of this extract may be explained by the high content in sesquiterpenes. A number of studies have attributed AChE inhibition to predominant occurrence of sesquiterpenes in extracts [9], [10], [11] and synergistic potential of sesquiterpenes [12]. Patel and Amin [12] although reported anti-cholinesterase activity in sesquiterpene rich extracts of Sphaeranthus indicus flower heads, the individual potency of predominant sesquiterpenes tested negative when tested for AChE inhibitory activity. The α- and β-caryophyllene constituted the sesquiterpenes with the highest occurrence and these have been identified as low molecular inhibitors. The low molecular weight feature is believed to confer an advantage to this compounds allowing for easy passage across the blood brain barrier [13]. The next potent extract was the leaf which had predominantly monoterpenes and sesquiterpenes in close ratios. The potency of A. melegueta leaf extracts at low concentrations may be attributed to the presence of limonene (monoterpene) as part of its major constituents. Reports have shown that limonene it can act synergistically with other terpenes to promote terpene absorption by facilitating trans-cellular membrane movement [14]. The weak potency of essential oil extracts of A. melegueta rhizome may be related to the concurrent occurrence of monoterpenes and sesquiterpenes in close ratios which may allow for antagonistic interactions. Yu et al. [15] reported that the anti-AChE activity of essential oils is strongly dependent on the interaction of different terpenoid contents. They reported different interactions including synergy between monterpenoids and antagonistic relationships between monoterpenoids and sesquiterepenes.
Extracts of M. myristica showed similar moderate potency for AChE inhibition as observed in majority of the extracts of A. melegueta. Also similarly these extracts were predominantly concentrated with bicyclic and monocyclic monoterpenes sesquiterpenes. A possible explanation for the similarity between the potency of these two groups of extracts may the presence of oxygen containing terpenes in their essential oils. Reports have shown that the presence of oxygenated functional groups in bicyclic terpenes can decrease its capacity to inhibit AChE [16].
The potency of O. gratissimum seed extract which exceeded the inhibitory capacity of the reference inhibitor may be attributed to its very high α-pinene content (48.19%). Pinene is notable for its ability to easily cross the blood–brain barrier where it inhibits activity of acetyl-cholinesterase, which destroys acetylcholine, resulting in better memory outcomes. The similar potency exhibited by O. gratissimum leaf extract may also be attributed to its high monoterpene content (terpinene: 52.86%). Miyazawa et al. [17] estimated the anti-AChE activity of 17 monoterpenoid compounds (hydrocarbons, alcohols and ketones) and documented a highest anti-AChE for terpenenes. The monoterpenes are natural products and main constituents of essential; they are lipophilic in nature thus allowing their selective movement into and across membrane structures [18], [19]. The monoterpene α-pinene has been demonstrated as a potent AChE inhibitor [16], [20] thus clearly explaining the potency demonstrated by O. gratissimum seed extract.
Essential oil extracts of C. crepidioides showed the most potent AChE inhibition after O. gratissimum. The AChE inhibition pattern of leaf and stem extracts of C. crepidioides these extracts demonstrates two scenarios of inhibition: extracts from the leaf had predominant concentrations of sesquiterpenes i.e. α-caryophyllene and β-caryophyllene while the stem extracts had predominant concentrations of monoterpenes i.e. thymol and 4-cyclohexabutyramide. This suggests the possibility of synergy between predominant bioactive components in essential oils to cause a significant inhibition in AChE activity irrespective of the subclass of the active compound. This possibility has also been documented [15]. Beyond the possibility of synergy between bioactive components, the potency of monoterpenes for AChE inhibition has been widely reported. The potency of monoterpenes has been highlighted on its interactions with cell membrane and their potential to influence the fluidity and porosity of membrane structure [21], [22] the antimicrobial action of essential oils have also been linked to their monoterpenoid components [14], [21], [23]. Sikkema et al. [24] showed that, as a result of their lipophilic character, cyclic monoterpenes will preferentially partition from an aqueous phase into membrane structures causing membrane expansion, increased membrane fluidity and inhibition of a membrane-embedded enzyme. In yeast cells and isolated mitochondria, α-pinene and β-pinene alter cellular integrity; inhibit respiration and ion transport processes and increase membrane permeability [14], [23]. Other reports on AChE inhibition and high content of monoterpenes mentioned that 1,8-cineole, camphor, α-pinene, β-pinene, borneol, linalool, menthone, carvone, anetole, anisole, have anticholinesterase activity [10], [25], [26], [27]. The anticholinergic activity of thymol has been reported [37] and other studies have suggested that the potency of thymol and its derivatives thymoquinone and thymohydroquinone as inhibitors of acetylcholinesterase (AChE) could be related or linked to its additional antioxidant potential [28].
From the pharmaco-dynamic perspective, the general patterns of AChE inhibition by essential oil extracts may be explained on relationship between the structure of the agonist (bioactive compounds) and receptor sites of AChE using the principle of drug concentration and effect. The model assumes that the drug interacts reversibly with its receptor and produces a maximal effect proportional to the number of receptors occupied, up to a maximal effect when all receptors are occupied [29]. In principle this implies that bioactive components of essential oil elicit AChE inhibition when the receptors are fully occupied by the compounds. Potency describes the relationship between receptor occupancy and the ability to initiate a response at the molecular, cellular, tissue or system level. Since all extracts showed 50% and 90% inhibition they could all be classified as being potent for inhibiting AChE.
A very important disparity in their inhibition potency is that although most extracts were potent for maximal effects i.e. IC90, only a few showed potency for minimal effects (IC10). The potency of essential oil extracts from C. crepidioides stem and leaf and rhizome of A. melegueta were detected at low effects. Clinical outcomes have shown that in cases when patients become tolerant to the effects of a drug, lowering the dosage or switching drugs are possible ways of addressing the problem [30]. Thus essential oil extracts with detectable potency at lower effects may have more clinical relevance, but recommendations cannot be made at this stage of study.
The results also suggest that extracts of O. gratissimum and C. crepidioides which showed strong AChE properties similar to galantamine (reference inhibitor) may be categorized as high potency agonists because they were able to elicit inhibition in smaller quantities than the reference substance. This ability to elicit inhibition at lower concentrations may be interpreted from the concentration–effect model that they produced maximal response while occupying a relatively low proportion of the total AChE receptor population. Other extracts particularly i.e. M. myristica and A. melegueta could be categorized as low potency agonists because they required higher concentrations to achieve the same level of inhibition observed in the high potency agonists.
5. Conclusion
This study has demonstrated different degrees of AChEI potency for the essential oil extracts, and could be considered as a first level screening stage for these extracts. We suggest further tests for therapeutic index (dose relationships between desired and undesired effects) and quantal dose relationships (percentage of individuals producing quantal effects) to aid clinical decisions and recommendations.
Furthermore this study has shown or demonstrated the considerable variability in the composition of the essential oil from different parts of the same plant. Given this variability, the overall effect of the essential oil of an aromatic plant may not be easily predicted, unless the exact composition and the type of interactions among its constituents are known. As such single and mixture inhibition assays using isolated bioactive compound from potent essential oils could provide information necessary for desirable pharmacological outcomes.
References
- 1.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils – a review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 2.Adams R.P. Allured Publishing Corporation; Illinois, USA: 1995. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; pp. 5–115. [Google Scholar]
- 3.de Souza A., Lopes E.M.C., da Silva M.C., Cordeiro I., Young M.C.M., Sobral M.E.G., Moreno P.R.H. Chemical composition and acetylcholinesterase inhibitory activity of essential oils of Myrceugenia myrcioides (Cambess.) O. Berg and Eugenia riedeliana O. Berg, Myrtaceae. Braz. J. Pharmacogn.Rev. Bras. Farmacogn. 2010;20(2):175–179. [Google Scholar]
- 4.Khadri A., Serralheiro M.L.M., Nogueira J.M.F., Neffati M., Smiti S., Araújo M.E.M. Antioxidant and antiacetylcholinesterase activities of essential oils from Cymbopogon schoenanthus L. Spreng. Determination of chemical composition by GC–mass spectrometry and 13C NMR. Food Chem. 2008;109(2008):630–637. [Google Scholar]
- 5.Vokou D., Margaris N.S. Variation of volatile oil concentration of Mediterranean aromatic shrubs, Thymus capitatus Hoffmanns et Link, Satureja thymbra L., Teucrium polium L. and Rosmarinus officinalis L. Int. J. Biometeorol. 1986;30:147–155. [Google Scholar]
- 6.Vokou D., Kokkini S., Bessiere J.M. Geographic variation of Greek oregano (Organum vulgare ssp. hirtum) essential oils. Biochem. Syst. Ecol. 1993;21:287–295. [Google Scholar]
- 7.Kokkini S., Vokou D. Mentha spicata (Lamiaceae) chemotypes growing wild in Greece. Econ. Bot. 1989;43:192–202. [Google Scholar]
- 8.Tarayre M., Thompson J.D., Escarre J., Linhart Y.B. Intraspecific variation in the inhibitory effects of Thymus vulgaris (Labitae) monoterpenes on seed germination. Oecologia. 1995;101:110–118. doi: 10.1007/BF00328907. [DOI] [PubMed] [Google Scholar]
- 9.Miyazawa M., Kakiuchi A., Watanabe H., Kameoka H. Inhibition of acetylcholinesterase activity by volatile α,β-unsaturated ketones. Nat. Prod. Lett. 1988;12:131–134. [Google Scholar]
- 10.Savelev S.U., Okello E.J., Perry E.K. Butyryl- and acetylcholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother. Res. 2004;18:315–324. doi: 10.1002/ptr.1451. [DOI] [PubMed] [Google Scholar]
- 11.Loizzo M.R., Tundis R., Conforti F., Menichini F., Bonesi M., Nadjafi F., Frega N., Menichini F. Salvia leriifolia Benth (Lamiaceae) extract demonstrates in vitro antioxidant properties and cholinesterase inhibitory activity. Nutr. Res. 2010;30:823–830. doi: 10.1016/j.nutres.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Patel M.B., Amin D. Sphaeranthus indicus flower derived constituents exhibits synergistic effect against acetylcholinesterase and possess potential antiamnestic activity. J. Complement. Integr. Med. 2012;9(1) doi: 10.1515/1553-3840.1618. (ISSN (Online) 1553-3840) [DOI] [PubMed] [Google Scholar]
- 13.Murata K., Matsumura S., Yoshioka Y., Ueno Y., Matsuda H. Screening of β-secretase and acetylcholinesterase inhibitors from plant resources. J. Nat. Med. 2015;69(1):123–129. doi: 10.1007/s11418-014-0859-3. [DOI] [PubMed] [Google Scholar]
- 14.Uribe S., Ramirez T., Pena A. Effects of β-pinene on yeast membrane functions. J. Bacteriol. 1985;161:1195–1200. doi: 10.1128/jb.161.3.1195-1200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Z.W., Wang B.C., Yang F.M., Sun Q.Y., Yang Z.N., Zhu L.C. Chemical composition and antiacetylcholinesterase activity of flower essential oils of Artemisia annua at different flowering stage. Iran. J. Pharm. Res. 2011;10(2):265–271. [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazawa M., Yamafuji C. Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids. J. Agric. Food Chem. 2005;53:1765–1768. doi: 10.1021/jf040019b. [DOI] [PubMed] [Google Scholar]
- 17.Miyazawa M., Watanabe H., Kameoka H. Inhibition of acetylcholinesterase activity by monoterpenoids with ap-menthane skeleton. J. Agric. Food Chem. 1997;45:677–679. [Google Scholar]
- 18.Gershenzon J., Crotaeu R. Terpenoids. In: Rosenthal G.A., Berenbaum M.R., editors. vol. I. Academic; New York: 1991. pp. 65–219. (Herbivores: Their Interactions With Secondary Plant Metabolites: The Chemical Participants). [Google Scholar]
- 19.De Sousa D.P. 1st ed. Nova Science Publishers; New York, NY, USA: 2012. Medicinal Essential Oils: Chemical, Pharmacological and Therapeutic Aspects; p. 236. [Google Scholar]
- 20.Perry N.S.L., Houghton P.J., Theobald A., Jenner P., Perry E.K. In vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J. Pharm. Pharmacol. 2000;52:895–902. doi: 10.1211/0022357001774598. [DOI] [PubMed] [Google Scholar]
- 21.Knobloch K., Pauli A., Iberl B., Weis N., Weigand H. Antibacterial activity and antifungal properties of essential oil components. J. Essent. Oils Res. 1988;1:119–128. [Google Scholar]
- 22.Sikkema J., De Bont J.A.M., Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrews R.E., Parks L.W., Spence K.D. Some effects of Douglas fir terpenes on certain microorganisms. Appl. Environ. Microbiol. 1980;40:301–304. doi: 10.1128/aem.40.2.301-304.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sikkema J., De Bont J.A.M., Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994;269:8022–8028. [PubMed] [Google Scholar]
- 25.Savelev S., Okello E., Perry N.S., Wilkins R.M., Perry E.K. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 2003;75:661–668. doi: 10.1016/s0091-3057(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 26.Sajjadi S.E., Shahpiri Z. Chemical composition of the essential oil of Salvia limbata C.A. Mey. Daru. 2004;12:94–97. [Google Scholar]
- 27.Picollo M.I., Toloza A.C., Mougabure C.G., Zygadlo J., Zerba E. Anticholinesterase and pediculicidal activities of monoterpenoids. Fitoterapia. 2008;79:271–278. doi: 10.1016/j.fitote.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Markesbery W.R. Oxidative stress hypothesis in Alzheimer's disease. Free Radic. Biol. Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 29.Walker M.G., Page C.P., Hoffman B.F., Curtis M. 3rd ed. Mosby; St. Louis: 2006. Integrated Pharmacology. [Google Scholar]
- 30.Soares-Weiser K., Rathbone J. Neuroleptic reduction and/or cessation and neuroleptics as specific treatments for tardive dyskinesia. Cochrane Database Syst. Rev. 2006;1:CD000459. doi: 10.1002/14651858.CD000459.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Albano S.M., Sofia Lima A., Graça Miguel M., Luis G., Pedro, José G., Barroso A. Cristina Figueiredo (2012) Antioxidant, Anti-5-lipoxygenase and Antiacetylcholinesterase Activities of Essential Oils and Decoction Waters of Some Aromatic Plants. Rec. Nat. Prod. 2012;6(1):35–48. [Google Scholar]
- 32.Brenner G.M. WB Saunders Company; Philadelphia: 2000. Pharmacology. [Google Scholar]
- 33.Dandlen S.A., Lima A.S., Mendes M.D., Miguel M.G., Faleiro M.L., Sousa M.J., Pedro L.G., Barroso J.G., Figueiredo A.C. Antimicrobial activity, cytotoxicity and intracellular growth inhibition of Portuguese Thymus essential oils. Braz. J. Pharmacogn. 2011;21(6):1012–1024. [Google Scholar]
- 34.DeKosky S.T., Harbaugh R.E., Schmitt F.A., Bakay R.A.E., Chui H.C., Knopman D.S., Reeder T.M., Shetter A.G., Senter H.J., Markesbery W.R. Cortical biopsy in Alzheimer's disease: diagnostic accuracy and neurochemical, neuropathological, and cognitive correlations. Ann. Neurol. 1992;32:625–632. doi: 10.1002/ana.410320505. [DOI] [PubMed] [Google Scholar]
- 35.Dohi S., Terasaki M., Makino M. Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J. Agric. Food. Chem. 2009;57:4313–4318. doi: 10.1021/jf804013j. [DOI] [PubMed] [Google Scholar]
- 36.Ellman G.L., Courtney K.D., Andres VJr, Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 37.Hisayama T., Takayanagi I. Increased 45Ca-efflux from smooth muscle microsomes by a rise in an extra microsomal Ca ion concentration, and the effect of thymol. J. Pharm. Pharmacol. 1983;35:532–533. doi: 10.1111/j.2042-7158.1983.tb04828.x. [DOI] [PubMed] [Google Scholar]
- 38.Melzer D. New drug treatment for Alzheimer's disease: lessons for healthcare policy. Brit. Med. J. 1998;316:762–764. doi: 10.1136/bmj.316.7133.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee P.K., Kumar V., Mal M., Houghton P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14(4):289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Murray A.P., Faraoni M.B., Castro M.J., Alza N.P., Cavallaro V. Natural AChE inhibitors from plants and their contribution to Alzheimer's disease therapy. Curr. Neuropharmacol. 2013;11:388–413. doi: 10.2174/1570159X11311040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh M.H., Houghton P.J., Whang W.K., Cho J.H. Screening of Korean herbal medicines used to improve cognitive function for anticholinesterase activity. Phytomedicine. 2004;11(6):544–548. doi: 10.1016/j.phymed.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Owokotomo I.A., Ekundayo O., Oladosu I.A., Aboaba S.A. Analysis of the Essential Oils of Leaves and Stems of Crassocephalum crepidioides Growing in South Western Nigeria. Int. J. Chem. 2012;4(2):34–37. [Google Scholar]
- 43.Owokotomo I.A., Ekundayo O., Dina O. Ocimum Gratissimum: the Brine Shrimps Lethality of a New Chemotype Grown in South Western Nigeria. Glob. J. Sci. Front. Res. Chem. 2012;12(6):44–49. [Google Scholar]
- 44.Politeo O., Irena Botica, Tea Bilusic, Mila Jukic, Ivana Carev, Franko Burcul, Mladen Milos Chemical composition and evaluation of acetylcholinesterase inhibition and antioxidant activity of essential oil from Dalmatian endemic species Pinus nigra Arnold ssp. dalmatica (Vis.) Franco. J. Med. Plant. Res. 2011;5(30):6590–6596. [Google Scholar]
- 45.Rahman A.U., Choudhary M.I. Bioactive natural products as a potential source of new pharmacophores a theory of memory. Pure Appl. Chem. 2001;73:555–560. [Google Scholar]
- 46.Schulz V. Ginkgo extract or cholinesterase inhibitors in patients with dementia: what clinical trial and guidelines fail to consider. Phytomedicine. 2003;10(Suppl. 4):74–79. doi: 10.1078/1433-187x-00302. [DOI] [PubMed] [Google Scholar]
- 47.Sims N.R., Bowen D.M., Allen S.J., Smith C.C., Neary D., Thomas D.J., Davison A.N. Presynaptic cholinergic degeneration in patients with dementia. J Neurochem. 1983;40:503–509. doi: 10.1111/j.1471-4159.1983.tb11311.x. [DOI] [PubMed] [Google Scholar]
- 48.Smail A., Badiâ Lyoussi, Maria G. Miguel (2011) Antioxidant and Antiacetylcholinesterase Activities of Some Commercial Essential Oils and Their Major Compounds. Molecules. 2011;16:7672–7690. doi: 10.3390/molecules16097672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wollen K.A. Alzheimer's disease: the pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Altern. Med. Rev. 2010;15(3):223–244. [PubMed] [Google Scholar]
- 50.Owokotomo I.A., Ekundayo O., Oladosun I.A., Aboaba S.A. Analysis of the essential oils of Crassocephallum crepidioides growing in South Western Nigeria. Int. J. Chem. 2011;3(4):34–37. [Google Scholar]
- 51.Owokotomo I.A., Ekundayo O. Comparative studies of the essential oils of Monodora myristica from Nigeria. Eur. Chem. Bull. 2012;1(6):263–265. [Google Scholar]
- 52.Owokotomo I.A., Ekundayo O., Dina O. Ocimum Gratissimum: the Brine Shrimps Lethality of a New Chemotype Grown in South Western Nigeria. Glob. J. Sci. Front. Res. Chem. 2012;12(6):45–48. [Google Scholar]
- 53.Owokotomo I.A., Ekundayo O., Oguntuase B.J. Chemical Constituents of the Leaf, Stem, Root and Seed Essential Oils of Aframomum melegueta (K. Schum) from South West Nigeria. Int. Res. J. Pure Appl. Chem. 2014;4(4):395–401. [Google Scholar]