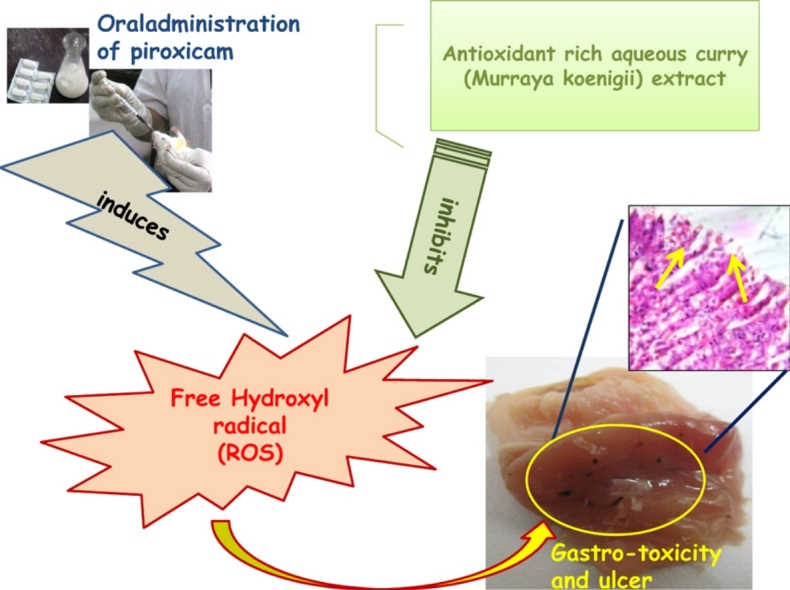
Graphical abstract
Keywords: Antioxidant, Curry leaves, Oxidative stress, Gastro-toxicity, Gastric ulcer, Piroxicam
Highlights
-
•
Piroxicam causes gastric ulceration through oxidative stress.
-
•
Curry leaf extract protects against piroxicam induced gastric injury.
-
•
Antioxidant mechanism(s) are involved in such protection.
-
•
The extract may have future therapeutic potential.
Abstract
Piroxicam (chemically 4-hydroxy-2-methyl-N-2-pyridinyl-2H-1,2-benzothiazine-3-carboxamide), a classical non-steroidal anti-inflammatory drug (NSAID) is orally administered to arthritic patients. Inhibition of prostaglandin E2 (PGE2) synthesis and subsequent free hydroxyl radical generation in vivo exert gastro-toxic side effects on piroxicam treatment. Leaves of curry plant are rich in antioxidants with prolific free radical scavenging activities. This led us to investigate the efficiency of the use of curry leaves in ameliorating piroxicam induced gastric damage. Piroxicam was orally (30 mg per kg body weight) administered in male albino Wistar rats to generate gastric ulcers. These rats were orally fed with graded doses of aqueous extract of curry or Murraya koenigii leaves (Cu LE) prior to piroxicam administration. Oxidative stress biomarkers, activities of antioxidant and pro-oxidant enzymes, mucin content and nature, PGE2 level, activities of mitochondrial enzymes and histomorphology of gastric tissues were studied. Piroxicam treatment altered all the above mentioned parameters whereas, curry leaf extract pre-treated animals were protected against piroxicam induced alterations. Hence, the protective action of the antioxidant rich Cu LE was investigated to propose a new combination therapy or dietary management to arthritic patients using piroxicam.
1. Introduction
Piroxicam, a classical NSAID, is a choice for most clinicians in arthritis and similar clinical conditions. The possible risk of gastro-toxic effects and ulceration of gastric mucosa imposed by this drug [1] has recently restricted its use. This drug induced gastric damage is possibly caused due to oxidative stress built up in vivo in the course of its metabolism or action. Earlier studies indicated gastro-protective PGE2 synthesis leads to decreased gastro-intestinal motility, reduced blood flow and ischaemia in the stomach [2]. Such changes generate free hydroxyl radical (•OH) in gastric tissues. Thus, the main causative factor behind piroxicam mediated gastro-toxicity and gastric ulceration has been recognized to be •OH [3].
Leaves of the curry plant (Murraya koenigii), well known for their culinary use, have been reported to be effective in many diseased conditions [4], [5]. Curry leaves may be medicinally useful for the treatment or prevention of diabetes, cancer and cardiovascular disease. Ingestion of curry leaves improved the plasma lipid profile in the rat feeding model. It also promoted both hypocholesterolemic effects and improved glycaemic status in obese mouse model [6]. There are reports suggesting that the leaves possess anti-oxidative and anti-lipid per-oxidative actions [7]. Thus, the leaves of the curry plant have the potential to provide protection against oxidative stress.
Association of high amount of •OH generation on oral administration of piroxicam has triggered the search for a nutritional component effective in •OH scavenging. Therefore, the remedy is sought to be located in the inclusion of antioxidants rich curry leaves in regular diet. In this study, we have in consequent phases determined the ulcer index, in vivo •OH titre, alterations oxidative stress biomarkers, alterations in activities of antioxidant and pro-oxidant enzymes and changes in nature and content of free gastric mucin. The present study investigates the efficacy of aqueous curry leaf extract in protecting piroxicam induced gastro-mucosal damage through anti-oxidative mechanisms.
2. Materials and methods
2.1. Chemicals
Piroxicam sold under the trade name Dolonex DT was purchased from the local chemist shop. All chemicals and solvents used in the present study were of analytical grade and procured from Sisco Research Laboratories (SRL), Mumbai, India; Qualigens (India/Germany); SD Fine Chemicals (India) and Merck Limited, Delhi, India.
2.2. Collection of curry leaves
Fresh green curry leaves were collected from different parts of the Burdwan district in West Bengal, India in between the months of August and November. The identity of the plant was confirmed by Mr. P. Venu, Scientist ‘F’, the Botanical Survey of India, Central National Herbarium (Government of India, Ministry of Environment and Forests), Botanic Garden, Howrah 711 103, West Bengal, India. The Herbarium of the plant was deposited in the BSI against voucher specimen No. CNH/I-I/42/2010/Tech.II/233.
2.2.1. Preparation of Cu LE
The leaves were separated, washed thoroughly in normal tap water and kept at room temperature in Borosil tray for 1 h with its bottom covered with a piece of blotting paper to soak any excess water. The leaves were then dried in a hot air oven at 35 °C for two days till the leaves were dry enough so that they could be crushed into a fine dust in a mechanical grinder and were stored in air tight Tarson bottles at normal room temperature.
For aqueous extract preparation, the dried leaf dust was soaked overnight in double distilled water (7.5 g per 100 ml), filtered through fine cotton cloth. The filtrate was centrifuged at 5000 rpm for 10 min (using a REMI cold-centrifuge). The supernatant, thus obtained, was filtered again through cotton cloth, collected in sterile polypropylene tubes and frozen at −20 °C. The contents of the tubes were then lyophilized and the resulting powdery material was then stored at −20 °C until further use. A definite amount of the lyophilized material was always freshly dissolved in double distilled water to give a particular concentration and an aliquot of this solution (not more than 0.5 ml) was fed to the rats with the help of a feeding needle. The yield of aqueous curry leaf extract (Cu LE) was 14.72 ± 0.36% (w/w).
2.3. Animals
Male Wistar rats of body weight 160–180 g were used throughout the experiments. The animals were handled as per the guidelines of institutional animal ethics committee (IAEC) of Department of Physiology, University of Calcutta in accordance with the committee for the purpose of control and supervision of experiment on animals (CPCSEA), Ministry of Environment and Forest, Government of India. All the experimental protocols had the approval (approved under proposal No. IAEC/PROPOSAL/DB-5/2010 dated 05/05/2010, approval date: 16/11/2011) of Institutional Animal Ethics Committee (IAEC) of the Department of Physiology, University of Calcutta. Prof. P.K. Samanta, M.Sc. (Vet.), Ph.D., Professor and Veterinary Surgeon and CPCSEA Nominee to Department of Physiology, University of Calcutta, acted as the advisor for animal care and handling.
2.4. Treatment and collection of tissue samples
For our present study, the animals were housed in galvanized wire cages, in well ventilated, air conditioned rooms of our animal house with 12 h light/dark cycle, at about 18 °C room temperature for 7 days to get adapted to laboratory condition. All rats had been given a standard diet containing 18% protein, 71% carbohydrate and vitamins which are considered to be an adequate (normal) dietary protein level [8].
The animals were released from quarantine and immediately they were kept on fasting condition in specially designed cages for the following 40 h. After that treatment of rats was carried out as per the schedule mentioned below.
The animals were divided into six groups as follows for the dose response study:
GROUP I: Control group (C). Rats were allowed to drink water supplied ad libitum.
GROUP II: Piroxicam treated group (Px). Rats were orally administered piroxicam at a dose of 30 mg/kg body weight dose with a feeding needle. The treatment was carried out immediately after 40 h fasting.
GROUP III: Cu LE pre-treated at a dose of 50 mg/kg body weight and piroxicam fed group (Cu LE1). Cu LE was administered at 50 mg/kg body weight at the onset of the experiment and immediately after 1 h, the animals were orally fed piroxicam at 30 mg/kg body weight.
GROUP IV: Cu LE pre-treated at a dose of 100 mg/kg body weight and piroxicam fed group (Cu LE2). Curry leaf aqueous extract was administered at 100 mg/kg bodyweight at the onset of the experiments and immediately after 1 h, the animals were orally fed piroxicam at 30 mg/kg body weight.
GROUP V: Cu LE pre-treated at a dose of 200 mg/kg body weight and piroxicam fed group (Cu LE3). Cu LE was administered at 200 mg/kg bodyweight at the onset of the experiments and immediately after 1 h, the animals were orally fed piroxicam at 30 mg/kg body weight.
GROUP VI: Cu LE pre-treated at a dose of 300 mg/kg body weight and piroxicam fed group (Cu LE4). Cu LE was administered at 300 mg/kg bodyweight at the onset of the experiments and immediately after 1 h, the animals were orally fed piroxicam at 30 mg/kg body weight.
In another separate set of experiment, animals were divided into the following four groups to ascertain the mechanism underlying Cu LE mediated protection against piroxicam induced gastric ulcer:
GROUP I: Control group (C). Rats were allowed to drink water supplied ad libitum.
GROUP II: Cu LE treated group (Cu LE200). Rats were orally administered Cu LE at 200 mg/kg body weight.
GROUP III: Piroxicam treated group (Px). Rats were orally administered piroxicam at dose of 30 mg/kg body weight. The treatment was carried out immediately after 40 h fasting.
GROUP IV: Cu LE pre-treated at 200 mg/kg body piroxicam fed group (Cu LE200 + Px). Cu LE was administered at 200 mg/kg bodyweight at the onset of the experiments and immediately after 1 h, the animals were orally fed piroxicam at 30 mg/kg body weight.
Each group of animals comprised of 6 rats. At the end of treatment, all the animals were allowed to drink water and kept undisturbed for four hours. The animals were sacrificed by cervical dislocation following light ether anaesthesia. The abdomen of each rat was surgically opened to collect the stomach for macroscopic observations, histological studies and biochemical estimations. The stomach tissue was kept in sterile plastic vial at −20 °C until further biochemical analysis. For histological studies, an appropriate portion of the fundic part of the stomach was placed immediately in formalin fixative. Prior to sacrifice blood was collected through cardiac puncture for determination of PG E2 in serum. Each set of experiment was repeated at least three times.
A separate set of experiment was carried out to determine the degree of inhibition of free hydroxyl radical generation in vivo with oral administration of Cu LE at a dose of 200 mg/kg body weight.
2.5. Determination of ulcer index
Stomach was flushed with saline and lesions in glandular portion were then exposed and examined under a magnifying glass. The grade of lesions was scored according to the following scale: 0, no pathology; 1, small 1–2 mm ulcers; 2, medium 3–4-mm ulcers; 4, large 5–6-mm ulcers; 8, ulcers greater than 6 mm. The sum of the total ulcer scores in each group of rats was divided by the number of animals in the group to give the mean ulcer index for that group [9].
2.6. Biochemical estimations
2.6.1. Determination of free mucin content
The free mucin content in the gastric tissues was estimated by measuring the amount of alcian blue bound to mucus [10]. The glandular stomach tissues were incubated with a 1% buffered sucrose solution of alcian blue in (0.1%) sodium acetate at 37 °C for 60 min. After incubation, the tissues were washed with sucrose and centrifuged. The supernatant was extracted with MgCl2, and the amount of alcian blue was estimated spectrophotometrically at 610 nm. The quantity (μg) of alcian blue/g of wet glandular tissue was then calculated.
2.6.2. Measurement of lipid peroxidation (LPO), protein carbonyl content (PCO), reduced glutathione (GSH) level and total sulfhydryl group (TSH) content
A portion of the fundic stomach was homogenized (5%) in the ice-cold 0.9% saline (pH 7.0) with a Potter–Elvehjem glass homogenizer for 30 s. The homogenate was centrifuged at 800 × g for 10 min and the supernatant was again centrifuged at 12,000 × g for 15 min in a RC-5B refrigerated Sorvall centrifuge to obtain the mitochondrial fraction. Lipid peroxides of this fraction were determined as thiobarbituric acid reactive substances (TBARS). Tetraethoxypropane (TEP) was used as standard [11].
Protein carbonyl content, an index of metal-catalyzed oxidative damage, was determined according to Levine et al., using 0.8 ml of the cell free (500 g) homogenate (10%) in 50 mM sodium phosphate buffer, pH 7.4 [12].
The GSH content (as acid-soluble sulfhydryl) was estimated by its reaction with DTNB (Ellman's reagent). After centrifugation of the 10% homogenate in 20 mM ice-cold ethylenediaminetetraacetic acid (EDTA) for 15 min at 12,000 × g, 1 ml aliquot of the supernatant was used to measure the GSH content [13].
2.6.3. Determination of gastric peroxidase (GPO), superoxide dismutase (SOD) and catalase (CAT) activities
A portion of the fundic stomach was homogenized (10% homogenate) in 0.25 M sucrose and 50 mM phosphate buffer (pH 7.2) and the mitochondrial fraction was prepared as described above. The GPO activity in this fraction was measured using iodide as an electron donor. The assay system contained in a final volume of 1 ml: 50 mM sodium acetate buffer pH 5.2, 1.7 mM KI, a suitable volume of enzyme, and 0.27 mM H2O2 added last to start the reaction. The enzyme activity was expressed as units/mg protein [14].
Superoxide dismutase activity of the mitochondrial fraction (Mn-type) and the post mitochondrial supernatant (Cu–Zn type) were measured by xanthine oxidase cytochrome c method [15] and haemotoxylin auto-oxidation method [16] respectively. In brief, for Mn-SOD a portion of the fundic stomach was homogenized (10%) in ice-cold 50 mM phosphate buffer, pH 7.8. The homogenate was then centrifuged at 500 × g for 10 min and the supernatant thus obtained was again centrifuged at 12,000 × g for 15 min to obtain the mitochondrial fraction. The mitochondrial pellet was suspended in buffer and used for the enzyme assay using a UV/VIS spectrophotometer at 550 nm with an O2•− generating system (xanthine/xanthine oxidase) in the presence of cytochrome c. The enzyme activity was expressed as Units/mg tissue protein.
To determine Cu–Zn SOD activity, a portion from the fundic stomach was homogenized (10%) in ice-cold 50 mM phosphate buffer containing 0.1 mM EDTA, pH 7.4. The homogenate was centrifuged at 12,000 × g for 15 min and supernatant collected. Inhibition of haematoxylin auto-oxidation by the cell free supernatant was measured at 560 nm using a UV-VIS spectrophotometer. The enzyme activity was expressed as units/min/mg of tissue protein.
Catalase was assayed by the method of [17]. A weighed amount of gastric tissue was homogenized (5%) in ice-cold 50 mM phosphate buffer, pH 7.0. The homogenate was centrifuged in cold at 12,000 × g for 12 min. The supernatant, thus obtained, was then collected and incubated with 0.01 ml of absolute ethanol at 4 °C for 30 min, after which 10% Triton X-100 was added so as to have a final concentration of 1%. The sample, thus obtained, was used to determine catalase activity by measuring the breakdown of H2O2 spectrophotometrically at 240 nm. The enzyme activity was expressed as μmol of H2O2 consumed/min/mg tissue protein.
2.6.4. Determination of the activities of glutathione reductase (GR), glutathione peroxidase (GPx) and glutathione-S-transferase (GST)
The activity of GR was determined according to the following method [18]. The assay mixture in a final volume of 3 ml contained 50 mM phosphate buffer, 200 mM KCl, 1 mM EDTA and water. The blank was set with this mixture. Then, 0.1 mM NADPH was added with suitable amount of homogenate (enzyme) into the cuvette. The reaction was initiated with 1 mM oxidized glutathione (GSSG). The decrease in NADPH absorption was monitored spectrophotometrically at 340 nm. The specific activity of the enzyme was calculated as units/min/mg tissue protein.
The GPx activity was measured according to the method of [19] with some modifications [20]. A weighed amount of gastric tissue was homogenized (10%) in ice-cold 50 mM phosphate buffer containing 2 mM EDTA, pH 7.0. The assay system in a final volume of 1 ml contained 0.05 M phosphate buffer with 2 mM EDTA, pH 7.0, 0.025 mM sodium azide, 0.15 mM glutathione, and 0.25 mM NADPH. The reaction was started by the addition of 0.36 mM H2O2. The linear decrease of absorbance at 340 nm was recorded using a UV/VIS spectrophotometer. The specific activity of the enzyme was expressed as nmol of NADPH produced/min/mg tissue protein.
The GST activity of the rat gastric tissue was measured spectrophotometrically according to the method as described by Habig et al. [21]. The enzymatic reaction was measured by observing the conjugation of 1-chloro,2,4-dinitrobenzene (CDNB) with reduced glutathione (GSH). One unit of enzyme conjugates 10.0 nmol of CDNB with reduced glutathione per minute at 25 °C. The rate where the reaction was linear was noted at 340 nm. The molar extinction of CDNB is 0.0096 μM−1/cm. The enzyme activity was expressed as units/min/mg of tissue protein.
2.6.5. Determination of tissue level of endogenous free hydroxyl radical (•OH) generation
The •OH generated in the stomach was measured using DMSO as •OH scavenger [22]. DMSO forms a stable product [methanesulfonic acid (MSA)] on reaction with fast blue BB salt. Four groups of rats containing six animals per group were used for each experiment. The first group served as control and the animals were injected (i.p.) with 0.4 ml of 25% DMSO in saline per 100 g body weight. The second group served as Cu LE administered group and the animals were injected DMSO in the earlier mentioned dose 30 min before oral administration of Cu LE at a dose of 200 mg/kg body weight. The third group was injected DMSO in the above mentioned dose exactly 30 min before feeding piroxicam only at 30 mg/kg body weight. The animals of the fourth group were injected DMSO and in an interval of 30 min and 1 h were orally administered Cu LE (at a dose of 200 mg/kg body weight) and piroxicam (at a dose of 30 mg/kg body weight) consecutively. They were kept at room temperature without any stress after the administration of DMSO. The stomach of each animal was processed for MSA, which was allowed to react with the fast blue BB salt to yield a yellow product. This was measured spectrophotometrically at 425 nm using benzenesulfonic acid as standard. Values obtained were expressed as nmol of •OH generated per g of stomach.
2.6.6. Indirect assessment of in vivo generation of superoxide anion free radical by determining the activities of the enzymes xanthine oxidase (XO) and xanthine dehydrogenase (XDH)
XO activity of the rat gastric tissue was assayed by measuring the conversion of xanthine to uric acid [23]. Briefly, the weighed amount of gastric tissue was homogenized in cold (10%) in 50 mM phosphate buffer, pH 7.8. The homogenates were centrifuged at 500 × g for 10 min. The resulting supernatant was further centrifuged at 12,000 × g for 20 min in cold. The supernatant, thus obtained, was collected and used for spectrophotometric assay of the enzyme at 295 nm using 0.1 mM xanthine in 50 mM phosphate buffer, pH 7.8, as the substrate. The enzyme activity was expressed as milli units/min/mg tissue protein.
The activity of XDH was measured by following the reduction of NAD+ to NADH according to the method of [24]. In brief, the weighed amount of rat gastric tissue was homogenized in cold (10%) in 50 mM phosphate buffer with 1 mM EDTA, pH 7.2. The homogenates were centrifuged in cold at 500 × g for 10 min. The supernatant, thus obtained, was further centrifuged in cold at 12,000 × g for 20 min. The final supernatant was used as the source of the enzyme, and the activity of the enzyme was measured spectrophotometrically at 340 nm with 0.3 mM xanthine as the substrate (in 50 mM phosphate buffer, pH 7.5) and 0.7 mM NAD+ as an electron donor. The enzyme activity was expressed as milli units/min/mg tissue protein.
2.6.7. Determination of the activities of pyruvate dehydrogenase (PDH) and Kreb's cycle enzymes
The weighed amount of rat gastric tissue was homogenized (10%) in ice-cold 50 mM phosphate buffer, pH 7.4 with a Potter Elvenjem glass homogenizer (Belco Glass Inc., Vineland, NJ, USA) for 30 s. The homogenates were then centrifuged at 500 × g for 10 min. The supernatant, thus obtained, was again centrifuged at 12,000 × g for 15 min to obtain a pellet containing mitochondria. This pellet was again suspended in the buffer and used for measuring the activities of the mitochondrial enzymes. The PDH activity was measured spectrophotometrically [25] with some modifications by following the reduction of NAD+ to NADH at 340 nm using 50 mM phosphate buffer, pH 7.4, 0.5 mM sodium pyruvate as the substrate and 0.5 mM NAD+ in addition to the enzyme. The enzyme activity was expressed as units/min/mg tissue protein.
Isocitrate dehydrogenase (ICDH) activity was determined by measuring the reduction of NAD+ to NADH at 340 nm with the help of a UV-VIS spectrophotometer [26]. One millilitre assay volume contained 50 mM phosphate buffer, pH 7.4, 0.5 mM isocitrate, 0.1 mM MnSO4, 0.1 mM NAD+ and the suitable amount of enzyme. The enzyme activity was expressed as units/min/mg tissue protein.
Alpha-ketoglutarate dehydrogenase (α-KGDH) activity was measured spectrophotometrically [26] by determining the reduction of 0.35 mM NAD+ to NADH at 340 nm using 50 mM phosphate buffer, pH 7.4 as the assay buffer and 0.1 mM alpha-ketoglutarate as the substrate. The enzyme activity was expressed as units/min/mg tissue protein.
Succinate dehydrogenase (SDH) activity was measured spectrophotometrically by following the reduction of potassium ferricyanide (K3FeCN6) at 420 nm [27] with some modifications. One millilitre assay mixture contained 50 mM phosphate buffer, pH 7.4, 2% (w/v) BSA, 4 mM succinate, 2.5 mM K3FeCN6 and a suitable aliquot of the enzyme. The enzyme activity was expressed as units/min/mg tissue protein.
2.6.8. Determination of the activities of some of the mitochondrial respiratory chain enzymes
NADH-cytochrome c oxidoreductase activity was measured spectrophotometrically by following the reduction of oxidized cytochrome c at 565 nm [28]. One millilitre of assay mixture contained in addition to the enzyme, 50 mM phosphate buffer, 0.1 mg BSA, 20 mM oxidized cytochrome c and 0.5 mM NADH. The activity of the enzyme was expressed as units/min/mg tissue protein.
The cytochrome c oxidase activity was determined spectrophotometrically by following the oxidation of reduced cytochrome c at 550 nm according to the method of [28]. One millilitre of assay mixture contained 50 mM phosphate buffer, pH 7.4, 40 mM reduced cytochrome c and a suitable aliquot of the enzyme. The enzyme activity was expressed as units/min/mg tissue protein.
2.6.9. Estimation of protein
The protein content of different samples was determined following the method of [29].
2.6.10. Prostaglandin E2 (PGE2) assay
100 mg of wet glandular gastric tissue was weighed and homogenized in 10 mM sodium phosphate buffer, pH 7.4 (1 ml). After centrifugation (9000 × g), PGE2 was measured in the supernatant by ELISA and in sera in similar way [30]. The values were expressed as pg/ml for serum and pg/100 mg gastric tissue for stomach PGE2 titre.
2.6.11. Histological studies
2.6.11.1. Studies using tissue sections stained with haematoxylin and eosin, per-iodo acid Schiff (PAS) and alcian blue
A portion from the fundic part of rat stomach was spread out on a wooden block, attached and fixed in formalin. Later an ulcerated part was separated out with the help of a surgical blade. The part of the stomach dissected out was embedded in paraffin following routine procedure and 5 μm thick sections were stained separately with haematoxylin–eosin, per-iodo-acid Schiff (PAS) reagent and Sirius red (Direct red 80; Sigma Chemical Co., St. Louis, MO, USA) respectively by a routine procedure [31].
Alcian blue dye staining was performed following another routine procedure [32]. Dewaxed tissue sections were brought to water medium and placed in alcian blue dye solution of pH 2.5 (prepared by dissolving 1 g alcian blue in 100 ml 3% acetic acid solution) for 5 min. The sections were washed in water to remove excess stain and counterstained with 0.5% neutral red stain for 2–3 min. Further washing with water and rinsing in absolute alcohol was carried out and the sections were mounted to observe under microscope.
2.6.11.2. Quantification of total collagen content by confocal microscopy
The rat gastric tissue sections (5 μm thick) were stained with Sirius red (Direct Red 80; Sigma Chemical Co., St. Louis, MO, USA) [33] and imaged with a laser scanning confocal system (Zeiss LSM 510 META, Germany) and the stacked images through multiple slices were captured. Four slides were prepared for each rat from each group and only the representative images are presented. The digitized images were then analyzed using image analysis system (ImageJ, NIH Software, Bethesda, MI) and the total collagen area fraction of each image was measured and expressed as the % collagen volume.
2.6.11.3. Quantification of pro-MMP 9 activity by gelatin zymography
The fundic part of the gastric mucosa was suspended in phosphate-buffered saline containing protease inhibitors, minced, and incubated for 10 min at 4 °C. It was centrifuged at 10,000 rpm for 10 min. The pellet was extracted in the lysis buffer (10 mM Tris–HCl pH 8.0, 150 mM NaCl, 1% Triton X-100 plus protease inhibitors) and centrifuged at 10,000 rpm for 10 min. Tissue extracts were preserved at −20 °C and used in future studies. For determination of pro-MMP-9 activity, mucosal extracts were electrophoresed in SDS-polyacrylamide gel containing 1 mg/ml gelatin under non-reducing conditions. To determine exactly the band of pro-MMP 9, rat uterine sample was loaded as a marker which has rich store of pro-MMP 9. The gels were washed in 2.5% Triton-X-100 and incubated in digestion buffer (40 mM Tris–HCl, pH 7.4, 0.2 M NaCl, 10 mM CaCl2) overnight at 20 °C and stained with 0.1% Coomassie Blue followed by destaining. The zones of gelatinolytic activity came as negative staining. Enzymatic activity was determined by measuring the area produced by each band at 92 kDa region with the help of Image J software. This procedure was adopted from [34] with slight modifications.
2.6.12. Quantitative determination of phytoconstituents of Cu LE
The methods of [35] were used to determine the total phenols and flavonoid content of the extract. Total phenols were expressed as mg gallic acid equivalents (GAE/g extract) where gallic acid was used as standard. Flavonoid content was expressed as mg catechin equivalents (CE/g extract) where catechin was used as standard. Alkaloid contents were estimated [36] and expressed as mg/g bismuth nitrate. Total tannins were determined according to the method of [37] and expressed as tannic acid equivalent (TAE/g extract).
2.6.13. Analysis of Cu LE through GC–MS
GC–MS analysis [38] was carried out using Agilent Technologies 6890 N Network GC system & interfaced to Agilent Technologies 5973 Inert Mass Selective Detector (MSD) employing the following conditions: column DB-1 ms fused silica capillary column (30 × 0.25 I.D. × 0.10 film, composed of 100% dimethylpolysiloxane) (chosen for improved signal to noise ratio for better sensitivity and mass spectral integrity), operating in electron impact mode; helium (5.0) was used as carrier gas at a constant flow of 1 ml/min. The injector, MS Source & MS quadrapole temperature was fixed at 250 °C, 230 °C and 150 °C, respectively, and turbo speed of the pump was 100%. The oven temperature was programmed from 50 °C (isothermal for 2 min), with an increase of 10 °C/min to 100 °C (isothermal for 5 min), then 10 °C/min to 300 °C (isothermal for 5 min). For tuning of the MSD in EI mode perfluorotributylamine (PFTBA) was used as tuning compound. Mass spectra were taken at 2235 EMvolts and fragments from 40 to 550.
Interpretation of the mass spectrum of GC–MS was conducted using the database of National Institute Standard and Technology (NIST) which consists of more than 62,000 patterns. The spectrum of the unknown component was compared with the spectrum of the known component inherent in the NIST library. The name, molecular weight and structure of the components of the test materials were ascertained.
2.7. Statistical evaluation
Data are represented by mean ± S.E.M. Significance of mean values of different parameters between the treated groups were analyzed using one way analysis of variances (ANOVA) after ascertaining the homogeneity of variances between the treatments. Paired comparisons were done by calculating the least significance. Statistical tests were performed using Microcal Origin 7.0 for Windows.
Each experiment was repeated at least three times with different rats.
3. Results
3.1. Dose standardization of aqueous curry leaf extract
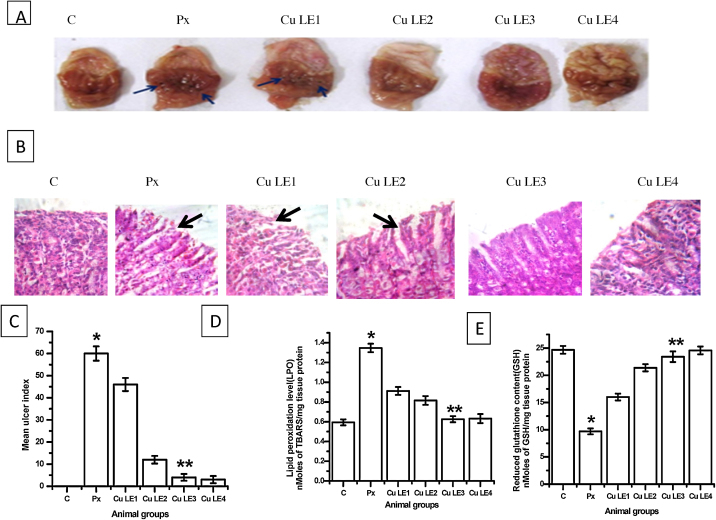
Fig. 1 shows that aqueous curry leaf extract provides protection to the gastric mucosa against piroxicam induced damage in a dose-dependent manner. Aqueous curry leaf extract pre-administered at 100 mg/kg body weight dose reduced ulcer index by 86.7% against piroxicam fed animal group (**P ≤ 0.001), but almost complete protection was rendered when 200 mg/kg BW and 300 mg/kg BW doses were administered. This is clearly indicating that the extract at 200 mg/kg BW dose is sufficient to provide protection against piroxicam induced gastric ulceration in rats.
Fig. 1.
Dose response studies with aqueous curry leaf extract. The rats were fed different doses of Cu LE (ranging from 50 to 300 mg/kg body weight) 1 h before oral administration of piroxicam at 30 mg/kg body weight. (A) Macroscopic view of rat gastric mucosal surface, blue arrow heads are pointing towards pin head ulcer spots. (B) Representative images of H&E stained gastric sections at magnification 400×, black arrow heads are pointing towards regions of mucosal erosion. (C) Bar graphs representing the changes in ulcer index. (D) Representative figure for changes in lipid peroxidation level. (E) Representative figure for changes in reduced glutathione content. All values in (C)–(E) are expressed as mean ± S.E.M. where *P ≤ 0.001 vs. control, **P ≤ 0.001 vs. piroxicam treated animals (one-way ANOVA followed by Scheffe’ multiple comparison test).
Parts A and B of Fig. 1 are representative photographs of macroscopic and microscopic changes in the rat stomach clearly indicating ulcerative damages on feeding rats with piroxicam at 30 mg/kg BW dose orally. Haematoxylin–eosin stained sections reveal that mucosal bleeding occurred on piroxicam feeding, which was protected when graded doses of the aqueous extract was administered before piroxicam feeding. Photographs of the inner surface of stomach show no ulcer spots in the rats fed 200 mg/kg BW and 300 mg/kg BW doses of the aqueous extract.
Biomarkers of oxidative stress altered in piroxicam induced gastro-toxicity. Lipid peroxidation level and reduced glutathione content were also protected in a dose dependent manner by aqueous curry leaf extract. In Fig. 1D and E curry leaf extract shows reduction in lipid peroxidation level by 53.7% (**P ≤ 0.001 vs. piroxicam fed group) and 1.4 fold increase (**P ≤ 0.001 vs. piroxicam fed group) in reduced glutathione content on administration of 200 mg/kg BW dose prior to oral administration of 30 mg/kg body weight dose of piroxicam.
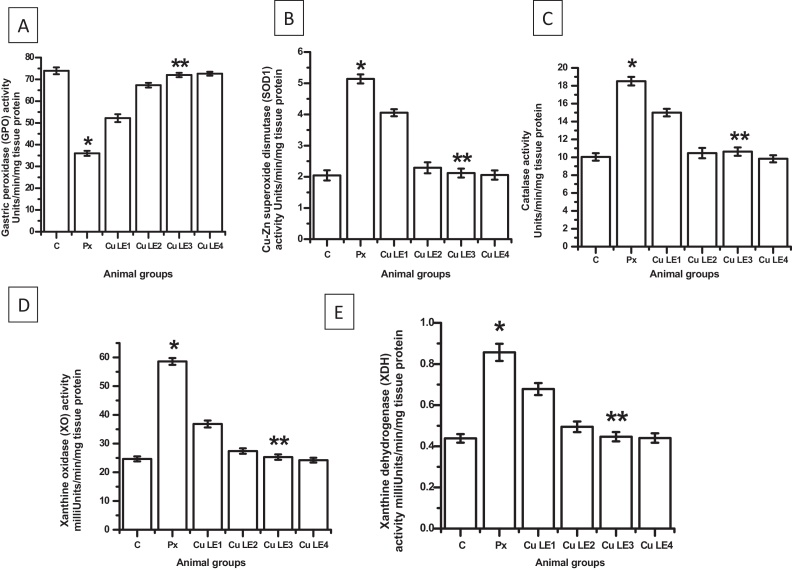
Key gastric antioxidant enzymes viz. gastric peroxidase, Cu–Zn superoxide dismutase and catalase altered in piroxicam fed animals and were near control levels in animal groups fed with aqueous curry leaf extract at doses as high as 200 mg/kg body weight and 300 mg/kg body weight dose prior to piroxicam administration. Fig. 2A–C clearly indicate alterations and protection of the antioxidant enzyme activities in piroxicam treatment and pre-treatment of graded doses of aqueous curry leaf extract in piroxicam-fed animals respectively. Fig. 2D and E showing increased activities of xanthine oxidase and xanthine dehydrogenase in piroxicam fed group indicate increased free superoxide anion radical generation in vivo on piroxicam feeding. Aqueous curry leaf extract at 200 mg/kg body weight dose maximally prevented such free radical generation by keeping the activities of the enzymes near control.
Fig. 2.
Dose dependent effects of aqueous curry leaf extract on activities of antioxidant (A–C) and pro-oxidant enzymes (D and E) in rat stomach. The rats were fed different doses of Cu LE (ranging from 50 to 300 mg/kg body weight) 1 h before oral administration of piroxicam at 30 mg/kg body weight. (A) The gastric peroxidase activity. (B) The Cu–Zn superoxide dismutase (SOD1) activity. (D) The catalase activity. (D) The xanthine oxidase activity. (E) The xanthine dehydrogenase activity. Bar graphs represent the values of activity levels of different enzymes and all the values are expressed as mean ± S.E.M. where *P ≤ 0.001 vs. control, **P ≤ 0.001 vs. piroxicam treated animals (one-way ANOVA followed by Scheffe’ multiple comparison test).
Repeating dose response studies thrice, it was concluded that 200 mg/kg body weight dose of the aqueous curry leaf extract administration one hour before piroxicam treatment can provide maximum protection and yield satisfactory results in piroxicam induced oxidative stress mediated toxicity and ulcerative damages. In the subsequent sections, results obtained with this selected 200 mg/kg BW (Cu LE) dose have been elaborated.
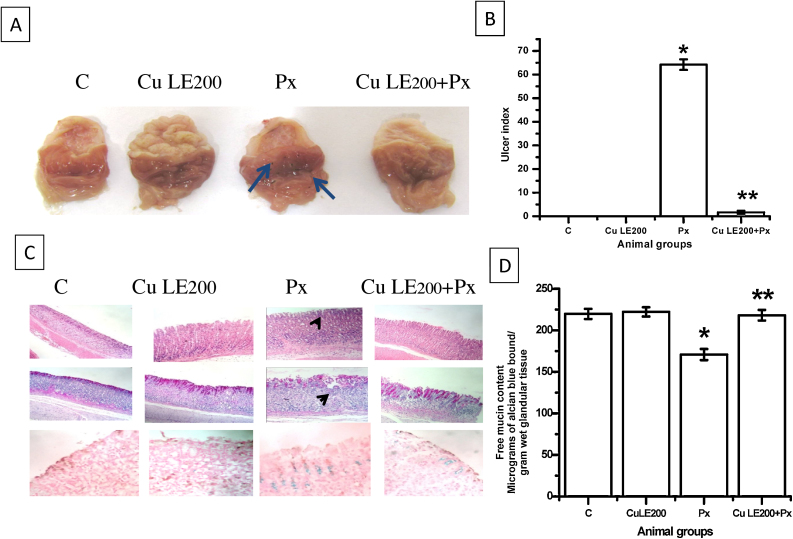
3.2. Anti-ulcer activity of Cu LE
Fig. 3A and B shows the haemorrhagic ulcers of the stomach mucosa and ulcer index determined respectively to ascertain the anti-ulcerative action of the selected dose of Cu LE. Macroscopic study clearly shows that there are no ulcer spots and the ulcer index has been reduced to a minimum of 1.67 ± 0.69 (**P ≤ 0.001 vs. piroxicam fed group) in 200 mg/kg body weight Cu LE pre-administered piroxicam-fed group.
Fig. 3.
Protective effective of 200 mg/kg body weight dose of aqueous curry leaf extract in piroxicam-fed rat stomach. (A) Macroscopic view of rat gastric mucosal surface, blue arrow heads are pointing towards pin head ulcer spots. (B) The bar graph representing changes in ulcer index values. (C) Upper panel showing photomicrographs of H&E stained gastric sections, middle panel consisting of PAS stained sections and lower panel represents alcian blue dye stained sections; captured as viewed under inverted phase contrast microscope at magnification 200×, black arrow heads are pointing towards regions of mucosal erosion. (D) Bar graph showing changes in free neutral mucin content. All values in (B) and (D) are expressed as mean ± S.E.M. where *P ≤ 0.001 vs. control, **P ≤ 0.001 vs. piroxicam treated animals (one-way ANOVA followed by Scheffe’ multiple comparison test).
Microscopic changes were studied using haematoxylin and eosin (H&E) staining, PAS staining and alcian blue dye staining and photomicrographs are represented in Fig. 3C. H&E stained gastric tissue sections of control group rats and only Cu LE treated group showed no prominent blood vessels in the mucosa and submucosa. Treatment of rats with oral administration of piroxicam with a dose of 30 mg/kg body weight resulted in marked changes in gastric tissue morphology. The mucosa of the gastro-oesophageal junction had few eosinophilic infiltration but submucosa showed to have both neutrophilic and eosinophilic infiltration in piroxicam treated animal group. Gastric tissue sections stained with H&E of Cu LE pre-treated animals showed no vascular congestion or specific cellular infiltration, thereby indicating protective effect of the extract against piroxicam induced ulcerative damage in rats. PAS stained gastric tissue sections of the control and only Cu LE treated animals showed a uniformly pink stained gastric mucosa. Tissue sections of piroxicam treated animals were discontinuously stained pink along the mucosal border due to degeneration and sloughing of mucosal cells. The uniformity in mucosal border staining of gastric tissue sections of Cu LE pre-treated animal group indicates a protective effect of the extract against piroxicam induced tissue damage.
Alcian blue (ACB) dye preferentially binds acidic mucin. Gastric tissue sections of control animals showed the blue dye binding intensity to be less when compared with sections from other animal groups. When ‘+1’ score is assigned to the control tissue sections, ACB stained piroxicam treated animal tissue sections scored ‘+3’. Such observation revealed a pathological change in mucin secretion type on piroxicam treatment. The histopathological finding clearly indicates that piroxicam treatment increases acid mucin secretion in otherwise neutral mucin secreting normal gastric tissue. Reduction in ACB staining intensity in tissue sections from only Cu LE treated group indicates that mucin secretion pattern did not alter significantly on pre-treatment with the extract. The free neutral mucin content depletes appreciably and the nature of secreted mucin turns acidic on ulcerated stomach. Fig. 3D shows that piroxicam also mediates its ulcerative damage through reduction in mucin level by 22.3% (*P ≤ 0.001 vs. control). Cu LE pre-treated piroxicam fed animals had no such reduction in mucin content clearly indicating protection from ulcerative damage rendered by the pre-feeding of the extract.
3.3. Effect of Cu LE on biomarkers of oxidative stress
Biomarkers of oxidative stress include lipid peroxidation level, protein carbonyl content, reduced glutathione (GSH), non enzymatic total sulfhydryl group content (TSH), oxidized glutathione (GSSG) content and GSH–GSSG ratio. Table 1 represents the changes in biomarkers of oxidative stress on piroxicam treatment and protection rendered on pre-treatment of rats with Cu LE at a dose of 200 mg/kg body weight. Lipid peroxidation level and protein carbonyl content increased in piroxicam treated rats by 2.16 folds and 5.57 folds respectively, compared to values obtained (P ≤ 0.001 vs. control) in control rats. Levels of TSH and GSH decreased significantly in piroxicam fed rats by 59.17% and 59.63% respectively from control rats (*P ≤ 0.001 vs. control in each case). GSSG content increased by 51.16% and the ratio (GSSG:GSH) increased by 4.3 folds from control in piroxicam treated rats (*P ≤ 0.001 vs. control in each case). Values in Table 1 clearly indicate that no biomarkers altered on feeding rats with only aqueous extract of curry leaves at 200 mg/kg BW dose. Table 1 further indicates that altered biomarkers were restored to control values when rats were pre-treated with aqueous curry leaf extract at 200 mg/kg BW dose before feeding piroxicam at 30 mg/kg BW dose.
Table 1.
Level of lipid peroxidation, protein carbonyl, total sulfhydryl, reduced glutathione and oxidized glutathione content in rat stomach.
| Parameters | Animal groups (Values expressed as mean ± S.E.M.) |
|||
|---|---|---|---|---|
| Control (C) | Aqueous curry leaf extract treated (Cu LE200) | Piroxicam treated (Px) | Aqueous curry leaf extract pre-treated + piroxicam treated (CuLE200 + Px) | |
| LPO (nmoles TBARS/mg protein) | 0.61 ± 0.03 | 0.57 ± 0.02 | 1.32 ± 0.06a | 0.65 ± 0.02b |
| PCO (nmoles carbonyl/mg protein) | 2.65 ± 0.29 | 2.61 ± 0.29 | 14.7 ± 0.31a | 2.76 ± 0.26b |
| Total sulfhydryl content (nmol TSH/mg of tissue protein) | 44.3 ± 0.99 | 44.5 ± 0.98 | 18.1 ± 0.56a | 45.1 ± 0.67b |
| Reduced glutathione content (nmol of GSH/mg of tissue protein) | 24.1 ± 0.88 | 24.3 ± 1.06 | 8.96 ± 0.34a | 22.1 ± 0.67b |
| Oxidized glutathione content (nmol of GSSG/mg of tissue protein) | 0.39 ± 0.02 | 0.41 ± 0.01 | 0.98 ± 0.06a | 0.41 ± 0.03b |
P ≤ 0.001 compared to control values using ANOVA.
P ≤ 0.001 compared to piroxicam treated values using ANOVA.
3.4. Protective effect of Cu LE on gastric antioxidant enzymes
Table 2 depicts the alterations in activities of different gastric antioxidant enzymes on piroxicam administration. Piroxicam feeding inhibits activities of key gastric antioxidant enzyme called gastric peroxidase and glutathione-S-transferase. Increased activities of gastric glutathione reductase, glutathione peroxidase, superoxide dismutases (Cu–Zn SOD and Mn SOD) and catalase are also observed on piroxicam feeding. Pre-treatment of piroxicam fed rats with Cu LE protected the activity of these antioxidant enzymes from being altered.
Table 2.
Alterations in the activities of key antioxidant enzymes in rat gastric tissue.
| Antioxidant enzymes (units of activity) | Animal groups (values of different antioxidant enzymes’ activities expressed as mean ± S.E.M) |
|||
|---|---|---|---|---|
| Control (C) | Aqueous curry leaf extract treated (Cu LE200) | Piroxicam treated (Px) | Aqueous curry leaf extract pre-treated + piroxicam treated (Cu LE200 + Px) | |
| Gastric peroxidase (units/min/mg of tissue protein) | 74.41 ± 1.71 | 74.98 ± 1.33 | 36.32 ± 1.11a | 64.55 ± 1.21b |
| Glutathione peroxidase (nmol of NADPH produced/min/mg of tissue protein) | 0.28 ± 0.01 | 0.27 ± 0.01 | 0.65 ± 0.02a | 0.31 ± 0.01b |
| Catalase (μmol of H2O2 consumed/min/mg of tissue protein) | 10.52 ± 0.52 | 10.57 ± 0.62 | 18.75 ± 0.52a | 11.23 ± 0.69b |
| Cu–Zn superoxide dismutase (units/min/mg of tissue protein) | 2.16 ± 0.21 | 2.05 ± 0.24 | 5.67 ± 0.24a | 2.44 ± 0.27b |
| Mn superoxide dismutase (units/min/mg of tissue protein) | 1.32 ± 0.12 | 1.37 ± 0.08 | 3.19 ± 0.21a | 1.56 ± 0.19b |
| Glutathione-S-transferase activity (units/min/mg of tissue protein) | 0.72 ± 0.02 | 0.72 ± 0.03 | 0.35 ± 0.03a | 0.63 ± 0.02b |
| Glutathione reductase activity (units/min/mg of tissue protein) | 0.29 ± 0.01 | 0.30 ± 0.01 | 0.57 ± 0.02a | 0.34 ± 0.01b |
P ≤ 0.001 compared to control values using ANOVA.
P ≤ 0.001 compared to piroxicam treated values using ANOVA.
Piroxicam was found to decrease the activities of on gastric tissue decreased gastric peroxidase and glutathione-S-transferase by 51.48% and 51.39% compared to activities observed in the control rats (*P ≤ 0.001 vs. control in each case). On the other hand, piroxicam feeding increased glutathione reductase, glutathione peroxidase, Cu–Zn SOD, Mn SOD and catalase by 96.5%, 56.92%, 2.62 folds, 55% and 78.23% respectively compared to respective controls (*P ≤ 0.001 vs. control in each case).
3.5. Protective effect of Cu LE on serum and tissue level PGE2
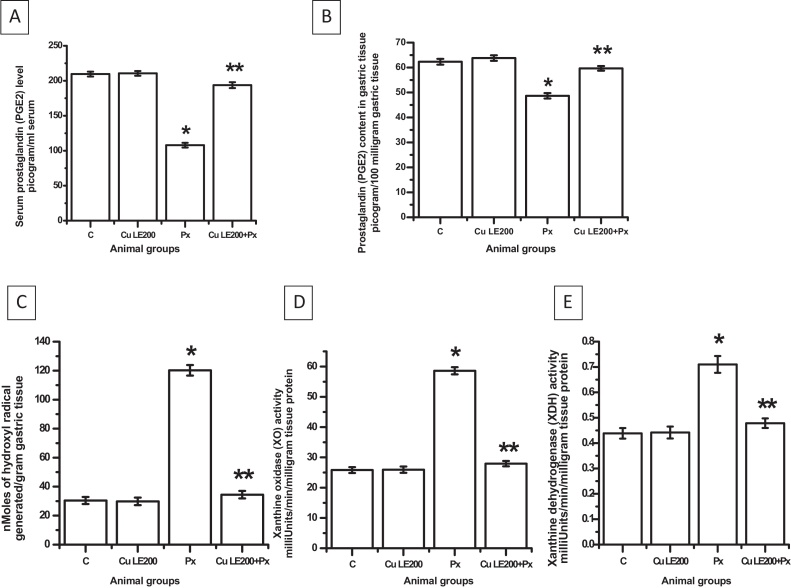
The serum level of PGE2 was decreased by 52.3% on piroxicam treatment (*P ≤ 0.001 vs. control). Piroxicam feeding also depleted tissue level of PGE2 by 21.9% (*P ≤ 0.001 vs. control). Both serum and tissue levels of PGE2 were found to be completely protected from being altered when the animals were pre-treated with Cu LE at a dose of 200 mg/kg body weight dose before piroxicam feeding (Fig. 4A and B). Administration of only Cu LE at 200 mg/kg BW dose did not alter PGE2 titre either in serum or in gastric tissue.
Fig. 4.
Protective mechanism of aqueous curry leaf extract at 200 mg/kg body weight dose against piroxicam induced oxidative stress in rat stomach. (A) The serum PGE level. (B) The gastric tissue PGE level. (C) The gastric tissue free hydroxyl radical level. (D) Xanthine oxidase activity. (E) Xanthine dehydrogenase activity. The bar graphs represent the values of the different parameters studied and all the values are expressed as mean ± S.E.M. where *P ≤ 0.001 vs. control, **P ≤ 0.001 vs. piroxicam treated animals (one-way ANOVA followed by Scheffe’ multiple comparison test).
3.6. Protective effect of Cu LE on the formation of reactive oxygen species in vivo
Treatment of rats with piroxicam results in huge amount of free radical generation in vivo. Measurement of free hydroxyl radical as represented in Fig. 4C in gastric tissues indicates a significant rise from control by 3.98 folds (*P ≤ 0.001 vs. control). Pre-treatment of rats with Cu LE significantly prevented the hydroxyl radicals from being increased (i.e., 73.85% [P < 0.001 vs. piroxicam fed group]).
Status of superoxide anion free radical was estimated indirectly by determining the activities of two pro-oxidant enzymes viz. XO and XDH (Fig. 4D and E). Rats treated with only piroxicam showed rise in XO activity and XDH activity by 2.27 folds and 61.36% respectively (*P ≤ 0.001 vs. control in each case), thereby clearly indicating significant elevation in tissue superoxide anion free radical. Pre-treatment of rats with Cu LE at 200 mg/kg BW dose before administering piroxicam showed significant protection in the activities of the two enzymes by 56.82% (for XO activity) and 38.03% (for XDH activity) when compared to only piroxicam fed group (*P ≤ 0.001 vs. piroxicam fed group in each case). Status of free oxygen radicals generated in tissues was found to remain unaltered in the animal group fed only Cu LE at a dose of 200 mg/kg body weight.
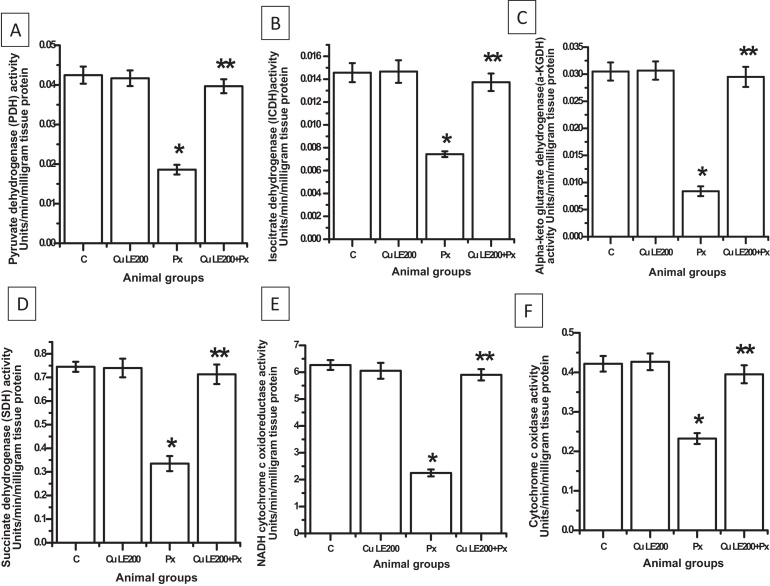
3.7. Protective effect Cu LE on the activities of PDH, ICDH, α-KGDH, SDH, NADH cytochrome c oxidoreductase and cytochrome c oxidase
Fig. 5 reveals that piroxicam treatment of rats with piroxicam at 30 mg/kg BW dose resulted in decrease in activities of PDH, ICDH, α-KGDH and SDH compared to control by 54.76%, 50%, 72.45% and 55.4% respectively (*P ≤ 0.01 vs. control). Rats treated with only Cu LE did not show any change in the activities of such enzymes compared to control. Pre-treatment of rats with Cu LE before piroxicam feeding also prevented any decrease in the activities of such mitochondrial Kreb's cycle enzymes.
Fig. 5.
Protective action of aqueous curry leaf extract at 200 mg/kg body weight on piroxicam induced altered activities of mitochondrial Kreb's cycle (A–D) and electron transport chain enzymes (E and F). (A) The pyruvate dehydrogenase activity. (B) The isocitrate dehydrogenase activity. (C) The alpha-ketoglutarate dehydrogenase activity. (D) The succinate dehydrogenase activity. (E) The NADH-cytochrome c oxido-reductase activity. (F) The cytochrome c oxidase activity. Bar graphs represent the values of activity levels of the enzymes and all the values are expressed as mean ± S.E.M. where *P ≤ 0.001 vs. control, **P ≤ 0.001 vs. piroxicam treated animals (one-way ANOVA followed by Scheffe’ multiple comparison test).
Alterations in mitochondrial respiratory chain enzymes namely NADH cytochrome c oxidoreductase and cytochrome c oxidase activities are represented in Fig. 5E and F respectively. On piroxicam treatment activity of NADH cytochrome c oxido reductase decreased by 60.13% and activity of cytochrome c oxidase activity reduced by 45.24% compared to respective control activities (*P ≤ 0.001 in each case). Rats were found to be protected against any such decreased activities of enzymes when pre-treated with Cu LE at a dose of 200 mg/kg body weight.
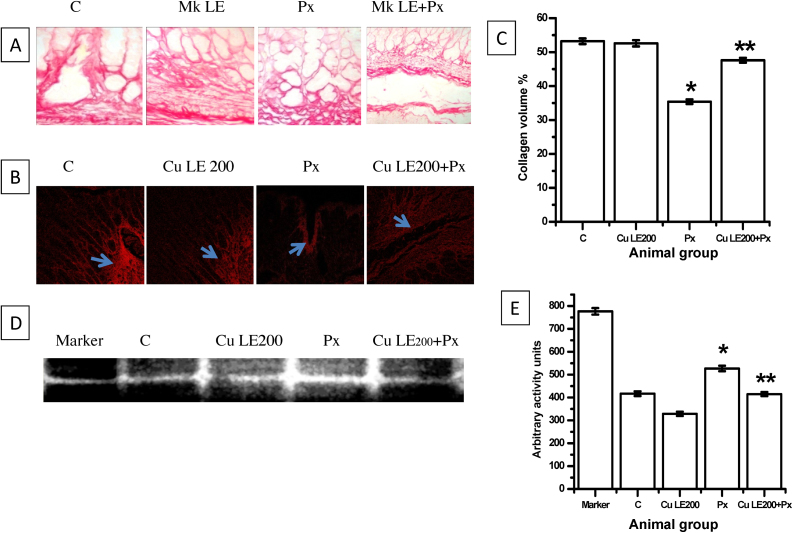
3.7.1. Protective effect of Cu LE on collagen content of rat gastric tissues
Fig. 6 indicates tissue disintegration and breakdown of cellular matrix to potentiate sloughing of mucosal cells on piroxicam administration. Photomicrographs of Sirius red stained sections and confocal microscopy done to determine tissue collagen volume reveal that piroxicam depleted tissue collagen significantly (33.4% decrease vs. control, *P ≤ 0.001 vs. control). Collagen volume did not decrease significantly in Cu LE pre-treated piroxicam administered group which indicates that tissue collagen depletion and gastric tissue damage can be well prevented if prior administration of Cu LE is done.
Fig. 6.
Protective effective of 200 mg/kg body weight dose of aqueous curry leaf extract in piroxicam-fed rat stomach. (A) The photomicrographs of picro-sirius red stained sections. (B) Representative figures of confocal images of picro-sirius red stained sections. (C) The bar graph showing changes in gastric tissue collagen volume. (D) The representative figure for determining pro-MMP 9 activity through gelatin zymography. (E) The bar graph representing arbitrary activity values of pro-MMP 9. All values in (C) and (E) are expressed as mean ± S.E.M. where *P ≤ 0.001 vs. control, **P ≤ 0.001 vs. piroxicam treated animals (one-way ANOVA followed by Scheffe’ multiple comparison test).
3.7.2. Protective effect of Cu LE on pro-MMP 9 activity in rat gastric tissue
Cu LE at a dose of 200 mg/kg body weight dose can effectively decrease pro-MMP 9 activity by 21.1% against activity in control animals. Therefore, when Cu LE was administered before piroxicam feeding, activity of pro-MMP 9 significantly decreased than the levels determined for only piroxicam administered animals. The activity levels of Pro-MMP 9 in Cu LE + Px treated animal group decreased by 21.3% against only piroxicam administered group.
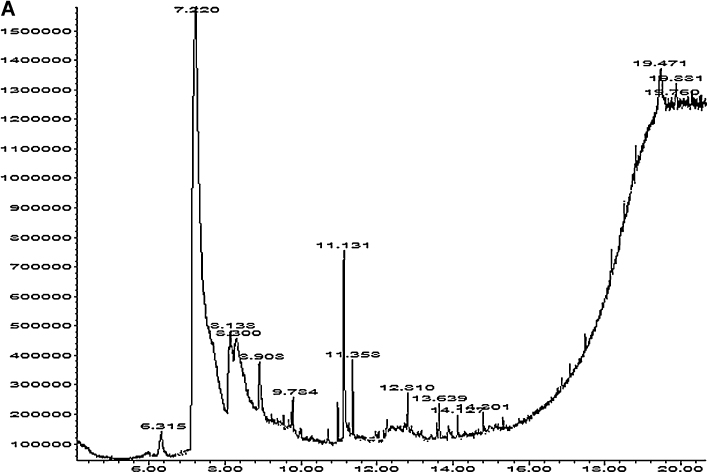
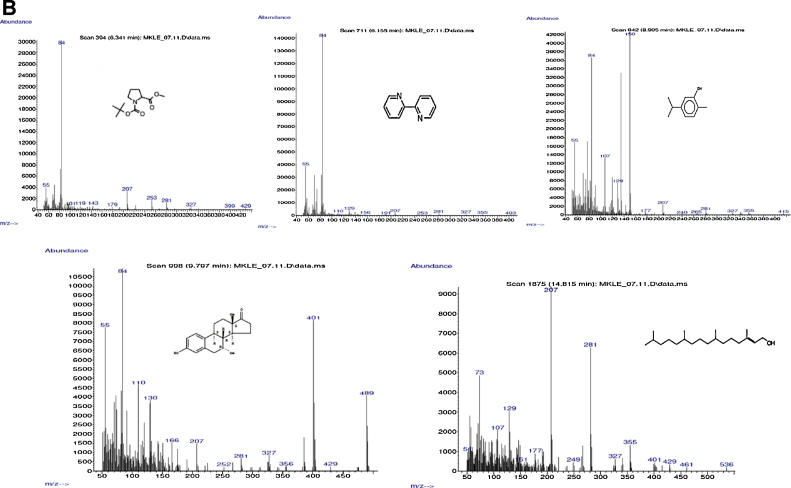
3.8. Chemical analysis of Cu LE
Dry curry leaf powder yielded 14.72% (by weight) water soluble components. Chemical characterization of the extract showed presence of polyphenol, flavonoid, alkaloid and tannin. Table 3 shows the amount of each substance in milligrams per gram extract. The extract contains protein and water soluble polyphenols in appreciable amount. Fig. 7A shows GCMS analysis of the extract and 7B bears the representative images of mass spectrometry of five important compounds present in the extract. Ten of the total fifteen compounds identified to be present in the extract include GC–MS reference compounds and metabolites from pestidicides. Therefore, five of the fifteen compounds determined to be relevant in the present study are pyrrolidine, [2-butyl-1-methyl-],2,2′-dipiperidine, phenol, [2-methyl-5-(1-methylethyl)], estra-1,3,5(10)-triene-17-one and phytol. Presence of these five compounds clearly supports the presence of alkaloids, polyphenols, flavonoids and chlorophyll respectively in Cu LE.
Table 3.
Extraction yield and phytochemical composition of aqueous curry leaf extract (Cu LE).
| Samples | Extraction yielda | Phytochemical content |
||||
|---|---|---|---|---|---|---|
| Total phenols (mg GAEb/g Cu LE) | Total flavonoid (mg CEc/g Cu LE) | Chlorophyll content (mg/g Cu LE) | Alkaloid content (mg BNd/g Cu LE) | Total tannin (mg TAEe/g Cu LE) | ||
| Curry (Murraya koenigii) leaf aqueous extract (Cu LE) | 14.72 ± 0.36 | 57.5 ± 0.05 | 5.2 ± 0.21 | 0.32 ± 0.006 | 38.6 ± 0.14 | 0.118 ± 0.006 |
Values represent the means of three replicates ± S.E.M.
Extraction yield (%) = (sample extract weight/sample weight) × 100.
GAE, gallic acid equivalent.
CE, catechin equivalent.
TAE, tannic acid equivalent.
BN, bismuth nitrate.
Fig. 7.
(A) The representative figure for gas chromatogram of the extract. (B) The representative figures for the mass spectrometry. Upper panel shows the mass spectrometric data of the chromatographic peaks obtained at 6.341 min, 7.245 min and 8.905 min. Lower panel shows the mass spectrometric data of the chromatographic peaks obtained at peak at 9.797 min and 14.815 min.
4. Discussion
Alternative medicine in management of different diseases is gaining in importance and emerging as an extensive field of research for the drug development industry. Different dietary factors and nutritional components are emerging in future therapeutics either as magical healers or as protective shields in ensuing fatal diseased conditions. Recent management of gastric pathology also relies more on the upcoming trend of using alternative medicine for protection and remedy. Considering the changes in disease management we searched for herbal nutritional sources effective in protecting against piroxicam induced gastro-ulcerative side effect.
Our present study aims to establish that aqueous leaf extract of curry plant (a popular South-Asian spice herb) has the potential to protect against piroxicam induced oxidative stress mediated gastric ulcer. This extract has been chemically characterized to be rich in alkaloids, polyphenols, flavonoids and chlorophyll. The antioxidant properties of alkaloids, polyphenols, flavonoids and phytol (obtained from breakdown of chlorophyll) from different herbal sources are used as nutritional supplements and alternative medicines in oxidative stress induced disease models [39], [40], [41]. This popular Indian spice herb with immense health benefits has been shown to possess prolific antioxidant activities. The leaf extract of M. koenigii has been shown to provide protection against oxidative stress induced in diabetes [4]. Aqueous extract of this leaf has been found to be effective in providing protection against cadmium and lead induced oxidative stress in rats [7], [42]. A number of in vitro [43], [44] and in vivo [45], [46] studies confirmed the free radical scavenging potential and antioxidant activities of leaf extracts of M. koenigii proposing its immediate ameliorative actions in oxidative stress models.
Considering the rich source of antioxidants in Cu LE, we studied the dose-dependent effect of the extract on piroxicam induced gastric oxidative stress and ulcer. Cu LE at 200 mg/kg BW dose maximally protected rat stomach against any oxidative damage mediated by 30 mg/kg BW dose of piroxicam. Our macroscopic and histopathological studies showed that almost no ulcerative damage occurred in rats when they were pre-treated with the antioxidant rich aqueous leaf extract. Collagen depletion, a marker for tissue disintegration and damage, was appreciably prevented on pre-treatment of piroxicam-fed rats with aqueous curry leaf extract. This is well exhibited in the confocal images of the Sirius red stained gastric tissue sections used for collagen volume determination by Image J software. Matrix metalloproteinases (MMPs) are enzymes secreted as zymogen granules called pro-MMPs. These zymogen granules are involved in extracellular matrix degradation and pro-MMP 9 and MMP-9 have been indicated as the primary factors in extracellular matrix degradation and epithelial cell denudation in NSAID(s) induced gastric ulcers [34]. Our present study also carried out gelatin zymography to determine whether pro-MMP 9 activity altered in piroxicam treatment and if the aqueous extract mediated protection was also through inhibition of matrix degrading enzyme. Quantitative determination of the changes in pro-MMP9 activity revealed that aqueous curry leaf extract pre-treatment inhibited significantly enhanced pro-MMP9 activity in piroxicam administered animals.
We observed increased accumulation of thiobarbituric acid reactive substances (TBARS) and protein carbonyls in gastric tissues of piroxicam treated rats indicating involvement of oxidative stress. Administration of piroxicam at 30 mg/kg BW dose further depleted reduced glutathione and non protein sulfhydryl compounds in gastric tissue. These findings were consistent with earlier reports on piroxicam induced gastric ulcer [9], [47]. Increase in lipid peroxidation and protein oxidation by 2.16 folds and 5.57 folds from control levels respectively resulted in increased consumption of glutathione. A significant increase in GSSG–GSH ratio in piroxicam-administered animals by 4.3 folds (P ≤ 0.001 vs. control) from control value established that glutathione consumption has markedly increased under stress conditions. Decrease in non-protein sulfhydryl compounds on piroxicam administration significantly indicates that such compounds might have been used in recycling endogenous antioxidants. Therefore, the findings support that antioxidant rich aqueous curry leaf extract can be immensely beneficial in suppressing oxidative damages in gastric tissue biomacromolecules like lipids and proteins through its direct free radical scavenging effects or some indirect antioxidant actions.
Significant decreases in the activities of antioxidant enzymes like gastric peroxidase and glutathione S-transferase and increase in the activities of glutathione reductase, glutathione peroxidase, catalase and superoxide dismutases indicate a growing imbalance in oxidants and antioxidants in gastric tissues after piroxicam administration. Aqueous curry leaf extract at 200 mg/kg BW dose protected against any such piroxicam induced alterations in activities of antioxidant enzymes. This well indicates that aqueous leaf extract has potentially scavenged the free radicals generated in vivo eliminating all adverse effects. This might have restored the oxidant–antioxidant balance in the stomach. Activities of mitochondrial Kreb's cycle enzyme and electron transport chain enzymes showed significant fall further supporting the fact that oxidative stress burden is the causative factor of gastro-mucosal erosion and bleeding. This finding indicates that building up of a reducing environment in the stomach results in accumulation of excess electrons that in turn generate reactive oxygen species (ROS) like superoxide anion radicals, hydroxyl radical etc. Free superoxide anion radicals and hydroxyl radicals have been indicated to be the major contributing factors in piroxicam and similar NSAIDs induced gastropathy and gastric ulcer. One study has clearly emphasized hydroxyl radical to be the principal causative agent in piroxicam mediated gastric ulcer [2]. In our present study we found that aqueous curry leaf extract is capable of scavenging free radicals. In vivo hydroxyl radical titre decreased significantly in rats pre-treated with aqueous curry leaf extract. Superoxide anion radical status determined indirectly by studying the activities of the pro-oxidant enzymes xanthine oxidase and xanthine dehydrogenase showed similar results. Under oxidative stress conditions selective proteolysis of dehydrogenase to oxidase results in formation of superoxide anion radical from molecular oxygen in the course of acting upon its substrates xanthine and hypoxanthine. A significant increase in activities of both the above mentioned enzymes in the present study suggests increased generation of superoxide anion radical in gastric tissues following piroxicam administration. Pre-treatment of rats with aqueous curry leaf extract protected the enhanced generation of superoxide anion radical by preventing the increase in activities of the pro-oxidant enzymes.
Gastric mucin is a pivotal factor in protecting gastric mucosa from physical damage and back diffusion of hydrogen ions. Depletion in mucin content in piroxicam-administered animals possibly occurred due to the adverse effects of free superoxide anion and hydroxyl radicals. Gastro-mucosal mucin depletion was protected on pre-administration of aqueous curry leaf extract in piroxicam-fed animals. Microscopic study of Alcian blue dye stained gastric sections puts forward the possibility that the leaf extract might have increased or changed the nature of mucous secreted in stomach. Stomach tissues of piroxicam fed animals showed increased acid mucin secretion, which was minimized to a great extent in aqueous extract pre-treated piroxicam-fed animals.
Piroxicam, a classical example of NSAID exerts its action like other NSAIDs by decreasing serum circulating and gastric tissue prostaglandins (PGE2) [30]. Such therapeutic action of piroxicam and other NSAIDs brings with it detrimental toxic actions in organs particularly the stomach where this PGE2 exerts its protective action. PGE2 stimulates mucous and bicarbonate secretion as well as mucosal blood flow, and induce angiogenesis. Serum and tissue level PGE2 were protected in aqueous curry leaf extract pre-administered rats further strengthening the idea to use this aqueous extract in combination therapy in piroxicam treatment.
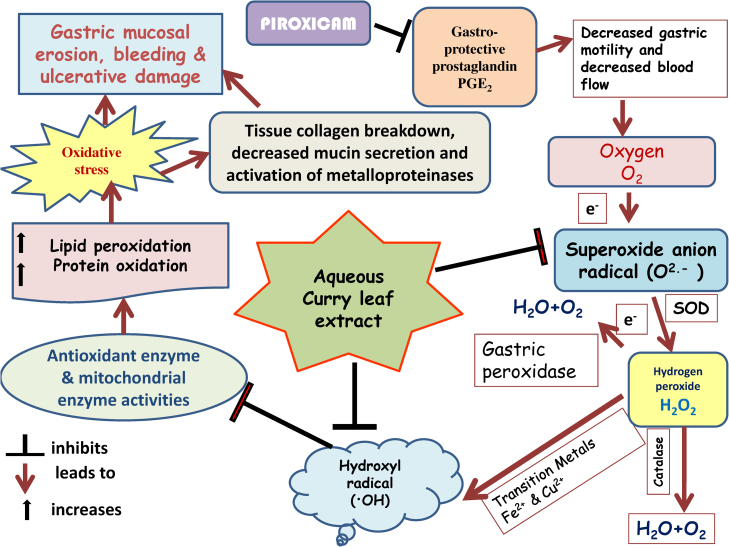
Fig. 8 proposes a model to explain the multi-step protection rendered by aqueous curry leaf extract in piroxicam induced gastric tissue damage. The figure clearly explains piroxicam mediated oxidative stress is the principal contributor in stomach tissue damage and ulcer. Aqueous extract pre-administration results in protection against all damaging effects through its antioxidant role, inhibitory action on pro-MMP9 activity and protective effects on quantity and nature of gastro-protective mucin secretion.
Fig. 8.
The possible mechanism of protection by Cu LE against piroxicam-induced oxidative stress mediated gastric ulcer.
Oral administration of piroxicam at a dose of 30 mg per kg body weight induced gastric ulcer in male Wistar rats. Pre-treatment with aqueous extract of curry leaves at a dose of 200 mg per kg body weight an hour before oral administration of piroxicam protected against piroxicam induced oxidative stress mediated gastric ulcer. Thus, curry leaves may be included in regular diet of patients undergoing piroxicam and similar NSAID treatment. It may be used either singly or in co-therapeutic treatment regimen. Piroxicam, otherwise safe drug with minimum side-effects may be effectively used by clinicians if necessary in combination with this aqueous extract.
Conflict of interest
The authors declare that there are no conflicts of interest.
Transparency document
Acknowledgements
Syed Benazir Firdaus gratefully acknowledges the receipt of University Research Fellowship from the University of Calcutta. D.G. is a DST INSPIRE SRF. A.C. is supported from her grants from UGC, Govt. of India grant no. PHW-105/11-12 (ERO) dated 02.11.2011. M.D. is a Woman Scientist under Women Scientists Scheme-A (WOS-A), Department of Science and Technology, Govt. of India. J.J. is a CSIR SRF. Dr. S.K.P. is supported from the funds available to him from RNTIICS, Kolkata and institutional fund. Dr. S.C. acknowledges the receipt of DST Ramanujan Fellowship. Dr. K.J. is supported by the fund of his institute. S.B.F. is thankful to Subir Chakraborty of RN Tagore International Institute of Cardiac Sciences and Barindra Nath Mandal (Technical Officer B, Div of Mol Med, Bose Institute) and Swaroop Biswas (Junior Lab assistant, CIF, Bose institute) for their technical assistance.
Footnotes
Available online 3 July 2014
References
- 1.Wolfe M.M., Lichenstein D.R., Singh G. Gastrointestinal toxicity of non-steroidal anti-inflammatory drugs. N. Engl. J. Med. 1999;340:1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay D., Biswas K., Bhattacharyya M. Gastric toxicity and mucosal ulceration induced by oxygen derived reactive species: protection by melatonin. Curr. Mol. Med. 2001;1:501–513. doi: 10.2174/1566524013363483. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay D., Chattopadhyay A. Reactive oxygen species-induced ulceration: protection by melatonin. Curr. Med. Chem. 2006;13:1187–1202. doi: 10.2174/092986706776360842. [DOI] [PubMed] [Google Scholar]
- 4.Arulselvan P., Subramanian S.P. Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultrastructural changes of pancreatic β-cell in experimental diabetes. Chem. Biol. Interact. 2007;165:155–164. doi: 10.1016/j.cbi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Kesari A.N., Kesari S., Singh S.K., Gupta R.K., Watal G. Studies on the glycemic and lipidemic effect of Murraya koenigii in experimental animals. J. Ethnopharmacol. 2007;112:305–311. doi: 10.1016/j.jep.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Singh A.P., Wilson T., Vorsa V., Luthria D., Freeman M.R., Scott R.M., Bilenker D., Shah S., Somasundaram S., Vorsa N. LC–MS–MS characterization of curry leaf flavonols and antioxidant activity. Food Chem. 2011;127:80–85. [Google Scholar]
- 7.Mitra E., Ghosh A.K., Ghosh D., Mukherjee D., Chattopadhyay A., Dutta S., Pattari S.K., Bandyopadhyay D. Protective effect of aqueous curry leaf (Murraya koenigii) extract against cadmium-induced oxidative stress in rat heart. Food Chem. Toxicol. 2012;50:340–353. doi: 10.1016/j.fct.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee A.K., Sadhu U., Dalal B.B., Chatterjee T. Studies on certain drug metabolising enzymes in deoxypyridoxine treated rats. Jpn. J. Pharmacol. 1984;34:367–373. doi: 10.1254/jjp.34.367. [DOI] [PubMed] [Google Scholar]
- 9.Bandyopadhyay D., Ghosh G., Bandyopadhyay A., Reiter R.J. Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal. Res. 2004;36:195–203. doi: 10.1111/j.1600-079x.2004.00118.x. [DOI] [PubMed] [Google Scholar]
- 10.Tariq M., Al Montaery A. Menadione protects gastric mucosa against ethanol-induced ulcers. Exp. Toxicol. Pathol. 2005;56:393–399. doi: 10.1016/j.etp.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Buege J.A., Aust S.G. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 12.Levine R.L., Williams J.A., Stadtman E.R., Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 13.Sedlak J., Lindsay R.H. Estimation of total, protein-bound, nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 14.Bandyopadhyay D., Bandyopadhyay A., Das P.K., Reiter R.J. Melatonin protects against gastric ulceration and increases the efficacy of ranitidine and omeprazole in reducing gastric damage. J. Pineal. Res. 2002;33:1–7. doi: 10.1034/j.1600-079x.2002.01107.x. [DOI] [PubMed] [Google Scholar]
- 15.McCord J.M., Keele B.B., Jr., Fridovich I. An enzyme based theory of obligate anaerobiosis: the physiological functions of superoxide dismutase. Proc. Natl. Acad. Sci. U.S.A. 1976;68:1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin J.P., Jr., Daily M., Sugarman E. Negative and positive assays of superoxide dismutase based on hematoxylin autooxidation. Arch. Biochem. Biophys. 1987;255:326–329. doi: 10.1016/0003-9861(87)90400-0. [DOI] [PubMed] [Google Scholar]
- 17.Beers R.F., Jr., Sizer I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 18.Krohne-Ehrich G., Schirmer R.H., Untucht-Grau R. Glutathione reductase from human erythrocytes. Isolation of the enzyme and sequence analysis of the redox-active peptide. Eur. J. Biochem. 1977;80:65–71. doi: 10.1111/j.1432-1033.1977.tb11856.x. [DOI] [PubMed] [Google Scholar]
- 19.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 20.Chattopadhyay A., Choudhury T.D., Bandyopadhyay D., Datta A.G. Protective effect of erythropoietin on the oxidative damage of erythrocyte membrane by hydroxyl radical. Biochem. Pharmacol. 2000;59:419–425. doi: 10.1016/s0006-2952(99)00277-4. [DOI] [PubMed] [Google Scholar]
- 21.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione-S-transferases, the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 22.Bandyopadhyay D., Biswas K., Bandyopadhyay U., Reiter R.J., Banerjee R.K. Melatonin protects against stress-induced gastric lesions by scavenging the hydroxyl radical. J. Pineal. Res. 2000;29:143–151. doi: 10.1034/j.1600-079x.2000.290303.x. [DOI] [PubMed] [Google Scholar]
- 23.Greenlee L., Handler P. Xanthine oxidase. IV. Influence of pH on substrate specificity. J. Biol. Chem. 1964;239:1090–1095. [PubMed] [Google Scholar]
- 24.Strittmatter C.F. Studies on avian xanthine dehydrogenases: properties and patterns of appearance during development. J. Biol. Chem. 1965;240:2557–2564. [PubMed] [Google Scholar]
- 25.Chretien D., Pourrier M., Bourgeron T., Séné M., Rötig A., Munnich A., Rustin P. An improved spectrophotometric assay of pyruvate dehydrogenase in lactate dehydrogenase contaminated mitochondrial preparations from human skeletal muscles. Clin. Chim. Acta. 1995;240:129–136. doi: 10.1016/0009-8981(95)06145-6. [DOI] [PubMed] [Google Scholar]
- 26.Duncan M.J., Fraenkel D.G. Alpha-ketoglutarate dehydrogenase mutant of Rhizobium meliloti. J. Bacteriol. 1979;137:415–419. doi: 10.1128/jb.137.1.415-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veeger C., DerVartanian D.V., Zeylemaker W.P. Succinate dehydrogenase. Methods Enzymol. 1969;13:81–90. [Google Scholar]
- 28.Goyal N., Srivastava V.M. Oxidation and reduction of cytochrome c by mitochondrial enzymes of Setaria cervi. J. Helminthol. 1995;69:13–17. doi: 10.1017/s0022149x00013778. [DOI] [PubMed] [Google Scholar]
- 29.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Adhikary B., Yadav S.K., Roy K., Bandyopadhyay S.K., Chattopadhyay S. Black tea and theaflavins assist healing of indomethacin-induced ulceration in mice by antioxidative action. J. Evid. Based. Complement. Altern. Med. 2010;2011:1–11. doi: 10.1155/2011/546560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basu A., Mitra E., Mukherjee D., Ghosh A.K., Firdaus S.B., Ghosh D., Chattyopadhyay A., Pattari S.K., Dutta S., Jana K., Bandhyopadhyay D. Aqueous tulsi leaf (Ocimum sanctum L.) extract protects against piroxicam-induced gastric ulceration: involvement of antioxidant mechanisms. Int. J. Pharm. Pharm. Sci. 2013;5:438–447. [Google Scholar]
- 32.Bancroft J.D., Gamble M. Elsevier Health Sciences; Philadelphia, USA: 2008. Theory and Practice of Histological Techniques; p. 179. [Google Scholar]
- 33.Mukherjee D., Roy S.G., Bandyopadhyay A., Chatyopadhyay A., Basu A., Mitra E., Ghosh A.K., Reiter R., Bandyopadhyay B. Melatonin protects against isoproterenol-induced myocardial injury in the rat: antioxidative mechanisms. J. Pineal. Res. 2010;48:251–262. doi: 10.1111/j.1600-079X.2010.00749.x. [DOI] [PubMed] [Google Scholar]
- 34.Swarnakar S., Ganguly K., Kundu P., Banerjee A., Maity P., Sharma A.V. Curcumin regulates expression and activity of matrix metalloproteinases 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. J. Biol. Chem. 2005;280:9409–9415. doi: 10.1074/jbc.M413398200. [DOI] [PubMed] [Google Scholar]
- 35.Sefi M., Fetoui H., Makni M., Zeghal N. Mitigating effects of antioxidant properties of Artemisia campestris leaf extract on hyperlipidemia, advanced glycation end products and oxidative stress in alloxan-induced diabetic rats. Food Chem. Toxicol. 2010;48:1986–1993. doi: 10.1016/j.fct.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Sreevidya N., Mehrotra S. Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff's reagent in plant materials. J. AOAC Int. 2003;86:1124–1127. [PubMed] [Google Scholar]
- 37.Polshettiwar S.A., Ganjiwale R.O., Wadher S.J., Yeole P.G. Spectrophotometric estimation of total tannins in some ayurvedic eye drops. Ind. J. Pharm. Sci. 2007;69:574–576. [Google Scholar]
- 38.Sermakkani M., Thangapandian V. GC–MS analysis of Cassia italica leaf methanol extract. Asian J. Pharm. Clin. Res. 2012;5:91–94. [Google Scholar]
- 39.Tachibana Y., Kikuzaki H., Lajis N.H., Nakatani N. Antioxidative activity of carbazoles from Murraya koenigii leaves. J. Agric. Food Chem. 2001;49:5589–5594. doi: 10.1021/jf010621r. [DOI] [PubMed] [Google Scholar]
- 40.Ningappa M.B., Dinesha R., Srinivas L. Antioxidant and free radical scavenging activities of polyphenol-enriched curry leaf (Murraya koenigii L.) extracts. Food Chem. 2008;106:720–728. [Google Scholar]
- 41.de Menezes Patrício Santos C.C., Salvanodori M.S., Mota V.G., Costa L.M., de Almeida A.A.C., de Oliveira G.A.L., Costa J.P., de Freitas R.M., de Almeida R.N. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J. 2013;1:1–9. doi: 10.1155/2013/949452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosh D., Firdaus S.B., Mitra E., Dey M., Chattopadhyay A., Pattari S.K., Dutta S., Jana K., Bandyopadhyay D. Hepatoprotective activity of aqueous leaf extract of Murraya koenigii against lead-induced hepatotoxicity in male Wistar rat. Int. J. Pharm. Pharm. Sci. 2013;5(1):285–295. [Google Scholar]
- 43.Rahman M.M., Gray A.I. A benzofuran derivative and carbazole alkaloids from Murraya koenigii and their antimicrobial activity. Phytochemistry. 2005;66(13):1601–1606. doi: 10.1016/j.phytochem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Roy M.K., Thalang V.N., Trakoontivakorn G., Nakahara K. Mechanism of mahenine induced apoptosis in human leukemia cell (HL-60) Biochem. Pharmacol. 2004;67(1):41–51. doi: 10.1016/j.bcp.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 45.Khan B.A., Abraham A., Leelamma S. Biochemical response in rats to the addition of Murraya koenigii and Brassica juncea to the diet. Plant Foods Hum. Nutr. 1996;49(4):295–299. doi: 10.1007/BF01091978. [DOI] [PubMed] [Google Scholar]
- 46.Iyer U.M., Mani U.V. A study on the effect of curry leaves supplementation on lipid profile, glycated proteins and amino acids in non-insulin-dependent patients. Plant Foods Hum. Nutr. 1990;40(4):275–282. doi: 10.1007/BF02193851. [DOI] [PubMed] [Google Scholar]
- 47.Das D., Bandyopadhyay D., Bhattacharjee M., Banerjee R.K. Hydroxyl radical is the major causative factor in stress-induced gastric ulceration. Free Radic. Biol. Med. 1997;23:8–18. doi: 10.1016/s0891-5849(96)00547-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.