Abstract
Flaxseed as well as its oil component possess antitumor activities against different types of cancer and have been used by some patients as complementary and/or alternative medicine. Linoorbitides (LOBs) are one family of flaxseed compounds that has implications for anticancer and antioxidant activity. The cytotoxicity of [1-9-NαC]-linusorb-B3 (LOB3), [1-9-NαC]-linusorb-B2 (LOB2), [1-9-NαC],[1-Rs,Ss-MetO]-linusorb-B2 ([MetO]-LOB2) and [1-8-NαC],[1-Rs,Ss-MetO]-linusorb-B1 ([MetO]-LOB1) was measured against human breast cancer Sk-Br-3 and MCF7 cell lines and melanoma A375 cell line. Overall cytotoxicity is cell-type specific. It scales as the hydrophobicity and concentration of the LOBs with the most abundant LOB3 being the most cytotoxic. Oral administration of LOB3 as a potential therapeutic agent might not be applicable as a much too high and/or frequent dose would be required to achieve a serum concentration of 400–500 μg/mL due to bioavailability and pharmacokinetic factors. However, LOB3 may be suitable for topical treatment formulations or as a lead compound in developing anticancer LOB derivatives.
Keywords: Flaxseed linoorbitide, Cytotoxicity, Breast cancer, Melanoma
1. Introduction
The nutraceutical effects of flaxseed have attracted significant research attention in recent years, leading to the identification and isolation of various families of bioactive compounds [1], [2], [3], [4]. Linoorbitides (LOBs) are a group of thermostable hydrophobic cyclopeptide compounds present in flaxseed oil. It has been noticed that cyclic peptides occur widely in both plants and animals and they can be broadly classified into two groups, cyclopeptides and cyclotides [5]. Various types of cyclopeptide have been isolated from plants and showed important biological functions such as anticancer, antidiabetic, cardio-protective and immunosuppressive properties, making them an abundant resource for future development of therapeutic agents [6], [7], [8], [9], [10], [11], [12], [13]. For example, sunflower trypsin inhibitor 1 (SFTI-1), a cyclopeptide containing 14-amino acids, has been used as the template to develop potent inhibitors of matriptase, which is present on the surface of certain types of cancer cells [14], [15].
Many cancer patients seek treatments with complementary and/or alternative medicine (CAM), especially when they have developed drug resistance towards chemotherapies. According to the 2007 Statistics on CAM Use in the United States, 23% of American cancer patients use CAM [16]. The actual number is likely to be higher as many cancer patients do not tell their physicians or oncologists about CAM usage. Flaxseed as well as its oil component have been observed to exhibit antitumor activities towards different types of cancer [12], [17], [18], [19], making flaxseed a CAM candidate for human cancer treatment. Flaxseed oil could even increase the therapeutic efficacy of trastuzumab against HER2-overexpressing breast cancer [20]. In spite of being a family of bioactive compounds in flaxseed oil, previous studies on flaxseed LOBs have mainly focused on the immunosuppressive and antimalarial activities of linoorbitide [1-9-NαC]-linusorb-B3 (LOB3) and its analogues. However, a recent study showed that LOBs increased the oxidative stability of flaxseed oil in their natural matrix and might possess anti-oxidative activity in vivo [21], implying that LOBs might possess anticancer activities. Furthermore, the calculated log P (logarithm of partition coefficient) values for the LOBs are between 2 and 5 (from ALOGPS, http://www.vcclab.org); making them suitable for both oral and topical administrations according to Lipinski's rule [22]. In the current study, we investigated whether flaxseed LOBs could impose cytotoxic effect towards human breast cancer and melanoma cells.
2. Materials and methods
2.1. Materials
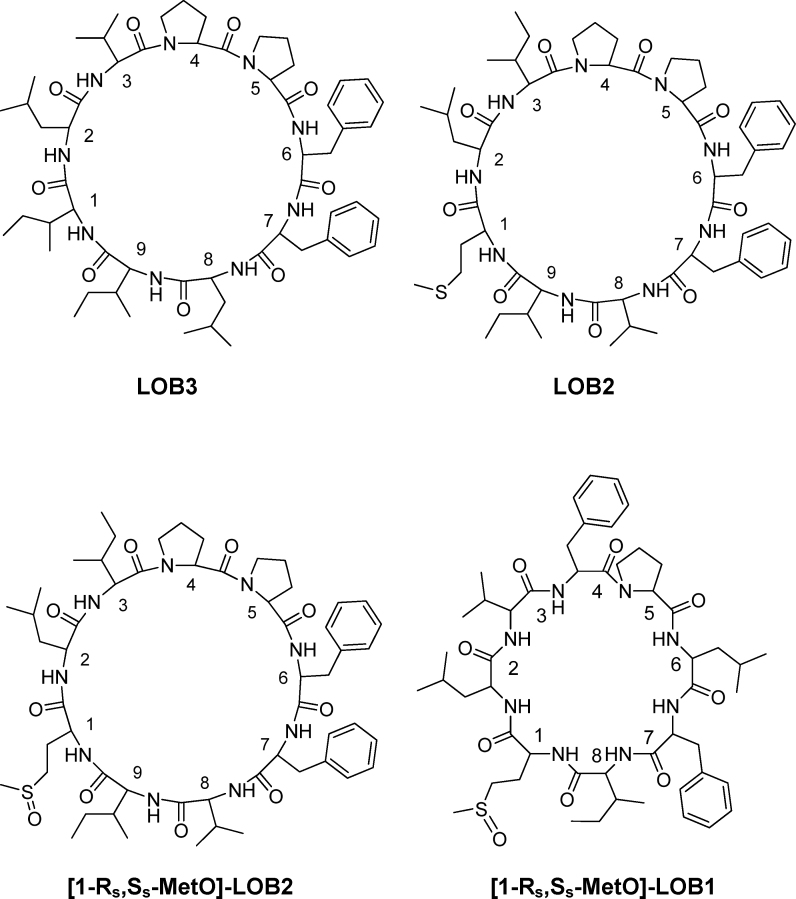
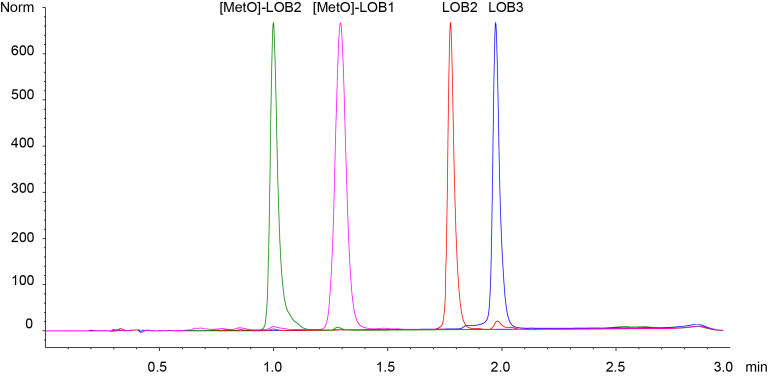
All chemicals used in the current study were purchased from Sigma–Aldrich Canada (Oakville, ON, Canada). The LOBs were a gift from Prairie Tide Chemicals (Saskatoon, SK Canada). Four linoorbitides LOB3, LOB2, [MetO]-LOB2 and [MetO]-LOB1 (Fig. 1) were used in this study. The elution order on a C18 reversed stationary phase liquid chromatograph column is [MetO]-LOB2 < [MetO]-LOB1 < LOB2 < LOB3 (Fig. 2). Of note is that both [MetO]-LOB2 and [MetO]-LOB1 contain methionine sulfoxide in their ring, which makes them less hydrophobic than their methionine containing counterparts [23]. Stock solutions of the LOBs were prepared in dimethyl sulfoxide (DMSO). Human melanoma cell line A375 and breast cancer cell lines Sk-Br-3 and MCF7 were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). ATCC-formulated cell culture medium for each cell line was purchased from Cedarlane Canada (Burlington, ON, Canada). The CytoTox 96® Non-Radioactive Cytotoxicity Assay was purchased from the Promega Corporation (Madison, WI, USA).
Fig. 1.
Chemical structures of linoorbitide LOB3 (NαC-[Ile-Leu-Val-Pro-Pro-Phe-Phe-Leu-Ile]), LOB2 (NαC-[Met-Leu-Ile-Pro-Pro-Phe-Phe-Val-Ile]), [MetO]-LOB2 (NαC-[MetO-Leu-Ile-Pro-Pro-Phe-Phe-Val-Ile]) and [MetO]-LOB1 (NαC-[Met-Leu-Val-Phe-Pro-Leu-Phe-Ile]).
Fig. 2.
Reversed phase HPLC chromatogram from a Chromolith® HighResolution RP-18e C-18 column (4.6 mm × 50 mm). The column was eluted by a linear gradient of water and acetonitrile (50:50–20:80 in 1.8 min, to 10:90 in 0.1 min, to 50:50 in 0.1 min and to equilibration for 1 min) with a flow rate of 2 mL/min at 32 °C.
2.2. Cell culture
Human melanoma cell line A375 and breast cancer cell lines Sk-Br-3 and MCF7 were cultured in T-75 culture flasks under ATCC-recommended cell culture conditions at 5% CO2 and 37 °C in a Thermo Forma™ Series II Water-Jacketed CO2 incubator (Thermo Fisher Scientific, Waltham, MA, USA). Cell culture media were changed every 2–3 d for each cell line.
2.3. Cytotoxicity assay
The cytotoxicity of LOBs was assayed in three independent experiments with each experiment carried out in triplicate. Briefly, the cultured cells were plated in 96-well plates with 5000 cells/well for cell line A375 and 10,000 cells/well for cell lines Sk-Br-3 and MCF7. The plated cells were allowed to grow to 70–80% confluence before being treated with LOBs for 24 h and 48 h, respectively. The administered concentrations for LOBs were 500 μM, 125 μM, 31.25 μM, 7.85 μM and 1.95 μM for A375 cells, 400 μg/mL, 200 μg/mL, 100 μg/mL, 50 μg/mL and 25 μg/mL for Sk-Br-3 cells, and 200 μg/mL, 100 μg/mL, 50 μg/mL, 25 μg/mL and 12.5 μg/mL for MCF7 cells. As molecular weights for all four LOBs are approximately 1000 daltons, their concentrations were approximately at 1 μM ≈ 1 μg/mL. Cells treated with DMSO (final concentration at 2%) were used as negative control. Cytotoxicity of the LOBs was measured using the CytoTox96® Non-Radioactive Cytotoxicity Assay.
2.4. Interaction between LOBs and phospholipid bubbles
Micron-sized phospholipid bubbles were generated by adding 0.5 mg of 1,2-distearoyl-sn-glycero-3-phosphocholine into 15 mL of H2O and vortexed (∼900 rpm) at room temperature for 30 s using a VWR analogue vortex mixer. LOB solutions were prepared by adding 1 mg of LOBs to 50 μL of 80% methanol. The interaction between LOBs and phospholipid bubbles was investigated by adding 10 μL of each respective LOB solution into 100 μL bubble suspension, vortexed (∼900 rpm) at room temperature for 30 s, and imaged immediately under a microscope at 100× magnification.
3. Results and discussion
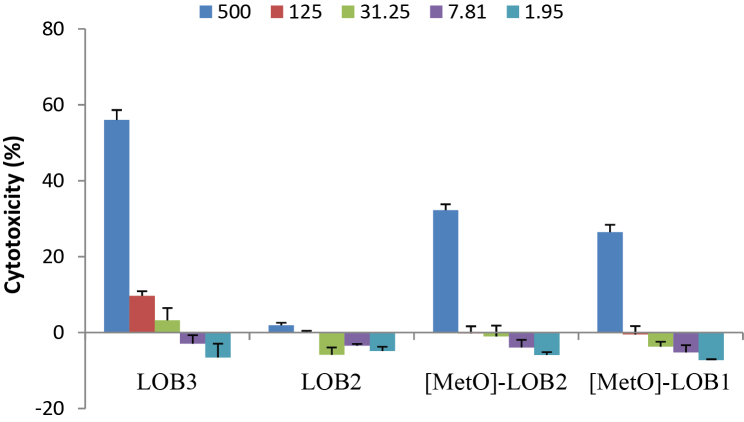
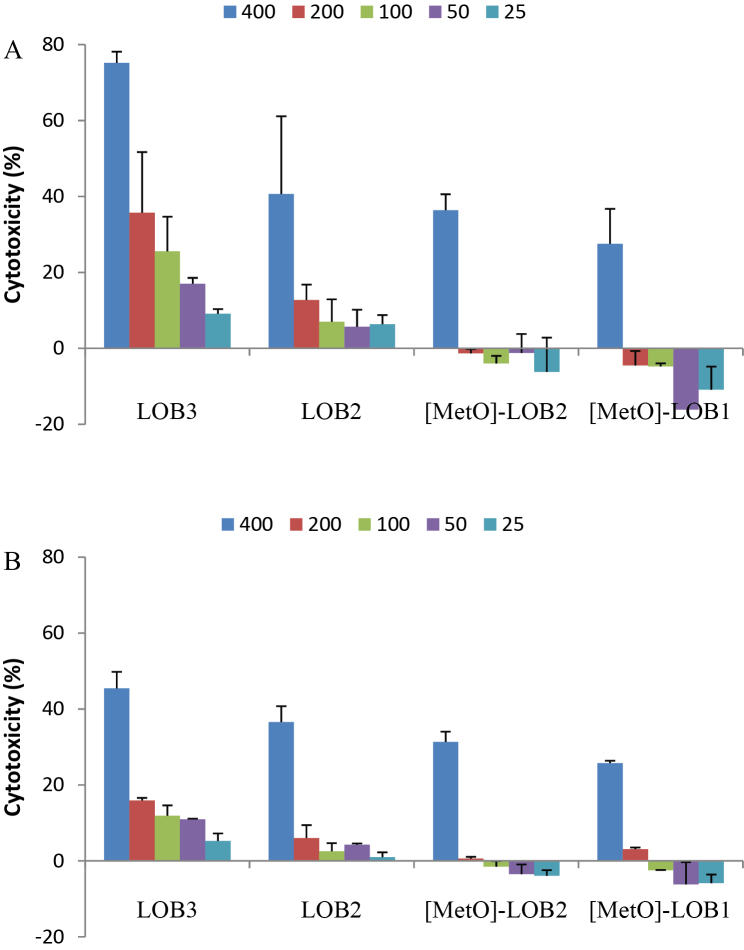
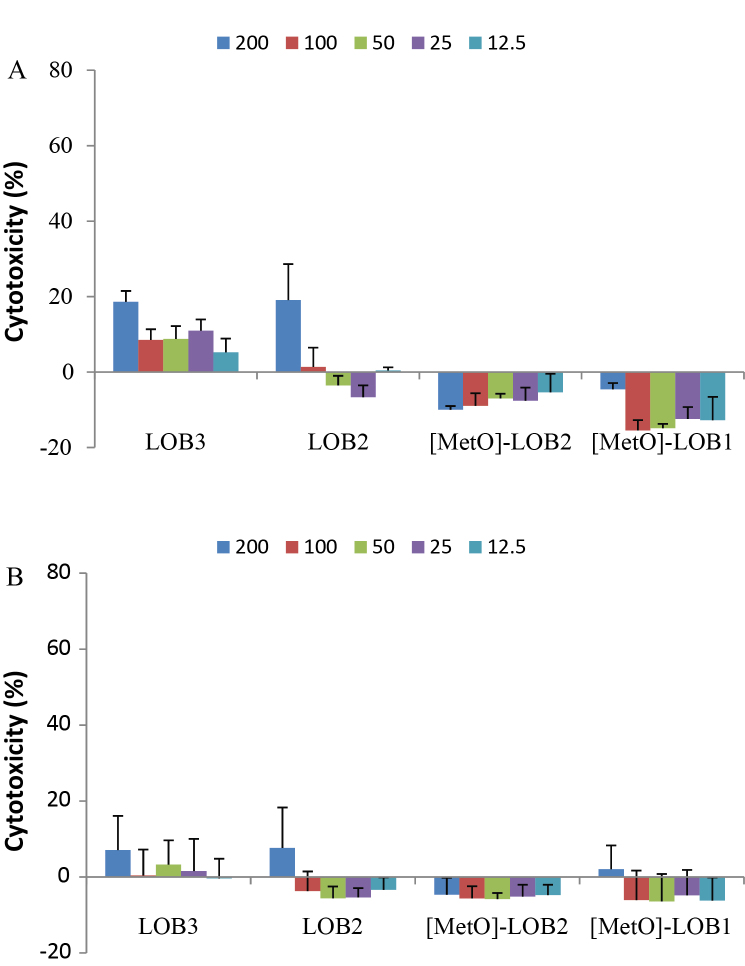
Cytotoxicity of LOB3, LOB2, [MetO]-LOB2 and [MetO]-LOB1 against melanoma A375 cells were measured at 24 h and 48 h of exposure time, respectively. At 24 h, no cytotoxicity was observed for any of the four LOBs. As the exposure was increased to 48 h, LOB3, [MetO]-LOB2 and [MetO]-LOB1 exhibited increased toxicity of 56%, 32% and 26%, respectively, compared to the control at concentration of 500 μM (Fig. 3). As LOB concentration decreased to 125 μM, only LOB3 showed a marginal cytotoxicity of 10%. However, no cytotoxicity was observed for LOB2 at any concentration (Fig. 3). Subsequently, the cytotoxicity of the four LOBs was measured against breast cancer Sk-Br-3 and MCF7 cells. As shown in Fig. 4, the respective cytotoxicity of LOB3, LOB2, [MetO]-LOB2 and [MetO]-LOB1 was at 75%, 41%, 36% and 28% towards the Sk-Br-3 cells after 24 h of exposure at 400 μg/mL. [MetO]-LOB2 and [MetO]-LOB1 only showed cytotoxicity at 400 μg/mL; however, both LOB3 and LOB2 exhibited a concentration-dependent cytotoxic response. As the concentration was reduced to 25 μg/mL, only 9% and 6% of cytotoxicity were remained for LOB3 and LOB2, respectively. When the exposure time was increased to 48 h, the cytotoxicity of LOB3 was significantly reduced to 45%, whereas LOB2, [MetO]-LOB2 and [MetO]-LOB1 maintained their respective cytotoxicity, at 400 μg/mL. For MCF7 cells, the maximum concentration of LOBs used for treatment was 200 μg/mL. As illustrated in Fig. 5, only LOB3 and LOB2 exhibited cytotoxicity at 18% and 19%, respectively, after 24 h of exposure and 7% and 8%, respectively, after 48 h of exposure. This observation was consistent with the experiment on breast cancer Sk-Br-3 cells where it was seen that cytotoxicity of both LOB3 and LOB2 was reduced due to prolonged treatment. We speculated that the cytotoxicity of LOB3 and LOB2 would be much higher at concentration of 400 μg/mL based on the experimental results on A375 and Sk-Br-3 cells. Interestingly, [MetO]-LOB2 and [MetO]-LOB1 showed small cytoprotective effects for the MCF7 cells instead of being cytotoxic.
Fig. 3.
Cytotoxicity (mean ± standard deviation) of linoorbitide LOB3, LOB2, [MetO]-LOB2 and [MetO]-LOB1 against human melanoma A375 cells at 48 h of exposure. The data were obtained from three independent experiments with each experiment carried out in triplicate.
Fig. 4.
Cytotoxicity (mean ± standard deviation) of linoorbitide LOB3, LOB2, [MetO]-LOB2 and [MetO]-LOB1 against human breast cancer Sk-Br-3 cells at 24 h (A) and 48 h (B) of exposure. The data were obtained from three independent experiments with each experiment carried out in triplicate.
Fig. 5.
Cytotoxicity (mean ± standard deviation) of linoorbitide LOB3, LOB2, [MetO]-LOB2 and [MetO]-LOB1 against human breast cancer MCF7 cells at 24 h (A) and 48 h (B) of exposure. The data were obtained from three independent experiments with each experiment carried out in triplicate.
In the current study, we showed that the cytotoxicity of LOBs was highly cell-type specific and concentration dependent. Since cancer cells normally retain high levels of reactive oxygen species (ROS), the anti-oxidative activity of LOBs might be important in eliciting their cytotoxic effects against cancer cells. Furthermore, we hypothesized that the hydrophobicity of LOBs might play an essential role in their interaction with cell membranes and this interaction would be the first step towards cytotoxicity. To test our hypothesis, micron-sized phospholipid bubbles were generated to mimic cell membranes for the study of their interaction with LOBs. As shown in Fig. 6, the order of interacting activity for this model system was [MetO]-LOB2 < [MetO]-LOB1 < LOB2 < LOB3, which was the same as the hydrophobicity order of the LOBs determined by HPLC (Fig. 2). Continuous observation under a microscope at 100× magnification showed that LOB3 attached to the phospholipid bubbles, which in turn caused rapid aggregation and breakage of the bubbles. LOB2 also caused quick phospholipid bubbles aggregation but broke them at a much slower rate. However, [MetO]-LOB2 exhibited only marginal activity in aggregating phospholipid bubbles. Thus, hydrophobic interaction with cell membrane might be another contributing factor, other than the anti-oxidative function, to the cytotoxic effects of LOBs against human cancer cells.
Fig. 6.
Images of the interactions between micron-sized phospholipid (1,2-distearoyl-sn-glycero-3-phosphocholine) bubbles and flaxseed LOB3, LOB2, [MetO]-LOB2 and [MetO]-LOB1 were taken under 100× magnification using a microscope attached to a Raman spectrophotometer at the Saskatchewan Structural Sciences Centre. The phospholipid bubbles were imaged at 100 μm × 100 μm, the interactions of the phospholipid bubbles with the LOBs were imaged at 50 μm × 50 μm, and the “zoom-in” views of phospholipid bubble interactions with LOB3 and LOB2 were imaged at 10 μm × 10 μm.
LOB3 possessed the highest in vitro antitumor activities against all three cancer cell lines at higher concentrations, which is consistent with the observation that LOB3 had higher immunosuppressive function than LOB2 [24]. Taking into consideration of the bioavailability and pharmacokinetic factors, high and/or frequent doses might be needed for oral administered LOB3 to reach a serum concentration of 400–500 μg/mL, which is high and uncommon for therapeutic drugs. Although it is impossible to predict how high the oral dose of LOB3 should be without any pharmacokinetic studies, much too high dose requirement would definitely eliminate further exploration on oral administration of LOB3. Intravenous infusion of LOB3 at 400–500 μg/mL is likely to be toxic to human and might not be suitable for the treatment of human breast cancer and melanoma. However, cream formulation of LOB3 might be applicable for topical usage against melanoma and perhaps other types of skin cancer. In addition, we observed the cytotoxicity of [MetO]-LOB2 and [MetO]-LOB1 against melanoma A375 and breast cancer Sk-Br-3 cells and LOB2 against breast cancer Sk-Br-3 and MCF7 cells at high concentrations. They experience the same formulation concern. In summary, pure LOBs, which are extracted from flaxseed oil, are unlikely to have any potential as anticancer agents due to the high concentration required for cytotoxicity. Synthetic derivatives of LOBs with better pharmacological activity, bioavailability and pharmacokinetic profile would likely be more applicable in developing LOB-based cancer therapy regimens. Further studies including human breast cancer and melanoma xenograft models are warranted in elucidating the anticancer function of flaxseed LOBs and their derivatives.
Acknowledgements
This work was supported by Agriculture Development Fund grants (20080205 and 20120099) from the Saskatchewan Ministry of Agriculture. Peptides were a generous gift from Prairie Tide Chemicals (Saskatoon, SK, Canada).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2015.06.011.
Contributor Information
Martin J.T. Reaney, Email: martin.reaney@usask.ca.
Jian Yang, Email: jian.yang@usask.ca.
Ramaswami Sammynaiken, Email: r.sammynaiken@usask.ca.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Bommareddy A., Zhang X.Y., Kaushik R.S., Dwivedi C. Effects of components present in flaxseed on human colon adenocarcinoma Caco-2 cells: possible mechanisms of flaxseed on colon cancer development in animals. Drug Discov. Ther. 2010;4:184–189. [PubMed] [Google Scholar]
- 2.Gui B., Shim Y.Y., Datla R.S., Covello P.S., Stone S.L., Reaney M.J. Identification and quantification of cyclolinopeptides in five flaxseed cultivars. J. Agric. Food Chem. 2012;60:8571–8579. doi: 10.1021/jf301847u. [DOI] [PubMed] [Google Scholar]
- 3.Mabrok H.B., Klopfleisch R., Ghanem K.Z., Clavel T., Blaut M., Loh G. Lignan transformation by gut bacteria lowers tumor burden in a gnotobiotic rat model of breast cancer. Carcinogenesis. 2012;33:203–208. doi: 10.1093/carcin/bgr256. [DOI] [PubMed] [Google Scholar]
- 4.Okinyo-Owiti D.P., Young L., Burnett P.G., Reaney M.J. New flaxseed orbitides: detection, sequencing, and 15N incorporation. Biopolymers. 2014;102:168–175. doi: 10.1002/bip.22459. [DOI] [PubMed] [Google Scholar]
- 5.Hu C.Q., Xu J.C. Amino acids and peptides. In: Xu R., Ye Y., Zhao W., editors. Introduction to Natural Products Chemistry. CRC Press; Boca Roton: 2012. pp. 147–167. [Google Scholar]
- 6.Górski A., Kasprzycka M., Nowaczyk M., Wieczoreck Z., Siemion I.Z., Szelejewski W., Kutner A. Cyclolinopeptide: a novel immunosuppressive agent with potential anti-lipemic activity. Transplant. Proc. 2001;33:553. doi: 10.1016/s0041-1345(00)02139-4. [DOI] [PubMed] [Google Scholar]
- 7.Drygała P., Olejnik J., Mazur A., Kierus K., Jankowski S., Zimecki M., Zabrocki J. Synthesis and immunosuppressive activity of cyclolinopeptide A analogues containing homophenylalanine. Eur. J. Med. Chem. 2009;44:3731–3738. doi: 10.1016/j.ejmech.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 8.Thevenard J., Ramont L., Devy J., Brassart B., Dupont-Deshorgue A., Floquet N., Schneider L., Ouchani F., Terryn C., Maquart F.X., Monboisse J.C., Brassart-Pasco S. The YSNSG cyclopeptide derived from tumstatin inhibits tumor angiogenesis by down-regulating endothelial cell migration. Int. J. Cancer. 2010;126:1055–1066. doi: 10.1002/ijc.24688. [DOI] [PubMed] [Google Scholar]
- 9.Yu R., Wang J., Li J., Wang Y., Zhang H., Chen J., Huang L., Liu X. A novel cyclopeptide from the cyclization of PACAP(1-5) with potent activity towards PAC1 attenuates STZ-induced diabetes. Peptides. 2010;31:1062–1067. doi: 10.1016/j.peptides.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Chang M., Li X., Sun Y., Cheng F., Li Y., Zhao W., Wang Q. A potential mechanism of a cationic cyclopeptide for enhancing insulin delivery across Caco-2 cell monolayers. Biol. Pharm. Bull. 2013;36:1602–1607. doi: 10.1248/bpb.b13-00487. [DOI] [PubMed] [Google Scholar]
- 11.Fang X.Y., Chen W., Fan J.T., Song R., Wang L., Gu Y.H., Zeng G.Z., Shen Y., Wu X.F., Tan N.H., Xu Q., Sun Y. Plant cyclopeptide RA-V kills human breast cancer cells by inducing mitochondria-mediated apoptosis through blocking PDK1-AKT interaction. Toxicol. Appl. Pharmacol. 2013;267:95–103. doi: 10.1016/j.taap.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Goyal A., Sharma V., Upadhyay N., Gill S., Sihag M. Flax and flaxseed oil: an ancient medicine & modern functional food. J. Food Sci. Technol. 2014;51:1633–1653. doi: 10.1007/s13197-013-1247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boivin V., Beyersdorf N., Palm D., Nikolaev V.O., Schlipp A., Müller J., Schmidt D., Kocoski V., Kerkau T., Hünig T., Ertl G., Lohse M.J., Jahns R. Novel receptor-derived cyclopeptides to treat heart failure caused by anti-β1-adrenoceptor antibodies in a human-analogous rat model. PLOS ONE. 2015;10:e0117589. doi: 10.1371/journal.pone.0117589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li P., Jiang S., Lee S.L., Lin C.Y., Johnson M.D., Dickson R.B., Michejda C.J., Roller P.P. Design and synthesis of novel and potent inhibitors of the type II transmembrane serine protease, matriptase, based upon the sunflower trypsin inhibitor-1. J. Med. Chem. 2007;50:5976–5983. doi: 10.1021/jm0704898. [DOI] [PubMed] [Google Scholar]
- 15.Quimbar P., Malik U., Sommerhoff C.P., Kaas Q., Chan L.Y., Huang Y.H., Grundhuber M., Dunse K., Craik D.J., Anderson M.A., Daly N.L. High-affinity cyclic peptide matriptase inhibitors. J. Biol. Chem. 2013;288:13885–13896. doi: 10.1074/jbc.M113.460030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes P.M., Bloom B., Nahin R. 2008. Complementary and Alternative Medicine Use Among Adults and Children: United States, 2007, CDC National Health Statistics Report #12. [PubMed] [Google Scholar]
- 17.Chen J., Stavro P.M., Thompson L.U. Dietary flaxseed inhibits human breast cancer growth and metastasis and downregulates expression of insulin-like growth factor and epidermal growth factor receptor. Nutr. Cancer. 2002;43:187–192. doi: 10.1207/S15327914NC432_9. [DOI] [PubMed] [Google Scholar]
- 18.Eilati E., Bahr J.M., Hales D.B. Long term consumption of flaxseed enriched diet decreased ovarian cancer incidence and prostaglandin E2 in hens. Gynecol. Oncol. 2013;130:620–628. doi: 10.1016/j.ygyno.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason J.K., Thompson L.U. Flaxseed and its lignan and oil components: can they play a role in reducing the risk of and improving the treatment of breast cancer? Appl. Physiol. Nutr. Metab. 2014;39:663–678. doi: 10.1139/apnm-2013-0420. [DOI] [PubMed] [Google Scholar]
- 20.Mason J.K., Fu M., Chen J., Thompson L.U. Flaxseed oil enhances the effectiveness of trastuzumab in reducing the growth of HER2-overexpressing human breast tumors (BT-474) J. Nutr. Biochem. 2015;26:16–23. doi: 10.1016/j.jnutbio.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Sharav O., Shim Y.Y., Okinyo-Owiti D.P., Sammynaiken R., Reaney M.J. Effect of cyclolinopeptides on the oxidative stability of flaxseed oil. J. Agric. Food Chem. 2014;62:88–96. doi: 10.1021/jf4037744. [DOI] [PubMed] [Google Scholar]
- 22.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 23.Gellman S.H. On the role of methionine residues in the sequence independent recognition of nonpolar protein surfaces. Biochemistry. 1991;30:6633–6636. doi: 10.1021/bi00241a001. [DOI] [PubMed] [Google Scholar]
- 24.Siemion I.Z., Cebrat M., Wieczorek Z. Cyclolinopeptides and their analogs – a new family of peptide immunosuppressants affecting the calcineurin system. Arch. Immunol. Ther. Exp. (Warsz) 1999;47:143–153. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.