Abstract
The anti-inflammatory potential of hydrophilic polyphenolic-rich extracts obtained from native Australian herbs: anise myrtle, lemon myrtle and Tasmannia pepper leaf, and a reference sample bay leaf, was evaluated using the lipopolysaccharide (LPS)-activated murine macrophage RAW 264.7 model. Pretreatment with all herbal extracts at non-cytotoxic concentrations reduced the LPS-induced protein levels of pro-inflammatory enzymes, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS). Concomitant decrease in accumulation of their products, prostaglandin E2 (PGE2) and nitric oxide (NO), respectively, was observed. A suppression of LPS-induced expression of COX-2 and iNOS and decrease of NO and PGE2 levels suggests potential anti-inflammatory properties of the extracts.
Anise myrtle, lemon myrtle and bay leaf selectively inhibited COX-2 and iNOS enzymes, while Tasmannia pepper leaf extract exhibited a pronounced inhibitory activity toward COX-1 and was the least effective inhibitor of iNOS. Anise myrtle and lemon myrtle are potentially more efficient anti-inflammatory agents than Tasmannia pepper leaf.
Keywords: Polyphenols, Herbs, Anise myrtle, Lemon myrtle, Tasmannia pepper leaf, COX-1, COX-2, iNOS
1. Introduction
Examining the indigenous use of plant foods may provide a wealth of potential candidates for isolation of health-promoting substances and native Australian plants provide an intriguing source. The Australian flora have developed in distinct isolation from other continents as a result of the separation of the Australian land masses from the supercontinent Gondwana and the rest of the world over 65 million years ago [1]. The conditions of geographic isolation, followed by a warming of the continent and subsequent onset of aridity, as well as the nutrient poor soils led to a unique adaptation of plants resulting in a more complex flora with approximately 85% of the vascular plants in Australia being endemic. Over centuries the Australian aborigines utilized a large body of edible plants. Some of these plants were reported to possess unique nutritious and organoleptic properties [10]. This characteristic offers opportunities to utilize them in the development of novel tastes and flavors.
Three endemic Australian herbs: Tasmannia pepper leaf, anise myrtle and lemon myrtle, which resemble a bay leaf, are produced commercially and are been incorporated in Australian cuisine. Hydrophilic polyphenolic-rich extracts obtained from these herbs contain highly bioactive flavonoids, phenolic acids and tannins [19]. The dominant compounds of anise myrtle and lemon myrtle hydrophilic extracts are ellagic acid and derivatives, ellagic acid glycosides and ellagitannins, whether chlorogenic acid, quercetin and derivatives are the major components of Tasmannia pepper leaf extract. These phytochemicals are reported to possess numerous health-enhancing properties. For example, ellagic acid is a potent antioxidant, exhibits estrogenic and/or anti-estrogenic activities, anti-inflammatory, antimicrobial and prebiotic effects [5], [14], [15], [18]. Chlorogenic acid possesses anti-diabetic, anti-lipidemic [13] and anti-inflammatory capacity [2], [21] and has radioprotective action [4]. Polyphenolic-rich extracts of anise myrtle, lemon myrtle and Tasmannia pepper leaf suppress the activities of isolated α-glucosidase, pancreatic lipase and angiotensin converting enzyme (ACE) [19]. The same extracts exhibit antioxidant, cytoprotective and pro-apoptotic activities [20].
Inflammation is the normal physiological and immune response to pathogen invasion and cell injury. During the inflammatory response, immunocytes, such as monocytes and macrophages, are activated and secrete inflammatory mediators, such as nitric oxide (NO) and prostaglandin E2 (PGE2), respectively, via the inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). iNOS and COX-2 are involved in tumor progression through various mechanisms, including inhibition of apoptosis, stimulation of angiogenesis and promotion of tumor cell proliferation [3]. Therefore inhibition of iNOS and COX-2 activities is a viable approach to inhibit inflammation and carcinogenesis and to prevent cancer.
To date no research data is available on anti-inflammatory activities of the indigenous Australian herbs. Therefore the objective of this study was to evaluate the anti-inflammatory properties of purified polyphenolic-rich extracts obtained from commercially produced anise myrtle, lemon myrtle and Tasmannia pepper leaf using the lipopolysaccharide (LPS)-activated murine macrophages RAW 264.7. Commercially available bay leaf was used as a reference sample.
2. Materials and methods
2.1. Plant material
Anise myrtle (Syzygium anisatum Vickery, Craven and Biffen; AM) and lemon myrtle (Backhousia citriodora F. Muell, Myrtaceae; LM) were supplied by the Australian Rainforest Products (Lismore, NSW, Australian). Tasmannia pepper leaf (Tasmannia lanceolata R. Br., Winteracea; TPL) was obtained from the Diemen Pepper (Birchs Bay, Hobart, Tasmania, Australia). The reference sample bay leaf (Laurus nobilis L., Lauraceae) was obtained from Hoyts Industries Pty. Ltd. (Moorabbin, Victoria, Australia).
2.2. Preparation and analysis of polyphenolic-rich extracts from plant sources
The polyphenolic-rich extracts were prepared and their composition evaluated as described previously [19].
2.3. Cell culture
RAW264.7 (murine macrophage) cell line was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in Dulbecco's Modified Eagle's medium (DMEM; Invitrogen) containing 10% fetal bovine serum (FBS, Invitrogen Corporation, Carlsbad, CA, USA), 100 μg/ml streptomycin and 100 units/ml penicillin (Invitrogen Corporation, Carlsbad, CA, USA) at 37 °C, humidified 5% carbon dioxide (CO2) atmosphere. The experimental cells were cultured no more than 40 passages.
2.4. Western blotting
RAW 264.7 cells (1 × 106 cells) were pre-cultured in 25 cm2 flasks for 24 h. After 24 h fresh serum-free medium was added for 2.5 h to eliminate the effect of FBS. Subsequently the cells were treated with different concentrations of polyphenolic extracts in PBS for 1 h before exposure to 40 ng/ml LPS for 12 h. Next cell lysates were prepared by adding lysis buffer (1 mg. ml−1 BPB, 0.1% glycerol, 0.1 M DTT, 0.04% pH7.2 SDS, 62.5 mM pH6.8 Tris–HCl). The whole-cell lysates were run on a 4–12% Bis-Tris gel (NuPAGE, Invitrogen Corporation) and transferred to PVDF membrane (Invitrogen Corporation) using iBLOT Gel Transfer System (Invitrogen Corporation). After blocking nonspecific sites with 5% non-fat milk powder in TBS/T, the membrane was washed, incubated with primary antibody, washed again, further incubated with secondary antibody, washed and incubated with chemiluminescent alkaline phosphatase substrate (Thermo Scientific SuperSignal West Femto Substrate, Thermo Fisher Scientific). Primary rabbit polyclonal antibodies against COX-1, COX-2, iNOS and α-tubulin, as well as horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody, purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), were added at concentrations of 1 μl per 1 ml TBS/T. The immunoactive proteins were detected and quantified using chemiluminescent imaging system (ImageQuant LAS 4000, GE Healthcare). The relative densities of COX-1, COX-2 and iNOS proteins presented in graphs were normalized to α-tubulin.
2.5. Measurement of nitrite concentration
Nitrite concentration in culture media was determined by the Griess reaction [24]. Briefly, RAW 264.7 cells (3 × 105 per well) were incubated for 24 h at 37 °C in 48-well plates. The medium was removed and fresh serum-free medium was added for 2.5 h. Subsequently, the cells were treated for 1 h with different concentrations of polyphenolic extracts, which did not suppress the proliferation, before exposure to 40 ng/ml LPS for 12 h. The culture supernatants were mixed with equal volumes of modified Griess reagent (Sigma–Aldrich) for 15 min at room temperature in the absence of light. Nitrite concentration was measured by absorbance levels at 540 nm against a sodium nitrite standard curve.
2.6. Measurement of prostaglandin E2 (PGE2) production
PGE2 concentration in culture supernatant was determined with a PGE enzyme immunoassay kit (Sapphire Biosciences, Redfern, Australia) according to the manufacturer's instructions. In brief, RAW 264.7 cells (5 × 105 per well) were incubated for 24 h in 6-well plates. The old medium was removed and fresh serum-free medium was added for 2.5 h and next the cells were treated for 1 h with different concentrations of polyphenolic extracts before exposure to 40 ng/ml LPS for 12 h. The level of PGE2 released into the culture medium was determined by measuring absorbance levels at 412 nm against a PGE2 standard curve.
2.7. Statistical analysis
The mean of results and standard deviations (SD) were calculated based on at least three independent evaluations (n = 3). One way ANOVA followed by Tukey's post hoc test were performed to assess differences between the samples at the level of p < 0.05. Statistical correlation analyses were performed using Graphpad Prism 5 (Graphpad Software, CA, USA).
3. Results
3.1. Effect of polyphenolic-rich extracts on iNOS and COX-2 expression in LPS-induced macrophages
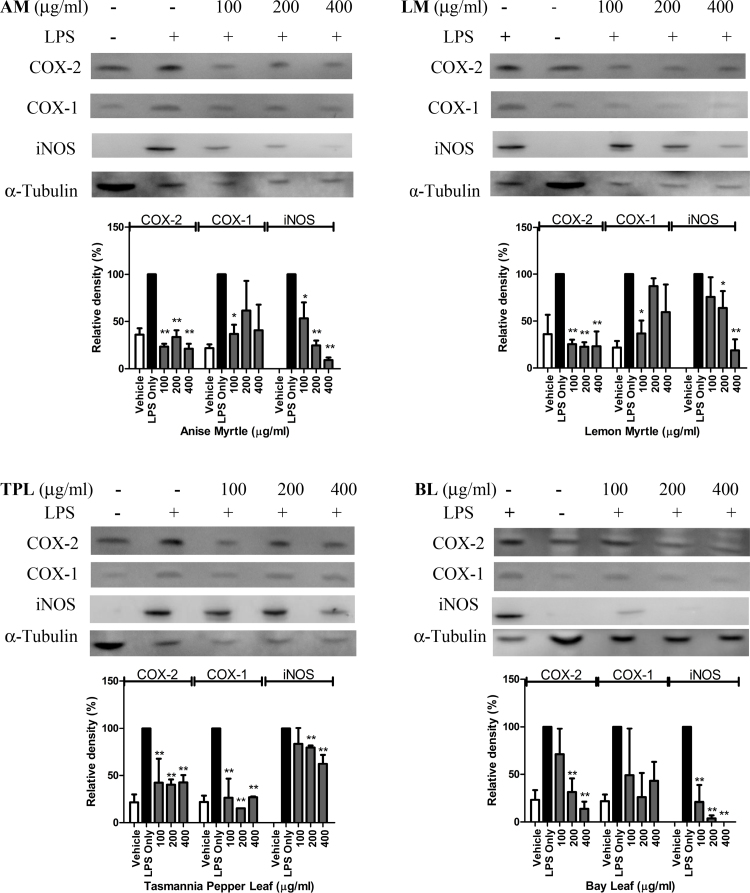
LPS challenge of the RAW 264.7 macrophage leads to activation of the key pro-inflammatory enzymes, iNOS and COX-2 [9], [17], [25]. In agreement, in the current study an enhanced expression of iNOS and COX-2 proteins was observed upon LPS activation (Fig. 1). Pre-treatment of the murine macrophage cells with polyphenolic-rich extracts of herbs applied at non-cytotoxic concentrations of 100, 200 and 400 μg/ml (as monitored by MTT assay using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; data not presented), inhibited the protein expression levels of iNOS and COX-2 in LPS-activated RAW 264.7 cells (Fig. 1). All native Australian herbs extracts exhibited a more pronounced inhibition of COX-2 than iNOS. Pre-treatment with the lowest concentration of extracts (100 μg/ml) decreased COX-2 accumulation to its level in non-activated macrophages. The same level of inhibition was observed at higher concentrations of the extracts of 200 and 400 μg/ml. In contrary, the reference sample bay leaf applied at the concentration of 100 μg/ml did not inhibit COX-2, but was active at higher concentrations. The effect was dose-dependent.
Fig. 1.
Effect of polyphenolic-rich extracts of native Australian herbs and a reference sample bay leaf on the expression levels of COX-2, COX-1 and iNOS in LPS-activated RAW 264.7 cells. AM: anise myrtle. LM: lemon myrtle. TPL: Tasmannia pepper leaf. BL: bay leaf. *Significant difference with LPS control at p < 0.05; **significant difference with LPS control at p < 0.01.
The expression of COX-1 enzyme has not been affected by pre-treatment of RAW 264.7 macrophage with bay leaf, anise myrtle and lemon myrtle extracts. The pre-treatment with anise myrtle and lemon myrtle extracts at the lowest concentration of 100 μg/ml showed some reduction of COX-1 accumulation in LPS-activated macrophages to its level in non-activated cells however this has not been confirmed at higher levels and is most probably an artifact introduced by the measuring technique. Tasmannia pepper leaf extract however exhibited a significant negative effect on COX-1 accumulation and this effect was stronger than the suppression of COX-2 (Fig. 1, TPL).
The inhibitory effects of herbal extracts toward iNOS accumulation were dose-dependent and statistically significant for all the evaluated extracts. The reference sample bay leaf showed a superior effect. Among the native Australian herbs, anise myrtle was the most effective with 90.9% inhibition at the highest concentration, followed by lemon myrtle (81.2% inhibition at the highest concentration) and Tasmannia pepper leaf (37.6% inhibition at the highest concentration).
3.2. Effect of polyphenolic-rich extracts on nitrite concentration and PGE2 production in LPS-induced macrophages
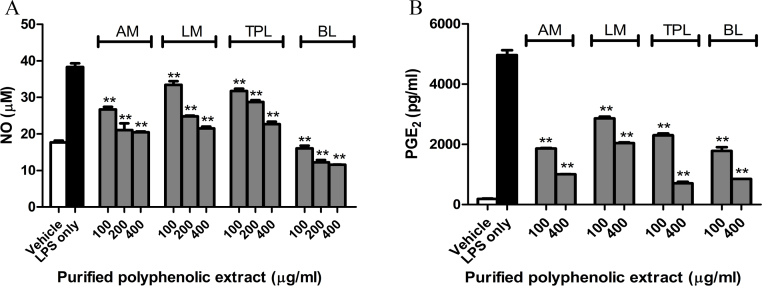
The increase of iNOS and COX-2 expression in LPS-activated murine macrophage RAW 264.7 was accompanied by the release of large amounts of their products, respectively, NO and PGE2 (Fig. 2). Exposure of the LPS-activated RAW 264.7 cells to the polyphenolic-rich herbal extracts resulted in a concentration-dependent reduction of NO (Fig. 2A). Bay leaf extract had the strongest inhibitory effect and was followed by anise myrtle. This result is in agreement with the suppression of iNOS expression by bay leaf showed above. Each extract produced also a concentration-dependent decrease in the levels of the COX-2 product, PGE2 (Fig. 2B), which confirms the inhibitory effect of herbal extracts toward the COX-2 enzyme.
Fig. 2.
Effect of polyphenolic-rich extracts from native Australian herbs and bay leaf on nitric oxide (NO) (A) and prostaglandin E2 (B) release in LPS-activated RAW 264.7 macrophage. AM: anise myrtle, LM: lemon myrtle, TPL: Tasmania pepper leaf, BL: bay leaf. **Significant difference with LPS control at p < 0.01.
3.3. Composition of polyphenolic-rich extracts
The compositions of the polyphenolic-rich extracts evaluated in this study were a subject of our earlier studies [19]. Table 1 presents the summary of the earlier results in order to discuss the relationship between the extracts composition and their potential physiological activities, as evaluated in the present study. Ellagic acid and derivatives were the major compounds of anise myrtle and lemon myrtle extracts, contributing, respectively 67% and 46% of the mixtures. Both extracts contained approximately 3% of quercetin and their glycosides as well as low levels of myricetin and hesperetin. The composition of Tasmannia pepper leaf extract was different, comprising of chlorogenic acid (29%) and quercetin and derivatives (11%). Traces of anthocyanins, cyanidin 3-glucoside and cyanidin 3-rutinoside, were also detected. Due to the fact that the leaf of the investigated native Australian herbs resemble the well known bay leaf and they are used in a similar way in the Australian cuisine as the bay leaf is used in western meals, we have selected bay leaf as a reference sample. The major phenolic compounds present in bay leaf are reported to be simple phenolic acids and flavan-3-ols [22].
Table 1.
Phenolic compounds in purified polyphenolic-rich extracts of anise myrtle, lemon myrtle and Tasmannia pepper leaf (mg/g DW).
| Compound | Anise myrtle | Lemon myrtle | Tasmannia pepper leaf |
|---|---|---|---|
| Ellagic acid | 153 ± 0.7 | 102 ± 5.8 | ND |
| Ellagic acid derivativesa | 514 ± 10.0 | 360 ± 27.0 | ND |
| Chlorogenic acid | ND | ND | 288.2 ± 10.2 |
| Catechin | 17.3 ± 4.5 | ND | ND |
| Quercetinb,c | 29.1 ± 4.9 | 31.3 ± 6.2 | 45.6 ± 4.4 |
| Quercetin 3-rutinosidec | ND | ND | 68.3 ± 9.4 |
| Myricetinc | 1.04 ± 0.2 | 1.20 ± 0.2 | ND |
| Hesperetinc | 4.10 ± 0.6 | 5.37 ± 1.1 | ND |
| Cyanidin 3-glucosided | ND | ND | 0.37 ± 0.01 |
| Cyanidin 3-rutinosided | ND | ND | 0.02 ± 0.001 |
Ellagitannins and ellagic acid glycosides were quantified as ellagic acid equivalent following hydrolysis based on the peak area at 250 nm.
Includes quercetin glycosides with the exception of quercetin 3-rutinoside.
Myricetin, hesperetin, quercetin and derivatives were quantified as quercetin 3-rutinoside equivalent based on the peak area at 370 nm.
Cyanidins were quantified as cyanidin 3-glucoside equivalent.
4. Discussion
The present study was undertaken to evaluate in vitro the potential anti-inflammatory activities of the leading commercially grown indigenous Australian herbs, anise myrtle, lemon myrtle and Tasmannia pepper leaf, using the accepted LPS-induced RAW 264.7 model system. The results showed that the evaluated polyphenolic-rich extracts successfully reduced the expression of inflammatory enzymes iNOS and COX-2 in LPS-activated murine macrophages, and this reduction was accompanied by the decrease of nitric oxide (NO) and prostaglandin E2 (PGE2) levels. COX-2 is an inducible enzyme upregulated at inflammatory sites [6], while its isoform COX-1 is constitutively expressed in most tissues, being responsible for maintaining basal levels of prostaglandins and many homeostatic functions [7]. Tasmannia pepper leaf extract exhibited inhibitory effect toward COX-1 simultaneously with the inhibition of iNOS and COX-2 enzymes. Anise myrtle and lemon myrtle also effectively inhibited the activities of iNOS and COX-2 and suppressed the release of the inflammatory mediators, NO and PGE2, without significant inhibition of COX-1. Therefore, anise myrtle and lemon myrtle are more suitable anti-inflammatory agents than Tasmannia pepper leaf.
Ellagitannins and ellagic acid were the major compounds of anise myrtle and lemon myrtle polyphenolic-rich extract. Similarly, polyphenolic compounds from native to Australia Tasmanian blue gum (Eucalyptus globulus) leaf or Malaysian honey, both containing ellagic acid, exhibited pronounced anti-inflammatory activities in vitro [12], [23]. The anti-inflammatory activity of ellagic acid based extracts has also been confirmed at in vivo level. Dietary supplementation of ellagic acid enriched pomegranate extract reduced chronic colonic inflammation in rats [18], while purified ellagic acid efficiently reduced carrageenan-induced inflammation in rats through inhibition of nuclear factor kappa B (NF-κB), inducible COX-2 and suppressing the accumulation of NO, pro-inflammatory cytokines IL-1β and TNF-α, as well as the induction of glutathione (GSH) and release of the anti-inflammatory cytokinin IL-10 [8].
Chlorogenic acid and quercetin derivatives are the major components of Tasmannia pepper leaf polyphenolic-rich extract (Table 1). This extract reduced LPS-induced inflammatory responses in RAW 264.7 cells. In agreement, Shan et al. [21] reported that chlorogenic acid decreased LPS-induced up-regulation of COX-2 at protein and mRNA levels in RAW 264.7 cells, which resulted in inhibition of PGE2 release by LPS-treated RAW 264.7 cells. The authors showed that chlorogenic acid significantly suppressed the LPS-induced activation of nuclear factor-kappaB (NF-κB) and c-Jun N-terminal kinase (JNK)-c-Jun-activator protein (AP-1) pathway [21]. Similarly, chlorogenic acid and quercetin derivatives isolated from pear, Rosa laevigata Michx, Tithonia diversifolia and blue honeysuckle (Lonicera caerulea L.) suppressed apoptosis and reduced inflammation in both in vitro and in vivo models [2], [11], [16], [26].
5. Conclusions
The present study demonstrates for the first time the potential anti-inflammatory activities of native Australian herbs polyphenols-rich extracts: anise myrtle, lemon myrtle and Tasmannia pepper leaf. The anti-inflammatory activities occurred through down-regulation of iNOS and COX-2 enzymes and inhibition of the accumulation of their respective products, NO and PGE2. This study has shown that anise myrtle and lemon myrtle potentially could be more efficient anti-inflammatory agents than Tasmannia pepper leaf.
Conflict of interest
There is no conflict of interest.
Transparency document
Footnotes
Available online 15 July 2014
References
- 1.Barr A. Greenhouse Publications; Richmond, Victoria, Australia: 1988. Traditional Bush Medicines: An Aboriginal Pharmacopoeia. [Google Scholar]
- 2.Chagas-Paula D.A., De Oliveira R.B., Da Silva V.C., Gobbo-Neto L., Gasparoto T.H., Campanelli A.P., Faccioli L.H., Da Costa F.B. Chlorogenic acids from Tithonia diversifolia demonstrate better anti-inflammatory effect than indomethacin and its sesquiterpene lactones. J. Ethnopharmacol. 2011;136(2):355–362. doi: 10.1016/j.jep.2011.04.067. [DOI] [PubMed] [Google Scholar]
- 3.Cianchi F., Perna F., Masini E. iNOS/COX-2 pathway interaction: a good molecular target for cancer treatment. Curr. Enzyme Inhib. 2005;1(2):97–105. [Google Scholar]
- 4.Cinkilic N., Cetintas S.K., Zorlu T., Vatan O., Yilmaz D., Cavas T., Tunc S., Ozkan L., Bilaloglu R. Radioprotection by two phenolic compounds: chlorogenic and quinic acid, on X-ray induced DNA damage in human blood lymphocytes in vitro. Food Chem. Toxicol. 2013;53:359–363. doi: 10.1016/j.fct.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Corbett S., Daniel J., Drayton R., Field M., Steinhardt R., Garrett N. Evaluation of the anti-inflammatory effects of ellagic acid. J. Perianesth. Nurs. 2010;25(4):214–220. doi: 10.1016/j.jopan.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Crofford L.J. COX-1 and COX-2 tissue expression: implications and predictions. J. Rheumatol. Suppl. 1997;49:15–19. [PubMed] [Google Scholar]
- 7.Greenhough A., Smart H.J., Moore A.E., Roberts H.R., Williams A.C., Paraskeva C., Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 8.E.I-Shitany N.A., E.I-Bastawissy E.A., E.I-Desoky K. Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 via an antioxidant mechanism. Int. Immunopharmacol. 2014;19(2):290–299. doi: 10.1016/j.intimp.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Herschman H.R. Prostaglandin synthase 2. Biochim. Biophys. Acta. 1996;1299:125–140. doi: 10.1016/0005-2760(95)00194-8. [DOI] [PubMed] [Google Scholar]
- 10.Hodgson J.M., Wahlqvist M.L. National Better Health Program; Melbourne, Australia: 1992. Koori Nutrition and Health: A Victorian Review. [Google Scholar]
- 11.Jin X.H., Ohgami K., Shiratori K., Suzuki Y., Koyama Y., Yoshida K., Ilieva I., Tanaka T., Onoe K., Ohno S. Effects of blue honeysuckle (Lonicera caerulea L.) extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 2006;82(5):860–867. doi: 10.1016/j.exer.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 12.Kassim M., Achoui M., Mustafa M.R., Mohd M.A., Yusoff K.M. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr. Res. 2010;30(9):650–659. doi: 10.1016/j.nutres.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Khang W.O., Annie H., Benny K.H.T. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by AMPK activation. Biochem. Pharm. 2013;85(9):1341–1351. doi: 10.1016/j.bcp.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Landete J.M. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, function and health. Food Res. Int. 2011;44:1150–1160. [Google Scholar]
- 15.Lee W.J., Ou H.C., Hsu W.C., Chou M.M., Tseng J.J., Hsu S.L., Tsai K-L., Sheu W.H-H. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J. Vasc. Surg. 2010;52(5):1290–1300. doi: 10.1016/j.jvs.2010.04.085. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Wang T.T., Zhou B., Gao W., Cao J., Huang L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.) Food Chem. 2014;152:531–538. doi: 10.1016/j.foodchem.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Lowenstein C.J., Alley E.W., Raval P., Snowman A.M., Snyder S.H., Russell S.W., Murphy W.J. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon. Proc. Natl. Acad. Sci. U. S. A. 1993;90:9730L 9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosillo M.A., Sánchez-Hidalgo M., Cárdeno A., Aparicio-Soto M., Sánchez-Fidalgo S., Villegas I., Alarcón de la Lastra C. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol. Res. 2012;66(3):235–242. doi: 10.1016/j.phrs.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Sakulnarmrat K., Konczak I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012;134(2):1011–1019. doi: 10.1016/j.foodchem.2012.02.217. [DOI] [PubMed] [Google Scholar]
- 20.Sakulnarmrat K., Fenech M., Thomas P., Konczak I. Cytoprotective and pro-apoptotic activities of native Australian herbs polyphenolic-rich extracts. Food Chem. 2013;136:9–17. doi: 10.1016/j.foodchem.2012.07.089. [DOI] [PubMed] [Google Scholar]
- 21.Shan J.H., Fu J., Zhao Z., Kong X., Huang H., Luo L., Yin Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NFκB and JNK/AP-1 activation. Int. Immunopharmacol. 2009;9(9):1042–1048. doi: 10.1016/j.intimp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Shan B., Cai Y.Z., Sun M., Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005;53(20):7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto K., Sakamoto S., Nakagawa K., Hayashi S., Harada N., Yamaji R., Nakano Y., Inui H. Suppression of inducible nitric oxide synthase expression and amelioration of lipopolysaccharide-induced liver injury by polyphenolic compounds in Eucalyptus globulus leaf extract. Food Chem. 2011;125(2):442–446. [Google Scholar]
- 24.Tan A.C., Hou D.X., Konczak I., Ramzan I., Tanigawa S., Sze D.M-Y. Native Australian fruit polyphenols inhibit COX-2 and iNOS expression in LPS-activated murine macrophages. Food Res. Int. 2011;44(7):2362–2367. [Google Scholar]
- 25.Wadleigh D.J., Reddy S.T., Kopp E., Ghosh S., Herschman H.R. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J. Biol. Chem. 2000;275:6259–6266. doi: 10.1074/jbc.275.9.6259. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S., Qi Y., Xu Y., Han X., Peng J., Liu K., Sun C.K. Protective effect of flavonoid-rich extract from Rosa laevigata Michx on cerebral ischemia-reperfusion injury through suppression of apoptosis and inflammation. Neurochem. Int. 2013;63(5):522–532. doi: 10.1016/j.neuint.2013.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.