Abstract
Coleus forskohlii extract (CFE), an herbal ingredient, is used for weight-loss products. CFE's alleged efficacy is attributed to forskolin. However, CFE has been shown to induce fatty liver in mice, with components other than forskolin playing a part in this effect. The present study addressed the underlying mechanism of CFE-induced fatty liver by analyzing changes in CFE-treated mice of lipid concentrations and of the levels of mRNAs encoding enzymes and transcription factors known to be related to fatty liver. Mice were fed a diet containing 0, 0.3 and 1% CFE for 2 weeks. CFE at 1% clearly induced fatty liver, as demonstrated by histological examination and confirmed by increases in triglyceride concentrations in liver. However, treated mice did not exhibit elevation in plasma levels of non-esterified fatty acids. Comprehensive analysis of liver mRNA levels revealed accumulation of multiple transcripts, including mRNAs encoding enzymes acetyl-CoA carboxylase and long-chain elongase; transcription factor peroxisome proliferator-activated receptor gamma (PPARγ); and lipid-droplet-associated fat-specific protein 27 (Fsp27). These findings suggest that the de novo synthesis and accumulation of triglyceride in the liver, through the enhanced expression of specific lipogenic mRNAs, is a major underlying mechanism of fatty liver induction by CFE.
Keywords: Coleus forskohlii, Forskolin, Fatty liver, De novo synthesis, PPARγ, Fsp27, Dietary supplement
1. Introduction
Obesity is an ongoing concern in the developed world, because this condition increases the risk of chronic diseases such as diabetes mellitus and cardiovascular disease [1]. To fight obesity, weight-loss dietary supplements often are used without adequate clinical evidence [2]. Among dietary supplements, herbal products are increasingly used [3], and are sometimes perceived as safe because such supplements are “natural”. However, recent studies have suggested that herbal products, especially those used for weight loss, can cause adverse events such as serious hepatic failure [4].
Coleus forskohlii extract (CFE) has been used for centuries in Ayurvedic medicine to treat various diseases of the cardiovascular, respiratory, gastrointestinal, and central nervous systems [5]. Currently, CFE has received attention as a popular herbal ingredient for weight-loss products, because CFE contains a diterpene compound, forskolin. This compound has been shown to activate adenylate cyclase [6], [7] to enhance lipolysis and fat loss in studies performed in cell culture [8], [9], rat [10], and human [11], [12]. Based on the activity of this component, CFE is generally standardized at 10% forskolin for use in dietary supplements.
In our previous studies, we observed that CFE induced hepatic cytochrome P450 (CYP) while also inducing fatty liver in mice, although these induction events were not seen with forskolin alone [13], [14]. The substance that induced fatty liver and hepatic CYP induction was soluble in ether and ethyl acetate [15]. CYP was induced by CFE at a dose lower than that needed to induce fatty liver, but both phenomena (CYP and fatty liver) seemed to be related. CFE-mediated CYP induction was clearly detected in high-carbohydrate diet [16]. To confirm the safety of CFE as a dietary supplement, we sought to examine the mechanism of action of CFE in the induction of fatty liver.
The development of fatty liver has been attributed to increased release of non-esterified fatty acids from adipose tissue; increased de novo synthesis of fatty acids; decreased beta-oxidation [17]; and decreased export of triglyceride as lipoprotein from liver [18].
In the present study, we examined the possible mechanism of action of CFE on fatty liver in mice by evaluating changes in lipid concentrations in plasma and liver, along with comprehensive profiling of hepatic mRNA expression of genes coding for lipogenic and triglyceride synthesis enzymes, transcription factors, and nuclear receptors.
2. Materials and method
2.1. Materials
Powdered CFE standardized to contain 10% forskolin was prepared as follows. Dried roots of C. forskohlii were obtained from the Bangalore in southern India, crushed, and subjected to extraction with supercritical CO2. The resulting forskolin-rich extract (20–30%) was combined with dextrin to yield a powder containing 10% forskolin. These extraction and preparation steps were outsourced to Tokiwa Phytochemical Co., Ltd. (Chiba, Japan). The final composition of the resulting CFE powder was as follows: water, 5.6%; protein, 0.3%; lipids, 22.7%; ash, 2.2%; and carbohydrates, 69.2%. The HPLC chromatographic profile has been reported elsewhere [19], and the analyzed contents of forskolin and 1,9-dideoxyforskolin, two substances available as standards in the CFE sample, were 10.37% and 1.71%, respectively. CFE was added in the proportions described below to an AIN93G purified diet purchased from Oriental Yeast Co., Ltd. (Tokyo, Japan). All other reagents were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
2.2. Animal experiment
Male ICR mice, aged 5 weeks (CLEA Japan, Inc., Tokyo, Japan), were housed in polypropylene cages at a constant temperature (22 ± 1 °C) with a 12-h/12-h light-dark cycle. After acclimation for 1 week, mice were allocated into three groups (5 mice per group), and provided with ad libitum access to AIN93G purified diet without CFE (0% CFE; control) or supplemented to 0.3% CFE or 1% CFE. After two weeks on the respective diet, animals were anesthetized with pentobarbital and exsanguinated from inferior vena cava with heparin as an anticoagulant. Livers were immediately removed, weighed, and assessed as follows. For all animals, a portion of the liver was stored in RNAlater (Applied Biosystems, Inc., Foster City, CA, USA) pending processing for mRNA analysis. For a subset of the animals, portions of the livers were fixed in 10% neutral buffered formalin pending processing for histopathological analysis. Other samples were snap frozen on dry ice and stored at −80 °C until analysis. All animal procedures were conducted in accordance with the Japan National Institute of Health and Nutrition Guidelines for the Care and Use of Laboratory Animals, and were approved by ethical committee of the Japan National Institute of Health and Nutrition.
2.3. Analysis of mRNA levels
Real time RT-PCR experiments were performed by the method previously described [20]. Briefly, total RNA was extracted using a QuickGene RNA tissue kit SII (Fuji Photo Film Co., Ltd., Tokyo, Japan), and the samples were subjected to real time RT-PCR using the One-Step SYBR RT-PCR kit (Perfect Real Time; Takara Bio Inc., Shiga, Japan) according to the manufacturer's protocol and Mx3000P® (STRATAGENE Co., La Jolla, CA, USA). The results were expressed as copy number ratio of the target mRNA to that of cyclophilin mRNA. The specific primers were synthesized via the Perfect Real Time Primer support system of Takara Bio (http://www.takara-bio.co.jp/prt/intro.htm); primer sequences are shown in Table 1. The mRNAs analyzed in the present study included transcripts encoding glycolytic enzymes Gck, Gapdh, Pklr1 and Pklr2; lipogenesis enzymes Acly, ACC1, ACC2, and Fasn; fatty acid elongation and desaturation enzymes Elovl6 and Scd1; triglyceride synthesis enzymes Gpam, Dgat1, and Dgat2; transcription factor and nuclear receptor proteins ChREBP, Srebp1, Nr1h2, Nr1h3, and PPARα, PPARγ, and PPARδ; and Cidea, Cideb, Cidec/Fsp27 [21].
Table 1.
Sequences of primers used for real-time RT-PCR analysis (5′–3′).
| Encoded protein | Forward | Reverse |
|---|---|---|
| Cyclophilin | ACGCCACTGTCGCTTTTC | CTGCAAACAGCTCGAAGGA |
| Gck | CTGGATGACAGAGCCAGGAT | CTCTGCCAGGATCTGCTCTAC |
| Gapdh | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
| Pklr1 | ACTTAGCAAAGTCTGCTTTAAGTGG | TGGCACGTCTCAGGTATCC |
| Pklr2 | GTGGAGGCTTCCTTCAAGTG | AGGTCGGTAGCGAGACAGAA |
| Acly | GCCCTGGAAGTGGAGAAGAT | CCGTCCACATTCAGGATAAGA |
| ACC1 | GCGTCGGGTAGATCCAGTT | CTCAGTGGGGCTTAGCTCTG |
| ACC2 | TGAATCTCACGCGCCTACTA | GCCTCTCTTCACCAGATGGA |
| Fasn | GCTGCTGTTGGAAGTCAGC | AGTGTTCGTTCCTCGGAGTG |
| Elovl6 | CAGCAAAGCACCCGAACTA | AGGAGCACAGTGATGTGGTG |
| Scd1 | TTCCCTCCTGCAAGCTCTAC | CAGAGCGCTGGTCATGTAGT |
| Gpam | GGAAGGTGCTGCTATTCCTG | TGGGATACTGGGGTTGAAAA |
| Dgat1 | TCGTGGTATCCTGAATTGGTG | AGGTTCTCTAAAAATAACCTTGCATT |
| Dgat2 | GGCGCTACTTCCGAGACTAC | TGGTCAGCAGGTTGTGTGTC |
| ChREBP | GGCCTGGCTGGAACAGTA | CGAAGGGAATTCAGGACAGT |
| Srebf1 | GGTTTTGAACGACATCGAAGA | CGGGAAGTCACTGTCTTGGT |
| Nr1h2 | GCTCTGCCTACATCGTGGTC | CTCATGGCCCAGCATCTT |
| Nr1h3 | TGTGCGCTCAGCTCTTGT | TGGAGCCCTGGACATTACC |
| Ppara | CTGAGACCCTCGGGGAAC | AAACGTCAGTTCACAGGGAAG |
| Pparg | GAAAGACAACGGACAAATCACC | GGGGGTGATATGTTTGAACTTG |
| Ppard | ATGGGGGACCAGAACACAC | GGAGGAATTCTGGGAGAGGT |
| Cidea | AAACCATGACCGAAGTAGCC | AGGCCAGTTGTGATGACTAAGAC |
| Cideb | CTGCCAGCCTCCAAGAACT | TAGCACTCCACGTAGCAGCA |
| Cidec | GATGGACTACGCCATGAAGTC | GTGCTCACTGCCACATGC |
Protein product abbreviations: Gck, glucokinase; Gapdh, glyceraldehyde-3-phosphate dehydrogenase; Pklr1 and Pklr2, pyruvate kinase liver and red blood cell (Pklr) 1 and 2; Acly, ATP-citrate synthase; ACC1 and ACC2, acetyl-coenzyme A carboxylase 1 and 2; Fasn, fatty acid synthase; Elovl6, long-chain elongase; Scd1, acyl-CoA desaturase 1; Gpam, glycerol-3-phosphate acyltransferase; Dgat1 and Dgat2, diacylglycerol acyltransferase 1 and 2; ChREBP, carbohydrate-responsive element-binding protein; Srebp1, sterol regulatory element binding factor 1; Nr1h2 and Nr1h3, liver X receptor (LXR) beta and alpha; Ppara, Pparg and Ppard, peroxisome proliferator-activated receptors alpha, gamma, and delta; Cidea, Cideb and Cidec/Fsp27, cell death–inducing DNA fragmentation factor 45-like effectors (CIDEs) A, B, and C.
2.4. Other measurements
Liver samples fixed with formalin were embedded in paraffin using standard procedures. The samples were sectioned at 3-μm thicknesses for staining with hematoxylin and eosin (H&E), or sectioned at 10-μm thicknesses for staining with Oil Red O. These morphological analyses were outsourced to Biosafety Research Center, Foods, Drugs and Pesticides (An-Pyo Center), Shizuoka, Japan. Measurement of plasma enzyme activities indicative of hepatic failure was outsourced to SRL Inc., Tokyo, Japan. The analyzed activities were alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP). Hepatic lipids were extracted by the methods of Bligh and Dyer [22]. Concentrations of triglyceride, cholesterol, phospholipid, and non-esterified fatty acid were measured using test kits from Wako Pure Chemical Industries, Ltd., Osaka Japan.
2.5. Statistical analyses
The data are presented as the mean and standard error (SE) for the individual groups. Statistical analysis of the data was carried out by one-way ANOVA with post hoc Dunnett's Multiple Comparisons Test where significance was indicated. Differences with p < 0.05 were considered to be significant. Statistical analyses were performed using Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
3. Results
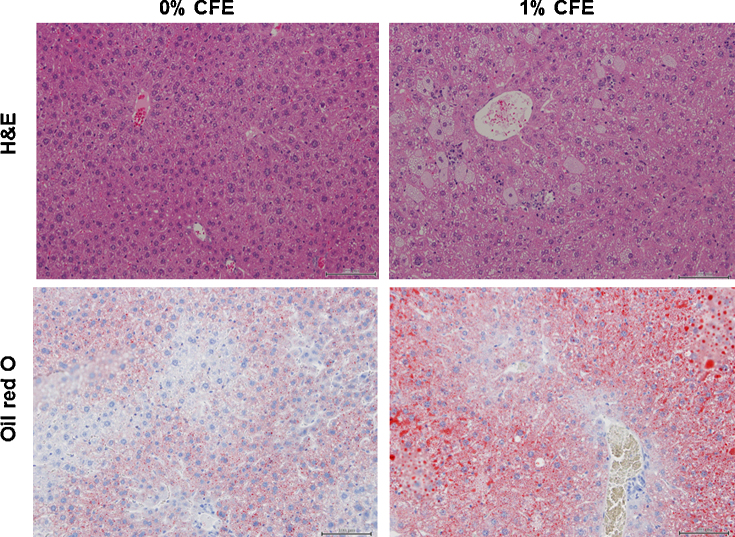
Body weight did not change with CFE treatment, but liver weight normalized to body weight (relative liver weight) increased in the 0.3% and 1% CFE groups. Hepatic lipid accumulation was confirmed by staining with Oil Red O. The liver tissue from the 0% CFE mice showed no-remarkable change, but that from the 1% CFE mice exhibited hepatic cellular damage, such as fatty change and necrosis (Fig. 1). Among the liver marker enzymes (AST, ALT, ALP) in plasma, ALP increased in the 1% CFE group; while other enzymes appeared to be elevated by CFE treatment, those changes were not statistically significant due to high standard error (Table 2). Plasma concentration of triglycerides was increased in the 1% CFE group; plasma levels of cholesterol, phospholipid, and non-esterified fatty acid were not changed by CFE exposure. Liver concentrations of triglyceride and cholesterol were increased in the 0.3% and 1% CFE groups; the increases in liver triglyceride were 2.3- and 3.2-fold in the respective CFE-treated groups compared with the control group.
Fig. 1.
Representative histopathologic changes in liver sections from mice stained with hematoxylin and eosin (H&E) and Oil red O (magnification 200×). Mice were maintained for 2 weeks on diet supplemented with 0, 0.3, or 1% CFE.
Table 2.
Changes in body weight, liver weight, plasma clinical parameters, and lipid concentration in plasma and liver of mice treated with CFE.
| CFE-treated |
|||
|---|---|---|---|
| Control (0%) | 0.3% | 1% | |
| Final body weight (g) | 36.0 ± 0.93 [1.0] | 34.5 ± 0.46 [0.96] | 33.6 ± 0.65 [0.93] |
| Liver weight (% body weight) | 4.33 ± 0.18 [1.0] | 5.91 ± 0.27 [1.4]* | 8.60 ± 0.40 [2.0]* |
| Plasma clinical parameters | |||
| AST (IU/L) | 48.0 ± 7.5 [1.0] | 113.4 ± 32.6 [2.4] | 160.0 ± 45.7 [3.3] |
| ALT (IU/L) | 17.0 ± 2.2 [1.0] | 64.2 ± 21.3 [3.8] | 100.6 ± 42.9 [5.9] |
| ALP (IU/L) | 264 ± 35 [1.0] | 380 ± 72 [1.4] | 512 ± 77 [1.9]* |
| Plasma lipids | |||
| Triglyceride (mg/dL) | 102 ± 19.0 [1.0] | 99 ± 28.2 [1.0] | 208 ± 34.6 [2.0]* |
| Cholesterol (mg/dL) | 208 ± 9.6 [1.0] | 146 ± 24.3 [0.7] | 189 ± 26.9 [0.9] |
| Phospholipid (mg/dL) | 277 ± 14.0 [1.0] | 214 ± 31.6 [0.8] | 268 ± 26.9 [1.0] |
| Non-esterified fatty acid (mequiv./L) | 1.49 ± 0.39 [1.0] | 1.23 ± 0.37 [0.8] | 1.85 ± 0.31 [1.2] |
| Hepatic lipids | |||
| Triglyceride (mg/g liver) | 14.1 ± 1.2 [1.0] | 32.9 ± 2.7 [2.3]* | 45.2 ± 4.9 [3.2]* |
| Cholesterol (mg/g liver) | 7.1 ± 0.51 [1.0] | 12.1 ± 1.5 [1.7]* | 13.4 ± 1.1 [1.9]* |
| Phospholipid (mg/g liver) | 13.9 ± 0.87 [1.0] | 13.1 ± 1.1 [0.95] | 13.4 ± 1.5 [0.97] |
Male ICR mice were maintained for 2 weeks on a diet supplemented with 0% CFE (control), 0.3% CFE, or 1% CFE. Each value is the mean and SE from 5 mice. Numbers in brackets indicate the ratio compared to the control group.
Significant difference from the level of control group is indicated by p < 0.05.
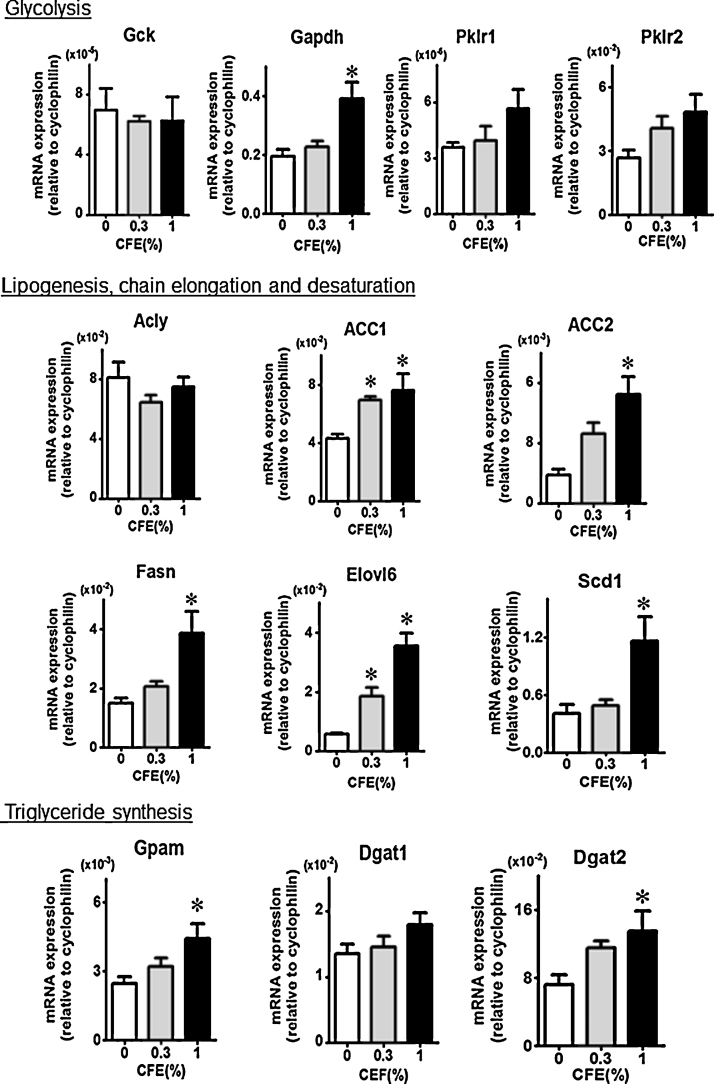
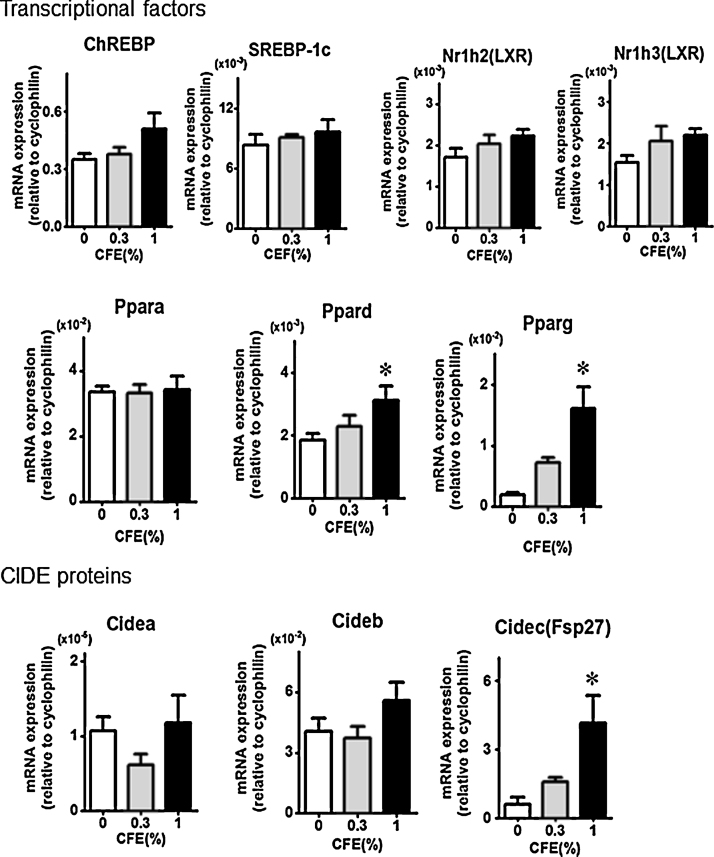
Expression in the liver of mRNAs encoding lipid synthesis enzymes and transcription factors was analyzed. Dose-related increases were detected in the levels of transcripts encoding various metabolic enzymes, especially those for lipogenesis enzyme ACC2 and fatty acid elongation enzyme Elovl6 (Fig. 2). Also, a clear increase was observed in the levels of the mRNAs encoding transcription factor PPARγ and lipid droplet protein Fsp27 (Fig. 3).
Fig. 2.
Expression of mRNAs encoding enzymes involved in glycolysis, fatty acid synthesis, chain elongation and desaturation, and triglyceride synthesis. Protein product abbreviations are defined in the main text and Table 1. Each value is the mean and SE from 5 mice. Significant difference from the level of control group (0% CFE) is indicated by *p < 0.05.
Fig. 3.
Expression of mRNAs encoding transcription factors and effectors involved in fatty liver. Each values is the mean and SE from 5 mice. Protein product abbreviations are defined in the main text and Table 1. Significant difference from the level of control group (0% CFE) is indicated by *p < 0.05.
4. Discussion
As shown by our results, CFE treatment induced obvious fatty liver even at the lower tested dose of 0.3% in the diet. The possible (non-exclusive) mechanisms of fatty liver induction include: (1) enhanced supply of non-esterified fatty acid from adipose tissue, (2) reduced secretion from liver, and (3) enhanced de novo lipogenesis. In an attempt to distinguish these hypotheses, we examined the possible mechanism of action of fatty liver due to CFE treatment.
An overflow of fatty acid derived from lipolysis has been proposed to be the main cause of the excess accumulation of triglyceride observed in hepatic steatosis [23]. The forskolin in CFE is thought to enhance lipolysis due to activation of adenylate cyclase [8], [9]. Therefore, enhanced lipolysis by forskolin is implicated as a source of increased fatty acid, leading to enhanced triglyceride synthesis in the liver. In fact, treatment of mice with 0.5% and 5% CFE for 3 weeks decreased fat tissue weight in our previous study [13]. However, in the present study, non-esterified fatty acid in plasma was not enhanced by 0.3% CFE treatment for 2 weeks, whereas a significant increase in liver triglyceride concentration was detected in this group. This finding suggests that an overflow of fatty acid derived from lipolysis is unlikely to be the mechanism of CFE-induced hepatic steatosis. Triglyceride is exported as lipoprotein from liver into blood. Reduced triglyceride secretion could lead to accumulation of triglyceride in the liver, as observed in the case of CCl4 administration [18]. However, in the present study, treatment with CFE did not yield a decrease in plasma lipid concentration; plasma triglyceride was rather high in 1% CFE group. This fact suggests that the effects of CFE exposure on fatty liver are not mediated by decreases in the secretion of liver triglycerides as in the case of CCl4 administration. Therefore, we speculate that enhanced de novo lipogenesis is involved in CFE-induced fatty liver.
In de novo lipogenesis, ACC and Fasn catalyze the rate-limiting and final steps, respectively [24]. Palmitoyl-CoA is elongated by Elovl6 and Scd1. Enzymes for triglyceride synthesis are transcriptionally regulated by ChREBP, SREBP-1c, and the LXRs (Nr1h2 and Nr1h3) in liver. ChREBP and SREBP-1c induce ACC, Fasn, Elovl6, and Scd1 genes in response to glucose and insulin, respectively. LXRs directly and indirectly activate transcription of the ACC-, Fasn-, and Scd1-encoding loci. In our present comprehensive RT-PCR analysis, expression of mRNAs encoding enzymes involved in de novo synthesis of fatty acid and triglyceride were increased, especially those coding for ACC, Fasn, and Elovl6. These changes in mRNA expression could contribute to enhance de novo lipogenesis due to CFE treatment, although mRNAs for the transcription factors themselves did not show significant accumulation.
Accumulating evidence suggests that nuclear receptor PPARs, which consist of PPARα, PPARγ, and PPARδ, are involved in lipid metabolism [25]. In the present study, expression of the transcript encoding PPARγ showed a clear increase in response to CFE treatment. PPARγ is expressed predominantly in adipose tissue, with low expression in liver [26], although this factor has been shown to play a critical role in hepatic steatosis in obese or diabetic mouse models [27], [28], [29]. Among CIDE proteins, which are involved in lipid droplet growth and lipoprotein lipidation [21], Fsp27/Cidec is an adipocyte lipid droplet protein [30]; the Fsp27-encoding gene is directly regulated by PPARγ in hepatic steatosis [28], [31]. In the present study, the mRNAs for Fsp27/Cidec and for PPARγ both accumulated following exposure to CFE. On the other hand, the PPARα gene is expressed predominantly in the liver and is a major activator of fatty acid oxidation pathways; elevated PPARα activity leads to decreased lipid levels. PPARδ is ubiquitously expressed in many tissues and has functions similar to those of PPARα. In the present study, expression of the PPARα-encoding mRNA did not exhibit change even at the higher tested dose of 1% CFE. Based on these findings, we conclude that enhanced accumulation of PPARγ- and Fsp27/Cidec-encoding transcripts might be a major contributor to CFE-induced fatty liver. Further detail study will be needed to confirm expression of PPARγ- and Fsp27/Cidec at protein level.
CFE is a natural herbal product, and composition may vary among products. We reported that two sources of CFE standardized with 10% forskolin showed similar increases in relative liver weight and CYP induction [14]. Also, we observed that CFE treatment induced fatty liver and hepatic CYP induction not only in ICR mice but also in C57BL mice (unpublished observation), suggesting fatty liver is commonly induced by CFE. It will be critical to identify the active substance(s) involved in the CFE induction of fatty liver. Notably, forskolin itself appears not to be involved in CFE induction of fatty liver and CYP [13], [14]. In ongoing research, we are seeking to identify the active substance. To date, we have shown that the substance is lipophilic, as demonstrated by solubility in ether and ethyl acetate [15]. Drug metabolism and energy metabolism pathways have been shown to interface through nuclear receptors [32]. The present work suggests that the active substance in CFE affects the expression of the PPARγ-encoding gene; future work will need to examine how the accumulation of PPARγ message is related to CYP induction following CFE treatment. It would be beneficial to seek unidentified substance by the expression of PPARγ in studies in vivo and in vitro.
In conclusion, this study indicated that CFE, a popular weight-loss dietary supplement, induces fatty liver by de novo lipogenesis, a process that may be mediated through enhanced expression of multiple enzymes and transcripts, in particular encoding PPARγ and Fsp27. Previous work suggested that fatty liver induction is the result of an undefined (non-forskolin) component of CFE. Thus, it will be necessary to identify unknown substance by focusing on the expression of PPARγ and Fsp27, and eliminate this activity from the extract to render CFE safe for use as a weight-loss dietary supplement.
Conflict of interest
The authors declare that there are no conflicts of interest.
Transparency document
Acknowledgement
This study was financially supported in part by a research grant from the Ministry of Health, Labour and Welfare, Japan (H24-006).
Footnotes
Available online 10 October 2014
References
- 1.Low S., Chin M.C., Deurenberg-Yap M. Review on epidemic of obesity. Ann. Acad. Med. Singapore. 2009;38:57–59. [PubMed] [Google Scholar]
- 2.Egras A.M., Hamilton W.R., Lenz T.L., Monaghan M.S. An evidence-based review of fat modifying supplemental weight loss products. J. Obes. 2011:2011. doi: 10.1155/2011/297315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gershwin M.E., Borchers A.T., Keen C.L., Hendler S., Hagie F., Greenwood M.R. Public safety and dietary supplementation. Ann. N. Y. Acad. Sci. 2010;1190:104–117. doi: 10.1111/j.1749-6632.2009.05270.x. [DOI] [PubMed] [Google Scholar]
- 4.Yellapu R.K., Mittal V., Grewal P., Fiel M., Schiano T. Acute liver failure caused by ‘fat burners’ and dietary supplements: a case report and literature review. Can. J. Gastroenterol. 2011;25:157–160. doi: 10.1155/2011/174978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ammon H.P., Muller A.B. Forskolin: from an ayurvedic remedy to a modern agent. Planta Med. 1985;51:473–477. doi: 10.1055/s-2007-969566. [DOI] [PubMed] [Google Scholar]
- 6.Metzger H., Lindner E. The positive inotropic-acting forskolin, a potent adenylate cyclase activator. Arzneimittelforschung. 1981;31:1248–1250. [PubMed] [Google Scholar]
- 7.Seamon K.B., Padgett W., Daly J.W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc. Natl. Acad. Sci. U. S. A. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen D.O., Ahmed B., Naseer K. Relationships between cyclic AMP levels and lipolysis in fat cells after isoproterenol and forskolin stimulation. J. Pharmacol. Exp. Ther. 1986;238:659–664. [PubMed] [Google Scholar]
- 9.Okuda H., Morimoto C., Tsujita T. Relationship between cyclic AMP production and lipolysis induced by forskolin in rat fat cells. J. Lipid Res. 1992;33:225–231. [PubMed] [Google Scholar]
- 10.Han L.K., Morimoto C., Yu R.H., Okuda H. Effects of Coleus forskohlii on fat storage in ovariectomized rats. Yakugaku Zasshi. 2005;125:449–453. doi: 10.1248/yakushi.125.449. [DOI] [PubMed] [Google Scholar]
- 11.Godard M.P., Johnson B.A., Richmond S.R. Body composition and hormonal adaptations associated with forskolin consumption in overweight and obese men. Obes. Res. 2005;13:1335–1343. doi: 10.1038/oby.2005.162. [DOI] [PubMed] [Google Scholar]
- 12.Henderson S., Magu B., Rasmussen C., Lancaster S., Kerksick C., Smith P., Melton C., Cowan P., Greenwood M., Earnest C., Almada A., Milnor P., Magrans T., Bowden R., Ounpraseuth S., Thomas A., Kreider R.B. Effects of Coleus forskohlii supplementation on body composition and hematological profiles in mildly overweight women. J. Int. Soc. Sports Nutr. 2005;2:54–62. doi: 10.1186/1550-2783-2-2-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virgona N., Taki Y., Yamada S., Umegaki K. Dietary Coleus forskohlii extract generates dose-related hepatotoxicity in mice. J. Appl. Toxicol. 2013;33:924–932. doi: 10.1002/jat.2770. [DOI] [PubMed] [Google Scholar]
- 14.Virgona N., Yokotani K., Yamazaki Y., Shimura F., Chiba T., Taki Y., Yamada S., Shinozuka K., Murata M., Umegaki K. Coleus forskohlii extract induces hepatic cytochrome P450 enzymes in mice. Food Chem. Toxicol. 2012;50:750–755. doi: 10.1016/j.fct.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 15.Yokotani K., Chiba T., Sato Y., Kubota Y., Watanabe Y., Murata M., Umegaki K. Estimation of components which induce mice cytochrome P450 in Coleus forskohlii extract. Pharmacometrics. 2012;82:67–73. [Google Scholar]
- 16.Yokotani K., Chiba T., Sato Y., Nakanishi T., Murata M., Umegaki K. Influence of dietary macronutrients on induction of hepatic drug metabolizing enzymes by Coleus forskohlii extract in mice. J. Nutr. Sci. Vitaminol. (Tokyo) 2013;59:37–44. doi: 10.3177/jnsv.59.37. [DOI] [PubMed] [Google Scholar]
- 17.Postic C., Girard J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 2008;34:643–648. doi: 10.1016/S1262-3636(08)74599-3. [DOI] [PubMed] [Google Scholar]
- 18.Pan X., Hussain F.N., Iqbal J., Feuerman M.H., Hussain M.M. Inhibiting proteasomal degradation of microsomal triglyceride transfer protein prevents CCl4-induced steatosis. J. Biol. Chem. 2007;282:17078–17089. doi: 10.1074/jbc.M701742200. [DOI] [PubMed] [Google Scholar]
- 19.Yokotani K., Chiba T., Sato Y., Taki Y., Yamada S., Shinozuka K., Murata M., Umegaki K. Hepatic cytochrome P450 mediates interaction between warfarin and Coleus forskohlii extract in vivo and in vitro. J. Pharm. Pharmacol. 2012;64:1793–1801. doi: 10.1111/j.2042-7158.2012.01563.x. [DOI] [PubMed] [Google Scholar]
- 20.Taki Y., Yamazaki Y., Shimura F., Yamada S., Umegaki K. Time-dependent induction of hepatic cytochrome P450 enzyme activity and mRNA expression by bilobalide in rats. J. Pharmacol. Sci. 2009;109:459–462. doi: 10.1254/jphs.08198sc. [DOI] [PubMed] [Google Scholar]
- 21.Xu L., Zhou L., Li P. CIDE proteins and lipid metabolism. Arterioscler. Thromb. Vasc. Biol. 2012;32:1094–1098. doi: 10.1161/ATVBAHA.111.241489. [DOI] [PubMed] [Google Scholar]
- 22.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 23.Ferre P., Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010;12(Suppl. 2):83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 24.Kawano Y., Cohen D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013;48:434–441. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications – a review. Nutr. J. 2014;13:17. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadian M., Suh J.M., Hah N., Liddle C., Atkins A.R., Downes M., Evans R.M. PPARgamma signaling and metabolism: the good, the bad and the future. Nat. Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavrilova O., Haluzik M., Matsusue K., Cutson J.J., Johnson L., Dietz K.R., Nicol C.J., Vinson C., Gonzalez F.J., Reitman M.L. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 28.Matsusue K., Kusakabe T., Noguchi T., Takiguchi S., Suzuki T., Yamano S., Gonzalez F.J. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 2008;7:302–311. doi: 10.1016/j.cmet.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schadinger S.E., Bucher N.L., Schreiber B.M., Farmer S.R. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am. J. Physiol. Endocrinol. Metab. 2005;288:E1195–E1205. doi: 10.1152/ajpendo.00513.2004. [DOI] [PubMed] [Google Scholar]
- 30.Puri V., Konda S., Ranjit S., Aouadi M., Chawla A., Chouinard M., Chakladar A., Czech M.P. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J. Biol. Chem. 2007;282:34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 31.Matsusue K. A physiological role for fat specific protein 27/cell death-inducing DFF45-like effector C in adipose and liver. Biol. Pharm. Bull. 2010;33:346–350. doi: 10.1248/bpb.33.346. [DOI] [PubMed] [Google Scholar]
- 32.Gao J., Xie W. Pregnane X receptor and constitutive androstane receptor at the crossroads of drug metabolism and energy metabolism. Drug Metab. Dispos. 2010;38:2091–2095. doi: 10.1124/dmd.110.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.