Abstract
Chrysin (5,7-dihydroxyflavone) is a flavonoid, natural component of traditional medicinal herbs, present in honey, propolis and many plant extracts. The objective of this study was to investigate the hypolipidemic properties of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Triton WR-1339 was administered intraperitoneally (400 mg/kg) to overnight-fasted mice to develop acute hyperlipidemia. Chrysin was administered orally (10 mg/kg) 30 min before Triton WR-1339. At 24 h after Triton WR-1339 injection, blood samples were collected to measure plasma lipid levels. The hepatic thiobarbituric acid reactive substances (TBARS), carbonyl content, non-protein sulfhydryl (NPSH) and ascorbic acid (AA) levels, as well as catalase (CAT) and superoxide dismutase (SOD) activity were recorded. Chrysin administration significantly decreased total cholesterol levels. In addition, it partially decreased non-high density lipoprotein-cholesterol and triglycerides levels in plasma of hyperlipidaemic mice. In addition chrysin administration prevented the increase on TBARS levels and prevented the decrease in SOD activity induced by Triton WR-1339. These findings indicated that chrysin was able to decrease plasma lipids concentration and that its antioxidant properties was, at least in part, involved in the hypolipidaemic action of chrysin.
Keywords: Chrysin, Triton WR-1339, Hyperlipidemia, Oxidative stress, Female C57BL/6 mice
1. Introduction
The liver is a key organ for lipid metabolism since hepatic cholesterol uptake from serum, coupled with intracellular processing and biliary excretion is an important feature in removal of excess cholesterol from the body, beyond synthesis and metabolism of cholesterol, bile acids and phospholipids [1]. Cholesterol is an essential constituent of most biological membranes, besides acting as a precursor for the synthesis of bile acids, hormones and vitamins [2].
High circulating cholesterol is associated with hypercholesterolemia, atherosclerosis, stroke [3] and increases the risk of cardiovascular diseases, fatty liver, and carcinogenesis [4]. Clinically, statins effectively lower plasma cholesterol by inhibiting HMG-CoA reductase activity [5]. Simvastatin is one of the lipid-lowering drugs used to inhibit this enzyme [6].
Taking into account that the liver plays a central role in the maintenance of systemic lipid homeostasis, it can be susceptible to damage by reactive oxygen species (ROS) [47]. Studies have demonstrated that hyperlipidemia reduces the hepatic antioxidant defense system [7], [8].
Humans are constantly exposed to free radicals created by several factors [9]. The excessive free radical generation may induce a number of alterations of cell constituents, including inactivation of enzymes, generation of reactive nitrogen species, damage of nucleic acid bases and proteins, and peroxidation of membrane lipids [10]. Antioxidants defense systems co-evolved along with aerobic metabolism to counteract oxidative damage from free radicals. Antioxidant is further supported with antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR) and glutathione peroxidase (GPx), as well as by naturally occurring antioxidants like, vitamin E, β-carotene, ascorbate, urate, and many others [48] that exert synergistic actions in removing free radicals [9]. Kumar et al. [8] demonstrated that there was a significant increase in hepatic markers of oxidative damage, such as lipid peroxidation, accompanied by deteriorating enzymatic and non enzymatic antioxidant status in rats fed a high cholesterol diet for 30 days.
The nonionic detergent, Triton WR1339 (Tyloxapol or an oxyethylated tertiary octyl phenol formaldehyde polymer), is used by several studies to induce hypercholesterolemia in animals [11], [12]. Its function is to inhibit the activity of the enzyme lipoprotein lipase, resulting in the accumulation of triglycerides and VLDL in plasma, beyond causes a significant increase in hepatic cholesterol biosynthesis by stimulating the activity of the enzyme HMG-CoA reductase [13].
Chrysin (5,7-dihydroxyflavone) is a natural flavonoid which is contained in many plant extracts, flowers such as the blue passion flower (Passiflora caerulea), honey and propolis [14], beyond is the major component of some traditional medicinal herbs [15]. It possesses anti-inflammatory and antioxidant properties and it is used as a dietary supplement [16]. Most studies show the effects of chrysin in the regulation of the reproductive system and hormones, it was effective in antagonizing the enzyme aromatase, thus preventing the conversion of testosterone to estradiol, a desirable effect for body builders to increase their muscle mass [17]. Chrysin has also been shown to inhibit tumour angiogenesis in vivo [18], it presents anti-viral [19], and anxiolytic [20] anti-allergic [21] and anti-estrogenic [22] activity. It has remarkably beneficial pharmacological effects [23], the most important and most reported is its anti-oxidant capacity [24]. Chrysin also alter hyperlipidemia induced by other stimulants. Pushpavalli et al. [15] demonstrated that chrysin was able to decrease the levels of plasma lipids caused by d-galactosamine in rats.
Considering that liver is the major organ responsible for cholesterol transport, metabolism and excretion it is reasonable to study hepatic lipaemic-oxidative disturbances in hypercholesterolemia [25]. The aim of this study was to investigate hypolipidemic properties of chrysin and its antioxidant effect in a model of hyperlipidemia induced by Triton WR-1339 in female C57BL/6 mice.
2. Materials and methods
2.1. Chemicals
Chrysin, simvastatin and Triton WR-1339 (Tyloxapol) (Fig. 1) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Simvastatin and Triton WR-1339 were dissolved in saline solution (pH 7.4) and chrysin was dissolved in PEG (Polyetylenoglicol 20%) and saline solution (pH 7.4). The doses of the compounds used in this study are: Triton WR-1339 (400 mg/kg, 2.5 ml/kg, i.p.) based on [7]; Chrysin (10 mg/kg; 10 ml/kg, p.o.), based on a pilot study previously conducted by our research group in C57BL/6 mice, that demonstrated that the compound is safe in this dose; and Simvastatin (10 mg/kg body W.T.) was used as the reference standard drug for evaluating the antihyperlipidemic activity, based on Sikarwar and Patil [26], since it is effective in reduces plasma cholesterol by inhibiting HMG-CoA reductase activity and reduces the risk of coronary events during treatment. All other chemicals were obtained from analytical grade or from standard commercial suppliers.
Fig. 1.
Structure of chrysin and Triton WR-1339.
2.2. Animals
Adult female C57BL/6 mice (16–25 g) were used. The animals were kept on a 12 h light/dark cycle, at room temperature (22 ± 2 °C), with free access to food and water. All experiments were approved by Ethics Committee on Animal Use (CEUA) of Universidade Federal do Pampa (number 011/2013). All efforts were made to minimize suffering and to reduce the number of animals used in the experiments. Mice were fasted for 12 h and then divided into six groups. Group 1 – control (n = 7): mice received canola oil (10 ml/kg, p.o.) 30 min before saline (2.5 ml/kg, i.p.). Group 2 – chrysin (n = 6): mice received chrysin (10 mg/kg; 10 ml/kg, p.o.) 30 min before saline (2.5 ml/kg, i.p.). Group 3 – simvastatin (n = 6): mice received simvastatin (10 mg/kg; 10 ml/kg, p.o.) 30 min before saline (2.5 ml/kg, i.p.). Group 4 – Triton WR-1339 (n = 7): mice received canola oil (10 ml/kg, p.o.) 30 min before Triton WR-1339 (400 mg/kg, 2.5 ml/kg, i.p.); Group 5 – chrysin + Triton WR-1339 (n = 5): mice received (10 mg/kg; 10 ml/kg, p.o.) 30 min before Triton WR-1339 (400 mg/kg, 2.5 ml/kg, i.p.). Group 6 – simvastatin + Triton WR-1339 (n = 6): mice received simvastatin (10 mg/kg; 10 ml/kg, p.o.) 30 min before Triton WR-1339 (400 mg/kg, 2.5 ml/kg, i.p.). All animals remained in a fasted state for the duration of the experiment (36 h) [25].
2.3. Experiments
At 24 h after the Triton WR-1339 injection, blood samples were collected directly from the ventricle of the heart in anaesthetized animals, using heparin as the anticoagulant, and plasma was separated by centrifugation (2400 × g) for 15 min. Subsequently mice were euthanized by decapitation. The livers were quickly removed and homogenized in 50 mM Tris–HCl, pH 7.4 (1/10, w/v). The homogenate was centrifuged at 2400 × g at 4 °C for 15 min and a low-speed supernatant fraction (S1) was used for assays.
2.4. Plasma Lipid levels
Plasma total cholesterol, high-density lipoprotein (HDL)-cholesterol and triglycerides were determined by enzymatic colorimetric methods using commercial kits (Labtest Diagnostica, MG, Brazil). Non-HDL values were obtained by the difference between total cholesterol and HDL-cholesterol levels. Plasma lipid levels were expressed as mg/dl.
2.5. Oxidative stress markers
Thiobarbituric acid reactive species (TBARS), a measure of lipid peroxidation, were determined using an aliquot (200 μl) of S1, 500 μl thiobarbituric acid (0.8%), 200 μl sodium dodecil sulfate (SDS, 8.1%) and 500 μl acetic acid, the mixture was incubated at 95 °C for 2 h. TBARS (thiobarbituric acid reactive species) were determined as described by Ohkawa et al. [27]. TBARS levels were expressed as nmol MDA/mg protein.
2.5.1. Protein carbonyl content
Carbonyl content was determined by a method based on the reaction of protein carbonyls with dinitrophenylhydrazine forming dinitrophenylhydrazone, a yellow compound [28]. Briefly, homogenized the liver tissue were diluted 1:10 (v/v) and an aliquot of 1 ml was added to the reaction mixture containing 200 μl of 10 mM dinitrophenyl hydrazine (prepared in 2 M HCl). The samples were kept in the dark for 1 h and the tubes were shaken with a Vortex mixer each 15 min. After that, 500 μl of denaturation buffer, 1.5 ml of ethanol and 1.5 ml of hexane were added to each tube. The tubes were shaken with a Vortex mixer for 40 s and centrifuged for 15 min to 3000 rpm. The supernatants obtained were discarded. The pellets were washed two times with 1 ml ethanol: ethyl acetate (1:1, v/v) and ressuspended in 1 ml of denaturation buffer. The sample tubes were shaken with a Vortex mixer for 5 min. These samples were used to measure absorbance at 370 nm (UV). Results were reported as carbonyl content (nmol/mg protein).
2.6. Enzymatic antioxidant defenses
SOD activity was assayed spectrophotometrically as described by Misra and Fridovich [29]. This method is based on the capacity of SOD to inhibit autooxidation of epinephrine to adrenochrome. Enzymatic reaction was initiated by adding an aliquot (20–60 μl) of the S1 and the substrate (epinephrine) to a concentration of 60 mM in a medium containing 50 mM glycine buffer, pH 10.3. The colour reaction was measured at 480 nm. One unit of enzyme was defined as the amount of enzyme required to inhibit the rate of epinephrine autooxidation by 50% at 26 °C. The enzymatic activity was expressed SOD activity as units (U)/mg protein.
CAT activity was assayed spectrophotometrically by the method of Aebi [30], which involves monitoring the disappearance of H2O2 in the S1 at 240 nm. Enzymatic reaction was initiated by adding an aliquot of 20 μl of the S1 and the substrate (H2O2) to a concentration of 0.3 mM in a medium containing 50 mM phosphate buffer, pH 7.0. The enzymatic activity was expressed in Units (one Unit decomposes 1 μmole of H2O2 per min at pH 7 at 25 °C).
2.7. Non enzymatic antioxidant defenses
2.7.1. Non-protein sulfhydryl (NPSH) content
To determine NPSH, S1 was mixed (1:2) with 10% trichloroacetic acid. After the centrifugation, the protein pellet was discarded and free —SH groups were determined in the clear supernatant. An aliquot of supernatant was added in 1 M potassium phosphate buffer pH 7.4 and 10 mM DTNB [31]. The colour reaction was measured at 412 nm. NPSH levels were expressed as mmol NPSH/g tissue.
2.7.2. Ascorbic acid (AA) determination
AA determination was performed as described by Jacques-Silva et al. [32]. Proteins were precipitated in 10 volumes of a cold 4% trichloroacetic acid solution. An aliquot of the sample at a final volume of 1 ml of the solution was incubated at 38 °C for 3 h then 1 ml H2SO4 65% (v/v) was added to the medium. The reaction product was determined using a colour reagent containing 4.5 mg/ml dinitrophenyl hydrazine and CuSO4 (0.075 mg/ml) at 520 nm. The content of ascorbic acid is related to tissue amount (μmol ascorbic acid/g wet tissue).
2.8. Protein determination
Protein concentration was measured by the method of Bradford [33], using bovine serum albumin as the standard.
2.9. Statistical analysis
Statistical analysis of data was performed using a two-way analysis (Chrysin × Triton WR-1339 and simvastatin × Triton WR-1339) of variance (ANOVA), followed by post hoc comparisons using Newman Keuls test when appropriate. Main effects are presented only when the second order interaction was not significant. To compare treatments, chrysin and simvastatin, the Student t test was applied. Experiments were expressed as mean ± S.D. Differences between groups were considered statistically significant when P < 0.05.
3. Results
3.1. Plasma lipids
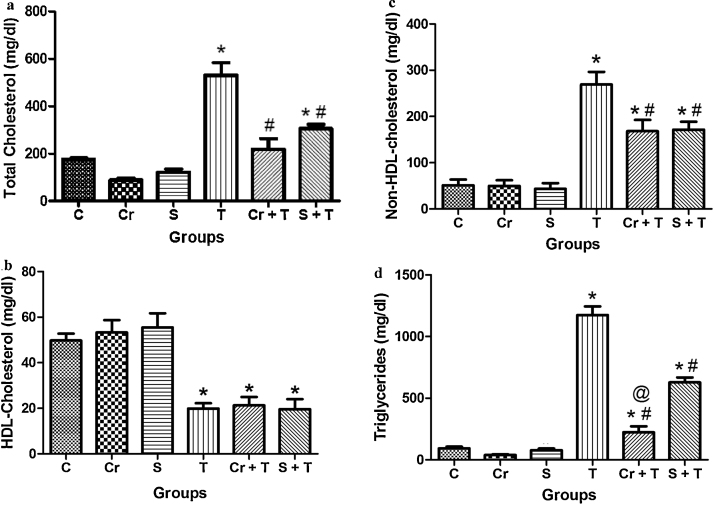
Two-way ANOVA of total cholesterol levels data yielded a significant Triton WR-1339 × chrysin interaction. Post hoc comparison demonstrated that Triton WR-1339 increased plasmatic total cholesterol levels in mice. Chrysin pretreatment was effective in preventing the increase of total cholesterol levels caused by Triton WR-1339 injection in mice. Two-way ANOVA of cholesterol total data yielded a significant Triton WR-1339 × simvastatin interaction. Simvastatin pretreatment was effective in partial preventing the increase of cholesterol total levels caused by Triton WR-1339 injection in mice. T-test demonstrated that there is no significant difference between simvastatin and Triton WR 1339 × chrysin and Triton WR-1339 groups (Fig. 2a).
Fig. 2.
Effect of Triton WR-1339 (T), simvastatin (S) and chrysin (Cr) on plasma lipid levels in C57BL/6 mice. (a) total cholesterol, (b) high-density lipoprotein (HDL)-cholesterol, (c) non-HDL-cholesterol, and (d) triglyceride levels from plasma of C57BL/6 mice. Data are reported as mean ± S.D. for five to six animals per group. *Compared to control group (C); #compared to Triton WR-1339 group (T) (P < 0.05-two-way analysis of variance/Newman Keuls) and @compared to simvastatin + Triton (S + T) (P < 0.05 – Student's t test).
Two-way ANOVA of HDL-cholesterol levels showed a significant Triton WR-1339 main effect. Post hoc comparison demonstrated that Triton WR-1339 decreased plasmatic HDL-cholesterol levels in mice. Pretreament with chrysin and simvastatin did not protect the decrease on HDL-cholesterol levels caused by Triton WR-1339 injection in mice. Two-way ANOVA of HDL-cholesterol levels showed a significant Triton WR-1339 main effect. T-test demonstrated that there is no significant difference P > 0.05 between simvastatin and Triton WR-1339 × chrysin and Triton WR-1339 groups (Fig. 2b).
Two-way ANOVA of non-HDL-cholesterol levels yielded a significant Triton WR-1339 × chrysin interaction. Post hoc comparison demonstrated that Triton WR-1339 increased plasma non-HDL-cholesterol levels in mice. Chrysin pretreatment partially decreased non-HDL-cholesterol levels caused by Triton WR-1339 injection in mice. Two-way ANOVA of non-HDL-cholesterol levels showed a significant Triton WR-1339 × simvastatin interaction. Simvastatin pretreatment partially decreased non-HDL-cholesterol levels caused by Triton WR-1339 injection in mice. T-test demonstrated that there isn’t significant difference (P > 0.05) between simvastatin and Triton WR-1339 × chrysin and Triton WR-1339 groups (Fig. 2c).
Two-way ANOVA of triglyceride data revealed a significant Triton WR-1339 × chrysin interaction. Post hoc comparison demonstrated that Triton WR-1339 increased the plasma triglycerides levels in mice. Oral administration of chrysin in mice partially blocked the increase of triglyceride levels induced by Triton WR-1339 injection in mice. Two-way ANOVA of triglyceride data revealed a significant Triton WR-1339 × simvastatin interaction. Simvastatin pretreatment partially decreased triglyceride levels caused by Triton WR-1339 injection in mice. T-test demonstrated that there is a significant different (P < 0.05) between simvastatin and Triton WR-1339 × chrysin and Triton WR-1339 groups (Fig. 2d).
3.2. Oxidative stress markers
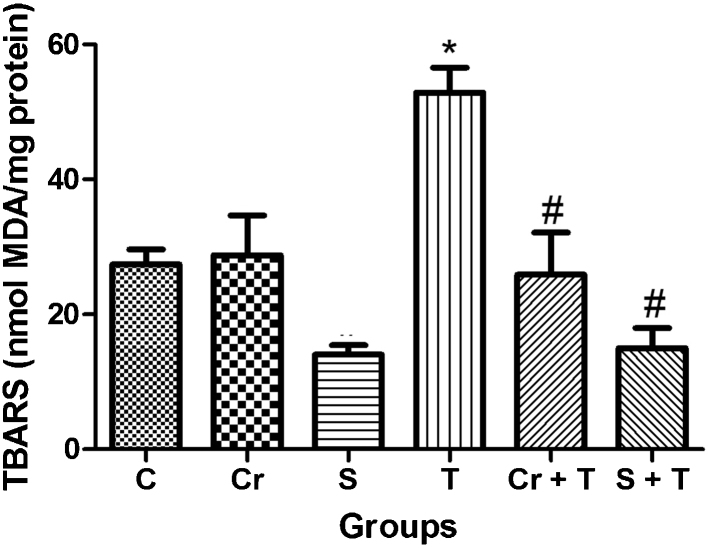
Two-way ANOVA of TBARS data revealed a significant Triton WR-1339 × Chrysin interaction. Post hoc comparison demonstrated that Triton WR-1339 increased TBARS levels in mice. Oral administration of chrysin significantly prevented the increase of TBARS levels induced by Triton WR-1339 injection in mice. Two-way ANOVA of TBARS data revealed a significant Triton WR-1339 × simvastatin interaction. Simvastatin pretreatment decreased TBARS levels caused by Triton WR-1339 injection in mice. T-test demonstrated that there is no significant difference P > 0.05 between simvastatin and Triton WR-1339 × chrysin and Triton WR-1339 groups (Fig. 3).
Fig. 3.
Effect of Triton WR-1339 (T), simvastatin (S) and chrysin (Cr) on markers of oxidative stress from liver of C57BL/6 mice, TBARS content from liver of C57BL/6 mice. Data are reported as mean ± S.D. for five to six animals per group. *Compared to control group (C); #compared to Triton WR-1339 group (T) (P < 0.05-two-way analysis of variance/Newman Keuls).
Chrysin pretreatment per se decreased carbonyl levels in mice compared with group control. However, there was no change in carbonyl protein in any of the other groups.
3.3. Enzymatic antioxidant defenses
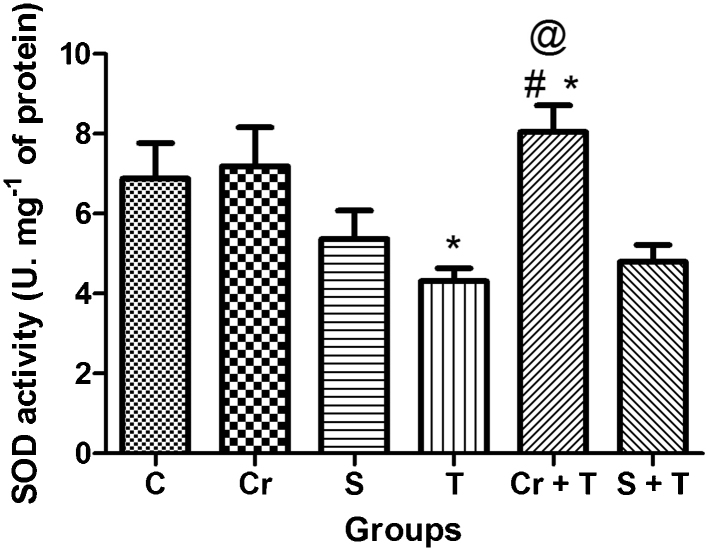
Two-way ANOVA of SOD data revealed significant Triton WR-1339 × chrysin interaction. Post hoc comparison demonstrated that Triton WR-1339 decreased SOD levels in mice. Oral administration of chrysin significantly prevented the decrease of SOD levels induced by Triton WR-1339 injection in mice. Simvastatin pretreatment did not protect the decrease on SOD levels caused by Triton WR-1339 injection in mice. T-test demonstrated that there is significant difference P < 0.05 between simvastatin and Triton WR-1339 × chrysin and Triton WR-1339 groups (Fig. 4)
Fig. 4.
Effect of Triton WR-1339 (T), simvastatin (S) and chrysin (Cr) on antioxidant enzyme defense, superoxide dismutase from liver of C57BL/6 mice. Data are reported as mean ± S.D. for five to six animals per group. *Compared to control group (C); #compared to Triton WR-1339 group (T) (P < 0.05-two-way analysis of variance/Newman Keuls) and @compared to simvastatin + Triton (S + T) (P < 0.05 – Student's t test).
Two-way ANOVA of catalase activity showed a significant Triton WR-1339 main effect. Post hoc comparison demonstrated that Triton WR-1339 decreased catalase activity in mice. Chrysin and simvastatin pretreatment was not able in protect the decrease on catalase activity caused by Triton WR-1339 injection in mice. T-test demonstrated that there is no significant difference P > 0.05 between simvastatin and Triton WR-1339 × chrysin and Triton WR-1339 groups.
3.4. Non enzymatic antioxidant defenses
NPSH and ascorbic acid data showed that chrysin, simvastatin and Triton WR-1339 did not change these parameters in livers of mice.
4. Discussion
The purpose of this study was to demonstrate the hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. A single oral dose (10 mg/kg) of chysin was able to prevent the increase of total cholesterol levels, partially prevent the increase on non-HDL-cholesterol and triglyceride levels, but it was not able to prevent the decrease on HDL-cholesterol levels induced by Triton WR 1339 administration in this experimental protocol. Additionally, we observed the ability of chrysin in act on parameters of oxidative stress, protecting the alteration on lipid peroxidation levels and SOD activity induced by Triton WR 1339 in mice.
In our study, we observed the increase in the levels of total cholesterol (3.0-times higher than the control group), non-HDL-cholesterol (5.3-times higher than the control group) and triglycerides (12.5-times higher than the control group) and decrease on HDL-cholesterol levels (2.5-times lower than the control group) 24 h after a single Triton WR-1339 injection in mice. The results demonstrated here were in accordance with those reported by others [11], [25]. Several studies have shown that systemic administration of Triton WR-1339 (nonionic surfactant) in fasted rats and mice causes increased level of lipids in plasma [25]. Initially there is a sharp increase in lipid level reaching a peak in two to three times the control value 24 h after the injection of Triton WR-1339 phase I (synthesis phase), this falls within hyperlipidemia next 24 h or 48 h after administration of Triton WR-1339, Phase II (the elimination phase) [26].
The nonionic detergent, Triton WR-1339, has been used widely to block the uptake of triacylglycerol-rich lipoproteins from plasma by peripheral tissues to produce acute hyperlipidemia in animal models, which are often used for a number of objectives, in particular for screening natural or chemical hypolipidemic drugs [11], [34]. Experimental evidence supports the concept that Triton WR-1339 physically alters very low density lipoproteins (VLDL), rendering them refractive to the action of lipolytic enzymes of blood and tissue [35]. This prevents or delays their removal from blood and secondarily stimulates the hepatic cholesterol biosynthesis, enhancing the hyperlipidemia [36]. There was marked increase in the level of serum total cholesterol, triglycerides, phospholipids, LDL, VLDL and decrease in the level of cholesterol carrier HDL in the rats treated with Triton WR-1339 [26].
It was observed that chrysin, given by the oral route to C57BL/6 female mice at a simple dose of 10 mg/kg, presented a hypolipidemic effect by preventing the augmentation of total cholesterol, and partly prevents the increase in non-HDL-cholesterol and triglyceride levels in a Triton WR-1339-induced hyperlipidemic model. These results are of great importance since the flavonoids like chrysin are viewed as important components of ‘functional foods’, acting as modifiers of cardiovascular disease [37]. Chrysin has effects cardioprotective against myocardial ischaemia/reperfusion injury in Langendorff-perfused rat hearts [38]. Furthermore, some patients under statin treatment cannot tolerate statins well or do not reach the low-density lipoprotein-cholesterol (LDL-C) goal recommended by the US National Institutes of Health guidelines [39]. Therefore, it is desirable to develop natural drugs that have cholesterol-lowering effect comparable to statins, but could be tolerated well by the patients.
In addition, the results presented here were significant since it is the first study using female mice of line C57BL/6 that receiving treatment with chrysin in this model of hyperlipidemia. We chose female because it has more risk for cardiovascular disease. Schwab et al. [40] showed differences in the distribution of cardiovascular risk factors in disfavour of females, including higher cholesterol levels. In study of Kautzky-Willer et al. [41], women showed higher mean total cholesterol levels than men, and overall, women tended to feature hyperlipidemia more often than their male counterparts. In the obese and hypertonic subgroups, LDL cholesterol was also higher in the women than in the men.
Other studies involving anti-hyperlipidemic profile of chrysin have been performed in rats. Pushpavalli et al. [15] demonstrated that treatment with chrysin, at dose of 25 mg/kg in rats, was effective in decreased total cholesterol, triglycerides, free fatty acids, LDL-cholesterol and VLDL cholesterol and increased HDL cholesterol levels in a model d-galactosamine-induced hepatotoxicity. Moreover, higher doses (50 mg/kg and 100 mg/kg) of chrysin have resulted in the production of by-products, interfering with the hepatoprotective activity, and consequently, decreasing its effect [15]. In the study of Anandhi et al. [42] chrysin at the dose of 200 mg/kg protected against hypercholesterolemia induced by Triton WR-1339 in rats. In our study a single dose of 10 mg/kg body weight was able to protect an increase in plasma lipid levels, showing that even at a dose 10 times lower, chrysin has managed to have a positive effect against hyperlipidemia in this protocol model. Unlike previous studies, we demonstrated here the preventive effect of chrysin, at lower dose, against hyperlipidemia and encourage its consumption in the diet as a preventive agent for cardiovascular diseases.
An important point of this work is that chrysin had better effect than simvastatin in reducing plasma lipid levels, mainly triglycerides. A possible explanation for the hypolipidemic action of chrysin is related to HMG-CoA reductase activity, mechanism similar than statins, another hypotheses are related to lipoprotein lipase activity, responsible to decrease plasma triglycerides levels. However, more studies are needed to identify the mechanisms of chrysin in this hyperlipidemia model. Therefore, our results suggest that chrysin supplementation can be used as an adjuvant on treatment of dyslipidemia.
Knowing that chrysin has antioxidant effect and that an important property of a compound that may affect hyperlipidemia is its antioxidant capacity we investigated whether Triton WR-1339-induced acute hyperlipidemia in female C57BL/6 mice altered some parameters of oxidative damage in the hepatic tissue and whether the antioxidant effect of chrysin was related to this process.
In our study Triton WR-1339 administered to mice was able to increase the marker of stress oxidative TBARS and decrease the activity antioxidant by enzyme SOD. Stokes et al. [43] reported that ROS levels in hypercholesterolemia were higher than in the normal state. In agreement with this, Oh et al. [7], found that 18 h after Triton WR-1339 administration to mice, the level of plasma TBARS was increased and the activity of two hepatic detoxicating enzymes, catalase and GPx, were decreased compared with the control group. Thus, in this study the hepatic markers of oxidative stress were altered in mice treated with Triton WR-1339. Oxygen free radicals or, more generally, reactive oxygen species (ROS), are the products of normal metabolic and signal-transduction events within a cell but free radical oxidation is responsible for the degradation of fatty acids and their esters in biological membranes and lipoproteins [44], consequently, this oxidation may also play a role in pathologic processes.
Chysin prevented the alteration on oxidative stress markers, it significantly decreased TBARS level of plasma and prevented the decrease on enzymatic antioxidant defenses SOD changed by Triton WR-1339. These results demonstrated the antioxidant action of chrysin in this model and suggest this effect as a possible mechanism of chrysin hypolipidemic action. Rice-Evans [45], described the beneficial effects of chrysin by having the capability of free radicals scavenging. Ciftci et al. [24], showed that chrysin (50 mg kg, per oral route) significantly increase GSH, CAT, GSH-Px and CuZn-SOD levels, but did not change the formation of TBARS in rat tissues. Sirovina et al. [46], demonstrated that administration of quercetin and chrysin to diabetic mice resulted in a significant decrease in lipid peroxidation level in liver tissue.
5. Conclusions
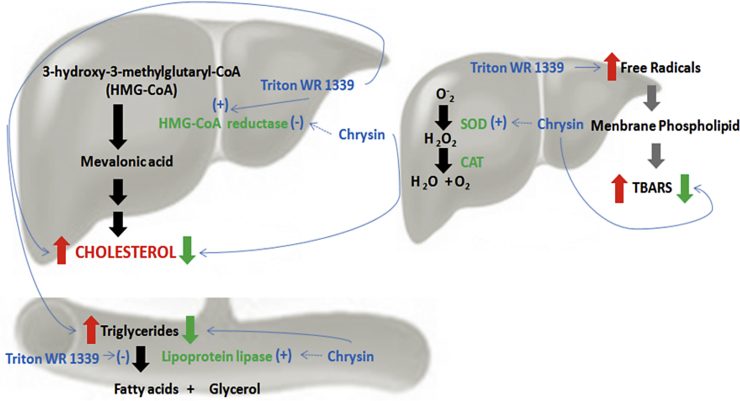
In conclusion, our findings demonstrated that chrysin (dose of 10 mg/kg), has antihyperlipidemic effect since it was able to prevent the augmentation of plasma total cholesterol, non-HDL cholesterol and triglyceride levels in the plasma of Triton WR-1339-induced hyperlipidemia in mice. Furthermore antihyperlipidemic action of chrysin demonstrated in this study was comparable to the standard drug simvastatin. The antioxidant effect of chrysin in liver was observed in this experimental protocol and it was suggested as a possible mechanism of chrysin hypolipidemic action (Fig. 5). Thus, our results suggest that chrysin supplementation can be used as an adjuvant on treatment of dyslipidemia diseases, since dyslipidemia is a major risk factor for coronary heart disease in women.
Fig. 5.
Scheme with potential targets of chrysin.
Conflict of interest
None declared.
Acknowledgements
The financial support by UNIPAMPA, FAPERGS, CAPES and CNPq is gratefully acknowledged.
Footnotes
Available online 12 May 2014
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.02.003.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Taniguchi H., Yomota E., Nogi K., Onoda Y. Effects of anti-ulcer agents on ethanol-induced gastric mucosal lesion in d-GalN-induced hepatitis rats. Arzneimittelforschung. 2002;52(8):600–604. doi: 10.1055/s-0031-1299937. [DOI] [PubMed] [Google Scholar]
- 2.Repa J.J., Mangelsdorf D.J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu. Rev. Cell Dev. Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 3.Sun X., Essalmani R., Day R., Khatib A.M., Seidah N., Prat A. Proprotein convertase subtilisin/kexin type 9 deficiency reduces melanoma metastasis in liver. Neoplasia. 2012;14(December (12)):1122–1131. doi: 10.1593/neo.121252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo M.N., Bok S.H., Choi M.S. Hypolipidemic and body fat-lowering effects of fatclean in rats fed a high-fat diet. Food Chem. Toxicol. 2009;47(August (8)):2076–2082. doi: 10.1016/j.fct.2009.05.041. (Epub 2009 June 14) [DOI] [PubMed] [Google Scholar]
- 5.Collins R., Armitage J., Parish S., Sleight P., Peto R. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(July (9326)):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 6.Jamal S.M., Eisenberg M.J., Christopoulos S. Rhabdomyolysis associated with hydroxymethylglutarylcoenzyme A reductase inhibitors. Am. Heart J. 2004;147(June (6)):956–965. doi: 10.1016/j.ahj.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 7.Oh P.S., Lee S.J., Lim K.T. Hypolipidemic and antioxidative effects of the plant glycoprotein (36 kDa) from Rhus verniciflua stokes fruit in Triton WR-1339-induced hyperlipidemic mice. Biosci. Biotechnol. Biochem. 2006;70(February (2)):447–456. doi: 10.1271/bbb.70.447. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S.A., Sudhahar V., Varalakshmi P. Protective role of eicosapentaenoate-lipoate (EPA-LA) derivative in combating oxidative hepatocellular injury in hypercholesterolemic atherogenesis. Atherosclerosis. 2006;189(November (1)):115–122. doi: 10.1016/j.atherosclerosis.2005.11.037. (Epub 2006 February 3) [DOI] [PubMed] [Google Scholar]
- 9.Uttara B., Singh A., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(March (1)):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ha B.J., Lee S.H., Kim H.J., Lee J.Y. The role of Salicornia herbacea in ovariectomy-induced oxidative stress. Biol. Pharm. Bull. 2006;29(July (7)):1305–1309. doi: 10.1248/bpb.29.1305. [DOI] [PubMed] [Google Scholar]
- 11.Harnafi H., Caid H.S., Bouanani N. el H., Aziz M., Amrani S. Hypolipemic activity of polyphenol-rich extracts from Ocimum basilicum in Triton WR-1339-induced hyperlipidemic mice. Food Chem. 2008;108:205–212. [Google Scholar]
- 12.Bertges L.C., Mourão CAJr, Souza J.B., Cardoso V.A.C. Hyperlipidemia induced by Triton WR1339 (Tyloxapol) in Wistar rats. Rev. Bras. Cien. Med. Saúde. 2011;1(1):32–34. [Google Scholar]
- 13.Janicki B.S., Aron S.A. Effect of Triton WR 1339 on lipoproteins and lipoprotein lipase of guinea pig plasma. Proc. Soc. Exp. Biol. Med. 1962;109(March):507–509. doi: 10.3181/00379727-109-27250. [DOI] [PubMed] [Google Scholar]
- 14.Phan T., Yu X.M., Kunnimalaiyaan M., Chen H. Antiproliferative effect of chrysin on anaplastic thyroid cancer. J. Surg. Res. 2011;170(September (1)):84–88. doi: 10.1016/j.jss.2011.03.064. (Epub 2011 April 20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pushpavalli G., Veeramani C., Pugalendi K.V. Influence of chrysin on hepatic marker enzymes and lipid profile against d-galactosamine-induced hepatotoxicity rats. Food Chem. Toxicol. 2010;48(June (6)):1654–1659. doi: 10.1016/j.fct.2010.03.040. (Epub 2010 March 31) [DOI] [PubMed] [Google Scholar]
- 16.Sobocanec S., Sverko V., Balog T., Saric A., Rusak G., Likic S., Kusic B., Katalinic V., Radic S., Marotti T. Oxidant/antioxidant properties of Croatian native propolis. J. Agric. Food Chem. 2006;54(October (21)):8018–8026. doi: 10.1021/jf0612023. [DOI] [PubMed] [Google Scholar]
- 17.Gambelunghe C., Rossi R., Sommavilla M., Ferranti C., Rossi R., Ciculi C., Gizzi S., Micheletti A., Rufini S.J. Effects of chrysin on urinary testosterone levels in human males. J. Med. Food. 2003;6(Winter (4)):387–390. doi: 10.1089/109662003772519967. [DOI] [PubMed] [Google Scholar]
- 18.Fu B., Xue J., Li Z., Shi X., Jiang B.H., Fang J. Chrysin inhibits expression of hypoxia-inducible factor-1alpha through reducing hypoxia-inducible factor-1alpha stability and inhibiting its protein synthesis. Mol. Cancer Ther. 2007;6(1):220–226. doi: 10.1158/1535-7163.MCT-06-0526. [DOI] [PubMed] [Google Scholar]
- 19.Critchfield J.W., Butera S.T., Folks T.M. Inhibition of HIV activation in latently infected cells by flavanoid compounds. AIDS Res. Hum. Retroviruses. 1996;12(January (1)):39–46. doi: 10.1089/aid.1996.12.39. [DOI] [PubMed] [Google Scholar]
- 20.Wolfman C., Violah P.A., Dajas F., Medina J.H. Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora coerulea. Pharmacol. Biochem. Behav. 1994;47(January (1)):1–4. doi: 10.1016/0091-3057(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 21.Pearce F.L., Befus A.D., Bienenstock J. Mucosal mast cells. III. Effect of quercetin and other flavonoids on antigen-induced histamine secretion from rat intestinal mast cells. J. Allergy Clin. Immunol. 1984;73(June (6)):819–823. doi: 10.1016/0091-6749(84)90453-6. [DOI] [PubMed] [Google Scholar]
- 22.Machala M., Kubinova R., Suchy V. Chemoprotective potentials of homoisoflavonoids and chalcones of Dracaena cinnabari: modulations of drug metabolizing enzymes and antioxidant activity. Phytother. Res. 2001;15(March (2)):114–118. doi: 10.1002/ptr.697. [DOI] [PubMed] [Google Scholar]
- 23.Li L., Wei D.-Q., Wang J.-F., Chou K.-C. Computational studies of the binding mechanism of calmodulin with chrysin. Biochem. Biophys. Res. Commun. 2007;358(July (4)):1102–1107. doi: 10.1016/j.bbrc.2007.05.053. (Epub 2007 May 22) [DOI] [PubMed] [Google Scholar]
- 24.Ciftci O., Ozdemir I., Aydin M., Beytur A. Beneficial effects of chrysin on the reproductive system of adult male rats. Andrologia. 2012;44(June (3)):181–186. doi: 10.1111/j.1439-0272.2010.01127.x. (Epub 2011 March 7) [DOI] [PubMed] [Google Scholar]
- 25.Rocha J.T., Sperança A., Nogueira C.W., Zeni G. Hypolipidaemic activity of orally administered diphenyl diselenide in Triton WR-1339-induced hyperlipidaemia in mice. J. Pharm. Pharmacol. 2009;61(December (12)):1673–1679. doi: 10.1211/jpp/61.12.0013. [DOI] [PubMed] [Google Scholar]
- 26.Sikarwar M.S., Patil M.B. Antihyperlipidemic activity of Salacia chinensis root extracts in Triton-induced and atherogenic diet-induced hyperlipidemic rats. Indian J. Pharmacol. 2012;44(January (1)):88–92. doi: 10.4103/0253-7613.91875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(June (2)):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 28.Reznick A.Z., Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 29.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(May (10)):3170–3175. [PubMed] [Google Scholar]
- 30.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 31.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(May (1)):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 32.Jacques-Silva M.C., Nogueira C.W., Broch L.C., Rocha J.B.T. Diphenyl diselenide and acorbic acid changes deposition of selenium and ascorbic acid in brain of mice. Pharmacol. Toxicol. 2001;88(March (3)):119–125. doi: 10.1034/j.1600-0773.2001.d01-92.x. [DOI] [PubMed] [Google Scholar]
- 33.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.Xie W., Wang W., Su H., Xing D., Cai G., Du L. Hypolipidemic mechanisms of Ananas comosus L. leaves in mice: different from fibrates but similar to statins. J. Pharmacol. Sci. 2007;103(March (3)):267–274. doi: 10.1254/jphs.fp0061244. [DOI] [PubMed] [Google Scholar]
- 35.Friedman M., Byers S.O. The mechanism responsible for the hypercholesteremia induced by Triton WR-1339. J. Exp. Med. 1953;97(January (1)):117–130. doi: 10.1084/jem.97.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldfarb S. Rapid increase in hepatic HMG-CoA reductase activity and in vivo cholesterol synthesis after Triton WR 1339 injection. J. Lipid Res. 1978;19(May (4)):489–494. [PubMed] [Google Scholar]
- 37.Johnston C. Functional foods as modifiers of cardiovascular disease. Am. J. Lifestyle Med. 2009;3(July (1 Suppl.)):39S–43S. doi: 10.1177/1559827609332320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Testai L., Martelli A., Cristofaro M., Breschi M.C., Calderone V. Cardioprotective effects of different flavonoids against myocardial ischaemia/reperfusion injury in Langendorff-perfused rat hearts. J. Pharm. Pharmacol. 2013;65(May (5)):750–756. doi: 10.1111/jphp.12032. (Epub 2013 February 26) [DOI] [PubMed] [Google Scholar]
- 39.Lenfant C., Cleeman J.I., Ganiats T.G., Graham G., Kleinman R.E. Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285(May (19)):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 40.Schwab K.O., Doerfer J., Naeke A., Rohrer T., Wiemann D., Marg W., Hofer S.E., Holl R.W. Influence of food intake, age, gender, HbA1c, and BMI levels on plasma cholesterol in 29,979 children and adolescents with type 1 diabetes – reference data from the German diabetes documentation and quality management system (DPV) Pediatr. Diabetes. 2009;10(May (3)):184–192. doi: 10.1111/j.1399-5448.2008.00469.x. (Epub 2008 October 10) [DOI] [PubMed] [Google Scholar]
- 41.Kautzky-Willer A., Stich K., Hintersteiner J., Kautzky A., Kamyar M.R., Saukel J., Johnson J., Lemmens-Gruber R. Sex-specific-differences in cardiometabolic risk in type 1 diabetes: a cross-sectional study. Cardiovasc. Diabetol. 2013;May (12):78. doi: 10.1186/1475-2840-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anandhi R., Annadurai T., Anitha T.S., Muralidharan A.R., Najmunnisha K., Nachiappan V., Thomas P.A., Geraldine P. Antihypercholesterolemic and antioxidative effects of an extract of the oyster mushroom, Pleurotus ostreatus, and its major constituent, chrysin, in Triton WR-1339-induced hypercholesterolemic rats. J. Physiol. Biochem. 2013;69:313–323. doi: 10.1007/s13105-012-0215-6. [DOI] [PubMed] [Google Scholar]
- 43.Stokes K.Y., Cooper D., Tailor A., Granger D.N. Hypocholesterolemia promotes inflammation and microvascular dysfunction: role of nitric oxide and superoxide. Free Radic. Biol. Med. 2002;33(October (8)):1026–1036. doi: 10.1016/s0891-5849(02)01015-8. [DOI] [PubMed] [Google Scholar]
- 44.Morgan M.J., Kim Y.S., Liu Z. Lipid rafts and oxidative stress-induced cell death. Antioxid. Redox Signal. 2007;9(September (9)):1471–1483. doi: 10.1089/ars.2007.1658. [DOI] [PubMed] [Google Scholar]
- 45.Rice-Evans C.A. Flavonoid antioxidants. Curr. Med. Chem. 2001;8(June (7)):797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- 46.Sirovina D., Oršolic N., Končic ´MZ, Kovačevic G., Benkovic V., Gregorovic G. Quercetin vs chrysin: effect on liver histopathology in diabetic mice. Human Exp. Toxicol. 2012:1–9. doi: 10.1177/0960327112472993. [DOI] [PubMed] [Google Scholar]
- 47.Hamelet J., Demluth K., Paul J.L., Delabar J.M., Janel N. Hyperhomocysteinemia due to cystathionine beta synthase deficiency induces dysregulation of genes involved in hepatic lipid homeostasis in mice. J. Hepatol. 2007;46(January (1)):151–159. doi: 10.1016/j.jhep.2006.07.028. (Epub 2006 September 22) [DOI] [PubMed] [Google Scholar]
- 48.Novo E., Parola M. The role of redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogen. Tissue Repair. 2012;5(June (Suppl. 1)):S4. doi: 10.1186/1755-1536-5-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.