Abstract
Despite the acclaimed phytotherapeutic attributes of Stigma maydis in folkloric medicine, there is paucity of information on its toxicity profile on hematological and lipid parameters. The toxicological effect of aqueous extract of corn silk at 100, 200 and 400 mg/kg body weight on hematological indices in Wistar rats were evaluated progressively at 24 h after 1, 7, 14, 21 and 28 days. Lipid parameters were also analyzed at the end of the experimental period. We observed that the extract did not exhibit any significant (p > 0.05) effect on red blood cells, hematocrit, hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and mean platelet volume at all the tested doses. The study however showed a significant increase in the serum levels of white blood cell, platelet, lymphocytes, high-density lipoprotein cholesterol; as well as feeding pattern in the animals, while the concentrations of total cholesterol, low-density lipoprotein cholesterol, and artherogenic index value were significantly lowered. These findings are suggestive of non-hematotoxic potential of the extract. Overall, the effect exhibited by corn silk extract in this study proved that, it is unlikely to be hematotoxic and could be a good candidature in the management of coronary heart diseases if consumed at the doses investigated.
Keywords: Anti-lipidemic, Hematotoxic, Lymphocyte, Metabolic, Phytotherapeutic, Thrombopoiesis
1. Introduction
Healing with medicinal plants is as old as mankind itself. The link between man and his quest for medicines in nature dates back to ancient times, when there were convincing evidences from written documents, monuments, and even original plant medicines [1]. Awareness of medicinal plants usage is a result of the many years of struggles against illnesses which has prompted man to seek medicines in leaves, roots, barks and other parts of plants [2]. The knowledge of the development of ideas related to the usage of medicinal plants as well as the evolution of awareness has increased the ability of health providers to respond to the challenges that have emerged with the spreading of professional services in enhancement of man's life. Until the advent of iatrochemistry in 16th century, plants had been the source of treatment and prophylaxis for many diseases [3]. Nonetheless, the decreasing efficacy of synthetic drugs, non-affordability and the increasing contraindications of their usage has re-awakened topical attention on natural medicines in recent years [2]. Particularly in Africa, about 80% of the world's population now relies on traditional medicinal system to augment and supplement the increasingly expensive orthodox medicine [4]. One among such plant's part finding applications in this regard is corn silk (CS).
Corn silks (Stigma maydis) are elongated stigmas from the female flowers of maize which look like a tuft of hairs. It is a waste material from corn cultivation and available in abundance [5]. The colors at first are usually light green and later turn into red, yellow or light brown. Its function is to trap pollens for pollination and is harvested at the same time as the corn cob. Each silk may be pollinated to produce one kernel of corn. The CS can be 30 cm long or longer with a faintly sweetish taste. It is rich in proteins, fixed and volatile oils, vitamins and carbohydrates. Calcium, potassium, magnesium, sodium salts, steroids, phenol and flavonoids are also believed to contribute to its pharmacological potentials [6]. Consistent with this report, our preliminary GC-MS analysis of the extract also revealed 22 active constituents including ascorbic acid, β-carotene, maysin, maizenic acid, and glycolic acid, among others. CS has long been used traditionally by the Chinese and Native Americans to treat many diseases. Its potential healthcare benefits as anti-fatigue, anti-depressant, anti-diabetic, and as hypoglycemic agent have been claimed in several reports [7], [8], [9]. Its efficacy in the treatment of cystitis, edema, kidney stones, prostate disorder, urinary infections, bedwetting, obesity, and as diuretic has also been reported [10]. Although not scientifically proven, CS tea has been claimed in traditional medicine practice to have many benefits to human health such as lowering blood pressure, and promote relaxation. Wang et al. [11] have also reported CS to be therapeutic in the management of pleurisy, and oxidative stress-induced inflammatory diseases.
The application of CS extract for the treatment of various ailments is increasing appreciably. Inspite of these profound therapeutic benefits exhibited by its various formulations, paucity of information exists in the literature about the safety of its aqueous extract. Accordingly, since non-toxicity is one of the criteria set by World Health Organization for the use of herbs as medicines [12], this present study was therefore, designed to investigate the effect of aqueous extract of CS on the hematological parameters and lipid profile after 28-day administration in Wistar rats.
2. Materials and methods
2.1. Assay kits
The assay kits for total cholesterol (TC), triacylglycerol (TAG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were products of Randox Laboratory Ltd, Co. Antrim, United Kingdom. All other reagents used were of analytical grade.
2.2. Plant collection and preparation of extract
Corn silks were harvested from a maize plantation in Malete, Ilorin, Kwara State, Nigeria between April and August, 2014. They were authenticated (from fresh whole maize plant) at the Plant Biology Unit, Department of Biosciences and Biotechnology, Kwara State University, Malete, Ilorin, Nigeria. Voucher specimen (No. KWASU/14-105) was prepared and deposited at the University's Herbarium. The CS was shade dried to constant weight prior to pulverizing with an electric blender (model MS-223; Blender/Miller III, Taiwan, China) to fine powder. The powdered sample (400 g) was suspended in distilled water (4 L) for 48 h with regular shaking by orbital shaker maintained at 300 rpm. The solution obtained was then filtered and the resulting filtrate lyophilized to give 22.5 g residue, corresponding to a yield of 5.6%. The lyophilized sample (CS extract) was reconstituted in distilled water to give doses of 100, 200, and 400 mg/kg body weight (b.w.) of the extract used in the study.
2.3. Animal treatment and extract administration
One hundred Wistar rats (150–180 g weight range) were used for the study. They were kept in metallic cages in a well ventilated room maintained at a temperature of 25 ± 2 °C with a 12-h light–dark cycle for one week to acclimatize, and allowed free access to food and water ad libitum. This conforms to the guidelines of the National Institute of Health [13] for laboratory animal care and use, and in accordance with the principles of good laboratory procedure [14]. The animals were thereafter randomized into 4 groups of 25 rats each. Rats in group 1 were given 1 mL distilled water and served as control. Groups 2–4 comprised animals administered with 1 mL of CS aqueous extract at 100, 200, and 400 mg/kg b.w. respectively. All administrations were done once daily via oral intubation with ad libitum provision of food and water throughout the experimental periods. Five (5) rats each from all the groups were sacrificed 24 h after extract administration on days 1, 7, 14, 21 and 28. The study was approved by the Departmental Independent Ethical Committee of Kwara State University, Malete, Nigeria, and a certified number KSU/IECCULA/002/08/014 was assigned and issued.
2.4. Determination of feed and water intake
The amount of feed and water intake were determined on daily basis [15], [16], [17], [18]. In brief, the weight and volume of daily feed, and water respectively administered and the left-overs by the following day were recorded and the differences were taken as the daily feed, and water intake. The average of the feed and water intake was calculated for every 7 days of the experimental period.
2.5. Blood collection and biochemical assays
The method described by Yakubu et al. [19] was adopted in the preparation of plasma. Under slight diethyl ether anesthesia, 2 mL of blood was collected into EDTA sample bottles by cardiac puncture using needle and syringe. Each specimen collected was carefully mixed with the anticoagulant (EDTA – 10%, w/v in distilled water) to prevent clotting. The Automated Haematologic Analyzer, Sysmex, KX-21 (Japan) was used to analyze hematological parameters.
A portion of the blood (0.5 mL) collected on day 29 (24 h after the last dose administration) was prepared and used as described above for hematological analysis, while the other portion collected in plain bottle was centrifuged at 15,000 × g for 5 min. The resulting serum was carefully aspirated with Pasteur pipette into sample bottles for lipid profile assays.
2.6. Statistical analysis
Plasma level of lymphocyte was expressed as percentage. Other data were expressed as means of five replicates ±SD and were subjected to one way analysis of variance (ANOVA) followed by Duncan Multiple Range Test. Statistical significance was considered at p < 0.05.
3. Results
Effects of administration of aqueous extract of CS at doses of 100, 200 and 400 mg/kg b.w. on the hematological indices of Wistar rats at days 1, 7, 14, 21, and 28 are as shown in Table 1, Table 2, Table 3, Table 4, Table 5 respectively.
Table 1.
Changes in hematological indices of Wistar rats orally given corn silk aqueous extract for day 1 (n = 5, X ± SD).
| Parameters | Control | Extract (mg/kg body weight) |
||
|---|---|---|---|---|
| 100 | 200 | 400 | ||
| Hb (g/dL) | 12.42 ± 0.34a | 12.65 ± 0.15a | 12.50 ± 0.45a | 12.56 ± 0.44a |
| HCT | 39.99 ± 1.45a | 39.95 ± 1.21a | 39.08 ± 1.35a | 40.65 ± 0.65a |
| RBC (×1012/L) | 7.50 ± 0.25a | 6.82 ± 0.17a | 6.89 ± 0.27a | 7.15 ± 0.25a |
| MCV (fl) | 54.85 ± 1.17a | 53.25 ± 1.04a | 53.65 ± 1.42a | 54.35 ± 1.11a |
| MCH (pg) | 17.20 ± 0.25a | 17.00 ± 0.10a | 17.90 ± 0.15a | 17.30 ± 0.21a |
| MCHC (g/dL) | 31.40 ± 0.23a | 31.10 ± 0.25a | 32.80 ± 0.55a | 31.70 ± 0.25a |
| WBC (×109/L) | 12.90 ± 0.24a | 9.10 ± 0.15b | 7.84 ± 0.38c | 5.89 ± 0.55d |
| Platelets (×109/L) | 769.05 ± 9.10a | 715.10 ± 13.21b | 793.20 ± 14.17c | 816.15 ± 12.25d |
| MPV | 9.60 ± 0.12a | 9.25 ± 0.44a | 9.52 ± 0.34a | 9.60 ± 0.25a |
| Lymphocytes (%) | 50.50 ± 1.85a | 58.15 ± 1.20b | 58.75 ± 1.16b | 65.23 ± 1.22c |
Values with different superscripts along the same row for each parameter are significantly different (p < 0.05). Hb, hemoglobin; HCT, hematocrit; PCV, packed cell volume; RBC, red blood cell; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cell; MPV, mean platelet volume.
Table 2.
Changes in hematological indices of Wistar rats orally given corn silk aqueous extract for day 7 (n = 5, X ± SD).
| Parameters | Control | Extract (mg/kg body weight) |
||
|---|---|---|---|---|
| 100 | 200 | 400 | ||
| Hb (g/dL) | 12.50 ± 0.14a | 12.60 ± 0.25a | 12.50 ± 0.15a | 12.80 ± 0.25a |
| HCT | 40.75 ± 1.45a | 40.15 ± 1.20a | 40.09 ± 1.15a | 40.60 ± 0.65a |
| RBC (×1012/L) | 7.44 ± 0.15a | 6.31 ± 0.12a | 6.99 ± 0.25a | 7.25 ± 0.15a |
| MCV (fl) | 55.00 ± 1.15a | 54.35 ± 1.15a | 54.65 ± 1.22a | 54.00 ± 1.15a |
| MCH (pg) | 17.70 ± 0.21a | 17.30 ± 0.15a | 17.50 ± 0.16a | 17.60 ± 0.21a |
| MCHC (g/dL) | 30.80 ± 0.13a | 31.80 ± 0.05a | 32.40 ± 0.25a | 32.10 ± 0.12a |
| WBC (×109/L) | 9.75 ± 0.25a | 11.15 ± 0.23b | 12.92 ± 0.34c | 13.15 ± 0.15c |
| Platelets (×109/L) | 785.05 ± 9.10a | 795.10 ± 10.35a | 699.45 ± 11.10b | 800.11 ± 06.23c |
| MPV | 9.90 ± 0.15a | 9.70 ± 0.41a | 9.25 ± 0.14a | 9.30 ± 0.30a |
| Lymphocytes (%) | 52.90 ± 1.70a | 76.00 ± 1.10b | 78.15 ± 1.11b | 75.19 ± 1.20b |
Values with different superscripts along the same row for each parameter are significantly different (p < 0.05). Hb, hemoglobin; HCT, hematocrit; PCV, packed cell volume; RBC, red blood cell; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cell; MPV, mean platelet volume.
Table 3.
Changes in hematological indices of Wistar rats orally given corn silk aqueous extract for day 14 (n = 5, X ± SD).
| Parameters | Control | Extract (mg/kg body weight) |
||
|---|---|---|---|---|
| 100 | 200 | 400 | ||
| Hb (g/dL) | 13.80 ± 0.10a | 13.58 ± 0.15a | 13.75 ± 0.15a | 13.50 ± 0.15a |
| HCT | 40.90 ± 1.20a | 40.99 ± 1.65a | 40.00 ± 1.05a | 40.90 ± 0.15a |
| RBC (×1012/L) | 7.29 ± 0.10a | 7.00 ± 0.15a | 7.55 ± 0.25a | 7.20 ± 0.21a |
| MCV (fl) | 54.80 ± 1.19a | 54.40 ± 1.25a | 54.90 ± 1.00a | 54.50 ± 1.15a |
| MCH (pg) | 17.20 ± 0.23a | 6.90 ± 0.20a | 17.01 ± 0.23a | 17.85 ± 0.29a |
| MCHC (g/dL) | 31.30 ± 0.25a | 31.50 ± 0.05a | 32.30 ± 0.15a | 31.80 ± 0.22a |
| WBC (×109/L) | 9.50 ± 0.22a | 11.10 ± 0.25b | 13.02 ± 0.35c | 13.15 ± 0.15c |
| Platelets (×109/L) | 676.15 ± 8.11a | 695.17 ± 9.15a | 701.10 ± 9.00b | 805.15 ± 15.20c |
| MPV | 9.10 ± 0.10a | 9.30 ± 0.20a | 9.40 ± 0.11a | 9.70 ± 0.10a |
| Lymphocytes (%) | 51.88 ± 1.00a | 67.23 ± 1.50b | 63.44 ± 1.30b | 65.22 ± 1.10b |
Values with different superscripts along the same row for each parameter are significantly different (p < 0.05). Hb, hemoglobin; HCT, hematocrit; PCV, packed cell volume; RBC, red blood cell; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cell; MPV, mean platelet volume.
Table 4.
Changes in hematological indices of Wistar rats orally given corn silk aqueous extract for day 21 (n = 5, X ± SD).
| Parameters | Control | Extract (mg/kg body weight) |
||
|---|---|---|---|---|
| 100 | 200 | 400 | ||
| Hb (g/dL) | 13.00 ± 0.15a | 13.35 ± 0.10a | 13.75 ± 0.15a | 13.49 ± 0.10a |
| HCT | 41.50 ± 1.10a | 41.30 ± 1.15a | 40.01 ± 1.00a | 41.00 ± 0.15a |
| RBC (×1012/L) | 7.11 ± 0.19a | 7.35 ± 0.42a | 7.59 ± 0.15a | 7.25 ± 0.21a |
| MCV (fl) | 55.10 ± 1.25a | 54.90 ± 1.11a | 54.20 ± 1.12a | 55.20 ± 1.15a |
| MCH (pg) | 16.99 ± 0.25a | 16.90 ± 0.54a | 17.35 ± 0.14a | 17.05 ± 0.29a |
| MCHC (g/dL) | 31.50 ± 0.33a | 30.95 ± 0.15a | 31.80 ± 0.11a | 31.95 ± 0.12a |
| WBC (×109/L) | 9.80 ± 0.26a | 13.22 ± 0.19b | 15.25 ± 0.18c | 18.15 ± 0.10d |
| Platelets (×109/L) | 680.15 ± 9.20a | 701.24 ± 10.00b | 697.35 ± 9.13b | 705.25 ± 11.20b |
| MPV | 9.00 ± 0.17a | 9.10 ± 0.25a | 9.25 ± 0.19a | 9.20 ± 0.10a |
| Lymphocytes (%) | 52.50 ± 1.27a | 79.92 ± 1.00b | 73.23 ± 1.23b | 71.25 ± 1.25b |
Values with different superscripts along the same row for each parameter are significantly different (p < 0.05). Hb, hemoglobin; HCT, hematocrit; PCV, packed cell volume; RBC, red blood cell; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cell; MPV, mean platelet volume.
Table 5.
Changes in hematological indices of Wistar rats orally given corn silk aqueous extract for day 28 (n = 5, X ± SD).
| Parameters | Control | Extract (mg/kg body weight) |
||
|---|---|---|---|---|
| 100 | 200 | 400 | ||
| Hb (g/dL) | 12.99 ± 0.25a | 13.15 ± 0.19a | 13.80 ± 0.11a | 13.50 ± 0.10a |
| HCT | 41.70 ± 1.15a | 41.30 ± 1.20a | 40.00 ± 1.23a | 41.10 ± 0.19a |
| RBC (×1012/L) | 7.17 ± 0.25a | 7.44 ± 0.45a | 7.63 ± 0.25a | 7.35 ± 0.11a |
| MCV (fl) | 55.00 ± 1.15a | 54.71 ± 1.43a | 54.00 ± 1.25a | 55.10 ± 1.13a |
| MCH (pg) | 17.00 ± 0.15a | 16.99 ± 0.51a | 17.25 ± 0.34a | 17.07 ± 0.30a |
| MCHC (g/dL) | 31.60 ± 0.22a | 30.72 ± 0.20a | 31.67 ± 0.43a | 31.73 ± 0.23a |
| WBC (×109/L) | 9.75 ± 0.22a | 13.15 ± 0.20b | 15.00 ± 0.13c | 18.01 ± 0.15d |
| Platelets (×109/L) | 680.05 ± 9.00a | 701.09 ± 10.11b | 695.20 ± 9.13b | 706.05 ± 13.25b |
| MPV | 9.00 ± 0.23a | 9.11 ± 0.15a | 9.20 ± 0.17a | 9.21 ± 0.15a |
| Lymphocytes (%) | 52.70 ± 1.34a | 79.99 ± 1.43b | 74.75 ± 1.35b | 71.79 ± 1.10b |
Values with different superscripts along the same row for each parameter are significantly different (p < 0.05). Hb, hemoglobin; HCT, hematocrit; PCV, packed cell volume; RBC, red blood cell; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cell; MPV, mean platelet volume.
Administration of the extract at the tested regimens (100, 200 and 400 mg/kg b.w.) had no significant (p > 0.05) effect on the hemoglobin (Hb), hematocrit (HCT), red blood cell (RBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and mean platelet volume (MPV) within the period of investigation (Table 1, Table 2, Table 3, Table 4, Table 5). Except for day 1 where a significant reduction in the level of WBCs was observed (Table 1), the extract at all the tested doses significantly increased (p < 0.05) this parameter on other days when compared with the control group (Table 2, Table 3, Table 4, Table 5). Evaluation of CS aqueous extract administration revealed a considerable increase (p < 0.05) in the percentage of serum level of lymphocytes throughout the experimental periods for all the tested doses compared to control group (Table 1, Table 2, Table 3, Table 4, Table 5). The platelet count also significantly increased (p < 0.05) following administration of the extract at 400 mg/kg b.w. throughout the investigation periods. Similar trends were also observed for the platelet count on days 1, 21 and 28 for the 200 mg/kg b.w. extract-treated rats (Table 1, Table 4, Table 5). However, administration of CS extract at 100 mg/kg b.w. produced values that competed favorably (p < 0.05) with the control on days 7 and 14 (Table 2, Table 3).
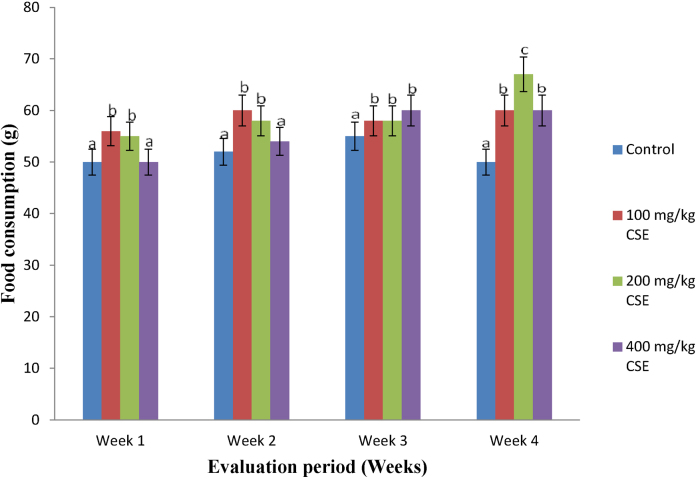
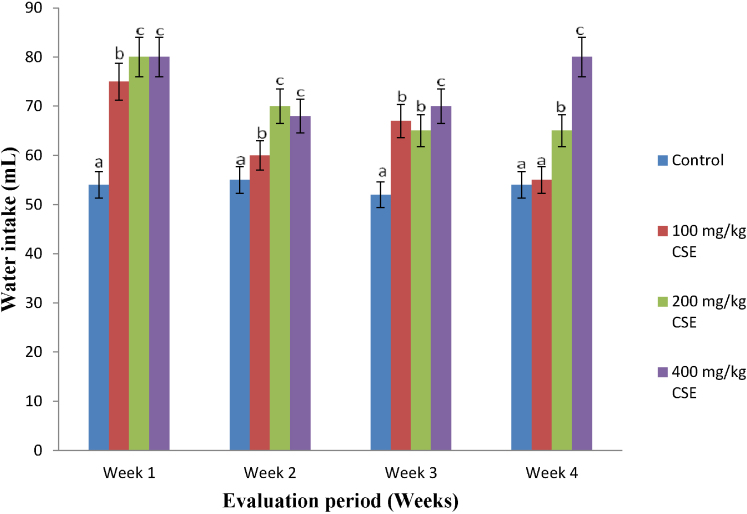
Compared with the control group, the feed and water taken by the extract-treated (at all doses) animals increased significantly throughout the experimental periods (Fig. 1, Fig. 2). It is worthy of note that, the feed consumed by the animals placed on 400 mg/kg b.w. of CS extract compared favorably well with the control in the first 2 weeks of the study, while steady and fairly constant consumption patterns were maintained throughout the remaining part of the feeding periods (Fig. 1).
Fig. 1.
Feed intake of Wistar rats orally given corn silk aqueous extract for 4 weeks (n = 20, 15, 10 and 5 respectively for weeks 1–4, X ± SD). Bars with different superscripts for the parameter are significantly different (p < 0.05). CSE: corn silk extract.
Fig. 2.
Water intake of Wistar rats orally given corn silk aqueous extract for 4 weeks (n = 20, 15, 10 and 5 respectively for weeks 1–4, X ± SD). Bars with different superscripts for the parameter are significantly different (p < 0.05). CSE: corn silk extract.
At all the concentrations of the extract investigated, a significant decrease in the serum level of TC and TAG was recorded. Administration of the extract also revealed a significant reduction in artherogenic index across all groups, with animals administered with 400 mg/kg b.w. of the extract comparing favorably well with the normal control group. The artherogenic index (ratio of LDL-C/HDL-C) was a product of significant moderation in the levels of HDL-C and LDL-C by the CS extract (Table 6).
Table 6.
Effect of corn silk aqueous extract on serum lipid profile of Wistar rats (n = 5, X ± SD).
| Indices | Control | Extract (mg/kg body weight) |
||
|---|---|---|---|---|
| 100 | 200 | 400 | ||
| TC (mmol/L) | 255.00 ± 17.30a | 204.02 ± 18.20b | 183.13 ± 15.50c | 203.54 ± 19.20b |
| TAG (mmol/L) | 355.21 ± 15.19a | 190.91 ± 10.31b | 252.50 ± 10.66c | 260.04 ± 18.45c |
| HDL-C (mmol/L) | 131.25 ± 11.47a | 165.02 ± 15.25b | 175.33 ± 17.10b | 141.27 ± 10.14a |
| LDL-C (mmol/L) | 75.02 ± 7.30a | 29.51 ± 4.20b | 33.01 ± 5.50b | 70.20 ± 7.20a |
| Artherogenic index | 0.57 ± 0.07a | 0.18 ± 0.01b | 0.19 ± 0.06b | 0.50 ± 0.05a |
Values with different superscripts along the same row for each parameter are significantly different (p < 0.05). TC, total cholesterol; TAG, triacylglycerol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
4. Discussion
Toxicity testing in animals is commonly used to assess potential health risk of pharmacological agents or plant extracts on humans [20]. Biochemical analysis on hematological parameters are often employed to determine the safety profile and influence of foreign compounds, including plant extracts, on the blood constituents as well as blood related functions in humans [21]. Such assessment on the blood is crucial and the results thereof can be harnessed to establish how pharmacologically safe an agent is, on the well being of humans. The parameters investigated in this study are useful indices in ascertaining the toxic potentials of botanicals in living systems [22]. In this study, except for WBC, platelets and lymphocytes, the non-significant difference in the hematological parameters following administration of the extract could be an indication that it is unlikely to be toxic to the blood. Specifically, the non-significant effect of the extract on RBCs and Hb might be a reflection of fairly unaltered erythropoiesis. This suggests that the release of erythropoietin was not stimulated in the kidney, thus maintaining the rates of production and destruction of blood corpuscles at equilibrium [23]. This non-significant effect on RBCs and Hb may also imply that, the morphology and osmotic fragility of the RBCs, as well as Hb incorporation into the RBCs were not affected. This further reveals that there was no change in the oxygen-carrying capacity of the blood and amount of oxygen delivered to the tissues following the extract administration [24]. Analysis on parameters (MCV, MCH and MCHC) relating to the status of RBCs may be of utmost importance in the diagnosis of anemia in animals [25]. The non-significant effect on these parameters is an indication that the extract at the tested doses had no effect on RBCs’ microcytes and Hb weight per RBCs. This suggests that CS extract does not predispose the animals to anemic condition throughout the investigation period. These submissions are in agreement with the finding of Ashafa and Olunu [26], where Murinda lucida was reported to be non-heamatotoxic in Wistar rats. The serum level of WBCs may indicate an organism's defensive capability against infections [27]. The decrease in the level of WBCs observed on Day 1 may be due to redress in rats’ system to respond to foreign compound, that is attributable to initial expose to the extract. However, the significantly increased WBC levels following CS extract administration on other days (7–28) indicates an enhanced vascular permeability and immune system boost. Additionally, since the extract exhibited a considerable increase in the levels of lymphocytes, this perhaps may be a tenable fact for its stimulatory effect on the effectors cells of the immune system at the tested doses. This could mean that the extract at the investigated doses and exposure period do not exert deleterious impact on the effectors cells of the immune system, thus further substantiating the non-haematotoxic tendency of the extract. Our findings agree with Yakubu et al. [19], who gave similar submission on administration of Fadogia agrestis stem on hematological indices of rats. The observed increase in platelet especially at 400 mg/kg b.w. of the extract throughout the experimental period may be adduced to its stimulatory effect on thrombopoietin. This implies that the extract can promote thrombopoiesis, repair the minute vascular damage that occurs with daily life and considerably manage thrombocytopenia in animals [28].
Water is predominantly life's essential nutrient occupying about 70% of the human bodily fluids, and provides effective metabolic milieu [29]. Hypothalamic stimulations of thirst and appetite occur respectively, in the paraventricular and ventromedial nuclei of the brain [30], [31]. Thus, factors that influence water intake will also impact on feeding pattern. In this study, the increase in feed and water consumption following administration of CS extract suggests that the investigated doses might have stimulated taste sense and enhanced appetite of the rats via co-ordinated action of the hypothalamus in the brain. This is believed to have aided the relatively improved performance and general well being of the animals. This agrees with earlier reports by Kiers et al. [32], Imafidon and Okunrobo [33]. These authors opined that improved performance is closely associated with feeding pattern in experimental animals.
Alteration in the concentration of major lipids such as TC, HDL-C, LDL-C, and TAG can give useful information on lipid metabolism as well as predisposition of the heart to atherosclerosis and its associated coronary heart diseases. TAG, LDL-C and HDL-C are associated with lipolysis, carrier of plasma cholesterol and atherosclerotic tendency, respectively [34]. The reduction in the serum levels of TC, TAG and LDL-C coupled with an increase in HDL-C at all the doses investigated could suggest that the extract may not predispose the animals to cardiovascular risk. The significant improvement in the lipid profile could also suggest that CS extract is anti-lipidemic. This is also buttressed by the reduction in the calculated artherogenic index, suggesting that the extract could be exploited in the management of hypercholesterolemia related disorders.
In conclusion, the observations following administration of CS extract at 100, 200 and 400 mg/kg body weight on the hematological and lipid parameters indicate that, it could be relatively safe as oral remedy if not consumed beyond the investigated experimental period.
Conflict of interest
The authors declared that there are no conflicts of interest.
Acknowledgements
This study was funded by the Center for Community Development, Kwara State University, Malete, Nigeria, through provision of an Action Research Grant (no. KWASUCCD/2014AR/1/32). The authors are very thankful for the accorded gesture.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2015.04.001.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Stojanoski N. Development of health culture in Veles and its region from the past to the end of the 20th century. Veles: Soc. Sci. Art. 1999:13–34. [Google Scholar]
- 2.Biljana B.P. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012;6(11):1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly K. Facts on file; New York: 2009. History of Medicine; pp. 29–50. [Google Scholar]
- 4.WHO . World Health Organization; Harare, Zimbabwe: 2000. Promoting the Role of Traditional Medicine in Health Systems: A Strategy for the African Region 2001–2010. Document Reference AFR/RC50/Doc. 9/R. [Google Scholar]
- 5.Maksimović Z., Dobric S., Kovacevic N., Milovanovic Z. Diuretic activity of Maydis stigma extract in rats. Pharmazie. 2004;59(12):967–971. [PubMed] [Google Scholar]
- 6.Ebrahimzadeh M.A., Pourmorad F., Hafe S. Antioxidant activities of Iranian corn silk. Turk. J. Biol. 2008;32:43–49. [Google Scholar]
- 7.Farsi D.A., Harris C.S., Reid L., Bennett S.A.L., Haddad P.S., Martineau L.C., Arnason J.T. Inhibition of non-enzymatic glycation by silk extracts from a mexican land race and modern inbred lines of maize (Zea mays) Phytother. Res. 2008;22:108–112. doi: 10.1002/ptr.2275. [DOI] [PubMed] [Google Scholar]
- 8.Jianyou G., Tongjun L., Linna H., Yongmei L. The effects of corn silk on glycaemic metabolism. Nutr. Metab. 2009;6:47. doi: 10.1186/1743-7075-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao W., Yin Y., Yu Z., Liu J., Chen F. Comparison of anti-diabetic effects of polysaccharides from corn silk on normal and hyperglycemia rats. Int. J. Biol. Macromol. 2012;50:1133–1137. doi: 10.1016/j.ijbiomac.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Grases F., March J.G., Ramis M., Costa-Bauzá A. The influence of Zea mays on urinary risk factors for kidney stones in rats. Phytother. Res. 1993;7:146–149. [Google Scholar]
- 11.Wang G.Q., Lu S., Liu J., Wang C. Anti-inflammation effects of corn silk in a rat model of carrageenin-induced pleurisy. Inflammation. 2012;35(3):822–827. doi: 10.1007/s10753-011-9382-9. [DOI] [PubMed] [Google Scholar]
- 12.WHO . WHO; Geneva: 1978. The Promotion and Development of Traditional Medicine Technical Report Series no. 615; pp. 1–15. [PubMed] [Google Scholar]
- 13.NIH . National Institute of Health Publication; 1985. Care and Use of Laboratory Animals; pp. 85–123. [Google Scholar]
- 14.WHO . World Health Organization; Geneva, Switzerland: 1998. Basic OECD Principles of GLP. [Google Scholar]
- 15.Salawu O.A., Chindo B.A., Tijani A.Y., Obidike I.C., Salawu T.A., Akingbasote A.J. Acute and sub-acute toxicological evaluation of the methanolic stem bark extract of Crossopteryx febrifuga in rats. Afr. J. Pharm. Pharmacol. 2009;3(12):621–626. [Google Scholar]
- 16.Oyedemi S.O., Adewusi E.A., Aiyegoro O.A., Akinpelu D.A. Antidiabetic and haematological effect of aqueous extract of stem bark of Afzelia africana (Smith) on streptozotocin-induced diabetic Wistar rats. Asian Pac. J. Trop. Biomed. 2011;1(5):353–358. doi: 10.1016/S2221-1691(11)60079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anofi O.T.A., Latifat O.O., Musa T.Y. Toxicity profile of ethanolic extract of Azadirachta indica stem bark in male Wistar rats. Asian Pac. J. Trop. Biomed. 2012;2(10):811–817. doi: 10.1016/S2221-1691(12)60234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalid G., Bashir A.G., Seema A., Khan M., Showkat A.D., Mohammad Y.D., Mudasir A.T. Antidiabetic activity of Artemisia amygdalina Decne in streptozotocin-induced riabetic rats. Biomed. Res. Intern. 2014 doi: 10.1155/2014/185676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yakubu M.T., Akanji M.A., Oladiji A.T. Haematological evaluation in male rats following chronic administration of aqueous extract of Fadogia agrestis stem. Pharmacogn. Mag. 2007;3(1):34–38. [Google Scholar]
- 20.Ajani E.O., Sabiu S., Bamisaye F.A., Ibrahim S., Salau B.A. Evaluation of the acute and sub-acute toxicity effect of ethanolic leaves extract of Lagenaria brevifolia (Bitter gourd) on hepatic and renal function of rats. J. Pharm. Biol. Sci. 2014;9:61–68. [Google Scholar]
- 21.Ashafa A.O.T., Yakubu M.T., Grierson D.S., Afolayan A.J. Evaluation of leaves extract of Chrysocomo ciliata L. on some biochemical parameters in Wistar rats. Afr. J. Biotechnol. 2008;6:1425–1430. [Google Scholar]
- 22.Sunmonu T.O., Ashafa A.O., Afolayan A.J. Toxicological evaluation of aqueous leaf and berry extracts of Phytolacca dioica L. in male Wistar rats. Food Chem. Toxicol. 2010;48:1886–1889. doi: 10.1016/j.fct.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Polenakovic M., Sikole A. Is erythropoietin a survival factor for red blood cells? J. Am. Soc. Nephrol. 1996;7(8):1178–1182. doi: 10.1681/ASN.V781178. [DOI] [PubMed] [Google Scholar]
- 24.Gruchy G.C. Blackwell Scientific Publication; Oxford, London: 1976. Clinical Haematology in Medical Practice; pp. 33–57. [Google Scholar]
- 25.Coles E.H. W. B Saunders; Philadelphia, USA: 1986. Veterinary Clinical Pathology; pp. 10–42. [Google Scholar]
- 26.Ashafa A.O.T., Olunu O.O. Toxicological evaluation of ethanolic root extract of Morinda lucida (L.) Benth. (Rubiaceae) in male Wistar rats. J. Nat. Pharm. 2011;2(2):108–114. [Google Scholar]
- 27.Aboyade O.M., Yakubu M.T., Grierson D.S., Afolayan A.J. Studies on the toxicological effect of the aqueous extract of fresh, dried, and boiled berries of Solanum aculeastrum Dunal in male Wistar rats. Hum. Exp. Toxicol. 2009;28:765–775. doi: 10.1177/0960327109354545. [DOI] [PubMed] [Google Scholar]
- 28.Geddis A.E. Inherited thrombocytopenias: an approach to diagnosis and management. Int. J. Lab. Hematol. 2013;35(1):14–25. doi: 10.1111/j.1751-553X.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 29.Wiggins P.M. Role of water in some biological processes. Microbiol. Rev. 1990;54:432. doi: 10.1128/mr.54.4.432-449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theologides A. Anorexia-producing intermediary metabolites. Am. J. Clin. Nutr. 1976;29:552–558. doi: 10.1093/ajcn/29.5.552. [DOI] [PubMed] [Google Scholar]
- 31.Nosiri C.I., Anuka J.A., Radindadi A.H. Endosulfan induced polydyspia in adult Wistar rats. Int. J. Pharmacol. 2010;9(1):1531–1576. [Google Scholar]
- 32.Kiers J.L., Meijer J.C., Nout M.J., Rombouts F.M., Nabuurs M.J., Van der Meulen J. Effect of fermented soya beans on diarrhoea and feed efficiency in weaned piglets. J. Appl. Microbiol. 2003;95:545–552. doi: 10.1046/j.1365-2672.2003.02011.x. [DOI] [PubMed] [Google Scholar]
- 33.Imafidon K.E., Okunrobo L.O. Study on biochemical indices of liver function tests of albino rats supplemented with three sources of vegetable oils. Nig. J. Basic Appl. Sci. 2012;19(2):105–110. [Google Scholar]
- 34.Oyedemi S.O., Yakubu M.T., Afolayan A.J. Effect of aqueous extract of Leonotis leonorus (L.) leaves in male Wistar rats. Hum. Exp. Toxicol. 2010;29:377–384. doi: 10.1177/0960327110363864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.