Abstract
The present work investigated repeated dose and reproductive toxicity of Calebin A in Wistar rats. A study for assessing the mutagenic potential of Calebin A through an AMES test is also described. Calebin A was orally administered to groups of 10 male and/or 10 female Wistar rats each, assigned to three dose levels (20, 50 and 100 mg/kg/body weight) once daily for 90 consecutive days. None of the animals in any of the treatment/control groups exhibited any abnormal clinical signs/behavioral changes, reproductive as well as developmental parameters, or gross and microscopic changes in both male and female rats. Calebin A was also evaluated for its ability to induce reverse mutations at selected loci of Salmonella typhimurium in the presence and absence of Aroclor 1254 induced rat liver S9 cell lines. In conclusion, 100 mg/kg/d of Calebin A is not likely to produce any significant toxic effects in male and female Wistar rats and no reproductive or developmental toxicity was observed at the same dose and hence Calebin A at 100 mg/kg was determined as “No Observed Adverse Effect Level (NOAEL)” under the test conditions.
Keywords: Calebin A, Reproductive, Toxicological, Histo-pathological, NOAEL
1. Introduction
The genus Curcuma is a well-known spice of India. More than 130 species and subspecies of it are found across the world. Curcuma caesia popularly called as black turmeric, is an erect rhizomatous perennial herb native to North-East and Central India. Its aromatic fresh rhizomes are applied for sprains and bruises [1]. In the Asian countries, turmeric powder derived from the rhizomes of Curcuma longa and C. caesia (both belong to Zingiberaceae family), served as a culinary aid as well as an ayurvedic medicine of extreme versatility for several thousand years. Due to lack of any noticeable toxicity, extensive research has been done on the useful medicinal properties of the Curcuma constituents [2], [3].
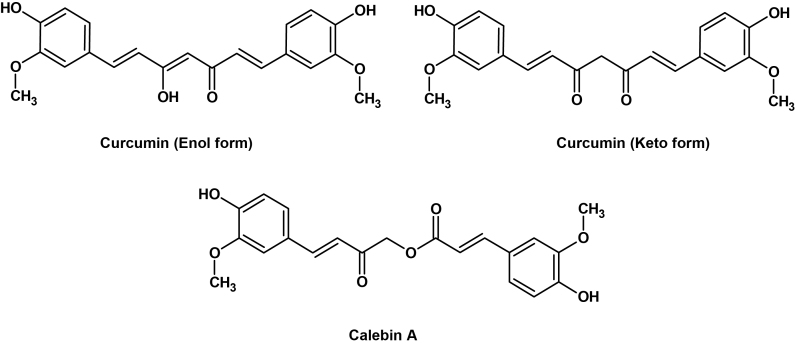
Calebin A is one of the minor components that co-occur along with the fabled curcuminoids in C. longa. It was first isolated and identified by Kim [4] from C. longa and it resembles curcuminoids closely in structure yet perceptibly different (Fig. 1). Calebin A, being a ferulate ester, lacks the characteristic feature of curcuminoids i.e., 1,3-diketonic structure. It was detected in quantities in other Curcuma species also, especially in C. caesia (Unpublished data).
Fig. 1.
Structural comparison of Curcumin and Calebin A.
In addition to Calebin A, isomeric Calebenoids have also been isolated and identified from C. longa [5]. Calebin A was found to be a more powerful protector of PC-12 and other susceptible cells from the β-amyloid insult [6] than their structural cousins namely curcuminoids. Calebin A might be an effective compound for the treatment of human gastric and other multi-drug resistant cancer cells [7]. Calebin A at 25 μmol/L was also shown to inhibit the growth of human hepatoma cell line HepG2 cells than curcumin in a dose and time dependent manner [8].
Paucity of Calebin A occurrence in Curcuma precluded the possibility of use of naturally occurring Calebin A in a pure form for toxicity studies. Hence an efficient method of synthesis of Calebin A was devised. The synthetic material matched in every respect the natural material in all the physical and spectral properties. Production wise, briefly, 27 L of isopropyl alcohol was added into 250 L stainless steel (SS) reactor. Intermediate −1, i.e., feruloylmethane of 3 kg was stirred with cupric oxide 1.6 kg together for 10 min. Water of 90 L was added to the above reaction mixture, stirred for 30 min, filtered and weighed. The filtered solid was dissolved in 33 L of ethyl acetate and stirred for 15 min. Potassium carbonate (3.24 kg) was mixed with water (48 L) and stirred in 250 L SS reactor. Calebin A, intermediate 2 – ferulic acid (9.12 kg) was added to the above with stirring. Once the solution becomes homogenous, tetrabutylammonium bromide (900 g) was added to this solution. The reaction mixture was filtered to get crystallized Calebin A. This was dried at 80 °C in vacuum tray drier, milled, sieved and packed. The yield of Calebin A was 1.3 kg. The sample of Calebin A that was used in this study was pale yellow powder in color with a molecular formula C21H20O7 and with a correct molecular weight as obtained from mass spectra. This powder had melting point of 138–140 °C, with satisfactory proton and carbon NMR and mass spectra, in total agreement with literature data [4]. The HPLC purity of the sample was 97.6%. The heavy metal contents were <10 ppm and the residual solvent content was conforming to USP specifications. No microbial contamination was observed. In spite of the useful pharmacological and health-promoting properties of Calebin A, there is very little information on its toxicity data. In continuation of our previous oral acute and repeated dose 28 day toxicity studies on Wistar rats (Majeed et al., Unpublished results), the current study targeted 90 day oral toxicity as well as a reproductive/developmental toxicity that could be considered as an extension of toxicity studies on Calebin A.
2. Materials and methods
2.1. Chemicals
For reverse mutation assay, methyl methane sulphonate (MMS), sodium azide, Nitrofluorene and 2-aminoanthracene were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 9-Amino Acridine was purchased from Fluka – Sigma Aldrich (St. Louis, MO, USA). The Salmonella typhimurium strains TA98, TA100, TA1535 and TA1537 strain WP2uvrA were purchased from Molecular Toxicology Inc. (Boone, NC, USA). A rat liver S9 fraction induced by aroclor in male Sprague–Dawley rats was purchased from Molecular Toxicology Inc. (Boone, NC, USA). The chemicals and solvents used throughout the experiments were of analytical grade.
2.2. Animals
For repeated dose and reproductive toxicity studies, rats were housed in an environment controlled room at 22 ± 3 °C and relative humidity of 30–70% with the photoperiod of 12 h light and 12 h darkness. Five rats of each sex per cage were housed in sterilized standard polycarbonate cages with steam sterilized clean paddy husk changed along with the cage twice a week. Pelleted rat diet manufactured by M/s Provimi Animal Nutrition India Pvt. Ltd., Bangalore with fixed amount of 25 g pellet diet was given to each animal daily and water given ad libitum.
2.3. Ethics
Both repeated dose (90 days) toxicity and reproductive and developmental toxicity studies were performed in accordance with the recommendation of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines for laboratory animal facility published in the gazette of India, December 15th 1998.
2.4. Test item preparation for dosing and procedure
In this study, the test item Calebin A was administered orally after suspending it in corn oil at daily doses of 20, 50 and 100 mg/kg/body weight, the doses that were in line with our earlier repeated dose (28 day) toxicity data with 100 mg/kg/d body weight had reported ‘No Observed Adverse Effect Level’ (NOAEL).
2.4.1. Repeated 90 day oral toxicity study [9]
The dose formulations and vehicle was administered at the dose volume of 10 mL/kg body weight of animals, in Main and Recovery Group animals (Table 1). Homogeneity of the test item in the corn oil vehicle was maintained during dose administration by constant stirring using a magnetic stirrer. Dose formulations or vehicle was administered by oral gavage to the respective groups once daily at approximately the same time each day (varying by ±2 h) for a period of 90 days. There was no administration of test item formulation/vehicle during the 30-day recovery period. All treated and control rats were observed once daily for a total of 90 days in the main treatment groups. The body weights, food consumption of all rats were recorded on test day 1 (immediately prior to oral application) and then on weekly basis for 90 days in main treatment groups (immediately prior to the sacrifice for necropsy). Observations for neuro-behavioral toxicity symptoms e.g., visual (response to external light source), auditory (response to external noise source), motor (gait, ataxia) and proprioceptive (righting reflex, threat perception) were made after the last dose and prior to the sacrifice for necropsy in main treatment groups of rats.
Table 1.
Grouping for repeated dose (90 day) oral toxicity study.
| Treatment groups | Number of rats/group | Sex (n) | |
|---|---|---|---|
| Main groups | |||
| Vehicle control (corn oil) | 20 | M (10) | F (10) |
| Low dose | 20 | M (10) | F (10) |
| Mid dose | 20 | M (10) | F (10) |
| High dose | 20 | M (10) | F (10) |
| Recovery groups | |||
| Vehicle control (corn oil) | 10 | M (5) | F (5) |
| High Dose | 10 | M (5) | F (5) |
M, male; F, female; n, number.
Blood samples were collected at the end of the treatment period from main groups. All rats were fasted overnight (water allowed) before blood collection. Blood was collected into K2 EDTA tubes for hematology and tubes without anticoagulant for clinical chemistry. Hematological parameters were determined using Sysmex XT 1800iv hematology analyzer and serum was separated in a refrigerated centrifuge at approximately 5000 rpm for 10 min and analyzed using Rx Daytona (Randox) automatic analyzer for the biochemistry parameters. Rats from all groups in the study were subjected to detailed necropsy and findings were recorded, the animals were examined visually for external abnormalities including palpable masses. On completion of the gross pathology examination the tissues and organs were collected from all animals and preserved in 10% formal saline.
2.4.2. Reproductive/developmental toxicity
The dose formulations of Calebin A were prepared once in 10 days during dosing period [10]. 1000, 2500 and 5000 mg of the test item was suspended after mixing in corn oil to get formulations of 2, 5 and 10 mg/mL, administered by oral gavage once daily at the dose volume of 10 mL/kg body weight, approximately the same time on each day (varying by ±2 h) for the period of 2 weeks while pre-mating, 2 weeks at the time while mating and in females for gestation and post-partum period. Group 1 (G1) rats received vehicle control while G2, G3 and G4 animals received low (20 mg/kg/d), mid (50 mg/kg/d) and high doses (100 mg/kg/d) respectively (Table 8). Homogeneity of the Calebin A in the vehicle was maintained during dose administration by constant stirring using a magnetic stirrer. One male to one female, 1:1 matings were allowed by keeping them in single cages in this study.
Table 8.
Grouping and dose formulation for reproductive/developmental toxicity study.
| Main groups | |||||
|---|---|---|---|---|---|
| Group no. | Treatment groups | Dose (mg/kg/d) | Concentration (mg/m) | No. of rats | Sex |
| G1 | Vehicle control | 0 | NA | 10 | M |
| 10 | F | ||||
| G2 | Low dose | 20 | 2 | 10 | M |
| 10 | F | ||||
| G3 | Mid dose | 50 | 5 | 10 | M |
| 10 | F | ||||
| G4 | High dose | 100 | 10 | 10 | M |
| 10 | F | ||||
M, male; F, female.
Clinical examination was done prior to the test item administration on day 1 and daily thereafter (±1 day) during treatment period. Rats were observed for changes in skin and fur, eyes, mucous membrane occurrence of secretions and excretions, changes in gait, posture and presence of any abnormal behavior. All rats were observed for morbidity and mortality once daily. Males and females were weighed on the first day of dosing, atleast weekly thereafter, and at termination. During pregnancy, females were weighed on days 0, 7, 14 and 20 and within 24 h of parturition (day 0 or 1 post-partum) and day 4 post-partum. Pups were weighed on day 0 and day 4. Food consumption was measured during in-life phase (pre-mating, pregnancy and lactation) of the experiment. Necropsy: the rats to be sacrificed at term, were anaesthetized with chloroform, weighed and exsanguinated. At necropsy, the animals were examined visually for external abnormalities including palpable masses. Special attention was paid to the organs of the reproductive system. The numbers of implantation sites were recorded. The counting of corpora lutea was done. The organs removed, examined and placed in 10% formal saline/Bouin's fixative. Histopathological examination was carried out on the preserved organs of vehicle control (G1) and high dose (G4) group rats. Since no organ/tissue showed test item related histopathological changes in the high dose groups as compared to the control animal tissues from the lower dose groups (G2 and G3) were not examined.
2.4.3. Bacterial reverse mutation assay
The experiments were carried out using the tester strains, plated in duplicates at each concentration [11]. The positive control factors were dissolved in DMSO vehicle and stored at −20 °C; these positive control factors were methyl methane sulphonate (MMS), sodium azide, 9-aminoacridine (9-AA), Nitrofluorene and 2-aminoanthracene (2-AA). A dose range-finding test was performed to determine the highest concentration for the present study, which was performed with the five tester strains at concentrations of 312.5, 625, 1250, 2500, and 5000 μg/plate, with and without the S9 mixture. The number of revertant colonies did not increase to more than twice the value observed in the controls for any of the tester stains. However, there were increased numbers of revertant colonies of TA1535 with the S9 mixture. Based on these results, a dose of 5000 μg/plate was selected as the maximum dose. Aroclor 1254 induced rat liver S9 was used as the metabolic activation system. The S9 mix was prepared immediately before its use and contained 10% S9, 1 M glucose-6-phosphate, 0.1 M nicotinamide-adenine dinucleotide phosphate, 0.4 M MgCl2 and 1.65 M KCl in a 0.2 M phosphate buffer at pH 7.4. 100 μl of tester strain, 50 μl of test article and 0.5 mL S9 mix were added to 2.0 mL molten selective top agar at 45 ± 2 °C. After mixing thoroughly, the mixture was overlaid onto the surface of minimal bottom agar. When plating the vehicle and positive controls, the test article aliquot was replaced by a 50 μl aliquot of vehicle used and appropriate positive control respectively. After the overlay had solidified, the plates were inverted and incubated for approximately 48–72 h at 37 ± 2 °C. Plates that were not counted immediately following the incubation period were stored at 2–8 °C until colony counting was conducted. The condition of the bacterial background lawn was evaluated for evidence of test article toxicity by using a colony counter. Revertant colonies for a given tester strain and activation condition were counted by colony counter unless the assay exhibits toxicity in the preliminary toxicity assay [11].
The study was conducted with and without metabolic activation (S9 fraction) prepared from Aroclor 1254 induced rat liver as per OECD Guidelines, Section 4, Test # 471, December, 1997. The solvent control and appropriate positive controls (methyl methane sulphonate, sodium azide, Nitroflourene, 9-aminoacridine employed for without metabolic activation and 9-Aminoanthracene employed for with metabolic activation) were tested simultaneously. Selection of dose levels for the mutagenecity assay was based upon the toxicity and precipitation profile of the test article.
The preliminary assay was conducted by exposing TA98, TA100, TA102, TA1535 and TA1537 to vehicle controls and to at least 7 concentrations of test article, one plate per dose, in both the presence and absence of S9 mix. Doses were selected such that precipitate does not interfere with manual scoring. A dose that caused >50% reduction in the mean number of revertants per plate relative to that of vehicle control or a reduction in the background lawn was considered as toxic. Hundred micorliters of tester strain and 50 μl of vehicle/test compound/positive control was added to 2.0 mL of molten top agar at 45 ± 2 °C. The mixture was mixed well and overlaid onto the surface of 25 mL of minimal bottom agar. Later, the plates were inverted and incubated for 48–72 h at 37 ± 2 °C. Plates that were not counted immediately following the incubation period were stored at 2–8 °C. On the day of its use, minimal top agar 0.6% agar (w/v) and 0.5% NaCl (w/v), was melted and supplemented with l-histidine and d-biotin solution to a final concentration of 0.5 mM each. Top agar without S9 was supplemented with 0.5 mL of water for each 2 mL of minimal top agar. For the preparation of media and reagents, all references to water imply sterile deionized water produced by the Milli-Q reagent Water system. Bottom agar plate was approximately 25 mL of Minimal glucose agar containing 50 X Vogel-Bonner minimal medium E (Vogel and Bonner, 1956) solution and 40% Glucose solution containing 1.5% (w/v) agar.
2.5. Statistical analysis
The data have been subjected to statistical evaluation for the significance of any test item induced changes and their interpretation of potential for toxicity. The statistical analysis of the experimental data was carried out by the Fishers Student's t-test. Statistical comparisons were evaluated at the 5% (P ≤ 0.05) significant level. For reproductive/developmental toxicity studies, all quantitative variables like laboratory investigations (pups size and pups survival ratio) were subjected to one-way ANOVA test. In the case of recovery groups also, data was analyzed using the methods stated above. Comparison of means between treatment groups was performed. Student's t-test was employed for bacterial reverse mutation assay results.
3. Results and discussion
In the 90 day toxicity study, all the rats appeared healthy and gained weight throughout the observation period. The body weight gain of experimental and control animals did not show any statistically significant variation. No statistically significant changes in food consumption were observed from week 0 to 13 in both male and female animals across control and treated groups (Table 2). There were no indications of any test-substance related neurobehavioral toxicity symptoms e.g., visual (response to external light source), auditory (response to external noise source), motor (gait, ataxia) and proprioceptive (righting reflex, threat perception), made after the last dose and prior to the sacrifice for necropsy between control and treatment receiving group animals (Table 3). The mean absolute organ weight of male and female rats at necropsy observed for liver, brain, kidney, spleen, heart, adrenal, testes, uterus, epididymis and ovary between control and treatment groups was not statistically significant (Table 4), and no abnormalities detected. Hematology (Table 5) and biochemistry (Table 6) profiles suggest that all the parameters therein were normal and the treatment group values were same as that of the control group values, with no statistically significant difference. Sporadic variations like decreased differential leukocyte counts (only lymphocytes) and increased PLT to male and female rats exposed to test item did not correlate with dose, sex or other physiologically significant parameters to be considered exposure related effect. The activities of serum enzymes like GOT and GPT, and other important biochemical constituents like glucose, creatinine, urea, total protein, triglyceride, albumin, uric acid, cholesterol and globulin, that are indicative of metabolic and pathological abnormalities, showed no significant change in experimental animals as compared to control animals. However, stray variations like GOT activity showing mild decrease in female rats of 50 and 100 mg/kg/d dose group and decrease level of cholesterol in rats of 50 mg/kg/d were observed. These changes, in absence of any physiological abnormality, appear to be spontaneous and may not be related to the exposure to the test item. In the individual gross morphology and histopathology of male and female rats (Table 7), no abnormality detected for majority of the animals in both control and high dose receiving group (G4). No necropsy was observed in both the animal groups with few commonly seen histopathological observations between control and high dose (G4) group animals (Table 7). The testis of one male revealed oligospermia and the epididymis of all animals and testis of other animals did not show any change in control group animals. Females of control group did not reveal any lesion whereas uterus of one female showed hydrometra. In the high dose group (G4), one male showed oligospermia in testis, ovary of one female rat revealed congestion. Lesions observed in control and high dose group animals were comparable to each other and also they appear to be incidental and spontaneous in nature and cannot be related to the test substance, Calebin A. There were no significant observations found in the recovery group (30 days) of animals in any of the parameters studied and hence not tabulated.
Table 2.
Effect of 90 days oral exposure to Calebin A on body weight (g) and food consumption in wistar rats.
| Dose groups | Parameters | Weeks |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 |
I |
V |
X |
XIII |
|||||||
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | ||
| Control | Body weight (g) | 143.5 ± 5.27 | 123.3 ± 3.21 | 185.0 ± 7.60 | 153.0 ± 5.06 | 296.0 ± 6.27 | 215.5 ± 3.90 | 372.0 ± 8.85 | 238.5 ± 3.72 | 399.5 ± 9.73 | 246.50 ± 3.16 |
| Food consumption (g/rat) | 14.75 ± 0.30 | 14.84 ± 0.17 | 14.83 ± 0.12 | 14.71 ± 0.25 | 15.94 ± 0.23 | 14.78 ± 0.15 | 16.20 ± 0.27 | 16.33 ± 0.12 | 16.23 ± 0.16 | 16.14 ± 0.16 | |
| Treated (20 mg/kg/d) | Body weight (g) | 142.5 ± 4.73 | 118.6 ± 2.98 | 197.5 ± 8.60 | 146.0 ± 4.45 | 319.5 ± 11.31 | 212.0 ± 5.92 | 399.5 ± 13.00 | 239.0 ± 6.66 | 431.5 ± 15.30 | 246.5 ± 5.67 |
| Food consumption (g/rat) | 15.05 ± 0.12 | 14.59 ± 0.16 | 14.65 ± 0.23 | 14.41 ± 0.15 | 15.85 ± 0.17 | 14.58 ± 0.11 | 16.46 ± 0.22 | 16.08 ± 0.16 | 16.78 ± 0.29 | 16.10 ± 0.21 | |
| Treated (50 mg/kg/d) | Body Weight (g) | 136.0 ± 4.81 | 127.1 ± 2.90 | 188.0 ± 5.97 | 158.0 ± 3.18 | 299.0 ± 12.62 | 222.7 ± 5.21 | 386.5± 14.79 | 251.1 ± 6.60 | 413.5 ± 17.79 | 256.6 ± 6.61 |
| Food consumption (g/rat) | 14.44 ± 0.28 | 14.46 ± 0.14 | 14.21 ± 0.19 | 14.50 ± 0.14 | 16.36 ± 0.24 | 15.08 ± 0.17 | 16.02 ± 0.22 | 15.59 ± 0.20 | 16.38 ± 0.23 | 15.71 ± 0.26 | |
| Treated (100 mg/kg/d) | Body weight (g) | 142.0 ± 2.49 | 120.1 ± 3.76 | 186.0 ± 10.16 | 148.5 ± 3.72 | 314.0 ± 14.62 | 210.0 ± 2.24 | 196.0 ± 16.68 | 238.5 ± 3.42 | 427.0 ± 17.41 | 241.5 ± 4.35 |
| Food consumption (g/rat) | 14.28 ± 0.12 | 14.42 ± 0.21 | 14.24 ± 0.20 | 14.53 ± 0.14 | 16.35 ± 0.21 | 15.11 ± 0.11 | 16.31 ± 0.15 | 16.15 ± 0.15 | 16.77 ± 0.16 | 16.08 ± 0.18 | |
Values are mean ± SE of 10 animals in each group.
Statistical significance was set at P < 0.05.
Table 3.
Effect of 90 days oral exposure to Calebin A on neurobehavioral response index of individual male and female wistar rats.
| Dose groups | No of rats | Neurobehavioural response |
|||
|---|---|---|---|---|---|
| Visual response | Auditory response | Motor response | Proprioceptive response | ||
| Control | 10 | NED | NED | NED | NED |
| Treated (20 mg/kg/d) | 10 | NED | NED | NED | NED |
| Treated (50 mg/kg/d) | 10 | NED | NED | NED | NED |
| Treated (100 mg/kg/d) | 10 | NED | NED | NED | NED |
NAD, no abnormality detected.
Table 4.
Effect of 90 days oral exposure to Calebin A on mean absolute organ weight (gm) of male and female wistar rats at necropsy.
| Organ | Sex | Control | Treated |
||
|---|---|---|---|---|---|
| (20 mg/kg/d) | (50 mg/kg/d) | (100 mg/kg/d) | |||
| Liver | Male | 13.95 ± 0.37 | 15.52 ± 0.85 | 14.39 ± 0.68 | 13.55 ± 0.83 |
| Female | 8.27 ± 0.44 | 8.31 ± 0.38 | 8.99 ± 0.32 | 8.36 ± 0.26 | |
| Brain | Male | 1.82 ± 0.04 | 1.77 ± 0.08 | 1.78 ± 0.06 | 1.86 ± 0.06 |
| Female | 1.76 ± 0.03 | 1.74 ± 0.03 | 1.82 ± 0.04 | 1.67 ± 0.06 | |
| Kidney | Male | 2.61 ± 0.07 | 3.02 ± 0.14 | 2.76 ± 0.03 | 2.77 ± 0.07 |
| Female | 1.67 ± 0.07 | 1.60 ± 0.03 | 1.65 ± 0.05 | 1.59 ± 0.04 | |
| Spleen | Male | 1.30 ± 0.34 | 1.05 ± 0.08 | 1.10 ± 0.04 | 1.04 ± 0.09 |
| Female | 0.87 ± 0.04 | 0.78 ± 0.04 | 0.91 ± 0.05 | 0.84 ± 0.04 | |
| Heart | Male | 1.27 ± 0.02 | 1.33 ± 0.06 | 1.30 ± 0.04 | 1.37 ± 0.14 |
| Female | 0.99 ± 0.06 | 0.88 ± 0.04 | 0.89 ± 0.03 | 0.94 ± 0.05 | |
| Adrenal | Male | 0.065 ± 0.001 | 0.063 ± 0.003 | 0.059 ± 0.001 | 0.064 ± 0.002 |
| Female | 0.091 ± 0.003 | 0.086 ± 0.002 | 0.081 ± 0.007 | 0.084 ± 0.001 | |
| Testes | Male | 3.46 ± 0.07 | 3.41 ± 0.12 | 2.99 ± 0.17 | 3.43 ± 0.08 |
| Uterus | Female | 0.538 ± 0.04 | 0.672 ± 0.005 | 0.670 ± 0.100 | 0.569 ± 0.042 |
| Epididymis | Male | 1.51 ± 0.03 | 1.67 ± 0.05 | 1.52 ± 0.07 | 1.55 ± 0.04 |
| Ovary | Female | 0.079 ± 0.002 | 0.081 ± 0.008 | 0.131 ± 0.030 | 0.120 ± 0.003 |
Values are mean ± SE of 10 animals.
Statistical significance was set at P < 0.05.
Table 5.
Effect of 90 days oral exposure to Calebin A on mean hematology data of male and female wistar rats at necropsy.
| Parameter | Sex | Control | Treated |
||
|---|---|---|---|---|---|
| 20 mg/kg/d | 50 mg/kg/d | 100 mg/kg/d | |||
| WBC (103/μl) | Male | 12.66 ± 1.34 | 10.52 ± 1.43 | 11.95 ± 2.16 | 9.38 ± 1.53 |
| Female | 5.80 ± 0.78 | 5.42 ± 0.70 | 4.75 ± 1.48 | 5.29 ± 0.62 | |
| RBC (106/μl) | Male | 8.74 ± 0.14 | 8.82 ± 0.14 | 8.46 ± 0.13 | 8.76 ± 0.15 |
| Female | 7.67 ± 0.11 | 8.04 ± 0.13 | 7.96 ± 2.65 | 7.95 ± 0.13 | |
| HGB (g/dl) | Male | 14.17 ± 0.16 | 14.29 ± 0.21 | 13.88 ± 0.21 | 14.56 ± 0.29 |
| Female | 12.14 ± 1.23 | 13.64 ± 0.13 | 13.82 ± 0.13 | 13.72 ± 0.19 | |
| HCT (%) | Male | 42.26 ± 0.49 | 42.66 ± 0.68 | 41.77 ± 0.63 | 42.99 ± 0.87 |
| Female | 38.94 ± 0.47 | 40.37 ± 0.41 | 40.87 ± 0.33 | 40.30 ± 0.59 | |
| MCV (fl) | Male | 48.39 ± 0.39 | 48.10 ± 0.78 | 49.38 ± 0.58 | 49.07 ± 0.35 |
| Female | 50.94 ± 0.56 | 50.23 ± 0.72 | 51.47 ± 0.57 | 50.54 ± 0.34 | |
| MCH (pg) | Male | 16.21 ± 0.14 | 16.22 ± 0.26 | 14.89 ± 1.50 | 16.62 ± 0.13 |
| Female | 17.39 ± 0.16 | 16.96 ± 0.22 | 17.30 ± 0.20 | 17.27 ± 0.15 | |
| MCHC (g/dl) | Male | 33.54 ± 0.15 | 33.67 ± 0.14 | 33.24 ± 0.16 | 33.92 ± 0.13 |
| Female | 34.16 ± 0.12 | 33.81 ± 0.15 | 33.70 ± 0.22 | 34.05 ± 0.13 | |
| PLT (103/μl) | Male | 803.00 ± 31.36 | 906.50 ± 32.91 | 881.63 ± 27.55 | 882.60 ± 40.92 |
| Female | |||||
| NEUT (%) | Male | 14.73 ± 1.95 | 21.52 ± 1.86 | 19.23 ± 1.54 | 21.64 ± 2.94 |
| Female | |||||
| LYMPH (%) | Male | 80.41 ± 1.98 | 71.78 ± 1.95 | 74.14 ± 1.70 | 69.60 ± 2.33 |
| Female | |||||
| MONO (%) | Male | 3.08 ± 0.23 | 3.83 ± 0.44 | 3.86 ± 0.51 | 3.29 ± 0.24 |
| Female | |||||
| EO (%) | Male | 1.68 ± 0.23 | 2.45 ± 0.37 | 2.70 ± 0.45 | 3.02 ± 0.80 |
| Female | |||||
| BASO (%) | Male | 0.10 ± 0.02 | 0.14 ± 0.05 | 0.07 ± 0.01 | 0.41 ± 0.02 |
| Female | |||||
Values are mean ± SE of 10 animals each.
Statistical significance was set at P < 0.05.
Table 6.
Effect of 90 days oral exposure to Calebin A on Mean serum biochemical data of male and female wistar rats at necropsy.
| Parameter | Sex | Control | Treated |
||
|---|---|---|---|---|---|
| 20 mg/kg/d | 50 mg/kg/d | 100 mg/kg/d | |||
| Glucose (mg/dl) | Male | 79.93 ± 9.00 | 74.16 ± 8.86 | 100.65 ± 9.05 | 86.66 ± 10.33 |
| Female | 93.21 ± 5.17 | 94.55 ± 7.03 | 80.14 ± 9.93 | 104.86 ± 7.13 | |
| Creatinine (mg/dl) | Male | 0.50 ± 0.06 | 0.43 ± 0.06 | 0.56 ± 0.07 | 0.44 ± 0.06 |
| Female | 0.56 ± 0.03 | 0.55 ± 0.03 | 0.38 ± 0.04 | 0.50 ± 0.05 | |
| GOT (U/L) | Male | 94.50 ± 9.91 | 91.90 ± 9.96 | 113.50 ± 12.36 | 93.00 ± 8.32 |
| Female | 128.20 ± 14.61 | 110.80 ± 11.65 | 69.44 ± 9.23* | 81.60 ± 6.78* | |
| Urea (mg/dl) | Male | 32.69 ± 2.57 | 30.08 ± 2.96 | 41.24 ± 3.23 | 40.53 ± 4.01 |
| Female | 32.84 ± 2.18 | 35.81 ± 2.66 | 31.82 ± 3.07 | 36.33 ± 2.69 | |
| Total protein (gm/dl) | Male | 6.36 ± 0.39 | 6.21 ± 0.33 | 6.63 ± 0.29 | 6.39 ± 0.19 |
| Female | 6.72 ± 0.28 | 6.35 ± 0.36 | 6.88 ± 0.15 | 6.36 ± 0.19 | |
| TG (mg/dl) | Male | 191.46 ± 8.96 | 143.16 ± 12.93 | 190.40 ± 14.10 | 159.97 ± 15.09 |
| Female | 203.92 ± 20.01 | 155.21 ± 9.16 | 154.45 ± 10.20 | 155.65 ± 10.69 | |
| Albumin (gm/dl) | Male | 3.27 ± 0.17 | 3.18 ± 0.18 | 3.41 ± 0.12 | 3.39 ± 0.20 |
| Female | 3.35 ± 0.13 | 3.23 ± 0.16 | 3.88 ± 0.04 | 3.55 ± 0.09 | |
| Uric acid (mg/dl) | Male | 0.89 ± 0.13 | 0.96 ± 0.13 | 0.83 ± 0.06 | 0.83 ± 0.07 |
| Female | 0.96 ± 0.12 | 0.98 ± 0.13 | 0.65 ± 0.12 | 0.75 ± 0.06 | |
| Cholesterol (mg/dl) | Male | 54.01 ± 6.18 | 40.15 ± 2.96 | 53.44 ± 6.07 | 54.53 ± 4.82 |
| Female | 56.21 ± 3.60 | 54.28 ± 4.76 | 41.46 ± 3.66* | 47.44 ± 3.65 | |
| Globulin (mg/dl) | Male | 3.08 ± 0.24 | 2.96 ± 0.25 | 3.21 ± 0.20 | 2.94 ± 0.09 |
| Female | 3.36 ± 0.17 | 3.15 ± 0.21 | 3.01 ± 0.12 | 2.71 ± 0.12 | |
| GPT (U/L) | Male | 57.80 ± 9.60 | 51.20 ± 5.30 | 55.30 ± 5.56 | 46.50 ± 4.99 |
| Female | 45.00 ± 3.41 | 45.31 ± 3.33 | 35.66 ± 3.39 | 41.50 ± 2.42 | |
Values are mean ± SE of 10 animals.
Significant at the level of P < 0.05.
Table 7.
Effect of 90 days oral exposure to Calebin A on Individual gross morphology and histopathology of male and female wistar rats.
| Dose group | Sex | Animal number | Mode of deatha | Period of study | Necropsy observation | Histopathological observation |
|---|---|---|---|---|---|---|
| Control | Male | 1 | TS | 90 Days | NED | Spleen: mild lymphoid hyperplasia |
| Female | 1 | TS | 90 Days | NED | NED | |
| Male | 2 | TS | 90 Days | NED | NED | |
| Female | 2 | TS | 90 Days | NED | NED | |
| Male | 3 | TS | 90 Days | NED | NED | |
| Female | 3 | TS | 90 Days | NED | NED | |
| Male | 4 | TS | 90 Days | NED | Lung: mild alveolar consolidation | |
| Female | 4 | TS | 90 Days | NED | NED | |
| Male | 5 | TS | 90 Days | NED | NED | |
| Female | 5 | TS | 90 Days | NED | Ovary: mild congestion | |
| Male | 6 | TS | 90 Days | NED | Testis: mild oligospermia | |
| Female | 6 | TS | 90 Days | NED | NED | |
| Male | 7 | TS | 90 Days | NED | NED | |
| Female | 7 | TS | 90 Days | NED | Lung: mild alveolar consolidation | |
| Male | 8 | TS | 90 Days | NED | Kidney: mild focal interstitial nephritis | |
| Female | 8 | TS | 90 Days | NED | NED | |
| Male | 9 | TS | 90 Days | NED | NED | |
| Female | 9 | TS | 90 Days | NED | NED | |
| Male | 10 | TS | 90 Days | NED | NED | |
| Female | 10 | TS | 90 Days | NED | NED | |
| Treated (100 mg/kg/d) | Male | 31 | TS | 90 Days | NED | NED |
| Female | 31 | TS | 90 Days | NED | Uterus: mild hydrometra | |
| Male | 32 | TS | 90 Days | NED | NED | |
| Female | 32 | TS | 90 Days | NED | NED | |
| Male | 33 | TS | 90 Days | NED | Lung: mild infiltration of inflammatory cells | |
| Female | 33 | TS | 90 Days | NED | NED | |
| Male | 34 | TS | 90 Days | NED | NED | |
| Female | 34 | TS | 90 Days | NED | Lung: mild alveolar consolidation | |
| Male | 35 | TS | 90 Days | NED | Testis: mild oligospermia | |
| Female | 35 | TS | 90 Days | NED | NED | |
| Male | 36 | TS | 90 Days | NED | Spleen: mild lymphoid hyperplasia | |
| Female | 36 | TS | 90 Days | NED | NED | |
| Male | 37 | TS | 90 Days | NED | NED | |
| Female | 37 | TS | 90 Days | NED | NED | |
| Male | 38 | TS | 90 Days | NED | Liver: mild hepatocellular degeneration | |
| Female | 38 | TS | 90 Days | NED | Kidney: mild focal interstitial nephritis | |
| Male | 39 | TS | 90 Days | NED | NED | |
| Female | 39 | TS | 90 Days | NED | NED | |
| Male | 40 | TS | 90 Days | NED | NED | |
| Female | 40 | TS | 90 Days | NED | NED |
FD, found dead; MS, moribund sacrifice; TS, terminal sacrifice; NAD, no abnormality detected.
In the reproductive and developmental toxicity study, the organ weight differences between control and treated group male rats for testes and epididymis are not statistically significant. Similarly in female rats, no gross changes observed in the weights of ovaries and uterus between control and other treatment receiving group animals (Table 9). All animals in all groups pups, both control and treated exhibited a comparable body weight gain during the course of the study (day 0 and day 4). There was no loss in body weight or difference in body weight gain between groups during the course of the study (Table 10). There were no significant changes observed in the average number of corpora lutea and percent of successful implantation between control and treatment group animals (Table 11). All group of animals showed no difference in achieving pregnancy, with majority of them conceiving in 1–5 days. All group female rats showed the preliminary signs of pregnancy in less than or equal to 22 days. No significant difference in mean values of implants between control and other treatment groups. The mean pups weight at day 4 was comparable between control and high dose receiving group animals (G4). No changes observed in pre-implantation, post natal and post natal loss of off-spring between all the groups of animals (Table 12).
Table 9.
Organ weights (g) of animals in reproductive/developmental toxicity study.
| Group | Male |
Female |
||
|---|---|---|---|---|
| Testes | Epididymis | Ovaries | Uterus | |
| Control | 3.09 | 1.22 | 0.34 | 0.93 |
| 20 mg/kg | 3.32 | 1.22 | 0.40 | 0.90 |
| 50 mg/kg | 2.97 | 1.10 | 0.38 | 0.93 |
| 100 mg/kg | 3.02 | 1.20 | 0.36 | 0.86 |
Table 10.
Body weight of male and female pups (g) in reproductive/developmental toxicity study.
| Group | Day 0 |
Day 4 |
||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Control | 5.63 | 5.62 | 10.15 | 9.90 |
| 20 mg/kg | 5.73 | 5.82 | 10.68 | 10.25 |
| 50 mg/kg | 5.54 | 5.40 | 10.39 | 10.10 |
| 100 mg/kg | 5.55 | 5.66 | 10.29 | 9.91 |
Table 11.
Corpora lutea (Cl) and implantation sites (Imp) in numbers in reproductive/developmental toxicity study.
| Group | Corpora lutea in average number |
Implantation sites in average number (IMP) | Percent of successful implantation % | ||
|---|---|---|---|---|---|
| Left | Right | Total | |||
| Control | 5.70 | 5.80 | 11.50 | 8.40 | 73.11 |
| 20 mg/kg | 5.50 | 5.70 | 11.20 | 8.30 | 73.48 |
| 50 mg/kg | 6.10 | 6.30 | 12.40 | 9.10 | 73.47 |
| 100 mg/kg | 6.50 | 6.30 | 12.90 | 9.60 | 73.67 |
Table 12.
Reports of effects on reproduction/development.
| Observation | Values |
|||
|---|---|---|---|---|
| Dosage (units) | Control 0 mg/kg/d |
Low 20 mg/kg/d |
Medium 50 mg/kg/d |
High 100 mg/kg/d |
| Pairs started (N) | 10 | 10 | 10 | 10 |
| Females showing evidence of copulation (N) | 10 | 10 | 10 | 10 |
| Females achieving pregnancy (N) | 9 | 9 | 9 | 10 |
| Conceiving days 1–5 (N) | 6 | 7 | 8 | 8 |
| Conceiving days 6–(N) | 3 | 2 | 1 | 2 |
| Pregnancy ≤ 21 days (N) | 3 | 2 | 2 | 3 |
| Pregnancy = 22 days (N) | 5 | 6 | 5 | 6 |
| Pregnancy ≥ 23 days (N) | 1 | 1 | 2 | 1 |
| Dams with live young born (N) | 9 | 9 | 9 | 10 |
| Dams with live young at day 4 pp (N) | 9 | 9 | 9 | 10 |
| Corpora lutea/dam (mean) | 11.50 | 11.20 | 12.40 | 12.90 |
| Implants/dam (mean) | 8.40 | 8.50 | 9.10 | 9.60 |
| Live pups/dam at birth (mean) | 8.33 | 8.35 | 8.95 | 9.00 |
| Live pups/dam at day 4 (mean) | 8.11 | 8.33 | 9.56 | 9.00 |
| Sex ratio (m/f) at birth (mean) | 1.08 | 1.2 | 1.12 | 1.25 |
| Sex ratio (m/f) at day 4 (mean) | 1.11 | 1.14 | 1.12 | 1.25 |
| Pup weight at birth (mean) | 5.62 | 5.77 | 5.47 | 5.61 |
| Pup weight at day 4 (mean) | 10.03 | 10.46 | 10.25 | 10.10 |
| Abnormal pups | ||||
| Dams with 0 | 0 | 0 | 0 | 0 |
| Dams with 1 | 0 | 0 | 0 | 0 |
| Dams with ≥2 | 0 | 0 | 0 | 0 |
| Loss of offspring | ||||
| Pre-implantation (corpora lutea minus implantations) | 3.10 | 2.90 | 3.30 | 3.30 |
| Pre-natal/post–implantations (implantations minus live births) | 0.27 | 0.26 | 0.57 | 0.60 |
| Post-natal (live births minus alive at post-natal day 4) | 0.22 | 0.22 | 0.11 | 0.00 |
N, number; PP, post partum.
The Ames test uses amino acid-requiring strains of S. typhimurium and E. coli to detect point mutations involving substitution, addition, or deletions of one or more DNA base pairs [12] The bacterial reverse mutation (Ames) test is a biological assay that is used to evaluate the mutagenic activity of medical compounds. For the initial screening, the Ames test is used worldwide to determine the mutagenic activity of new medicines as many carcinogenic materials were shown to have a high predictive value in rodents when a positive result is obtained [13], [14]. Mutagenecity testing using the Ames test is one of the most frequently used method and is recommended by regulatory agencies for determining genetic risk (Korea Food and Drug Administration, U.S. Food and Drug Administration, Organization for Economic Cooperation and Development). The experimental methods used in the study were based on the published reports by Maron and Ames with minor modifications. S. typhimurium tester strains TA 98 and TA1537 (to detect frame-shift mutagens), TA100 and TA1535 (to detect base pair-substitution mutagens) and TA102 for oxidative mutagens were obtained from Molecular Toxicology Inc. (Boone, USA) and were used as the tester strains. No positive mutagenic response was observed in any of the S. typhimurium tested compared with the concurrent vehicle control groups regardless of the presence or absence of the S9 mixture. The positive controls showed significantly increased numbers of revertant colonies, indicating that the assay was valid. In the results of the mutagenecity assay, the concentrations of Calebin A selected for the study ranged from 312.5 to 5000 μg/plate. Calebin A gave a negative response in the presence (Table 13) and absence (Table 14) of rat liver S9 in the assay up to 5000 μg/plate.
Table 13.
Effect of Calebin A on bacterial reverse mutation assay with (+S9) mix (Ames).
| Concentration (μg/plate) | Bacterial strains |
||||
|---|---|---|---|---|---|
| TA 98 | TA 100 | TA 102 | TA 1535 | TA 1537 | |
| Mean revertants/plate ± SEM | |||||
| Vehicle | 40 ± 3.5 | 191 ± 4 | 351 ± 1.5 | 15 ± 0.5 | 14 ± 0.5 |
| 312.5 | 43 ± 3 | 187 ± 2 | 330 ± 1 | 16 ± 2 | 11 ± 0.5 |
| 625 | 41 ± 2.5 | 179 ± 3.5 | 303 ± 4.5 | 19 ± 1.5 | 11 ± 1 |
| 1250 | 41 ± 4 | 182 ± 2.5 | 319 ± 1.5 | 17 ± 3 | 16 ± 1 |
| 2500 | 42 ± 2.5 | 188 ± 0.5 | 303 ± 4.5 | 11 ± 1 | 14 ± 2 |
| 5000 | 40 ± 1.5 | 197 ± 2.5 | 328 ± 2.5 | 14 ± 1.5 | 15 ± 2.5 |
| Positive control | 1050a ± 37.5 | 1226a ± 28.5 | 2394a ± 12.5 | 335a ± 16.5 | 203a ± 18.5 |
Aminoanthracene (2 μg/plate).
Data are presented as mean ± standard deviation.
Table 14.
Effect of Calebin A on bacterial reverse mutation assay without (+S 9) mix.
| Concentration (μg/plate) | Bacterial strains |
||||
|---|---|---|---|---|---|
| TA 98 | TA 100 | TA 102 | TA 1535 | TA 1537 | |
| Mean revertants/plate ± SEM | |||||
| Vehicle | 21 ± 1 | 111 ± 2 | 201 ± 3.5 | 10 ± 1 | 11 ± 1 |
| 312.5 | 21 ± 0 | 122 ± 2 | 203 ± 1.5 | 8 ± 1 | 9 ± 1 |
| 625 | 19 ± 2.5 | 119 ± 2.5 | 211 ± 0.5 | 9 ± 0 | 10 ± 1.5 |
| 1250 | 24 ± 2.5 | 112 ± 2.5 | 198 ± 3.5 | 11 ± 0.5 | 11 ± 1.5 |
| 2500 | 22 ± 2 | 105 ± 3.5 | 198 ± 0.5 | 12 ± 0.5 | 11 ± 2 |
| 5000 | 18 ± 3 | 115 ± 2 | 202 ± 3 | 9 ± 0.5 | 10 ± 1 |
| Positive control | 701a ± 8.5 | 723b ± 21 | 1654c ± 12.5 | 407d ± 8 | 123e ± 11 |
Data are presented as mean ± standard deviation.
Nitrofluorene (3 μg/plate).
Methyl methane sulphonate (1.5 μl/plate).
Methyl methane sulphonate (2 μl/plate).
Sodium azide (2 μg/plate).
9-Aminoacridine (85 μg/plate).
4. Conclusion
Calebin A, in the repeated dose 90 day oral toxicity study and in the reproductive/developmental toxicity study, showed no signs of toxicity and hence it could be concluded that a repeated oral exposure to test item Calebin A, up to 100 mg/kg/d does not produce any toxic effects and may be treated as ‘No Observed Adverse Effect Level’ (NOAEL) under the test conditions employed. Also, as Calebin A exhibited a negative response in the presence and absence of Aroclor 1254 induced rat liver S9 in the assay up to 5000 μg/plate, it was concluded that Calebin A was not mutagenic in the Bacterial Reverse Mutation Assay.
Funding
The author(s) disclose that financial support for the research described herein was provided by Sami Labs Limited.
Conflict of interest
Dr. Muhammed Majeed is the Founder and Managing Director of Sami Labs Limited; the remaining authors are either full time employees of Sami Labs or its subsidiary Sabinsa Corporation.
Transparency document
Acknowledgements
We thank CSIR funded – Indian Institute of Toxicology Research, Lucknow, India for conducting oral toxicology studies.
References
- 1.Wealth of India . vol. 2. CSIR – National Institute of Science Communication and Information Resources; New Delhi: 2001. p. 264. (A Dictionary of Indian Raw Materials and Industrial Products). [Google Scholar]
- 2.Aggarwal B.B., Young J.S., Shishodia S. The molecular targets and therapeutic uses of curcumin in health and disease. Adv. Exp. Med. Biol. 2007;XXI:489–577. [Google Scholar]
- 3.Aggarwal B.B., Yuan W., Li S., Gupta S.C. Curcumin-free turmeric exhibits anti-inflammatory and anti-cancer activities: identification of novel components of turmeric. Mol. Nutr. Food Res. 2013;57:1529–1542. doi: 10.1002/mnfr.201200838. [DOI] [PubMed] [Google Scholar]
- 4.Kim D.S.H.L., Park S.Y. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: drug discovery effort against Alzheimer's disease. J. Nat. Prod. 2002;65:1227–1231. doi: 10.1021/np010039x. [DOI] [PubMed] [Google Scholar]
- 5.Zeng Y., Qiu F., Takahashi K., Liang J., Qu G., Yao X. New sesquiterpenes and Calebin derivatives from Curcuma longa. Chem. Pharm. Bull. 2007;55:940–943. doi: 10.1248/cpb.55.940. [DOI] [PubMed] [Google Scholar]
- 6.Kim D.S.H.L., Kim J.Y. Total synthesis of Calebin A, preparation of its analogues, and their neuronal cell protectivity against β-amyloid insult. Bioorg. Med. Chem. Lett. 2001;11:2541–2543. doi: 10.1016/s0960-894x(01)00489-9. [DOI] [PubMed] [Google Scholar]
- 7.Li Y., Li S., Han Y., Liu J., Zhang J., Li F., Wang Y., Liu X., Yao L. Calebin-A induces apoptosis and modulates MAPK family activity in drug resistant human gastric cells. Eur. J. Pharmacol. 2008;591:252–258. doi: 10.1016/j.ejphar.2008.06.065. [DOI] [PubMed] [Google Scholar]
- 8.Chen L., Li S.Q., Liu C.-H., Guo H., Li Y. Comparison of inhibitive effects of curcumin and Calebin A on human hepatoma cell line HepG2. Disi. Junyi. Daxue. Xuebao. 2009;30(1):7–10. [Google Scholar]
- 9.O.E.C.D. 1998. OECD 408. Guidelines for Testing of Chemicals: Repeated Dose 90-Day Oral Toxicity Study in Rodents; pp. 1–13. (Section 4, No. 408, Adopted 03 October 1998) [Google Scholar]
- 10.O.E.C.D. 1995. OECD 421. Guidelines for testing of chemicals: Reproduction/Developmental Toxicity Screening Test; pp. 1–13. (Adopted 27 July 1995) [Google Scholar]
- 11.O.E.C.D. 1997. OECD 471. Guidelines for Testing of Chemicals: Bacterial Reverse Mutation Assay Test; pp. 1–11. (Adopted 21 July) [Google Scholar]
- 12.Demma J., Engidawork E., Hellman B. Potential genotoxicity of plant extracts used in Ethiopian traditional medicine. J. Ethnopharmacol. 2009;122:136–142. doi: 10.1016/j.jep.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Yahagi T., Nagao M., Seino Y., Matsushima T., Sugimura T. Mutagenicities of N-nitrosamines on Salmonella. Mutat. Res. 1977;48(2):121–129. doi: 10.1016/0027-5107(77)90151-8. [DOI] [PubMed] [Google Scholar]
- 14.Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113(3–4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.