Abstract
The purpose of the present study was to investigate the effects of phytic acid (IP6) on morphological and immunohistochemical parameters on intestinal explants exposed to deoxynivalenol (DON) and fumonisin B1 (FB1). The jejunal explants were exposed for 4 h to different treatments: control, DON (10 μM), DON plus 2.5 mM or 5 mM IP6, FB1 (70 μM), and FB1 plus 2.5 mM or 5 mM IP6. Both mycotoxins induced significant intestinal lesions and decreased villi height. The presence of 2.5 mM and 5 mM IP6 significantly inhibited the morphological changes caused by the mycotoxins. DON induced a significant increase in caspase-3 (83%) and cyclooxygenase-2 (71.3%) expression compared with the control. The presence of 5 mM IP6 induced a significant decrease in caspase-3 (43.7%) and Cox-2 (48%) expression compared with the DON group. FB1 induced a significant increase in caspase-3 expression (47%) compared to the control, whereas IP6 induced no significant change in this expression. A significant decrease in cell proliferation was observed when explants were exposed to 5 mM of IP6 in comparison with the DON and FB1 groups. The present data provide evidence that phytic acid modulates the toxic effects induced by DON and FB1 on intestinal tissue.
Keywords: IP6; Protective effect; DON; FB1, Jejunum; Swine
1. Introduction
The search for high-quality and healthy food is constantly increasing worldwide, leading to a great interest in natural antioxidants, mainly plant extracts and compounds, and their possible health benefits [1]. The inositol hexaphosphate (IP6, InsP6, phytic acid) is a natural antioxidant widely present in cereals, legumes, nuts, oil seeds, spores, needles and pollen and corresponds to 1–5% of the weight of the cereals [2], [3]. Several studies in human and animal models have demonstrated the preventive and therapeutic effects of IP6 in different diseases, including inhibition of platelet aggregation [4], reduction of serum lipids [5], protective effects in inflammatory bowel disease [6] and neurodegenerative diseases [7], prevention of cardiovascular diseases [8], prevention of kidney stone formation [9] and inhibition of cancer development [10], [11], [12], [13], [14], [15], [16], [17], [18]. The protective effect of IP6 has been related to its antioxidant potential in inhibiting radical oxygen species (ROS) production [6], [19].
The intestine is a complex organ with several cell types, functions, serves as the primary barrier against the ingestion of chemicals, antigens, natural toxins, contaminated food [20], [21]. Mycotoxins, such as deoxynivalenol (DON), fumonisin B1 (FB1), induce toxic, immunotoxic effects on the intestine of pigs [22]. Mycotoxins are the most common contaminants of cereal grains found worldwide [23], are considered a risk factor to human, animal health [24].
The toxic effects of fusariotoxins have been reported in humans, pigs, experimental animals and in vitro models [22], [25], [26], [27]. Upon acute exposure to high doses of DON, animals exhibit diarrhea, vomiting, leukocytosis and hemorrhage [28], whereas chronic exposure induces anorexia, reduced weight gain and nutritional efficiency, and changes to the neuroendocrine and immune systems [29]. At the molecular level, DON acts to inhibit protein synthesis by binding to the 28S ribosomal RNA peptidyltransferase site, inducing the phosphorylation of mitogen-activated protein kinases (MAPKs), promoting apoptosis, and inducing changes in cytokine gene expression [30], [31] and a decrease in the expression of cell adhesion proteins [22], [32], [33].
Exposure to cereals contaminated with FB1 causes pulmonary edema in pigs [34], leukoencephalomalacia in horses [35], liver and kidney cancer in rats [36], [37] and esophageal cancer and neural tube defects in humans [38], [39]. At the cellular level, FB1 inhibits ceramide synthase, blocking the synthesis of sphingolipids, a class of membrane lipids that plays an important role in cell signaling transduction pathways and cell growth, differentiation and death [40], [41].
In a previous study, we demonstrated that IP6 modulates the toxic effects induced by DON exposition on IPEC cells [42]. In this study, we are interested in evaluating the effects of phytic acid on an ex vivo model exposed to DON and FB1, the most common fusariotoxins that contaminate cereals. Furthermore, we focus on the search for compounds that can inhibit or inactivate the action of these mycotoxins. The beneficial effects of IP6 have been demonstrated in several diseases; however, there are few reports on IP6 action during mycotoxin exposure [42], [43]. Our hypothesis is that the antioxidant properties of IP6 contribute to reduce the ribotoxic stress and lipid peroxidation caused by DON and FB1, respectively. The choice of the swine as the experimental model was based on the physiological and morphological similarities with the human gastrointestinal tract as well as similarities in the absorption of IP6 [44]. The explant culture technique used in this experiment permits the evaluation of tissue morphology, maintaining the complex patterns of differentiation observed in vivo and permitting the use of fewer experimental animals [21]. The aim of this study was to investigate the effect of IP6 on jejunal explants exposed to DON and FB1, focusing on intestinal morphology, cell proliferation and apoptosis. The expressions of a cell junction protein and cyclooxygenase-2 were also analyzed.
2. Materials and methods
2.1. Animals
Six 24-day-old crossbred (Landrace × Large White × Duroc) piglets (7.9 kg ± 0.72) were used in the present study. All animal experimentation procedures were performed in accordance with the ethics committee on the use of animals (CEUA/UEL/Brazil-process n° 8022.2012.40).
2.2. Phytic acid
Phytic acid (inositol hexaphosphoric acid) dodecasodium salt from rice (MW: 1080) was purchased from Sigma–Aldrich (St. Louis, MO, USA). The salt was dissolved in distilled water and the pH was adjusted to 7.2 before the solution was passed through a membrane filter. The resultant solution was stored at −20 °C before dilution in explant culture media. The IP6 concentrations used in this study (2.5 mM and 5 mM) were chosen based in a previous experiment with swine jejunal explants where these doses improved the jejunal morphology compared to lower doses [44].
2.3. DON and FB1 mycotoxins
The purified DON (MW: 296.32) and FB1 (MW: 721.83) mycotoxins were purchased from Sigma–Aldrich (St. Louis, MO, USA) and the Cayman Chemical Company (MI, USA), respectively. The mycotoxins were dissolved in ultrapure water at final dilution of 10 μM for DON and 70 μM for FB1 and stored at −20 °C. The concentrations of mycotoxins used were equivalent to 3 mg kg−1 feed and 50.5 mg kg−1 of feed for DON and FB1, respectively.
The doses used in this experiment were based in previous experiments [46], [47] that have shown toxic effects with these concentrations in the explant model.
2.4. Jejunal explants technique
The jejunum was chosen because previous studies have identified this region as a target of the toxic action of DON and FB1 [22]. The piglets were euthanized by the administration of acepromazine 1% (0.1 mL/10 Kg) IM, sodium pentobarbital (40 mg/kg) IV and, subsequently, KCl 15% solution IV. The jejunum was rapidly excised, and samples 5 cm in length were collected, washed with buffer solution and opened longitudinally. The explants were collected using a punch 8 mm in diameter. From each animal six explants (replicates) were collected for each treatment. The total number of explants from each pig was 42. The explants collected were laid in six well plates (three explants/well) with 3 mL of the following treatments: control (A, B and C) – only culture media (DMEM – Dulbecco's modified Eagle's medium (Gibco) plus penicillin/streptomycin (1.25 μL/mL – Gibco), gentamicin (10 μL/mL – Novafarma), fetal bovine serum (100 μL/mL – Invitrogen) and l-glutamine (0.4 μL/mL – Sigma–Aldrich); culture media with IP6 2.5 mM (D and E); and culture media with IP6 5 mM (F and G). The explants were incubated at 37 °C under orbital shaking. After one hour, DON (10 μM) was added in the B, D and F treatments, and FB1 (70 μM) in the C, E and G treatments. The explants were returned to incubation for three more hours. The total time of incubation (4 h) was chosen to encompass the times previously used in an experiment with explants of swine jejunum [48].
2.5. Histological assessment
After the incubation period, the explants were fixed in 10% neutral buffered formalin, dehydrated in alcohols and embedded in paraffin. Sections of 5 μm were stained with hematoxylin-eosin (HE) for histopathological evaluation. An intestinal histological score previously described [47] was used to compare the histological changes between the treatments. The frequency and severity of each lesion was evaluated on a scale in which the maximal total score was 22 points (Table 1). The villi height was measured randomly on 10 villi using an image analysis system (Motic Image Plus, Motic Instruments, Richmond, Canada). Sections of the jejunum were submitted to alcian blue staining to evaluate goblet cell density. Positively stained goblet cells were counted randomly in 10 villi per explant at 400× magnification.
Table 1.
Histological criteria score used to establish the jejunal lesional score*
| Type of lesion | Severity score | Maximal total score |
|---|---|---|
| Villi atrophy | 0–2 | 22 |
| Villi fusion | 0–2 | |
| Interstitial edema | 0–2 | |
| Lymphatic vessel dilation | 0–2 | |
| Loss of apical enterocytes | 0–2 | |
| Cell vacuolation | 0–2 | |
| Necrotic debris | 0–2 | |
| Microvilli Homogeneity | 0–2 | |
| Enterocytes morphology# | 0–3 | |
| Number of villi• | 0–3 |
The jejunal score of each treatment was obtained by the sum of each lesion score. The severity score was determined as: 0-diffuse; 1-local; 2-absent.
Columnar epithelium (3); cuboid epithelium (2); flattened epithelium (1); no epithelium (0).
>25 villi/explant (3); 15–25 villi (2); <15 villi (1) and no villi (0).
2.6. Immunohistochemical assessment
The evaluation of cell junction expression, cell proliferation, apoptosis and cyclooxygenase-2 expression were performed using antibodies against E-cadherin (anti-4A2C7, 1:50, Zymed), Ki-67 (anti-7B11, 1:50 dilution, Zymed), cleaved caspase-3 (Ccasp-3) (anti-Asp 175, 1:200 dilution, Cell Signaling Technology, Inc.) and Cox-2 (anti-CX-294, 1:100 dilution, Dako), respectively. An anti-p53 antibody (anti-BP53.12, 1:50 dilution, Zymed) was used to evaluate the expression of p53. Samples of jejunum were analyzed on formalin-fixed and paraffin-embedded sections. Tissue sections were deparaffinized with xylene and dehydrated through a graded ethanol series. The positive and negative controls were used according to the manufacturer's instructions.
Heat-mediated antigen retrieval was performed by heating the sections immersed in EDTA buffer, pH 9.0, in a microwave oven (750 W) for 10 min for E-cadherin, Ki-67, Cox-2, and p53 staining and for 17 min for Ccasp-3 staining. Endogenous peroxidase activity was blocked by incubation in a methanol–H2O2 (100: 50 mL) solution, whereas for Ccasp-3 and Cox-2, a blocker protein (Dako) was also used for 30 min. The sections were incubated overnight at 4 °C with the primary antibody. The polymer secondary antibody (Nichirei Biosciences, Tokyo, Japan) was applied for 30 min, followed by the addition of a chromogen (3,3-diaminobenzidine). Finally, the tissue sections were counterstained with hematoxylin and mounted on coverslips using a permanent mounting medium.
The positive immunoexpression of Ccasp-3, p53 and Ki-67 was estimated by counting strongly positive immunostaining of the cytoplasm (Ccasp-3) and nucleus (p53, Ki-67) in five random fields in the crypt region/explant at 400× magnification. The expression of E-cadherin was estimated by the evaluation of five fields at 200× magnification. The staining was considered positive when homogeneous and strong basolateral membrane staining of the enterocytes was observed. The expression of Cox-2 was estimated by evaluating five fields in the crypt region at 200× magnification. Fields were considered positive when 75% or more of the cells were immunostained.
2.7. Statistical analysis
The experimental design used in the present study was entirely randomized with six replicates for each treatment (each explant represent one replicate). The means of the lesional score, intestinal morphometry, number of goblet cells, positive immunostaining for Ki-67, p53, and Ccasp-3 and the number of positive fields for E-cadherin and Cox-2 were used for statistical analysis. The data are presented as the means with their standard errors and were analyzed using the free software Action 2.3 (Campinas, SP, Brazil). One-way analysis of variance (ANOVA) followed by a multiple comparison procedure (Tukey test) was used for statistical analysis. Fisher's test was used to compare the results of E-cadherin and Cox-2 expression. P values of ≤0.05 were considered significant.
3. Results
3.1. Effect of IP6 on the histology and morphometry of jejunal explants exposed to DON and FB1mycotoxins
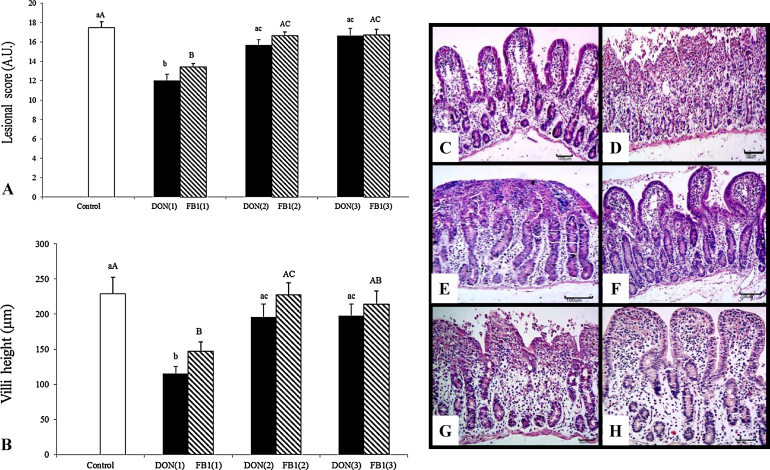
After 3 h of incubation with DON or FB1, the explants exhibited moderate to severe jejunal lesions. The main histological changes observed included multifocal to diffuse atrophy and villi fusion, the lack of apical epithelium, necrotic debris, cell vacuolation and the flattening of enterocytes (Fig. 1). Histological scores decreased significantly in the explants exposed to DON (31.4%) and FB1 (23%) compared with the control group. On the other hand, when the explants were pre-treated with 2.5 mM and 5 mM of IP6, the histological scores exhibited a significant increase (p ≤ 0.05) compared with explants exposed to mycotoxins alone (Fig. 1). Explants submitted to 2.5 mM IP6 exhibited an increase of 30.8% and 23.8% after DON and FB1 treatment, respectively, whereas 5 mM of IP6 induced an increase of 38.3% and 24.6% in histological score in explants exposed to DON and FB1, respectively.
Fig. 1.
The effects of IP6 on the histological morphology of jejunal explants exposed to DON and FB1. DON(1)-DON alone; DON(2)-DON plus 2.5 mMIP6; DON(3)-DON plus 5 mM IP6; FB1(1)-FB1 alone; FB1(2)-FB1 plus 2.5 mM IP6; FB1(3)-FB1 plus 5 mM IP6. (A) Effect of IP6 on the lesional score of explants exposed to DON and FB1 (AU-Arbitrary Units). (B) Effect of IP6 on the villi height of explants exposed to DON and FB1 (μm). Mean values with unlike letters were significantly different (p ≤ 0.05). (C) Control treatment with normal morphology. HE, bar 100 μm. (D) DON alone; villi atrophy and loss of the apical enterocytes. HE, bar 100 μm. (E) DON alone; severe villi fusion. HE, bar 100 μm. (F) DON plus 5 mM IP6 showing histological aspects similar to the control group, HE, bar 100 μm. (G) FB1 group with villi atrophy, fusion and loss of the apical enterocytes. HE, bar 50 μm. (H) FB1 plus 5 mM IP6 showing histological aspects similar to the control group, HE, bar 50 μm.
Explants exposed to DON exhibited a significant decrease (50%) in villi height compared with the control. However, explants treated with 2.5 mM or 5 mM of IP6 exhibited a significant increase in villi height (70.2% and 71.8%, respectively) compared with explants exposed to DON (Fig. 1). Explants incubated with FB1 exhibited a significant decrease in villi height (35%) compared with the control group, whereas pre-treatment with 2.5 mM or 5 mM of IP6 promoted an increase of 54.5% (p ≤ 0.05) and 45.5% (p ≥ 0.05) in villi height, respectively.
Goblet cell density was estimated by counting the number of goblet cells per villi. The mean number of goblet cells was 4, 3.4 and 3.8 for the control, DON and FB1 explants, respectively. Treatments with 2.5 and 5 mM of IP6 induced no significant changes in goblet cell density compared with explants treated with DON and FB1 alone (data not shown).
3.2. Effect of IP6 on cell proliferation and apoptosis in jejunal explants exposed to DON and FB1 mycotoxins
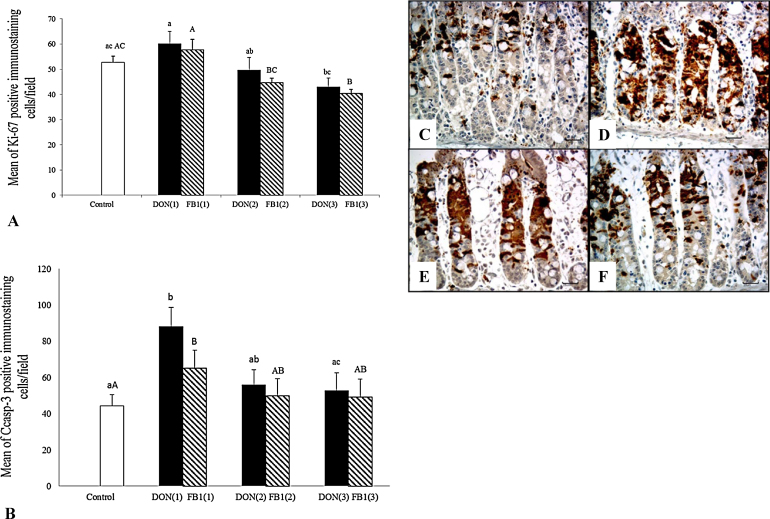
Cell proliferation and apoptosis were assessed using an immunohistochemical assay to detect Ki-67 and cleaved caspase-3, respectively. Explants exposed to DON and FB1 exhibited no significant change in cell proliferation compared with the control group. However, explants previously treated with 5 mM of IP6 exhibited a significant decrease (28.6%) in Ki-67 expression compared with the explants exposed to DON (Fig. 2). Moreover, IP6 treatment also induced a significant decrease in cell proliferation compared with explants exposed to FB1 alone (22% and 30% for 2.5 and 5 mM of IP6, respectively).
Fig. 2.
The effects of IP6 on the cell proliferation (ki-67) and apoptosis (Ccasp-3) on jejunal explants exposed to DON and FB1. DON(1)-DON alone; DON(2)-DON plus 2.5 mMIP6; DON(3)-DON plus 5 mM IP6; FB1(1)-FB1 alone; FB1(2)-FB1 plus 2.5 mM IP6; FB1(3)-FB1 plus 5 mM IP6. (A) Mean number of Ki-67 immunostained cells per field on explants exposed to the different treatments. (B) Mean number of Ccasp-3 immunostained cells per field on explants exposed to the different treatments. Mean values with unlike letters were significantly different (p ≤ 0.05). (C) Control treatment; mild Ccasp-3 cytoplasmic immunostaining in crypt cells. (D) DON alone; strong and diffuse Ccasp-3 immunostaining in crypt cells. (E) FB1 alone; moderate to accentuated Ccasp-3 immunostaining in crypt cells. (F) DON plus 5 mM IP6; reduction in Ccasp-3 immunostaining in crypt cells. (C)–(F) Immunoperoxidase method, bar 25 μm.
A significant increase in caspase-3 expression was observed in explants exposed to DON (83%) and FB1 (47%) compared with the control group. Nonetheless, when explants were exposed to DON plus 5 mM IP6, a significant decrease (43.7%) in cell apoptosis was observed compared with the DON group. Explants exposed to 2.5 mM IP6 plus DON exhibited no changes in caspase-3 expression compared with the DON group. No significant change in caspase-3 expression was observed when the explants were treated with IP6 plus FB1 compared with the FB1 group (Fig. 2).
The expression of p53 was assessed to evaluate the relationship of p53 with apoptosis induction. No immunostaining was observed in control explants or explants exposed to mycotoxins alone or with IP6 treatments.
3.3. Effect of IP6 on the expression of junction proteins and cyclooxygenase-2 on jejunal explants exposed to DON and FB1 mycotoxins
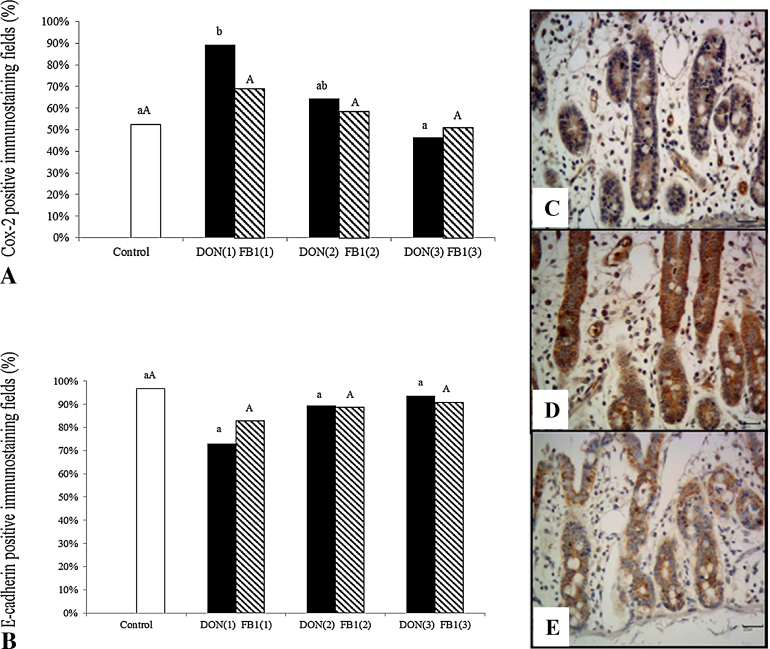
Oxidative stress was evaluated by the immunoexpression of Cox-2. Explants exposed to DON alone exhibited a significant increase (71.3%) in Cox-2 expression compared with the control. However, in explants treated with IP6 (5 mM) plus DON, we observed a significant decrease (48%) in Cox-2 expression in comparison with the DON group. Exposure to FB1 alone or in addition to IP6 induced no significant change in Cox-2 immunoexpression in the explants (Fig. 3).
Fig. 3.
The effects of IP6 on cyclooxygenase and E-cadherin expression in jejunal explants exposed to DON and FB1. DON(1)-DON alone; DON(2)-DON plus 2.5 mMIP6; DON(3)-DON plus 5 mM IP6; FB1(1)-FB1 alone; FB1(2)-FB1 plus 2.5 mM IP6; FB1(3)-FB1 plus 5 mM IP6. (A) Effect of IP6 on Cox-2 expression in explants exposed to the different treatments. (B) Effect of IP6 on E-cadherin expression in explants exposed to the different treatments. Percentage values with unlike letters were significantly different (p ≤ 0.05). (C) Control treatment; mild cytoplasmic immunostaining for Cox-2. (D) DON alone; strong and diffuse immunostaining for Cox-2. (E) DON plus 5 mM IP6; moderate cytoplasmic immunostaining for Cox-2. (C)–(E) Immunoperoxidase method, bar 25 μm.
The expression of E-cadherin was assessed to evaluate adherens junction integrity. Explants exposed to DON and FB1 alone or with the addition of IP6 exhibited no significant changes in E-cadherin expression (Fig. 3).
4. Discussion
Phytic acid is a potent natural antioxidant component of cereal diets that can modulate inflammatory and carcinogenic diseases [19], [48]. Most studies on phytic acid thus far have focused on the antineoplastic effects of IP6, whereas the effects on inflammation are unknown, and the mechanisms of anti-inflammatory action remain poorly understood.
The effects of IP6 on inflammatory diseases have been associated with the inhibition of reactive oxygen species (ROS) production, the increase in TGF-β (transforming growth factor-beta) gene expression, the decrease in TNF-α (tumor necrosis factor-alpha) transcription and the modulation of proinflammatory cytokine secretion in the intestinal epithelium [49], [50]. Previous studies have indicated that the intestinal tissue is a target for deoxynivalenol [22] and fumonisin B1 [51]. Efforts to alleviate the toxic effects of mycotoxins have been an area of research interest for our group [52]. In the present study, we present evidence that phytic acid promotes a protective effect on jejunal explants exposed to Fusarium mycotoxins.
The main histological changes observed in jejunal explants exposed to DON and FB1 alone included atrophy and fusion of the villi, loss of apical enterocytes, necrotic debris and changes in enterocytes morphology. Similar lesions were observed in previous studies with in vivo and ex vivo exposure to DON and FB1 [22], [47]. In this study, we observed a protective effect of IP6. This effect could be evaluated by an increase in the histological score and the villi height in explants exposed to IP6. In a previous experiment, we observed that doses of 2.5 mM and 5 mM IP6 improved the intestinal morphology and villi height of jejunal explants submitted to hypoxia [45]. IP6 likely acts on intestinal morphology by inhibiting the ribotoxic stress and lipid peroxidation caused by DON and FB1, respectively, through its antioxidant action. Moreover, the increase in intracellular IP6 may promote cellular differentiation and morphology preservation through the modulation of apoptosis and proliferation signal transduction, as observed in colon cancer studies [10], [11]. In the present study, no significant change in goblet cell density was observed in explants submitted to the different treatments. In in vivo studies, pigs fed diets with FB1 and DON exhibited significant changes in goblet cells density [22], [53]. The difference in these results is likely related to the short period of exposure to mycotoxins (3 h) in this ex vivo model. The interactions between IP6 and intestinal mucin production remain to be investigated.
Studies in vitro and in vivo exhibited contradictory results with respect to the proliferation of intestinal cells after DON [22], [33], [54] and FB1 [22], [55] exposure. In this experiment, the explants exposed to DON and FB1 alone exhibited proliferative cell indexes similar to control explants. Interestingly, the combination of 5 mM IP6 plus the mycotoxins promoted a significant decrease of 28% and 30% in cell proliferation compared with DON and FB1 mycotoxin treatment alone, respectively. Phytic acid can decrease cell proliferation by binding to the insulin-like growth factor II (IGF-II) receptor, decreasing DNA synthesis and proliferating cell nuclear antigen (PCNA) and blocking ERK 1/2 signaling to arrest the cells in the G1 phase of the cell proliferation cycle [16]. However, no data concerning the interaction between IP6 and Ki-67 expression in cell proliferation are available. We hypothesized that similar to the action reported on PCNA, IP6 inhibits the expression of Ki-67, leading to cell quiescence.
In the present study, cell apoptosis was evaluated by the positive immunoexpression of Ccasp-3. In the crypt region, we observed an increase of 83% and 47% in caspase-3 expression in explants exposed to DON and FB1, respectively. Such changes reflected on the decrease in villi height in explants exposed to DON and FB1. It has been established that DON activates p38 MAPKs through a ribotoxic stress response and the generation of reactive oxygen species (ROS), inducing cell apoptosis [31], [46], [56]. In this apoptotic pathway, Ccasp-3 is activated, and mutation of the p53 gene may also occur [31]. The mechanisms for apoptosis induction by FB1 are unclear but likely involve the disruption of many regulatory cell pathways, including the inhibition of protein kinase C and the activation of MAPKs and Ccasp-3 due the intracellular accumulation of sphingolipids and consequent lipid peroxidation [57], [58]. In an in vitro study with cancer cells, IP6 decreased the activated levels of ERK1/2, JNK1/2 and p38 and induced apoptosis together with a decrease in cell proliferation [12]. However, phytic acid has the ability to modulate apoptosis through prevention or induction to protect the cells and prevent diseases [19]. In this experiment, 5 mM IP6 was able to significantly reduce the cell apoptosis induced by DON. We hypothesize that IP6 modulated the decrease in cell apoptosis by inhibiting the activation of MAPKs induced by the oxidative stress caused by mycotoxins. We observed no significant p53 expression in the explants exposed to DON and FB1. It is likely that in this experiment, the short exposure time, even at high doses of mycotoxin, was not sufficient to induce apoptosis linked to activation of the p53 gene. Taken together, these results suggest that DON and FB1 induce apoptosis via the caspase-3 pathway.
In addition to triggering the activation of MAPKs, both DON [31], [32], [54] and FB1 [22], [59], [60] can decrease cellular adhesion and transepithelial electrical resistance (TEER) as well as increase Cox-2 expression, mainly by upregulation of TNF-α. Furthermore, the decrease of cellular adhesion and the upregulation of Cox-2 expression have been related to ROS production and MAPK activation [61], [62], [63]. Changes in cell junction proteins were described in explants exposed to DON and FB1 [47]. In the present study, no significant changes in E-cadherin expression were observed in any of the treatments. However, when the explants were exposed to 5 mM IP6, the E-cadherin expression improved 28% and 9% compared with the DON and FB1 groups, respectively. It is likely that, the short period of incubation with the mycotoxins (3 h) was insufficient to induce a significant change in the E-cadherin expression. We believe that longer periods of IP6 exposure could confer higher protection against the injuries caused by mycotoxins on cell adhesion proteins.
The expression of Cox-2 has increased 71% in explants exposed to DON compared with the control, whereas the incubation with 5 mM IP6 plus DON induced a decrease of 48%. Similar effects were observed in explants incubated with FB1 and IP6 (5 mM) plus FB1, although the differences were not significant. There are few reports of the effects of DON and FB1 on Cox-2 expression [60], [64]. The decrease of Cox-2 expression and TNF-α transcription has been reported in in vivo [65] and in vitro [49], [66] associated to IP6 exposure in colon inflammatory disease and cancer. Similarly, we observed a significant reduction in Cox-2 expression when we compared explants exposed to DON with explants treated with IP6. It is well established that DON induces the direct production of ROS by a ribotoxic stress pathway [31], [56]. The production of ROS leads to the expression of Cox-2, an enzyme that plays an important role in inflammatory reactions [67]. Phytic acid binds to the coordination sites for iron, preventing the redox activation necessary to catalyze OH formation by a Fenton reaction [48]. Therefore, we hypothesize that the decrease in Cox-2 expression observed in this study was associated with the ability of IP6 to inhibit ROS production. Fumonisin induced no significant changes in Cox-2 expression. ROS production induced by FB1 occurs indirectly via the intracellular accumulation of sphingolipids [57], [58]. We believe that the short period of incubation with the FB1 mycotoxin was not sufficient to promote an accumulation of sphingolipids that induced a significant increase in the Cox-2 expression.
In conclusion, the present study demonstrated that phytic acid alleviates the toxic effects induced by DON and FB1 on intestinal tissue. The protective effects were demonstrated by immunohistological and morphometrical assays. Ingestion of phytic acid could contribute to the maintenance of intestinal homeostasis, the absorption of nutrients and the defense against toxic agents, however further studies are necessary to understand the mechanism of action of this effect in mycotoxin contamination.
Conflict of interest
None declared.
Acknowledgements
This study was supported by a grant from CNPq (474691/2012-8) and Fundação Araucária, Brazil. E.O.S. and A.P.F.R.L.B. were supported by a research fellowship financed by CNPq.
Footnotes
Available online 29 May 2014
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.05.001.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Kulawik P., Ozogul F., Glew R., Ozogul Y. Significance of antioxidants for seafood safety and human health: a review. J. Agric. Food Chem. 2013;61:475–491. doi: 10.1021/jf304266s. [DOI] [PubMed] [Google Scholar]
- 2.Lolas G.M., Palamidis N., Markakis P. The phytic acid-total phosphorus relation in barley, oats, soybeans and wheat. Cereal Chem. 1976;53:867–871. [Google Scholar]
- 3.Graf E., Eaton J.W. Antioxidant functions of phytic acid. Free Radic. Biol. Med. 1990;8:61–69. doi: 10.1016/0891-5849(90)90146-a. [DOI] [PubMed] [Google Scholar]
- 4.Vucenik I., Podezasy J.J., Shamsuddin A.M. Antiplatelet activity of inositol hexaposphate (IP6) Anticancer. 1999;19:3689–3693. [PubMed] [Google Scholar]
- 5.Onomi S., Okazaki Y., Katayama T. Effect of dietary level of phytic acid on hepatic and serum lipid status in rats fed a high-sucrose diet. Biosci. Biotechnol. Biochem. 2004;68:1379–1381. doi: 10.1271/bbb.68.1379. [DOI] [PubMed] [Google Scholar]
- 6.Graf E., Eaton J.W. Dietary suppression of colonic cancer. Fiber or phytate? Cancer. 1985;56:717–718. doi: 10.1002/1097-0142(19850815)56:4<717::aid-cncr2820560402>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Anekonda T.S., Wadsworth T.L., Sabin R., Frahler K., Harris C., Petriko B., Ralle M., Woltjer R., Quinn J.F. Phytic acid as a potential treatment for Alzheimer's pathology: evidence from animal and in vitro models. J. Alzheimers Dis. 2011;23:21–35. doi: 10.3233/JAD-2010-101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grases F., Sanchis P., Perello J., Isern B., Prieto R.M., Fernadez-Palomeque C., Fiol M., Bonnin O., Torres J.J. Phytate (myo-inositol hexakisphophate) inhibits cardiovascular calcifications in rats. Front. Biosci. 2006;11:136–142. doi: 10.2741/1786. [DOI] [PubMed] [Google Scholar]
- 9.Grases F., Garcia-Ferragut L., Costa-Bauzá A., March J.G. Study of the effects of different substances on the early stages of papillary stone formation. Nephron. 1996;73:561–568. doi: 10.1159/000189141. [DOI] [PubMed] [Google Scholar]
- 10.Jenab P.M., Thompson L.U. Phytic acid in wheat bran affects colon morphology. cell differentiation and apoptosis, Carcinogenesis 21. 2000:1547–1552. [PubMed] [Google Scholar]
- 11.Challa A., Rao D.R., Reddy B. Interactive suppression of aberrant crypt foci induced by azoxymethene in rat colon by phytic acid and green tea. Carcinogenesis. 1997;18:2023–2026. doi: 10.1093/carcin/18.10.2023. [DOI] [PubMed] [Google Scholar]
- 12.Gu M., Raina K., Agarwal R. Inositol hexaphosphate down-regulates constitutive and ligand-induced mitogenic and cell survival signaling, and causes caspase-mediated apoptotic death of human prostate carcinoma PC-3 cells. Mol. Carcinog. 2010;49:1–12. doi: 10.1002/mc.20560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapral M., Wawszyk J., Jurzak M., Hollek A., Weglarz L. The effect of inositol hexaphosphate on the expression of selected metalloproteinase and their tissue inhibitors in IL-1β-stimulated colon. Int. J. Colorectal Dis. 2012;27:1419–1428. doi: 10.1007/s00384-012-1445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khatiwada J., Verghese M., Davis S., Williams L.L. Green tea, phytic acid, and inositol in combination reduced the incidence of azoxymethane-induced colon tumor in fisher 344 male rats. J. Med. Food. 2011;14:1313–1320. doi: 10.1089/jmf.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H.J., Lee S.A., Choi H. Dietary administration of inositol and/or inositol-6-phosphate prevents chemically-induced rat hepatocarcinogenesis. Asian Pac. J. Cancer. 2005;6:41–47. [PubMed] [Google Scholar]
- 16.Shamsuddin A.M. Metabolism and cellular functions of IP6: a review. Anticancer Res. 1999;19:3733–3736. [PubMed] [Google Scholar]
- 17.Shamsuddin A.M., Vucenik I. Ip6 and inositol in cancer prevention and therapy. Curr. Cancer Ther. Res. 2005;1:259–269. [Google Scholar]
- 18.Tantivejkul K., Vucenik I., Shamsuddin A.M. Inositol hexaphosphate (IP6) inhibits key events of cancer metastasis: I. In vitro studies of adhesion, migration and invasion of MDA-MB 231 human breast cancer cells. Anticancer Res. 2003;23:3671–3680. [PubMed] [Google Scholar]
- 19.Vucenik I., Shamsuddin A.M. Protection against cancer by dietary IP6 and Inositol. Nutr. Cancer. 2006;55:109–125. doi: 10.1207/s15327914nc5502_1. [DOI] [PubMed] [Google Scholar]
- 20.Bouhet S., Oswald I.P. The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Vet. Immunol. Immunopathol. 2005;108:199–209. doi: 10.1016/j.vetimm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Randall K.J., Turton J., Foster J.R. Explant culture of gastrointestinal tissue: a review of methods and applications. Cell Biol. Toxicol. 2011;27:267–284. doi: 10.1007/s10565-011-9187-5. [DOI] [PubMed] [Google Scholar]
- 22.Bracarense A.-P.F.L., Lucioli J., Grenier B., Pacheco G.D., Moll W.-D., Schatzmayr G., Oswald I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012;10:1776–1786. doi: 10.1017/S0007114511004946. [DOI] [PubMed] [Google Scholar]
- 23.Binder E.M., Tan L.M., Chin L.J., Handl J., Richard J. Worldwide occurrence of mycotoxins in commodities, feed and feed ingredients. Anim. Feed Sci. Technol. 2007;137:265–282. [Google Scholar]
- 24.Rodrigues I., Naehrer K. A three-year survey on the worldwide occurrence of mycotoxin in feedstuffs and feed. Toxins. 2012;4:663–675. doi: 10.3390/toxins4090663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouhet S., Le Dorze E., Peres S., Fairbrother J.M., Oswald I.P. Mycotoxin fumonisin B1 selectively down-regulates the basal IL-8 expression in pig intestine: in vivo and in vitro studies. Food Chem. Toxicol. 2006;44:1768–1773. doi: 10.1016/j.fct.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Ueno Y., Iijima K., Wang S.-D., Sugiura Y., Sekijima M., Tanaka T., Chen C., Yu S.-Z. Fumonisin as a possible contributory risk factor for primary liver cancer: a 3-year study of corn harvested in Haimen, China by HPLC and ELISA. Food Chem. Toxicol. 1997;35:1143–1150. doi: 10.1016/s0278-6915(97)00113-0. [DOI] [PubMed] [Google Scholar]
- 27.Zhou H.R., Islam Z., Pestka J.J. Rapid, sequential activation of mitogen-activated protein kinases and transcription factors precedes proinflammatory cytokine mRNA expression. In spleens of mice exposed to the trichothecene vomitoxin. Toxicol. Sci. 2003;72:130–142. doi: 10.1093/toxsci/kfg006. [DOI] [PubMed] [Google Scholar]
- 28.Ueno Y. Toxicological features of T-2 toxin and related trichothecenes. Fundam. Appl. Toxicol. 1984;4:S124–S132. doi: 10.1016/0272-0590(84)90144-1. [DOI] [PubMed] [Google Scholar]
- 29.Pestka J.J., Smolinski A.T. Deoxynivalenol: toxicology and potential effects on humans. J. Toxicol. Environ. Health B. Crit. 2005;8:39–69. doi: 10.1080/10937400590889458. [DOI] [PubMed] [Google Scholar]
- 30.Chen C.Y., Del Gatto-Konczak F., Wu Z., Kacin M. Stabilization of interleukin-2 mRNA by the C-Jun NHZ-terminal kinase. Science. 1998;280:1945–1949. doi: 10.1126/science.280.5371.1945. [DOI] [PubMed] [Google Scholar]
- 31.Pestka J.J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. A Chem. Anal. Control Expo. Risk Assess. 2008;25:1128–1140. doi: 10.1080/02652030802056626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinton P., Braicu C., Nougayrede J.-P., Laffitte J., Taranu I., Oswald I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen activated protein kinase-dependent mechanism. J. Nutr. 2010;140:1956–1962. doi: 10.3945/jn.110.123919. [DOI] [PubMed] [Google Scholar]
- 33.Van De Walle J., Sergent T., Piront N., Toussaint O., Schneider Y.-J., Larondelle Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl. Pharmacol. 2010;245:291–298. doi: 10.1016/j.taap.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Gumprecht L.A., Beasley V.R., Weigel R.M., Parker H.M., Tumbleson M.E., Bacon C.W., Meredith F.I., Haschek W.M. Development of fumonisin-induced hepatotoxicity and pulmonary edema in orally dosed swine: morphological and biochemical alterations. Toxicol. Pathol. 1998;26:777–788. doi: 10.1177/019262339802600610. [DOI] [PubMed] [Google Scholar]
- 35.Kellerman T.S., Marasas W.F.O., Thiel P.G., Gelderblom W.C.A., Cawood M.E., Coetzer J.A.W. Leukoencephalomalacia in two horses induced by oral dosing of Fumonisin B1. Onderstepoort J. Vet. Res. 1990;57:269–275. [PubMed] [Google Scholar]
- 36.Gelderblom W.C.A., Abel S., Smuts C.M., Marnewick J., Marasas W.F.O., Lemmer E.R., Ramljak D. Fumonisin-induced hepatocarcinogenesis: mechanisms related to cancer initiation and promotion. Environ. Health Perspect. 2001;109(Suppl. 2):291–300. doi: 10.1289/ehp.01109s2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voss K.A., Riley R.T., Norred W.P., Bacon C.W., Filmore I.M., Howard P.C., Plattner R.D., Collins T.F.X., Hansen D.K., Porter J.K. An overview of rodent toxicities: liver and kidney effects of fumonisinas and Fusarium moniliforme. Environ. Health Perspect. 2001;109(Suppl. 2):259–266. doi: 10.1289/ehp.01109s2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gelineau-van Waes J., Rainey M.A., Maddox J.R., Voss K.A., Sachs A.J., Gardner N.M., Wilberding J.D., Riley R.T. Increase sphingoid base-1-phosphates and failure of neural tube closure after exposure to Fumonisin or FTY720. Birth Defects Res. A: Clin. Mol. Teratol. 2012;94:790–803. doi: 10.1002/bdra.23074. [DOI] [PubMed] [Google Scholar]
- 39.Wang H., Wei H., Ma J., Luo X. The Fumonisin B1 content in corn from North China, a high-risk area of esophageal cancer. J. Environ. Pathol. Toxicol. Oncol. 2000;19:139–141. [PubMed] [Google Scholar]
- 40.Merril A.H., Jr., Van Echten G., Wang E., Sandhoff K. Fumonisin B1 inhibits sphingosine (sphinganine) N-acyltransferase and de novo sphingolipids biosynthesis. J. Biol. Chem. 1993;268:27299–27305. [PubMed] [Google Scholar]
- 41.Soriano J.M., González L., Catalá A.I. Mechanism of action of sphingolipids and their metabolites in the toxicity of Fumonisin B1. Prog. Lipid Res. 2005;44:345–356. doi: 10.1016/j.plipres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Pacheco G.D., Silva C.A., Pinton P., Oswald I.P., Bracarense A.P.F.R.L. Phytic acid protects porcine intestinal epithelial cells from deoxynivalenol (DON) cytotoxicity. Exp. Toxicol. Pathol. 2012;64:345–347. doi: 10.1016/j.etp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Abu El Saad A.S., Mahmoud H.M. Phytic acid exposure alters aflatoxin B1-induced reproductive and oxidative toxicity in albino rats (Rattus norvegicus) Evid. Based Complement. Altern. Med. 2009;6:331–341. doi: 10.1093/ecam/nem137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlemmer U., Jany K.D., Berk A., Schulz E., Rechkemmer G. Degradation of phytate in the gut of pigs-pathway of gastro-intestinal inositol phosphate hydrolysis and enzymes involved. Arch Tierernahr. 55. 2001:255–280. doi: 10.1080/17450390109386197. [DOI] [PubMed] [Google Scholar]
- 45.Silva E.O., Gerez J.R., Bracarense A.P.F.R.L. Effect of phytic acid from rice and corn on morphology, cell proliferation, apoptosis and cyclooxygenase-2 expression in swine jejunal explants. Ciênc. Agrotec. 2014;38 (in press) [Google Scholar]
- 46.Lucioli J., Pinton P., Patrick C., Laffitte J., Grosjean F., Kolf-Clauw M., Oswald I.P., Baracarense A.P.F.R.L. The food contaminant deoxynivalenol activates the mitogen activated protein kinases in the intestine: interest of ex vivo models as an alternative to in vivo experiments. Toxicon. 2013;66:31–36. doi: 10.1016/j.toxicon.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 47.Basso K., Gomes F., Bracarense A.P. Deoxynivanelol and fumonisin, alone or in combination, induce changes on intestinal junction complexes and in E-cadherin expression. Toxins. 2013;5:2341–2352. doi: 10.3390/toxins5122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamp D.W., Israbian V.A., Yeldandi A.V., Panos R.J., Graceffa P., Weitzman S.A. Instillation of asbestos phytic acid, an iron chelator, attenuates pulmonary inflammation and fibrosis in rats after intratracheal instillation of asbestos. Toxicol. Pathol. 1995;23:689–695. doi: 10.1177/019262339502300606. [DOI] [PubMed] [Google Scholar]
- 49.Cholewa K., Parfiniewicz B., Berdnarek I., Swiatkowska L., Jezienicka E., Kierot J., Weglarz L. The influence of phytic acid on TNF-alpha and its receptor genes expression in colon cancer Caco-2 cells. Acta Pol. Pharm. 2008;65:75–79. [PubMed] [Google Scholar]
- 50.Kapral M., Wawszczyk J., Hollek A., Weglarz L. Induction of the expression of genes encoding TGF-beta isoforms and their receptors by inositol hexaphosphate in human colon cancer cells. Acta Pol. Pharm. 2013;70:357–363. [PubMed] [Google Scholar]
- 51.Grenier B., Bracarense A.P., Schwartz H.E., Lucioli J., Cossalter A.M., Moll W.D., Schatzmayr G., Oswald I.P. Biotransformation approaches to alleviate the effects induced by Fusarium mycotoxins in swine. J. Agric. Food Chem. 2013;61:6711–6719. doi: 10.1021/jf400213q. [DOI] [PubMed] [Google Scholar]
- 52.Grenier B., Bracarense A.P.F.L., Schwartz H.E., Trumel C., Cossalter A.M., Schatzmayr G., Kolf-Clauw M., Moll W.D., Oswald I.P. The low intestinal and hepatic toxicity of hydrolyzed fumonisin B1 correlates with its inability to alter the metabolism of sphingolipids. Biochem. Pharmacol. 2012;83:1465–1473. doi: 10.1016/j.bcp.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Piva A., Casadei G., Pagliuca G. Activated carbon does not prevent the toxicity of culture material containing Fumonisin B1 when fed to weanling piglets. J. Anim. Sci. 2005;83:1939–1947. doi: 10.2527/2005.8381939x. [DOI] [PubMed] [Google Scholar]
- 54.Diesing A.-K., Nossol C., Danicke S., Walk N., Post A., Kahlert S., Rothkotter H.-J., Kluess J. Vulnerability of polarized intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application. PLoS ONE. 2011;6:e17472. doi: 10.1371/journal.pone.0017472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Theumer M.G., Lopez A.G., Chulze S.N., Rubinstein H.R. Immunobiological effects of Fumonisin B1 in experimental subchronic mycotoxicoses in rats. Clin. Diagn. Lab. Immunol. 2002;9:149–155. doi: 10.1128/CDLI.9.1.149-155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang G.-H., Jarvis B.B., Chung Y.-J., Pestka J.J. Apoptosis induction by the Satratoxins and other trichothecene mycotoxins: relationship to ERK, p38 MAPK, and SAPK/JNK activation. Toxicol. Appl. Pharmacol. 2000;164:149–160. doi: 10.1006/taap.1999.8888. [DOI] [PubMed] [Google Scholar]
- 57.Desai K., Sullards M.C., Alleggod J., Wang E., Schmelmz E.M., Hartl M., Humpf H.-U., Liotta D.C., Peng Q., Merril A.H., Jr. Fumonisins and fumonisin analogs as inhibitors of ceramide synthase and inducers of apoptosis. Biochim. Biophys. Acta. 2002;1585:188–192. doi: 10.1016/s1388-1981(02)00340-2. [DOI] [PubMed] [Google Scholar]
- 58.Gopee N.V., He Q., Sharma R.P. Fumonisin B1 induced apoptosis is associated with delayed inhibition of protein kinase C, nuclear factor κB and tumor necrosis factor α in LLC-PK1 cells. Chem. Biol. Interact. 2003;146:131–145. doi: 10.1016/s0009-2797(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 59.Bouhet S., Hourcade E., Loiseau N., Fikry A., Martinez S., Roselli M., Galtier P., Mengheri E., Oswald I.P. The mycotoxin Fumonisin B1 alters the proliferation and the barrier function of porcine intestinal epithelial cells. Toxicol. Sci. 2004;77:165–171. doi: 10.1093/toxsci/kfh006. [DOI] [PubMed] [Google Scholar]
- 60.Meli R., Ferrante M.C., Raso G.M., Cavaliere M., Di Carlo R., Lucisano A. Effect of Fumonisin B1 on inducible nitric oxide synthase and cyclooxygenase-2 in LPS-stimulated J774A.1 cells. Life Sci. 2000;67:2845–2853. doi: 10.1016/s0024-3205(00)00871-7. [DOI] [PubMed] [Google Scholar]
- 61.Huet O., Laemmel E., Fu Y., Dupic L., Aprico A., Andrews K.L., Moore S.L., Harrois A., Meikle P.L., Vicaut E., Chin-Dusting J.P., Duranteau J. Interleukin 10 antioxidant effect decreases leukocytes/endothelial interaction induced by tumor necrosis factor α. Shock. 2013;39:83–88. doi: 10.1097/SHK.0b013e318278ae36. [DOI] [PubMed] [Google Scholar]
- 62.Kim H., Bae S., Kim Y., Cho C.H., Kim S.J., Kim Y.J., Lee S.P., Kim H.R., Hwang Y.I., Kang J.S., Lee W.J. Vitamin C prevents stress-induced damage on the heart caused by the death of cardiomyocytes, through down-regulation of the excessive production of catecholamine, TNF-α, and ROS production in Gulo (−/−) Vit C-insufficient mice. Free Radic. Biol. Med. 2013;65C:573–583. doi: 10.1016/j.freeradbiomed.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 63.Sánchez-Fidalgo S., da Silva M.S., Cárdeno A., Aparicio-Soto M., Frankland-Sawaya A.C., Souza-Brito A.R., de Ia Lastra C.A. Abarema cochliacarpos reduces LPS-induced inflammatory response in murine peritoneal macrophages regulating ROS-MAPK signal pathway. J. Ethnopharmacol. 2013;149:140–147. doi: 10.1016/j.jep.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 64.Krishnaswamy R., Devaray S.N., Padma V.V. Lutein protects HT-29 cells against deoxynivalenol-induced oxidative stress and apoptosis: prevention of NF-kappa B nuclear localization and down regulation of NF-kappa B and cyclo-oxygenase-2 expression. Free Radic. Biol. Med. 2010;49:50–60. doi: 10.1016/j.freeradbiomed.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 65.Saad N., Esa N.M., Ithnin H. Suppression of β-catenin and Cyclooxygenase-2 expression and Cell proliferation in Azoxymethane-induced colonic cancer in rats by rice bran phytic acid (PA) Asian Pac. J. Cancer Prev. 2013;14:3093–3099. doi: 10.7314/apjcp.2013.14.5.3093. [DOI] [PubMed] [Google Scholar]
- 66.Parfiniewicz B., Pendzich J., Kapral M., Bednarek I., Weglarz L. The influence of TNF-alpha on concentration of soluble adhesion molecules in cultures of HT-29 cells exposed to inositol hexaphosphate. Acta Pol. Pharm. 2012;6:1291–1297. [PubMed] [Google Scholar]
- 67.Korbecki J., Baranowska-Bosiacka I., Gutowska I., Chlubek D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J. Physiol. Pharmacol. 2013;64:409–421. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.