Highlights
-
•
Evaluate the effects of hesperidin in iron induced hepatic and renal function.
-
•
Hesperidin has significant antioxidant property.
-
•
Hesperidin shows reduced lipid profile.
-
•
Administration of hesperidin averts oxidative stress in liver and kidney tissues.
-
•
Rescue the liver and kidney tissues from pathology.
Keywords: Hesperidin, Iron, Liver, Kidney, Oxidative stress, Antioxidant, Lipid peroxidation
Abstract
The present study was to evaluate the protective role of hesperidin (HDN) against iron-induced hepatic and renal toxicity in rats. Administration of iron (30 mg/kg body weight) intraperitoneally for 10 days, the levels of serum hepatic markers, renal functional markers, lipid profile, lipid peroxidation markers and iron concentration in blood were significantly (p < 0.05) increased. The toxic effect of iron was also indicated by significant (p < 0.05) decrease in the levels of plasma, liver and kidney of enzymatic and non-enzymatic antioxidants. Administration of hesperidin at different doses (20, 40 and 80 mg/kg body weight) significantly (p < 0.05) reversed the levels of serum hepatic markers, renal functional markers, lipid profile, lipid peroxidation markers, restored the levels of hepatic, renal enzymatic antioxidants and non-enzymatic antioxidants with decrease in iron concentration in blood. Hesperidin at a dose of 80 mg/kg body weight exhibits significant protection on hepatic and renal when compared with other two doses (20 and 40 mg/kg body weight). All these changes were corroborating by histological observations of liver and kidney. This study demonstrated the protective role of hesperidin in reducing toxic effects of iron in experimental rats.
1. Introduction
Heavy metals can be classified as potentially toxic (arsenic, cadmium, lead, etc.), probably essential (vanadium, cobalt) and essential (copper, zinc, iron, manganese, etc.). Toxic elements can be very harmful even at low concentration when ingested over a long time period [1]. They might come from the soil, environment, fertilizers and/or metal-containing pesticides, introduced during the production process or by contamination from the metal processing equipment. Food consumption had been identified as the major pathway of human exposure to toxic metals, compared with other ways of exposure such as inhalation and dermal contact [2].
Humans are constantly exposed to hazardous pollutants in the environment-for example, in the air, water, soil, rocks, diet or workplace. Trace metals are important in environmental pathology because of the wide range of toxic reactions and their potential adverse effects on the physiological function of organ systems. Exposures to toxic trace metals have been the subject of numerous environmental and geochemical investigations, and many studies have been published on the acute and/or chronic effects of high-level exposures to these types of agents; however, much fewer data are available concerning the health effects of low-dose chronic exposure to many trace metals [3].
Iron is an important trace element of the body, being found in functional form in hemoglobin, myoglobin, cytochrome enzymes with iron sulphur complexes [4]. Liver is one of the largest organs in the human body and the main site for intense metabolism and excretion [5]. Hepatotoxicity is the most common finding in patients with iron overloading as liver is mainly the active storage site of iron in our body [6]. Hydroxy radical may form due to excess iron concentration in kidney that leads to progression of tubular injury. Clinical evidence showed that iron deposition in kidney associated with the anemia during kidney diseases [7].
Although an optimum level of iron is always maintained by the cells to balance between essentiality and toxicity, in some situations it is disrupted, resulting in iron overload which is associated to the oxidative stress induced disorders including anemia, heart failure, hepatocellular necrosis and cirrhosis [8]. In iron overload-induced diseases, iron removal by iron chelation therapy is an effective life-saving strategy. Iron overload increases the formation of reactive oxygen species (ROS) which involves the initiation of lipid peroxidation, protein oxidation and liver fibrosis. However, excess iron is stored as Fe3+ in ferritin and iron overload sustains for long period and released depends on the efficiency of iron chelating drugs [9]. The currently available iron-chelating agents used clinically are deferoxamine, 1,2-dimethyl-3-hydroxypyrid-4-one (deferiprone, L1), and deferasirox [10]. The body lacks to excrete excessive iron and therefore the interest has been focused to develop the potent chelating agent capable of complexing with iron and promoting its excretion.
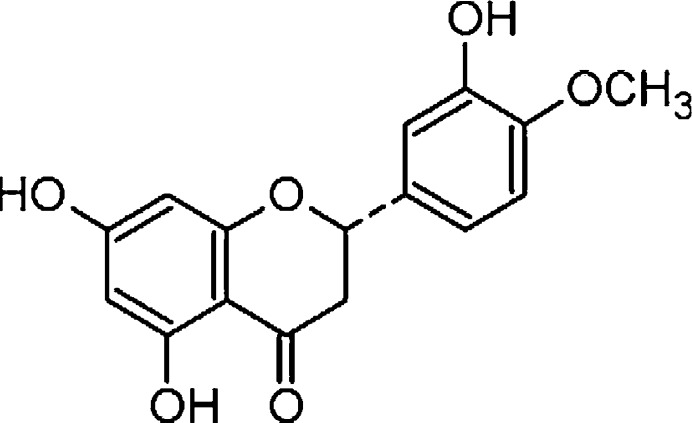
Flavonoids are phenolic compounds abundantly distributed in plants. It has been reported that most of them are effective antioxidants [11]. They were suggested to present a good scavenger to iron ions [12]. Hesperidin (3,5,7-trihydroxy flavanone-7-rhamnoglucoside) is a pharmacologically active bioflavonoid found in citrus fruits, with good free radical scavenging as well as anti-lipid peroxidation properties in biological membranes [13]. Hesperidin (Fig. 1) possesses highest reducing power, chelating activity on Fe2+, hydrogen radical scavenging and hydrogen peroxide scavenging activities when compared with natural and synthetic antioxidants such as α-tocopherol, ascorbic acid, butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA) and trolox [14]. Clinical and experimental data showed the antihypertensive, lipid-lowering, insulin-sensitizing, antioxidative and anti-inflammatory properties of hesperidin [15]. However, the protective role of hesperidin against iron-induced liver and kidney injury has not been investigated. Hence we proposed to investigate whether administration of hesperidin offers protection against iron-induced liver and kidney injury.
Fig. 1.
Structure of hesperidin.
2. Materials and Methods
2.1. Chemicals and drugs
Hesperidin (PubChem CID: 10621); ferrous sulfate (PubChem CID: 24393); 2-thiobarbituric acid (PubChem CID: 2723628); butylated hydroxytoluene (PubChem CID 31404); reduced glutathione (PubChem CID:745); 2,2′-dipyridyl (PubChem CID: 1474); xylenol orange (PubChem CID: 73041); 2,4-dinitrophenylhydrazine (PubChem CID:CID: 3772977); γ-glutamyl-p-nitroanilide (PubChem CID: 3772977); 5,5′-dithiobis(2-nitrobenzoic acid) (PubChem CID: 6254); trichloroacetic acid (PubChem CID: 6421); phenazine methosulfate (PubChem CID 9285); nitroblue tetrazolium (PubChem CID: 9281); reduced nicotinamide adenine dinucleotide (PubChem CID: 439153); 1-chloro-2,4-dinitrobenzene (PubChem CID: 6) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The rest of the chemicals were obtained from S.D. Fine Chemicals Mumbai, India and were of analytical grade.
2.2. Experimental animals
Adult male albino rats of Wistar strain (200–220 g) were used for the experiment. The animals were housed in polypropylene cages and maintained in 12-h light/12-h dark cycle, 50% humidity and 25 ± 2 °C. The animals had free access to standard pellet diet (M/S. Pranav Agro Industries Ltd., Bangalore, India) and water ad libitum. This study was approved (Vide. No. 644, 2009) by Institutional Animal Ethics Committee of Annamalai University and the study conducted in accordance with the “Guide for the Care and Use of Laboratory Animals”.
2.3. Experimental design
Ferrous sulfate (30 mg/kg body weight) was dissolved in isotonic saline and injected intraperitoneally (i.p). Hesperidin powder was dissolved in 0.1% carboxy methyl cellulose and each rat received daily 1 ml at a dose of 20, 40 and 80 mg/kg body weight orally by intragastric tube throughout the experimental period.
The animals were randomly divided into six groups of six rats in each group.
Group I: served as control (isotonic saline).
Group II: animals were orally administered with hesperidin alone (80 mg/kg body weight).
Group III: animals received ferrous sulfate (30 mg/kg body weight).
Group IV–VI: animals were treated with ferrous sulfate (30 mg/kg body weight) following oral administration of hesperidin (20, 40, 80 mg/kg body weight) for 10 days.
At the end of the experimental period, animals in different groups were sacrificed by cervical decapitation. Blood samples were collected without heparin for serum separation. Serum separated by centrifugation was used for various biochemical estimations.
2.4. Preparation of tissue homogenate
Rats were anesthetized by ketamine (28 mg/kg body weight, intra muscularly) and the animals were sacrificed by cervical decapitation. The liver and kidney was quickly excised, rinsed with isotonic saline, blotted dry on filter paper, weighed and then 10% (w/v) homogenates of tissue was prepared in buffer (0.1 M Tris-HCL buffer (pH 7.4) and centrifuged at 3000 × g for 20 min at 4 °C. The resulting tissue homogenate was used for various biochemical assays.
2.5. Assessment of serum hepatic marker enzymes
The activities of serum aspartate aminotransferase (E.C.2.6.1.1), alanine aminotransferase (E.C.2.6.1.2), alkaline phosphatase (E.C.3.1.3.1) and lactate dehydrogenase (E.C.3.1.3.1) were assayed using commercially available diagnostic kits (Sigma diagnostics (I) Pvt. Ltd., Baroda, India). Gamma glutamyl transferase (E.C.2.3.2.2) activity was determined by the method of Rosalki et al. [16] using γ-glutamyl-p-nitroanilide as substrate. Based on Vanden Berg reaction, serum bilirubin was estimated by the method of Malloy and Evelyn [17].
2.6. Assessment of renal functional marker enzymes
The activities of urea, creatinine and were estimated by Agappe Diagnostic (I) Pvt. Ltd., Kerala, India. Haemoglobin was estimated by Drabkin and Austin [18]. Creatinine clearance as an index of glomerular filtration rate was calculated from creatinine level in serum and creatinine level in 24 h urine sample.
2.7. Assessment of iron concentration
For determination of iron in blood, 1 ml of blood was digested with nitric acid in microwave oven. After digestion, iron was continuously pre concentrated and determined by flame atomic absorption spectrophotometry. A Perkin-Elmer 5000 atomic absorption spectrometer furnished with an iron hollow-cathode lamp (lamp current 4 mA) was used to determine the iron concentration. The instrument was set at 228.8 nm with a slit width of 0.5 nm. The acetylene flow rate was 2.0 l/min and an airflow rate of 17.0 l/min was employed to ensure an oxidizing flame.
2.8. Assessment of lipid profile
Lipids extracted from the tissues using by the method of Folch et al. [19]. The levels of total cholesterol, triglycerides and free fatty acids in the serum and tissues were estimated by the methods of Zlatkis et al., Fossati and Prencipe, Falholt et al. [20], [21], [22], respectively. The phospholipids estimation was done by the method of Zilversmit and Davis [23].
2.9. Assessment of lipid peroxidation
Lipid peroxidation in plasma, liver and kidney was estimated spectrophotometrically by measuring thiobarbituric acid reactive substances and lipid hydroperoxides by the method of Niehius and Samuelson, Jiang et al. [24], [25], respectively.
2.10. Assessment of enzymatic antioxidants
Superoxide dismutase activity was determined by the method of Kakkar et al. [26]. The activity of catalase was determined by the method of Sinha et al. [27]. Glutathione peroxidase activity was estimated by the method of Rotruck et al. [28]. Glutathione S-transferase activity was determined by the method of Habig et al. [29].
2.11. Assessment of non-enzymatic antioxidants
Vitamin C concentration was measured as previously reported Omaye et al. [30]. Vitamin E (α-tocopherol) was estimated by the method of Desai et al. [31]. Reduced glutathione was determined by the method of Ellman et al. [32].
2.12. Histological Observation
The liver and kidney sample fixed for 48 hr in 10% formalin were dehydrated by passing successfully in different mixture of ethyl alcohol–water, cleaned in xylene and embedded in paraffin. Sections of liver and kidney (5–6 μm thick) were prepared and then stained with hematoxylin and eosin dye, which mounted in neutral DPX medium for microscopic observations.
2.13. Statistical Analysis
Values are given as means ± S.D for six rats in each group. Data were analyzed by one-way analysis of variance followed by Duncan's Multiple Range Test (DMRT) using SPSS version 13 (SPSS, Chicago, IL). The limit of statistical significance was set at (p < 0.05) and the values sharing a common superscript did not differ significantly.
3. Results
3.1. Effect of hesperidin on serum hepatic markers
Table 1 depicts the levels of serum hepatic markers in control and experimental rats. In Fe treated rats, the activities of serum hepato-specific enzymes such as aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, lactate dehydrogenase, gamma glutamyl transferase and the levels of bilirubin were significantly increased (p < 0.05). Administration of hesperidin significantly reversed these changes in a dose dependent manner.
Table 1.
Effect of hesperidin on iron-induced activities of serum hepatic markers in control and experimental rats.
| Groups | Control | Normal + HDN (80 mg/kg) | Normal + Fe (30 mg/kg) | Fe (30 mg/kg) + HDN (20 mg/kg) | Fe (30 mg/kg) + HDN (40 mg/kg) | Fe (30 mg/kg) + HDN (80 mg/kg) |
|---|---|---|---|---|---|---|
| AST (IU/l) | 56.61 ± 4.25a | 57.11 ± 4.51a | 87.70 ± 6.31b | 79.44 ± 6.01c | 72.23 ± 5.59d | 64.02 ± 4.83e |
| ALT (IU/l) | 27.34 ± 2.06a | 28.27 ± 2.62a | 46.61 ± 3.15b | 40.73 ± 2.97c | 35.41 ± 2.75d | 31.79 ± 2.70e |
| ALP (IU/l) | 90.17 ± 8.14a | 91.63 ± 8.08a | 144.31 ± 11.34b | 131.74 ± 11.50c | 119.70 ± 9.96d | 105.51 ± 9.66e |
| LDH (IU/l) | 107.60 ± 8.58a | 107.46 ± 8.60a | 167.65 ± 14.16b | 151.52 ± 11.87c | 136.94 ± 10.63d | 121.36 ± 10.09e |
| GGT (IU/l) | 0.68 ± 0.05a | 0.69 ± 0.05a | 1.21 ± 0.13b | 1.08 ± 0.06c | 0.97 ± 0.06d | 0.83 ± 0.05e |
| Bilirubin (mg/dl) | 0.74 ± 0.06a | 0.72 ± 0.06a | 1.25 ± 0.10b | 1.06 ± 0.09c | 0.94 ± 0.65d | 0.84 ± 0.07e |
Values are mean ± SD for 6 rats in each group. Values are not sharing a common superscript letter (a, b, c, d and e) differ significantly at p < 0.05 (DMRT). HDN—hesperidin, Fe—ferrous sulfate.
3.2. Effect of hesperidin on renal functional markers
Table 2 presents the levels of renal functional markers in control and experimental rats. In Fe treated rats, the activities of renal functional markers such as urea, creatinine, creatinine clearance and hemoglobin were significantly increased (p < 0.05). Administration of hesperidin significantly (p < 0.05) reversed these changes in a dose dependent manner. Our results indicate that hesperidin at a dose of 80 mg/kg body weight was more effective than other doses (20 and 40 mg/kg body weight). Hence, hesperidin 80 mg/kg body weight was used for further biochemical studies.
Table 2.
Effect of hesperidin on the levels renal functional markers in control and experimental rats.
| Groups | Control | Normal + HDN (80 mg/kg) | Normal + Fe (30 mg/kg) | Fe (30 mg/kg) + HDN (20 mg/kg) | Fe (30 mg/kg) + HDN (40 mg/kg) | Fe (30 mg/kg) + HDN (80 mg/kg) |
|---|---|---|---|---|---|---|
| Urea in serum (mg/dl) | 36.64 ± 2.52a | 35.56 ± 2.45a | 63.41 ± 5.33b | 57.72 ± 4.76c | 51.21 ± 4.89d | 43.54 ± 3.96e |
| Creatinine in serum (mg/dl) | 0.46 ± 0.05a | 0.45 ± 0.05a | 0.92 ± 0.09b | 0.84 ± 0.06c | 0.76 ± 0.04d | 0.55 ± 0.05e |
| Creatinine clearance (ml/min) | 0.29 ± 0.05a | 0.29 ± 0.05a | 0.10 ± 0.03b | 0.14 ± 0.03c | 0.20 ± 0.04d | 0.23 ± 0.04e |
| Hemoglobin (g/dl blood) | 10.90 ± 0.71a | 11.18 ± 0.93a | 6.60 ± 0.63b | 7.70 ± 0.50c | 8.61 ± 0.58d | 10.74 ± 0.65e |
Values are mean ± SD for 6 rats in each group. Values are not sharing a common superscript letter (a, b, c, d and e) differ significantly at p < 0.05 (DMRT). HDN—hesperidin; Fe—ferrous sulfate.
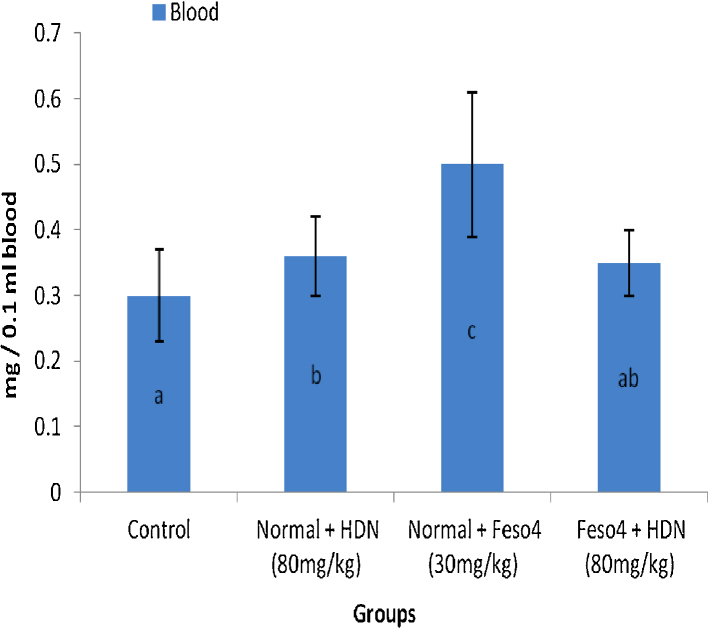
3.3. Iron concentration in blood
The concentration of iron has been depicted in Fig. 2. Fe administration to normal rats resulted in a significant (p < 0.05) increase in concentrations of Fe in blood. However, HDN restored the elevated levels significantly (p < 0.05) to within normal range in these animals when compared to their respective control groups.
Fig. 2.
Effect of hesperidin (HDN) on the accumulation of Fe in blood of control and. experimental rats. Values are mean ± SD for 6 rats in each group; values are not sharing a common superscript letter (a, b and c) differ significantly at p < 0.05 (DMRT).
3.4. Effect of hesperidin on lipid profile
The changes in the levels of serum and tissue lipids in normal and experimental rats are illustrated in Table 3. The levels of serum and tissue (liver & kidney) total cholesterol, triglycerides (TGs), free fatty acids (FFAs) and phospholipids (PLs) were highly altered in Fe treated rats when compared with control group. Oral administration of HDN to Fe intoxicated rat changes in the levels of serum and tissue total cholesterol, TGs, FFAs and PLs were near to normal.
Table 3.
Changes in the levels of Cholesterol, TGs, and PLs in serum and tissues of control and experimental rats.
| Groups | Control | Normal + HDN (80 mg/kg) | Normal + Fe (30 mg/kg) | Fe (30 mg/kg) + HDN (80 mg/kg) |
|---|---|---|---|---|
| Cholesterol | ||||
| Serum | 83.45 ± 8.27a | 82.14 ± 7.81a | 115.97 ± 10.96b | 94.57 ± 8.40c |
| Liver | 281.71 ± 20.16a | 277.77 ± 20.15a | 386.22 ± 30.21b | 312.11 ± 25.78c |
| Kidney | 350.71 ± 27.57a | 347.190 ± 27.06a | 477.91 ± 34.72b | 394.30 ± 32.14c |
| Triglycerides | ||||
| Serum | 73.96 ± 6.59a | 71.17 ± 6.57a | 120.80 ± 10.16b | 85.44 ± 8.13bc |
| Liver | 283.66 ± 16.75a | 280.21 ± 16.10a | 379.78 ± 3.05b | 315.51 ± 22.5c |
| Kidney | 325.11 ± 25.93a | 319.56 ± 25.38a | 466.38 ± 37.85b | 365.83 ± 30.78c |
| Free fatty acids | ||||
| Serum | 77.64 ± 6.43a | 75.74 ± 6.24a | 132.90 ± 13.16b | 89.58 ± 7.47c |
| Liver | 708.42 ± 52.01a | 706.31 ± 51.67a | 871.61 ± 72.67b | 788.37 ± 59.67c |
| Kidney | 325.11 ± 25.93a | 319.56 ± 25.38a | 466.38 ± 37.85b | 365.83 ± 30.78c |
| Phospholipids | ||||
| Serum | 13.92 ± 0.83a | 14.01 ± 0.96a | 7.80 ± 0.94b | 12.57 ± 0.72c |
| Liver | 8.87 ± 1.02a | 9.05 ± 0.92a | 3.87 ± 0.37b | 7.49 ± 0.74c |
| Kidney | 5.29 ± 0.25a | 5.90 ± 0.54a | 1.88 ± 0.65b | 4.49 ± 0.71c |
Values are mean ± SD for 6 rats in each group. Values are not sharing a common superscript letter (a, b and c) differ significantly at p < 0.05 (DMRT). Cholesterol—mg/dl serum and mg/g tissue; triglycerides—mg/dl serum and mg/g tissue; free fatty acids—mg/dl serum and mg/g tissue; phospholipid–mg/dl serum and mg/g tissue. HDN—hesperidin, Fe—ferrous sulfate.
3.5. Effect of hesperidin on lipid peroxidation
Table 4 shows the levels of lipid peroxidative markers (measured by the levels of thiobarbituric acid reactive substances and lipid hydroperoxides) were significantly increased in the plasma and tissue (liver & kidney) of Fe treated rats. Administration of HDN significantly (p < 0.05) decreased the levels of thiobarbituric acid reactive substances and lipid hydroperoxides on iron intoxicated rats.
Table 4.
Changes in the levels of TBARS, lipid hydroperoxides in plasma and tissues of control and experimental rats.
| Groups | Control | Normal + HDN (80 mg/kg) | Normal + Fe (30 mg/kg) | Fe (30 mg/kg) + HDN (80 mg/kg) |
|---|---|---|---|---|
| TBARS | ||||
| Plasma | 0.35 ± 0.01a | 0.33 ± 0.01a | 0.46 ± 0.04b | 0.40 ± 0.03c |
| Liver | 8.09 ± 0.64a | 7.56 ± 0.56a | 15.42 ± 1.42b | 9.46 ± 0.78c |
| Kidney | 16.76 ± 1.12a | 16.01 ± 0.90a | 28.41 ± 2.41b | 19.85 ± 1.31c |
| Lipid hydroperoxides | ||||
| Plasma | 13.02 ± 1.38a | 12.85 ± 1.29a | 19.77 ± 2.13b | 15.91 ± 1.68c |
| Liver | 0.83 ± 0.06a | 0.81 ± 0.05a | 1.32 ± 0.08b | 0.96 ± 0.06c |
| Kidney | 0.66 ± 0.05a | 0.64 ± 0.05a | 0.97 ± 0.07b | 0.76 ± 0.06c |
Values are mean ± SD for 6 rats in each group. Values are not sharing a common superscript letter (a, b and c) differ significantly at p < 0.05 (DMRT). The levels of TBARS were expressed as mM/dl plasma and mM/g tissue; Lipid hydroperoxides—×10−5 mM/dl plasma and mM/g tissue. HDN—hesperidin, Fe—ferrous sulfate.
3.6. Effect of hesperidin on enzymatic antioxidants
Table 5 illustrates the activities of enzymatic antioxidants namely superoxide dismutase, catalase, glutathione peroxidase, glutathione-S-transferase in tissue (Liver & Kidney) of control and experimental rats. A significant (p < 0.05) depletion in the activities of enzymatic antioxidants in Fe treated rats was observed. Treatment of HDN along with Fe increased the levels of enzymatic antioxidants in tissue (liver & kidney).
Table 5.
Changes in the activities of enzymatic antioxidants in control and experimental rats.
| Groups | Control | Normal + HDN (80 mg/kg) | Normal + Fe (30 mg/kg) | Fe (30 mg/kg) + HDN (80 mg/kg) |
|---|---|---|---|---|
| SOD | ||||
| Liver | 7.74 ± 0.56a | 8.30 ± 0.81a | 5.45 ± 0.32b | 6.75 ± 0.45c |
| Kidney | 12.07 ± 0.92a | 12.51 ± 1.06a | 8.09 ± 0.56b | 10.41 ± 0.73c |
| CAT | ||||
| Liver | 90.51 ± 6.67a | 94.42 ± 5.92a | 55.04 ± 4.13b | 75.21 ± 5.00c |
| Kidney | 48.25 ± 4.06a | 51.07 ± 4.28a | 32.11 ± 1.91b | 40.34 ± 3.19c |
| GPx | ||||
| Liver | 7.03 ± 0.46a | 7.28 ± 0.49a | 4.61 ± 0.25b | 6.02 ± 0.32c |
| Kidney | 5.46 ± 0.35a | 5.52 ± 0.48a | 3.22 ± 0.16b | 4.76 ± 0.29c |
| GST | ||||
| Liver | 7.98 ± 0.46a | 8.19 ± 0.40a | 5.95 ± 0.26b | 7.08 ± 0.34c |
| Kidney | 6.09 ± 0.34a | 6.15 ± 0.42a | 3.97 ± 0.28b | 5.54 ± 0.28c |
Values are mean ± SD for 6 rats in each group. Values are not sharing a common superscript letter (a, b and c) differ significantly at p < 0.05 (DMRT). SOD–one unit of enzyme activity was taken as the enzyme reaction, which gave 50% inhibition of NBT reduction in one minute/mg protein; CAT—μmol of H2O2 consumed/min/mg protein; GPx—μg of GSH consumed/min/mg protein; GST—μmoles of CDNB-GSH conjugate formed/min/mg protein. HDN—hesperidin, Fe—ferrous sulfate.
3.7. Effect of hesperidin on non-enzymatic antioxidants
Table 6 shows the changes in the levels of plasma and tissue (liver & kidney) non-enzymatic antioxidants namely reduced glutathione, vitamin C and vitamin E. A significant (p < 0.05) decrease in the levels of non-enzymatic antioxidants was noticed in rats treated with Fe when compared to control rats. Treatment with HDN (80 mg/kg body weight) along with Fe restored the levels of non-enzymatic antioxidants to near normal.
Table 6.
Changes in the levels of non-enzymatic antioxidants in control and experimental rats.
| Groups | Control | Normal + HDN (80 mg/kg) | Normal + Fe (30 mg/kg) | Fe (30 mg/kg) + HDN (80 mg/kg) |
|---|---|---|---|---|
| GSH | ||||
| Plasma | 19.93 ± 1.31a | 20.07 ± 1.71a | 15.22 ± 1.19b | 18.07 ± 1.11c |
| Liver | 4.20 ± 0.21a | 4.29 ± 0.30a | 2.83 ± 0.16b | 3.52 ± 0.23c |
| Kidney | 2.44 ± 0.19a | 2.57 ± 0.19a | 1.22 ± 0.18b | 1.77 ± 0.23c |
| Vitamin C | ||||
| Plasma | 1.86 ± 0.08a | 1.94 ± 0.11a | 1.54 ± 0.06b | 1.70 ± 0.07c |
| Liver | 1.59 ± 0.05a | 1.62 ± 0.05a | 1.14 ± 0.04b | 1.44 ± 0.06c |
| Kidney | 0.94 ± 0.08a | 1.01 ± 0.09a | 0.61 ± 0.06b | 0.82 ± 0.07c |
| Vitamin E | ||||
| Plasma | 1.34 ± 0.07a | 1.39 ± 0.12a | 0.90 ± 0.07b | 1.21 ± 0.09c |
| Liver | 0.78 ± 0.06a | 0.83 ± 0.07a | 0.49 ± 0.04b | 0.62 ± 0.05c |
| Kidney | 0.69 ± 0.05a | 0.73 ± 0.05a | 0.46 ± 0.01b | 0.55 ± 0.03c |
Values are mean ± SD for 6 rats in each group. Values are not sharing a common superscript letter (a, b and c) differ significantly at p < 0.05 (DMRT). The level of GSH was expressed as mg/dl plasma and μg/mg tissue protein; The levels of vitamin C and vitamin E were expressed as mg/dl plasma and μM/mg tissue. HDN—hesperidin, Fe—ferrous sulfate.
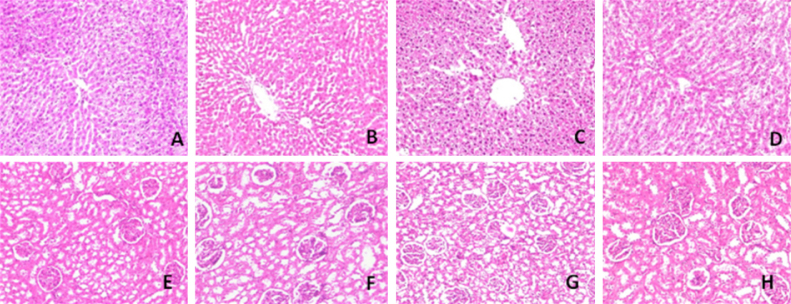
3.8. Histological analysis of liver and kidney
Histological analysis showed that Fe administration induces the pathological changes in liver. The liver of control rats (Fig. 3A) and HDN (Fig. 3B) treated rats showed a normal architecture. Fe exposure resulted in changes in liver architecture as indicated by focal necrosis, inflammatory cell infiltration and giant cell formation (Fig. 3C). Fe along with HDN administration (Fig. 3D) showed near normal hepatocytes with mild portal inflammation.
Fig. 3.
Histopathology of liver and kidney.
Histological studies showed that Fe administration induces the pathological changes in kidney. The focal areas of hemorrhage and inflammation of renal cells (Fig. 3E) were observed in Fe alone intoxicated rats. Rats administered with HDN along with Fe showed near normal appearance of glomerulai and tubules (Fig. 3F). Administration of HDN to normal rats did not produce any pathological changes in kidney (Fig. 3G) when compared with normal control rats (Fig. 3H).
4. Discussion
The objective of the present work was to investigate the protective effects of hesperidin on iron induced toxicity in rats. It has been demonstrated for their protective effect against iron induced toxicity in rats. In the present study, Liver damage by iron had been assessed by leakage of enzymes such as aspartate aminotransferase and alanine aminotransferase, into blood [33], [34]. In the present study, higher activities of serum, aspartate aminotransferase, alanine aminotransferase (an indicator of hepatocytes mitochondrial damage) have been found in response to iron overload-induced oxidative stress. Such increased activities might be attributed to the leakage of these enzymes from the injured liver cells into the blood stream because of the altered liver membrane permeability [35]. Increase in serum alkaline phosphatase activities is the indicative of cellular damage due to loss functional integrity of cell membranes. Lactate dehydrogenase is a sensitive intracellular enzyme, which increase in serum is also an indicator of cell damage [36] reported that releasing of transaminases (aspartate aminotransferase and alanine aminotransferase) and lactate dehydrogenase from the cell cytosol can occur secondary to cellular necrosis. Serum Gamma glutamyl transferase has been widely used as an index of liver dysfunction. Recent studies indicating that serum gamma glutamyl transferase might be useful in studying oxidative stress related issues. The products of the gamma glutamyl transferase reaction may themselves lead to increased free radical production, particularly in the presence of iron [37], [38], [39]. Bilirubin is other well known indicators of tissue damage by toxic substance and their levels are also substantially increased in iron intoxicated rats. Hesperidin (80 mg/kg body weight) may stabilize the hepatic cellular membrane damage and protect the hepatocytes against toxic effects of iron, which may decrease the leakage of the enzymes into blood stream. In this context, the membrane protective effect of hesperidin has already been reported [40].
The accumulation of iron in blood was effectively reduced by hesperidin, which revealed that hesperidin chelate the iron. Moreover, the hydroxyl groups of hesperidin or its active metabolites might bind with iron and enhanced the excretion of iron, which in consequence decrease accumulation of iron and reduce the toxic effects of iron. It is quite well known that hesperidin, a citrus flavonoid act as antioxidant molecule [41], which can scavenge the excess iron in biological system. High dose of Fe might lead to alterations in lipid metabolism and changes in the levels of serum and tissue lipids. It may be due to accumulation of Fe in liver, which plays a central role in lipid homeostasis. In our study, we have observed increased concentrations of serum and tissue lipids such as cholesterol, TGs, FFAs and PLs in Fe treatment. The observed increase in the levels of FFAs could due to Fe induced disturbances of mitochondrial function, which in turn may lead to the inhibition of β-oxidation and increased accumulation of FFA in tissues. The Fe induced rise of cholesterol in serum and tissues may be due to changes in the gene expression of hepatic enzymes mainly HMG-COA reductase. Heavy metal induced change in the gene expression of HMG-COA reductase has already been reported [42]. The increased PLs content in Fe intoxicated rats may be due to elevation in the levels of FFAs and cholesterol. The antioxidant property could also contribute to the protection of membrane lipids from free radical thereby HDN attenuated the abnormal dispersion of membrane lipids in circulation as well as reduced the excessive generation of more toxic peroxides, which cause drastic changes in cells and tissues. Reduced risk of cardiovascular disease is often attributed to the intake phytochemicals, which lower excessive cholesterol and/or TGs concentrations [43].
Lipid peroxidation is the process of oxidative degradation of poly unsaturated fatty acid and the products of lipid peroxidation inactivate cell constituents by oxidation or cause oxidative stress by undergoing radical chain reaction ultimately leading to the cell damage [44], [45]. Iron is the most common cofactor within the oxygen handling biological machinery and, specifically, lipid peroxidation of biological membranes is the main pathogenic mechanism of iron overload induced tissue damage [46]. The mitochondrion is a target for iron toxicity, with oxidative mitochondrial damage and poisoning of enzymes of the tri carboxylic acid cycle and energy metabolism recognized as potential targets [47]. Iron is also an essential element whose redox properties and coordination chemistry suits it for a number of catalytic and transport functions in living cells [48]. However, these same properties render iron toxic, to a large extent due to its ability to generate reactive oxygen species [49], [50]. Iron is a well known inducer of reactive oxygen species. Its ability to accelerate lipid peroxidation is well established [51], [52]. Harmful effects of extreme iron deposition in liver are likely during iron overload, which has been associated with the initiation and propagation of ROS induced oxidative damage to all biomacromolecules (proteins, lipids, sugar and DNA) that can lead to a critical failure of biological functions and ultimately cell death [53]. Free radicals such as superoxide anion, hydrogen peroxide, hydroxyl radical, which cause lipid peroxidation, can lead to cell death [54]. It is well known that excess free iron induces the expression of nitric oxide, releases the nitric oxide which combines with superoxide anions to form “peroxynitrite”, a very toxic mediator of lipid peroxidation as well as oxidative damage to cellular membrane [55], [56]. Earlier studies have demonstrated the critical role of iron in the formation of reactive oxygen species that ultimately cause peroxidative damage to vital cell structures [57]. An effective therapeutic approach can play a double role in reducing the rate of oxidation - one by sequestering and chelating cellular iron stores and other as radical trap (i.e., antioxidant activity) [58]. Since HDN has shown antioxidant and free radical scavenging activity [59], the present study primarily ameliorating the effect of HDN on iron accumulation and oxidative damage in the liver of iron overloaded rat is studied. Oral administration of hesperidin significantly inverse the iron induced peroxidative damage in liver which is evidenced from the lowered levels of thiobarbituric acid reactive substances and lipid hydroperoxides. This may be due to the antioxidative effect of hesperidin [60].
An antioxidant is a molecule capable of slowing or preventing the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons from a substance to an oxidizing agent. Oxidation reaction can produce free radicals, which start chain reactions that damage cells. Antioxidants terminate these chain reactions by removing free radical intermediates, and inhibit other oxidation reactions by being oxidized themselves. As a result are often reducing agents such as thiols, ascorbic acid or polyphenols [61].
The enzymatic antioxidants superoxide dismutase, catalase and glutathione peroxidase and glutathione-S-transferase play a vital role during the process of scavenging reactive oxygen species or preventing their formation [62]. Superoxide dismutase, catalase and glutathione peroxidase constitute the major enzymatic antioxidant defenses which convert active oxygen molecules in to non-toxic compounds [60]. Superoxide dismutase is a ubiquitous enzyme with an essential function in protecting aerobic cells against oxidative stress. It is primarily mitochondrial enzyme usually found in the plasma membrane [63]. Catalase is a tetrameric heme protein that undergoes alternative divalent oxidation and reduction at its active site in the presence of hydrogen peroxide [64]. As a substrate for the antioxidant enzyme glutathione peroxidase, reduced glutathione protects cellular constituents from the damaging effects of peroxides formed in metabolism and other reactive oxygen species reaction [65]. Glutathione peroxidase catalyzes the reaction of hydroperoxides with reduced glutathione to form glutathione disulphide and the reduction product of the hydroperoxide [66]. The glutathione-S-transferase is a group of isoenzyme is capable of detoxifying various endogenous and exogenous substances by conjugating reduced glutarhione. In this context, the decreased activities of superoxide dismutase, catalase and glutathione peroxidase and Glutathione-S-transferase were observed in tissues of Fe-treated rats. Hesperidin offers protection against oxidative damage due to the ability of enhanced antioxidant activity [67].
The non-enzymatic antioxidants such as vitamin C, vitamin E and reduced glutathione are closely interlinked with each other and play an excellent role in protecting the cell from lipid peroxidation [68]. Vitamin C is a naturally occurring free radical scavenger which decreases free radical ability and lipid peroxidation sequence [69]. It regenerates membrane bound alpha-tocopherol radical and removes the radical from the lipid to the aqueous phase. It also protect tissues from lipid peroxidation both in vivo and in vitro [70]. Vitamin E is the most important lipo soluble antioxidant [71] and has the potential to improve tolerance of iron supplementation and prevent further tissue damage. Excess iron imbalances their levels with excess ROS production thus resulting oxidative stress, followed by peroxidative decomposition of cellular membrane lipids which is a postulated mechanism of hepatocellular injury in iron overload [72]. Vitamin E scavenges ROS, such as peroxyl radicals and suppresses lipid peroxidation [73]. The tripeptide GSH is an important endogenous antioxidant which has a major role in restoring other free radical scavengers and antioxidants such as vitamin C and E to their reduced state [71], [74]. A number of researchers have examined the antioxidant activity and radical scavenging properties of hesperidin using a variety of assay systems [75], [76], [77]. Treatment with hesperidin in iron-intoxicated rats protects the depletion of non-enzymatic antioxidants via its metal-chelating and antioxidant property [78] and may minimize the usage of these antioxidants, thus restoring their levels.
In the present study, the hepatic histoarchitecture of the iron treated rats resulted in focal necrosis, inflammatory cell infiltration and giant cell formation. It might be due to the formation of highly reactive radicals because of oxidative threat induced by iron. The accumulated hydroperoxides can cause cytotoxicity, which is associated with peroxidation of membrane phospholipids by lipid hydro peroxides, the basis for cellular damage. The necrotic conditions coincide with our biochemical studies, which show increased levels of lipid peroxidation. Administration of hesperidin reduced the histological alterations induced by iron. It can be attributed to the antioxidant and chelating ability of hesperidin, which significantly reduced the oxidative threat leading to reduction of pathological changes and restoration of normal physiological functions.
Histopathological observations in the kidney showed that Fe induced multiple foci of hemorrhage, necrosis and cloudy swelling of the tubules. The accumulation of Fe and its contents in the tissues is the basis for cellular damage. It is well established that the free radicals and intermediate products of peroxidation are capable of damaging the membrane integrity and altering their function, which can lead to the development of various pathological processes. Fe preferentially binds to the membrane and disturbs the redox state of the cells. Hence, the long retention of Fe in the tissues and increased oxidative state promoted by Fe might lead to a collapse in membrane integrity and other pathological changes in liver and kidney.
In conclusion, our results indicates that HDN may play a protective role in reducing the toxic effects of Fe-induced oxidative damage in liver and kidney, which could be due to its antioxidant potential by scavenging the free radicals. The present study therefore provides biological evident supporting the efficacy of HDN against Fe-induced toxicity in rats.
Transparency document
Transparency document
References
- 1.Unak P., Lambrecht F.Y., Biber F.Z., Darcan S. Iodine measurements by isotope dilution analysis in drinking water in Western Turkey. J. Radio Anal. Nucl. Chem. 2007;273:649–665. [Google Scholar]
- 2.Jamali M.K., Kazi T.G., Arain M.B., Afridi H.I., Jalbani N., Sarfraz R.A., Baig J.A. A multivariate study: variation in uptake of trace and toxic elements by various varieties of Sorghum bicolor L. J. Hazard. Mater. 2008;158:644–651. doi: 10.1016/j.jhazmat.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Centeno Jose A., Mullick Florabel G., Ishak Kamal G., Franks Teri J., Burke Allen P., Koss Michael N., Perl Daniel P., Tchounwou Paul B., Pestaner Joseph P., Selinus O., editors. Environmental pathology, Essentials of Medical Geology: Revised Edition. Springer Science+Business Media Dordrecht; 2013. [Google Scholar]
- 4.Pulla Reddy A.C., Lokesh B.R. Effect of curcumin and eugenol on iron-induced hepatic toxicity in rats. Toxicology. 1996;1073:9–45. doi: 10.1016/0300-483x(95)03199-p. [DOI] [PubMed] [Google Scholar]
- 5.Ward F.M. Daly Hepatic disease. In: Walker R., Edwards C., editors. Clinical Pharmacy and Therapeutics. Churchill Livingstone; New York: 1999. pp. 195–212. [Google Scholar]
- 6.Papanastasiou D.A., Vayenas D.V., Vassilopoulos A., Repanti M. Concentration of iron and distribution of iron and transferrin after experimental iron overload in rat tissues in vivo: study of the liver, the spleen, the central nervous system and other organs. Pathol. Res. Pract. 2000;196:47–54. doi: 10.1016/S0344-0338(00)80021-7. [DOI] [PubMed] [Google Scholar]
- 7.Madhusudhan K.S., Oberoi R. Renal iron deposition in aplastic anemia: magnetic resonance imaging appearance. Indian J. Nephrol. 2011;21:134–135. doi: 10.4103/0971-4065.82145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman L.S., Gilman A. 11th ed. McGraw-Hill; New York: 2006. The Pharmacological Basis of Therapeutics. [Google Scholar]
- 9.Bridges K.R., Hoffman K.E. The effects of ascorbic acid on the intracellular metabolism of iron and ferritin. J. Biol. Chem. 1986;261:14273–14277. [PubMed] [Google Scholar]
- 10.Pardo-Andreu G.L., Barrios C. Curtietal Protective effects of Mangifera indica L. extract (Vimang), and its major component mangiferin, on iron-induced oxidative damage to rat serum and liver. Pharmacol. Res. 2008;57:5779–5786. doi: 10.1016/j.phrs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 12.Shackelford R.E., Manuszak R.P., Johnson C.D. Iron chelators increase the resistance of Ataxia telangeictasia cells to oxidative stress. DNA Repair (Amst.) 2004;3:1263–1272. doi: 10.1016/j.dnarep.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Suarez J., Marhuenda Herrera M.D. In vitro scavenger and antioxidant properties of hesperidin and neohesperidin dihydrochalcone. Phytomedicine. 1998;5:469–473. doi: 10.1016/S0944-7113(98)80044-5. [DOI] [PubMed] [Google Scholar]
- 14.Hussein M., Othman S. Structure activity relationship of antioxidative property of hesperidin. Int. J. Pharm. Dev. 2011;3:19–29. [Google Scholar]
- 15.Chanet A., Milenkovic D., Manach C., Mazur A., Morand C. Citrus flavanones: what is their role in cardiovascular protection. J. Agric. Food Chem. 2012;60:8809–8822. doi: 10.1021/jf300669s. [DOI] [PubMed] [Google Scholar]
- 16.Rosalki S.B., Rav D., Lehman D., Prentice M. Determination of serum gamma-glutamyl trans peptidase activity and its clinical applications. Ann. Clin. Biochem. 1970;7:143–147. [Google Scholar]
- 17.Malloy E., Evelyn K. The determination of bilirubin with the photoelectric colorimeter. J. Biol. Chem. 1937;119:481–487. [Google Scholar]
- 18.Drabkin D.L., Austin J.M. Spectrophotometric constants for common haemoglobin derivatives in human, dog and rabbit blood. J. Biol. Chem. 1932;98:719–733. [Google Scholar]
- 19.Folch J., Lees M., Sloane S.G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 20.Zlatkis A., Zak B., Boyle A.J. A new method for the direct determination of serum cholesterol. J. Lab. Clin. Med. 1953;41:486–492. [PubMed] [Google Scholar]
- 21.Fossati P., Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- 22.Falholt K., Lund B., Falholt W. An easy colorimetric micromethod for routine determination of free fatty acids in plasma. Clin. Chim. Acta. 1973;46:105–111. doi: 10.1016/0009-8981(73)90016-8. [DOI] [PubMed] [Google Scholar]
- 23.Zilversmit B.B., Davis A.K. Micro determination of plasma phospholipids by trichloroacetic acid precipitation. J. Lab. Clin. Med. 1950;35:155–160. [PubMed] [Google Scholar]
- 24.Niehius W.G., Samuelson B. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Z.Y., Hunt J.V., Wolff S.D. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low-density lipoprotein. Anal. Biochem. 1992;202:384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- 26.Kakkar P., Das B., Viswanathan P.N. A modified spectroscopic assay of superoxide dismustase. Indian J. Biochem. Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 27.Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 28.Rotruck J.T., Pope A.L., Ganther H.E. Selenium, biochemical role as a component of glutathione peroxidase purification assay. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 29.Habig W.H., Pabst M.J., Jakpoby W.B. Glutathione transferase, a first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 30.Omaye S.T., Turnbull J.D., Sauberlich H.E. Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol. 1979;62:1–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- 31.Desai I.D. Vitamin E analysis method for animal tissues. Methods Enzymol. 1984;105:138–143. doi: 10.1016/s0076-6879(84)05019-9. [DOI] [PubMed] [Google Scholar]
- 32.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 33.Suzumura K., Hashimura Y., Kubota H. Antioxidative property of T-0970, a new ureido phenol derivative. Free Rad. Res. 2000;32:255–264. doi: 10.1080/10715760000300261. [DOI] [PubMed] [Google Scholar]
- 34.Manjunatha H., Srinivasan K. Protective effect of dietary curcumin and capsaicin on induced oxidation of low-density lipoprotein, iron-induced hepatotoxicity and carrageenan-induced inflammation in experimental rats. J. FEBS. 2006;73:4528–4537. doi: 10.1111/j.1742-4658.2006.05458.x. [DOI] [PubMed] [Google Scholar]
- 35.Shohda A., Maraghy E.L., Sherine M. Hepatoprotective potential of crocin and curcumin against iron overload-induced biochemical alterations in rat. AJBR. 2009;3:215–221. [Google Scholar]
- 36.Gaskill C.L., Miller L.M., Mattoon J.S., Hoffmann W.E. Liver histopathology and liver serum alanine aminotransferase and alkaline phosphatase activities in epileptic dogs receiving Phenobarbital. Vet. Pathol. 2005;42:147–160. doi: 10.1354/vp.42-2-147. [DOI] [PubMed] [Google Scholar]
- 37.Drozdz R., Parmentier C., Hachad H. Glutamyltransferase dependent generation of reactive oxygen species from a glutathione/transferrin system. Free Radic. Biol. Med. 1998;25:786–792. doi: 10.1016/s0891-5849(98)00127-0. [DOI] [PubMed] [Google Scholar]
- 38.Whitfield J.B. Gamma glutamyl transferase. Crit. Rev. Clin. Lab. Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 39.Lee D.H., Blomhoff R., Jacobs D.R. Is serum gamma glutamyltransferase a marker of oxidative stress. Free Radic. Res. 2004;38:535–539. doi: 10.1080/10715760410001694026. [DOI] [PubMed] [Google Scholar]
- 40.Horie Y., Miyaji M., Yokoyama K., Udagawa S.I. Neosartorya tatenoi, a new species from Brazilian soil. Trans. Mycol. Soc. Jap. 1992;33:395–399. [Google Scholar]
- 41.Chanet A., Milenkovic D., Manach C., Mazur A., Morand C. Citrus flavanones: what is their role in cardiovascular protection. J. Agric. Food Chem. 2012;60:8809–8822. doi: 10.1021/jf300669s. [DOI] [PubMed] [Google Scholar]
- 42.Kojima R., Randall J.D., Ho E., Manshio H., Suzuki Y., Gullans S.R. Regulation of expression of the stress response gene. Biochem. J. 2004;380:783–794. doi: 10.1042/BJ20040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howard B.V., Kritchevsky D. Phytochemicals and cardiovascular disease. A statement for healthcare professionals from American heart association. Circulation. 1997;95:2591–2593. doi: 10.1161/01.cir.95.11.2591. [DOI] [PubMed] [Google Scholar]
- 44.Tribble D.L., Jones D.P. The pathophysiological significance of lipid peroxidation in oxidative cell injury. Hepatology. 1987;7:377–386. doi: 10.1002/hep.1840070227. [DOI] [PubMed] [Google Scholar]
- 45.Comporti M. Lipid peroxidation and cellular damage in toxic liver injury. Lab. Invest. 1985;53:599–623. [PubMed] [Google Scholar]
- 46.Bonkovsky H.L. Iron and the liver. Am. J. Med. Sci. 1991;301:32–43. doi: 10.1097/00000441-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Hershko C., Link G., Cabantchik I. Pathophysiology of iron overload. Ann. N.Y. Acad. Sci. 1998;850:191–201. doi: 10.1111/j.1749-6632.1998.tb10475.x. [DOI] [PubMed] [Google Scholar]
- 48.Harris W.R. Marcel Dekker, Inc.; New York: 2002. Iron chemistry in Molecular and Cellular Iron Transport. Templeton (Edn) pp. 1–40. [Google Scholar]
- 49.Halliwell B., Gutteridge J.M.C. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990;186:1–88. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 50.Halliwell B., Gutteridge J.M.C. Biologically relevant metal ion dependent hydroxyl radical generation. An update. FEBS Lett. 1992;307:108–112. doi: 10.1016/0014-5793(92)80911-y. [DOI] [PubMed] [Google Scholar]
- 51.Aust S.D., Marehouse L.A., Thomas C.E. Role of metals in oxygen radical reactions. J. Free Radic. Biol. Med. 1985;1:3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- 52.Valko M., Morris H., Cronin M.T. Metal, toxicity and oxidative. Curr. Med. Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 53.Sayre L.M., Moreira P.I., Smith M.A., Perry G. Metal ions and oxidative protein modification in neurological disease. Ann. Ist. Super Sanita. 2005;41:143–164. [PubMed] [Google Scholar]
- 54.Butterfield D.A., Kanski J. Brain protein oxidation in age related neuro degenerative disorders that are associated with aggregated proteins. Mech. Ageing Dev. 2001;122:945–962. doi: 10.1016/s0047-6374(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 55.Chen L., Zhang B.H. Evidence suggesting that nitric oxide mediates iron-induced toxicity in cultured proximal tubule cells. Am. J. Physiol. Ren. 1998;274:18–25. doi: 10.1152/ajprenal.1998.274.1.F18. [DOI] [PubMed] [Google Scholar]
- 56.Chen L., Wang Y. Molecular mechanisms by which iron induces nitric oxide synthesis in cultured proximal tubule cells. Exp. Nephrol. 2001;9:198–204. doi: 10.1159/000052612. [DOI] [PubMed] [Google Scholar]
- 57.Toyokuni S. Iron and carcinogenesis: from Fenton reaction to target gene. Redox Rep. 2002;7:189–197. doi: 10.1179/135100002125000596. [DOI] [PubMed] [Google Scholar]
- 58.Rothman R.J., Serroni A., Farber J.L. Cellular pool of transient ferric iron, chelatable by deferoxamine and distinct from ferritin that is involved in oxidative cell injury. Mol. Pharmacol. 1992;42:703–710. [PubMed] [Google Scholar]
- 59.Wilmsen P.K., Spada D.S., Salvador M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J. Agric. Food Chem. 2005;53:4757–4761. doi: 10.1021/jf0502000. [DOI] [PubMed] [Google Scholar]
- 60.Kannampalli P., Sang H.P., Kyong C.K. Hesperidin a flavanoglycone protects against γ-irradiation induced hepatocellular damage and oxidative stress in Sprague Dawley rats. Eur. J. Pharmacol. 2008;587:273–280. doi: 10.1016/j.ejphar.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 61.Sies H. Oxidative stress, oxidants and antioxidants. Exp. Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 62.Veerappan R.M., Senthil S., Rao M.R., Ravikumar R. Redox status and lipid peroxidation in alcoholic hypertensive patients and alcoholic hypertensive patients with diabetes. Clin. Chem. Acta. 2004;340:207–212. doi: 10.1016/j.cccn.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Mccord J.M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 64.Deisseroth A., Dounce A.L. Catalase: physical and chemical properties, mechanism of catalysis. Physiological role. Physiol. Rev. 1970;50:319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- 65.Deneke S.M., Fanburg B.L. Regulation of cellular glutathione. Am. J. Physiol. Lung Cell Mol. Physiol. 1989;257:163–173. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- 66.Eaton J.W. Catalase, glutathione peroxidase and hydrogen peroxidase. J. Clin. Lab. Med. 1991;118:3–4. [Google Scholar]
- 67.Tirkey N., Pilkhwal S., Kuhad A., Chopra K. Hesperidin, a citrus bioflavonoid, and decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol. 2005;5:2. doi: 10.1186/1471-2210-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winker B.S. Unequivocal evidence in support of non enzymatic redox coupling between glutathione/glutathione disulphide and ascorbic acid/dehydroascorbic acid. Biophys. Acta. 1992;117:287–290. doi: 10.1016/0304-4165(92)90026-q. [DOI] [PubMed] [Google Scholar]
- 69.Choi S.W., Benzic I.F.F., Collins A.R., Hannigan B.M. Vitamin C and E: acute interactive effects on biomarkers of antioxidant defence and oxidative stress. Mut. Res. 2004;551:109–117. doi: 10.1016/j.mrfmmm.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Mccay P.B. Vitamin E, interactions with free radicals and ascorbate. Ann. Rev. Nutr. 1985;5:323–340. doi: 10.1146/annurev.nu.05.070185.001543. [DOI] [PubMed] [Google Scholar]
- 71.Burton G.W., Ingold K.U. Vitamin E: application of the principles of physical organic chemistry to the exploration of structure and function. Acc. Chem. Res. 1986;19:194–201. [Google Scholar]
- 72.Bonkowsky H.L., Healey J.F., Sinclair P.R. Iron and the liver. Acute and long-term effects of iron-loading on hepatic haem metabolism. Biochem. J. 1981;196:57–64. doi: 10.1042/bj1960057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burton G.W., Joyce A., Ingold K.U. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch. Biochem. Biophys. 1983;221:281–290. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- 74.Gaganjit K., Naveen T., Kanwaljit C. Beneficial effect of hesperidin on lipopolysaccharide-induced hepatotoxicity. Toxicol. 2006;226:152–160. doi: 10.1016/j.tox.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 75.Jovanovic S.V., Steeden S., Tosic M. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994;116:4846–4851. [Google Scholar]
- 76.Fraga C.G., Martino V.S., Ferraro G.E., Coussio J.D., Boveris A. Flavonoids as antioxidants evaluated by in vitro and in situ liver chemiluminescence. Biochem. Pharmacol. 1987;36:717–720. doi: 10.1016/0006-2952(87)90724-6. [DOI] [PubMed] [Google Scholar]
- 77.Miller N.J., Rice-Evans. C.A. The relative contribution of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange, apple fruit juices and black currant drink. Food Chem. 1997;60:331–337. [Google Scholar]
- 78.Cody V., Middleton E., Harbone H.B. Alan E Liss; New York: 1986. Plant Flavonoids in Biology and Medicine-Biochemical, Pharmacological and Structure activity Relationships. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document