Abstract
This study aimed to investigate potential acute and subchronic toxicity of rhodium (II) citrate in female Balb/c mice after intraperitoneal injections. In the acute test, independent groups received five doses; the highest dose (107.5 mg/kg) was equivalent to 33 times that used in our previous reports. The other doses were chosen as proportions of the highest, being 80.7 (75%), 53.8 (50%), 26.9 (25%) or 13.8 mg/kg (12.5%). Animals were monitored over 38 days and no severe signs of toxicity were observed, according to mortality, monitoring of adverse symptoms, hematological, biochemical and genotoxic parameters. We conclude that the median lethal dose (LD50) could be greater than 107.5 mg/kg. In the subchronic test, five doses of Rh2Cit (80, 60, 40, 20 or 10 mg/kg) were evaluated and injections were conducted on alternate days, totaling five applications per animal. Paclitaxel (57.5 mg/kg) and saline solution were controls. Clinical observations, histopathology of liver, lung and kidneys and effects on hematological, biochemistry and genotoxic records indicated that Rh2Cit induced no severe toxic effects, even at an accumulated dose up to 400 mg/kg.We suggest Rh2Cit has great potential as an antitumor drug without presenting acute and subchronic toxicity.
Keywords: Histopathology, Biochemical and hematological analysis, Genotoxicity, Toxicological analysis, lethal dose, Acute and subchronic toxicity
1. Introduction
The use of metals or metal containing compounds in the therapeutics of cancer has been reported since the sixteenth century. From the 1960s onwards, with the discovery of cisplatin, many researchers have investigated the anticancer properties of a large number of other metal complexes. Platinum-based drugs have revolutionized the treatment of testicular and ovarian solid tumors, despite inducing high toxicity and cell chemoresistance [22], [36]. Interestingly, rhodium carboxylates, a class of metal complexes, have presented promising antitumor activity in cisplatin-resistant cell lines. These complexes showed cytostatic activity in tumors L1210, Ehrlich ascites carcinoma [2], [51], [40] sarcoma 180 and P388 and melanoma B16 [19], [20]. However, further investigations have discouraged researchers due to their toxicity in normal cells [17]. Recent structural studies suggest that the antitumor activity of dirhodium (II) carboxylates may occur by binding to adjacent guanines in DNA, in a similar manner to cisplatin [22]. Others studies indicated that the effect of rhodium (II) acetate, propionate and methoxyacetate is to irreversibly inhibit all enzymes that have a sulfhydryl group in or near their activity site [19], [20].
Among the class of rhodium carboxylates, rhodium (II) citrate (Rh2Cit) presented antitumor, cytotoxic, and cytostatic activity on Ehrlich ascites carcinoma. Prominently, this complex has shown itself to be a promising antitumor agent for chemotherapy since it induces lower toxicity to normal cells when compared to analogous rhodium carboxylates [50], [51]. Rh2Cit structure is similar to that of other rhodium (II) carboxylates, with dinuclear Rh (II) ions bound by four bridging citrate ligands [9]. This complex has a molecular weight of 941.74 and solubility of 7 mg/mL at room temperature [1]. Recently, our research group has demonstrated that Rh2Cit induced significant cytotoxic effects on 4T1 and MCF-7 mammary carcinoma cell lines [6], [7], and it presented antitumor effect on an orthotopic 4T1 carcinoma model after intratumoral and intravenous administration in Balb/c mice [6], [7], [37], [38]. Although these studies have demonstrated the great potential of these complexes, there are limited reports about their toxicity. In order to explore the prospects for Rh2Cit in biomedical applications, we designed the present study to assess its acute and subchronic toxicity profiles in mice and, thus, to obtain safety information regarding its use as a therapeutic compound.
It is important that promising drugs be widely evaluated, in particular to allow the development of procedures and practices, which can prevent further injury and/or disease as well as lead to the introduction of more effective and safer therapies for patients [39]. Pre-clinical tests involving animal models are fundamental to test a new therapeutic drug since they have been validated as relatively accurate predictors of hazards to which humans may be exposed [41], [23], [42].
Adverse drug effects related to toxicity depend on many parameters such as dose, route of administration and duration of exposure. Acute toxicity, for instance, can be determined by the effects of a single exposure to a substance, and subchronic toxicity evaluation is useful to determine the presence of toxic effects after repeated exposure and extended duration. On the other hand, it is important to investigate the reactions of animals after repeated administrations because this approach can enhance the clinical efficacy, but it can also cause toxicity and severe reactions [5], [41]. Drug toxicity often manifests in bone marrow, causing a range of serious side effects, including leucopenia (especially neutropenia), bone marrow suppression (especially myelosupression) or immunotoxicity. Thus, the assessment of the potentially toxic impact of new compounds on bone marrow is important during drug discovery, since drug toxicity often manifests in this tissue [39].
Thereby, the choice of therapeutic strategy must consider clinical signs of toxicity (such as pain or discomfort in animals), hematological and biochemical information, pathological observations in tissues and organs and potential evaluation of genotoxicity. These toxicological evaluations may predict potential adverse effects of a studied drug in humans [41] . In this context, the aim of this study was to investigate the acute and subchronic toxicity induced by Rh2Cit in female Balb/c mice through its effects on the mortality, clinical status, gross and microscopic pathology, hematological and biochemical parameters and its genotoxic potential in the bone marrow.
2. Materials and methods
2.1. Preparation of rhodium (II) citrate
Rhodium (II) citrate (Rh2Cit) was prepared and characterized as previously described. Briefly, Rh2Cit was synthesized by exchanging trifluoroacetate ligands from the precursor rhodium (II) trifluoroacetate with citrate ligands. The compound was obtained as a green aqueous solution with a standardized concentration of 0.054 mol/L.
2.2. Animals
Ninety female Balb/c mice (12 weeks old) were purchased from the Multidisciplinary Center for Biological Investigation on Laboratory Animal Science (Cemib) of the State University of Campinas (Unicamp, SP/Brazil). Upon arrival, all animals were examined for health condition to confirm their suitability for study. All mice were housed in polycarbonate cages with ventilation under standard conditions (temperature: 23 ± 3 °C; relative humidity: 50 ± 10%) and 12 h dark/light cycle. After an acclimatization period of 20 days, the animals, with ages of 12–16 weeks and a weight of 25.6 ± 2.02 g, were randomly distributed in treatment groups (n = 6 or 8/per group). The animals had free access to water and food.
All care and procedures were conducted according to the guidelines of the Animal Research Ethics Committee of the University of Brasilia — Institute of Biological Sciences, Brazil (process no 109,434/2008).
2.3. Experimental design: treatment and conduct of animals in acute and subchronic toxicity experiments
The antitumor effect of free Rh2Cit on mice bearing breast cancer has been demonstrated in our previous articles by two routes: intratumoral [6], [7] and intravenous [37]. The best results were obtained by the intratumoral route. In this present study, we accessed the intraperitoneal route (i.p.) because it is the most similar route to on-site therapies (intramammary injection) in relation to pharmacokinetic parameters, such as absorption, distribution and elimination. Direct administration of cytotoxic drugs into the peritoneal cavity is a strategy designed to enhance locoregional drug delivery while abrogating systemic toxicities. Furthermore, this route allows concentrations to be attained at the site of the tumor that are many times higher than would be tolerated in the systemic circulation; these can easily exceed concentrations shown in vitro to be required to overcome clinical drug resistance. This strategy has been developed and described in several Phase 1 clinical trials, especially for ovarian tumors and peritoneal metastasis, with wide extended action and reduced side effects.
3. Animal treatment
3.1. Acute toxicity
A total of 42 mice were assigned randomly to six groups of eight animals each. The rhodium (II) citrate (Rh2Cit) solution was injected via intraperitoneal route in mice in a single dose containing 107.5 mg/kg Rh2Cit or proportional doses of it as 80.7 (75%), 53.8 (50%), 26.9 (25%) or 13.8 (12.5%), while the control group was exposed to saline solution (0.9% w/v).
3.2. Subchronic toxicity
A total of 42 mice were assigned randomly to seven groups of six animals each. The mice were treated with 300 μL of solution containing different concentrations of Rh2Cit (80, 60, 40, and 20 or 10 mg/kg) or paclitaxel (57.8 mg/kg, equivalent to clinical dose used in humans). The negative control group was injected with the same volume (300 μL) of saline solution (0.9% w/v). The mice of Rh2Cit or saline experimental groups received repeated doses via intraperitoneal injections every two days, totalizing five injections, and the total maximum accumulated dose of Rh2Cit was 400 mg/Kg. The mice treated with paclitaxel received only two injections during all the experimental period (5th and 28th day), totalizing an accumulated dose of 115.6 mg/kg.
3.3. Clinical sign observation
After the medication, clinical symptoms indicative of changes in normality with respect to eyes, diarrhea, skin ulcers, abdominal cramps, hyper/hypoactivity, lethargy, neurological behavior (changes in motor activity) and/or deaths were recorded daily.
For the acute toxicity assay, the general behavior of mice and signs of toxicity were observed continuously for two hours after injection. Further, the mice were monitored once a day up to 38 days, when the animals were euthanized. The body weights and food consumption were monitored on the first (day 0) and final experimental day (day 38).
For the subchronic toxicity experiment, mice were closely monitored every day for any behavioral changes, toxicity signs and lethality rate from dosing (day 0) to the end of the experiment (day 44). Body weights and food consumption were recorded on the first day of the study (day 0) prior to dosing and then 11, 25, 32 and 43 days thereafter.
3.4. Toxicity analysis
For the analysis of both acute and chronic toxicity induced by Rh2Cit, at the end of the experiments (days 38 and 44, respectively), the mice were anesthetized with ketamine (80 mg/kg) and xylazine (10 mg/kg) by the intraperitoneal route. Then, blood samples (1 mL) were collected by intracardiac puncture to carry out the hemogram and biochemical dosages of serum glutamic pyruvic transaminase (GPT, also known as alanine aminotransferase or ALT) and creatinine. Afterwards, animals were immediately euthanized by cervical dislocation, according to the American Veterinary Medical Association (AVMA) guidelines. Subsequently, lungs, kidneys and liver were surgically collected and processed for the histological analyses, and bone marrow was removed for genotoxicity analyses (DNA fragmentation and cell cycle).
3.5. Hematological parameters
The blood samples, collected in microtubes containing EDTA, were analyzed using an auto-hematology analyzer (XZ 2100 Sysmex equipment) for white blood cells (WBC), red blood cells (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and platelets (PLT) analysis.
3.6. Serum biochemistry parameters
For the biochemical analyses, blood samples collected without EDTA were transferred to vacutainer tubes and centrifuged at 3000 rpm for five minutes. Then, GPT and creatinine levels were determined in the automated chemistry analyzer ADVIA 2400 (Siemens), using the appropriate ADVIA reagents, protocols and controls.
3.7. Bone marrow genotoxicity analysis
In order to evaluate genotoxicity potential induced by Rh2Cit, bone marrow cells (BM) were collected from femurs and resuspended in one milliliter of fetal bovine serum (FBS, Gibco). These cells were used to perform DNA fragmentation and cell cycle analyses by flow cytometry, which is a rapid detection method of DNA damage and interference with cell mitosis caused by several agents. In this experimental approach, fluorochromes (e.g., propidium iodide) interact with DNA molecules present in the cell in a stoichiometric basis; i.e., DNA from cells in G2 phase (4n) will have proportionally more dye and will fluoresce more brightly than DNA from G1 phase cells (2n) or fragmented DNA (<2n). Thus, the proportion of cells in each cell cycle phase or the presence of fragmented DNA can be classified according to the fluorescent bright pattern of the analyzed samples [10], [11].
Briefly, cells were fixed in cold 70% ethanol, and stored overnight at −30 °C. Afterwards, the cells were centrifuged and incubated with 300 μL of lysis buffer (0.1% sodium citrate, 0.1% Triton X-100 and 20 μg/mL of propidium iodide, diluted in PBS pH 7.4) for 30 min at room temperature and protected from light. DNA fragmentation and cell cycle were analyzed using a FACSCalibur® flow cytometry (Becton & Dickenson, USA) and a total of 10,000 events were collected per sample.
3.8. Histopathological analysis
Firstly, liver, lung and kidneys were macroscopically examined in respect to their color, consistency and size. Histopathology analysis of these organs was also performed in order to verify possible toxic effects induced by treatments, to examine general aspects of organ preservation, as well as evaluations of presence of inflammatory infiltrate (all organs), nuclear pyknosis (all organs), cell desquamation (kidneys) and other histological pathologies. Thus, tissues were fixed in paraformaldehyde (4% at room temperature) for 24 h, transferred to 70% ethanol and included in paraffin using an automatic tissue processor (OMA® DM-40, São Paulo, Brazil). Then, sections were cut to 5 μm of thickness in a Leica RM2235 manual microtome (Leica Microsystems, Nussloch, Germany) and stained with hematoxilin-eosin (HE) for histological analyses (light microscopy). Sections were photographed with an MC 80 DX camera coupled to a Zeiss Axiophot light microscope (Carl Zeiss).
In addition to the evaluated general aspects of parenchyma integrity, and with the aim of making the examination more objective, sensitive and accurate, some morphometric parameters (Table 1) were measured by means of image analysis using the software Image Pro-Plus 6.0 (Media Cybernetics, Silver Spring, USA). Proper calibration of the digital tools was performed before each analysis. Details of sampling are described along with the respective results.
Table 1.
Histomorphometric parameters analyzed in mice for the assessment of eventual toxicity of rhodium (II) citrate subchronic treatment.
| Organ | Parameters |
|---|---|
| Kidney | Number of glomeruli Area of the Bowman’s capsule interspace (μm2) |
| Liver | Area of the central vein (μm2) Number of infiltrating mononuclear cells |
| Lung | Area of the alveolar air space (μm2) Thickness of the alveolar wall (μm) |
3.9. Statistical analysis
Statistical analysis was carried out using the SPSS (Statistical Package for the Social Sciences) version 17.0 and Prism version 5.0 softwares. Data were expressed as mean ± SEM (standard error of mean) and values of p < 0.05 were considered statistically significant. Quantitative variables were tested for normal distribution with the Shapiro–Wilk test. Possible differences among groups were investigated by performing ANOVA or the Kruskal–Wallis test (data not normally distributed), followed respectively by Bonferroni’s or Dunn’s multiple comparison tests. The Wilcoxon test (data not normally distributed) was used to verify differences between initial and final body weight inside each group. For the analyses of body weight over time (subchronic toxicity), the Friedman Test was used, followed by the Wilcoxon Test (2-to-2 comparisons).
4. Results
4.1. Clinical signs and mortality
In Table 2, the ratio of animals with clinical signs and the mortality after the different treatments are shown. According to the acute toxicity analysis, the animals that received Rh2Cit had no severe signs of toxicity or mortality in any experimental group. This indicates that the median lethal dose (LD50) of rhodium citrate is more than 107.5 mg/kg. Although animals from the group treated with the single dose of 107.5 mg/kg (the highest dose) presented clinical symptoms such as lethargy and mild abdominal cramps in the first two hours after the treatments, these reactions were transitory. During this period, we observed, in the 80.7 mg/Kg Rh2Cit and 53.8 mg/Kg Rh2Cit groups, that the treatment caused hyperactivity in almost all the mice, and one animal in the 53.8 mg/Kg Rh2Cit group had hypoactivity. However, these clinical signs of toxicity and/or abnormalities in neurological function were not observed thereafter until day 38 (end of the experimental period).
Table 2.
The ratio of animals with clinical signs and the mortality after different treatments.
| Treatment | N animals | Animals with clinical sign/treated animals | Died animals/treated animals | |
|---|---|---|---|---|
| Acute toxicity | Negative control | 8 | 0/8 | 0/8 |
| Rh2Cit 107.57 mg/Kg | 8 | 5/8 | 0/8 | |
| Rh2Cit 80.7 mg/Kg | 8 | 6/8 | 0/8 | |
| Rh2Cit 53.8 mg/Kg | 8 | 6/8 | 0/8 | |
| Rh2Cit 26.9 mg/Kg | 8 | 0/8 | 0/8 | |
| Rh2Cit 13.8 mg/Kg | 8 | 1/8 | 0/8 | |
| Subchronic toxicity | Negative control | 6 | 0/6 | 0/6 |
| Rh2Cit 80 mg/Kg | 6 | 0/6 | 0/6 | |
| Rh2Cit 60 mg/Kg | 6 | 0/6 | 0/6 | |
| Rh2Cit 40 mg/Kg | 6 | 0/6 | 0/6 | |
| Rh2Cit 20 mg/Kg | 6 | 0/6 | 0/6 | |
| Rh2Cit 10 mg/Kg | 6 | 0/6 | 0/6 | |
| Paclitaxel 57.80 mg/Kg | 6 | 6/6 | 0/6 | |
In the subchronic toxicity study, no clinical signs were seen of systemic toxicity such as diarrhea, ataxia, spasms, bleeding, vomiting, dyspnea, lethargy, hypoactivity, sweating and appearance of spots or alopecia, or abnormalities in neurological functions after administration of Rh2Cit. However, in animals treated with paclitaxel (57.8 mg/kg), hypoactivity was observed during the two subsequent hours after treatment; and, three days after the first injection of paclitaxel, hair loss was noticed until the end of the experiment.
4.2. Body weight and food consumption
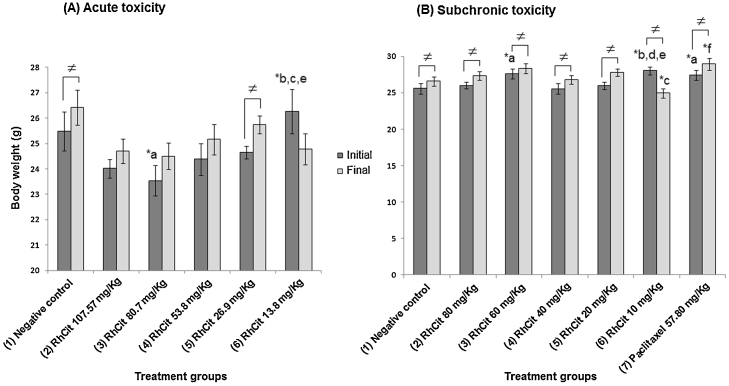
In relation to the acute toxicity study, we verified a significant increase in body weight of mice treated with saline (negative control) and 26.9 mg/kg Rh2Cit between the beginning (day 0) and the end of the experiment (day 38) (Fig. 1A). However, comparing the percentage of body weight gain or loss it was observed that all groups had an average increase of about 5% (Supplementary data 1). Food and water consumption was also not significantly different between the control and Rh2Cit tested groups (data not shown).
Fig. 1.
Body weight of the Balb/c female mice in the acute (A) and subchronic (B) toxicity tests of rhodium citrate (Rh2Cit) administration evaluated for 38 and 44 days respectively. Negative control received saline solution by intraperitoneal injection.
Data are presented as mean ± SEM (standard error of mean). The lowercase letters indicate significant differences detected by Dunn’s multiple comparison test, with a = significant compared to group 1; b = significant compared to group 2; c = significant compared to group 3; d = significant compared to group 4; e = significant compared to group 5; f = significant compared to group 5. Asterisks indicate significant differences at *p < 0.05. The Wilcoxon test was used to verify differences among the initial and final body weight inside each group (≠).
In the subchronic toxicity test, an average increase was observed in the final body weight (day 44) compared to the start of the experiment (day 0), except for the 10 mg/kg Rh2Cit group, which had a reduction in the final body weight of mice (Fig. 1B). In general, we observed an average gain in body weight of mice beneath 8%, except in the 10 mg/kg Rh2Cit group, which showed an average reduction of 11%, between day 0 and day 44 (Supplementary data 1). However, this group, like the other treated groups, showed no significant differences compared to the negative control at the end of the experiment (Fig. 1B).
4.3. Hematological analyses
As compared to the negative control, although some significant differences have been observed in the erythrogram of the acute and subchronic toxicity assays, values were inside the reference intervals (Table 3).
Table 3.
Erythrogram of the Balb/c female mice in the acute and subchronic toxicity tests of rhodium citrate (Rh2Cit). Negative control received saline solution by intraperitoneal injection.
| Treatment | Group | RBC (x106/μL) |
HGB (g/dL) |
HCT (%) |
MCV (fL) |
MCH (pg) |
MCHC (g/dL) |
|
|---|---|---|---|---|---|---|---|---|
| Acute toxicity | Negative control | 1 | 9.17 ± 0.17 | 14.78 ± 0.21 | 46.15 ± 0.45 | 50.37 ± 0.81 | 16.13 ± 0.24 | 32.05 ± 0.30 |
| RhCit 107.57 mg/Kg | 2 | 9.46 ± 0.12 | 14.52 ± 0.26 | 46.64 ± 0.84 | 49.32 ± 0.74 | 15.36 ± 0.28 | 31.10 ± 0.19 | |
| RhCit 80.7 mg/Kg | 3 | 9.29 ± 0.07 | 14.46 ± 0.12 | 46.44 ± 0.30 | 49.96 ± 0.28 | 15.56 ± 0.07 | 31.12 ± 0.17 | |
| RhCit 53.8 mg/Kg | 4 | 9.91 ± 0.10**a*c | 15.50 ± 0.16*bc | 47.90 ± 0.54 | 48.38 ± 0.06 | 15.65 ± 0.10 | 32.35 ± 0.21*bc | |
| RhCit 26.9 mg/Kg | 5 | 9.44 ± 0.13 | 14.56 ± 0.23*d | 45.52 ± 0.78 | 48.20 ± 0.28 | 15.42 ± 0.09*a | 32.00 ± 0.10 | |
| RhCit 13.8 mg/Kg | 6 | 9.62 ± 0.07 | 14.84 ± 0.07 | 46.20 ± 0.38 | 48.02 ± 0.26 | 15.44 ± 0.10*a | 32.14 ± 0.27 | |
| P-values | 0.007 | 0.017 | 0.192 | 0.017 | 0.091 | 0.001 | ||
| – | ||||||||
| Subchronic toxicity | Negative control | 1 | 9.14 ± 0.2 | 14.80 ± 0.25 | 45.88 ± 0.44 | 50.26 ± 0.98 | 16.20 ± 0.29 | 32.26 ± 0.26 |
| RhCit 80 mg/Kg | 2 | 9.38 ± 0.11 | 14.63 ± 0.17 | 44.85 ± 0.62 | 47.85 ± 0.72 | 15.63 ± 0.20 | 32.63 ± 0.09 | |
| RhCit 60 mg/Kg | 3 | 9.65 ± 0.17 | 14.86 ± 0.26 | 45.40 ± 0.70 | 47.10 ± 0.15**a | 15.42 ± 0.10 | 32.76 ± 0.22 | |
| RhCit 40 mg/Kg | 4 | 9.69 ± 0.11 | 15.04 ± 0.25 | 45.82 ± 0.61 | 47.28 ± 0.37**a | 15.52 ± 0.13 | 32.80 ± 0.21*a | |
| RhCit 20 mg/Kg | 5 | 9.44 ± 0.40 | 14.27 ± 0.58 | 44.50 ± 1.48 | 47.20 ± 0.64*a | 15.13 ± 0.09*a | 32.03 ± 0.28 | |
| RhCit 10 mg/Kg | 6 | 9.01 ± 0.20 | 13.77 ± 0.42*d | 42.23 ± 1.29 | 46.90 ± 0.7*a | 15.27 ± 0.24 | 32.60 ± 0.06 | |
| P-values | 0.131 | 0.288 | 0.069 | 0.035 | 0.016 | 0.209 | ||
Data are presented as mean ± SEM (standard error of mean). RBC = Red Blood Cells; HGB = Hemoglobin; HCT = Hematocrit; MCV = Mean Corpuscular volume; MCH = Mean Corpuscular hemoglobin; MCHC = Mean corpuscular hemoglobin concentration; g/dL = grams per deciliter; fl = fentoliters; pg = picograms. For the acute toxicity, p-value of MCH was generated by the Kruskall–Wallis test, while p-values of the other parameters were generated by ANOVA; for the chronic toxicity, RBC, HCT and MCH were generated by ANOVA, while p-values of the other parameters were generated by the Kruskall–Wallis test. The superscript letters indicate significant differences detected by Bonferroni’s or the Dunn’s multiple comparison tests, with a = significant compared to group 1; b = significant compared to group 2; c = significant compared to group 3; d = significant compared to group 4; e = significant compared to group 5. Asterisks indicate significant differences at *p < 0.05 and **p < 0.01.
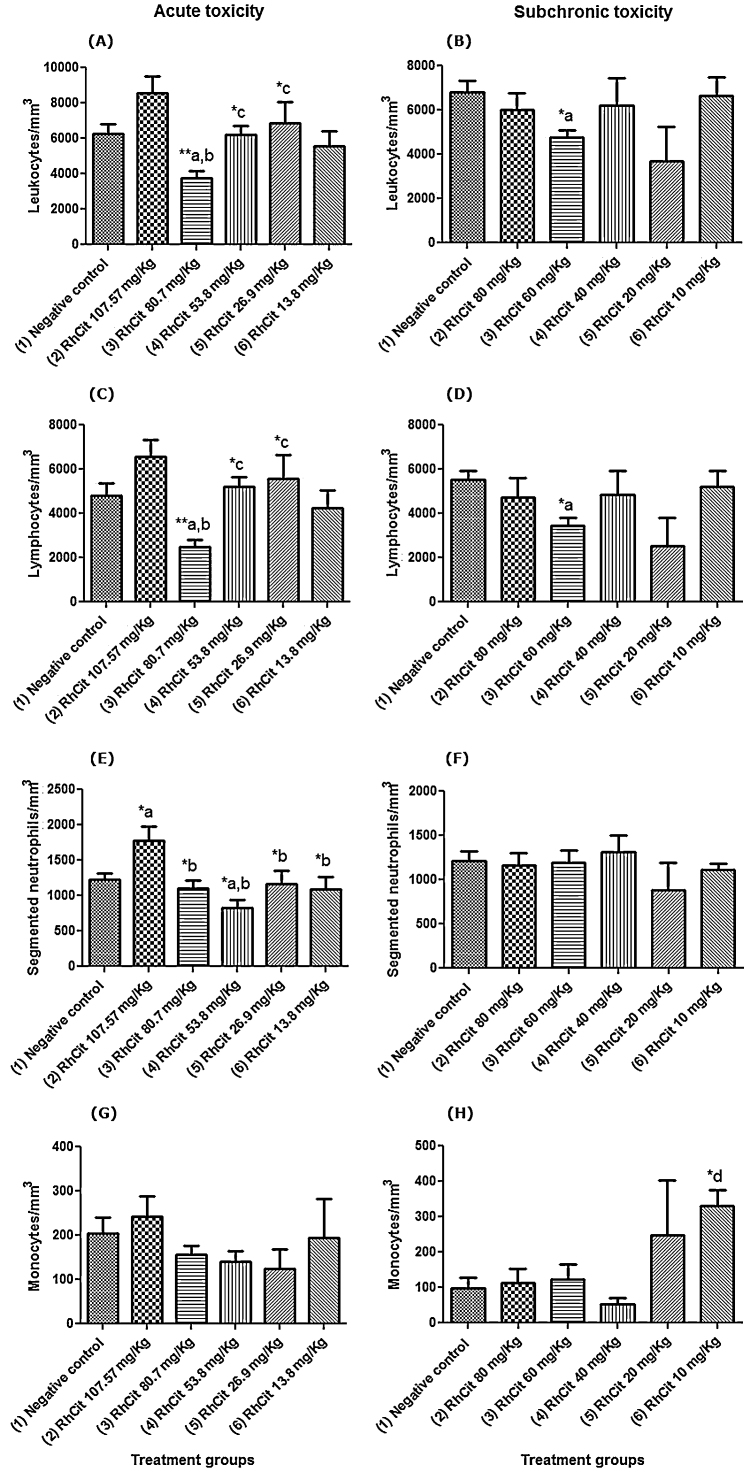
In respect to the leukogram of the acute toxicity tests, in the group treated with 80.7 mg/kg Rh2Cit, there was a significant decrease in the number of white blood cells (WBC) as compared to the negative control (saline group), which was mainly related to a reduction in lymphocytes, although inside the reference intervals [14]. The treatment with 100.57 mg/kg Rh2Cit promoted a significant increase in the number of segmented neutrophils, while that with 53.8 mg/kg Rh2Cit showed a reduction in the number of these cells, also inside the reference intervals for mice [14].
In the analyses of subchronic toxicity, the only group in which a significant reduction of the WBC occurred, also related to the lymphocyte number, was that treated with 60 mg/kg Rh2Cit. However, as in the acute toxicity, both WBC and lymphocyte quantities were inside the reference intervals for mice (Fig. 2) [14]. A significant increase was also observed in the number of monocytes in the group treated with 10 mg/kg Rh2Cit. There were no significant changes in rods, basophils or eosinophils for the acute and subchronic tests, the same occurring with the plateletgram (data not showed).
Fig. 2.
Leukogram of the Balb/c female mice in the acute and subchronic toxicity tests of rhodium citrate (Rh2Cit). Negative control received saline solution by intraperitoneal injection.
Data are presented as mean ± SEM (standard error of mean). WBC = White Blood Cells. The lowercase letters indicate significant differences detected by Bonferroni’s (segmented neutrophils and monocytes) or Dunn’s (WBC and lymphocytes) multiple comparison tests, with a = significant compared to group 1; b = significant compared to group 2; c = significant compared to group 3; d = significant compared to group 4; e = significant compared to group 5. Asterisks indicate significant differences at *p < 0.05 and **p < 0.01.
4.4. Biochemical analyses
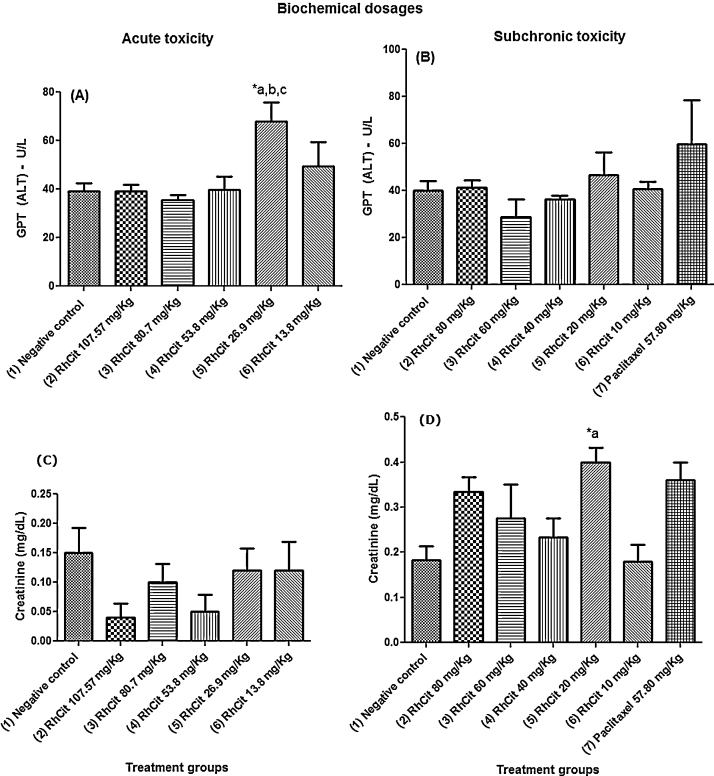
In the tests of acute toxicity, the only observed biochemical alteration was related to an increase in serum glutamic pyruvic transaminase (GPT) above the reference values [38] in the group treated with 26.9 mg/kg Rh2Cit. For the subchronic toxicity tests, the treatment with 20 mg/kg Rh2Cit was the only one that promoted a significant elevation in creatinine values, not affecting GPT levels (Fig. 3). However, such increased creatinine levels were still inside the reference values described for female Balb-C mice [46].
Fig. 3.
Biochemical dosages of serum glutamic pyruvic transaminase (GPT) and creatinine in Balb/c female mice of the acute and subchronic toxicity tests of rhodium citrate (Rh2Cit). Negative control received saline solution by intraperitoneal injection.
Data are presented as mean ± SEM (standard error of mean). The lowercase letters indicate significant differences detected by Bonferroni’s (GPT) or Dunn’s (creatinine) multiple comparison tests, with a = significant compared to group 1; b = significant compared to group 2; c = significant compared to group 3. Asterisks indicate significant differences at *p < 0.05.
4.5. Bone marrow genotoxicity analysis
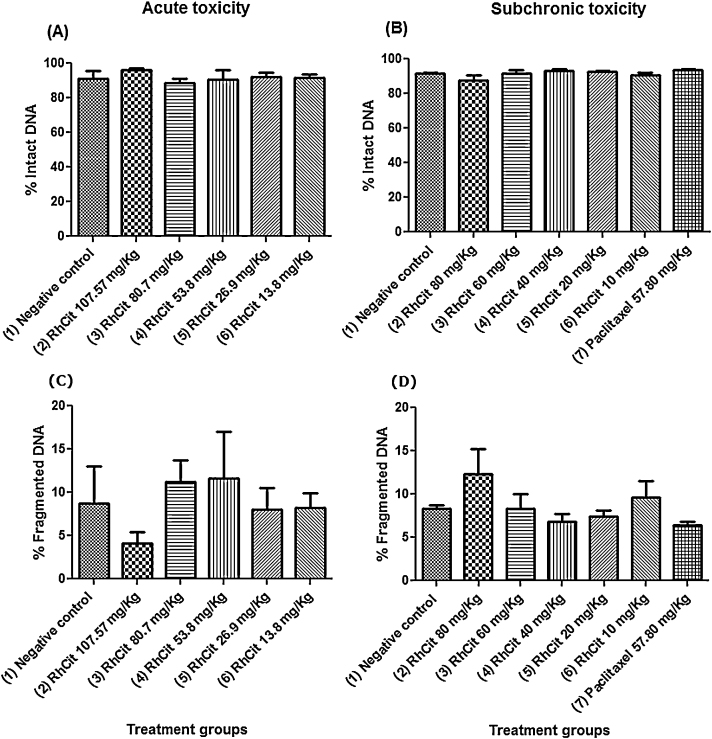
In order to investigate whether an acute or subchronic exposure to rhodium citrate (Rh2Cit) induces genotoxicity, bone marrow cells of treated mice were collected and had their DNA evaluated by flow cytometry. The percentages of intact and fragmented DNA of the experimental groups were similar to those found in the negative control group, indicating no significant alterations in either acute or subchronic exposures (Fig. 4).
Fig. 4.
Evaluation of genotoxicity by DNA fragmentation analyses of bone marrow cells under acute and subchronic exposure after intraperitoneal administration of rhodium citrate (Rh2Cit) in Balb/c female mice. Negative control received saline solution by intraperitoneal injection.
Data are presented as mean ± SEM (standard error of mean).
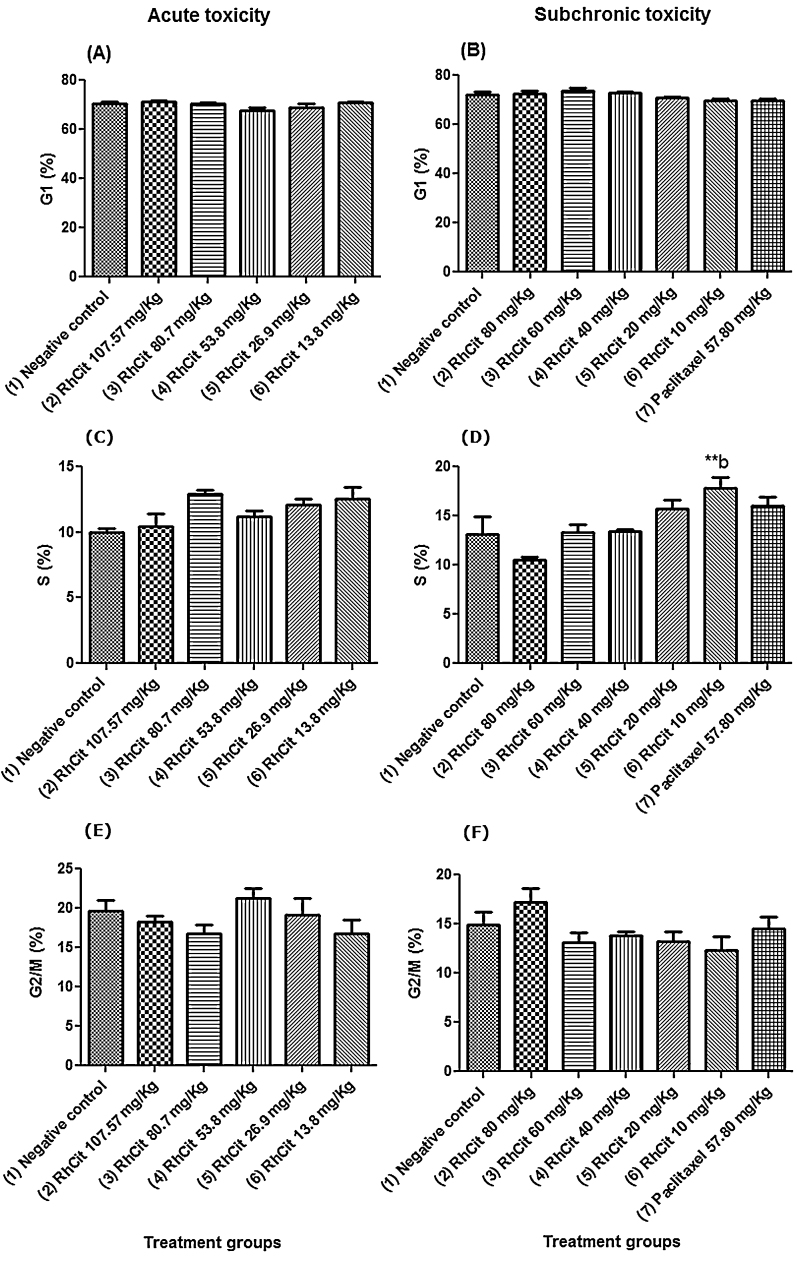
Regarding differences between the treated groups, a significant increase was observed between the groups treated with 80 mg/kg Rh2Cit or 10 mg/kg Rh2Cit in cell cycle profile in the chronic toxicity test.
In general, cell cycle profile analysis of bone marrow cells extracted from Rh2Cit treated mice showed no significant alterations when compared to the negative control group (Fig. 5). Cell cycle profile of mice submitted to a subchronic exposure of 80 mg/Kg Rh2Cit presented a proportion of cells that was slightly different in the S and G2/M phases (10.5% and 17.2%, respectively), showing that there was a tendency for the cell cycle to be arrested (Figs. 5D and F). Nevertheless, statistical analysis showed that this difference was not significant. Only mice treated with 10 mg/Kg Rh2Cit (group 6) had a significantly different proportion of cells in the S phase (17.8%), showing a discreet increase when compared to group 2 (80 mg/Kg) after subchronic exposure (Fig. 5D). Overall, these data indicate that acute or subchronic exposure to Rh2Cit showed no biologically significant alterations regarding bone marrow cell cycle profile.
Fig. 5.
Cell cycle profile of bone marrow cells in acute and subchronic exposure after intraperitoneal administration of rhodium citrate (Rh2Cit) in Balb/c female mice. Negative control received saline solution by intraperitoneal injection.
Data are presented as mean ± SEM (standard error of mean). The lowercase letter b indicates significant difference compared to group 2 detected by the Bonferroni test.
4.6. Macroscopic and histopathological analyses of lung, liver and kidneys
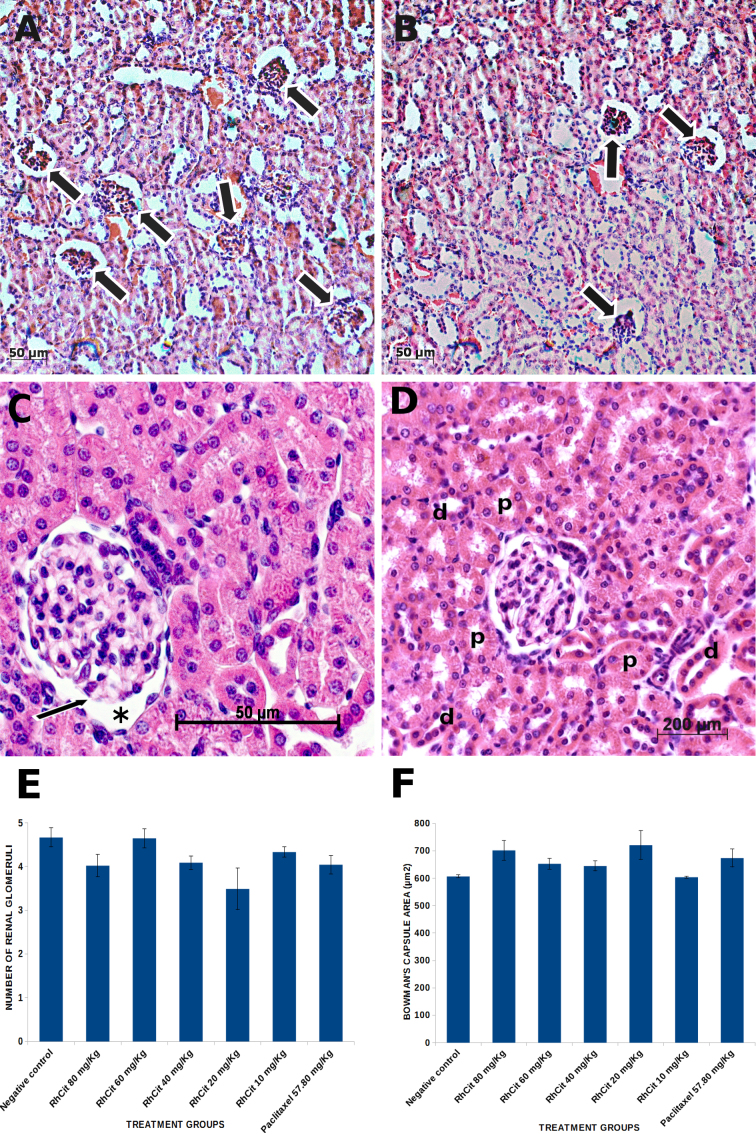
Evaluation of rhodium (II) citrate subchronic toxicity on the parenchyma of kidney, liver and lung by means of histopathology did not reveal any lesion or significant alteration in these organs. With respect to the renal cortex, tissue morphology of mice treated with any of the dosages of rhodium (II) citrate was preserved and similar to that of control animals (Fig. 6A and B). Normal Malpighian corpuscles comprising a glomerulus enclosed within a Bowman's capsule were homogeneously distributed throughout the parenchyma among many proximal and distal convoluted tubules (Fig. 6C and D), which often contained eosinophilic (proteinaceous) filtrate. A reduction in the number of glomeruli counted on 15 randomly analyzed visual fields (at 200 × magnification, for each of three studied animals per group) was noticed in some of the treatment groups as compared to the control, especially in animals that received 20 mg/kg rhodium (II) citrate, which displayed the lowest numbers (Fig. 6E). Nevertheless, this decrease was not significant (p < 0.05). On the other hand, an increase in the Bowman’s capsule interspace area was detected in mice from some treatment groups, but again differences were not significant (Fig. 6F).
Fig. 6.
Histological sections of renal cortex from Balb/C mice treated with rhodium (II) citrate at various concentrations. (A) Representative photomicrograph of kidney parenchyma of animals from the negative control group; the large arrows indicate renal corpuscles. (B) Histological pattern of kidney cortex in mice treated with 20 mg/kg rhodium (II) citrate; although no pathological alteration was noticed (as well as in the other treatments), a lesser number of glomeruli (large arrows) was counted in 15 randomly analyzed microscopic fields (200x magnification) as compared to the control, but this reduction was not significant (E). (C) A renal corpuscle of a control animal is shown in detail, with an intact glomerulus (thin arrow) enclosed within Bowman's capsule; interspace areas (asterisk) were measured along with glomeruli count (F). (D) Photomicrograph of proximal (p) and distal (d) convoluted tubules surrounding an intact renal corpuscle of an animal treated with 20 mg/kg rhodium (II) citrate, which is representative of the histological pattern of all groups. Data are presented as mean ± SEM (standard error of mean).
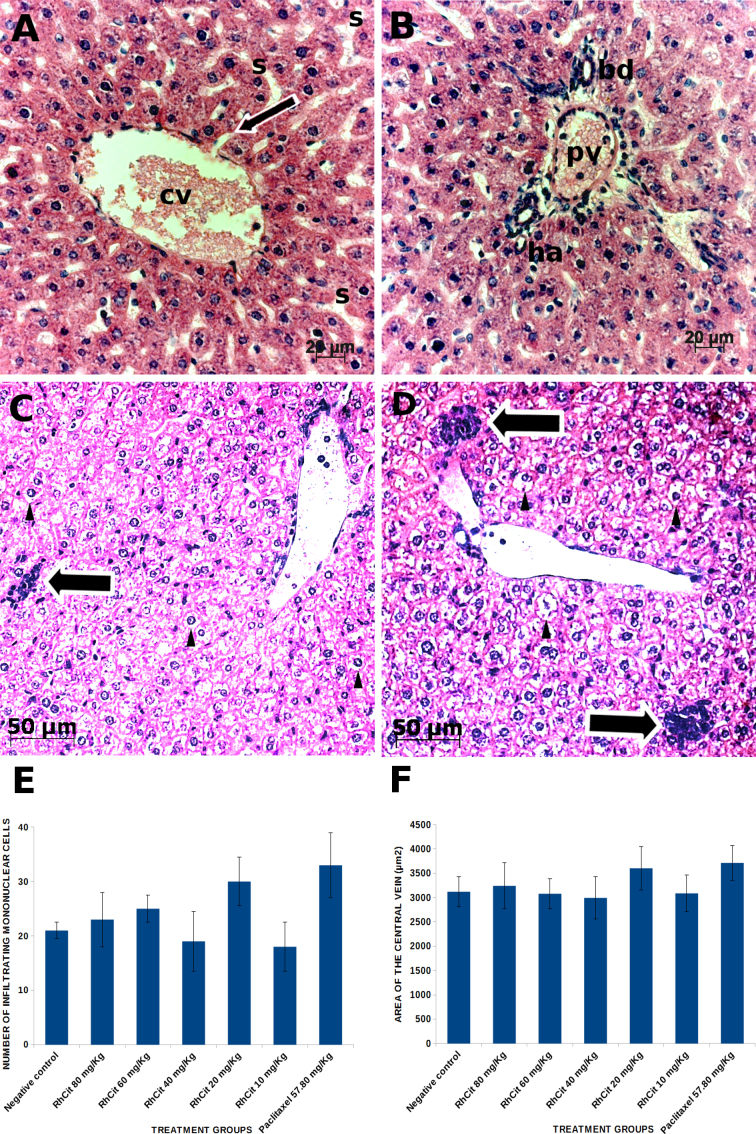
With regard to liver parenchyma, normal morphology of hepatic lobules and their components was observed in all the experimental groups. Hepatocytes were radially arranged in cords around the central vein and displayed large, round and euchromatic nuclei (Fig. 7A). Some vacuolated hepatocytes were equally seen in samples of all treatments, including the negative control (saline-treated animals). Well preserved portal tracts comprising a branch of the hepatic artery, a portal venule and a bile duct were found in all the examined animals regardless of their groups (Fig. 7B). Infiltration of mononuclear cells was occasionally detected both in control and treated animals (Fig. 7C and D) with no significant differences (p < 0.05) in numbers among groups (Fig. 7E). The area of central veins was measured in 12 randomly analyzed microscopic fields (at 200 × magnification) for each of the three studied animals per group (the two images with the highest and lowest mean values were discarded). Although an increase in the mean area of central veins was observed in some of the treatment groups as compared to the control animals, especially in animals that received 20 mg/kg rhodium (II) citrate or 57.80 mg/kg paclitaxel, this alteration was not significant (Fig. 7F).
Fig. 7.
Histological sections of liver from Balb/C mice treated with rhodium (II) citrate at various concentrations. Morphology remained unaltered after administration of any of the tested dosages. Therefore, unless otherwise stated, photomicrographs are representative of all experimental groups. (A) Central vein (cv) surrounded by cords of hepatocytes radially arranged; the thin arrow indicates one of the sinusoids (s) opening into the central vein. (B) Portal area comprising branches of the portal vein (pv), hepatic artery (ha) and bile duct (bd). Vacuolation of hepatocytes (arrow head) was occasionally observed in a few lobules both in the negative control (C) and in all treatment groups (D) and was associated to the presence of infiltrating mononuclear cells (large arrows), which were counted in 12 randomly analyzed microscopic fields. No significant differences in the numbers of these cells were detected among all groups (E). Similarly, central vein area was not significantly changed after any of the treatments as compared to the negative control (F). Data are presented as mean ± SEM (standard error of mean).
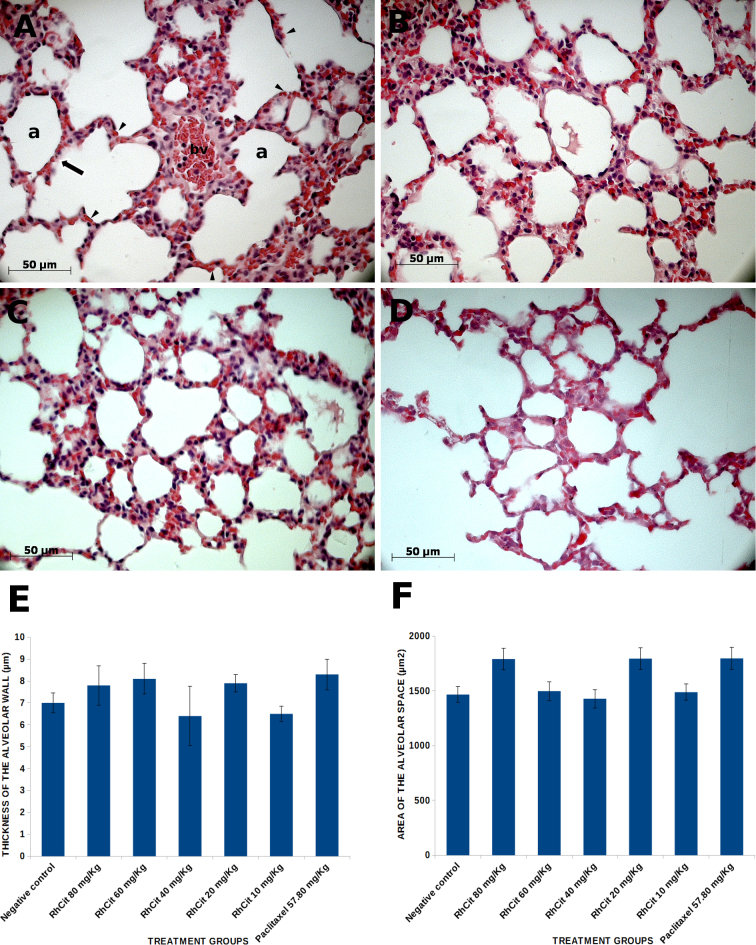
Lung histopathology was also performed to assess the effects of rhodium on the alveoli, the functional portion of this organ. Nevertheless, no alteration was detected and samples of all the experimental groups exhibited the same pattern of several alveolar air spaces separated by very thin alveolar septa (Fig. 8A–D). The thickness of the alveolar walls was measured in 10 randomly analyzed microscopic fields (at 200 × magnification) for each of three studied animals per group (Fig. 8E), and no significant difference could be detected among all treatments (p > 0.05). Similarly, the area of alveolar air spaces remained unchanged after rhodium administration as compared to the control (Fig. 8F).
Fig. 8.
Histological sections of lung from Balb/C mice treated intraperitoneally with rhodium (II) citrate at various concentrations. Morphology remained unaltered after administration of any of the dosages. (A) Alveoli of a control animal with a blood vessel (bv) containing several erythrocytes and some mononuclear cells; erythrocytes are also visible circulating through capillaries (arrowheads) within the alveolar septum (arrow) which surrounds each alveolus (a). The same pattern of alveoli was observed in mouse treated with 80 mg/kg rhodium (II) citrate (B), which is representative of all other rhodium treatments, and paclitaxel (C). Some areas with a slight reduction in the alveolar wall (septum) thickness were equally seen both in control and treated animals (D). This parameter was assessed in 10 randomly analyzed mice. Data are presented as mean ± SEM (standard error of mean).
5. Discussion
Metal complexes have played an important role in increasing the possibilities of chemotherapeutic compounds. However, the implementation of treatments is limited for safety reasons, including the threat of severe adverse effects and intrinsic or acquired resistance of tumor cells to the drugs [18]. Therefore, the search for compounds of rhodium, which have lower toxicity and significant antitumor activity, has led researchers to synthesize and test complexes with different carboxylate ligands, including citrate [30].
In cancer therapy, optimized dosing is crucial for establishing nontoxic dose and safety for clinical use. Acute toxicity values such as LD50 and adverse symptoms due to treatment are often used as the basis for classifying chemicals into toxicity categories, and for their subsequent regulation [49], [24], [34], [22].
In this respect, the compound Rh2Cit showed promising results for cancer therapy, with LD50 superior to 107.5 mg/kg in a single dose and superior to 400 mg/kg in accumulated doses, corroborating the literature [1]. The lethal dose to 50% of a population of mice (LD50) of citrate rhodium (II) has been estimated to be greater than 260 mg/kg [1]. However, no toxicity study of this compound has been systematically reported.
Unlike Rh2Cit, other complexes of rhodium, such as acetate and butyrate are highly toxic in mice, presenting values of DL10 of 0.7 mg/kg and 19 mg/kg, respectively [3]. Our study showed that doses of up to than 107.5 mg/kg are clinically safe with no lethality, suggesting that Rh2Cit may be classified as a low-toxic substance according to the toxicity categories of chemicals [35].
Nevertheless, studies on the effect of Rh2Cit treatment in accumulated doses and over prolonged time have not been conducted. Our study is the first report about the subchronic toxicity of Rh2Cit. We demonstrated that this complex did not produce any mortalities or clinical signs of systemic toxicity in Balb/c mice after five doses of up to 80 mg/kg/each (total dose of up to 400 mg/kg).
Severe intoxication symptoms and relevant changes in organ appearance, histopathology, biochemical, hematological and genotoxicity parameters were not observed after accumulated dosing. The observed clinical symptoms of lethargy and mild abdominal cramps, in the first two hours after the treatments, were transitory reactions. Andrade et al. [1] showed that 6 mg/kg Rh2Cit caused an inflammatory response that was characterized by a persistent edema for 24 h when this complex was injected into mice pads. Moreover, these researchers found an increase in neutrophilic segmented cells in the peritoneal exudate, as well as a significant increase in the number of spread monocytes when it was injected in the peritoneal cavity of the mice. However, these effects could probably be correlated to transitory reactions in response to drugs. We found that the therapeutic dose (about 12 mg/kg, administered in seven injections) used in our previous studies [6], [7] is at least 33 times less than the highest dose used in this study (400 mg/kg, administered in five injections). Thus, we considered that Rh2Cit could be considered a low toxic drug.
Studies on genotoxicity risk assessment are among the parameters required by international regulatory agencies to guide the safe use of pharmaceuticals [12]. Approaches and methods comprising DNA damage evaluations in bone marrow have often been conducted for this purpose since it is a complex organ containing a variety of haematopoietic cells (including stem cells) that are essential to give rise to nearly 10 different cell types present in the peripheral blood [12], [16]. It is known that cancer adjuvant therapies using radiation, platinum compounds, antimetabolites, alkylating agents, and anthracyclines induce unwanted side effects such as myelotoxicity, leading to non- repaired DNA damage, alterations in cell cycle, chromosomal instability, apoptosis, and mutations in normal bone marrow cells [45], [33], [26], [31], [27]. In the present work, bone marrow cells collected from mice exposed to acute and subchronic doses of Rh2Cit were submitted to DNA damage analysis to evaluate changes in the percentage of DNA integrity/fragmentation and cell cycle profile.
Acute and subchronic treatments with different doses of Rh2Cit did not induce significant alterations in DNA fragmentation ratio of bone marrow cells. Considering that DNA fragmentation is a hallmark associated with cell death, particularly apoptosis [10], [11], the present data suggest that Rh2Cit treatment did not affect bone marrow cell viability. Precise and strict cell cycle regulation is required for haematopoietic cells during normal blood cell development [31], whereas, disruptions in this regulation may lead to failure in blood production [16]. It has been reported that rhodium (II) carboxylates, particularly sodium butyrate, inhibit DNA synthesis in vivo and that cells in S phase are the ones mainly affected [2], [3]. Conversely, in the present work, the cell cycle profile of bone marrow cells did not show significant alterations after Rh2Cit acute and subchronic treatments, suggesting that the Rh2Cit doses and treatment regimens studied did not disturb the proliferation regulation of these cells.
Myelosuppression is a common adverse effect of several chemotherapy treatments, and it affects proliferation of bone marrow cells, leading to temporary anemia, leucopenia, and thrombocytopenia in peripheral blood [45], [26]. Cisplatin-induced myelosupression is considered mild and dose-dependent. Carboplatin induces a more severe myelosupression, when compared to cisplatin, showing intense incidence of neutropenia and thrombocytopenia of 18 and 25%, respectively [13]. Paclitaxel is another chemotherapeutic drug known to induce genotoxicity in bone marrow cells [28] and was used in the present study as a positive control for DNA damage. Nevertheless, no significant alterations were observed in DNA fragmentation or cell cycle profile after subchronic exposure. Churin et al. [8] reported that paclitaxel bone marrow toxicity is evidenced a few days after administration, showing a reverse and recovered pattern 14 days later. Thus, considering that genotoxicity and hematological parameters of paclitaxel treated mice were evaluated on the 44th day, it is possible that bone marrow features were reestablished to normal values. Altogether, data obtained by DNA damage analysis of bone marrow cells indicate that acute and subchronic treatments of Rh2Cit, in the doses and treatment regimens studied, are non-myelosuppressive. These results are supported by blood analysis of treated mice showing hematological parameter values within established reference intervals [14], [38].
The kidney is one of the most commonly affected organs after exposure to toxic compounds and such susceptibility can mainly be attributed to its function. Concentrations of the filtrate components are greatly increased across the tubular structure of each nephron and reach very high levels (in some cases, there is a >100-fold rise) at distal tubules and collecting ducts [4]. Therefore, since kidneys filter plasma continuously, toxic substances are rapidly and progressively accumulated at very elevated concentrations in renal tissues, which make histopathological analysis of this organ a fundamental requirement in drug safety evaluation. In the present study, no alteration of the renal parenchyma morphology was observed after any of the treatments with Rh2Cit as compared to the negative control animals. This is another promising finding that favors the use of this compound in cancer therapy, since platin derivatives are usually nephrotoxic.
Norrgren et al. [32] identified signs of tubular necrosis with chronic inflammation, fibrosis and missing tubuli in kidneys of Wistar rats treated intraperitoneally with 5 mg/kg cisplatin (in three of five animals), whilst 5 mg/kg radioactive (191Pt) cisplatin seemed to have a lower but still relevant toxicity in renal tissues, with one (20%) individual affected from a group of five rats. Similarly, Sherif 2014 [43] observed, in Sprague–Dawley Rats injected intraperitoneally with 7 mg/kg cisplatin, tubular damage characterized by cellular degeneration and detachment, which was ameliorated, but not avoided, by the use of Arjunolic acid, a compound extracted from a plant in Indian medicine. In addition to the rigorous pathological evaluation of the tissue samples in a blinded fashion, we performed the assessment of morphometric parameters (number of renal corpuscles per a defined area and Bowman's capsule area), and significant differences among treatments were still not present.
Thus, the increased creatinine levels in the group treated with 20 mg/Kg Rh2Cit may be due to causes not related to the kidney parenchyma rather than to a nephrotoxic effect of this dosage. A rise in serum creatinine concentration can be caused by pre-renal, renal and post-renal conditions. While pre-renal causes are related to increased protein catabolism, renal causes are usually associated with conditions that compromise 70–75% of functional renal mass, and post-renal causes include any cause that results in the obstruction of the lower urinary system [15]. In addition, although no evidence of renal lesions was present, the analysis of nephrotoxicity biomarkers such as the kidney injury molecule-1 (Kim-1), which is a reliable marker for tubular epithelial injury prior to morphological changes [44], may contribute, in future studies, to assess early adverse effects of our and other chemotherapeutic compounds on renal parenchyma.
With respect to liver, histological patterns in mice treated with any of the Rh2Cit dosages were similar to those found in animals that received only PBS. This organ is also very prone to xenobiotic-induced injury because of its central role in xenobiotic metabolism and its anatomic and physiologic structure [21]. Therefore, this absence of effects corroborates the safety of anticancer therapy with Rh2Cit. Conversely, intraperitoneal administration of cisplatin at 10 mg/kg to Swiss mice led to hepatic tissue degeneration, with widespread apoptosis and vacuolization. Co-treatment of these animals with extract of Boldo (Peumus boldus) reduced, but did not completely avoid, such hepatotoxicity of cisplatin [29]. In the present study, along with a careful examination of liver sections with regard to tissue architecture and cell morphology and counts of infiltrating mononuclear cells of what described above (5–10 mg/kg groups), which exerted toxic effects in these sites.
On the other hand, the hypothesis of hormesis should not be discarded; by which exposure to a low dose of a chemical agent or environmental factor that is damaging at higher doses induces an adaptive beneficial effect on the cell or organism [28]. In other words, 26.9 mg/kg Rh2Cit (acute toxicity) and 20 mg/Kg Rh2Cit (subchronic toxicity) doses increased the body’s tolerance for greater toxicity, such as those of 107.57 and 80 mg/Kg Rh2Cit dosage.
Lung parenchyma injury has often been associated with the use of cisplatin in human subjects. Leo et al. [25] described the areas of central veins and confirmed that none of the treatments caused any pathological alteration. Furthermore, it is noteworthy that even the highest Rh2Cit dosage used in our investigation (80 mg/kg) did not damage pivotal organs such as kidneys and liver, and is several-fold higher than those of cisplatin in the studies described above (5–10 mg/kg), which exerted toxic effects in these sites.
Moreover, Leo et al. [25] described the occurrence of pulmonary tissue alterations such as foci of fibroblastic proliferation filling alveolar airspaces, diffuse alveolar damage with interstitial/alveolar edema, presence of mild to moderate interstitial fibrous thickening, accumulation of macrophages and neutrophils in the alveolar spaces and many other findings, in patients who received preoperative chemotherapy with cisplatin. Therefore, we also microscopically analyzed lung samples of treated animals with the aim of determining whether Rh2Cit can also represent a safer alternative to cisplatin with respect to pulmonary toxicity. Besides the general histopathological evaluation, we also measured the mean area of alveolar air spaces and mean thickness of the alveolar wall in order to improve objectivity, accuracy and sensitivity of the comparison between lung parenchyma from the control and experimental groups. No alteration could be noticed in the lungs of any of the Rh2Cit-treated animals, nor were any significant differences among the morphometric parameters of all groups detected. However, for better understanding of these histopathological data, compound biodistribution studies will be performed later.
6. Conclusion
In general, according to our acute and subchronic tests, in all of the tested parameters, Rh2Cit- treated mice were similar to the negative control, suggesting that this complex was nontoxic. Rh2Cit did not induce mortality and its LD50 was superior to 400 mg/kg. The intraperitoneal administration of Rh2Cit at the tested doses induced no relevant behavioral alterations, toxicological signs, or any other adverse effects on Balb/c mice during all experimental periods (38 and 44 days after dosing). Moreover, our study revealed that this complex does not have notable genetic toxicity. Thus, this study provides evidence that indicates that Rh2Cit offers a good potential as a novel antitumor drug for cancer treatment without acute and subchronic toxic effects.
Conflict of interests
All the authors declare no conflict of interests.
Authors’ contributions
All authors have effectively contributed to this work. Marcella L.B. Carneiro was the principal investigator, performed the laboratory work for this study and takes primary responsibility for the paper. Márcia R. Mortari and Izabel C.R. da Silva participated in the design of the study and Sônia N. Báo coordinated the research; Aparecido R. de Souza was responsible for the chemical synthesis of the rhodium citrate; Cláudio A.P. Lopes conducted the histopathological analyses of the organs and morphometric analyses; Graziella A. Joanitti did the analyses of the genotoxicity; Ana L. Miranda-Vilela was responsible for statistical analysis and interpretation of data. Marcella L.B Carneiro, Ana L. Miranda-Vilela, Cláudio A.P Lopes, Graziella A. Joanitti and Izabel C.R. da Silva wrote the manuscript and all authors revised the document.
Acknowledgements
We are grateful to Sabin Institute/Sabin Laboratories for technical support in the hematology and biochemical dosages, and the Brazilian National Council for Technological and Scientific Development (CNPq), the Foundation to Support Research in the Federal District (FAPDF), the Coordination for Further Training of Graduate Staff (CAPES), the CAPES-Rede CON-NANO, NCT-Nanobiotecnologia, and CNANO-UnB for financial support.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2015.07.010.
Appendix A. Supplementary data
The following are Supplementary data to this article:
Changes in body weight over the days of the Balb/c female mice in the acute (A) and subchronic (B and C) toxicity tests of rhodium citrate (Rh2Cit). (A) Relative mean body weight changes of mice injected with different concentrations of rhodium (II) citrate (Rh2Cit) in the acute toxicity tests. (B) Absolute and (C) relative mean body weight changes of mice injected with different concentrations of rhodium (II) citrate (Rh2Cit) in the subchronic toxicity tests. Negative controls received saline solution by intraperitoneal injection. Error bars represent SEM (standard error of mean).
References
- 1.Andrade Júnior A.F., Cabral M., Moreira R.d.C., Feder D., Sudo L.S. Citrato do rúdio: II Modelo de agente inflamatúrio. Arq. mód. ABC. 1988;11:11–15. [Google Scholar]
- 2.Bear J.L. Precious metals. In: Zysk E.E., Bonucci J.A., editors. Proceedings of the ninth international precious metals conference, Int Precious Metals; Allentown, PA; 2015. pp. 337–344. [Google Scholar]
- 3.Bear J.L., Gray H.B., Jr., Rainen L., Chang I.M., Howard R., Serio G., Kimball A.P. Interaction of Rhodium(II) carboxylates with molecules of biologic importance. Cancer Chemother. Rep. 1975;59:611–620. [PubMed] [Google Scholar]
- 4.Bonventre J.V., Vaidya V.S., Schmouder R., Feig P., Dieterle F. Next- generation biomarkers for detecting kidney toxicity. Nat. Biotechnol. 2010;28:436–440. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce R.D. An up-and-down procedure for acute toxicity testing. Fundam. Appl. Toxicol. 1985;5:151–157. doi: 10.1016/0272-0590(85)90059-4. [DOI] [PubMed] [Google Scholar]
- 6.Carneiro M.L., Nunes E.S., Peixoto R.C., Oliveira R.G., Lourenco L.H., da Silva I.C., Simioni A.R., Tedesco A.C., de Souza A.R., Lacava Z.G., Bao S.N. Free Rhodium (II) citrate and rhodium (II) citrate magnetic carriers as potential strategies for breast cancer therapy. J. Nanobiotechnol. 2011;9:11. doi: 10.1186/1477-3155-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carneiro M.L., Peixoto R.C., Joanitti G.A., Oliveira R.G., Telles L.A., Miranda- Vilela A.L., Bocca A.L., Vianna L.M., da Silva I.C., de Souza A.R., Lacava Z.G., Bao S.N. Antitumor effect and toxicity of free rhodium (II) citrate and rhodium (II) citrate-loaded maghemite nanoparticles in mice bearing breast cancer. J. Nanobiotechnol. 2013;11:4. doi: 10.1186/1477-3155-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Churin A.A., Gol’dberg V.E., Karpova G.V., Voronova O.L., Feodorova E.P., Kolotova O.V., Skurikhin E.G., Pershina O.V. Reaction of bone marrow hematopoiesis to the toxic effect of paclitaxel. Bull. Exp. Biol. Med. 2008;145:213–217. doi: 10.1007/s10517-008-0053-2. [DOI] [PubMed] [Google Scholar]
- 9.da Silva Nunes E., Carneiro M.L.B., de Oliveira R.G.S., Báo S.N., de Souza A.R. Colloidal stability, surface characterisation and intracellular accumulation of Rhodium (II) citrate coated superparamagnetic iron oxide nanoparticles in breast tumour: a promising platform for cancer therapy. J. Nanopart. Res. 2013;15:1–15. [Google Scholar]
- 10.Darzynkiewicz Z., Huang X. Analysis of cellular DNA content by flow cytometry. Current protocols in immunology. 2004 doi: 10.1002/0471142735.im0507s60. 5.7. 1-5.7. 18. [DOI] [PubMed] [Google Scholar]
- 11.Darzynkiewicz Z., Zhao H., Halicka H.D., Rybak P., Dobrucki J., Wlodkowic D. DNA damage signaling assessed in individual cells in relation to the cell cycle phase and induction of apoptosis. Crit. Rev. Clin. Lab. Sci. 2012;49:199–217. doi: 10.3109/10408363.2012.738808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dearfield K.L., Cimino M.C., McCarroll N.E., Mauer I., Valcovic L.R., Agency U.S.E.P. Genotoxicity risk assessment: a proposed classification strategy. Mutat. Res. 2002;521:121–135. doi: 10.1016/s1383-5718(02)00236-x. [DOI] [PubMed] [Google Scholar]
- 13.Decatris M.P., Sundar S., O'Byrne K.J. Platinum- based chemotherapy in metastatic breast cancer: current status. Cancer Treat. Rev. 2004;30:53–81. doi: 10.1016/S0305-7372(03)00139-7. [DOI] [PubMed] [Google Scholar]
- 14.Everds N.E. Hematology of the laboratory mouse. In: Fox J.G., Davisson B.S., Newcomer M.T., Quimby C.E., Smith F.W.A.L., editors. III. Elsevier; San Diego, California, USA: 2007. pp. 133–170. (The Mouse in Biomedical Research: Normative Biology, Husbandry, and Models). [Google Scholar]
- 15.Fox J.G., Barthold S., Davisson M., Newcomer C.E., Quimby F.W., Smith A. 2006. The Mouse in Biomedical Research: Normative Biology, Husbandry, and Models. Academic Press. [Google Scholar]
- 16.Furukawa Y. Cell cycle control genes and hematopoietic cell differentiation. Leuk Lymphoma. 2002;43:225–231. doi: 10.1080/10428190290005973. [DOI] [PubMed] [Google Scholar]
- 17.Hall L.M., Speer R.J., Ridgway H.J. Synthesis and antitumor activity of certain rhodium(II) carboxylates. J. Clin. Hematol. 1980:25–27. [Google Scholar]
- 18.Heffeter P., Jungwirth U., Jakupec M., Hartinger C., Galanski M., Elbling L., Micksche M., Keppler B., Berger W. Resistance against novel anticancer metal compounds: differences and similarities. Drug Resist. Update. 2008;11:1–16. doi: 10.1016/j.drup.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Howard R.A., Kimball A.P., Bear J.L. Mechanism of action of tetra-mu-carboxylatodirhodium(II) in L1210 tumor suspension culture. Cancer Res. 1979;39:2568–2573. [PubMed] [Google Scholar]
- 20.Howard R.A., Sherwood E., Erck A., Kimball A.P., Bear J.L. Hydrophobicity of several rhodium(II) carboxylates correlated with their biologic activity. J. Med. Chem. 1977;20:943–946. doi: 10.1021/jm00217a016. [DOI] [PubMed] [Google Scholar]
- 21.Jones A.L. Hepatology; a textbook of liver disease: 1990. Anatomy of the normal liver; pp. 3–30. [Google Scholar]
- 22.Katsaros N., Anagnostopoulou A. Rhodium and its compounds as potential agents in cancer treatment. Crit. Rev. Oncol. Hematol. 2002;42:297–308. doi: 10.1016/s1040-8428(01)00222-0. [DOI] [PubMed] [Google Scholar]
- 23.Kelland L. Of mice and men: values and liabilities of the athymic nude mouse model in anticancer drug development. Eur. J. Cancer. 2004;40:827–836. doi: 10.1016/j.ejca.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Klaassen C.D., Eaton D.L. Principles of toxicology. Casarett Doull’s Toxicol. 1991;4:12–49. [Google Scholar]
- 25.Leo F., Pelosi G., Sonzogni A., Chilosi M., Bonomo G., Spaggiari L. Structural lung damage after chemotherapy: fact or fiction? Lung Cancer. 2010;67:306–310. doi: 10.1016/j.lungcan.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Makin G. Principles of chemotherapy. Paediatr. Child Health. 2014;24:161–165. [Google Scholar]
- 27.Marin A., Martin M., Linan O., Alvarenga F., Lopez M., Fernandez L., Buchser D., Cerezo L. Bystander effects and radiotherapy. Rep. Pract. Oncol. Radiother. 2015;20:12–21. doi: 10.1016/j.rpor.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattson M.P. Hormesis defined. Ageing Res. Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mondal J., Bishayee K., Panigrahi A.K., Khuda-Bukhsh A.R. Low doses of ethanolic extract of Boldo (Peumus boldus) can ameliorate toxicity generated by cisplatin in normal liver cells of mice in vivo and in WRL-68 cells in vitro, but not in cancer cells in vivo or in vitro. J. Integrative Med. 2014;12:425–438. doi: 10.1016/S2095-4964(14)60045-5. [DOI] [PubMed] [Google Scholar]
- 30.Najjar R., dos Santos F.S., Seidel W. 1987. Synthesis and characterization of the rhodium (II) citrate complex. [Google Scholar]
- 31.Nakamura-Ishizu A., Takizawa H., Suda T. The analysis, roles and regulation of quiescence in hematopoietic stem cells. Development. 2014;141:4656–4666. doi: 10.1242/dev.106575. [DOI] [PubMed] [Google Scholar]
- 32.Norrgren K., Sjölin M., Björkman S., Areberg J., Johnsson A., Johansson L., Mattsson S. Comparative renal, hepatic, and bone marrow toxicity of cisplatin and radioactive cisplatin (191Pt) in Wistar rats. Cancer Biother. Radiopharm. 2006;21:528–534. doi: 10.1089/cbr.2006.21.528. [DOI] [PubMed] [Google Scholar]
- 33.Nurgalieva Z., Liu C.C., Du X.L. Chemotherapy use and risk of bone marrow suppression in a large population-based cohort of older women with breast and ovarian cancer. Med. Oncol. 2011;28:716–725. doi: 10.1007/s12032-010-9512-5. [DOI] [PubMed] [Google Scholar]
- 34.Oecd Oecd. OECD Guidelines for the Testing of Chemicals. Org. Economic. 1994 [Google Scholar]
- 35.Parasuraman S. Toxicological screening. J. Pharmacol. Pharmacother. 2011;2:74. doi: 10.4103/0976-500X.81895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasetto L.M., D'Andrea M.R., Brandes A.A., Rossi E., Monfardini S. The development of platinum compounds and their possible combination. Crit. Rev. Oncol. Hematol. 2006;60:59–75. doi: 10.1016/j.critrevonc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Peixoto R.C., Miranda- Vilela A.L., Filho J.S., Carneiro M.L., Oliveira R.G., da Silva M.O., de Souza A.R., Bao S.N. Antitumor effect of free rhodium (II) citrate and rhodium (II) citrate-loaded maghemite nanoparticles on mice bearing breast cancer: a systemic toxicity assay. Tumour Biol. 2015;36:3325–3336. doi: 10.1007/s13277-014-2966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quimby F.W., Luong R.H. Clinical chemistry of the laboratory mouse. In: Fox J.G., Davisson M.T., Newcomer C.E., Quimby F.W., editors. III. Elsevier; San Diego, California, USA: 2007. pp. 133–170. (The Mouse in Biomedical Research: Normative Biology, Husbandry, and Models). [Google Scholar]
- 39.Rahman H.S., Rasedee A., Othman H.H., Chartrand M.S., Namvar F., Yeap S.K., Abdul Samad N., Andas R.J., Muhammad Nadzri N., Anasamy T. Acute toxicity study of zerumbone-loaded nanostructured lipid carrier on BALB/c mice model. BioMed Res. Int. 2014. 2014 doi: 10.1155/2014/563930. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Reibscheid E.M., Zyngier S., Maria D.A., Mistrone R.J., Sinisterra R.D., Couto L.G., Najjar R. Antitumor effects of rhodium (II) complexes on mice bearing Ehrlich tumors. Braz. J. Med. Biol. Res. 1994;27:91–94. [PubMed] [Google Scholar]
- 41.Sass N. Humane endpoints and acute toxicity testing. ILAR J. 2000;41:114–123. doi: 10.1093/ilar.41.2.114. [DOI] [PubMed] [Google Scholar]
- 42.Sharma S.V., Haber D.A., Settleman J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat. Rev. Cancer. 2010;10:241–253. doi: 10.1038/nrc2820. [DOI] [PubMed] [Google Scholar]
- 43.Sherif I.O. Amelioration of cisplatin-induced nephrotoxicity in rats by triterpenoid saponin of Terminalia arjuna. Clin. Exp. Nephrol. 2014:1–7. doi: 10.1007/s10157-014-1056-0. [DOI] [PubMed] [Google Scholar]
- 44.Song L., Xue L., Yu J., Zhao J., Zhang W., Fu Y. Kidney injury molecule-1 expression is closely associated with renal allograft damage. Bosn. J. Basic Med. Sci. 2013;13:170. doi: 10.17305/bjbms.2013.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sridhar T., Symonds R.P. Principles of chemotherapy and radiotherapy. Obstetr. Gynaecol. Reprod. Med. 2009;19:61–67. [Google Scholar]
- 46.Stechman M.J., Ahmad B.N., Loh N.Y., Reed A.A., Stewart M., Wells S., Hough T., Bentley L., Cox R.D., Brown S.D. Establishing normal plasma and 24-hour urinary biochemistry ranges in C3H, BALB/c and C57BL/6J mice following acclimatization in metabolic cages. Lab. Animals. 2010;44:218–225. doi: 10.1258/la.2010.009128. [DOI] [PubMed] [Google Scholar]
- 49.Zbinden G., Flury-Roversi M. Significance of the LD50-test for the toxicological evaluation of chemical substances. Arch. Toxicol. 1981;47:77–99. doi: 10.1007/BF00332351. [DOI] [PubMed] [Google Scholar]
- 50.Zhang C.X., Lippard S.J. New metal complexes as potential therapeutics. Curr. Opin. Chem. Biol. 2003;7:481–489. doi: 10.1016/s1367-5931(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 51.Zyngier S., Kimura E., Najjar R. Antitumor effects of rhodium (II) citrate in mice bearing Ehrlich tumors. Braz. J. Med. Biol. Res. 1989;22:397–401. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes in body weight over the days of the Balb/c female mice in the acute (A) and subchronic (B and C) toxicity tests of rhodium citrate (Rh2Cit). (A) Relative mean body weight changes of mice injected with different concentrations of rhodium (II) citrate (Rh2Cit) in the acute toxicity tests. (B) Absolute and (C) relative mean body weight changes of mice injected with different concentrations of rhodium (II) citrate (Rh2Cit) in the subchronic toxicity tests. Negative controls received saline solution by intraperitoneal injection. Error bars represent SEM (standard error of mean).