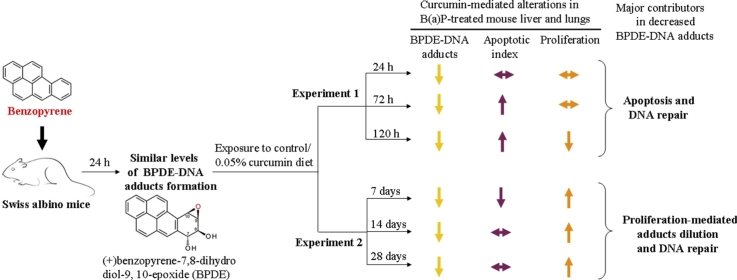
Graphical abstract
Abbreviations: B(a)P, benzo(a)pyrene; BPDE, benzo(a)pyrenediol-epoxide; CYP450, cytochrome P450; HRP, horseradish peroxidase; IHC, immunohistochemical; PAH, polycyclic aromatic hydrocarbons; PVDF, polyvinylidene difluoride; TBS, tris-buffered saline; TBST, TBS containing 0.1% Tween-20
Keywords: Benzo(a)pyrene, BPDE-DNA adducts, Apoptosis, Liver/lungs, IHC staining, Curcumin
Highlights
-
•
Effects of dietary curcumin on disappearance of DNA adducts were studied.
-
•
Curcumin post-treatment enhanced the disappearance of BPDE-DNA adducts in liver/lungs.
-
•
Curcumin post-treatment augmented B(a)P-induced apoptosis during 24–120 h.
-
•
Curcumin post-treatment increased PCNA in B(a)P-treated tissues during 7–28 days.
-
•
Curcumin-mediated increase in apoptosis and cell proliferation decreased DNA adducts.
Abstract
To study the post-treatment effects of dietary curcumin on the levels of benzo(a)pyrene [B(a)P]-induced DNA adducts, mice were administered oil or B(a)P and randomized into 7 subgroups after 24 h. One of the subgroups from both the oil and B(a)P groups was killed at 24 h while the remaining 6 subgroups were shifted to powdered control or 0.05% curcumin diet and killed after 24, 72 and 120 h (experiment 1), and 7, 14, and 28 days (experiment 2). Quantitative comparisons of BPDE-DNA nuclear adducts (area and intensity) in immunohistochemically stained lungs and liver sections was carried out by IHC profiler. A time-dependent decrease in the levels of adducts in B(a)P-treated animals was further enhanced by curcumin exposure compared to the levels in time-matched controls. To assess the contribution of apoptosis and cell proliferation in observed curcumin-mediated enhanced decrease of BPDE-DNA adducts, comparative evaluation of apoptosis and cell proliferation markers was undertaken. Results suggested enhancement of B(a)P-induced apoptosis in liver and lungs by curcumin during 24–120 h while no such enhancement was observed at 7–28 days. Results suggest curcumin-mediated enhancement in apoptosis (experiment 1) and adduct dilution (experiment 2) to be the reason for the observed higher decrease of BPDE-DNA adducts.
1. Introduction
Polycyclic aromatic hydrocarbons (PAH) are ubiquitous environmental agents, many of which have been identified as toxic, mutagenic and/or potent human carcinogens [1], [2]. PAH occur widely in the environment as a result of incomplete combustion of fossil fuels and other organic matter, and human exposure to PAH is therefore unavoidable [3]. Humans are exposed to complex mixtures of PAH, which have been implicated in inducing skin, lung and breast cancer. Benzo(a)pyrene [B(a)P], a well-known ubiquitous carcinogen belonging to the PAH group of compounds, is metabolically activated by phase I enzymes [or CYP1A class of cytochrome P450 (CYP450) enzymes] to form a highly mutagenic reactive electrophile, benzo(a)pyrenediol-epoxide (BPDE). Though phase II enzymes catalyze the detoxification of BPDE, some of the reactive electrophiles interact covalently with DNA to form adducts that mark an early initiation event. Unrepaired/misrepaired adducts lead to mutation in genes involved in proliferation, growth, apoptosis and finally to a disease condition such as cancer [4].
Plant-derived natural compounds have been receiving increased attention as chemopreventives because of their low toxicity and high tolerability. The efficacy of polyphenols when administered before or after the carcinogen treatment has been established and shown to modulate carcinogen-induced incidence/multiplicity/latency period of tumor development [5]. Curcumin/turmeric has been shown to possess chemopreventive activity at both initiation and promotion stages of chemical-induced carcinogenesis [6], [7], [8], [9], [10]. Earlier studies have shown that dietary curcumin pre-treatment decreases the formation of B(a)P-derived DNA adducts in mouse tissues by inhibiting carcinogen-induced phase I enzymes and directly inducing phase II enzymes [7]. Effects of turmeric/curcumin after exposure to carcinogens on the repair or disappearance of adducts, if any, are not known. Hence, in the present study, the post-treatment effect of curcumin on the disappearance of BPDE-DNA adducts in tissues of mice have been evaluated. Herein, we show that dietary curcumin treatment subsequent to B(a)P exposure enhances the disappearance of BPDE-DNA adducts. This could possibly be due to the curcumin-mediated enhancement of apoptosis of DNA adduct-containing cells and/or repair of DNA-adducts in mouse tissues.
2. Materials and methods
2.1. Materials
Benzo(a)pyrene [B(a)P] (purity ∼98%) and curcumin (purity ∼65–70%) were obtained from Sigma–Aldrich (St. Louis, MO, USA). Antibodies for Bax, Bcl-2, cyclin D1, β-actin, anti-mouse horseradish peroxidase (HRP) conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and caspase-3 from Abcam (Cambridge, MA, USA). Monoclonal antibody for BPDE-DNA adduct clone 5D11 was obtained from Hycult Biotechnology (Uden, Netherlands). The monoclonal antibody for proliferating cell nuclear antigen (PCNA) was procured from BD Pharmingen (San Diego, CA, USA). The anti-rabbit HRP conjugated secondary antibodies were obtained from Amersham Biosciences (Buckinghamshire, UK).
2.2. Animal treatment
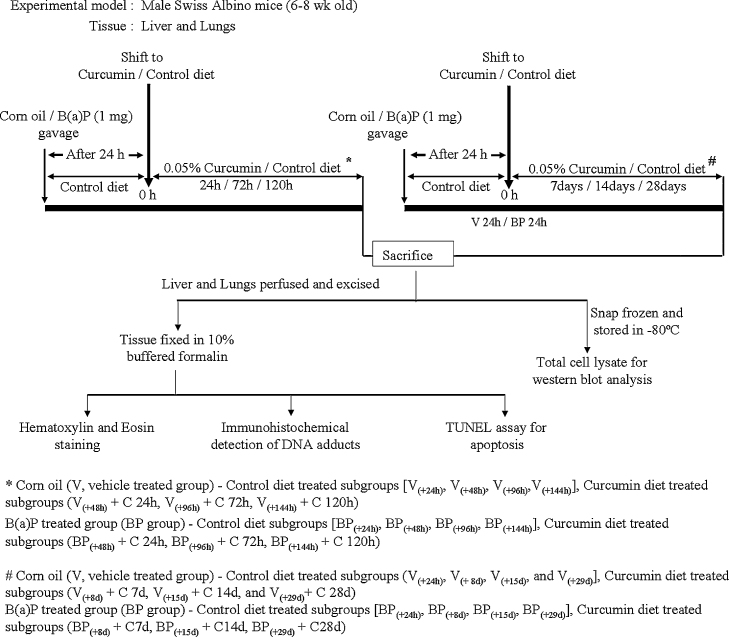
All animal studies were conducted with approval from the Institutional Animal Ethics Committee endorsed by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India guidelines. Inbred male Swiss albino mice (6–8 week old; Animal house, ACTREC, India) were randomized and housed in polypropylene cages, maintained under standard conditions of 22 ± 2 °C, 50% ± 10% relative humidity, and 12 h light/dark cycles. They received a control/experiment diet and drinking water ad libitum during the experimental period. Two different sets of experiments (1 and 2) were conducted at different times. Initially, corn oil (0.1 ml, V group) or B(a)P (1 mg in 0.1 ml corn oil, BP group) was administered by gavage to all animals that were maintained on standard laboratory diet (Fig. 1). After 24 h of corn oil or B(a)P administration, mice were randomized into seven subgroups. One of the subgroups (from both the groups V and BP) was killed at 24 h time point [subgroups V(+24 h) and BP(+24 h)] whereas half of the 6 subgroups (from both the groups) were continued on the powdered control diet (standard laboratory diet) and the other half were shifted to powdered experimental diet (0.05% curcumin in standard laboratory diet), which was prepared as described [11]. In experiment 1, mice shifted to control/experimental diets were killed after 24, 72 and 120 h [BP(+48 h), BP(+96 h), BP(+144 h) (control diet)/BP(+48 h) + C 24 h, BP(+96 h) + C 72 h, BP(+144 h) + C 120 h (experimental diet)] whereas in experiment 2, they were killed after 7, 14 and 28 days [BP(+8 d), BP(+15 d), BP(+29 d) (control diet)/BP(+8 d) + C 7 d, BP(+15 d) + C 14 d, BP(+29 d) + C 28 d (experimental diet)]. Both the experiments 1 and 2 had independent V(+24 h) and BP(+24 h) groups. Animals in all subgroups were observed for any apparent signs of toxicity such as weight loss or mortality during the entire study period. Animals were killed by CO2 asphyxiation and their liver and lungs were perfused and excised, and a part of the liver and lungs tissue were fixed in 10% buffered formalin for histopathological evaluation and immunohistochemical (IHC) staining, while the rest of the tissues were snap frozen in liquid nitrogen and stored at −80 °C until preparation of extract. The experimental conditions, i.e. dose, route of B(a)P administration, sampling time, dose and route of curcumin exposure employed in the present study, were chosen on the basis of our earlier studies demonstrating the effect of curcumin on the formation of BPDE-DNA adducts in mouse liver and lungs [7], [12].
Fig. 1.
Experimental design for studying the effect of curcumin post-treatment on disappearance of BPDE-DNA adducts in mouse tissues.
2.3. Protein immunoblotting
Total cell lysates from the tissues were prepared by previously described cell fractionation procedure [13]. The lysates were aliquoted, their protein content was determined, and they were stored at −80 °C. The total cell proteins (50–100 μg) were resolved on 8–12% sodium-dodecylsulphate polyacrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking with 5% non-fat skimmed milk in Tris-buffered saline (TBS, pH 7.4) containing 0.1% Tween-20 (TBST), the membranes were probed with antibodies for Bax, Bcl-2, caspase-3, PCNA, cyclin D1 overnight at 4 °C. All primary and secondary antibodies were first standardized for their dilution and then used accordingly. Blotting membranes were then washed three times with TBST and incubated with 1:4000 dilutions of anti-rabbit, anti-mouse, or anti-goat HRP conjugated secondary antibodies. Immunoreactive bands were visualized using a chemiluminescence reagent (Amersham Biosciences, Buckinghamshire, UK), followed by autoradiography. β-actin was used as the loading control. Densitometry of various analyte proteins and their respective loading controls from the same blot was performed using Image J 1.43 (NIH) software. Relative optical density was calculated by dividing the densitometry of analyte(s) protein with the respective loading control.
2.4. BPDE-DNA adduct measurement
The levels of BPDE-DNA adducts were detected by immunohistochemical staining for BPDE-DNA adducts in formalin-fixed, paraffin embedded 5 μm tissue sections as described previously [14]. Sections were incubated with anti-BPDE antibody (1:30 dilution). Detection was done using Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA). Diaminobenzidine (DAB) was employed as the chromogenic substrate, and slides were counterstained with Mayer's hematoxylin. Images were captured with Zeiss Microscope (Imager Z1) to which an Axiocam MRc5 digital camera was attached. Quantitative analysis of the images (magnification ×400) was performed by IHC profiler [15], which is an open source plug-in for the quantitative evaluation and automated scoring of immunohistochemistry images of tissue samples. This modified digital image analysis is based on protocols adopted earlier [16].
2.5. Digital image analysis
IHC photomicrographs were used for developing semi-automated analysis protocol, namely IHC profiler [15]. As a first step, a color de-convolution plug-in was used to un-mix the pure DAB and hematoxylin stained areas that left a complimentary image. The pixel intensities of separated DAB images range from 0 to 255. Value 0 represents the darkest shade whereas 255 represents the lightest shade of the DAB brown color in the image. To select the DAB-stained (brown) nuclei, the threshold feature of the Image J 1.43 (NIH) software was used. Further to assign an automated percentage of pure DAB staining patterns in the nucleus, a macro was developed and plugged in the Image J 1.43 (NIH) software to obtain an automated counting of the pixel wise percentage contribution of high, medium and low positive pixels/intensity in an image, i.e. the number of pixels of a specific intensity value vs. their respective intensity zone. For measurement of BPDE-DNA adducts [similar areas of tissue sections (mm2) and number of cells (∼800 cells/section/animal)], total intensity (%) [of nuclei containing percentage of high, medium and low intensity] was analyzed within different treatment groups. However, apoptosis was measured in terms of total apoptotic nuclei intensity as well as percentage of apoptotic positive and negative cells in similar areas of tissue sections (mm2) and number of cells (∼800 cells/section/animal) in different treatment groups. In this method, pixel intensities were categorized into 4 zone(s) with pixel intensity value ranging from 0 to 60 for a score value of high positive (3+), 61–120 for medium positive (2+), and 121–170 for low positive (1+). Pixel values beyond 170 were empirically analyzed and were found to be negative (0, blue stained nuclei) cells. After determining these numbers, the program applied them to a simple algebraic formula as shown below to determine the actual number of high/medium/low positive intensity.
In order to determine the total percentage intensity (of adducts containing nuclei and/or apoptotic nuclei), the following formula was used.
Quantitative analysis was performed in photomicrographs of 10 randomly selected fields per section with at least three mice per group. More than 800 cells were counted per section.
2.6. Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay
Apoptosis was assayed in formalin-fixed, paraffin embedded 5 μm tissue sections employing in situ TUNEL assay kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. The nuclei of the apoptotic cells were stained brown in color. Levels of apoptosis/apoptotic index were computed in two ways: (1) quantitative comparison of the images (magnification ×400) in terms of percentage intensity was done by modified digital image analysis protocols as described above and (2) by counting the number of positively stained cells × 100/total number of cells in the photomicrographs of tissue sections (without taking into account the color intensity) in the same image by using cell counter plug-in of Image J 1.43 (NIH) software [15], of at least 10 different randomly selected fields per section with at least three mice per group. More than 800 cells were counted per section.
2.7. Statistical analysis
Densitometry and quantitative analysis of images were performed using Image J 1.43 (NIH) software. Statistical analysis was performed using SPSS 15.0 software (IBM, Inc., Chicago, IL, USA) and STATA 12 software (StataCorp, TX, USA). Data are presented as mean ± SE. Means of (western blot analysis) data were compared using ANOVA with post hoc testing. Statistical comparisons of levels of BPDE-DNA adducts and TUNEL positivity among the groups were made using Poisson regression, which is specific for data representing counts or number of events and can handle cases in which few or no events occur. A p ≤ 0.05 was considered statistically significant.
3. Results
Based on the net body weight gain and histopathological evaluation of tissues, no toxicity or mortality was observed in animals belonging to the various treatment groups during the experimental period (Supplementary Figs. 1 and 2).
Supplementary Figs. 1 and 2 related to this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.11.008.
Supplementary Fig. S1.
Representative photomicrograph of hematoxylin and eosin (H&E) stained (A) liver and (B) lungs sections from mice belonging to the various treatment groups in experiment 1.
Supplementary Fig. S2.
Representative photomicrograph of hematoxylin and eosin (H&E) stained (A) liver and (B) lungs sections from mice belonging to the various treatment groups in experiment 2.
3.1. Effect of dietary curcumin post-treatment on disappearance of BPDE-DNA adducts in mouse tissues
3.1.1. Measurement of BPDE-DNA adducts at 24, 72 and 120 h
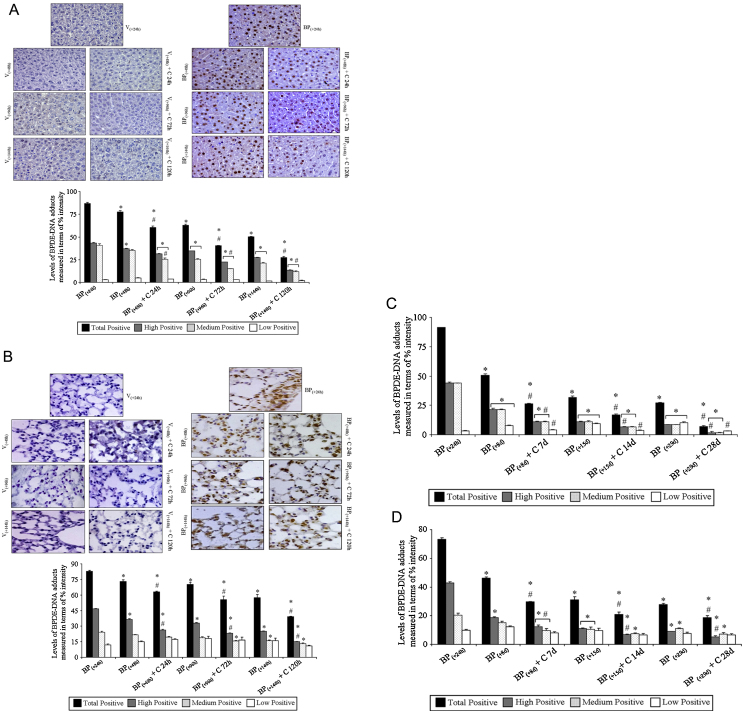
In animals receiving vehicle [V(+24 h), V(+48 h), V(+96 h), V(+144 h)] or vehicle + curcumin [V(+48 h) + C 24 h, V(+96 h) + C 72 h, V(+144 h) + C 120 h]-treated subgroups, BPDE-DNA adducts were not detected in liver and lungs of mice, while detectable levels of BPDE-DNA adduct(s) were observed by IHC staining following 24 h of B(a)P administration in these tissues [subgroup BP(+24 h), maintained on standard laboratory diet] (Fig. 2A and B) as reported [7], [12], suggesting specificity of reagent (antibody) and demonstrating a major difference in the levels of BPDE-DNA adducts between exposed and non-exposed animals/tissues. Levels of BPDE-DNA adducts were measured in a similar area of tissue sections (mm2) and number of cells (∼800 cells/section/animal) in terms of total adduct intensity as well as nuclei containing a percentage of high, medium and low intensity due to BPDE-DNA adducts. It was observed that with passage of time, mice on the control diet for 24, 72 and 120 h [subgroups BP(+48 h), BP(+96 h), BP(+144 h)] showed a time-related significant decrease in total adduct(s) intensity (levels) in the liver and lungs compared to BP(+24 h) and subgroup of preceding time point (Fig. 2, Fig. 3). Interestingly, mice that were shifted to 0.05% curcumin diet and killed at 24, 72 and 120 h [subgroups BP(+48 h) + C 24 h, BP(+96 h) + C 72 h, BP(+144 h) + C 120 h] showed significantly higher decrease in the levels of adducts (intensity) in the liver and lungs compared to BP(+24 h) and respective time-matched controls [subgroups BP(+48 h), BP(+96 h), BP(+144 h)] (Fig. 2, Fig. 3). This decrease was also evident when a comparison of percentage intensity of nuclei containing high, medium and low levels of adducts was made between curcumin-treated and respective time-matched controls. In the liver, the observed decrease in total adduct intensity in B(a)P [BP(+48 h), BP(+96 h), BP(+144 h)] and B(a)P + curcumin [BP(+48 h) + C 24 h, BP(+96 h) + C 72 h, BP(+144 h) + C 120 h]-treated subgroups appears to be attributed to the reduction in percentage intensity of nuclei containing high and medium levels of adducts. In the lungs, it was due to decrease in nuclei containing high levels of adducts both in B(a)P [BP(+48 h), BP(+96 h), BP(+144 h)] and B(a)P + curcumin [BP(+48 h) + C 24 h, BP(+96 h) + C 72 h, BP(+144 h) + C 120 h]-treated subgroups (Fig. 2A and B). Notably, the percentage intensity of nuclei containing low levels of adducts remained similar in all the subgroups, i.e. animals given B(a)P [BP(+24 h), BP(+48 h), BP(+96 h), BP(+144 h)] and B(a)P + curcumin [BP(+48 h) + C 24 h, BP(+96 h) + C 72 h, BP(+144 h) + C 120 h]-treated subgroups (Fig. 2A and B). Together, results suggest that dietary curcumin led to enhancement of decrease in nuclei containing high and medium levels of adducts in the liver whereas in the lungs a curcumin-mediated enhanced decrease was mainly observed in nuclei containing high levels of adduct(s).
Fig. 2.
Effect of curcumin post-treatment on levels of BPDE-DNA adducts in mouse tissues (experiments 1 and 2). Levels of BPDE-DNA adducts were measured by immunohistochemical detection in paraffin-embedded tissue sections of mouse (A) liver at 24, 72 and 120 h, (B) lungs at 24, 72 and 120 h, (C) liver at 7, 14 and 28 days, and (D) lungs at 7, 14 and 28 days; using a monoclonal antibody specifically recognizing BPDE-DNA adducts. Results are presented as representative photomicrographs at magnification ×400. Quantitative analysis was done by digital image analysis as described under Section 2. Data represent mean ± SE of three observations. Statistical analysis was performed using STATA 12 software using Poisson regression analysis. ‘*’ significant when compared to BP(+24 h) subgroup; ‘#’ significant when compared to respective time-matched controls.
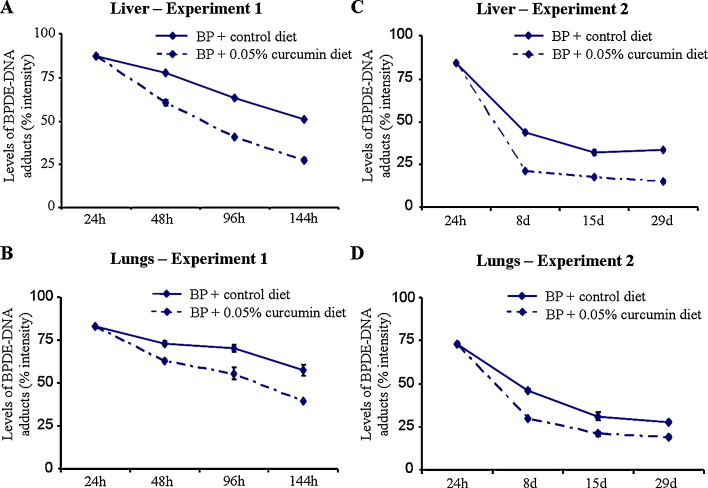
Fig. 3.
Levels of BPDE-DNA adducts in mouse liver and lungs (experiments 1 and 2).
3.1.2. Measurement of BPDE-DNA adducts at 7, 14 and 28 days
As observed in experiment 1, levels of BPDE-DNA adducts were not detected in vehicle [V(+24 h), V(+8 d), V(+15 d), V(+29 d)] or vehicle + curcumin [V(+8 d) + C 7 d, V(+15 d) + C 14 d, V(+29 d) + C 28 d]-treated subgroups in liver and lungs of mice while detectable levels of BPDE-DNA adducts were observed (stained nuclei) following 24 h of single dose of B(a)P in the liver and lungs of mice (Fig. 2C and D). Significant differences were not observed in subgroups [V(+24 h) and BP(+24 h)] in two different sets of experiments conducted at different times. As observed in experiment 1, mice on the control diet for 7, 14 and 28 days [subgroups BP(+8 d), BP(+15 d), BP(+29 d)] showed a time-related significant decrease in total adduct levels as seen by adduct intensity in the liver and lungs of mice compared to BP(+24 h) and subgroup of preceding time point. Interestingly, mice that were shifted to 0.05% curcumin diet and killed at 7, 14 and 28 days [subgroups BP(+8 d) + C 7 d, BP(+15 d) + C 14 d, BP(+29 d) + C 28 d] showed a significantly higher decrease in total levels of adduct intensity in the liver and lungs compared to BP(+24 h) and respective time-matched controls [subgroups BP(+8 d), BP(+15 d), BP(+29 d)] (Fig. 2, Fig. 3). This decrease was also evident when comparison of the percentage intensity of nuclei containing high, medium and low levels of adducts was made between curcumin-treated and time-matched controls. In the liver, the observed decrease in total adduct intensity appears to be attributed to reduction in percentage intensity of nuclei containing high and low levels of adducts. However, in lungs, it was mainly due to a decrease in intensity of nuclei containing high levels of adducts in mice shifted to 0.05% curcumin diet and killed at 7, 14 and 28 days [subgroups BP(+8 d) + C 7 d, BP(+15 d) + C 14 d, BP(+29 d) + C 28 d] compared to BP(+24 h) and respective time-matched controls [subgroups BP(+8 d), BP(+15 d), BP(+29 d)] (Fig. 2C and D). These results suggest that dietary curcumin further enhanced the decrease in total adduct intensity in the liver and lungs of mice although the extent of decrease varied.
3.2. Effect of dietary curcumin post-treatment on apoptosis in mouse tissues
3.2.1. Measurement of apoptotic index at 24, 72 and 120 h
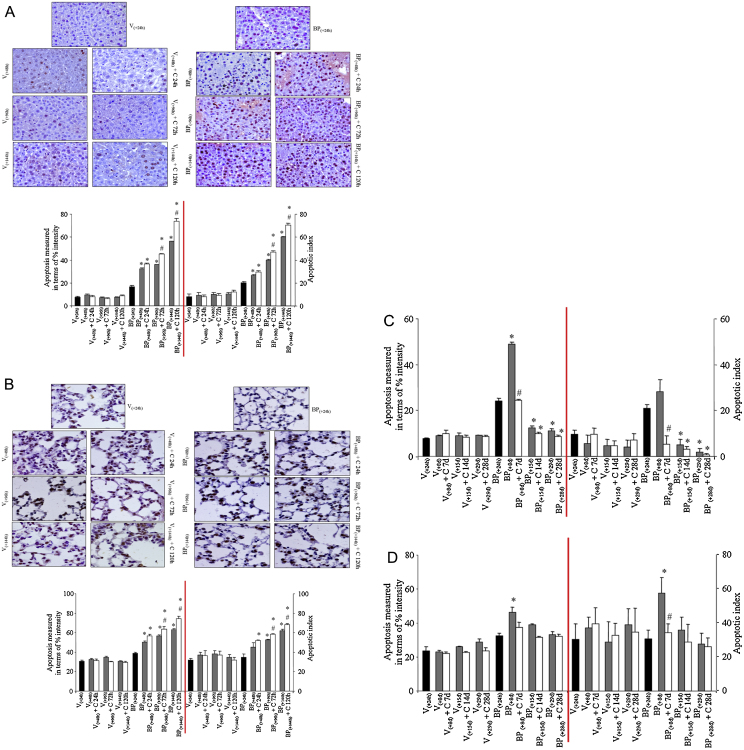
The observed decrease in levels of BPDE-DNA adducts in liver and lungs may be attributed to increased loss of adducts containing cells and/or enhanced DNA repair and/or dilution of adducted DNA by newly synthesized non-adducted DNA. To investigate the effect of dietary curcumin post-treatment on B(a)P-induced cell turnover in mouse liver and lungs, TUNEL assay was employed. Turnover of cells by apoptosis in the liver and lungs was measured in a similar area of tissue sections (mm2) and number of cells (∼800 cells/section/animal). Apoptotic index was measured in terms of total apoptotic nuclei intensity as well as the percentage of apoptotic positive and negative cells. Notably, 5–10% and 20–35% of total apoptotic nuclei were detected in the liver and lung tissues of vehicle [V(+24 h), V(+48 h), V(+96 h), V(+144 h)] or vehicle + curcumin [V(+48 h) + C 24 h, V(+96 h) + C 72 h, V(+144 h) + C 120 h]-treated subgroups, respectively (Fig. 4A and B). Compared to the vehicle treated group (V group), a significant increase in the percentage of positive cells/the percentage intensity of the total apoptotic nuclei was observed following 24 h of single dose of B(a)P [subgroup BP(+24 h)] in liver whereas in the lungs, it was similar to the vehicle treated group (Fig. 4A and B). It was observed that compared to subgroup BP(+24 h), mice on control diet for 24, 72 and 120 h [subgroups BP(+48 h), BP(+96 h), BP(+144 h)] showed an increase in apoptotic cells as judged by the percentage of TUNEL positive apoptotic cells (apoptotic index) and/or the percentage intensity of total apoptotic nuclei in the liver and lungs of mice. Interestingly, mice that were shifted to the 0.05% curcumin diet and killed at 72 and 120 h [subgroups BP(+96 h) + C 72 h, BP(+144 h) + C 120 h] showed further increase in B(a)P-mediated apoptosis as seen by an increase in numbers of apoptotic cells as well as the percentage intensity of total apoptotic nuclei compared to BP(+24 h) and respective time-matched controls [subgroups BP(+96 h) and BP(+144 h)] (Fig. 4, Fig. 5). These observations thus suggest that dietary curcumin further enhances the B(a)P-induced apoptosis, which would indirectly confer protection due to increased removal of adduct containing cells.
Fig. 4.
Effect of curcumin post-treatment on apoptosis/apoptotic index in B(a)P-treated mice (experiments 1 and 2). Levels of cell turn-over or extent of apoptosis was analyzed by TUNEL assay in paraffin-embedded tissue sections of mouse (A) liver at 24, 72 and 120 h, (B) lungs at 24, 72 and 120 h, (C) liver at 7, 14 and 28 days, and (D) lungs at 7, 14 and 28 days. Results are presented as representative photomicrographs at magnification ×400. Quantitative analysis was done by digital image analysis as described under Section 2. Data represent mean ± SE of five observations. Statistical analysis was performed using STATA 12 software using Poisson regression analysis. ‘*’ significant when compared to BP(+24 h) subgroup; ‘#’ significant when compared to respective time-matched controls.
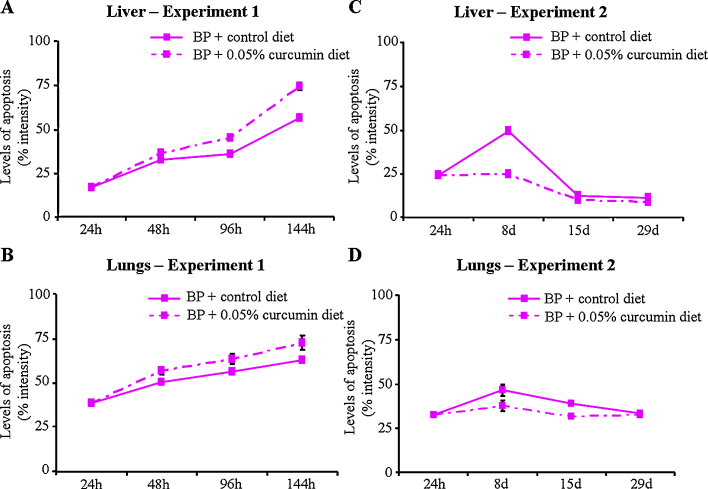
Fig. 5.
Levels of apoptosis in mouse liver and lungs (experiments 1 and 2).
3.2.2. Measurement of apoptotic index at 7, 14 and 28 days
As observed in experiment 1, 5–10% and 20–35% of total apoptotic cells (apoptotic index) were detected in the liver and lung tissues of vehicle [V(+24 h), V(+8 d), V(+15 d), V(+29 d)] or vehicle + curcumin [V(+8 d) + C 7 d, V(+15 d) + C 14 d, V(+29 d) + C 28 d]-treated subgroup, respectively (Fig. 4C and D). It was observed that compared to subgroup BP(+24 h), mice on the control diet for 7 days [subgroup BP(+8 d)] showed an increase in apoptosis as judged by an increased percentage of positive cells (apoptotic index) and/or the percentage intensity of total apoptotic nuclei in the liver and lungs of mice whereas a relative decrease in apoptosis in the liver was observed in mice on the control diet for 14 and 28 days [subgroups BP(+15 d), BP(+29 d)] (Fig. 4, Fig. 5). Interestingly, mice that were shifted to the 0.05% curcumin diet and killed at 7, 14 and 28 days did not show significant difference in the level of apoptosis in the liver and lungs of mice compared to BP(+24 h) and respective time-matched controls [subgroups BP(+8 d), BP(+15 d), BP(+29 d)]. However at 8 days, the liver of mice showed a decrease in the percentage of positive apoptotic cells (apoptotic index) and/or the percentage intensity of total apoptotic nuclei (Fig. 4, Fig. 5). An observed decrease in DNA adducts without enhancement in the levels of apoptosis in the liver and lungs suggests a role of DNA repair and/or dilution of BPDE-DNA adducts in tissue cells.
3.2.3. Measurement of apoptosis-related markers at 24, 72 and 120 h
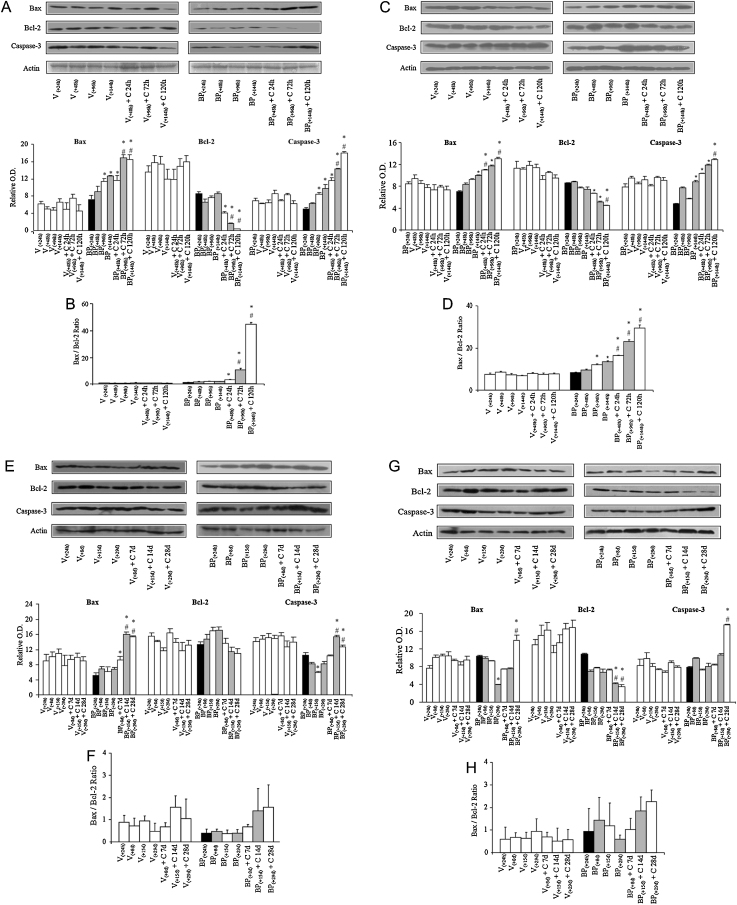
Further, to confirm and compliment the post-treatment effects of dietary curcumin in enhancement of apoptosis measured by TUNEL assay, protein levels of apoptosis-related markers were analyzed in the liver and lungs of mice by immunoblotting. Notably, the levels of apoptosis markers (Bax, Bcl-2 and caspase-3) remained similar in vehicle [V(+24 h), V(+48 h), V(+96 h), V(+144 h)] or vehicle + curcumin [V(+48 h) + C 24 h, V(+96 h) + C 72 h, V(+144 h) + C 120 h]-treated subgroups in the liver and lungs of mice (Fig. 6A and C). No change in levels of apoptosis markers (Bax, Bcl-2 and caspase-3) was observed following 24 h of a single dose of B(a)P [subgroup BP(+24 h)] in liver and lungs compared to vehicle treated group (V group). In comparison with subgroup BP(+24 h), mice on the control diet for 24, 72 and 120 h [subgroups BP(+48 h), BP(+96 h), BP(+144 h)] showed significant increase in the protein level of Bax in the liver (72 and 120 h) and lungs (120 h). Mice shifted to 0.05% curcumin diet [subgroups BP(+48 h) + C 24 h, BP(+96 h) + C 72 h, BP(+144 h) + C 120 h] showed a significant increase in the protein level of Bax in the liver (72 and 120 h) and lungs (24 and 120 h) compared to BP(+24 h) and respective time-matched controls (Fig. 6A and C). Concurrent to this, the protein level of Bcl-2 protein was unaltered in mice on the control diet [subgroups BP(+48 h), BP(+96 h), BP(+144 h)] compared to BP(+24 h). Importantly, mice that were shifted to 0.05% curcumin diet [subgroups BP(+48 h) + C 24 h, BP(+96 h) + C 72 h, BP(+144 h) + C 120 h] showed a decrease in the level of Bcl-2 in the liver (72 and 120 h) and lungs (120 h) compared to BP(+24 h) and respective time-matched controls (Fig. 6A and C). These observations together account for the progressive increment seen in the Bax/Bcl-2 ratio upon dietary curcumin post-treatment and thereby indicates that post-treatment with curcumin further enhances the apoptosis in B(a)P-treated mice (Fig. 6B and D). In addition, significant increase was also observed in the protein level of caspase-3 (the death executioner) at 72 and 120 h in the liver and at 120 h in the lungs of mice shifted to curcumin diet compared to respective time-matched controls (Fig. 6A and C). This correlates well with the enhancement observed in apoptotic index as well as in Bax/Bcl-2 ratio upon curcumin treatment. Overall, these results suggest that curcumin-mediated enhanced apoptosis in B(a)P-treated mice could be one of the plausible reasons contributing toward the decrease in BPDE-DNA adducts in liver and lungs of mice.
Fig. 6.
Effect of curcumin post-treatment on apoptosis-related biochemical markers in B(a)P-treated mice (experiments 1 and 2). Representative blot and relative levels of Bax, Bcl-2 and Caspase-3 protein were measured in total cell lysates prepared from mouse (A) liver at 24, 72 and 120 h, and (C) lungs at 24, 72 and 120 h, (E) liver at 7, 14 and 28 days, and (G) lungs at 7, 14 and 28 days analyzed by immunoblotting using specific antibodies. β-actin was used as the loading control. Extent of apoptosis was determined by calculating the ratio of normalized band intensity of Bax and Bcl-2 in (B) liver at 24, 72 and 120 h, (D) lungs at 24, 72 and 120 h, (F) liver at 7, 14 and 28 days, and (H) lungs at 7, 14 and 28 days of mice. Data represent mean ± SE of three observations. ‘*’ significant when compared to BP(+24 h) subgroup; ‘#’ significant when compared to respective time-matched controls (p ≤ 0.5, ANOVA followed by Bonferroni's test, p ≤ 0.05).
3.2.4. Measurement of apoptosis-related markers at 7, 14 and 28 days
Further, to confirm post-treatment effects of dietary curcumin on apoptosis measured by TUNEL assay, protein levels of apoptosis-related markers were analyzed in the liver and lungs of mice by immunoblotting. As observed in experiment 1, levels of apoptosis markers (Bax, Bcl-2 and Caspase-3) remained similar in vehicle [V(+24 h), V(+8 d), V(+15 d), V(+29 d)] or vehicle + curcumin [V(+8 d) + C 7 d, V(+15 d) + C 14 d, V(+29 d) + C 28 d]-treated subgroups in the liver and lungs of mice (Fig. 6E and G). No change in the levels of apoptosis markers (Bax, Bcl-2 and caspase-3) was observed in the liver and lungs of mice on the control diet for 8, 15 and 29 days [subgroups BP(+8 d), BP(+15 d), BP(+29 d)] compared to BP(+24 h). Mice shifted to 0.05% curcumin diet [subgroups BP(+8 d) + C 7 d, BP(+15 d) + C 14 d, BP(+29 d) + C 28 d] showed significant increase in the level of Bax protein in the liver (14 and 28 days) and lungs (28 d) compared to respective time-matched controls (Fig. 6E–H). Levels of Bcl-2 were similar in the liver of mice shifted to 0.05% curcumin diet [subgroups BP(+8 d) + C 7 d, BP(+15 d) + C 14 d, BP(+29 d) + C 28 d] compared to BP(+24 h) and respective time-matched controls whereas decrease was observed in the lungs (14 and 28 days) of mice shifted to curcumin diet compared to BP(+24 h) and respective time-matched controls (Fig. 6E and F). In addition, significant increase was noticed in the protein expression of caspase-3, the death executioner, at 14 and 28 days in the liver and at 28 days in the lungs of mice shifted to curcumin diet compared to BP(+24 h) and respective time-matched controls. Observed decrease in DNA adducts without enhancement in levels of apoptosis in liver and lungs suggest role of DNA repair and/or dilution of BPDE-DNA adducts in tissue cells.
3.3. Effect of dietary curcumin post-treatment on levels of cell proliferation markers in mouse tissues
3.3.1. Measurement of cell proliferation markers at 24, 72 and 120 h
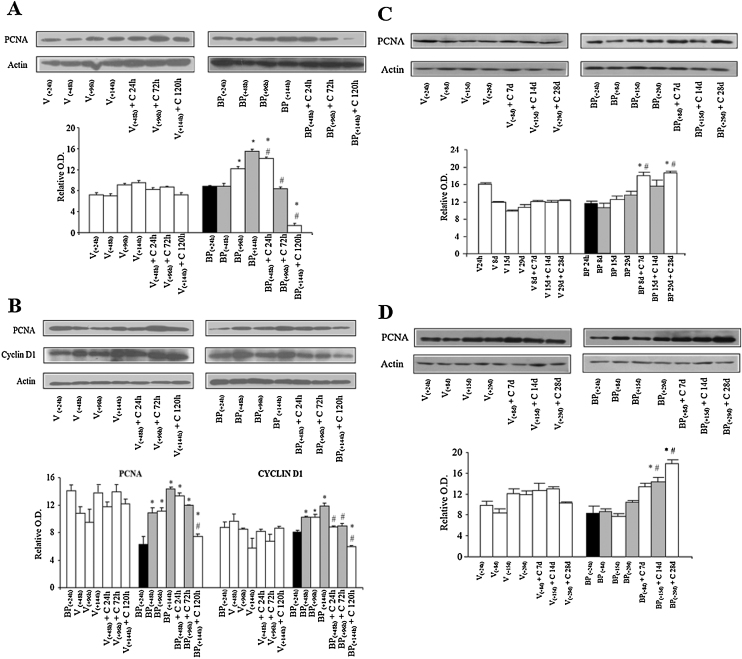
In addition to the role of apoptosis in disappearance of BPDE-DNA adducts, contribution of dilution of adduct containing DNA by newly synthesized non-adducted DNA, protein levels of cell proliferation markers such as PCNA in mouse liver and lungs were analyzed and compared by immunoblotting analysis. Levels of PCNA remained similar in vehicle [V(+24 h), V(+48 h), V(+96 h), V(+144 h)] or vehicle + curcumin [V(+48 h) + C 24 h, V(+96 h) + C 72 h, V(+144 h) + C 120 h]-treated subgroups in the liver and lungs of mice (Fig. 7, Fig. 8). Similarly, no significant change in the levels of PCNA was observed following 24 h of single dose of B(a)P [subgroup BP(+24 h)] in liver and lungs compared to vehicle treated group (V group) (Fig. 7, Fig. 8). Furthermore, mice on the control diet [subgroups BP(+48 h), BP(+96 h), BP(+144 h)] showed an increase in the levels of PCNA in the liver and lungs compared to subgroup BP(+24 h) except in the liver at 48 h. Interestingly, mice that were shifted to 0.05% curcumin diet [subgroups BP(+48 h) + C 24 h, BP(+96 h) + C 72 h, BP(+144 h) + C 120 h] showed significant decrease in the levels of PCNA in the liver (72 and 120 h) and lungs (120 h) compared to respective time-matched controls (Fig. 7, Fig. 8). As observed in the case of PCNA, a similar trend was observed in the levels of cyclin D1 wherein a significant curcumin-mediated decrease in the cyclin D1 level was observed in lungs of mice compared to respective time-matched controls (Fig. 7B).
Fig. 7.
Effect of curcumin post-treatment on cell proliferation markers in B(a)P-treated mice (experiments 1 and 2). Representative blot and relative levels of (A) PCNA in liver at 24, 72 and 120 h, (B) PCNA and Cyclin D1 in lungs at 24, 72 and 120 h, (C) PCNA in liver at 7, 14 and 28 days, and (D) PCNA in lungs at 7, 14 and 28 days measured in total cell lysates prepared from mouse tissues as analyzed by immunoblotting using specific antibodies, respectively. β-Actin was used as the loading control. Data represent mean ± SE of three observations. ‘*’ significant when compared to BP(+24 h) subgroup; ‘#’ significant when compared to respective time-matched controls (p ≤ 0.5, ANOVA followed by Bonferroni's test, p ≤ 0.05).
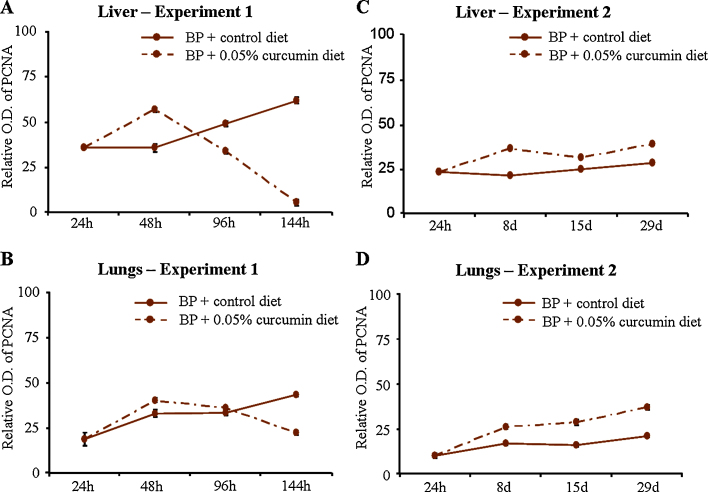
Fig. 8.
Levels of proliferation in mouse liver and lungs (experiments 1 and 2).
3.3.2. Measurement of cell proliferation markers at 7, 14 and 28 days
Similar comparative evaluations of cell proliferation markers were undertaken in the liver and lungs of mice at 7, 14 and 28 days. As analyzed in experiment 1, proliferation was assessed by comparing levels of PCNA. As observed in experiment 1, levels of PCNA remained similar in vehicle [V(+24 h), V(+8 d), V(+15 d), V(+29 d)] or vehicle + curcumin [V(+8 d) + C 7 d, V(+15 d) + C 14 d, V(+29 d) + C 28 d]-treated subgroups in the liver and lungs of mice (Fig. 7, Fig. 8). No change in the level of PCNA was observed in the liver and lungs of mice on control diet for 8, 15 and 29 days [subgroups BP(+8 d), BP(+15 d), BP(+29 d)] compared to BP(+24 h). Interestingly, mice that were shifted to 0.05% curcumin diet [subgroups BP(+8 d) + C 7 d, BP(+15 d) + C 14 d, BP(+29 d) + C 28 d] showed an increase in the levels of PCNA in the liver (7 and 28 days) and lungs (14 and 28 days) compared to BP(+24 h) and respective time-matched controls (Fig. 7, Fig. 8).
4. Discussion
Exposure to complex mixtures of PAH, which have been implicated in inducing skin, lung and breast cancer, is unavoidable. PAH-induced carcinogenesis involves a number of steps including: (i) the enzymatic activation of the PAH into metabolites, (ii) the covalent binding of the PAH metabolites to DNA, and (iii) the induction of mutations that serve to initiate the transformation process as a result of PAH-DNA adducts. Levels of DNA adducts measured at any point in time reflect tissue-specific rates of adducts formation and removal, which in turn, depends upon carcinogen activation/detoxification, DNA repair, adduct instability, tissue turnover, etc. The concept that cancer can be prevented or that certain diet-derived substances can postpone its onset is receiving increasing attention [17], [18]. Turmeric/curcumin pre-treatment has been demonstrated to decrease the formation of BPDE-DNA adducts in tissues of mice/rats as a result of a decrease in B(a)P-induced phase I enzymes and/or induction of phase II enzymes [7], [12]. In several studies curcumin post-treatment has been shown to decrease multiplicity of carcinogen-induced tumor formation in experimental models such as B(a)P-induced forestomach tumors, NDEA-induced hepatocarcinogenesis, DMBA-induced mammary tumorigenesis, AOM-induced colon tumors, etc. [10], [11], [19]. Even after exposure to carcinogen, a decrease in tumor multiplicity due to exposure to turmeric/curcumin was observed and is likely to be due to the decrease in cell proliferation and/or loss of initiated/DNA adduct containing cells. To understand the post-treatment effect of curcumin on disappearance of BPDE-DNA adducts, levels of BPDE-DNA adducts were measured at various time intervals in the liver and lungs of mice after allowing the formation of equal/similar levels of adducts and then exposing the animals to dietary curcumin. Levels of BPDE-DNA adducts were measured in tissue sections by IHC staining wherein an adduct-specific antibody was employed and levels were quantitated by measuring the adduct-intensity employing image analyses based on a principle adopted for analyzing nuclear staining typical for a DNA adduct staining pattern [16]. Although the method employed for quantitation of BPDE-DNA adducts does not provide absolute levels of DNA adducts per unit weight of DNA or defined number of normal nucleotides, it is capable of unbiased stratification and allows for relative comparison of adduct levels per equal area and/or similar number of cells. This non-destructive measurement method provides localization of adducts within cells in reasonable time and cost and multiple samples can be processed in a batch employing defined conditions.
Absence of BPDE-DNA adducts were observed in tissue sections from liver and lungs of mice receiving vehicle or dietary curcumin whereas significant as well as measurable levels of BPDE-DNA adducts were observed in these tissues following 24 h of B(a)P administration as reported in mouse skin, liver and lungs [7], [20], [21]. The time-dependent [BP(+48 h), BP(+96 h), BP(+144 h)/BP(+8 d), BP(+15 d), BP(+29 d)] decrease in the levels of BPDE-DNA adducts in the liver and lungs compared to BP(+24 h) following the single dose of carcinogen exposure was similar to that observed by others investigators in mouse/rat skin during 24 h–28 days [20], [22]. The time-related decrease in the levels of DNA adducts was relatively higher in the liver than the lungs compared to BP(+24 h). Our results clearly demonstrate that dietary curcumin (0.05%) post-treatment [subgroups BP(+48 h) + C 24 h, BP(+96 h) + C 72 h, BP(+144 h) + C 120 h and BP(+8 d) + C 7 d, BP(+15 d) + C 14 d, BP(+29 d) + C 28 d in experiments 1 and 2, respectively] further enhanced the decrease in the levels of BPDE-DNA adducts both in the liver and lungs at 48–144 h (experiment 1) and 8–28 days (experiment 2) compared to BP(+24 h) and respective time-matched controls [subgroups BP(+48 h), BP(+96 h), BP(+144 h) and BP(+8 d), BP(+15 d), BP(+29 d) in experiments 1 and 2, respectively]. In the present study the observation of high levels of BPDE-DNA adducts at 24 h after the carcinogen treatment and sharp decreases within 1 wk (∼8 days) is also in agreement with observations reported on mouse/rat skin [22]. The probable reasons for the observed time-related decrease in the levels of BPDE-DNA adducts in the liver and lungs could be due to loss or turnover of DNA adduct containing cells and/or repair of carcinogen-DNA adducts and/or dilution of adducted DNA with newly synthesized non-adducted DNA. The observed curcumin-mediated enhancement in the disappearance of BPDE-DNA adducts is likely to be due to modulation of one or more of the aforementioned processes. Analyses conducted to identify the reasons for curcumin-mediated enhanced disappearance of BPDE-DNA adducts showed that basal levels of apoptosis/turnover in the control liver (5–10%) and lungs (20–35%) were significantly enhanced by a single dose of B(a)P only in the liver (17–24%) but not in the lungs (32–38%). Subsequently, a time-related increase in the percentage apoptosis was observed in both the tissues, (subgroups BP(+48 h), BP(+96 h), BP(+144 h); 32–56%) and these rates or levels were further enhanced in B(a)P-treated animals exposed to dietary curcumin [subgroups BP(+48 h) + C 24 h, BP(+96 h) + C 72 h, BP(+144 h) + C 120 h; 37–73%] compared to BP(+24 h) and respective time-matched controls [subgroups BP(+96 h) and BP(+144 h)]. Apoptosis measured employing semi-quantitative and quantitative assays, and parameters showed good agreement in direction and extent of change that appears to be one of the major contributors in disappearance of BPDE-DNA adducts in tissues studied. Quantitative analysis and comparison of IHC staining measuring BPDE-DNA adducts and apoptosis in tissue sections have the advantage that preceding or subsequent paraffin-embedded sections from the same portion of the tissue are compared, and this comparison is likely to be relevant and meaningful. Curcumin-mediated enhancement of apoptosis in B(a)P-treated (normal liver and lung tissues) cells has some similarity with its effects in terms of apoptosis observed in transformed or immortalized cells in culture [23], [24], [25]. To the best of our knowledge, this is an initial in vivo report demonstrating that dietary curcumin augmented the expression of caspase-3 and increased the Bax/Bcl-2 ratio and apoptotic index in normal cells in response to B(a)P-induced DNA damage. This in turn probably accounts for the enhanced disappearance of adduct containing nuclei although the degree of responses varied.
The other potential contributor in observed relative decrease in BPDE-DNA adducts is cell proliferation, and its role was assessed by comparing the levels of PCNA by western blot analysis. It was seen from experiment 1 that levels of PCNA were enhanced post B(a)P-treatment especially at later time points [subgroups BP(+96 h) and BP(+144 h)], and B(a)P-mediated increases were significantly decreased by dietary curcumin when compared to time-matched B(a)P-treated controls in liver [subgroups BP(+96 h) + C 72 h, BP(+144 h) + C 120 h] and lungs [BP(+144 h) + C 120 h]. In experiment 2, levels of PCNA were not altered significantly at 8–28 days post B(a)P [BP(+8 d), BP(+15 d), BP(+29 d)] both in the liver and lungs while curcumin treatment resulted in significant increase in the levels of PCNA in liver [subgroups BP(+8 d) + C 7 d, BP(+29 d) + C 28 d] and lungs [BP(+15 d) + C 14 d, BP(+29 d) + C 28 d]. It may be noted that exposure to dietary curcumin alone does not alter the levels of PCNA in liver and lungs of mice.
After considering and comparing the slope of time-related and curcumin-mediated changes in BPDE-DNA adducts and numbers of cells undergoing apoptosis and cell proliferation, it is seen that the observed decrease in BPDE-DNA adducts in experiment 1 is mainly attributed to curcumin-mediated enhanced apoptosis. In experiment 2 dilution of BPDE-DNA adducts by newly synthesized non-adducted DNA due to cell proliferation appears to be the reason (Fig. 3, Fig. 5, Fig. 8). In both these experiments, apoptosis (experiment 1) and cell proliferation (experiment 2) alone may not be sufficient to result in the extent of decrease as potential contribution of DNA-repair may also be included. Although potential contribution if any, of DNA-repair in curcumin-mediated enhanced disappearance of BPDE-DNA adducts has not been studied/ruled out (in terms of activity and/or protein levels of DNA repair enzymes) in our study, this shortcoming does not diminish the importance of the convincing evidence presented on enhanced disappearance of BPDE-DNA adducts due to enhanced apoptosis and/or cell proliferation.
In conclusion, the present study suggested that curcumin post-treatment augments B(a)P-induced apoptosis, and this eventually resulted in increased loss of adducts containing cells in mice evaluated at 24–120 h, suggesting the role of apoptosis in removal of adduct containing cells. Curcumin-mediated enhanced loss in BPDE-DNA adduct containing cells probably results in reduction in the numbers of initiated cells in respective tissues, and this, along with curcumin-mediated inhibition of cell proliferation in these tissues leads to decrease in tumor multiplicity/tumor area/volume.
Transparency document
Acknowledgements
The authors thank ACTREC for financial support, ACTREC and Council of Scientific and Industrial Research for awarding fellowship to Gaurav Kumar. The authors thank Dr. Mary Carter, Coordinator, Health Sciences Writing Centre, University of Oklahoma for her critical reading of the manuscript and Mrs. Sadhana Kannan for assisting in statistical analysis. The authors also thank Mr. Prasad Phase and Mr. M. L. Jagtap for technical assistance.
Footnotes
Available online 14 November 2014
References
- 1.Goldman R., Enewold L., Pellizzari E., Beach J.B., Bowman E.D., Krishnan S.S., Shields P.G. Smoking increases carcinogenic polycyclic aromatic hydrocarbons in human lung tissue. Cancer Res. 2001;61:6367–6371. [PubMed] [Google Scholar]
- 2.Mastrangelo G., Fadda E., Marzia V. Polycyclic aromatic hydrocarbons and cancer in man. Environ. Health Perspect. 1996;104:1166–1170. doi: 10.1289/ehp.961041166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips D.H. Polycyclic aromatic hydrocarbons in the diet. Mutat. Res. 1999;443:139–147. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 4.Boysen G., Hecht S.S. Analysis of DNA and protein adducts of benzo[a]pyrene in human tissues using structure-specific methods. Mutat. Res. 2003;543:17–30. doi: 10.1016/s1383-5742(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 5.Maru G.B., Kumar G., Ghantasala S., Tajpara P. Polyphenol(s)-mediated in vivo cellular responses during carcinogenesis. In: Watson R.R., Preedy V.R., Zibadi S., editors. Polyphenols in Health and Diseases. Elsevier; 2014. pp. 1141–1179. [Google Scholar]
- 6.Garg R., Ramchandani A.G., Maru G.B. Curcumin decreases 12-O-tetradecanoylphorbol-13-acetate-induced protein kinase C translocation to modulate downstream targets in mouse skin. Carcinogenesis. 2008;29:1249–1257. doi: 10.1093/carcin/bgn114. [DOI] [PubMed] [Google Scholar]
- 7.Garg R., Gupta S., Maru G.B. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: mechanism of its anti-initiating action. Carcinogenesis. 2008;29:1022–1032. doi: 10.1093/carcin/bgn064. [DOI] [PubMed] [Google Scholar]
- 8.Maru G.B., Ramchandani A.G., Kumar G., Garg R. Curcumin-mediated cellular responses in chemical carcinogenesis. In: Watson R.R., Preedy V.R., editors. Bioactive Foods and Extracts. CRC Press; 2010. pp. 181–203. [Google Scholar]
- 9.Thapliyal R., Maru G.B. Inhibition of cytochrome P450 isozymes by curcumins in vitro and in vivo. Food Chem. Toxicol. 2001;39:541–547. doi: 10.1016/s0278-6915(00)00165-4. [DOI] [PubMed] [Google Scholar]
- 10.Thapliyal R., Naresh K.N., Rao K.V., Maru G.B. Inhibition of nitrosodiethylamine-induced hepatocarcinogenesis by dietary turmeric in rats. Toxicol. Lett. 2003;139:45–54. doi: 10.1016/s0378-4274(02)00440-x. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande S.S., Ingle A.D., Maru G.B. Inhibitory effects of curcumin-free aqueous turmeric extract on benzo[a]pyrene-induced forestomach papillomas in mice. Cancer Lett. 1997;118:79–85. doi: 10.1016/s0304-3835(97)00238-3. [DOI] [PubMed] [Google Scholar]
- 12.Thapliyal R., Deshpande S.S., Maru G.B. Mechanism(s) of turmeric-mediated protective effects against benzo(a)pyrene-derived DNA adducts. Cancer Lett. 2002;175:79–88. doi: 10.1016/s0304-3835(01)00675-9. [DOI] [PubMed] [Google Scholar]
- 13.Afaq F., Saleem M., Aziz M.H., Mukhtar H. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion markers in CD-1 mouse skin by oleandrin. Toxicol. Appl. Pharmacol. 2004;195:361–369. doi: 10.1016/j.taap.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y.J., Weksler B.B., Wang L., Schwartz J., Santella R.M. Immunohistochemical detection of polycyclic aromatic hydrocarbon-DNA damage in human blood vessels of smokers and non-smokers. Atherosclerosis. 1998;140:325–331. doi: 10.1016/s0021-9150(98)00136-1. [DOI] [PubMed] [Google Scholar]
- 15.Varghese F., Bukhari A.B., Malhotra R., De A. IHC profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLOS ONE. 2014;9:e96801. doi: 10.1371/journal.pone.0096801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee S., Malhotra R., Varghese F., Bukhari A.B., Patil A., Budrukkar A., Parmar V., Gupta S., De A. Quantitative immunohistochemical analysis reveals association between sodium iodide symporter and estrogen receptor expression in breast cancer. PLOS ONE. 2013;8:e54055. doi: 10.1371/journal.pone.0054055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhuri T., Pal S., Agwarwal M.L., Das T., Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–340. doi: 10.1016/s0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 18.Surh Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 19.Rao C.V., Rivenson A., Simi B., Reddy B.S. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–266. [PubMed] [Google Scholar]
- 20.Alexandrov K., Rojas M., Bourgeois Y., Chouroulinkov I. The persistence of benzo[a]pyrene diol-epoxide deoxyguanosine adduct in mouse skin and its disappearance in rat skin. Carcinogenesis. 1983;4:1655–1657. doi: 10.1093/carcin/4.12.1655. [DOI] [PubMed] [Google Scholar]
- 21.Ashurst S.W., Cohen G.M., Nesnow S., DiGiovanni J., Slaga T.J. Formation of benzo(a)pyrene/DNA adducts and their relationship to tumor initiation in mouse epidermis. Cancer Res. 1983;43:1024–1029. [PubMed] [Google Scholar]
- 22.Bjelogrlic N.M., Makinen M., Stenback F., Vahakangas K. Benzo[a]pyrene-7,8-diol-9,10-epoxide-DNA adducts and increased p53 protein in mouse skin. Carcinogenesis. 1994;15:771–774. doi: 10.1093/carcin/15.4.771. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal B.B., Kumar A., Bharti A.C. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 24.Jiang M.C., Yang-Yen H.F., Yen J.J., Lin J.K. Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutr. Cancer. 1996;26:111–120. doi: 10.1080/01635589609514468. [DOI] [PubMed] [Google Scholar]
- 25.Shankar S., Srivastava R.K. Involvement of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int. J. Oncol. 2007;30:905–918. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.