Abstract
Arsenic and nicotine exposure has been a major health concern globally. Individually both these toxicants increase the risk to various diseases including cancers. However, limited information exists on the co-exposure. In this study, we evaluate the effects of their individual and combined exposure and if co-exposure to these toxicants might have a synergism or antagonism. Male rats were exposed to a very low dose of arsenic (25 ppm in drinking water) or nicotine (0.25 mg/kg, sub-cutaneously) for a period of 5 months and post exposure various biochemical variables indicative of oxidative stress and apoptosis evaluated. Almost all glutathione linked enzymes showed marked alteration in individual as well as co-exposure treated groups. While serum creatinine and apoptosis indicator, lactate dehydrogenase (LDH) were significantly increased in both treatments, an additive effect was noted in co-exposure group. A similar trend was also seen in brain and liver but not in kidneys. Gene expression studies showed marked reduction in catalase, Cu-Zn SOD, GST, there was a significant up regulation in Bax, caspase 3 in various tissues along with urinary 8-OHdG levels, indicative of DNA damage and apoptosis. Interestingly, a decrease in liver arsenic concentration was noted in co-exposed group compared to arsenic alone exposed group. In conclusion, the present study suggests that arsenic and nicotine exhibited significant toxicity during individual exposure whereas co-exposure to these toxins showed variable conditions (indicative of both synergism and antagonism) in male rats.
Keywords: Arsenic nicotine co-exposure, DNA damage, Oxidative stress, Apoptosis, Synergism
1. Introduction
Arsenic is predominately present in water, soil, and air from natural and anthropogenic sources, however drinking of contaminated drinking water is the main cause of acute and chronic adverse health effects in humans [12], [21]. Over 200 million people worldwide are at risk, out of which more than 100 million are residing in West Bengal, India ([43] and Bangladesh); areas where groundwater arsenic concentrations exceed the World Health Organization maximum permissible level of 50 mg/L [60]. Moreover, many states of United States too have reported significant arsenic groundwater concentrations (up to 50 ppm) [63], [30]. Epidemiological studies have suggested a strong correlation between chronic arsenic exposure and various human diseases such as hyperkeratosis, atherosclerosis, diabetes, obstructive pulmonary diseases [12], [64], [63], [44]. Long term arsenic exposure has also been linked to cancer of skin, lung, colon and rectal [70], [22], [29]. While the mechanism of arsenic induced toxicity is not clearly defined, several mechanisms have been proposed of which arsenic-induced oxidative stress is among most widely accepted and studied [12].
Nicotine, the most important constituent of tobacco, is responsible for habit forming properties of tobacco chewing and cigarette smoking. Nicotine poisoning produces nausea, vomiting, abdominal pain, diarrhoea, headaches, sweating, and pallor however, more severe poisoning results in dizziness, weakness, and confusion, progressing to convulsions, hypotension, and coma [28]. Like arsenic, Nicotine also induces oxidative stress which ultimately results in several pathological conditions that needs further assessment.
Although arsenic and nicotine have been studied individually many times, there are only a few co-exposure studies. We recently demonstrated that pre-exposure to nicotine before arsenic exposure revealed interesting toxicokinetic and oxidative stress modulating interactions in the brain and liver of rats [47]. Co-exposure studies have reported synergism between both compounds [17], impaired arsenic methylation and metabolism [19], [34], [33], increase risk of lung cancer [4], [9] and oxidative stress [12], [24]. Due to ever increasing exposure to these environmental toxicants particularly in developing countries, there is increased interest to investigate the role of these toxicants individually and also interactive if co-exposure takes place [5]. In the present study we studied the individual and combined exposure to arsenic and nicotine on number of biochemical variables indicative of oxidative stress. Further we also studied if there is any synergism between arsenic and nicotine during co-exposure which would alleviate changes upon tobacco smoking in arsenicosis areas.
2. Material and method
2.1. Chemicals
Sodium meta arsenite was obtained from E. Merck (Darmstadt, Germany), nicotine as nicotine hydrogen tartarate was procured from Sigma–Aldrich (St. Louis, MO, USA), while all other chemicals were of “AnalaR” or “Extra pure” grade and obtained from BDH chemicals (Mumbai, India), Merck (Darmstadt, Germany) or Sigma (St. Louis, MO, USA).
2.2. Experimental design
Forty-eight male Wistar rats (60–80 g, ∼5 weeks) were obtained from the animal facility of Defence Research and Development Establishment (DRDE). They were maintained on ad libitum pellet diet (Lipton’s India Ltd) and water in an air-conditioned room with regular alternate cycles of 12 h light and darkness. The metal contents of the animal feed (in ppm dry wt.) were Cu 10, Mn 55, Co 5, Zn 45, and Fe 70. The animals were weighed every week and the doses were adjusted accordingly. All animals received humane care in compliance with the guidelines of the “Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA)”. The animals were acclimatized for 7 days prior to their use in experiments and were allowed a standard diet and water throughout the study. All 48 animals were randomized into 4 groups of 12 rats each and were treated as below for 5 months-
Group 1: Control (treated as normal and received normal water)
Group 2: Arsenic as sodium meta-arsenite (25 ppm in drinking water)
Group 3: Nicotine as nicotine dihydrogen tartarate (0.25 mg/kg body weight subcutaneously)
Group 4: Arsenic + Nicotine (same as in group 2 and 3, respectively)
After 5 months, rats were anesthetized under light ether and the blood samples were collected using a needle via intra-cardiac puncture to evaluate blood and serum biochemical variables after 15 days, 1 month and thereafter every month till the date of sacrifice. After 5 months, exposure was stopped and blood was collected in heparinized vials and serum was collected in non-heparinized tubes for biochemical estimation. Animals were quickly dissected under light ether anaesthesia, 24 h after the last dosing. Brain, liver and kidney was removed, washed thoroughly with chilled normal saline and stored at −80 °C until use for biochemical estimation. Liver and brain tissue required for gene expression analysis are washed with ice cold DEPC (diethyl pyrocarbonate)—treated water to remove extraneous material and kept at −20 °C in RNAlater®.
2.3. Biochemical assays
Amount of ROS in blood and tissues was measured using 2′,7′-dichlrofluorescin diacetate (DCF-DA) as described by Socci et al. [59]. Analysis of blood GSH concentration was performed by method of Ellman et al. [8] slightly modified by Jollow et al. [26]. Reduced glutathione (GSH) and oxidized glutathione (GSSG) content in tissue samples were measured as described by Hissin and Hilf [18]. SOD activity was assayed by the method of Kakkar et al. [27]. Catalase activity in tissue was assayed following the procedure of Sinha et al. [57]. Measurement of lipid peroxidation was done by the method described by Ohkawa et al. [46]. Glutathione peroxidase was determined by the literature method [10]. GST activity was determined by Habig et al. [16]. Glutathione reductase was determined by the literature method of Worthington and Rosemeyer [68]. Glutamic oxaloacetic transaminase (GOT), Glutamic pyruvic transaminase (GPT), Urea, Creatinin and Lactate dehydrogenase (LDH) activities were measured in serum using Merck kits. 8-OHdG concentration in the urine was estimated using highly sensitive ELISA kit. Arsenic was estimated using a Hydride Vapor Generation System (PerkinElmer model MHS-10) fitted with an atomic absorption spectrophotometer (AAS, PerkinElmer model AAnalyst 100) by Parker et al. [49].
2.4. RNA isolation and RT-PCR
RNA stabilized whole brain was minced and 35 mg liver, 50 mg kidney and 75 mg of minced brain was used for RNA isolation. Total RNA was isolated from brain, liver and kidney as previous reported [39]. All RNA samples were stored at −80 °C for further experiments.
Two micrograms of RNA was converted to cDNA using the First strand synthesis kit as described previously [38]. For semi-quantitative RT-PCR, 5 ng cDNA template was used for each sample and PCR was performed for 30 cycles 95 °C for 15 s, varying annealing temperature for 30 s and 72 °C for 30 s, with initial deactivation at 95 °C for 5 min and final extension at 72 °C for 7 min in GeneAmp PCR system 2700 (Applied Biosystems, USA). PCR products were electrophoresed on 1.5% agarose gel with ethidium bromide (Sigma–Alrich, MO, USA) and bands were visualized and recorded using Geldoc XR (Bio-Rad, Hercules, California, USA). List of primers used in the study are described in Table 1.
Table 1.
List of primers used in the study.
| Genes | Primer sequence (5′ to 3′) | Ta | Product size |
|---|---|---|---|
| β-actin | F'-GAGAGGGAAATCGTGCGTGACR'-CATCTGCTGGAAGGTGGACA | 65 °C | 453 bp |
| Catalase | F'-TGGCCTCCGAGATCTTTTCAATGR'-GCGCTGAAGCTGTTGGGGTAGTA | 63 °C | 452 bp |
| GPx | F'-CGCTCATGACCGACCCCAAGTR'-GCCAGCCATCACCAAGCCAATA | 65 °C | 221 bp |
| GST | F-5′-CTAGTAAGTTCGCGCCGCCCAG-3′R- 5′-AGGGAGCAGAGCCTTGCAACC-3′ | 65 °C | 350 bp |
| Cu Zn SOD | F'-GCGGCTTCTCTCGTCTCCTTGCR'- TTGATGGACATGGAACCCATGCTCG | 65 °C | 201 bp |
| Mn SOD | F'-ACAGCAAGCACCACGCGACCR'- AACACCCACCACGGGCCTGA | 67 °C | 560 bp |
| Bax | F-5′-GGATGGCTGGGGAGACACCTGAG -3′R-5-CGGCCCCAGTTGAAGTTGCCATCAG-3′ | 65 °C | 217 bp |
| Bcl2 | F-5′-TGAGAGCAACCGAACGCCCG-3′R-5′-GCACCCAGAGTGATGCAGGCC-3′ | 65 °C | 534 bp |
| Caspase 3 | F-5′-GCAGAGCCATGGGCACGTCTT-3′R-5′-TGGGCTGCAGGGCCTATTGC-3′ | 63 °C | 315 bp |
2.5. Statistical analysis
Experimental results were expressed as the mean ± SEM and were accompanied by number of observations. Data was assessed by the method of one-way analysis of variance (ANOVA) followed by Bonferroni test. Values with different symbols were significantly different from one another at the 5% level of probability.
3. Results
3.1. Effect on body weight index
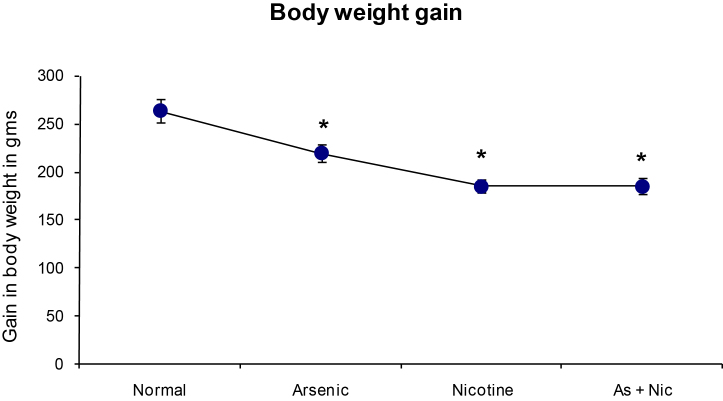
Gain in body weight was recorded every week and the change in body weight (after completion of exposure—before exposure started) was presented in Fig. 1. There was significant decrease in the body weight gain in all the exposed groups as compared to normal animals.
Fig. 1.
Effect of arsenic and nicotine on body weight index in exposed rats after 6 months.
Units: gain in body weight is expressed as grams (gms)
Values are mean ± SE; n = 5.
*p < 0.05 Compared to normal animals.
3.2. Effect on blood oxidative stress variable
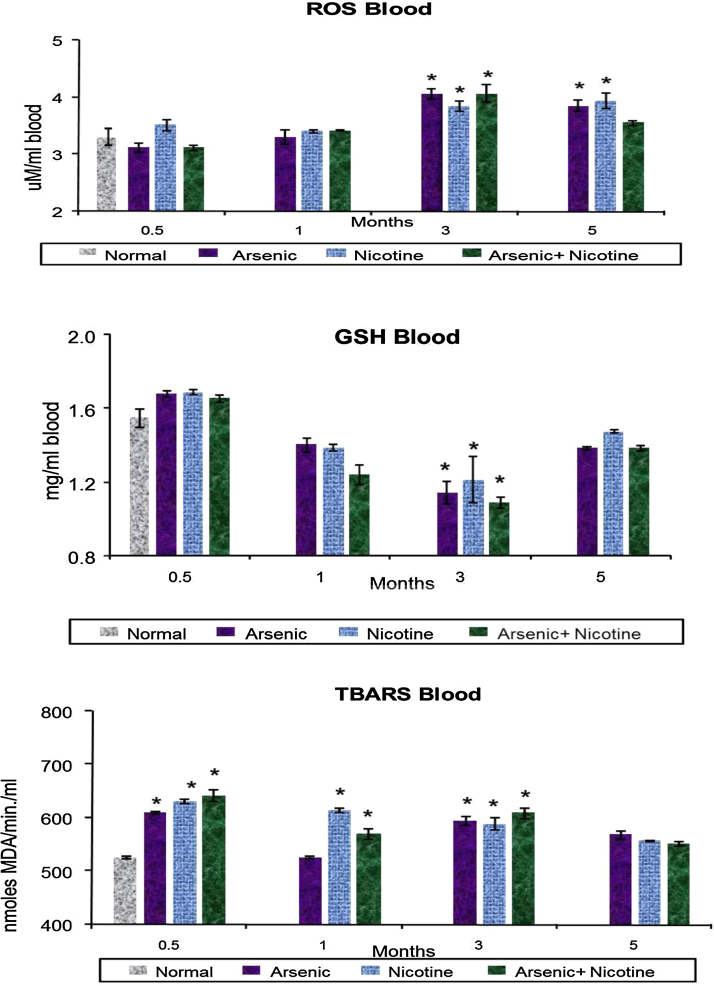
Individual arsenic and nicotine exposure in animals demonstrated consistent time dependent increase in blood reactive oxygen species (ROS) levels. However, in the combined exposure group, this increase was only observed up to 3 months with no further increase (Fig. 2). Interestingly, an increase after 15 days followed by subsequent decline up to 3 months in GSH was noted in all the exposed groups. After 3 months an elevated level of GSH was found in all exposed group. Blood TBARS levels increased at various stages of the study following various exposures, however, there was no particular trend observed (Fig. 2).
Fig. 2.
Effect of co-exposure of arsenic and nicotine on ROS, GSH and TBARS concentration in blood.
Abbreviations and units: Reactive Oxygen Species (ROS) as μM ml−1 blood; Reduced Glutathione (GSH) as mg/ml blood and TBARS—nmoles MDA produced/ml blood.
Values are mean ± SE; n = 5.
*p < 0.05 Compared to normal animals.
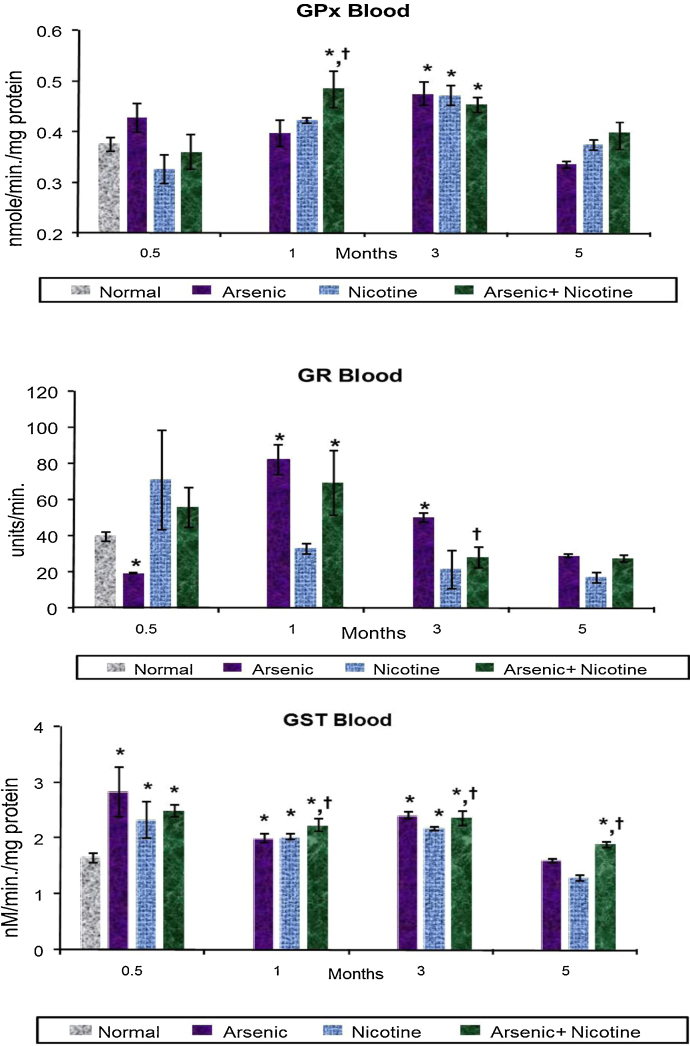
Co-exposure of As and nicotine demonstrated a significant increase in GPx activity by 1 month that continued till 3 months, however, in individual treatment increased GPx activity was only noted by 3 months. However, GR activity demonstrated a significant increase in 1 month followed by a significant decrease in its activity. This increase at 1 month was mainly contributed by arsenic and not nicotine (Fig. 3). Unlike GPx and GR, GST levels remained significantly elevated throughout the study in individual as well as co-exposure groups (Fig. 3).
Fig. 3.
Effect of co-exposure of arsenic and nicotine on GPx, GR and GST activity in blood.
Abbreviations and units: Glutathione Peroxidase as GPx-mM/ml blood; Glutathione Reductase as GR-mg/ml blood and Glutathione S-Transferase as GST—nM conjugate/min/mg protein.
Values are mean ± SE; n = 5.
*p < 0.05 Compared to normal animals.
†p < 0.05 Compared to arsenic exposed group.
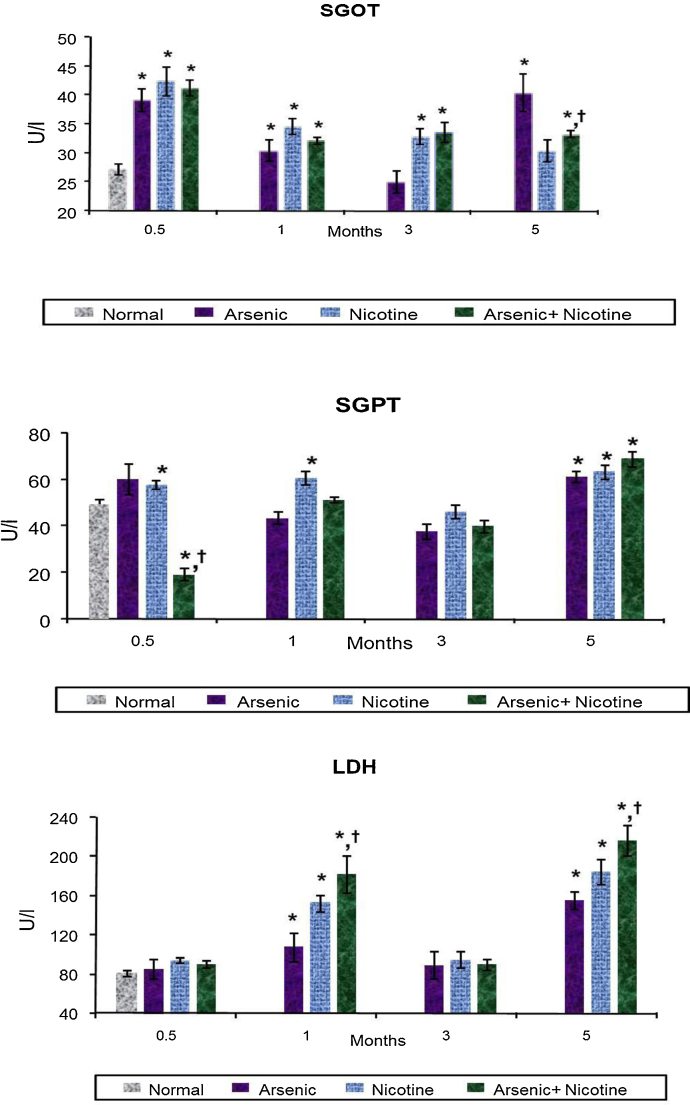
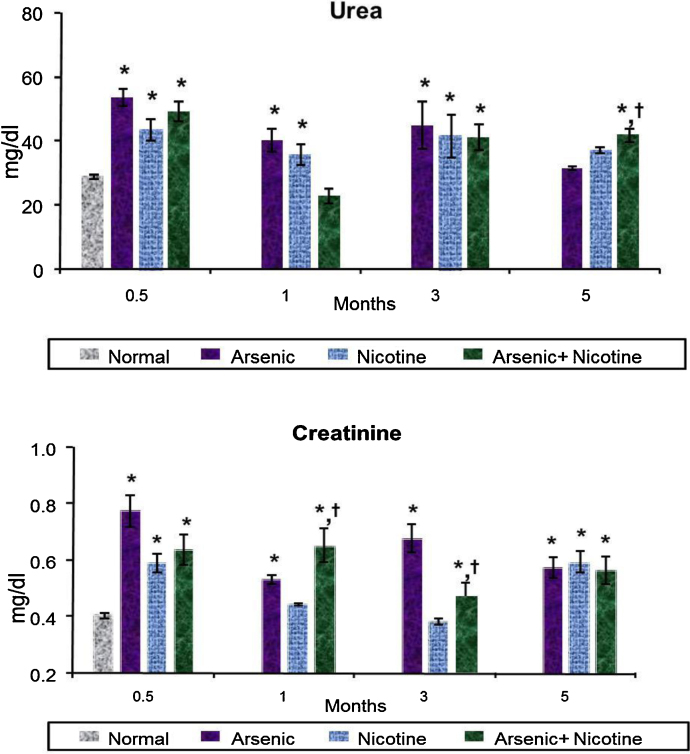
3.3. Effect on serum biochemical variables indicative of liver, kidney and membrane damage (Fig. 4, Fig. 5)
Fig. 4.
Effect of co-exposure of arsenic and nicotine on GOT, GPT and LDH activity in serum.
Abbreviations and units: Serum Glutamic oxaloacetic transaminase (GOT) as U/l; Serum Glutamic pyruvic transaminase (GPT) as U/l and Lactate dehydrogenase (LDH) as U/l.
Values are mean ± SE; n = 5.
*p < 0.05 Compared to normal animals.
† p < 0.05 Compared to arsenic exposed group.
Fig. 5.
Effect of co-exposure of arsenic and nicotine on urea and creatinine level in serum.
Abbreviations and units: serum urea as mg/dl and Creatinine as mg/dl.
Values are mean ± SE; n = 5.
*p < 0.05 Compared to normal animals.
† p < 0.05 Compared to arsenic exposed group.
There was a significant increase in SGOT, SGPT activity in all treated groups as compared to controls. However, the levels fluctuated over various time points but were highest at 5 months in all treated groups. Unlike SGOPT and SGPT, LDH levels increased in a time dependent manner where the highest levels were reached by 5 months of exposure. Co-exposure of arsenic and nicotine showed more damage as compared to the individual treatments (Fig. 4). Arsenic, nicotine and co-exposed animals all showed an elevated urea level during the entire experimental period (Fig. 5). However, there was no time dependent trend observed. Likewise creatinine too showed an increased response following various treatments over the study period (Fig. 5).
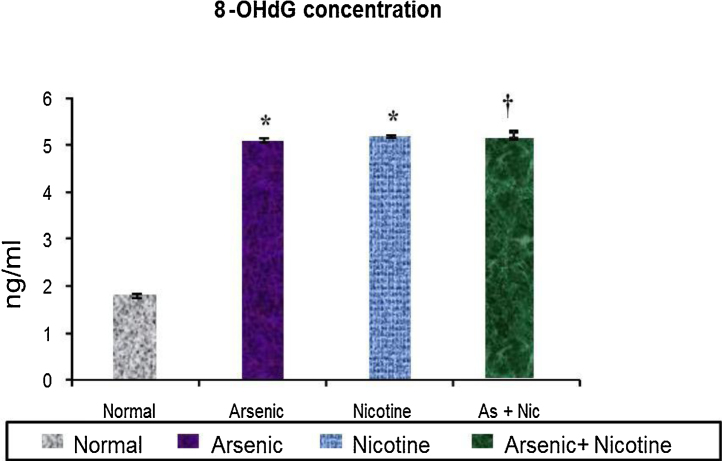
3.4. Effect on 8-OHdG concentration in urine (Fig. 6)
Fig. 6.
Effect of co-exposure of arsenic and nicotine on 8-OHdG concentration in urine as an indicator of DNA damage.
Abbreviations and units: 8-oxo 2′ deoxy Guanosine is expressed as 8-OHdG.
Values are mean ± SE; n = 5.
*p < 0.05 Compared to normal animals.
† p < 0.05 Compared to arsenic exposed group.
There was an equal increase in urine 8-OHdG concentration upon arsenic or nicotine exposure. In the combination group, an elevated concentration of 8-OHdG was observed as compared to controls however, these levels were lower than the individual treatment groups .
3.5. Effect on brain biochemical variables (Table 2)
Table 2.
Effect of co-exposure of arsenic and nicotine on brain oxidative stress variables in rats.
| Normal | Arsenic | Nicotine | As + Nicotine | |
|---|---|---|---|---|
| ROS | 272.7 ± 1.7 | 285.4 ± 0.7* | 293.6 ± 5.9* | 308.3 ± 0.4*, ** |
| GSH | 5.48 ± 0.08 | 4.65 ± 0.11* | 5.31 ± 0.14 | 6.16 ± 0.20*, ** |
| GSSG | 2.13 ± 0.03 | 1.92 ± 0.14 | 2.64 ± 0.09 | 2.81 ± 0.20*, ** |
| GSH/GSSG | 2.59 ± 0.03 | 2.20 ± 0.08* | 2.09 ± 0.09* | 2.07 ± 0.11* |
| TBARS | 2.43 ± 0.09 | 2.83 ± 0.24 | 2.87 ± 0.18 | 3.04 ± 0.13 |
| SOD | 2.09 ± 0.23 | 1.42 ± 0.09* | 1.28 ± 0.14* | 0.876 ± 0.08* |
| Catalase | 4.19 ± 0.14 | 3.16 ± 0.34* | 3.00 ± 0.10* | 3.05 ± 0.13* |
| GPx | 0.501 ± 0.007 | 0.405 ± 0.031* | 0.417 ± 0.014* | 0.429 ± 0.008 |
| GR | 8.10 ± 0.33 | 7.68 ± 0.16 | 7.20 ± 0.25* | 8.49 ± 0.06 |
| GST | 4.70 ± 0.06 | 4.15 ± 0.11* | 4.63 ± 0.10 | 4.35 ± 0.05* |
Abbreviations and units: Reactive Oxygen Species as ROS—FIU; Reduced glutathione as GSH—mg/gm, Oxidized glutathione as GSSG—mg/gm, Thiobarbituric acid reactive substances as TBARS——μg/gm, Superoxide dismutase as SOD—units/min/mg protein; Catalase—μmoles H2O2 produced/min./mg protein; Glutathione Peroxidase as GPx—nmole conjugate produced/min/mg protein; Glutathione reductase as GR—units/l and Glutathione S-transferase as GST—μmole/min/mg protein.
Values are mean ± SE; n = 5.
p < 0.05 Compared to normal control group.
p < 0.05 Compared to normal arsenic group.
In brain, arsenic exposure led to a significant increase in ROS level and a decrease in enzymes regulating GSH and maintaining ROS balance, except GR suggesting oxidative stress. Interestingly, nicotine only affected GSH regulating enzymes (GPx and GR) more pronouncedly as compared to control animals during the exposure period. Co-exposure to arsenic + nicotine led to a more pronounced increase ROS and GSSG while to our surprise there was an increase in GSH level (Table 2).
3.6. Effect on hepatic biochemical variables (Table 3)
Table 3.
Effect of co-exposure of arsenic and nicotine on liver oxidative stress variables in rats.
| Normal | Arsenic | Nicotine | As + Nicotine | |
|---|---|---|---|---|
| ROS | 713.1 ± 10.3 | 821.4 ± 21.7* | 948.3 ± 9.6* | 852.8 ± 10.5* |
| GSH | 11.1 ± 0.33 | 8.23 ± 0.31* | 12.0 ± 0.26 | 10.3 ± 0.38** |
| GSSG | 3.72 ± 0.07 | 2.76 ± 0.10* | 3.62 ± 0.15 | 3.06 ± 0.15* |
| GSH/GSSG | 3.24 ± 0.07 | 2.74 ± 0.07* | 2.94 ± 0.06* | 3.13 ± 0.05** |
| TBARS | 4.71 ± 0.06 | 6.17 ± 0.17* | 8.92 ± 0.17* | 7.12 ± 0.38*, ** |
| SOD | 3.95 ± 0.08 | 3.22 ± 0.14* | 3.14 ± 0.19* | 3.15 ± 0.21* |
| Catalase | 6.75 ± 0.33 | 6.30 ± 0.21 | 4.34 ± 0.27* | 4.11 ± 0.22* |
| GPx | 0.513 ± 0.018 | 0.380 ± 0.016* | 0.297 ± 0.015* | 0.336 ± 0.009* |
| GR | 38.9 ± 0.14 | 38.2 ± 1.17 | 33.1 ± 1.03* | 36.1 ± 0.2 |
| GST | 11.1 ± 0.1 | 12.6 ± 0.3 | 14.6 ± 1.0* | 10.3 ± 0.9 |
Abbreviations and units: Reactive Oxygen Species (ROS)—FIU; Reduced glutathione (GSH)—mg/gm, Oxidized glutathione (GSSG)—mg/gm, TBARS—μg/gm, Superoxide dismutase (SOD)—units/min./mg protein; Catalase—μmoles H2O2 produced/min/mg protein; Glutathione Peroxidase as GPx—nmole conjugate produced/min/mg protein; Glutathione reductase as GR—units/l and Glutathione S-transferase as GST—μmole/min/mg protein.
Values are mean ± SE; n = 5.
*p < 0.05 When all the exposed groups (arsenic, nicotine and arsenic + nicotine) were compared with normal animals.
**p < 0.05 When co-exposed group was compared to arsenic group.
Most of the hepatic oxidative stress variables demonstrated marked alteration following treatment of arsenic and nicotine alone or in combination. However, there were subtle differences between the treated groups. While arsenic exposure alone did not significantly affect the levels of catalase, GR and GST, nicotine exposure alone depleted catalase and GR and increased GST levels (Table 3), suggesting differential effects of compounds.
3.7. Effect on renal biochemical variables (Table 4)
Table 4.
Effect of co-exposure of arsenic and nicotine on renal oxidative stress variables in rats.
| Normal | Arsenic | Nicotine | As+ Nicotine | |
|---|---|---|---|---|
| ROS | 479.2 ± 5.3 | 492.8 ± 3.3* | 565.5 ± 28.5* | 543.4 ± 17.3* |
| GSH | 8.45 ± 0.35 | 6.68 ± 0.49* | 7.91 ± 0.24* | 8.79 ± 0.32** |
| GSSG | 2.57 ± 0.08 | 2.39 ± 0.07 | 2.51 ± 0.05 | 2.95 ± 0.14*, ** |
| GSH/GSSG | 3.43 ± 0.12 | 2.65 ± 0.17* | 2.87 ± 0.16* | 3.22 ± 0.03** |
| TBARS | 2.29 ± 0.02 | 3.04 ± 0.28* | 3.10 ± 0.15* | 3.15 ± 0.09* |
| SOD | 2.53 ± 0.06 | 2.11 ± 0.11 | 1.92 ± 0.15* | 1.95 ± 0.06* |
| Catalase | 4.88 ± 0.62 | 3.49 ± 0.30* | 3.25 ± 0.39* | 2.89 ± 0.12* |
| GPx | 0.487 ± 0.02 | 0.371 ± 0.03* | 0.37 ± 0.02* | 0.372 ± 0.02* |
| GR | 38.1 ± 1.8 | 34.7 ± 1.3 | 31.7 ± 1.5 | 35.7 ± 1.1 |
| GST | 9.8 ± 0.8 | 4.71 ± 0.3* | 6.60 ± 0.3* | 6.32 ± 0.4* |
Abbreviations and units: Reactive Oxygen Species (ROS)—FIU; Reduced glutathione (GSH)—mg/gm, Oxidized glutathione (GSSG)—mg/gm, TBARS—μg/gm, Superoxide dismutase (SOD)—units/min/mg protein; Catalase—μmoles H2O2 produced/min/mg protein; Glutathione Peroxidase as GPx—nmole conjugate produced/min/mg protein; Glutathione reductase as GR—units/l and Glutathione S-transferase as GST—μmole/min/mg protein.
Values are mean ± SE; n = 5.
*p < 0.05 When all the exposed groups (arsenic, nicotine and arsenic + nicotine) were compared with normal animals.
**p < 0.05 When co-exposed group was compared to arsenic group.
Renal oxidative stress variables indicated similar alterations in oxidative stress variables in all the exposed groups with an increase in ROS and TBARS on arsenic and nicotine exposure. On the other hand there was a significant depletion in the level of GSH, GSH/GSSG ratio, catalase, GPx and GST activities. Interestingly, these changes during individual exposure do not led to a synergistic effects as there was increase in ROS but it was almost the same as in nicotine exposed group while there was only a marginal synergistic effects in GSSG and GSH/GSSG ratio. The changes in TBARS, catalase, GPx and GST were almost the same as observed with individual exposure .
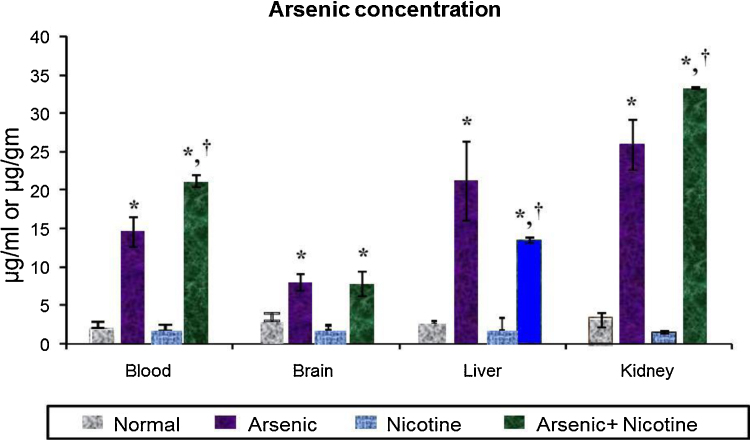
3.8. Effect on arsenic concentration in blood and tissues (Fig. 7)
Fig. 7.
Effect of co-exposure of arsenic and nicotine on arsenic concentration in blood, brain, liver and kidney.
Units: arsenic in blood is expressed as μg/ml blood, arsenic in brain, liver and kidney are expressed as μg g−1 tissue weight.
Values are mean ± SE; n = 5.
*p < 0.05 Compared to normal animals.
† p < 0.05 Compared to arsenic exposed group.
As expected, significant accumulation of arsenic was observed in blood and all tissues studied following chronic exposure to arsenic. However, co-exposure of arsenic and nicotine together demonstrated a highly significant arsenic accumulation in blood and kidney and a marked reduction in liver concentration in comparison to only arsenic exposed groups, indicating the influence of nicotine on the arsenic levels .
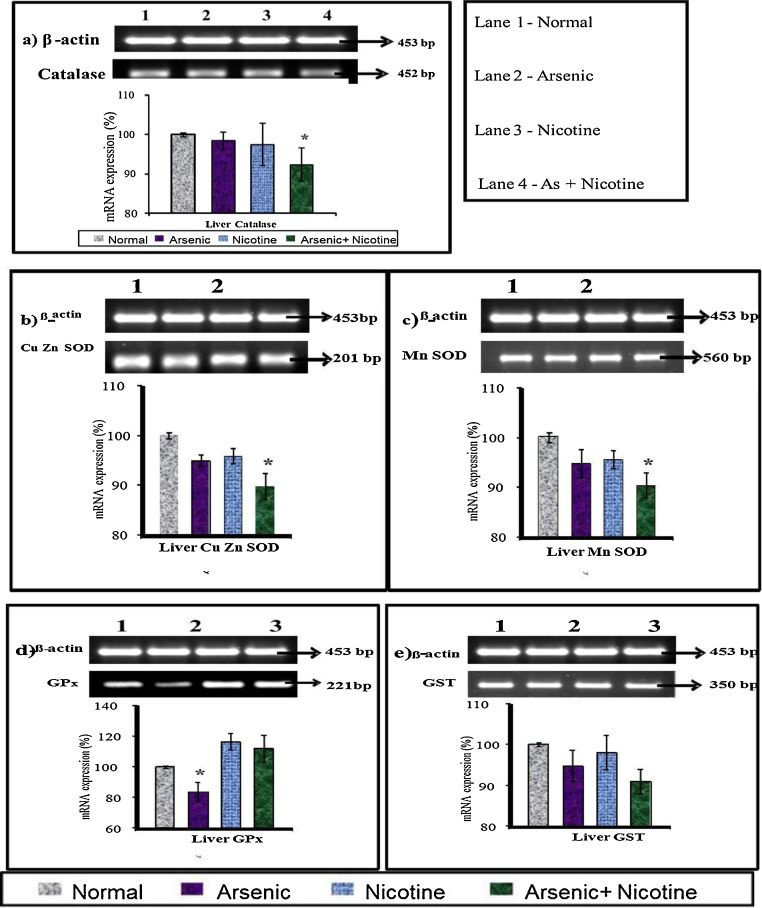
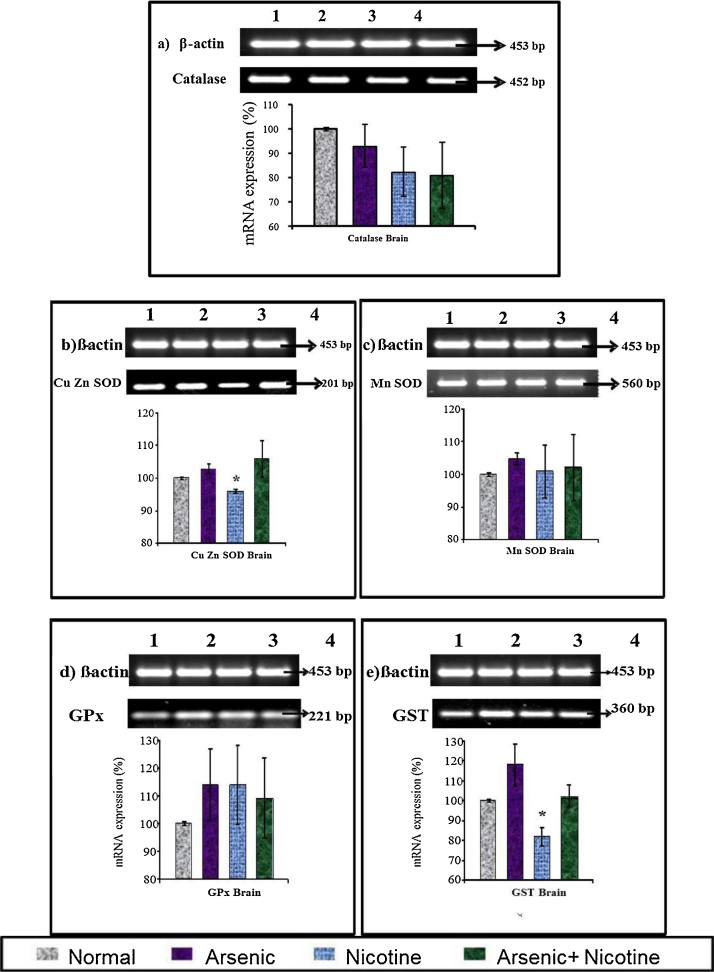
3.9. Effect on liver and brain antioxidant mRNA expression in rats (Fig. 8, Fig. 9, Fig. 10)
Fig. 8.
Total RNA isolation from (A) liver and (B) brain of control and exposed animals after 6 months of exposure. Lane 1—Group 1 (Normal) Lane 2—Group 2 (Arsenic) Lane 3—Group 3 (Nicotine) Lane 4—Group 4 (Nicotine + As).
Fig. 9.
mRNA expression of β-actin (453 bp), catalase (452 bp), Cu–Zn SOD (201 bp), MnSOD (560 bp), GPx (221 bp) and GST (350 bp) in arsenic and nicotine exposed rat liver as compared with control after 6 months of exposure. Lane 1—Group 1 (Normal) Lane 2—Group 2 (Arsenic) Lane 3—Group 3 (Nicotine) Lane 4—Group 4 (Nicotine + As).
*p < 0.05 Compared to normal animals.
Fig. 10.
mRNA expression of β-actin (453 bp), catalase (452 bp), Cu–Zn SOD (201 bp), MnSOD (560 bp), GPx (221 bp) and GST (350 bp) in arsenic and nicotine exposed rat brain as compared with control after 6months of exposure. Lane 1—Group 1 (Normal) Lane 2—Group 2 (Arsenic) Lane 3—Group 3 (Nicotine) Lane 4—Group 4 (Nicotine + As).
*p < 0.05 Compared to normal animals.
Brain catalase transcript level showed decreased expression but it was not statistically significant. Nicotine exposure on the other resulted in significant decrease in Cu-Zn SOD and GST transcript levels. No change in other mRNA expression of other antioxidants was however noted in brain .
Liver catalase, Cu-Zn SOD and MnSOD mRNA expression levels were down-regulated in co-exposed group compared to normal group. Arsenic exposure however led a down regulation of GPx transcript while no change in other groups were noted.
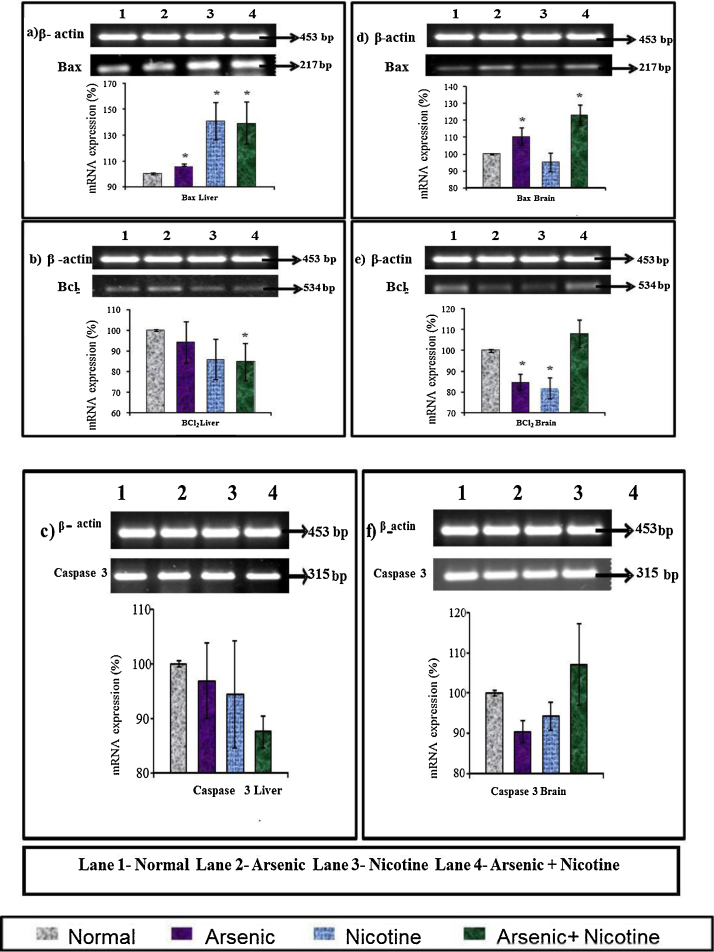
3.10. Effect on liver and brain apoptotic gene mRNA expression in rats (Fig. 11)
Fig. 11.
mRNA expression of β-actin (453 bp), Bax (217 bp), Bcl-2 (534 bp) and Caspase-3 (315 bp) in arsenic and nicotine exposed rat liver and brain as compared with control after 6months of exposure. Lane 1—Group 1 (Normal) Lane 2—Group 2 (Arsenic) Lane 3—Group 3 (Nicotine) Lane 4-Group 4 (Nicotine + As).
*p < 0.05 compared to normal animals.
There was an increase in hepatic Bax transcript level in all of the exposed groups but was more prominent in nicotine and co-exposed group as compared to controls. Hepatic Bcl2 (anti-apoptotic protein) and Caspase 3 mRNA expression also showed a slight down regulation in co-exposed group .
An increase in brain Bax expression in arsenic and co-exposed group along with an increased caspase 3 expression in co-exposed group was also observed. In contrary a decreased Bcl2 mRNA transcript level was found in both the individually exposed arsenic and nicotine groups.
4. Discussion
Arsenic and nicotine are known to induce oxidative stress. Arsenic induces oxidative stress via generation of reactive oxygen species, reactive nitrogen species and also other reactive metabolites of arsenic such as dimethyl arsenic peroxyl radicals, dimethyl arsenic radicals etc [12]. Enhanced generation of reactive oxygen and nitrogen species is associated with a deficient antioxidant system leading to increased oxidative stress and is largely accepted as one of the common mechanisms of arsenic-induced neurotoxicity [48], [11], [56], [58], [6]. Arsenic is also known to induce carcinogenic effects in skin, lungs, liver, kidneys and bladder [66]. On the other hand, nicotine toxicity depends largely on the dose and duration of exposure. Exposure to a low dose and for a shorter duration exhibits antioxidant properties [41] while, prolonged exposure to a higher dose generates free radicals resulting in oxidative stress and reduction in antioxidant enzymes [15]. Interestingly, not only these compounds generated free radicals during their metabolism but also altered the gene expression levels of anti-oxidant enzymes [52], [1], [3], [69]
Arsenic and nicotine individually and in combination demonstrated significant decrease in body weight which could be due to (i) increased metabolic rate [13], (ii) activation of lipoprotein lipase [67] or (iii) suppression of glycolysis [37]. We observed a significant increase in ROS in the exposed groups, however, animals co-exposed to arsenic and nicotine demonstrated comparatively less pronounced increase in ROS compared to the individual exposures, suggesting that effects on ROS were not cumulative but demonstrate largely antagonistic effects which may thus be attributed to a mechanism which currently is unknown and require further investigation.
Arsenic induced changes in glutathione have been reported previously [24], [12]. All the exposed groups showed an initial increase in GSH concentration but gradually it started showing a decrease compared to normal animals. After three months of exposure GSH level again showed a marked increase suggesting an adaptive mechanism might have been developed. An increase in lipid peroxidation was observed in all the exposed groups and the changes were consistent throughout the duration of exposure [51], [62]. Glutathione peroxidase and glutathione reductase activities showed a similar trends after month 1 and 3 in all exposed groups compared to normal animals. A moderate synergism however was noted in GPx activity after combined exposure to arsenic and nicotine. There was masking of nicotine effects by arsenic in the combination group in blood GR activity. An increase in GST activity in all the exposed groups may be attributed to the involvement of GST in arsenic and nicotine detoxification [25], [65].
Elevated serum transaminases activities suggested hepatic injury. Elevated serum GOT activity was noted in all the exposed groups throughout the exposure period (from day 15 to 6 month) indicating liver injury. Interestingly, serum GPT activity decreased in animals co-exposed to nicotine and arsenic compared to normal, arsenic or nicotine group after day 15 and 2 month suggesting antagonism which needs to be explored further. Increased serum urea and creatinine levels indicated renal damage. Previous studies have reported arsenic or nicotine induced hepatic injury and renal dysfunction on continuous exposure [61], [23], [35], [65], [55]. Serum urea increased in all the exposed groups throughout the exposure period except in the first month when we noted a moderate decrease in the combination group. A similar trend in the serum creatinin level was also noted. Lactate dehydrogenase activity in the serum is considered as marker of membrane damage, and it was found elevated on arsenic or nicotine exposure [32], [42]. We noted no changes in LDH activity after day 15 and 3 months in any of the exposed groups studied. However, a similar trend was observed after month 1, 5 and 6. During this period there was a prominent increase in the serum LDH activity in the combination group as compared to individual exposed group.
8-oxo 2′deoxy Guanosine (8-OHdG) concentration was evaluated in the urine as an indicator of oxidative DNA damage. An increased concentration of 8-OHdG in the urine suggest DNA damage. No difference however was noted between the exposed group. 8-OHdG has been reported to be marker for age related chronic diseases [50], [31].
Brain biochemical variables suggested an increased oxidative stress in animals co-exposed to arsenic and nicotine compared to normal animals. Arsenic or nicotine induced increased brain reactive oxygen species and a decreased antioxidant level has been reported earlier [48], [20]. We report no significant difference in the toxicity among the two exposed groups except the brain SOD activity exhibited a more pronounced depletion in co-exposed group compared to normal and individual exposed group. These findings are in consistent with the brain arsenic concentration which was same as in arsenic alone group. Semi quantitative gene expression analysis in the present study of certain antioxidant enzymes also indicated a decrease in the level of antioxidant mRNA transcript level in the exposed groups but the reason behind changes in the expression is not clear
The extend of oxidative injury in brain of arsenic exposed animals was comparatively less pronounced compared to nicotine exposed group. Arsenic and nicotine induced hepatic injury particularly oxidative stress has been extensively studied [12], [7] suggesting that both arsenic and nicotine induced oxidative stress targets liver. It was thus expected that the level of toxicity would be higher in combination group compared to individual exposure groups.
However, it was not seen in the present study possibly due to decreased uptake of arsenic in the co-exposed groups. Decreased liver arsenic level in co-exposed group is possibly due to nicotine induced altered arsenic metabolism. It has been reported earlier that all forms of tobacco use is associated with less efficient arsenic methylation as the smoking inhibits the specific AS3MT involved in arsenic methylation [33]. Cigarette smoking is also known to increase serum homocysteine concentration [45], [53], which, via the concurrent accumulation of S-adenosylhomocysteine, exerts a strong inhibition of S-adenosylmethionine-dependent transmethylation reactions, including those of arsenic [14], [36]. Smokers also tend to have lower levels of folate and vitamin B6 and B12 [45], which are essential for homocysteine metabolism and thus increasing its accumulation. Altogether, these alterations to decreased liver arsenic concentration in co-exposed group and thus less pronounced oxidative stress than expected. Liver antioxidant mRNA expression was also studied and the down regulation of certain antioxidant mRNA transcript was observed in arsenic and co-exposed group but the exact mechanism behind these alterations need to be understood. All of these changes ultimately resulted in decreased protein expression in liver of exposed groups.
Arsenic or nicotine exposure leads to renal oxidative stress during its metabolism suggesting nephrotoxicity [2], [54]. In the present study, all the exposed groups showed renal injury in terms of increased oxidative stress. Increased kidney arsenic concentration in co-exposed group was also possibly due to nicotine induced altered arsenic metabolism in liver leading to enhanced elimination by kidney.
Arsenic or nicotine stimulated apoptosis was investigated in earlier studies and it was observed that both toxicants are responsible for apoptosis related cell death [40], [71]. An increased liver Bax (apoptotic protein) mRNA expression in the nicotine alone and combination group compared to normal group suggest susceptibility to apoptosis in arsenic exposed group. On the other hand in the co-exposed group a slight decreased caspase 3 expression was found. A more detailed study is thus required to unequivocally establish the effects of combined exposure on apoptotic pathway. We observed an increased brain Bax level in arsenic and arsenic + nicotine co-exposed animals while, increased caspase 3 was noted in arsenic + nicotine co-exposed rat brain suggesting an increased apoptosis. However, we admit that apoptotic gene expression data did not provide a final conclusion.
To our knowledge this is the first study to suggest possible synergism between arsenic and nicotine co-exposed animals. However, this was established based only on few variables. Thus there is need to have a more detailed study to establish mechanism that nicotine is a major factor behind synergism earlier reported between tobacco and arsenic. We further suggest a possibility that other than nicotine there might possibly be other constituent(s) moieties responsible for the reported synergism. There was a significant alteration in antioxidant gene expression levels but the mechanisms behind these changes are not known. It can thus be concluded that both arsenic and nicotine if given individually or in combination are toxic at the present dose and duration. Further studies need to be done in this direction to study the exact mechanism behind synergism between arsenic and nicotine on oxidative stress or apoptosis.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgment
One of us (SA) thanks Department of Science and Technology for the award of Junior Research Fellowship.
References
- 1.Bera A.K., Rana T., Bhattacharya D., Das S., Pan D., Das S.K. Sodium arsenite-induced alteration in hepatocytes function of rat with special emphasis on superoxide dismutase expression pathway and its prevention by mushroom lectin. Basic Clin. Pharmacol. Toxicol. 2011;109:240–244. doi: 10.1111/j.1742-7843.2011.00718.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya S., Haldar P.K. Trichosanthes dioica fruit ameliorates experimentally induced arsenic toxicity in male albino rats through the alleviation of oxidative stress. Biol. Trace Elem. Res. 2012;148:232–241. doi: 10.1007/s12011-012-9363-3. [DOI] [PubMed] [Google Scholar]
- 3.Bruin J.E., Petre M.A., Lehman M.A., Raha S., Gerstein H.C., Morrison K.M. Maternal nicotine exposure increases oxidative stress in the offspring. Free Radic. Biol. Med. 2008;44:1919–1925. doi: 10.1016/j.freeradbiomed.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Chen C.L., Hsu L.I., Chiou H.Y., Hsueh Y.M., Chen S.Y., Wu M.M. Blackfoot Disease Study Group. Ingested arsenic, cigarette smoking, and lung cancer risk: a follow-up study in arseniosis endemic areas in Taiwan. J. Am. Med. Assoc. 2004;292:2984–2990. doi: 10.1001/jama.292.24.2984. [DOI] [PubMed] [Google Scholar]
- 5.Daughton C.G. 2008. In: International Encyclopedia of Public Health K., Heggenhougen Kris., Quah Stella, editors. Vol. 5. Academic Press; Oxford: 2008. pp. 66–102. (Pharmaceuticals as Environmental Pollutants: the Ramifications for Human Exposure). [Google Scholar]
- 6.Das J., Ghosh J., Manna P., Sinha M., Sil P.C. Arsenic-induced oxidative cerebral disorders: protection by taurine. Drug Chem. Toxicol. 2010;32:93–102. doi: 10.1080/01480540802564171. [DOI] [PubMed] [Google Scholar]
- 7.Dey S.K., Roy S. Role of reduced glutathione in the amelioration of nicotine-induced oxidative stress. Bull. Environ. Contam. Toxicol. 2010;84:385–389. doi: 10.1007/s00128-010-9948-5. [DOI] [PubMed] [Google Scholar]
- 8.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 9.Ferreccio C., González C., Milosavjlevic V., Marshall G., Sancha A.M., Smith A.H. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology. 2000;11:673–679. doi: 10.1097/00001648-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Flohe L., Gunzler W.A. Assays of glutathione-peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 11.Flora S.J., Gupta R. Beneficial effects of Centella asiatica aqueous extract against arsenic-induced oxidative stress and essential metal status in rats. Phytother. Res. 2007;21:980–988. doi: 10.1002/ptr.2208. [DOI] [PubMed] [Google Scholar]
- 12.Flora S.J.S. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011;51:257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Frankish H.M., Dryden S., Wang Q., Bing C., Mocfarlane I.A., Willians G. Nicotine administration reduces neuropeptide Y and neuropeptide Y mRNA concentrations in the rat hypothalamus: NPY may mediate nicotine’s effects on energy balance. Brain Res. 1995;694:139–146. doi: 10.1016/0006-8993(95)00834-d. [DOI] [PubMed] [Google Scholar]
- 14.Gamble M.V., Liu X., Ahsan H., Pilsner R., Ilievski V., Slavkovich V. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ. Health Perspect. 2005;113:1683–1688. doi: 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan Z.Z., Yu W.F., Nordberg A. Dual effects of nicotine on oxidative stress and neuroprotection in PC12 cells. Neurochem. Int. 2003;43:243–249. doi: 10.1016/s0197-0186(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 16.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases: first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 17.Hays A.M., Srinivasan D., Witten M.L., Carter D.E., Lantz R.C. Arsenic and cigarette smoke synergistically increase DNA oxidation in the lung. Toxicol. Pathol. 2006;34:396–404. doi: 10.1080/01926230600824926. [DOI] [PubMed] [Google Scholar]
- 18.Hissin P.J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 19.Hopenhayn-Rich C., Biggs M.L., Fuchs A., Bergoglio R., Tello E.E., Nicolli H., Smith A.H. Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology. 1996;7:117–124. doi: 10.1097/00001648-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Hritcu L., Ciobica A., Gorgan L. Nicotine-induced memory impairment by increasing brain oxidative stress. Cent. Eur. J. Biol. 2009;4:335–342. [Google Scholar]
- 21.Hughes M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002;33:1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 22.IARC . (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans) International Agency for Research on Cancer; Lyon: 1987. Summaries & evaluations: Arsenic and arsenic compounds (Group 1) p. 100. Supplement 7. [Google Scholar]
- 23.Islam K., Haque A., Karim R., Fajol A., Hossain E., Salam K.A. Dose-response relationship between arsenic exposure and the serum enzymes for liver function tests in the individuals exposed to arsenic: a cross sectional study in Bangladesh. Environ. Health. 2011;10:64. doi: 10.1186/1476-069X-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain A., Flora S.J.S. Age dependent changes in arsenic and nicotine induced oxidative stress in male rat. Inter. Med. Appl. Sci. 2011;3:195–202. [Google Scholar]
- 25.Jain A., Yadav A., Bozhkov A.I., Padalko V.I., Flora S.J.S. Therapeutic efficacy of silymarin and naringenin in reducing arsenic-induced hepatic damage in young rats. Ecotoxicol. Environ. Saf. 2011;74:607–614. doi: 10.1016/j.ecoenv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Jollow D.J., Mitchell J.R., Zamppaglione Z., Gillette J.R. Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolites. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 27.Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide-dismutase. Int. J. Biochem. Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 28.Karaconji I.B. Facts about nicotine toxicity. Arch. Ind. Hyg. Toxicol. 2005;56:363–371. [PubMed] [Google Scholar]
- 29.Kayajanian G. Arsenic, cancer, and thoughtless policy. Ecotoxicol. Environ. Saf. 2003;55:139–142. doi: 10.1016/s0147-6513(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 30.Knobeloch L.M., Zierold K.M., Anderson H.A. Association of arsenic-contaminated drinking-water with prevalence of skin cancer in Wisconsin’s Fox River Valley. J. Health Popul. Nutr. 2006;24:206–213. [PubMed] [Google Scholar]
- 31.Li X., Pi J., Li B., Xu Y., Jin Y., Sun G. Urinary arsenic speciation and its correlation with 8-OHdG in Chinese residents exposed to arsenic through coal burning. Bull. Environ. Contam. Toxicol. 2008;81:406–411. doi: 10.1007/s00128-008-9471-0. [DOI] [PubMed] [Google Scholar]
- 32.Liao Y.T., Chen C.J., Li W.F., Hsu L.I., Tsai L.Y., Huang Y.L. Elevated lactate dehydrogenase activity and increased cardiovascular mortality in the arsenic-endemic areas of southwestern Taiwan. Toxicol. Appl. Pharmacol. 2012;262:232–237. doi: 10.1016/j.taap.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 33.Lindberg A.L., Sohel N., Rahman M., Persson L.A., Vahter M. Impact of smoking and chewing tobacco on arsenic-induced skin lesions. Environ. Health Perspect. 2010;118:533–538. doi: 10.1289/ehp.0900728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindberg A.L., Ekström E.C., Nermell B., Rahman M., Lönnerdal B., Persson L.A., Vahter M. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ. Res. 2008;106:110–120. doi: 10.1016/j.envres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Liu L.Z., Jiang Y., Carpenter R.L., Jing Y., Peiper S.C., Jiang B.H. Role and mechanism of arsenic in regulating angiogenesis. PLoS One. 2011;6:e20858. doi: 10.1371/journal.pone.0020858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marafante E., Vahter M. The effect of methyltransferase inhibition on the metabolism of [74As] arsenite in mice and rabbits. Chem. Biol. Interact. 1984;50:49–57. doi: 10.1016/0009-2797(84)90131-5. [DOI] [PubMed] [Google Scholar]
- 37.Maritz G.S. Maternal nicotine exposure and carbohydrate metabolism of fatal and neonatal lung tissue: response to nicotine with drawl. Respiration. 1987;51:232–240. doi: 10.1159/000195206. [DOI] [PubMed] [Google Scholar]
- 38.Mehta A., Verma V., Nandihalli M., Ramachandra C.J., Sequiera G.L., Sudibyo Y., Chung Y., Sun W., Shim W. A systemic evaluation of cardiac differentiation from mRNA reprogrammed human induced pluripotent stem cells. PLoS One. 2014;9:e103485. doi: 10.1371/journal.pone.0103485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta A., Chung Y.Y., Ng A., Iskandar F., Atan S., Wei H., Dusting G., Sun W., Wong P., Shim W. Pharmacological response of human cardiomyocytes derived from virus-free induced pluripotent stem cells. Cardiovasc. Res. 2011;91:577–586. doi: 10.1093/cvr/cvr132. [DOI] [PubMed] [Google Scholar]
- 40.Mishra D., Mehta A., Flora S.J.S. Reversal of arsenic-induced hepatic apoptosis with combined administration of DMSA and its analogues in guinea pigs: role of glutathione and linked enzymes. Chem. Res. Toxicol. 2008;21:400–407. doi: 10.1021/tx700315a. [DOI] [PubMed] [Google Scholar]
- 41.Mizrak S., Turan V., Caglayan O., Ercan G. The effect of long term pre/postnatal low/high dose nicotine exposure on tissue oxidant/antioxidant status and DNA damage in rats. Drug Res. (Stuttg). 2015 doi: 10.1055/s-0034-1387739. In Press. [DOI] [PubMed] [Google Scholar]
- 42.Muthukumaran S., Sudheer A.R., Menon V.P., Nalini N. Protective effect of quercetin on nicotine-induced prooxidant and antioxidant imbalance and DNA damage in Wistar rats. Toxicology. 2008;243:207–215. doi: 10.1016/j.tox.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 43.National Research Council (US) Arsenic in Drinking Water: 2001 Update. National Academies Press (US); Washington (DC): 2001. Subcommittee to update the 1999 arsenic in drinking water report. [PubMed] [Google Scholar]
- 44.Navas-Acien A., Silbergeld E.K., Streeter R.A., Clark J.M., Burke T.A., Guallar E. Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ. Health Perspect. 2006;114:641–648. doi: 10.1289/ehp.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Callaghan P., Meleady R., Fitzgerald T., Graham I. Smoking and plasma homocysteine. Eur. Heart J. 2002;23:1580–1586. doi: 10.1053/euhj.2002.3172. [DOI] [PubMed] [Google Scholar]
- 46.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 47.Pachauri V., Flora S.J.S. Effect of nicotine pre-treatment on arsenic-induced oxidative stress in male Wistar rats. Hum. Exp. Toxicol. 2013;32:972–982. doi: 10.1177/0960327112474833. [DOI] [PubMed] [Google Scholar]
- 48.Pachauri V., Mehta A., Mishra D., Flora S.J.S. Arsenic induced neuronal apoptosis in guinea pigs is Ca2+ dependent and abrogated by chelation therapy: role of voltage gated calcium channels. Neurotoxicology. 2013;35:137–145. doi: 10.1016/j.neuro.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Parker M.M., Humoller F.L., Mahler D.J. Determination of copper and zinc in biological material. Clin. Chem. 1967;13:40–48. [PubMed] [Google Scholar]
- 50.Prabhulkar S., Li C.Z. Assessment of oxidative DNA damage and repair at single cellular level via real-time monitoring of 8-OHdG biomarker. Biosens. Bioelectron. 2010;26:1743–1749. doi: 10.1016/j.bios.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 51.Ramos O., Carrizales L., Yanez L., Mejia J., Batres L., Ortiz D. Arsenic increased lipid-peroxidation in rat-tissues by a mechanism independent of glutathione levels. Environ. Health Perspect. 1995;103:85–88. doi: 10.1289/ehp.95103s185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rana T., Bera A.K., Das S., Bhattacharya D., Pan D., Das S.K. Metabolic adaptations to arsenic-induced oxidative stress in male Wistar rats. J. Biochem. Mol. Toxicol. 2012;26:109–116. doi: 10.1002/jbt.20416. [DOI] [PubMed] [Google Scholar]
- 53.Refsum H., Nurk E., Smith A.D., Ueland P.M., Gjesdal C.G., Bjelland I. The Hordaland homocystein study: a community based study of homocystein: its determinants and association with disease. J. Nutr. 2006;136:17315–17405. doi: 10.1093/jn/136.6.1731S. [DOI] [PubMed] [Google Scholar]
- 54.Sener G., Sehirli A.O., Ipci Y., Cetinel S., Cikler E., Gedik N. Chronic nicotine toxicity is prevented by aqueous garlic extract. Plant Foods Hum. Nutr. 2005;60:77–86. doi: 10.1007/s11130-005-5103-x. [DOI] [PubMed] [Google Scholar]
- 55.Sener G., Toklu H.Z., Cetinel S. β-Glucan protects against chronic nicotine-induced oxidative damage in rat kidney and bladder. Environ. Toxicol. Pharmacol. 2007;23:25–32. doi: 10.1016/j.etap.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Shila S., Kokilavani V., Subathra M., Panneerselvam C. Brain regional responses in antioxidant system to alpha-lipoic acid in arsenic intoxicated rat. Toxicology. 2005;210:25–36. doi: 10.1016/j.tox.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 58.Sinha M., Manna P., Sil P.C. Taurine, a conditionally essential amino acid, ameliorates arsenic-induced cytotoxicity in murine hepatocytes. Toxicol. In Vitro. 2007;21:1419–1428. doi: 10.1016/j.tiv.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Socci D.J., Bjugstad K.B., Jones H.C., Pattisapu J.V., Arendash G.W. Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp. Neurol. 1999;155:109–117. doi: 10.1006/exnr.1998.6969. [DOI] [PubMed] [Google Scholar]
- 60.Steinmaus C.M., Yuan Y., Smith A.H. The temporal stability of arsenic concentrations in well water in western Nevada. Environ. Res. 2005;99:164–168. doi: 10.1016/j.envres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Tan M., Schmidt R.H., Beier J.I., Watson W.H., Zhong H., States J.C. Chronic sub hepatotoxic exposure to arsenic enhances hepatic injury caused by high fat diet in mice. Toxicol. Appl. Pharmacol. 2011;257:356–364. doi: 10.1016/j.taap.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taysi S., Gumustekin K., Demircan B., Aktas O., Oztasan N., Akcay F. Hippophae rhamnoides attenuates nicotine-induced oxidative stress in rat liver. Pharm. Biol. 2010;48:488–493. doi: 10.3109/13880200903179707. [DOI] [PubMed] [Google Scholar]
- 63.Tchounwou P.B., Patlolla A.K., Centeno J.A. Carcinogenic and systemic health effects associated with arsenic exposure—a critical review. Toxicol. Pathol. 2003;31:575–588. doi: 10.1080/01926230390242007. [DOI] [PubMed] [Google Scholar]
- 64.Tseng C.H. An overview on peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Angiology. 2002;53:529–537. doi: 10.1177/000331970205300505. [DOI] [PubMed] [Google Scholar]
- 65.Vijayan V., Helen A. Protective activity of Bacopa monniera Linn.: on nicotine-induced toxicity in mice. Phytother. Res. 2007;21:378–381. doi: 10.1002/ptr.2073. [DOI] [PubMed] [Google Scholar]
- 66.Waalkes M.P., Liu J., Ward J.M., Diwan B.A. Animal models for arsenic carcinogenesis: inorganic arsenic is a transplacental carcinogen in mice. Toxicol. Appl. Pharmacol. 2004;198:377–384. doi: 10.1016/j.taap.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 67.Winders S.E., Grunberg N.E. Effects of nicotine on body weight food consumption and body composition in male rats. Life Sci. 1990;46:1523–1530. doi: 10.1016/0024-3205(90)90425-q. [DOI] [PubMed] [Google Scholar]
- 68.Worthington D.J., Rosemeyer M.A. Human glutathione reductase: purification of crystalline enzyme from erythrocytes. Eur. J. Biochem. 1974;48:167–177. doi: 10.1111/j.1432-1033.1974.tb03754.x. [DOI] [PubMed] [Google Scholar]
- 69.Wuenschell C., Kunimi M., Castillo C., Marjoram P. Nicotine-responsive genes in cultured embryonic mouse lung buds: interaction of nicotine and superoxide dismutase. Pharmacol. Res. 2004;50:341–350. doi: 10.1016/j.phrs.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Z., Wang X., Cheng S., Sun L., Son Y.O., Yao H., Li W., Budhraja A., Li L., Shelton B.J., Tucker T., Arnold S.M., Shi X. Reactive oxygen species mediate arsenic induced cell transformation and tumorigenesis through Wnt/β-catenin pathway in human colorectal adenocarcinoma DLD1 cells. Toxicol. Appl. Pharmacol. 2011;256:114–121. doi: 10.1016/j.taap.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 71.Zhou X., Sheng Y., Yang R., Kong X. Nicotine promotes cardiomyocyte apoptosis via oxidative stress and altered apoptosis-related gene expression. Cardiology. 2010;115:243–250. doi: 10.1159/000301278. [DOI] [PubMed] [Google Scholar]