Highlights
-
•
A mycological and toxigenic screening was carried out on soy samples for feed use.
-
•
Aspergillus spp. and Fusarium spp. were found to frequently colonize soy.
-
•
Aflatoxin was the most common mycotoxin detected.
-
•
No DON producers were isolated despite DON occurrence.
Keywords: Fumonisins, Fusarium verticillioides, Aflatoxins, Deoxynivalenol, Toxin co-occurrence, Animal health
Abstract
Soy products are a main component of animal feed. Because mycotoxins may harm farm animals, undermining productivity and health, a mycological and toxigenic screening was carried out on 36 batches used in animal feed, collected in 2008, 2009 and 2010 in Italy. The investigated mycoflora of a subset of soy seed (n = 6) suggested that Aspergillus spp. and Fusarium spp. frequently colonize soy seeds. Aflatoxins, fumonisins and deoxynivalenol were detected in 88.9%, 72.2% and 30.6% of samples, respectively. Co-occurrence of at least two toxins was observed in 72% of cases. The molecular analysis of the Fusarium spp. population identified Fusarium verticillioides as potential producers of fumonisins, but no known deoxynivalenol producers were detected. It is suggested that the widespread presence of toxins can be due to non-optimal storing conditions of the feed. Moreover, our results suggest that mycotoxin thresholds should be adapted to consider the frequent case of toxin co-occurrence. This approach would better reflect the real toxigenic risk of feedstuffs.
1. Introduction
Mycotoxins are secondary metabolites produced by several fungi mainly belonging to the genera Fusarium, Aspergillus and Penicillium. Their global occurrence is considered to be a major risk factor, affecting human and animal health. It is estimated that up to 25% of the world's crop production is contaminated to some extent by mycotoxins [11], [12], [19], [28], [35], [39]. Mycotoxin contamination may occur in the field before harvest, during harvesting, or during storage and processing. Environmental factors such as substrate composition, humidity and temperature govern the mycotoxin production and thus the degree of contamination of feed and food commodities. According to their various chemical structure, mycotoxins have a wide spectrum of toxicological effects. The nature and intensity of these effects is related to the dose and duration of exposure [18]. A major concern is chronic low-dose contamination that may even remain undetected, but may result in reduced weight gain, reduced reproduction and increased susceptibility to infections [27].
A large number of predominant mycotoxins are produced by the Fusarium fungi, probably constituting the most prevalent toxin-producing fungi found on cereals in the northern temperate regions of Europe, America and Asia [10]. There is compelling evidence for the implication of fusariotoxins in livestock disorders in different parts of the world. Outbreaks of fusariotoxicoses have been reported for Europe, Asia, New Zealand and South America. Moreover, chronic intake of these mycotoxins is reported on a regular and more widespread basis due to continuing global contamination of cereal grains and animal feed [12].
The most important fusariotoxins with respect to animal health and productivity are deoxynivalenol (DON) and fumonisins (FBs) [35]. Co-occurrence of Fusarium mycotoxins [9] has also become an important issue, with complex and indeterminate implications on animal health and welfare [35].
Exposure to these mycotoxins has been positively linked with a number of specific syndromes in farm livestock [6]. In spite of enhanced awareness of the debilitating effects of these mycotoxins and chronic exposure of farm animals to DON, the risk of exposure to fusariotoxins has not diminished in the past years, presenting a continuous hazard in continental Europe, Canada and the USA [12].
DON, also known as vomitoxin due to its emetic effects in pigs, is produced principally by Fusarium graminearum and Fusarium culmorum and is considered to be a major cause of economic losses due to reduced growth performance. The mode of action of DON is explained by its ability to bind to the 60S ribosomal subunit and to inhibit protein synthesis. Moreover, DON activates mitogen-activated protein kinases (MAPKs) and cause apoptosis through a process known as “ribotoxic stress response” [34]. DON exposure is generally associated with feed refusal, depressed feed intake, and possibly impaired immune function in many animal species [30]. The European Commission (EC) has published guidance levels for DON in products intended for animal feed. These guidance values for DON are 8 mg kg−1 in cereals and cereal products, 12 mg kg−1 in maize by-products and 5 mg kg−1 in complementary and complete feeding stuffs with the exception of feeding stuffs for pigs (0.9 mg kg−1), calves (<4 months) and lambs (2 mg kg−1) [16].
FBs are a group of mycotoxins produced primarily by Fusarium verticillioides and Fusarium poliferatum. The known forms are FB1, FB2 and FB3, of which in particular FB1 is considered the most common and harmful [7].
Due to their structural similarity to the sphingoid bases, FBs interfere with the de novo biosynthesis of ceramide and sphingolipid metabolism by specifically inhibiting sphingosine N-acyltransferase (ceramide synthase). Ceramide synthase inhibition leads to accumulation of the sphingoid bases (sphinganine and sphingosine) in tissues that exert proapoptotic, cytotoxic, and growth inhibitory effects [40].
FBs are likely involved in the incidence of many diseases such as leukoencephalomalacia in horses and lung edema in pigs, and they are also suspected to be a cause of esophageal tumors in certain human populations [36], [6]. Regulatory authorities have established guidance levels for FBs (total including FB1, FB2 and FB3) in animal feed. These guidance values, concerning complementary and complete feeding stuffs, are 5 mg kg−1 for horses, rabbit, pigs and pet animals, 10 mg kg−1 for fish, 20 mg kg−1 for poultry, calves (<4 months) and lambs and 50 mg kg−1 for adult ruminants (>4 months) and mink [16].
Animal exposure to a mixture of several mycotoxins from commercial feed, derived not only from Fusarium but also from Aspergillus, has been reported [3]. However, the occurrence of single-mycotoxin contamination seems to be rare [4]. Generally, data on possible interactions between mycotoxins upon ingestion are poor and often outdated. The effects on some intestinal parameters, including morphology, histology, expression of cytokines and junction proteins, induced by a combined exposure to DON and FBs, were investigated in piglets [4]. In the gastrointestinal tract of piglets for example, four different interactions at different levels of the intestine were reported for the combined effects of DON and FB1: synergistic (number of goblet cells and eosinophils in the ileum), additive (expression of IL-10, TNF-α and adherent proteins), less-than-additive (histological lesions and expression of IFN-γ) and antagonistic effects (some cell populations such as goblet cells, plasma cells, eosinophils and lymphocytes in the jejunum and some cytokine expression such as IL-1β and IL-6) [4]. Synergistic and additive effects are potentially mediated by both DON and FBs [4] through the activation of MAPKs that are known to be involved in several physiological processes such as cell growth, apoptosis and immune response [13]. No explanations were found for the antagonistic effects [4].
An experimental interaction between aflatoxins (AFs) and DON was reported in broiler chickens, and additive toxicity was demonstrated on broiler performance and health [25].
AFs, a group of mycotoxins able to infect a wide range of crops, are produced by several different species of Aspergillus, including A. flavus, A. parasiticus, A. nomius, A. pseudotamarii, A. flavus being the most common. Four different forms of AFs have been identified, including AFB1, AFB2, AFG1, AFG2 [7]. AFs cause liver injury in a wide variety of animal species, and may have effects on production aspects (eggs, milk and weight gains) and on the immune system. AFs are also carcinogenic, teratogenic and mutagenic, with AFB1 being the most toxic [36]. AFB1 is responsible for hepatic cancer by inducing DNA adducts in the target cells that consequently undergo genetic changes [23]. The limits of AFB1 established by the European Community concerning complete feeding stuffs, are 20 μg kg−1 for cattle, sheep and goats, 5 μg kg−1 for dairy animals, 10 μg kg−1 for calves and lambs and 20 μg kg−1 for pigs and poultry [17].
The study of mycological composition of feed may help guiding the detection of toxins [33] despite the impossibility to predict the amount of toxins produced, given the fact that mycotoxin production is linked to different environmental factors such as climate [38]. Few studies have so far focused on the potential contamination of soy by multiple types of mycotoxins [26]. The aim of this work was to assess the mycotoxigenic risk of soy samples used for animal feed by a combined study of the mycological composition of soy samples and their toxin content with special attention to potential co-occurrence of fusariotoxins.
2. Materials and methods
2.1. Sampling and mycological analysis
Soy samples were collected randomly from a feed manufacturing company located in the Lombardy region (Northern Italy). The samples were obtained during 2008–2010 (Supplementary Table 1). All samples were stored at 4 °C in sealed plastic bags until mycological and mycotoxin analysis. Fifty seeds were randomly selected, surface sterilized in 0.37% NaOCl (VWR Prolabo, Briar France) and immersed in 0.1% ‘Tween 20’ (Acros New Jersey, USA) for 10 min before being dried on sterile filter paper in a laminar flow hood.
In the case of soy flour, 10 g were used and mixed with 1, 10, or 100 ml of water. 1 ml of each dilution was then used for plating. From the original batch of isolates (Table 1), a liquid suspension of the soy seed or flour was plated on 20 potato dextrose agar (PDA) Petri dishes and left at 24 °C for up to 7 days. Colonies were screened according to their phenotype on PDA. Genus or order attribution was carried out according to standard taxonomic procedures as described by [31] for Fusarium spp. [20], for Penicillium spp., and [2] for Aspergillus spp. The procedure of Fusarium selection carried out for the seed batches listed in Table 2 followed the protocol described by [21]. After 6–12 days, Fusarium resembling colonies were transferred to PDA (Merck Darmstadt Germany) and incubated at 22 ± 2 °C for 6 days. Single-colonies were then produced by washing off spores with sterile deionized distilled water and by serial dilutions on PDA plates. Spores produced were stored at −80 °C until further use.
Table 1.
Seed lot ID of soy feed samples obtained from Italy in 2008–2010, and number of strains obtained for each fungal group from 20 plates for each seed lot.
| Seed lot ID | Black aspergilli | Other Aspergillus spp. | Fusarium spp. | Penicillium spp. | Microdochium spp. | Other fungi |
|---|---|---|---|---|---|---|
| S01 | – | – | – | – | – | 3 |
| S02 | – | 5 | – | 3 | – | – |
| S03 | 2 | 6 | – | – | 1 | 2 |
| S04 | – | – | – | – | – | – |
| S05 | 5 | 23 | – | – | – | 5 |
| S06 | 3 | 2 | 6 | 1 | – | – |
Table 2.
Isolate ID, seed lot from where the strain was obtained, NCBI temporary deposited sequence number and species attribution.
| Isolate ID | Seed lot | NCBI sequence number | Species |
|---|---|---|---|
| S06_1 | S06 | JQ354942 | Fusarium verticillioides |
| S06_2 | S06 | JQ354947 | Fusarium verticillioides |
| S06_3 | S06 | JQ354954 | Fusarium verticillioides |
| S06_4 | S06 | JQ354943 | Fusarium verticillioides |
| S06_5 | S06 | JQ354955 | Fusarium verticillioides |
| S06_6 | S06 | JQ354949 | Fusarium verticillioides |
| S07_1 | S07 | JQ354945 | Fusarium verticillioides |
| S07_2 | S07 | JQ354958 | Fusarium verticillioides |
| S07_3 | S07 | JQ354959 | Fusarium verticillioides |
| S07_4 | S07 | JQ354950 | Fusarium verticillioides |
| S08_1 | S08 | JQ354951 | Fusarium verticillioides |
| S08_2 | S08 | JQ354944 | Fusarium verticillioides |
| S08_3 | S08 | JQ354946 | Fusarium verticillioides |
| S08_6 | S08 | JQ354952 | Fusarium verticillioides |
| S09_1 | S09 | JQ354956 | Fusarium verticillioides |
| S09_2 | S09 | JQ354960 | Fusarium verticillioides |
| S09_3 | S09 | JQ354957 | Fusarium verticillioides |
| S09_4 | S09 | JQ354948 | Fusarium verticillioides |
| S09_5 | S09 | JQ354953 | Fusarium verticillioides |
2.2. Mycotoxin analysis
Samples were analyzed for the presence of FBs, total AFs and DON using commercially available quantitative ELISA assay kits (Helica Biosystems, Inc., Fullerton, CA, USA). Mycotoxin extraction and analysis were performed according to the manufacturer's instructions as follows. A total of 20 g of the samples was mixed with 40 ml of 90% methanol (VWR International, Milan, Italy) for FBs, 100 ml of 70% methanol for AFs and 100 ml of deionized water from a Milli-Q system (Millipore, Bedford, MA, USA) for DON. The extract was then filtered through filter paper (Whatman No. 1 – Whatman Inc., Clifton, NJ, USA) and the filtrate was used directly for AF ELISA analysis, while for FB and DON detection the filtrate was diluted with deionized water (1:20 and 1:10, respectively). The ELISA procedure was performed according to the manufacturer's instructions. The optical density (OD) was measured at 450 nm by an ELISA reader (Labsystems Multiskan Plus, Labsystems, Helsinki, Finland). A calibration curve using OD values was constructed from five standard concentrations between 0.1–6 mg kg−1 for FBs, 1–20 μg kg−1 for AFs and 0.5–10 mg kg−1 for DON. The limits of detection were 0.1 mg kg−1 for FBs, 1 μg kg−1 for AFs and 0.15 mg kg−1 for DON. The mycotoxin concentrations in the samples were measured by interpolation from the corresponding calibration curves.
2.3. Molecular analysis
Fungal cultures from specified seed lots (Table 2) were grown for 5 days in PDB (Sigma) and DNA was extracted according to the protocol described by [21]. The identity of the strains isolated was confirmed by sequencing of the elongation factor 1α (EF-1α), using primers and PCR conditions as described by [14]. The sequences were then blasted using both the Fusarium database (http://isolate.fusariumdb.org/blast.php) and the NCBI (National Center for Biotechnology Information) blast tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome), in order to determine the species. Sequences of EF-1α were analyzed using the CLC main workbench 6.01 software (CLC BIO, Aarhus, DK).
3. Results
3.1. Fungal characterization
Morphological observations on PDA plates were carried out on an original batch of seed lots from 2008 (Table 1) in order to guide the potential search for dangerous toxins that are regulated at the European level. The identification on PDA plates of colonies resembling Aspergillus and Fusarium species suggested testing for AFs, DON and FBs. In addition, other potentially mycotoxigenic fungi were identified, such as Pennicilium spp.
3.2. Mycotoxin contamination
FBs were detected in 30.6% of the samples with an average concentration of 0.4 mg kg−1; the maximum level recorded was 2.5 mg kg−1 (Table 3). AFs occurred in 88.9% of the samples with an average concentration of 3 μg kg−1; the maximum level recorded was 5.9 μg kg−1 (Table 3). DON was detected in 72.2% of the samples with an average concentration of 2.6 mg kg−1; the maximum level recorded was 6.4 mg kg−1 (Table 3).
Table 3.
Occurrence of fumonisins, aflatoxins and deoxynivalenol in 36 soy feed samples.
| Mycotoxins | Positive samples (%) | Concentrations |
|
|---|---|---|---|
| Average ± SD (mg kg−1) | Range (mg kg−1) | ||
| Fumonisins | 30.6 | 0.40 ± 0.70 | 0.1–2.5 |
| Aflatoxins | 88.9 | 3.0·10−3 ± 1.36·10−3 | 0.8·10−3–5.9·10−3 |
| Deoxynivalenol | 72.2 | 2.60 ± 1.37 | 0.8–6.4 |
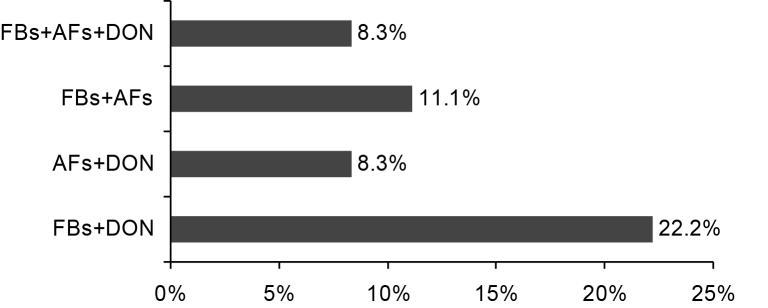
Co-occurrence of FBs, AFs and DON was found in 8.3% of the samples analyzed. The most frequent mycotoxin combination was FBs + DON (22.2%) followed by FBs + AFs (11%) (Fig. 1).
Fig. 1.
Co-occurrence of fumonisins (FBs), aflatoxins (AFs) and deoxynivalenol (DON) in soy feed samples (n = 36) collected in Italy between 2008 and 2010.
3.3. Molecular identification
To precisely determine which species of Fusarium were obtained from the isolation procedure on PDA, monoconidial strains were produced. The partial EF-1α sequences were obtained from the 19 Fusarium isolates obtained from 4 feed batches. Sequences were deposited at the NCBI gene bank with accession numbers as specified in Table 2.
All the strains were classified as Fusarium verticillioides, member of the Gibberella fujikuroi species complex [32]. No Fusarium species known to be able to produce DON could be isolated. On the contrary, FBs producers (Gibberella fujikuroi species complex) were isolated.
4. Discussion
Soy material can be contaminated by various mycotoxins, but systematic investigations of toxigenicity in soy are lacking. Because soy is a widely used component of animal feeds, but also employed for human consumption, we explored the level of contamination of mycotoxins present in soy samples used for animal feed by combining mycotoxin measures and mycological determination of colonizing fungi.
Fusarium species were reported to infect soybean [5], [24], and contamination of soy by fusariotoxins such as DON was shown in Brazil [29]. In this work, it is suggested that species from the Gibberella fujikuroi complex are probably the main cause of FB accumulation. Surprisingly, no DON producers have been identified, despite our attempts of isolation. The results can be explained by the presence of unknown fungal strains able to produce DON that cannot be isolated with traditional methods or more likely by the presence of chemicals that cross react in the ELISA test. Further studies applying LC–MS methods are warranted to elucidate the cause for this observation. The most frequently found toxin in soy was AF, which is produced by Aspergillus spp. Indeed a large set of isolates obtained from soy were classified as belonging to the latter genus. This result differs from a previous survey by Escobar and Reguero [15] in Cuba who found less than 5% of the soybean contaminated by AFs. The overall levels of mycotoxin contamination observed were not extremely high, and all concentrations were below the EU guidance levels. Nonetheless, the co-occurrence of different toxins may be a cause of concern that requires further attention.
Soy contamination may have occurred in the storage facilities, because it was not possible to obtain isolates from the inside of the intact soy seeds. However, it was not possible nor the aim of this study to establish whether Fusarium contamination occurred in the field or during storage. This can indeed be the case, given the fact that, often, different cereals are stocked in the same location. The importance of correct storage of grains in order to diminish the risk of cross-contamination and toxin diffusion is fundamental to preserve healthy feed.
Combinations of AFs and DON were shown to be extremely toxic and to have an impact on feed intake [8], therefore attention toward mycotoxin co-occurrence should be increased. To conclude, the work represents the first description of soy material used as animal feed contaminated by multiple mycotoxins. As reported for corn [37] and other crops [1], co-occurrence of toxins produced by a diverse set of fungal species can be frequent. Soy material used for animal feed showed relatively low levels of contamination but large screening campaigns for detecting the co-occurrence of mycotoxins that could enhance overall toxicity due to synergistic effects [22] are warranted.
Conflict of interest
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2015.01.006.
Transparency document
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Beg M.U., Al-Mutairi M., Beg K.R., Al-Mazeedi H.M., Ali L.N., Saeed T. Mycotoxins in poultry feed in Kuwait. Arch. Environ. Contam. Toxicol. 2006;50:594–602. doi: 10.1007/s00244-005-2094-0. [DOI] [PubMed] [Google Scholar]
- 2.Bennett J.W. An overview of the genus Aspergillus. In: Machida M., Gomi K., editors. Aspergillus: Molecular Biology and Genomics. Caister Academic Publisher; USA: 2010. pp. 4–37. [Google Scholar]
- 3.Binder E.M., Tan L.M., Chin L.J., Handl J., Richard J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007;137:265–282. [Google Scholar]
- 4.Bracarense A.P.F.L., Lucioli J., Grenier B., Drociunas Pacheco G., Moll W.D., Schatzmayr G., Oswald I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012;107:1776–1786. doi: 10.1017/S0007114511004946. [DOI] [PubMed] [Google Scholar]
- 5.Broders K.D., Lipps P.E., Dorrance A.E. Evaluation of F. graminearum as a seed and seedling pathogen of corn and soybean in Ohio. Phytopathology. 2007;97(Suppl.):S159. [Google Scholar]
- 6.Caloni F., Cortinovis C. Effects of fusariotoxins in the equine species. Vet. J. 2010;186:157–161. doi: 10.1016/j.tvjl.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Chaytor A.C., Hansen J.A., van Heugten E., See M.T., Kim S.W. Occurrence and decontamination of mycotoxins in swine feed. Asian Australas. J. Anim. Sci. 2011;5:723–738. [Google Scholar]
- 8.Chaytor A.C., See M.T., Hansen J.A., de Souza A.L.P., Middleton T.F., Kim S.W. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs. J. Anim. Sci. 2011;89:124–135. doi: 10.2527/jas.2010-3005. [DOI] [PubMed] [Google Scholar]
- 9.Cote L.M., Beasley V.R., Bratich P.M., Swanson S.P., Shivaprasad H.L., Buck W.B. Sex-related reduced weight gains in growing swine fed diets containing deoxynivalenol. J. Anim. Sci. 1985;61:942–950. doi: 10.2527/jas1985.614942x. [DOI] [PubMed] [Google Scholar]
- 10.Creppy E.E. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002;127:19–28. doi: 10.1016/s0378-4274(01)00479-9. [DOI] [PubMed] [Google Scholar]
- 11.Diekman M.A., Green M.L. Mycotoxins and reproduction in domestic livestock. J. Anim. Sci. 1992;70:1615–1627. doi: 10.2527/1992.7051615x. [DOI] [PubMed] [Google Scholar]
- 12.D’Mello J.P.F., Placinta C.M., MacDonald A.M.C. Fusarium mycotoxins: a review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999;80:183–205. [Google Scholar]
- 13.Dong C., Davis R.J., Flavell R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 14.Dubos T., Pasquali M., Pogoda F., Hoffmann L., Beyer M. Evidence for natural resistance towards trifloxystrobin in Fusarium graminearum. Eur. J. Plant Pathol. 2011;130:239–248. [Google Scholar]
- 15.Escobar A., Regueiro O.S. Determination of Aflatoxin B1 in food and feedstuffs in Cuba (1990 through 1996) using an immunoenzymatic reagent kit (Aflacen) J. Food Prot. 2002;65:219–221. doi: 10.4315/0362-028x-65.1.219. [DOI] [PubMed] [Google Scholar]
- 16.European Commission Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (2006/576/EC) Off. J. Eur. Union L. 2006;229:7–9. [Google Scholar]
- 17.FAO Worldwide regulations for mycotoxins in food and feed in 2003. Food Nutr. Pap. 2004;81:1–7. [PubMed] [Google Scholar]
- 18.Fink-Gremmels J. Mycotoxins: their implications for human and animal health. Vet. Q. 1999;21:115–120. doi: 10.1080/01652176.1999.9695005. [DOI] [PubMed] [Google Scholar]
- 19.Fink-Gremmels J., Georgiou N.A. Risk assessment of mycotoxins for the consumers. In: Ennen G., Kuiper H.A., Valentin A., editors. Residues of Veterinary Drugs and Mycotoxins in Animal Products. NL-Wageningen Press; Wageningen: 1996. pp. 159–174. [Google Scholar]
- 20.Frisvad J.C., Samson R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 2007;49:1–174. [Google Scholar]
- 21.Giraud F., Pasquali M., El Jarroudi M., Vrancken C., Brochot C., Cocco E., Hoffmann L., Delfosse P., Bohn T. Fusarium head blight and associated mycotoxin occurrence on winter wheat in Luxembourg in 2007/2008. Food Addit. Contam. A. 2010;27:825–835. doi: 10.1080/19440040903567232. [DOI] [PubMed] [Google Scholar]
- 22.Gutleb A.C., Morrison E., Murk A.J. Cytotoxicity assays for mycotoxins produced by Fusarium strains – a review. Environ. Toxicol. Pharmacol. 2002;11:307–318. doi: 10.1016/s1382-6689(02)00020-0. [DOI] [PubMed] [Google Scholar]
- 23.Hamid A.S., Tesfamariam I.G., Zhang Y., Zhang Z.G. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: geographical distribution, mechanism of action and prevention. Oncol. Lett. 2013;5:1087–1092. doi: 10.3892/ol.2013.1169. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington T.C., Steimel J., Workneh F., Yang X.B. Molecular identification of fungi associated with vascular discoloration of soybean in the north central United States. Plant Dis. 2000;84:83–89. doi: 10.1094/PDIS.2000.84.1.83. [DOI] [PubMed] [Google Scholar]
- 25.Huff W.E., Kubena L.F., Harvey R.B., Hagler W.M., Jr., Swanson S.W., Phillips T.D., Creger C.R. Individual and combined effects of aflatoxin and deoxynivalenol (DON, vomitoxin) in broiler chickens. Poult. Sci. 1986;65:1291–1298. doi: 10.3382/ps.0651291. [DOI] [PubMed] [Google Scholar]
- 26.Jajić I., Jurić V., Abramović B. First survey of deoxynivalenol occurrence in crops in Serbia. Food Control. 2008;19:545–550. [Google Scholar]
- 27.Kuiper-Goodman T. Mycotoxins: risk assessment and legislation. Toxicol. Lett. 1995;82–83:853–859. doi: 10.1016/0378-4274(95)03599-0. [DOI] [PubMed] [Google Scholar]
- 28.Larsen J.C., Hunt J., Perrin I., Ruckenbauer P. Workshop on trichothecenes with a focus on DON: summary report. Toxicol. Lett. 2004;153:1–22. doi: 10.1016/j.toxlet.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Martinelli J.A., Bocchese C.A.C., Xie W., O’Donnell K., Kistler H.C. Soybean pod blight and root rot caused by lineages of the Fusarium graminearum and the production of mycotoxins. Fitopatol. Bras. 2004;29:492–497. [Google Scholar]
- 30.Morgavi D.P., Riley R.T. A historical overview of field disease outbreaks known or suspected to be caused by consumption of feeds contaminated with Fusarium toxins. Anim. Feed Sci. Technol. 2007;137:201–212. [Google Scholar]
- 31.Nelson P.E., Dignani M.C., Anaissie E.J. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 1994;7:479–504. doi: 10.1128/cmr.7.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Donnell K., Cigelnik E., Nirenberg H.I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia. 1998;90:465–493. [Google Scholar]
- 33.Pasquali M., Giraud F., Brochot C., Cocco E., Hoffmann L., Bohn T. Genetic Fusarium chemotyping as a useful tool for predicting nivalenol contamination in winter wheat. Int. J. Food Microbiol. 2010;137:246–253. doi: 10.1016/j.ijfoodmicro.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Pestka J.J. Deoxynivalenol: toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007;137:283–298. [Google Scholar]
- 35.Placinta C.M., D’Mello C.P.F., MacDonald A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999;78:21–37. [Google Scholar]
- 36.Richard J.L. Some major mycotoxins and their mycotoxicoses – an overview. Int. J. Food Microbiol. 2007;119:3–10. doi: 10.1016/j.ijfoodmicro.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Rocha L.O., Nakai V.K., Braghini R., Reis T.A., Kobashigawa E., Corrêa B. Mycoflora and co-occurrence of fumonisins and aflatoxins in freshly harvested corn in different regions of Brazil. Int. J. Mol. Sci. 2009;10:5090–5103. doi: 10.3390/ijms10115090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt-Heydt M., Parra R., Geisen R., Magan N. Modelling the relationship between environmental factors, transcriptional genes and deoxynivalenol mycotoxin production by strains of two Fusarium species. J. R. Soc. Interface. 2011;8:117–126. doi: 10.1098/rsif.2010.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schollenberger M., Muller H.M., Rufle M., Terry-Jara H., Suchy S., Plank S., Drochner W. Natural occurrence of Fusarium toxins in soy food marketed in Germany. Int. J. Food Microbiol. 2007;113:142–146. doi: 10.1016/j.ijfoodmicro.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 40.Voss K.A., Voss G.W., Haschek W.M. Fumonisins: toxicokinetics, mechanism of action and toxicity. Anim. Feed Sci. Technol. 2007;137:299–325. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.