Abstract
Fetal/neonatal exposure to the endocrine disruptor bisphenol A (BPA) has induced obesity and increased glucose intolerance. We hypothesized that chronic BPA exposure would worsen the obesity and glucose intolerance induced by a high fat diet (HFD). The drinking water of C57bl/6n dams was treated with vehicle (VEH) or BPA (25 ng/ml) from gestation day 11.5 to postnatal day 21. Another group was treated with oral diethylstilbestrol (DES, 1 μg/kg/day) during gestation. Progeny were treated with VEH (VEH and DES groups) or BPA (2.5 ng/ml) in the drinking water and fed either a control diet (CD) or HFD from weaning until euthanasia at 4 months of age. CD-fed mice were similar in size; however HFD-BPA males and HFD-DES mice were smaller than HFD-VEH mice. No CD-fed mice were glucose intolerant. All HFD-fed mice were glucose intolerant. Cholesterol and triglyceride were increased in HFD-VEH mice and HFD-BPA males. Total fat weight and adipocyte area were similar in HFD-VEH and HFD-BPA mice and reduced in HFD-DES mice. HFD-BPA females increased perirenal and reduced gonadal fat weights. Reduced leptin and increased IL-6 in CD-BPA and CD-DES mice were not found in their HFD-cohorts. Adiponectin levels were similar. Thus, although chronic BPA exposure did not increase body size or increase glucose intolerance, it induced an adipokine imbalance in CD-fed mice and sex-specifically altered the lipid response and adipose deposition when fed the HFD.
Abbreviations: AUC, area under the curve; BL, body length; BPA, bisphenol A; BMI, body mass index; BSA, body surface area; BW, body weight; CD, control diet; DES, diethylstilbestrol; GD, gestation day; GTT, glucose tolerance test; HFD, high fat diet; IL-6, interleukin 6; VEH, vehicle
Keywords: Chronic bisphenol A exposure, Glucose intolerance, Obesity, C57bl/6n mice, High fat diet
1. Introduction
Exposure to various man-made compounds is continuous, widespread and begins early in life [1], [2], [3]. National surveys have demonstrated the near ubiquitous presence of many compounds, including the estrogenic endocrine disruptor compound bisphenol A (BPA), in the urinary profiles of most people [3], [4], [5]. Exposure to endocrine disruptor compounds especially in early life stages may have long term consequences beyond their impact on reproductive health. Although a link between higher BPA exposures and greater chronic disease incidence has been challenged [6], most epidemiological analyses suggest that higher BPA exposure may enhance cardiovascular disease, obesity and diabetes morbidity in humans [7], [8], [9], [10], [11]. The impact of BPA might also be found before adulthood. Higher urinary BPA levels were linked to greater obesity in children [12], [13]. One major concern is that increased exposure to endocrine disruptor compounds such as BPA may be driving an increase in obesity and type 2 diabetes in children and adults [3], [14].
Supporting the human data, BPA exposure during the fetal/neonatal period has led to glucose intolerance in the adult progeny in most rodent studies [15], [16], [17], [18], [19]. When adult male and female rats and mice were compared, BPA exposed males showed glucose intolerance, while similarly treated females were unaffected [15], [16], [17]. However, other studies did not find a correlation between early life BPA exposure and glucose intolerance in adults of either sex [20], [21]. BPA exposure is not restricted to gestation or lactation; rather BPA exposure is lifelong [3]. Thus, it was intriguing that glucose intolerance was not observed in adult male or female outbred CD-1 mice treated with dietary from gestation day 0 until euthanasia on postnatal day 70 [22]. Overall, such data argue that the development of adult glucose intolerance is greatly influenced by sex and the time of BPA exposure.
Obesity is associated with the development of glucose intolerance and type 2 diabetes [23]. In rodent models, studies linking BPA exposure with induced obesity and greater glucose intolerance have resulted in conflicting results. A correlation between BPA-induced obesity and glucose intolerance in the adult progeny was identified in some, but not all, epidemiological studies [15], [19], [20], [21], [22], [24], [25], [26]. In experimental studies, obesity can be induced by feeding mice a diet high in fat. When outbred CD-1 mice were exposed to low dose BPA during the fetal/neonatal period and then fed a control diet or high fat diet for 5 weeks beginning at 9 weeks of age, no glucose intolerance was observed in male or female mice fed either diet [20]. In contrast, using a similar design and outbred CD-1 mice, but initiating control or high fat diet feeding at 3 months of age, glucose intolerance was found in BPA exposed males fed the control and high fat diets, but not in the similarly exposed and fed females [16]. Although these data found no consensus for an additional impact of a high fed diet on mice exposed to BPA, they suggest the potential for an additive effect for high fat diet and BPA and suggest that male mice may be especially susceptible.
In the present experiments, we extended these studies to a common inbred mouse line, C57bl/6n, and continuously treated the mice with oral BPA from gestation day 11.5 to euthanasia at 4 months of age. We tested the hypothesis that chronic BPA exposure would worsen the impact of obesity started in early life due to high fat feeding and that males would be particularly vulnerable to the combined effect of BPA and a high fat diet. We report the impact of chronic BPA exposure on body growth, organ fat deposits, mesenteric adipocyte size, glucose tolerance, serum lipid and adipokine parameters of male and female mice fed a control or high fat diet.
2. Materials and methods
2.1. Animal manipulation
The animal use protocol was reviewed by the Lady Davis Institute Animal Care Committee and animal experiments were performed according to the guidelines of the Canadian Council on Animal Care. The timeline of experimental handling is shown in the schematic in Fig. 1A. C57bl/6n female mice (Charles River, St. Constant, Que.) were mated and the day of vaginal plug detection designated gestation day 0.5. Dams were fed a Harlan Teklad Global 2018 diet (3.1 kcal/g, 6.2% fat, 18% calories fat) before mating and during gestation and lactation. All mice were housed in polycarbonate cages with ¼ in. corn cob bedding in a 12 h dark/light schedule. Food and water intake was ad libitum.
Fig. 1.
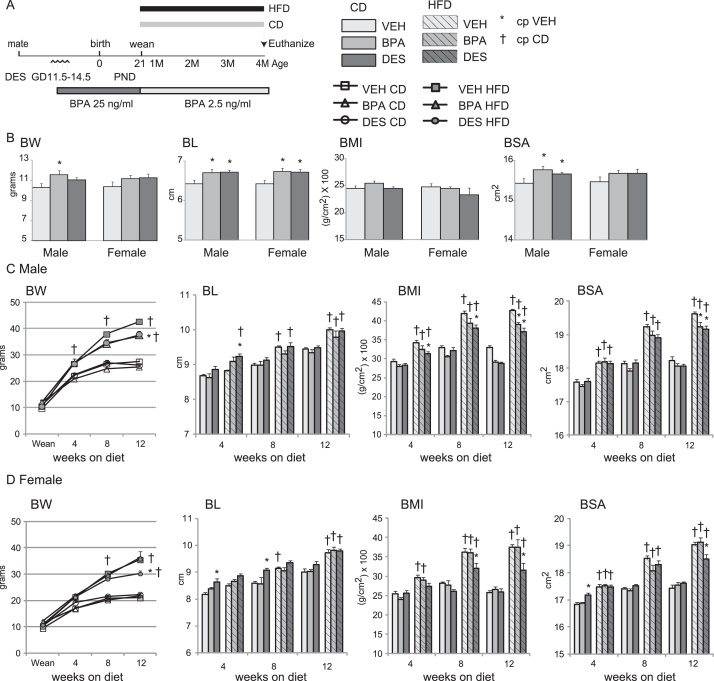
(A) Schematic of experimental design. C57bl/6n dams, n = 6–7 per treatment, were given drinking water containing BPA, 25 ng/ml, or an equal volume of ethanol (vehicle, VEH) beginning on gestation day 11.5 and continuing until weaning on postnatal day 21. The drinking water of progeny contained 2.5 ng/ml BPA beginning at weaning until euthanasia at 4 months of age. A separate group of dams were orally treated with DES (1 μg/kg/day) using a micropipette on gestation days 11.5–14.5. VEH, BPA and DES progeny were randomly selected and fed either a control (CD) or high fat diet (HFD) from weaning until euthanasia. (B–D) Physiological parameters at weaning (B) or with time on diets in male (C) and female (D) mice. BL is body length in cm; BW is body weight in grams; BMI is body mass index using the formula BMI = [BW/(BL)2] x 100 where BW is in g and BL in cm. Body surface area (BSA) was calculated using the formula). Data are expressed as mean ± SEM. Significance indicated by p < 0.05 when compared with * VEH; † CD.
BPA (>99% pure, 2396558, CAS 80-05-7) was purchased from Sigma–Aldrich (Oakville, Ont.). BPA was dissolved in ethanol and added to a concentration of 25 ng BPA/ml sterile-filtered drinking water. This amount replicates that used in previous studies that demonstrated BPA-mediated glucose intolerance in outbred CD-1 mice [19], [22]. An equal volume of ethanol (final concentration 0.1%) was added to the drinking water of vehicle (VEH) treated dams and their progeny. Dams, n = 6–7/group, were exposed to the treatments in glass bottles from gestation day 11.5 until pup weaning on postnatal day 21.
Diethylstilbestrol (DES), a non-steroidal estrogen, has been used as an estrogenic reference compound for BPA [27] and in studies exploring the impact of endocrine disruptor compounds in rodents [16], [20], [27], [28], [29], [30], [31], [32]. DES (99% pure, D4628, CAS 56-53-1) was purchased from Sigma–Aldrich (Oakville, Ont.). Dams, n = 6, were treated once daily with DES (1.0 μg/kg body weight in peanut oil) on gestation days 11.5–14.5 using a micropipette. This amount is similar to that which previously induced glucose intolerance in outbred CD-1 mice [19].
At weaning on postnatal day 21, 1–2 progeny from each dam were randomly selected to be placed on either a CD (Teklad TD6416, 3.7 kcal/g, 4.2% fat, 10% of calories from fat) or a HFD (Teklad TD6414, 5.1 kcal/g, 34% fat, 60% calories from fat) until euthanasia at 4 months of age. Within treatment groups, the BW of those selected for the CD or HFD were not statistically different. Also at weaning, the concentration of BPA in the water was reduced ten-fold to 2.5 ng BPA/ml. The dose of BPA was reduced at weaning to simulate reduced BPA exposure in adulthood [3] and is similar to the exposures used in previous studies [16], [20]. Mice, n = 7–8/treatment/sex, were randomly selected for each test unless otherwise noted.
2.2. Physiological parameters
Body weight (BW) and body length (BL) measurements were collected at weaning on postnatal day 21 and monthly thereafter until euthanasia at 4 months of age. BL was measured from nose to anus using calipers.
At euthanasia, the weight of the testes, all prostate lobes, seminal vesicles including contents, uterus and ovaries were measured. In addition, the BW, BL and the wet weight of adipose deposits surrounding the kidneys, testes, ovaries and mesentery was collected. Body mass index (BMI) was calculated using the formula BMI = [BW/(BL)2] × 100 where BW is in grams and BL in cm. Body surface area (BSA) was calculated using the formula where K is 10.5 and W is the BW in grams [33], [34].
2.3. Glucose and insulin tolerance tests
At ∼15 weeks of age, after 12 weeks on the respective diets, food was removed from the cages for 5 h prior to glucose tolerance testing [35]. Blood was collected from the saphenous vein, applied to OneTouch Ultra Test Strips and glucose levels read using a OneTouch SureStep Glucometer (DiabetesShop, Brampton, Ont.) according to the manufacturer's instructions. After baseline glucose measurement, mice were injected IP with dextrose (2.0 g/kg BW) and glucose was quantified 15, 30, 60 and 120 min later. The area under the curve (AUC) was calculated using the trapezoidal rule [35].
2.4. Serum analyses
Blood was collected by cardiac puncture after a 4 h fast. The blood was allowed to clot, the serum collected and frozen at −80 °C prior to analyses. Measurement of cholesterol and triglyceride was performed by the McGill Comparative Medicine Department (Montreal, Que.) using a VITROS 350 chemistry analysis system. ELISA kits specific for mouse serum samples were used to measure leptin and adiponectin (ALPCO Diagnostics, Salem, NH) and interleukin 6 (IL-6) (eBioscience, Inc., San Diego, CA) using standard curves and according to the manufacturer's instructions.
2.5. Mesenteric adipocyte area measurement
A portion of the mesenteric fat collected at euthanasia from VEH, BPA and DES treated mice fed the HFD was fixed immediately in 4% formaldehyde. Tissue from n = 4 mice/group was cryoprotected in 30% sucrose in PBS overnight, equilibrated in PBS for ∼4 h and then frozen in OCT (Tissue Tek, Torrance, CA) overnight at −20 °C. Sections, 5 μm, were cut using a cryostat at −24 °C and stained with Oil Red O [36]. Photographs were taken of 5 random fields/section using Infinity Capture software (Lumenera, Ottawa, Ont.). The outline of at least 100 fat cells/mouse was measured using Image J software. The cross-sectional area of VEH male mice adipocytes was arbitrarily designated 100%.
2.6. Statistical analyses
The Kolmogorov–Smirnov test was used to verify that the data had equal variance about the group mean prior to ANOVA analyses. Significance for all parameters was evaluated using two-way ANOVA with the statistical program SigmaStat 3.1 and the Student–Newman–Keuls post hoc test. Physiology parameters were tested for statistical significance using ANCOVA and litter size as a covariate and the SPSS version 20 Statistical package. A p-value of <0.05 was considered significant.
3. Results
3.1. BPA exposure and growth
To mimic continuous human BPA exposure via the oral route and to approximate the higher BPA exposure of children and adolescents versus the lower exposure of adults [2], [3], we added BPA to the drinking water and treated the mice with a higher dose of BPA during gestation and lactation, followed by a lower dose after weaning. Dams weighed ∼22 g at mating and had increased body weight (BW) to ∼25 g by gestation day 11.5. Calculating an average water intake of 5 ml/day/25 g mouse [37], we estimate that dams were exposed to 5 μg/kg/day BPA at the beginning of treatment. The number of pups weaned from VEH (8.3 ± 0.7), BPA (8.0 ± 1.1) or DES (8.2 ± 0.4) dams was similar suggesting that neither BPA nor DES treatment had an impact on pregnancy maintenance.
Previous studies with early life exposure to BPA or DES reported an increase in body size at weaning [26], [38]. To verify that our treatments increased body size at weaning, we measured BW and body length (BL), and calculated body mass index (BMI) and body surface area (BSA), Fig. 1B. We found weanling male pups from BPA dams had increased body size with increased BW, BL and BSA. Female pups from BPA dams were longer than VEH female pups. Male and female pups from DES dams were longer and male pups had a greater BSA than pups from VEH dams. Thus, as expected [26], exposure to BPA and DES alone increased weanling body size.
To test whether chronic exposure to BPA altered body growth with time and whether increased dietary fat immediately after weaning would alter the growth trajectory, we changed the diet of the mice at weaning on post-natal day 21 to one containing either 10% kcal or 60% kcal from fat. At this time, we also reduced the amount of BPA in the drinking water 10-fold for reasons described earlier. We measured BW and BL, and calculated BMI and BSA in males and females on each of the diets, Fig. 1C and D. Water intake is a function of BW in mice, including C57bl/6 mice [37]. We measured BW at the 4-, 8- and 12-week intervals in mice fed the CD and HFD and calculated water intake. Based on the predicted water intake predicted by their measured BW, we estimate that male progeny fed the CD were exposed to 0.66 ± 0.1, 0.61 ± 0.1 and 0.59 ± 0.1 μg/kg/day BPA, and that female progeny fed the CD were exposed to 0.75 ± 0.1, 0.67 ± 0.1 and 0.65 ± 0.1 μg/kg/day BPA at the end of the 4-, 8- and 12-week intervals, respectively. Similarly, we estimate that male progeny fed the HFD were exposed to 0.58 ± 0.1, 0.51 ± 0.1 and 0.49 ± 0.1 μg/kg/day BPA, and female progeny fed the HFD exposed to 0.66 ± 0.1, 0.59 ± 0.1 and 0.53 ± 0.1 μg/kg/day BPA at the end of the 4-, 8- and 12-week intervals, respectively. These doses approximate human exposure levels [39] and are similar to doses used previously to assess the impact of BPA on glucose tolerance in CD-1 mice [16], [19], [20], [22].
Body size increased similarly with time in VEH, BPA and DES males fed the CD, Fig. 1C. In female mice, CD-VEH and CD-BPA females grew similarly, Fig. 1D. The body length (BL) of CD-DES females was longer and this group had a greater BSA at early times than CD-VEH females. HFD-fed mice increased body size with time in all mice when compared with their CD-fed cohort. HFD-BPA males had reduced BW, BMI and BSA when compared with HFD-VEH males by 12 weeks fed the HFD. In contrast, female growth was unaffected by BPA exposure and HFD-VEH and HFD-BPA females had similar body size parameters. HFD-DES male and females had reduced BW, BMI and BSA than HFD-VEH males and females by 12 weeks fed the HFD. Overall, BPA exposure did not change growth when fed the CD, and reduced body growth in males fed the HFD.
To confirm that sufficient BPA was ingested to induce predicted changes in reproductive organ weights, we measured prostate weight at euthanasia. CD-BPA males had significantly increased prostate weight when compared with CD-VEH males, 61.2 ± 9 versus 35.1 ± 3.1, respectively. As expected, prostate weight in CD-DES males, 36.3 ± 6, was similar to that of CD-VEH males [40], [41]. Testes, seminal vesicles, ovary and uterus weights did not differ in VEH, BPA or DES treated mice. These data support the idea that the BPA treatment was sufficient to induce a well established effect on prostate tissue [42].
3.2. Serum lipids
To verify expected increases in cholesterol and triglycerides upon HFD feeding in VEH mice and to determine whether BPA and DES altered the lipid response to the diet, we measured circulating total cholesterol and triglyceride in serum samples collected at euthanasia, Table 1. In CD-fed mice, serum cholesterol was reduced in CD-BPA males and increased in CD-BPA females when compared with CD-VEH mice. Serum cholesterol and triglyceride were increased in HFD-VEH males and females versus their CD-fed cohorts suggesting that the HFD induced a predicted increase in serum lipids. HFD-BPA males had increased cholesterol and triglycerides when compared with their CD-fed cohorts, suggesting that BPA did not ablate the lipid response to a high fat diet in males. HFD-DES males increased cholesterol only when compared with their CD-DES cohort. In females, although HFD-VEH females increased cholesterol and triglycerides, HFD-BPA females had reduced cholesterol and unchanged triglyceride when compared with CD-BPA females. Also unlike VEH females, HFD-DES females did not increase cholesterol or triglycerides when compared with CD-DES females. Thus, BPA and DES exposures sex-specifically altered the levels of circulating cholesterol and triglycerides in response to control and high fat diet fed mice.
Table 1.
Serum lipid analyses.
| Male |
Female |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD |
HFD |
CD |
HFD |
|||||||||
| VEH | BPA | DES | VEH | BPA | DES | VEH | BPA | DES | VEH | BPA | DES | |
| Cholesterol (mmol/l) | 4.16 ± 0.42 | 2.05 ± 0.31a | 3.7 ± 0.22 | 5.41 ± 0.23b | 4.18 ± 0.41b | 4.94 ± 0.55b | 2.66 ± 0.14c | 5.21 ± 0.21a,c | 3.28 ± 0.11 | 3.84 ± 0.14b,c | 3.62 ± 0.19b | 3.94 ± 0.25c |
| Triglyceride (mmol/l) | 1.0 ± 0.20 | 0.59 ± 0.09 | 0.83 ± 0.05 | 1.64 ± 0.21b | 1.34 ± 0.13b | 1.16 ± 0.16 | 0.96 ± 0.14 | 1.04 ± 0.24 | 1.18 ± 0.37 | 1.88 ± 0.27b | 1.07 ± 0.15a | 1.27 ± 0.11 |
Significance is when p < 0.05:
compared with VEH within diet;
compared with CD within treatment;
compared with males within diet.
3.3. Glucose homeostasis
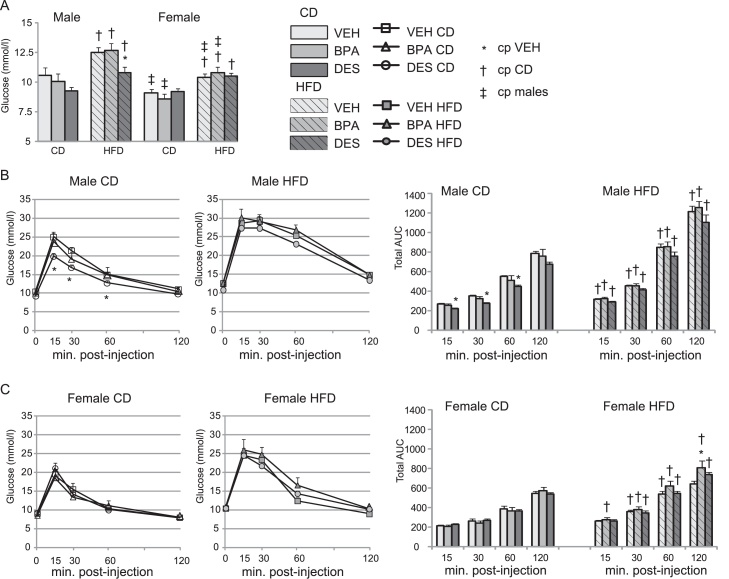
To determine whether continued BPA exposure would impact glucose regulation and whether a HFD would reveal additional glucose homeostasis abnormalities, we measured glucose levels at baseline and after glucose challenge (GTT) in mice fed the CD and HFD. VEH, BPA and DES mice fed the CD had similar baseline glucose levels within the male group and within the female group, Fig. 2A. All HFD-fed mice had significantly increased baseline glucose compared with their CD-fed cohort. HFD-DES males had a lower baseline glucose level than HFD-VEH males. When males and females were compared, HFD-VEH and HFD-BPA females, but not HFD-DES females, had lower baseline glucose than similarly treated males. Overall, BPA and DES exposure did not increase baseline glucose in mice fed a HFD.
Fig. 2.
(A) Fasting glucose. Food was removed for 5 h and serum glucose was measured in n = 5–7 mice/group. (B and C) Glucose tolerance test and area under the curve calculation in male (B) and female (C) mice. Serum glucose was measured at the times indicated after an IP injection of dextrose in n = 7 mice/group. The total area under the curve was calculated for each time period. Data are expressed as mean ± SEM. Significance, indicated by p < 0.05 when compared with * VEH; † CD, ‡ males.
CD-VEH and CD-BPA males responded similarly to GTT, Fig. 2B. CD-DES males had lower glucose at early times than CD-VEH males. No differences were detected in CD-fed females, regardless of treatment. All HFD-fed mice were more glucose intolerant than their CD cohort. When the AUC was calculated for each time period, HFD-BPA females had a minor, but significant, increase in AUC in the last hour of the GTT than HFD-VEH females, Fig. 2C. Thus, BPA had no impact on glucose intolerance in males or females when fed the CD whereas DES induced an improved glucose tolerance in CD-fed males. We conclude that continued BPA exposure induced a minor reduction in glucose tolerance and only at a late time in HFD-fed females.
3.4. Impact of BPA on adipose tissues
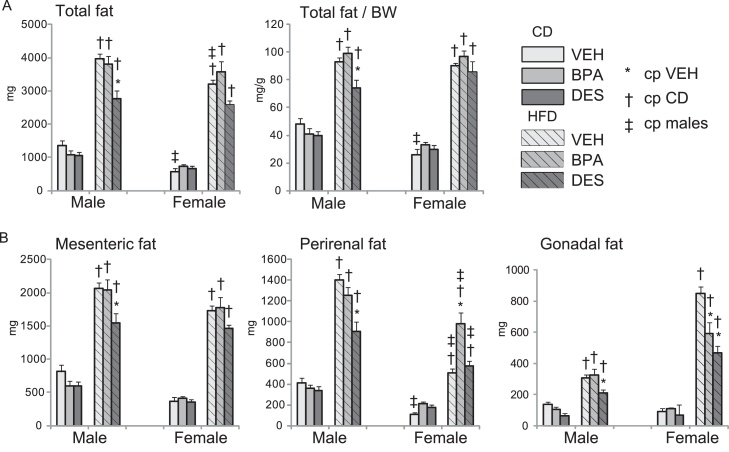
To test whether continued BPA exposure would alter adipose deposition, we weighed total adipose tissue surrounding the mesentery, kidneys and gonads, and indexed total fat to BW (total fat/BW), Fig. 3. Total fat and total fat/BW were similar in CD-fed mice, regardless of treatment, Fig. 3A. HFD feeding increased total fat and total fat/BW in all mice when compared with their CD-fed cohorts. Within the HFD groups, HFD-DES males had lower total fat and reduced indexed total fat compared with HFD-VEH mice. Therefore, chronic BPA exposure did not increase total fat accumulation and DES exposure reduced total fat accumulation.
Fig. 3.
Mesenteric, perirenal and gonadal fat deposits in n = 6–8 mice/group were weighed. (A) Total weight of the fat deposits (left) and total fat indexed to BW (right). (B) Individual fat deposit weights. Data are expressed as mean ± SEM. Significance, indicated by p < 0.05 when compared with * VEH; † CD, ‡ males.
The amount of fat surrounding the mesentery, perirenal fat surrounding both kidneys, and gonadal fat surrounding both testes and both ovaries was measured to identify whether the site specific organ fat deposition was altered by BPA, Fig. 3B. CD-fed males and females had similar mesenteric, perirenal and gonadal fat weights, regardless of treatment. HFD feeding increased fat weight at all sites examined over that present in their CD-fed cohorts. However, whereas HFD-VEH and HFD-BPA males had similar fat weights, HFD-DES males had reduced mesenteric, perirenal and gonadal fat. All HFD females had similar amounts of mesenteric fat, regardless of drug treatment. HFD-BPA females had increased perirenal fat compared with HFD-VEH females. In contrast, gonadal fat was reduced in HFD-BPA and HFD-DES females compared with HFD-VEH mice. We conclude that BPA and DES sex specifically altered organ fat deposition.
To determine whether mesenteric fat adipocyte size was altered by BPA, we measured the area of Oil Red O stained mesenteric adipocytes in HFD-fed mice, Fig. 4A. We found the mesenteric fat adipocyte area of HFD-VEH and HFD-BPA males was similar whereas it was reduced significantly in HFD-DES males, Fig. 4B. There were no differences in adipocyte size in female mice. Thus, BPA did not increase the mesenteric fat weight or adipocyte area in males or females. Both mesenteric fat weight and adipocyte area were reduced in HFD-DES males.
Fig. 4.
(A) Sections of mesenteric fat isolated from HFD mice were stained with Oil Red O to identify adipocytes. Stained sections from male mice are shown at 100× magnification. (B) The area of 100 adipocytes per mouse was measured in n = 4 mice/group. VEH adipocyte area was artificially designated as 100%. Data are expressed as mean ± SEM. Significance, indicated by p < 0.05 when compared with * VEH.
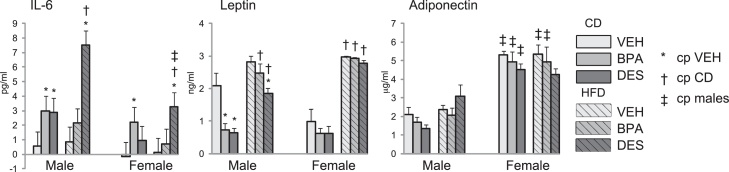
Increased fat may be accompanied by increased secretion of inflammatory cytokines [43]. To test whether treatment and/or diet increased inflammatory cytokine secretion, we measured the amount of interleukin-6 (IL-6) in serum collected at euthanasia, Fig. 5. We found circulating IL-6 was increased in CD-BPA male and female mice, and in CD-DES males when compared with CD-VEH mice. IL-6 was further increased in HFD-DES males and females. Thus, BPA and DES exposure increased circulating IL-6 when fed the CD and IL-6 was further increased in HFD-DES mice.
Fig. 5.
Serum IL-6, leptin and adiponectin analyses were measured in serum collected at euthanasia from n = 5 mice/group. Data are expressed as mean ± SEM. Significance, indicated by p < 0.05 when compared with * VEH; † CD, ‡ males.
To characterize adipokine secretion from fat, we measured circulating leptin and adiponectin, Fig. 5. Leptin levels usually reflect adiposity. Leptin was reduced in CD-BPA and CD-DES males compared with CD-VEH males. In CD-fed females, there were no differences in leptin level among the treatment groups. HFD feeding induced increased circulating leptin in male and female groups. Within the HFD-fed group, HFD-DES males had reduced leptin compared with HFD-VEH males. Adiponectin levels were higher in females than males, and were not altered by treatment or diet. Therefore, although BPA and DES reduced leptin levels when fed the CD, neither BPA nor DES treatment ablated or accentuated HFD-induced increases in circulating leptin.
4. Discussion
Greater BPA exposure in humans has been linked to an increased risk for obesity and insulin resistance [10], [44], [45]. We found that the BPA exposed C57bl/6n mice were larger than VEH mice at weaning and continued BPA exposure increased adult prostate weight. These results are in agreement with an earlier study [38]. However, we found that chronic BPA exposure which began on GD11.5 and continued until euthanasia of 4 months of age, did not contribute to greater body size in male or female mice. Further, this chronic BPA exposure did not induce greater insulin resistance in male or female mice fed a CD. Consistent with earlier studies [46], HFD-VEH mice had more insulin resistance than those fed a CD and greater resistance was found in males than in females. This pattern was also present in HFD-BPA male and female mice. HFD-DES males showed some improved glucose tolerance over that of HFD-VEH mice. Supporting the comparable GTT responses, total fat deposition and mesenteric fat adipocyte sizes were similar in VEH and BPA mice. DES mice with some improvement in GTT also had less fat deposition and smaller mesenteric fat adipocytes. Despite the similar mesenteric fat loads in VEH and BPA mice, CD-BPA male and female mice had increased IL-6, reduced leptin and similar adiponectin arguing that some lipodystrophy was induced by chronic BPA exposure. Based on our combined results, we conclude that neither chronic BPA exposure nor gestational DES exposure substantially worsened the impact of obesity or obesity-induced glucose intolerance due to HFD feeding in males. Rather, we found chronic BPA and gestational DES exposures induced an adipokine imbalance in response in mice fed the CD and sex specifically altered the lipid response and adipose deposition response to a HFD.
4.1. Chronic BPA exposure did not substantially change glucose tolerance
Our data support the argument that chronic oral BPA exposure at a human relevant level, given either in the diet or in the drinking water, and in either outbred or inbred mice, has little impact on adult male or female glucose tolerance which is in line with the results of earlier studies [16], [20], [21], [22]. Our studies used inbred C57bl/6n mice with orally delivered BPA from GD11.5 to euthanasia at 4 months. Similar to our study which used C57bl/6n mice and at similar doses of BPA, GTT was not altered in CD-1 or Avy male or female mice exposed to oral BPA during gestation and lactation [16], [20], [21] or in CD-1 male or female mice treated with BPA up to postnatal day 70 [22]. In contrast, subcutaneously injected BPA induced glucose intolerance in male progeny of OF1 dams [17] and C57bl/6 dams [15]. Complicating direct comparison of these studies is the fact that dietary delivery and subcutaneous delivery of BPA may not be equivalent. Although oral and subcutaneous injection of BPA led to increased prostate weight arguing some effectiveness of both delivery modes, at least for this activity [47], the physiological relevance of subcutaneous injection for metabolic effects remains a question, especially given that most BPA exposure occurs through the diet.
Besides delivery route, the differing impacts of BPA on glucose homeostasis may also be influenced by dose and the exposure time relative to pancreatic and fat development. Higher BPA doses given during gestation and lactation have induced glucose intolerance in the adult progeny [15], [16], [17], [19]. Regarding the timing of BPA exposure in mice, the murine pancreas begins development around GD 9–10 and has rudimentary insulin secreting abilities by GD18, and rapidly matures in the neonate [48]. Thus, gestational BPA exposure would be expected to have a greater impact on early development of the pancreas and exposure after gestation would have more impact on later maturation events. In contrast to exposure which is limited to the developmental period, chronic exposure has the potential to modulate differentiation as well as maturation of the pancreas. Adult C57bl/6n males and females exposed to DES during GD11.5 to 14.5 in our experiments did not develop an increase in glucose intolerance whereas adult CD-1 males treated with the similar dose from GD9-18 developed glucose intolerance [19]. The differing results could reflect either the sensitivity of the mouse strain or the more extended developmental period of DES exposure in the latter study. The differing outcomes in adults of chronic versus only early-life BPA exposures are consistent with the developmental origins of adult disease hypothesis [49]. This hypothesis predicts that exposure to BPA in early life would drive increased disease susceptibility in the adult, whereas continued adult exposure would not be associated with any increased disease susceptibility.
The impact of BPA on glucose homeostasis is unlikely to be influenced by the amount of fat in the diet. Glucose tolerance was similar in untreated mice and BPA exposed mice when they were fed control diets which were high in fat, such as the AIN93G diet [20], [21], [22] or low in fat such as the one used in our study or by Angle et al. [19]. To the best of our knowledge, two other studies have combined BPA exposure with a HFD. HFD feeding, whether at weaning as described here, or at 9 weeks of age [20] or 12 weeks of age [16] and a duration of either 12 weeks, 5 weeks or 10 weeks, respectively, led to no change in glucose tolerance in mice exposed to low dose BPA. These data suggest that BPA exposure does not worsen the response to a glucose load when under a metabolic stress such as a HFD.
4.2. BPA modified circulating lipid levels, adipose tissue deposition and adipocyte secretion
In CD-fed mice, BPA exposure increased serum cholesterol in females, and reduced it in males, suggesting some sex specific impact. In contrast, no impact of low dose BPA on cholesterol or triglyceride was detected in CD-1 male and female mice fed the CD [22]. As expected, cholesterol and triglyceride were increased by the HFD in VEH males and females, and were also increased by the HFD in BPA and DES males. Increases in cholesterol and triglyceride were also found in Wistar rats fed a HFD after exposure to BPA during gestation and lactation [18]. These results suggest that gestational exposure to DES is sufficient to modify lipids permanently. However, neither BPA nor DES exposure precluded an increase in circulating cholesterol and triglyceride increase in males fed the HFD.
Our data indicate no increase in total fat accumulation and suggest no increase in mesenteric adipocyte size after chronic BPA exposure. This result is similar to the lack of increase in body size and fat accumulation found in outbred CD-1 or Avy mice exposed to low dose dietary BPA [21], [22]. Visceral obesity and increased glucose intolerance are risk factors for the metabolic syndrome. The lack of increased total and mesenteric fat accumulation is in agreement with the lack of any increase in glucose intolerance in BPA or DES exposed mice. Together these data support the idea that chronic BPA exposure did not induce a metabolic syndrome-like phenotype in C57bl/6n mice. Unlike visceral or mesenteric fat, increased ectopic fat surrounding organs such as the kidney or gonads is thought to contribute primarily to local toxic effects and not systemic metabolic disease [50], [51]. HFD feeding revealed sex-specific changes in ectopic fat deposition in BPA exposed mice. BPA exposure did not alter ectopic fat deposition surrounding the testes or kidneys in males. Rather, BPA exposure induced increases in perirenal fat and reduced gonadal fat accumulation in females compared with VEH females when fed the HFD. It is unclear whether these altered deposits have any physiological impact on ovary function or renal function in these mice. Increased perirenal fat is an indicator of impaired kidney function [52] suggesting that the higher amount of perirenal fat in HFD-BPA females might prove detrimental, and the lower amount in HFD-DES males might be correlated with preserved kidney function.
Leptin is produced in proportion to the amount of body fat by mature adipocytes, especially those in visceral fat [53]. We found leptin was reduced in C57Bl/6n mice exposed to BPA and DES and fed the CD. This result is in contrast to earlier studies which found increased leptin in BPA exposed OLF [17] and ICR mice [54]. No change in leptin level was detected in outbred CD-1 mice exposed to BPA (16). These data suggest that the mouse strain used may have a significant effect on the ability of BPA to impact leptin secretion. We measured circulating leptin and adiponectin after 12 weeks on the HFD. Thus, although it is likely that the changes in leptin and not adiponectin levels are due to adaptations to the HFD and BPA, we cannot comment on any hypothalamic signals that might affect feeding behavior because we did not measure food consumption. Reduced leptin is associated with reduced body fat and/or lipodystrophy [53]. The reduced leptin in CD-BPA and CD-DES males which had similar amounts of total body fat argues for some baseline lipodystrophy with both treatments. This is supported by the increase in circulating IL-6 in BPA and DES exposed mice. IL-6, secreted by macrophages and adipocytes, is associated with low grade inflammation and the metabolic syndrome [55]. The IL-6 increase in CD-BPA males and females supports the idea that BPA exposure induces a functional defect in fat and that this is also present in HFD-DES mice. Similar to other studies, leptin was increased in response to a protracted HFD and neither BPA nor DES exposure altered this response [16], [18], [56]. Adiponectin is a key regulator of insulin sensitivity [57]. Circulatory adiponectin levels were unaffected by BPA or DES exposure suggesting no change in insulin sensitivity. These data are consistent with the lack of any substantial glucose intolerance in the BPA or DES exposed mice. Overall, the increased IL-6, reduced leptin and unchanged adiponectin suggest that chronic BPA exposure beginning in gestation and continuing into adulthood impairs fat function creating an adipokine imbalance.
Thus, our study shows evidence for chronic BPA exposure inducing sex-specific changes in serum cholesterol, triglyceride, leptin and adiponectin as well as site-specific fat accumulation which were also diet dependent. The data support the notion that chronic exposure to other endocrine disrupting compounds might also influence rodent, and potentially human, glucose homeostasis and adipocyte function. There are many physiological differences between mice and humans; however, our data supports the idea that obesity and glucose control in humans might be influenced by chronic exposure to BPA, and that this exposure may increase metabolic disease.
Funding
This work was supported by operating grants from the Canadian Institutes for Health Research and the Heart and Stroke Foundation of Quebec to LEC.
Conflict of interest
Chalifour reports grants from Canadian Institute for Health Research, grants from Heart and Stroke Foundation of Quebec, during the conduct of the study. Patel and Di Iorio declare no conflict of interest.
Transparency document
Acknowledgement
We gratefully acknowledge the excellent animal care staff and expert animal handling of Kathy Ann Forner.
Footnotes
Available online 7 August 2014
References
- 1.Lakind J.S., Naiman D.Q. Bisphenol A (BPA) daily intakes in the United States: estimates from the 2003–2004 NHANES urinary BPA data. J. Expos. Sci. Environ. Epidemiol. 2008;18(6):608–615. doi: 10.1038/jes.2008.20. [DOI] [PubMed] [Google Scholar]
- 2.Vandenberg L.N. Exposure to bisphenol A in Canada: invoking the precautionary principle. Can. Med. Assoc. J. 2011;183(11):1265–1270. doi: 10.1503/cmaj.101408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman Å., Heindel J.J., Jobling S., Kidd K.A., Zoeller R.T., Jobling S.K. World Health Organization; 2013. State of the Science of Endocrine Disrupting Chemicals 2012: An Assessment of the State of the Science of Endocrine Disruptors Prepared by a Group of Experts for the United Nations Environment Programme and World Health Organization. [Google Scholar]
- 4.Woodruff T.J., Zota A.R., Schwartz J.M. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ. Health Perspect. 2011;119(6):878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mortensen M.E., Calafat A.M., Ye X., Wong L.-Y., Wright D.J., Pirkle J.L. Urinary concentrations of environmental phenols in pregnant women in a pilot study of the National Children's Study. Environ. Res. 2014;129(0):32–38. doi: 10.1016/j.envres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaKind J.S., Goodman M., Naiman D.Q. Use of NHANES data to link chemical exposures to chronic diseases: a cautionary tale. PLoS ONE. 2012;7(12):e51086. doi: 10.1371/journal.pone.0051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turer A.T., Hill J.A., Elmquist J.K., Scherer P.E. Adipose tissue biology and cardiomyopathy. Circ. Res. 2012;111(12):1565–1577. doi: 10.1161/CIRCRESAHA.111.262493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inadera H. Developmental origins of obesity and type 2 diabetes: molecular aspects and role of chemicals. Environ. Health Prev. Med. 2013;18:185–197. doi: 10.1007/s12199-013-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemp M.W., Kallapur S.G., Jobe A.H., Newnham J.P. Obesity and the developmental origins of health and disease. J. Paediatr. Child Health. 2012;48(2):86–90. doi: 10.1111/j.1440-1754.2010.01940.x. [DOI] [PubMed] [Google Scholar]
- 10.Melzer D., Osborne N.J., Henley W.E., Cipelli R., Young A., Money C. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women/clinical perspective. Circulation. 2012;125(12):1482–1490. doi: 10.1161/CIRCULATIONAHA.111.069153. [DOI] [PubMed] [Google Scholar]
- 11.Melzer D., Gates P., Osborn N.J., Henley W.E., Cipelli R., Young A. Urinary bisphenol A concentration and angiography-defined coronary artery stenosis. PLoS ONE. 2012;7(8):e43378. doi: 10.1371/journal.pone.0043378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trasande L., Attina T., Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA: J. Am. Med. Assoc. 2012;308(11):1113–1121. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- 13.Eng D.S., Lee J.M., Gebremariam A., Meeker J.D., Peterson K., Padmanabhan V. Bisphenol A and chronic disease risk factors in US children. Pediatrics. 2013;132(3):e637–e645. doi: 10.1542/peds.2013-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haslam D.W., James W.P.T. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 15.Liu J., Yu P., Qian W., Li Y., Zhao J., Huan F. Perinatal bisphenol A exposure and adult glucose homeostasis: identifying critical windows of exposure. PLoS ONE. 2013;8(5):e64143. doi: 10.1371/journal.pone.0064143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacKay H., Patterson Z.R., Khazall R., Patel S., Tsirlin D., Abizaid A. Organizational effects of perinatal exposure to bisphenol-A and diethylstilbestrol on arcuate nucleus circuitry controlling food intake and energy expenditure in male and female CD-1 mice. Endocrinology. 2013;154(4):1465–1475. doi: 10.1210/en.2012-2044. [DOI] [PubMed] [Google Scholar]
- 17.Alonso-Magdalena P., Vieira E., Soriano S., Menes L., Burks D., Quesada I. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ. Health Perspect. 2010;118(9):1243–1250. doi: 10.1289/ehp.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei J., Lin Y., Li Y., Ying C., Chen J., Song L. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. 2011;152(8):3049–3061. doi: 10.1210/en.2011-0045. [DOI] [PubMed] [Google Scholar]
- 19.Angle B.M., Do R.P., Ponzi D., Stahlhut R.W., Drury B.E., Nagel S.C. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod. Toxicol. 2013;42:256–268. doi: 10.1016/j.reprotox.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan K.K., Haller A.M., Sorrell J.E., Woods S.C., Jandacek R.J., Seeley R.J. Perinatal exposure to bisphenol-A and the development of metabolic syndrome in CD-1 mice. Endocrinology. 2010;151(6):2603–2612. doi: 10.1210/en.2009-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson O.S., Peterson K.E., Sanchez B.N., Zhang Z., Mancuso P., Dolinoy D.C. Perinatal bisphenol A exposure promotes hyperactivity, lean body composition, and hormonal responses across the murine life course. FASEB J. 2013;27(4):1784–1792. doi: 10.1096/fj.12-223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kendig E.L., Buesing D.R., Christie S.M., Cookman C.J., Gear R.B., Hugo E.R. Estrogen-like disruptive effects of dietary exposure to bisphenol A or 17α-ethinyl estradiol in CD1 mice. Int. J. Toxicol. 2012;31(6):537–550. doi: 10.1177/1091581812463254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haslam D.W., James W.P.T. Life expectancy. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 24.Migliarini B., Piccinetti C., Martella A., Maradona F., Giacacchini G., Carnegali O. Perspectives on endocrine disruptor effects on metabolic sensors. Gen. Comp. Endocrinol. 2011;173(3):416–423. doi: 10.1016/j.ygcen.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 25.vom Saal F.S., Nagel S.C., Coe B.L., Angle B.M., Taylor J.A. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell. Endocrinol. 2012;354(1–2):74–84. doi: 10.1016/j.mce.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin B.S., Soto A.M. Bisphenol A perinatal exposure and body weight. Mol. Cell. Endocrinol. 2009;304(1–2):55–62. doi: 10.1016/j.mce.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vandenberg L.N., Colborn T., Hayes T.B., Heindel J.J., Jacobs D.R., Lee D.-H. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman A., Schorge J., Greene M.F. The long-term effects of in utero exposures—the DES story. N. Engl. J. Med. 2011;364(22):2083–2084. doi: 10.1056/NEJMp1104409. [DOI] [PubMed] [Google Scholar]
- 29.Henley D.V., Korach K.S. Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function. Endocrinology. 2006;147(6):s25–s32. doi: 10.1210/en.2005-1117. [DOI] [PubMed] [Google Scholar]
- 30.Richter C., Birnbaum L., Farabollini F., Newbold R., Rubin B., Talsness C. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newbold R., Padilla-Banks E., Snyder R.J., Phillips T.M., Jefferson W.N. Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod. Toxicol. 2007;23(3):290–296. doi: 10.1016/j.reprotox.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swedenborg E., Ruegg J., Makela S., Pongratz I. Endocrine disruptive chemicals: mechanisms of action and involvement in metabolic disorders. J. Mol. Endocrinol. 2009;43(1):1–10. doi: 10.1677/JME-08-0132. [DOI] [PubMed] [Google Scholar]
- 33.Farriol M., Rosselló J., Schwartz S. Body surface area in Sprague-Dawley rats. J. Anim. Physiol. Anim. Nutr. 1997;77(1–5):61–65. [Google Scholar]
- 34.Cheung M.C., Spalding P.B., Gutierrez J.C., Balkan W., Namias N., Koniaris L.G. Body surface area prediction in normal, hypermuscular, and obese mice. J. Surg. Res. 2009;153(2):326–331. doi: 10.1016/j.jss.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Andrikopoulos S., Blair A.R., Deluca N., Fam B.C., Proietto J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1323–E1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 36.Goodpaster B.H., Theriault R., Watkins S.C., Kelley D.E. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49(4):467–472. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 37.Bachmanov A.A., Reed D.R., Beauchamp G.K., Tordoff M.G. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 2002;32(6):435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin B.S. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011;127(1–2):27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Chapin R.E., Adams J., Boekelheide K., Gray L.E., Hayward S.W., Lees P.S.J. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res. B: Dev. Reprod. Toxicol. 2008;83(3):157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- 40.LaRocca J., Boyajian A., Brown C., Smith S.D., Hixon M. Effects of in utero exposure to bisphenol A or diethylstilbestrol on the adult male reproductive system. Birth Defects Res. B: Dev. Reprod. Toxicol. 2011;92(6):526–533. doi: 10.1002/bdrb.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu X., Zhang N., Lee M.M. In: The Influence of Endocrine Disruptors on Male Pubertal Timing. Endocrine Disruptors and Puberty. Diamanti-Kandarakis E., Gore A.C., editors. Humana Press; 2012. pp. 339–355. [Google Scholar]
- 42.Prins G.S., Tang W.-Y., Belmonte J., Ho S.-M. Perinatal exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin. Pharmacol. Toxicol. 2008;102(2):134–138. doi: 10.1111/j.1742-7843.2007.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gastaldelli A., Basta G. Ectopic fat and cardiovascular disease: what is the link? Nutr. Metab. Cardiovasc. Dis. 2010;20(7):481–490. doi: 10.1016/j.numecd.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Bae S., Kim J.H., Lim Y.-H., Park H.Y., Hong Y.-C. Associations of bisphenol A exposure with heart rate variability and blood pressure/novelty and significance. Hypertension. 2012;60(3):786–793. doi: 10.1161/HYPERTENSIONAHA.112.197715. [DOI] [PubMed] [Google Scholar]
- 45.Shankar A., Teppala S., Sabanayagam C. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ. Health Perspect. 2012;120(9):1297–1300. doi: 10.1289/ehp.1104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Louwe M.C., van der Hoorn J.W.A., van den Berg S.A.A., Jukema J.W., Romijn J.A., Willems van Dijk K. Gender-dependent effects of high-fat lard diet on cardiac function in C57 Bl/6 J mice. Appl. Physiol. Nutr. Metab. 2012;37(2):214–224. doi: 10.1139/h11-153. [DOI] [PubMed] [Google Scholar]
- 47.Prins G.S., Ye S.-H., Birch L., Ho S.-m., Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague–Dawley rats. Reprod. Toxicol. 2011;31(1):1–9. doi: 10.1016/j.reprotox.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benitez C.M., Goodyer W.R., Kim S.K. Deconstructing pancreas developmental biology. Cold Spring Harbor Perspect. Biol. 2012;4(6) doi: 10.1101/cshperspect.a012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barker D.J.P. The origins of the developmental origins theory. J. Intern. Med. 2007;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 50.Britton K.A., Fox C.S. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124(24):e837–e841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 51.Després J.-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 52.Lamacchia O., Nicastro V., Camarchio D., Valente U., Grisorio R., Gesualdo L. Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol. Dial. Transplant. 2011;26(3):892–898. doi: 10.1093/ndt/gfq522. [DOI] [PubMed] [Google Scholar]
- 53.Coppari R., Bjorbaek C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat. Rev. Drug Discov. 2012;11(9):692–708. doi: 10.1038/nrd3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyawaki J., Sakayama K., Kato H., Yamamoto H., Masuno H. Perinatal and postnatal exposure to bisphenol A increase adipose tissue mass and serum cholesterol level in mice. J. Atheroscler. Thromb. 2007;14(5):245–252. doi: 10.5551/jat.e486. [DOI] [PubMed] [Google Scholar]
- 55.Spranger J., Kroke A., Möhlig M., Hoffmann K., Bergmann M.M., Ristow M. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the Prospective Population-Based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52(3):812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 56.Fang C.X., Dong F., Thomas D.P., Ma H., He L., Ren J. Hypertrophic cardiomyopathy in high-fat diet-induced obesity: role of suppression of forkhead transcription factor and atrophy gene transcription. Am. J. Physiol.: Heart Circ. Physiol. 2008;295(3):H1206–H1215. doi: 10.1152/ajpheart.00319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ben-Jonathan N., Hugo E.R., Brandebourg T.D. Effects of bisphenol A on adipokine release from human adipose tissue: implications for the metabolic syndrome. Mol. Cell. Endocrinol. 2009;304(1–2):49–54. doi: 10.1016/j.mce.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.