Highlights
-
•
We assessed perinatal or peripubertal exposure to daidzein in rats.
-
•
Perinatal exposure to daidzein does not confer a positive effect on BMD.
-
•
Peripubertal exposure to daidzein protects against a decline in BMD.
-
•
Exposure to daidzein does not have serious adverse effects on sexual development.
Abbreviations: AGD, anogenital distance; BMD, bone mineral density; BW, body weight; ER, estrogen receptor; PND, postnatal day
Keywords: Daidzein, Bone mineral density, Safety, Perinatal period, Timing of exposure
Abstract
Neonatal exposure to isoflavones improved bone health in thereafter in previous animal studies. However, since isoflavones possess hormonal activity, it may interfere with reproductive development. In the present study, we assessed the safety and efficiency of perinatal or peripubertal exposure to daidzein on bone and reproductive organ development at early adulthood in rats. Sprague-Dawley pregnant rats (n = 18) were divided into 3 groups: (1) dams and their offspring were fed the control diet. (2) Dams were fed the daidzein diet (0.5 g daidzein/kg diet) during pregnancy and then the control diet at postnatal day 13 and their offspring were fed the control diet. (3) Dams and their offspring were fed the daidzein diet through the experiment. While perinatal exposure to daidzein did not confer a positive effect on bone mineral density on postnatal day 35, peripubertal exposure to daidzein protected against a decline in bone mineral density. Meanwhile, exposure to daidzein during the perinatal or peripubertal period did not affect reproductive organ weights at early adulthood in rats. Further investigations should assess the mechanisms underlying these responses of bone metabolism to daidzein, as well as the safety of daidzein exposure during the perinatal period and throughout life.
1. Introduction
The recent growing interest in health and diet has led to an increased focus on soy foods and their functional components, such as isoflavones. Soy isoflavones have a structure similar to that of estrogen and have weak estrogenic activity [1]. Therefore, isoflavones have been used by some postmenopausal women as a natural alternative to estrogen replacement therapy for prevention of postmenopausal osteoporosis [2]. Equol is a metabolite of daidzein. Equol is formed by microbiota in the intestine and it binds to estrogen receptors (ERs) and induces transcription more strongly than soy isoflavones [3]. Lampe [4] suggested that the clinical effectiveness of soy isoflavones is due to their ability to produce equol.
Estrogen plays a critical role in the skeletal development of female and male mammals throughout life [5]. However, the effects of isoflavone exposure during early life on bone development have not been extensively studied. Injecting pregnant mice with diethylstilbestrol during pregnancy results in positive, long-lasting effects on the skeleton of female offspring [5]. This finding suggests that in utero exposure to estrogen can result in programming of bone cells. Moreover, short-term exposure to estrogen or compounds with estrogen-like activity during early stages of development has positive effects on bone health in adulthood in mice [6]. Female offspring of mice exposed to soy isoflavones have a higher bone mineral density (BMD) compared with control group and improved femur structure at 4 months of age, the time when maximal bone mass is established in CD-1 mice [7], [8], [9]. However, Kaludjerovic and Ward [10] reported that short-term neonatal exposure to isoflavones provides protection against deterioration of bone tissue in female mice, but not in male mice, after a decline in endogenous sex steroid production. We previously reported that consumption of a diet with 0.08% (w/w) isoflavones stimulates bone formation in immature male mice and exerts the opposite effect in female mice [11]. These results suggest that daidzein has a specific, sexually dimorphic effect on bone formation and BMD during growth periods.
Consumption of soy isoflavone supplements during early life is frequently associated with beneficial effects on bone development in mice [7], [8], [9]. However, there is concern regarding isoflavones as endocrine-disrupting chemicals because of their estrogenic activity [12], [13]. Pregnancy and lactation are hormone-sensitive and important periods in the development of reproductive capacity of offspring. Therefore, the fetus or nursing offspring may be more affected by estrogen or food components with potential hormonal activity than adults. Several studies have shown that perinatal exposure to isoflavones in rodents induces adverse reproductive effects on the offspring, such as a change in reproductive organ weight and serum hormone levels in mice and rats [12], [13], [14].
We previously reported that isoflavones were present in the milk of dams that were exposed to isoflavones in rats [15]. The maternal–fetal intrauterine transfer of isoflavones in rats fed a diet supplemented with isoflavones elicits high serum isoflavone levels in rat pups. These high levels are maintained throughout the suckling period by the passage of isoflavones into maternal milk [15], [16]. Therefore, human infants can be exposed to elevated levels of isoflavones throughout nursing if the mother is consuming a diet that is abundant in soy foods or supplements [17]. However, few studies have assessed the transfer of isoflavone and its metabolite equol from mothers to the nursing offspring. Therefore, the objective of the present study was to assess the safety and efficiency of perinatal or peripubertal exposure to daidzein on bone development and on reproductive organs at early adulthood in rats.
2. Materials and methods
2.1. Animals
Female Sprague-Dawley rats at day 5 of gestation were purchased from Charles River Laboratories Japan, Inc. (Yokohama, Japan). Rats were housed individually in clear plastic cages with wire-mesh covers and paper chip bedding (ALPHA-DriTM, Shepherd Specialty Papers, Inc., Richland, MI). The weaned offspring were housed individually in stainless-steel cages. Rats were maintained in a temperature- and humidity-controlled room (23 ± 1 °C and 60% ± 5% relative humidity) with a 12-h light–dark cycle. The offspring were kept in the same groups as their respective dams. Dams were pair-fed their respective diet and offspring were pair-fed their respective diets among males or females. This experiment complied with the guidelines of the Standards Relating to the Care and Management of Experimental Animals and Relief of Pain of the Japanese government (Notice No. 88 of the Ministry of the Environment, 2006).
2.2. Diets and chemicals
Pregnant rats were fed diet mixtures containing daidzein at dose levels of 0 or 0.5 g/kg. These diets were based on the modified AIN-93G diet with replacement of corn oil for soybean oil [18]. All ingredients were purchased from Oriental Yeast Co., Ltd. (Tokyo, Japan). Purified daidzein (>98% purity) and equol were purchased from LC Laboratories Co., Ltd. (Woburn, MA).
2.3. Experimental design
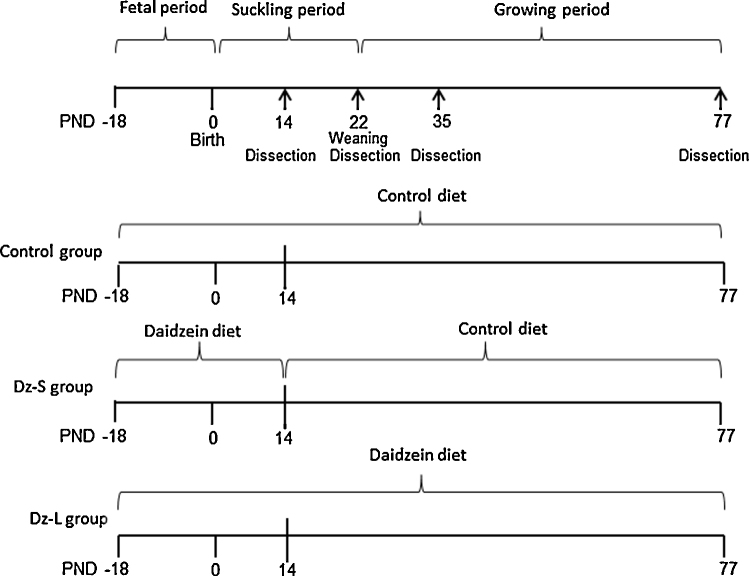
The experimental design is shown in Fig. 1. Pregnant rats at day 5 of gestation (n = 18) of random body weights were divided into three groups of six rats each: dams and their offspring were fed the control diet (Control); dams were fed the daidzein diet (0.5 g/kg diet) during pregnancy and then the control diet at postnatal day (PND) 13 and their offspring were fed the control diet (Dz-S); and dams and their offspring were fed the daidzein diet (Dz-L) through the experiment. Accordingly, pups in the Dz-S group were exposed to daidzein only through the placenta and the milk from the dams. The day of birth was designated as PND 1. On PND 1, the number of pups was adjusted to eight (4 males and 4 females) for each dam. Litter size, birth weight, and the sex ratio were measured as pregnancy outcomes. Pups were weaned on PND 22, and offspring were housed in individual cages. From PNDs 22 to 77, offspring were weighed weekly and food intake was measured daily. Offspring (1 female and 1 male per litter) were anesthetized by exposure to diethyl ether (Wako, Osaka, Japan) on PNDs 14, 22, 35, and 77, and dams were anesthetized by exposure to diethyl ether on PND 22. After laparotomy, the stomach was removed on PND 14. Whole blood was collected by cardiac puncture, and the testes, seminal vesicles, ovaries, and uteri were removed and weighted on PNDs 22, 35, and 77. Serum was separated by centrifugation at 3000 rpm for 20 min and frozen at −80 °C. For measurement of BMD, the right femur was removed and stored in 70%/30% ethanol/water (Sigma–Aldrich Japan, Tokyo, Japan).
Fig. 1.
Experimental design. PND: postnatal day
2.4. Pup development
Hormonal imbalances in male or female pups due to the diet treatments were determined by comparing their anogenital distance (AGD) measurements with those of rats [12]. AGD, which is the length of the perineum from the base of the genital tubercle to the proximal edge of the anus under natural extension without stretching, was measured at PNDs 14 and 22.
2.5. Quantification of BMD and bone metabolism
Femoral BMD was measured by dual-energy X-ray absorptiometry using a specialized software program for small animals (Model DCS-600EX-R III, Aloka, Ltd., Tokyo, Japan). BMD was calculated using the bone mineral content of the measured area. Intra-assay and inter-assay coefficients of variation were less than 1.0% and 4.8%, respectively. The detection limit of BMD is 15 mg/cm2. Serum osteocalcin levels were measured with a commercial kit, the Rat Osteocalcin EIA Kit (Biomedical Technologies, Inc., Stoughton, MA).
2.6. Serum daidzein and equol concentrations in offspring and the stomach contents of pups
Serum daidzein and equol concentrations were measured by the methods reported by Gamache and Acworth [19]. An aliquot of the pretreated sample solution was used for high-performance liquid chromatography (HPLC) (Shimadzu LC10AD) with an electrochemical detector (Coulochem III, ESA Laboratories Inc., Chelmsford, MA) that was equipped with analytical cells (detector 1, 300 mV; detector 2, 600 mV) (ESA) and a guard cell (650 mV) (ESA). The HPLC conditions were as follows: column, Nova-Pac C18 (Waters, 3.9 mm × 150 mm; Nihon Waters K.K., Tokyo, Japan); column temperature, 40 °C; mobile phase, 50/35/15% (v/v/v) 50 mM sodium acetate (pH 4.8):methanol:acetonitrile (Wako, Osaka, Japan); and flow rate, 0.65 ml/min.
2.7. Statistical analysis
Data are expressed as means ± SEM. All statistical tests were conducted with SPSS, version 15.0 (SPSS Inc., Chicago, IL). Significance of differences in BMD was determined by single-factor analysis of covariance and Fisher's protected least significant difference tests. Body weight was used as a covariate in the analysis of BMD to adjust for possible confounding. Body weight, AGD, reproductive organ weight, and serum isoflavone and osteocalcin concentrations were analyzed using analysis of variance (ANOVA). Differences between treatment groups were assessed by Tukey's test. Differences were considered significant at p < 0.05.
3. Results
3.1. Pregnancy outcomes
There was no difference in the number of pups per litter among the groups (Control = 13.3 ± 1.0; Dz-S = 12.2 ± 1.4, and Dz-L; 12.3 ± 0.9). There were also no differences in the number of males and females per litter (m, f: Control = 6.5, 6.8; Dz-S = 6.7, 5.5; and Dz-L = 7.0, 5.3) and birth weight among the groups (Control = 7.0 ± 0.3 g; Dz-S = 7.4 ± 0.2 g; Dz-L = 7.5 ± 0.2 g).
3.2. Body weight, food intake, AGD, and sex organ weight
The mean body weight for males on PND 1 (day of birth) and PND 22 (weaning day) was not significantly different among the groups (Table 1). The mean body weight for males on PND 35 was significantly higher in the Dz-L group than in the Control and Dz-S groups, but there was no significant difference among groups on PND 77. There was no difference in total weight gained among the groups (Table 1). There was no difference in food intake in dams and offspring among the groups (data not shown). There were no significant differences in mean body weight and the total weight gained in females at any of the time points among the groups (Table 1).
Table 1.
Body weight and total weight gain of offspring on PNDs 1, 22, 35, and 77.
| Group | Control | Daidzein Short | Daidzein Long |
|---|---|---|---|
| Male | |||
| Body weight (g) | |||
| PND 1 | 7.32 ± 0.36 | 7.60 ± 0.29 | 7.90 ± 0.32 |
| PND 22 | 67.5 ± 1.2 | 63.6 ± 1.6 | 63.1 ± 1.7 |
| PND 35 | 151.4 ± 1.7b | 148.2 ± 1.0b | 158.6 ± 2.0a |
| PND 77 | 433.0 ± 6.7 | 430.8 ± 11.8 | 439.8 ± 14.8 |
| Total weight gain (g) |
425.7 ± 6.5 |
423.2 ± 12.0 |
431.9 ± 15.0 |
| Female | |||
| Body weight (g) | |||
| PND 1 | 3.56 ± 0.40 | 3.30 ± 0.51 | 3.03 ± 0.26 |
| PND 22 | 65.0 ± 0.3 | 61.0 ± 1.3 | 61.0 ± 1.9 |
| PND 35 | 130.7 ± 2.2 | 127.5 ± 3.8 | 135.5 ± 5.4 |
| PND 77 | 243.3 ± 4.3 | 242.6 ± 7.5 | 233.0 ± 17.0 |
| Total weight gain (g) | 239.8 ± 4.4 | 239.2 ± 7.3 | 230.0 ± 6.8 |
All values are means ± SE (n = 6). Values that do not share the same superscript letters are significantly different from each other (p < 0.05). Data were analyzed using one-way ANOVA. Differences between the groups were assessed by Tukey's test.
Dams and their offspring fed the control diet (Control); dams fed the daidzein diet (0.5 g daidzein/kg diet) during pregnancy then the Control diet at postnatal day (PND) 13 and their offspring fed the Control diet (Dz-S); and dams and their offspring fed the daidzein diet (Dz-L).
PND: postnatal day.
Table 2 shows AGD and sex organ weight. The AGD of male and female offspring on PNDs 14 and 22 was not different among the groups. On PNDs 22, 35 and 77, treatment with daidzein had no effect on the mean weight of testes and seminal vesicles. The weight of the ovaries and uterus in female offspring on PNDs 22, 35, and 77 was not altered by daidzein exposure to the dams or offspring.
Table 2.
Anogenital distance and sex organ weight of offspring on PNDs 14, 22, 35, and 77.
| Group | Control | Daidzein Short | Daidzein Long |
|---|---|---|---|
| Male | |||
| Anogenital distance (mm) | |||
| PND 14 | 3.72 ± 0.09 | 3.96 ± 0.18 | 3.77 ± 0.11 |
| PND 22 | 3.14 ± 0.10 | 3.14 ± 0.11 | 3.21 ± 0.13 |
| Testis weight (mg) | |||
| PND 22 | 277 ± 10 | 264 ± 14 | 262 ± 14 |
| PND 35 | 1304 ± 42 | 1346 ± 40 | 1277 ± 43 |
| PND 77 | 3353 ± 27 | 3319 ± 44 | 3233 ± 75 |
| Seminal vesicle (mg) | |||
| PND 22 | 43 ± 2 | 45 ± 2 | 44 ± 2 |
| PND 35 | 161 ± 12 | 149 ± 9 | 169 ± 25 |
| PND 77 |
922 ± 49 |
922 ± 37 |
967 ± 55 |
| Female | |||
| Anogenital distance (mm) | |||
| PND 14 | 2.61 ± 0.10 | 2.89 ± 0.11 | 2.72 ± 0.03 |
| PND 22 | 2.01 ± 0.04 | 2.15 ± 0.08 | 2.12 ± 0.09 |
| Ovaries + uterus (mg) | |||
| PND 22 | 65 ± 3 | 67 ± 5 | 68 ± 3 |
| PND 35 | 210 ± 33 | 167 ± 21 | 233 ± 27 |
| PND 77 | 515 ± 48 | 546 ± 41 | 541 ± 54 |
All values are means ± SE (n = 6). Data were analyzed using one-way ANOVA. Differences between the groups were assessed by Tukey's test (p < 0.05).
Dams and their offspring fed the control diet (Control); dams fed the daidzein diet (0.5 g daidzein/kg diet) during pregnancy then the Control diet at postnatal day (PND) 13 and their offspring fed the Control diet (Dz-S); and dams and their offspring fed the daidzein diet (Dz-L).
PND: postnatal day.
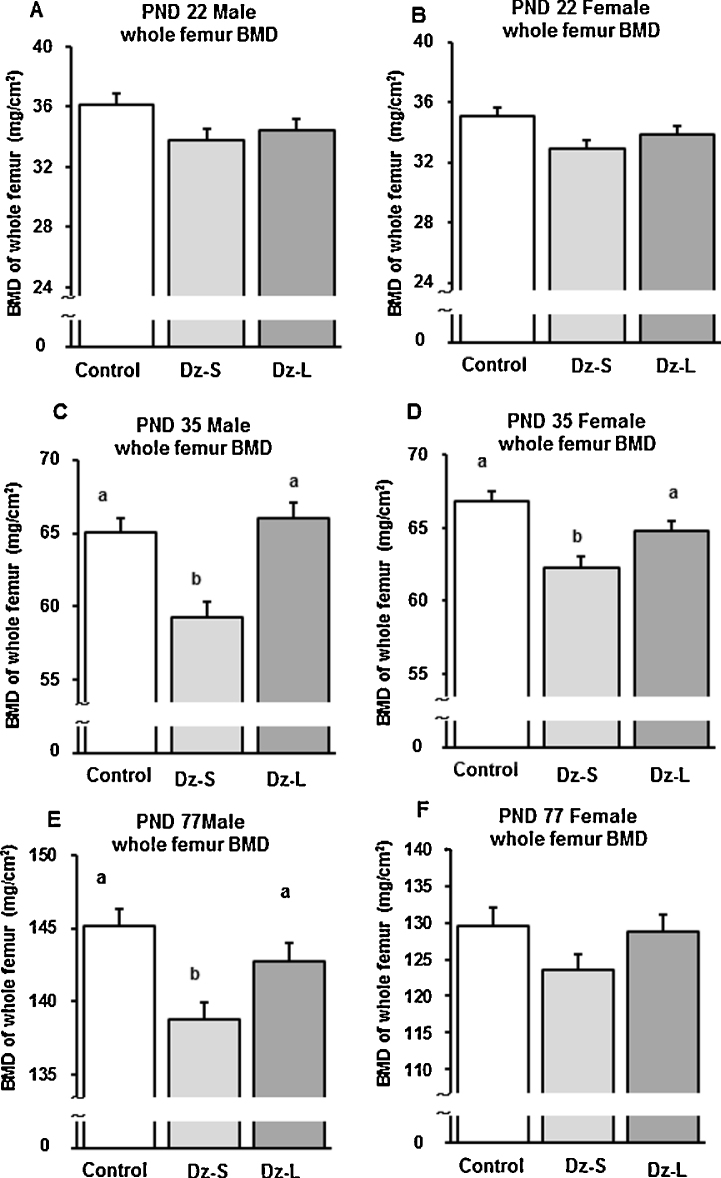
3.3. BMD of the femur
On PND 22, there was no difference in femur BMD in male and female rats among the groups (Fig. 2A and B). On PND 35, femur BMD in male and female rats in the Dz-S group was significantly lower than that in the Control and Dz-L groups (Fig. 2C and D). On PND 77, femur BMD in male rats in the Dz-S group was significantly lower than that in the Control and Dz-L groups (Fig. 2E). However, femur BMD in female rats was not different among the groups on PND 77 (Fig. 2F).
Fig. 2.
Bone mineral density of the right femur in offspring of dams that were fed the control diet or the diet containing 0.5 g daidzein/kg during pregnancy and lactation and/or the post-weaning period. Data are shown for PNDs 22, 35, and 77. All values are means ± SEM (n = 6). Values that do not share the same superscript letters are significantly different from each other (p < 0.05). Significant differences in BMD were determined by one-factor ANCOVA and Fisher's protected least significant difference test. Control group: rats were fed a control diet; Dz-S group: rats were fed a daidzein diet (0.5 g daidzein/kg diet) during pregnancy and lactation; Dz-L group: rats were fed a daidzein diet (0.5 g daidzein/kg diet) during pregnancy, lactation, and post-weaning. PND: postnatal day.
3.4. Serum osteocalcin concentrations
Table 3 shows serum osteocalcin concentrations, which are a biochemical marker of bone formation. In male rats, mean serum osteocalcin concentrations on PND 22 were significantly lower in the Dz-S and Dz-L groups (1.48 ± 0.08 and 1.51 ± 0.08 ng/ml, respectively) than in the Control group (1.91 ± 0.12 ng/ml). On PND 35, the mean serum osteocalcin concentration in the Dz-S group was significantly lower than that in the Dz-L group (4.45 ± 0.20 ng/ml) in male rats. On PND 77, there was no difference in serum osteocalcin concentrations in male rats among the groups.
Table 3.
Serum osteocalcin concentrations in rat offspring on PNDs 22, 35, and 77.
| Group | Control | Daidzein Short | Daidzein Long |
|---|---|---|---|
| Osteocalcin (ng/ml) | |||
| Male | |||
| PND 22 | 1.91 ± 0.12a | 1.48 ± 0.08b | 1.51 ± 0.08b |
| PND 35 | 4.08 ± 0.22ab | 3.34 ± 0.30b | 4.45 ± 0.20a |
| PND 77 |
5.04 ± 0.33 |
4.23 ± 0.17 |
4.84 ± 0.22 |
| Female | |||
| PND 22 | 1.64 ± 0.04 | 1.62 ± 0.06 | 1.63 ± 0.05 |
| PND 35 | 3.95 ± 0.10a | 3.37 ± 0.09b | 3.67 ± 0.06ab |
| PND 77 | 4.23 ± 0.11 | 4.16 ± 0.14 | 4.11 ± 0.13 |
All values are means ± SE (n = 6). Values that do not share the same superscript letters are significantly different from each other (p < 0.05). Data were analyzed using one-way ANOVA. Differences between the groups were assessed by Tukey's test.
Control; the group fed the control diet, Daidzein Short; the group fed the daidzein diet (0.5 g daidzein/kg diet) during both pregnancy and lactation periods, Daidzein Long; the group fed the daidzein diet (0.5 g daidzein/kg diet) during pregnancy, lactation and postweaning periods.
PND: postnatal day.
In female rats, mean serum osteocalcin concentrations were significantly lower in the Dz-S group (3.37 ± 0.09 ng/ml, p = 0.001) and tended to be lower (p = 0.085) in the Dz-L group (3.67 ± 0.06 ng/ml) than in the Control group (3.95 ± 0.10 ng/ml) on PND 35 (Table 3). There was no difference in mean serum osteocalcin concentrations in female rats among the groups on PNDs 22 and 77.
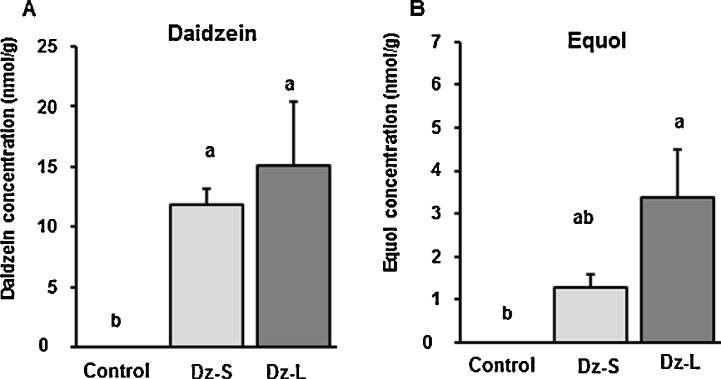
3.5. Stomach content and serum daidzein and equol concentrations
Serum daidzein and equol concentrations were detected in dams in the Dz-L group (4.30 ± 0.45 and 5.71 ± 1.26 μmol/L, respectively). Stomach contents of daidzein and equol in each offspring were measured and combined the values from male and female. Daidzein (11.86 ± 1.28 and 15.12 ± 5.32 nmol/g, respectively) and equol (1.29 ± 0.29 and 3.37 ± 1.14 nmol/g, respectively) were detected in the stomach contents of the suckling pups of dams in the Dz-S and Dz-L groups (Fig. 3). Serum daidzein and equol concentrations were observed in the offspring on PNDs 22 (2.03 ± 0.40 and 1.58 ± 0.18 μmol/L, respectively), 35 (4.23 ± 0.91 and 4.40 ± 1.08 μmol/L, respectively), and 77 (1.17 ± 0.33 and 4.31 ± 0.76 μmol/L, respectively) in the Dz-L group.
Fig. 3.
Daidzein and equol concentrations in the stomach contents of suckling pups of dams that were fed a control diet or diet containing 0.5 g daidzein/kg during pregnancy and lactation. Data are shown for PND 14. All values are means ± SEM (n = 6–9). Values that do not share the same superscript letters are significantly different from each other (p < 0.05). Data were analyzed using one-way ANOVA. Differences between the groups were assessed by Tukey's test. Control group: rats were fed a control diet; Dz-S group: rats were fed a daidzein diet (0.5 g daidzein/kg) during pregnancy and lactation; Dz-L group: rats were fed a daidzein diet (0.5 g daidzein/kg) during pregnancy, lactation, and post-weaning.
4. Discussion
This study showed that perinatal exposure to daidzein inhibited an increase in BMD and serum osteocalcin concentrations in male and female rats on PND 35. However, peripubertal exposure to daidzein protected against the decline of these bone parameters in male and female rats on PND 35. On PND 77, femur BMD in male rats that were exposed to daidzein perinatally was lower than that in control rats or those with peripubertal exposure to daidzein. However, femur BMD in female rats was not different among the groups. These results suggest that the effects of daidzein on bone metabolism during development depend on the timing of exposure and sex. However, perinatal or peripubertal exposure to daidzein did not affect development of reproductive organs by early adulthood in rats.
On PND 35, femur BMD in male and female rats in the Dz-S group was significantly lower than that in the Control and Dz-L groups (Fig. 2C and D). Similarly, serum osteocalcin concentrations, a biochemical marker of bone formation, tended to be lower in male and female rats in the Dz-S group than in the Control and Dz-L groups on PND 35 (Table 3). On PND 77 in male rats, femur BMD in the Dz-S group was significantly lower than that in the Control and Dz-L groups (Fig. 2E), but female BMD was not different among the groups (Fig. 2F). Our results suggest that perinatal and postnatal daidzein exposure show different effects on bone metabolism. Mardon et al. [20] reported that rats with perinatal or lifelong exposure to an isoflavone-rich diet had increased femoral and metaphyseal BMD at 24 months, but there was no significant effect at 3, 6, or 12 months. However, mice that are treated with daidzein during the prenatal period have lower femur and lumbar spine BMD than controls at 4 months of age [21]. Endogenous hormone status and the age at BMD measurement may affect experimental outcomes. The age at which BMD is most altered needs to be determined to fully understand how bone mineral accumulation is affected by prenatal or postnatal exposure to isoflavones. Bone quality is also important for assessing bone development. Further studies are required to confirm the effects of daidzein on bone development, such as measurement of biomechanical strength and bone microarchitecture.
In male rats in the Dz-S and Dz-L groups, mean serum osteocalcin concentrations on PND 22 were significantly lower than those in the Control group, but there was no difference in female rats among the groups. These sex-specific findings are consistent with those of previous studies using a mouse model in which exposure to daidzein (2 mg/kg body weight/day) from PNDs 1 to 5 improved BMD [8] and microarchitecture in the femur of female mice at 4 months of age, but it had minimal effects on bone development in male mice [9]. Reports on men with administration of aromatase and/like compounds have shown that estrogen is crucial for bone growth and development [22], [23], [24]. Estrogen withdrawal prevents epiphyseal fusion, which stops bones from lengthening, up-regulates bone resorption, and decreases BMD [25]. Therefore, exposure to estrogen-like compounds is crucial for healthy skeletal development in male mammals. In our study, daidzein showed different effects on bone metabolism in female rats compared with male rats. The reason for this finding may be because of the difference in types of sex hormones [11]. Additionally, the change in BMD in male rats on PND 77 observed in our study may have occurred as a consequence of programming effects in the fetal period. “The concept of programming” is that a stimulus or insult during a critical or sensitive period of development can have long-term or lifetime effects on an organism [26]. Therefore, hormonal signals operating during critical windows have numerous programming effects. Estrogen-signaling pathways and modes of epigenetic programming have provided some insight into the potential mechanism of action. By binding and activating nuclear receptors, isoflavones may interfere with hormonal signaling and/or the production of enzymes and transcription factors [27], [28]. During development, such as fetal and/or perinatal periods, endocrine hormones and enzymes can alter epigenetic regulation that controls gene transcription [29]. It is speculated that exposure to daidzein during the fetal period has induced irreversible effects on bone metabolism in this study. Further studies are required to confirm the effects of perinatal and peripubertal exposure to daidzein on bone development, as well as to determine the underlying mechanism.
Safety is an important issue to consider when extrapolating these findings to isoflavone supplements or soy-based infant formulas. We investigated the effect of daidzein on safety in addition to bone metabolism. We found no marked differences in the outcome of pregnancy, including the number of pups per litter, the sex ratio, and birth weight. Our findings are consistent with those of Ward et al. [9] who reported that no effects on those parameters were observed in exposure of pregnant mice to genistein (3.75 mg/day) and/or daidzein (3.75 mg/day) subcutaneously. Consequently, exposure of dams to daidzein does not appear to have a remarkable effect on pregnancy outcomes.
In our study, perinatal or peripubertal exposure to daidzein did not affect body weight in female rats. However, peripubertal exposure to daidzein in the Dz-L group increased body weight in male rats on PND 35 compared with the Control and Dz-S groups (Table 1). There was no significant difference in male offspring body weight among the groups on PND 77 (Table 1). In contrast to our findings, Ronis et al. [30] showed that male rats exposed to dietary isoflavones (80 mg/kg body weight/day) from PNDs 15 to 33 had reduced body weight at PND 33. In the present study, the daidzein dose for offspring in the Dz-S and Dz-L groups was calculated as approximately 51 mg/kg body weight/day, which was lower than that in the study by Ronis et al. [30]. Although a large number of studies have shown that exposure to isoflavones during the prenatal and/or postnatal period influence growth, the results are inconsistent.
In our study, the sex organ weights and AGD in male and female rats exposed to daidzein during the perinatal or peripubertal periods did not change with treatment (Table 2). AGD is used as a measure of endocrine disruption. A longer AGD at earlier onset of puberty has been observed in female and male mice that were exposed to estrogen [31]. Our study indicated that exposure to daidzein during the perinatal period or during the perinatal period and after weaning might not affect parameters of endocrine disruption.
In our study, pregnant rats in the Dz-S and Dz-L groups were fed approximately 50 mg daidzein/kg body weight/day (approximately 15 mg/day), and daidzein and equol were detected in the stomach contents of suckling pups (Fig. 3). Although daidzein is transferred from lactating mothers to suckling pups [15], our results suggest that equol is also transferred from mothers to suckling pups via the breast milk of dams that are fed a diet containing daidzein. Moreover, a similar transfer from mother to fetus has been reported in rats and humans [15], [16], [17]. Daidzein is rapidly transferred from the maternal circulation to the fetus, and elevated serum daidzein levels are present in rat fetuses from mothers that were fed isoflavones [15], [16], [17], [32]. One of the main metabolites of daidzein is equol, which is thought to be the most potent modifier of the effects of isoflavones. Equol might mediate the effects of daidzein in sex organ development and bone metabolism in rat fetuses and offspring.
In our study, serum daidzein and equol concentrations in offspring on PNDs 22, 35, and 77 were detected. A previous study showed that circulating daidzein levels in rats on PND 21 were similar to those in human infants (1.16 ± 0.09 μmol/L) who were fed a soy protein-based formula [32]. Serum daidzein concentrations in rat offspring on PNDs 35 and 77 in our study are several times higher than those in Asian people [33], [34]. The dose of isoflavones that was administered in the study was much higher than those from ordinary soy products. However, the dose of soy isoflavones used in this study could be obtained by isoflavones supplements. Our results suggest that excessive intake of isoflavones during the prenatal and postnatal periods should be carefully assessed in further studies. Further investigation concerning the safety and efficacy of soy products fortified with isoflavones and extracted supplements remains a research priority, particularly because of their widespread availability and growing use.
5. Conclusions
In conclusion, we assessed the safety and efficiency of perinatal or peripubertal exposure to daidzein on bone and reproductive organ development at early adulthood in rats. Perinatal exposure to daidzein does not confer a positive effect on BMD in either male or female rats on PND 35. However, peripubertal exposure to daidzein protects against a decline in BMD in male and female rats on PND 35. Perinatal exposure to daidzein leads to reduced femoral BMD in male rats, but not female rats, on PND 77. Therefore, the effects of daidzein on bone metabolism during development may depend on the timing of exposure and sex. Exposure to daidzein during the perinatal or peripubertal period does not have serious adverse effects on sexual development of offspring. Further investigations should assess the mechanisms underlying these responses of bone metabolism to daidzein, as well as the safety of daidzein exposure during the perinatal period and throughout life.
Conflict of interest
The authors have no conflicts of interest.
Transparency document
Acknowledgements
This study was supported by grants from the MEXT-Supported Program for the Strategic Research Foundation at Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (S1101015). Excellent technical assistance was provided by Chiharu Yamazaki. The authors would like to thank Dr Sachie Ikegami, Otsuma Woman's University, for her advice on the research.
Contributor Information
Yuko Tousen, Email: tousen@nih.go.jp.
Hajimu Ishiwata, Email: ishiwata@tenor.ocn.ne.jp.
Ken Takeda, Email: takedak@rs.noda.tus.ac.jp.
Yoshiko Ishimi, Email: ishimi@nih.go.jp.
References
- 1.Schmitt E., Dekant W., Stopper H. Assaying the estrogenicity of phytoestrogens in cells of different estrogen sensitive tissues. Toxicol. In Vitro. 2001;15:433–439. doi: 10.1016/s0887-2333(01)00048-0. [DOI] [PubMed] [Google Scholar]
- 2.Taku K., Melby M.K., Takebayashi J., Mizuno S., Ishimi Y., Omori T., Watanabe S. Effect of soy isoflavone extract supplements on bone mineral density in menopausal women: meta-analysis of randomized controlled trials. Asia Pac. J. Clin. Nutr. 2010;19:33–42. [PubMed] [Google Scholar]
- 3.Morito K., Hirose T., Kinjo J., Hirakawa T., Okawa M., Nohara T., Ogawa S., Inoue S., Muramatsu M., Masamune Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol. Pharm. Bull. 2001;24:351–356. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 4.Lampe J.W. Is equol the key to the efficacy of soy foods? Am. J. Clin. Nutr. 2009;89:1664S–1667S. doi: 10.3945/ajcn.2009.26736T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Migliaccio S., Newbold R.R., Bullock B.C., Jefferson W.J., Sutton F.G., Jr., McLachlan J.A., Korach K.S. Alterations of maternal estrogen levels during gestation affect the skeleton of female offspring. Endocrinology. 1996;137:2118–2125. doi: 10.1210/endo.137.5.8612556. [DOI] [PubMed] [Google Scholar]
- 6.Migliaccio S., Newbold R.R., Teti A., Jefferson W.J., Toverud S.U., Taranta A., Bullock B.C., Suggs C.A., Spera G., Korach K.S. Transient estrogen exposure of female mice during early development permanently affects osteoclastogenesis in adulthood. Bone. 2000;27:47–52. doi: 10.1016/s8756-3282(00)00286-6. [DOI] [PubMed] [Google Scholar]
- 7.Piekarz A.V., Ward W.E. Effect of neonatal exposure to genistein on bone metabolism in mice at adulthood. Pediatr. Res. 2007;61:48–53. doi: 10.1203/01.pdr.0000250200.94611.03. [DOI] [PubMed] [Google Scholar]
- 8.Kaludjerovic J., Ward W.E. Neonatal exposure to daidzein, genistein, or the combination modulates bone development in female CD-1 mice. J. Nutr. 2009;139:467–473. doi: 10.3945/jn.108.100115. [DOI] [PubMed] [Google Scholar]
- 9.Ward W.E., Piekarz A.V., Fonseca D. Bone mass, bone strength, and their relationship in developing CD-1 mice. Can. J. Physiol. Pharmacol. 2007;85:274–279. doi: 10.1139/y07-020. [DOI] [PubMed] [Google Scholar]
- 10.Kaludjerovic J., Ward W.E. Neonatal administration of isoflavones attenuates deterioration of bone tissue in female but not male mice. J. Nutr. 2010;140:766–772. doi: 10.3945/jn.109.116343. [DOI] [PubMed] [Google Scholar]
- 11.Fujioka M., Sudo Y., Okumura M., Wu J., Uehara M., Takeda K., Hosokawa Y., Yamada K., Ikegami S., Ishimi Y. Differential effects of isoflavones on bone formation in growing male and female mice. Metabolism. 2007;56:1142–1148. doi: 10.1016/j.metabol.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Dinsdale E.C., Ward W.E. Early exposure to soy isoflavones and effects on reproductive health: a review of human and animal studies. Nutrients. 2010;2:1156–1187. doi: 10.3390/nu2111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cederroth C.R., Zimmermann C., Nef S. Soy, phytoestrogens and their impact on reproductive health. Mol. Cell. Endocrinol. 2012;355:192–200. doi: 10.1016/j.mce.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 14.Napier I.D., Simon L., Perry D., Cooke P.S., Stocco D.M., Sepehr E., Doerge D.R., Kemppainen B.W., Morrison E.E., Akingbemi B.T. Testicular development in male rats is sensitive to a soy-based diet in the neonatal period. Biol. Reprod. 2014;90:40. doi: 10.1095/biolreprod.113.113787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikegami S., Tousen Y., Ishimi Y., Umegaki K., Nakashima Y. Possible adverse effects of soy isoflavone mixture on pregnant and lactating rats and their suckling pups. J. Health Sci. 2006;52:558–567. doi: 10.3177/jnsv.52.174. [DOI] [PubMed] [Google Scholar]
- 16.Brown N.M., Setchell K.D. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab. Invest. 2001;81:735–747. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- 17.Franke A.A., Custer L.J. Daidzein and genistein concentrations in human milk after soy consumption. Clin. Chem. 1996;42:955–964. [PubMed] [Google Scholar]
- 18.Reeves P.G., Nielsen F.H., Fahey G.C., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 19.Gamache P.H., Acworth I.N. Analysis of phytoestrogens and polyphenols in plasma, tissue, and urine using HPLC with coulometric array detection. Proc. Soc. Exp. Biol. Med. 1998;217:274–280. doi: 10.3181/00379727-217-44232. [DOI] [PubMed] [Google Scholar]
- 20.Mardon J., Mathey J., Kati-Coulibaly S., Puel C., Davicco M.J., Lebecque P., Horcajada M.N., Coxam V. Influence of lifelong soy isoflavones consumption on bone mass in the rat. Exp. Biol. Med. (Maywood) 2008;233:229–237. doi: 10.3181/0707-RM-202. [DOI] [PubMed] [Google Scholar]
- 21.Ward W.E., Piekarz A.V. Effect of prenatal exposure to isoflavones on bone metabolism in mice at adulthood. Pediatr. Res. 2007;61:438–443. doi: 10.1203/pdr.0b013e3180332d67. [DOI] [PubMed] [Google Scholar]
- 22.Morishima A., Grumbach M.M., Simpson E.R., Fisher C., Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J. Clin. Endocrinol. Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 23.Maffei L., Murata Y., Rochira V., Tubert G., Aranda C., Vazquez M., Clyne C.D., Davis S., Simpson E.R., Carani C. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J. Clin. Endocrinol. Metab. 2004;89:61–70. doi: 10.1210/jc.2003-030313. [DOI] [PubMed] [Google Scholar]
- 24.Bilezikian J.P., Morishima A., Bell J., Grumbach M.M. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N. Engl. J. Med. 1998;339:599–603. doi: 10.1056/NEJM199808273390905. [DOI] [PubMed] [Google Scholar]
- 25.Carani C., Qin K., Simoni M., Faustini-Fustini M., Serpente S., Boyd J., Korach K.S., Simpson E.R. Effect of testosterone and estradiol in a man with aromatase deficiency. N. Engl. J. Med. 1997;337:91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 26.Lucas A. Long-term programming effects of early nutrition – implications for the preterm infant. J. Perinatol. 2005;25:S2–S6. doi: 10.1038/sj.jp.7211308. [DOI] [PubMed] [Google Scholar]
- 27.Mueller S.O., Simon S., Chae K., Metzler M., Korach K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol. Sci. 2004;80:14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- 28.Wiegand H., Wagner A.E., Boesch-Saadatmandi C., Kruse H.P., Kulling S., Rimbach G. Effect of dietary genistein on Phase II and antioxidant enzymes in rat liver. Cancer Genomics Proteomics. 2009;6:85–92. [PubMed] [Google Scholar]
- 29.Godfrey K.M., Barker D.J. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000;71:1344S–1352S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- 30.Ronis M.J., Chen Y., Badeaux J., Badger T.M. Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling. J. Nutr. 2009;139:1431–1438. doi: 10.3945/jn.109.107029. [DOI] [PubMed] [Google Scholar]
- 31.Amstislavsky S.Y., Kizilova E.A., Golubitsa A.N., Vasilkova A.A., Eroschenko V.P. Preimplantation exposures of murine embryos to estradiol or methoxychlor change postnatal development. Reprod. Toxicol. 2004;18:103–108. doi: 10.1016/j.reprotox.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Setchell K.D., Zimmer-Nechemias L., Cai J., Heubi J.E. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;5(350):23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 33.Adlercreutz H., Markkanen H., Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 34.Lee N. Phytoestrogens as bioactive ingredients in functional foods: Canadian regulatory update. J. AOAC Int. 2006;89:1135–1137. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.