Abstract
The aim of the present work was to develop a biochemical, histologic and immunohistochemical study about the potential hepatotoxic effect of d-limonene – a component of volatile oils extracted from citrus plants. Blood alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) from d-limonene-treated animals were determined and compared to morphologic hepatic lesions in order to investigate the possible physiopathologic mechanisms involved in the liver toxicity, in experimental animals treated with d-limonene. Wistar rats were randomly divided into seven groups: two control groups (untreated or receiving only vehicle, tween-80); one positive control (vehicle); two experimental groups treated with d-limonene at doses of 25 mg/kg/day and 75 mg/kg/day for 45 days, and two other groups treated with the same doses for 30 days and kept under observation during 30 more days. Biochemical data showed significant reduction in ALT levels in the animals treated with 75 mg/kg of d-limonene. Histological analysis revealed some hepatocyte morphological lesions, including hydropic degeneration, microvesicular steatosis and necrosis, Kupffer cell hyperplasia and incipient fibrosis. By immunohistochemistry, influx of T (CD3+) and cytotoxic (CD8+) lymphocytes was observed in the rats treated with d-limonene at both dose levels. In conclusion, it is possible that d-limonene has been directly responsible for hepatic parenchymal and matrix damage following subchronic treatment with d-limonene.
Abbreviations: ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HE, hematoxylin–eosin
Keywords: Limonene, Essential oils, Hepatotoxicity, Histopathology, Rodent
1. Introduction
d-Limonene(1-methyl-4-(1-methylethenyl)-cyclohexene) is a monocyclic monoterpene found as the major constituent in several citrus oils such as lemon and orange [29], and other essential oils [5] known to be employed as flavoring agents and fragrances in household products [4], [22], [9]. It is used clinically to dissolve cholesterol-containing gallstones and for relief of gastroesophageal reflux and heartburn due to its gastric acid neutralizing effect. It also shows chemopreventive activity against many types of cancers, including breast and colorectal cancers [8], [23], and has anti-oxidant property [19], [28]. Orally administered d-limonene is rapidly absorbed in the gastrointestinal tract in animals and humans, being subsequently distributed to different parts of the body, such as the liver, lung and kidney, where it is readily metabolized [7], [23].
The liver is the most important organ for metabolization and elimination of substances, but it is also a frequent target for toxic aggressions. Over the years, there has been increasing evidence that plant-derived components can cause hepatotoxicity. For instance, the occurrence of acute hepatitis and acute liver failure has been attributed to the use of some plants, such as the popular kava-kava [15]. Acute and chronic experiment protocols have evidenced that d-limonene exhibits fairly low toxicity [11], although (+)-limonene was shown to cause renal toxicity in male rats, probably related to the high expression of α2u-globulin in the liver [14], [22]. In view of the widespread use of d-limonene in food products, this work was aimed at investigating the cytotoxic effects and identifying hepatocellular lesions of this substance after administration to Wistar rats.
2. Materials and methods
2.1. Animals and treatments
Fifty-eight male Wistar rats (Rattus norvegicus Berkenhout, 1769) (90 days old and weighing about 280–350 g) were obtained from the vivarium Prof. Dr. Thomas George of the Federal University of Paraíba (UFPB, João Pessoa, Paraíba, Brazil), where they were born and bred. The animals were housed in polyethelene cages and kept under standard laboratory conditions at a temperature of 23 ± 2° C, with a 12 h light/12 h dark photoperiod. They were fed on rat chow pellets (Purina) and received water ad libitum. All animals were deprived of food and water 60 min before the beginning of the experiments. The experimental protocol was approved by the Ethical Committee of UFPB, which follows the international principles in ethics for animal experimentation.
Wistar rats were randomly divided into seven groups as shown in Table 1, and treated by gavage and once daily for 30 or 45 days. The treatment groups received d-limonene (Sigma-Aldrich, USA), diluted in 0.2% tween-80 (Sigma-Aldrich, USA), at the dose levels of 25 mg/kg and 75 mg/kg of body weight. The lower dose was based on the lowest dose showing activity on the central nervous system reported in the literature, while the higher dose was related to the occurrence of hepatomegaly as reported previously [12]. Another treatment group used as a positive control received ethanol (diluted in 20% distilled water) at a dose of 3.5 ml/kg of body weight following the same protocol as the other treatment groups. The control group comprised one group that received no treatment and another that received tween-80.
Table 1.
Animal groups and treatments.
| Group | Drug | N | Duration of treatment (days) |
|---|---|---|---|
| I | – | 8 | – |
| II | Tween-80 | 10 | 45 |
| III | 20% ethanol | 10 | 45 |
| IV | 25 mg/kg of d-limonene | 10 | 45 |
| V | 75 mg/kg of d-limonene | 10 | 45 |
| VI | 25 mg/kg of d-limonene | 5 | 30 |
| VII | 75 mg/kg of d-limonene | 5 | 30 |
2.2. Bioassay
During the experiment, the animals were observed for detection of clinical signs of toxicity. The animals from group I to V were sacrificed on the 46th day, while animals from groups VI and VII were sacrificed 30 days after the end of treatment. The animals were killed by cervical dislocation and, immediately after death, they underwent laparotomy for the removal, weighing and histopathological analysis of liver, kidneys, lungs and heart. The organs were fixed in formalin (10% formaldehyde solution), embedded in paraffin, and sectioned at 3.0 μm thickness for routine hematoxylin–eosin (HE) staining. The staining with picrosirius red was employed for collagen detection and the Perls’ technique was used for the investigation of hepatosiderosis. The morphological evaluation was carried out in a light microscope (Nikon Eclipse E200). Prior to autopsy, blood was drawn by cardiac puncture for biochemical analysis of serum alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) using kinetic methods (Toxicology Laboratory, UFPB).
The hepatic lesions were identified according to the following criteria: (1) hydropic degeneration, (2) microvesicular steatosis, (3) necrosis (hypostained or absent nucleus, intense cytoplasmic eosinophilia, and destruction or loss of the architecture of the hepatocyte cord), and (4) apoptosis. A system of scores to grade liver lesions was employed following the criteria set by the Brazilian Society of Pathology and the Brazilian Society of Hepatology (1999) (Table 2).
Table 2.
Criteria for histological evaluation of the degree of fibrosis (A), degree of portal lymphocyte infiltration (B), degree of periportal and periseptal lymphocyte infiltration (C) and degree of necroinflammatory activity in the hepatic tissue (D).
| A |
B |
||
|---|---|---|---|
| Score | Lobular architecture | Degree | Septal and portal inflammatory infiltrate |
| F0 | Normal (absence of fibrosis) | 0 | Absent of rare portal lymphocytes |
| F1 | Fibrous extension of portal spaces | 1 | Discreet increase in the number of portal lymphocytes |
| F2 | Fibrous extension of portal spaces with portal-portal septa | 2 | Moderate increase in the number of portal lymphocytes |
| F3 | Fibrous extension of portal spaces with portal-portal and portal-central septa, with possible nodule formation | 3 | Marked increase in the number of portal lymphocytes |
| F4 | Cirrhosis with predominant nodular areas in relation to the remaining lobules | 4 | Strongly marked increase in the number of portal lymphocytes |
| C |
D |
||
|---|---|---|---|
| Degree | Septal and portal inflammatory activity | Degree | Necroinflammatory activity |
| 0 | Absence of lesions in the interface portal space/parenchyma | 0 | Normal and isomorphic hepatocytes |
| 1 | Lymphocyte extravasations not characterizing piecemeal necrosis | 1 | Discreet hepatocytic alterations (tumefaction or acidophilic retraction) with lymphohistiocytic infiltrate and rare necrotic areas |
| 2 | Discreet piecemeal necrosis | 2 | Focal hepatocyte necrosis surrounded by lymphohistiocytic aggregate |
| 3 | Moderate piecemeal necrosis | 3 | Focal hepatocyte necrosis surrounded by lymphohistiocytic aggregate associated with limited areas of confluent necrosis |
| 4 | Piecemeal necrosis in extensive areas of portal spaces | 4 | Focal hepatocyte necrosis surrounded by lymphohistiocytic aggregate associated with limited areas of extensive/multiple confluent necrosis |
The degree of hepatic siderosis was evaluated according to [21] as follows: (1) absence of hepatic iron, (2) iron particles visible at 400× magnification, (3) iron particles visible at 100× magnification, (4) iron particles visible at 25× magnification, and (5) accumulation of iron particles visible at 10× magnification or with the naked eye.
Immunohistochemistry was performed in order to verify the exact nature of the inflammatory mononuclear populations. The histological sections underwent antigenic recovery by using citrate buffer (pH 6.0), heated in water bath at a temperature of 95 °C for 20 min. After cooling, they were blocked with peroxidase blocking solution (3% hydrogen peroxide) following primary antibody incubation for 12 h (dilution 1:200). The antibodies used were: CD3 (anti-human T cells, clone UCHT1), CD8 (anti-human cytotoxic T cells, clone M 707), and macrophage marker (clone HAM-56). The sections were then incubated with the secondary antibody from LSAB kit for 30 min and the amplifier from the same kit for another 30 min. The immunoreactions were developed with diaminobenzidine and counterstained with Harris hematoxylin for observation under a light microscope.
2.3. Statistical analysis
The histopathological data were analyzed for statistical significance using one-way analysis of variance (ANOVA) followed by Kruskal–Wallis. The multiple comparison of the weights and biochemical scores were analyzed by ANOVA followed by Tukey's test. The difference between groups was considered significant when P < 0.05.
3. Results
3.1. Biochemical analysis
After treatment, ALT levels were significantly reduced in animals exposed to the highest dose of d-limonene (75 mg/kg/day for 45 days) when compared to control values, while AST and ALP serum levels were not altered. Serum levels of AST, ALT and ALP 45 days after the end of treatment were also comparable to that of control animals (Table 3).
Table 3.
AST, ALT and ALP serum levels in control, ethanol and d-limonene-treated rats after 45 days of treatment.
| Enzyme (UI/L) | Group |
|||
|---|---|---|---|---|
| II | III | IV | V | |
| AST | 168.6 ± 15.8 | 171.2 ± 8.7 | 150.2 ± 10.4 | 147.2 ± 11.4 |
| ALT | 66.7 ± 4.5 | 65.1 ± 3.7 | 63.2 ± 3.7 | 50.8 ± 3.0* |
| ALP | 153.1 ± 20.7 | 123.1 ± 5.4 | 118.7 ± 8.5 | 104.5 ± 11.5 |
Data expressed by mean ± standard error (S.E.M).
P < 0.05, Tukey's test II – control (tween-80), III – 20% ethanol, IV – 25 mg/kg, and V – 75 mg/kg.
3.2. Morphological evaluation
The necroscopic examination of the abdominal cavity did not reveal any signs of portal hypertension. Kidneys, lungs and heart were macroscopically and microscopically normal. No change in the weight of these organs as well as the weight of the liver was observed in the d-limonene-treated groups when compared to the control groups (data not shown). During the experimental procedure, no deaths, piloerection, locomotor activity alteration or any other clinical sign of toxicity were observed in the treated animals.
3.3. Liver histopathology
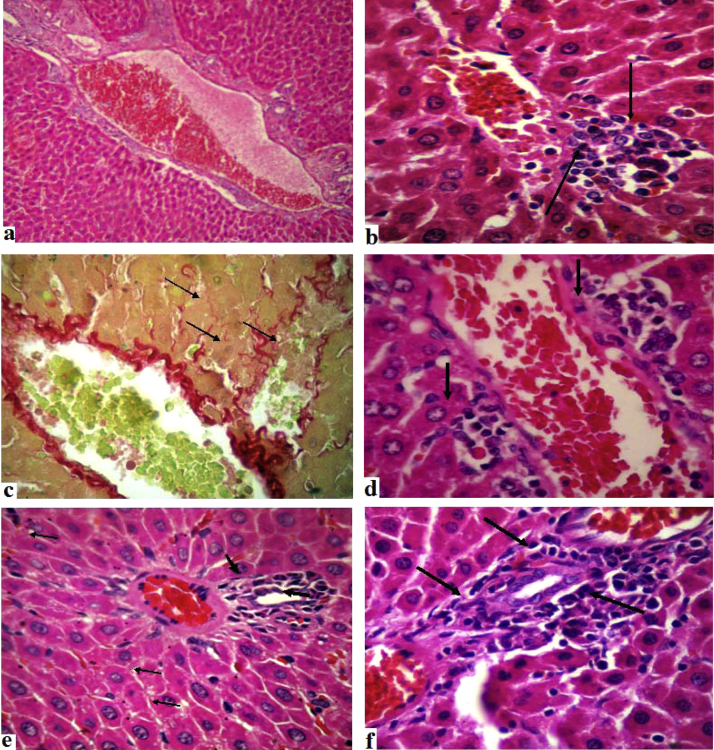
The liver of control rats showed normal histological architecture and the presence of a small number of resident lymphocytes in the portal connective stroma (Fig. 1a). In the 25 and 75 mg/kg d-limonene-treated, the histopathological analysis revealed the occurrence of hepatocellular lesions, such as hydropic degeneration, microvesicular steatosis, apoptosis, necrosis (Fig. 1b) or stromal fibrosis, showing the formation of fine connective septa in perisinusoidal spaces (Fig. 1c). Discreet (degree 1) or moderate (degree 2) inflammation was observed in the hepatic lobes (parenchyma) (Fig. 1d) and/or portal spaces (Fig. 1e and f). The lesions were always associated with inflammatory processes characterized by mononuclear cells exudation (lymphocytes and histiocytes) in the portal and lobular compartments.
Fig. 1.
Liver of control and d-limonene treated rats.
According to the scores presented in Table 2, the lymphocytary reaction restricted to the portal space was considered as discreet (degree 1) in more than 50% of the livers analyzed. No portal/septal degree 3 or 4 of inflammation was observed (Table 4).
Table 4.
Degree of portal inflammation and parenchymal (lobular) of control, ethanol and d-limonene-treated rats during 30 days and 45 days.
| Group | Degree of portal hepatic inflammation |
Degree of portal hepatic inflammation |
Treatment (days) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 or 4 | 0 | 1 | 2 | 3 or 4 | ||
| I | 2 (25%) | 6 (75%) | 0 | 0 | 5 (62.5%) | 3 (37.5%) | 0 | 0 | - |
| II | 3 (30%) | 7 (70%) | 0 | 0 | 6 (60%) | 4 (40%) | 0 | 0 | 45 |
| III | 0 | 6 (60%) | 4 (40%) | 0 | 4 (40%) | 2 (20%) | 4 (40%) | 0 | 45 |
| IV | 0 | 5 (50%) | 5 (50%) | 0 | 1 (10%) | 3 (30%) | 6 (60%) | 0 | 45 |
| V | 2 (20%) | 7 (70%) | 1 (10%) | 0 | 0 | 5 (50%) | 5 (50%) | 0 | 45 |
| VI | 0 | 3 (60%) | 2 (40%) | 0 | 1 (20%) | 4 (80%) | 0 | 0 | 30 |
| VII | 1 (20%) | 4 (80%) | 0 | 0 | 0 | 3 (60%) | 2 (40%) | 0 | 30 |
I – control (without treatment), II – tween-80, III – 20% ethanol, IV – 25 mg/kg, V – 75 mg/kg, VI – 25 mg/kg, VII – 75 mg/kg.
The intensity of lymphocytary inflammatory infiltration in the hepatic parenchyma (lobular inflammation) was characterized as discreet (degree 1), occurring predominantly in groups VI and VII (treated with 25 mg/kg and 75 mg/kg of d-limonene, respectively, for 30 days and sacrificed 30 days after the end of treatment), and as moderate (degree 2) specially in animals from group IV (treated with 25 mg/kg d-limonene for 45 days) (Table 4).
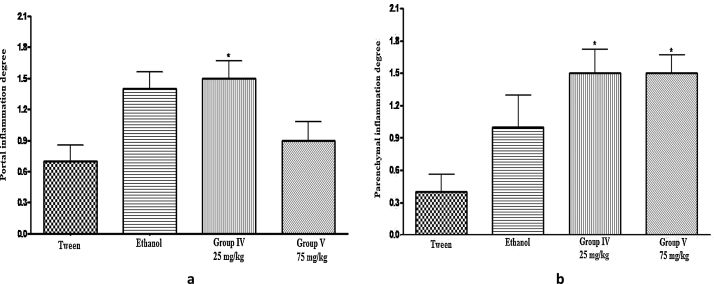
The degree of portal inflammatory response was significantly different in group IV (treated with 25 mg/kg d-limonene for 45 days) when compared to group II (control – tween-80) (Fig. 2), while the degree of lobular inflammatory activity was significant between groups IV and V (treated with 25 mg/kg and 75 mg/kg of d-limonene, respectively, for 45 days) and the control groups (without treatment and tween-80) (Fig. 2). None of the animals from control or treated groups exhibited hepatic siderosis, according to the classification adopted in this experimental protocol.
Fig. 2.
Degree of hepatic portal inflammation (a) and parenchymal inflammation (b).
3.4. Immunohistochemical analysis
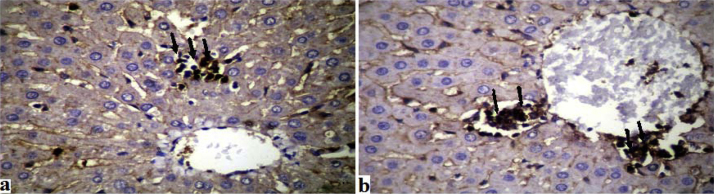
The results revealed that the inflammatory infiltrates in the hepatic tissue exhibited histiocytic elements associated to slight hyperplasia of Kupffer cells as evidenced by the macrophage marker (clone HAM-56). The infiltrate consisted predominantly of T cells as indicated by CD3 (anti-human T cells, clone UCHT1), and CD8 (anti-human cytotoxic T cells, clone M 707) (Fig. 3a and b).
Fig. 3.
Histological section.
4. Discussion
Food is the major source of exposure to limonene (96%). According to the Committee of Experts of the FAO, WHO Food Additives values for daily intake of d-limonene range of 0–1.5 mg/kg body weight per day. Citrus juices represented a daily intake of 1 mg/kg body weight per day in adults and 2 mg/kg for children [10].
In this study, the serum levels of ALT, AST and ALP obtained in all of the d-limonene-treated animals do not indicate hepatic disease. The alterations found in these animals would only be considered significant if the aminotransferases values were at least three times higher than the values obtained in the control groups [15]. However, the alterations in serum levels of the biochemical indicators do not exclude the possibility of occurrence of hepatic lesions when the organism is exposed to potential hepatotoxic agents.
The histological assessment of the liver showed the presence of discreet or moderate lesions exhibiting leukocyte infiltration, hydropic degeneration and/or necrosis. The hydropic degeneration refers to the occurrence of reversible intracellular edema as a consequence of toxic, infectious or immune aggressions. The increase in cell volume and clearness is associated with the formation of microvacuoles or clear cytoplasmic spaces as a result of the action of injurious agents that cause intracellular accumulation of sodium and increase in water content [13]. These lesions were observed in the liver of all d-limonene-treated rats at both dose levels (25 and 75 mg/kg), but not in control and ethanol-treated animals. The rats from the d-limonene treated group killed 30 days after the end of treatment also failed to exhibit these features, reflecting the reversibility of this type of cellular lesion. The hydropic degeneration observed in all of the groups treated with d-limonene elicits a possible direct cellular aggression; therefore, it is reasonable to assume that the metabolization of the terpene d-limonene generated a potential noxious substance to the mitochondrial functioning, stimulating specific immune reactions. Hepatotoxic metabolites can be produced through the oxidative metabolism of drugs (phase 1) with the participation of CYP450 enzymatic system, and the conjugative metabolism of drugs (phase 2) [1], [2], [26]. Phase 1 mechanisms are probably implicated in the toxic aggressions induced by d-limonene since this terpene is metabolized in rats and humans into oxygenated compounds such as dihydroperillic acid, perillic acid, and limonene-8-9-diol [3], [17], [26], [20]. Moreover, earlier studies have shown the increase in CYP450 activity in rodents treated orally and intraperitoneally with d-limonene [1], [2]. Reactive metabolites that damage mitochondrial respiration and ATP production, and generate oxygen reactive species, inhibiting β-oxidation, can cause steatosis; however, this kind of lesion was not significantly expressed in this study.
Steatosis or fatty liver was observed in a small number of animals from the d-limonene- and ethanol-treated groups. On the other hand, the occurrence of significant inflammatory reactions with the presence of mononuclear cells was detected in most of the animals treated with d-limonene during 30 or 45 days. The inflammatory cells were found especially in the parenchymal, intermediate and periportal areas, as well as the portal spaces, indicating a possible hepatotoxic effect of d-limonene in rats. The drug-induced liver injury can cause primary damage, i.e. necrosis or apoptosis, or even cell death as specific immune response with the participation of cytotoxic T lymphocytes. The hepatic necroinflammatory lesions observed in the d-limonene-treated groups probably resulted from the hypersensitivity process as evidenced by the immunohistochemical evaluation which demonstrated the prevalence of T lymphocytes (CD3 positive) in the parenchymal mononuclear infiltration. These finding, however, do not agree with the reports by [27], which do not indicate the occurrence of abnormal liver histopathology in rats treated orally with doses of 30 and 75 mg/kg of d-limonene. As the mobilization of T cells represents one aspect of d-limonene hepatic inflammatory lesion, it is expected that a specific immune response involving cytotoxic lymphocytes with concomitant release of cytokines may occur as a consequence of the covalent binding between reactive metabolites and proteins, resulting in the formation of neo-antigens or haptens [18], [16]. In the present experimental model, the hepatic parenchymal inflammatory lesions were less significant in the animals from groups VI and VII (treated with 25 and 75 mg/kg d-limonene and killed 30 days after the end of treatment), giving further support to the hypothesis that in the rat d-limonene has an intrinsic hepatotoxic potential.
Insipient fibrosis was observed in d-limonene-treated animals without the occurrence of connective tissue growth of the portal spaces (degree F0). Special staining methods (picrosirius) evidenced the presence of fine perisinusoidal collagen septa associated to the lobular inflammatory process. Such alterations were similar to those observed in parenchymal hepatic inflammation caused by various factors, including alcoholic intoxication [6].
However, these findings cannot be relevant to humans, considering that the doses used were higher, as compared to No-observed-adverse-effect level (NOAEL) reported by [11]. In practice, human exposure to limonene, via inhalation of the environment, pharmaceuticals, among other uses, it would not be sufficient to produce such hepatotoxic effects.
Liver injury as a result of alcohol consumption has been experimentally demonstrated in rodents and humans to the extent that in rodents the intragastric administration of ethanol has been shown to cause steatosis, necrosis, inflammation and hepatic fibrosis [24], [25]. Ethanol-treated rats (3.5 ml/kg/day) were used as a positive control, and like the d-limonene-treated group, a small number of animals exhibited steatosis, showing isolated small fat droplets in the hepatocytes. Furthermore, the necroinflammatory activity detected in the hepatic parenchyma of the ethanol group was also similar to that displayed by the d-limonene treatment groups.
5. Conclusion
In conclusion, the hepatocellular injuries reported in this study are probably related to the direct toxic effects of d-limonene reactive metabolites. The inflammatory mononuclear afflux can be interpreted as a manifestation of the immune response induced by these metabolites, and the hyperplasia of Kupffer cells could be indicated as a contributing factor to the stimulation of the lymphocytic immune response.
Conflict of interest
The authors declare that there are no conflicts of interest.
Transparency document
Acknowledgements
The authors thank José C. Duarte and Gilmário M. Lima for technical assistance and the Brazilian agencies for financial support: CAPES, CNPq (Grant number: Processo 306004/2010-0).
References
- 1.Ariyoshi T., Arakaki M., Ideguchi K., Ishizuka Y., Noda K., Ide H. Studies on the metabolism of d-limonene (p-mentha-1,8-diene) III. Effects of d-limonene on the lipids and drug metabolizing enzymes in rat livers. Xenobiotica. 1975;5:33–38. doi: 10.3109/00498257509056091. [DOI] [PubMed] [Google Scholar]
- 2.Austin C.A., Shepard E.A., Pike S.F., Rabin B.R., Phillips I.R. The effect of terpenoid compounds on cytochrome P-450 levels in rat liver. Biochem. Pharmacol. 1988;37:2223–2229. doi: 10.1016/0006-2952(88)90585-0. [DOI] [PubMed] [Google Scholar]
- 3.Crowell P.L., Lin S., Vedejs E., Gould M.N. Identification of metabolites of the antitumor agent d-limonene capable of inhibiting protein isoprenylation and cell growth. Cancer Chemother. Pharmacol. 1992;31:205–212. doi: 10.1007/BF00685549. [DOI] [PubMed] [Google Scholar]
- 4.Crowell P.L., Gould M.N. Chemoprevention of mammary carcinogenesis by hydroxylated derivatives of d-limonene. Crit. Rev. Oncog. 1994;5:1–22. doi: 10.1615/critrevoncog.v5.i1.10. [DOI] [PubMed] [Google Scholar]
- 5.Gomide M.S., Lemos F.O., Lopes M.T.P., Alves T.M.A., Viccini L.F., Coelho C.M. The effect of the essential oils from five different Lippia species on the viability of tumor cell lines. Rev. Bras. Farmacogn. 2013;23:895–902. [Google Scholar]
- 6.Goodmann Z.D., Ishak K.G. Occlusive venous lesion in alcoholic liver disease. A study of 200 cases. Gastroenterology. 1982;83:786–796. [PubMed] [Google Scholar]
- 7.Igimi H., Nishimura M., Kodama R., Ide H. Studies on the metabolism of d-limonene (p-mentha-1,8-diene) I. The absorption, distribution and excretion of d-limonene in rats. Xenobiotica. 1974;4:77–84. doi: 10.3109/00498257409049347. [DOI] [PubMed] [Google Scholar]
- 8.Igimi H., Hisatsugu T., Nishimura M. The use of d-limonene preparation as a dissolving agent of gallstones. Am. J. Dig. Dis. 1976;21:926–939. doi: 10.1007/BF01071903. [DOI] [PubMed] [Google Scholar]
- 9.Jayaprakasha G.K., Murthy K.N.C., Uckoo R.M., Patil B.S. Chemical composition of volatile oil from Citrus limettioides and their inhibition of colon câncer cell proliferation. Ind. Crops Prod. 2013;45:200–207. [Google Scholar]
- 10.JECFA . Toxicological Evaluation of Certain Food Additives and Contaminants. Annex 4. World Health Organization, Joint AO/WHO Expert Committee on Food Additives (WHO Food Additives Series 32); Geneva: 1993. Limonene; pp. 299–301. [Google Scholar]
- 11.Kim Y.W., Kim M.J., Chung B.Y., Bang D.Y., Lim S.K., Choi S.M., Lim D.S., Cho M.C., Yoon K., Kim H.S., Kim K.B., Kim Y.S., Kwack S.J., Lee B.-M. Safety evaluation and risk assessment of d-limonene. J. Toxicol. Environ. Health B: Crit. Rev. 2013;16:17–38. doi: 10.1080/10937404.2013.769418. [DOI] [PubMed] [Google Scholar]
- 12.Kodama R., Yano T., Furukawa K., Noda K., Ide H. Studies on the metabolismo f d-limonene (p-mentha-1,8-diene) IV. Isolation and characterization of new metabolites and species differences in metabolism. Xenobiotica. 1976;6:377–389. doi: 10.3109/00498257609151649. [DOI] [PubMed] [Google Scholar]
- 13.Kosif R., Yılmaz F., Evrendilek G.A., Dıramalı M. Histopathological effects of Aloe barbadensis and soybean oil on rat liver. Int. J. Morphol. 2010;28:1101–1106. [Google Scholar]
- 14.Lehman-Mckeeman L.D., Rodriguez P.A., Takigiku R., Caudill D., Fey M.L. d-Limonene-induced male rat-specific nephrotoxicity: evaluation of the association between d-limonene and α2u-globulin. Toxicol. Appl. Pharmacol. 1989;99:250–259. doi: 10.1016/0041-008x(89)90007-0. [DOI] [PubMed] [Google Scholar]
- 15.Maddrey W.C. Drug-induced hepatotoxicity. J. Clin. Gastroenterol. 2005;39:83–89. doi: 10.1097/01.mcg.0000155548.91524.6e. [DOI] [PubMed] [Google Scholar]
- 16.Njoku D.B., Greenberg R.S., Bourdi M., Borkowf C.B., Dake E.M., Martin J.L., Pohl L.R. Autoantibodies associated with volatile anesthetic hepatitis found in the sera of a large cohort of pediatric anesthesiologists. Anesth. Analg. 2002;94:243–249. doi: 10.1097/00000539-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Poon G.K., Vigushin D., Griggs L.J. Identification and characterization of limonene metabolites in patients with advanced cancer by liquid chromatography/mass spectrometry. Drug Metab. Dispos. 1996;24:565–571. [PubMed] [Google Scholar]
- 18.Robin M.A., Le Rot M., Descatoire V., Pessayre D. Plasma membrane cytochromes P450 as neoantigens and autoimmune targets in drug-induced hepatitis. J. Hepatol. 1997;26(Suppl. 1):23–30. doi: 10.1016/s0168-8278(97)82329-x. [DOI] [PubMed] [Google Scholar]
- 19.Safari M.R., Heidari A.A., Shams S. The effects of volatile oils on low density lipoprotein glycation by model system in vitro. Clin. Chem. Lab. Med. 2001;45:S1–S473. [Google Scholar]
- 20.Schmidt L., Belov V.N., Göen T. Sensitive monitoring of monoterpene metabolites in human urine using two-step derivatisation and positive chemical ionisation-tandem mass spectrometry. Anal. Chim. Acta. 2013;793:26–36. doi: 10.1016/j.aca.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 21.Searle J., Leggett B.A., Crawford D.H.G. Iron storage disease. In: MacSween R.N.M., Burt A.D., Portmann B.C., Ishak K.G., Scheuer P.J., Anthony P.P., editors. Pathology of the Liver. 4th ed. Churchill Livingstone; Edinburgh: 2002. pp. 257–272. [Google Scholar]
- 22.Shimada T., Shindo M., Miyazawa M. Species differences in the matabolism of (+)- and (−)-limonenes and their metabolites, carveols and carvones, by cytochrome P450 enzymes in liver microsomes of mice, rats, guinea pigs, rabbits dogs, monkeys, and humans. Drug Metab. Pharmacokinet. 2002;17:507–515. doi: 10.2133/dmpk.17.507. [DOI] [PubMed] [Google Scholar]
- 23.Sun J. d-Limonene: safety and clinical applications. Altern. Med. Rev. 2007;12:259–262. [PubMed] [Google Scholar]
- 24.Tipoe G.L., Liong E.C., Leung T.M., Nanji A.A. A voluntary oral-feeding rat model for pathological alcoholic liver injury. Methods Mol. Biol. 2008;447:11–31. doi: 10.1007/978-1-59745-242-7_2. [DOI] [PubMed] [Google Scholar]
- 25.Tipoe G.L., Liong E.C., Casey C.A., Donohue T.M., Jr., Eagon P.K., So H., Leung T.M., Fogt F., Nanji A.A. A voluntary oral ethanol-feeding rat model associated with necroinflammatory liver injury. Alcohol Clin. Exp. Res. 2008;32:669–682. doi: 10.1111/j.1530-0277.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- 26.Vigushin D.M., Poon G.K., Bodd Y.A. Phase I and pharmacokinetic study of d-limonene in patients with advanced cancer. Cancer Research Campaign Phase I/II Clinical Trials Committee. Cancer Chemother. Pharmacol. 1998;42:111–117. doi: 10.1007/s002800050793. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization . World Health Organization; 1998. International Programme On Chemical Safety. Concise International Chemical Assessment Document. [Google Scholar]
- 28.Yang X., Zhao H., Wang J., Meng Q., Zhang H., Yao L., Zhang Y., Dong A., Ma Y., Wang Z. Chemical composition and antioxidant activity of essential oil of pine cones of Pinus armandii from the southwest region of China. J. Med. Plants Res. 2010;4:1668–1672. [Google Scholar]
- 29.Zhang Z., Vriesekoop F., Yuan Q., Liang H. Effects of nisin on the antimicrobial activity of d-limonene and its nanoemulsion. Food Chem. 2014;150:307–312. doi: 10.1016/j.foodchem.2013.10.160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.