Abstract
Phytoestrogens are plant-derived estrogen-like compounds that are increasingly used for their suggested health promoting properties, even by healthy, young women. However, scientific concerns exist regarding potential adverse effects on female reproduction. In this study, naringenin (NAR), 8-prenylnaringenin (8-PN), genistein (GEN), coumestrol (COU), quercetin (QUE) and resveratrol (RSV) up-regulated steroidogenic acute regulatory protein (StaR) mRNA levels in KGN human granulosa-like tumor cells. Most of the phytoestrogens tested also increased CYP19A1 (aromatase) mRNA levels via activation of ovary-specific I.3 and II promoters. Yet, only NAR (3 and 10 μM), COU (10 and 30 μM) and QUE (10 μM) also statistically significantly induced aromatase activity in KGN cells after 24 h. 8-PN, aromatase inhibitor letrozole and estrogen receptor antagonist ICI 182,780 concentration-dependently inhibited aromatase activity with IC50 values of 8 nM, 10 nM and 72 nM, respectively. Co-exposure with ICI 182,780 (0.1 μM) statistically significantly attenuated the induction of aromatase activity by QUE and COU, but not NAR. Cell cycle status and proliferation of KGN cells were not affected by any of the phytoestrogens tested. Nonetheless, the migration of KGN cells was significantly reduced with approximately 30% by COU, RSV and QUE and 46% by GEN at 10 μM, but not NAR and 8-PN. Our results indicate that phytoestrogens can affect various pathways in granulosa-like cells in vitro at concentrations that can be found in plasma upon supplement intake. This implies that phytoestrogens may interfere with ovarian function and caution is in place regarding the use of supplements with high contents of phytoestrogens.
Keywords: Phytoestrogens, KGN cells, Ovarian-specific CYP19A1 promoters, CYP19, Cell migration, Tumor progression
1. Introduction
Phytoestrogens are naturally occurring plant compounds that are omnipresent in our daily diet. Over the past years, phytoestrogens have attracted much attention due to their estrogenic or anti-estrogenic properties and their potential use as alternatives for hormone replacement therapy in postmenopausal women. In addition, supplements that contain high levels of phytoestrogens are commercially available for breast enhancement and relieve of menstrual complaints. Phytoestrogens are diverse plant-derived group of compounds and they consist of several groups of different chemical classes classified according to their chemical structure including flavanoids, coumestans and lignans. Most flavanoids are present in plants in the conjugated glycoside forms and can be readily hydrolysed by gastrointestinal bacteria to biologically active aglycones [1]. There is considerable interest in whether human exposure to phytoestrogens has any health risks or benefits due to the increase of nutritional and pharmaceutical use of dietary phytoestrogenic compounds [2], [3]. Epidemiologic evidence mainly based on Asian population studies, supports a protective effect of high phytoestrogen diets to reduce the incidence of certain hormone-responsive cancers, such as breast and prostate cancer. Contrary, there have been concerns that phytoestrogens, through their estrogenic properties, may increase the risk of recurrence or stimulate the growth of existing tumors. In addition, numerous in vivo and in vitro studies have demonstrated altered ovarian function and changes in the developing female reproductive system following exposure to phytoestrogens in laboratory animal studies [4], [5], [6]. Phytoestrogens were able to affect female reproductive function by modulating the female cycles that in turn resulted in infertility in animals [7], [8] and humans [9], [10]. In humans, the prevalence of precocious puberty was significantly higher in Korean girls with high serum isoflavone levels [11]. Despite the numerous studies, the molecular mechanisms underlying the adverse effects of phytoestrogens on ovarian function still remain elusive.
It is well known that phytoestrogens may disrupt endocrine-dependent processes by acting as estrogen receptor (ER) agonists or antagonist due to their bi-phenolic structure required for ligand–receptor association. Phytoestrogens can bind weakly to ERs, typically with affinities that are 1000 times less than that of 17β-estradiol (E2) [12]. ERβ receptor is a classical steroid receptor predominantly expressed in granulosa cells. In contrast, ERα protein is expressed at low levels in granulosa cells [13]. Several phytoestrogens are selective estrogen receptor modulators that have greater affinity for ERβ than ERα [14]. In addition to classical estrogen receptors, phytoestrogens were shown to be ligands for the non-classical estrogen receptor G-protein coupled protein receptor 1 GPER1 [15]. Moreover, it has become clear that phytoestrogens can exert endocrine disrupting properties by inhibiting key steroidogenic enzymes. During puberty, E2 that is synthesized and secreted by granulosa cells in the ovaries, modulates the structure and function of female estrogen-sensitive tissues and contributes to maintaining a proper menstrual cycle pattern and female sexual behavior. Ovarian steroidogenesis is initiated by the delivery of cholesterol from cytosol into the mitochondria by the steroidogenic acute regulatory protein (StAR) [16], [17]. The final step in estrogen synthesis is catalyzed by aromatase (CYP19A1), which converts androgens into estrogens. Human CYP19A1 comprises of ten exons including exons II–X that encode the aromatase protein and 3′-untranslated region of the mRNA. Alternative first exons encode unique 5′-untranslated regions of the aromatase mRNA transcripts in different estrogen-producing tissues [18], [19]. Aromatase transcripts in gonads, brain, adipose and placenta contain different first exons (II, If, I.4/I.3 and I.1, respectively) and the expression of CYP19A1 in each of these organs is controlled by alternatively spliced tissue-specific promoters regulated by distinct signaling pathways in a hormone-specific manner [19], [20], [21], [22], [23]. In ovarian granulosa cells, aromatase expression is FSH-driven and is regulated via the ovary-specific PII promoter [21]. Many studies with various models have shown the inhibitory effects of phytoestrogens on aromatase activity [24], [25], [26], [27], [28], [29], while other phytoestrogens induce aromatase activity [30], [31].
The aim of our work was to study the effects of several potent phytoestrogens that are frequently used in dietary supplements as possible modulators of ovarian function and cellular behavior in vitro. It should be noted that many phytoestrogens are omnipresent in our daily diet, albeit at much lower concentrations than can be found in dietary supplements. The data from these studies were used to assess the potential risk for ovarian dysfunction in humans upon high intake levels of phytoestrogens including naringenin (NAR), 8-prenylnaringenin (8-PN), genistein (GEN), coumestrol (COU), quercetin (QUE) and resveratrol (RSV) (for chemical structures see supplementary data Fig. S1). These phytoestrogens were selected for their previously reported induction (GEN and QUE) [30], [31] or inhibition (NAR and 8-PN) [29], [30], [31] and/or their reported effects on ovarian tumor cell behavior (RSV, GEN, QUE, COU) [32], [33], [34]. In our study, we used the KGN granulosa-like tumor cell line of human origin. KGN cells were previously shown to maintain many of the physiological features of normal human granulosa cells, including steroidogenesis [35] and secretion of estrogens [36], [37]. We show here that KGN cells display the ovarian-specific PII/I.3-driven aromatase expression, similarly to normal granulosa cells surrounding the preovulatory follicle. KGN cells have also been reported to express both ERs [38]. Moreover, several studies have demonstrated that KGN cells respond similarly to primary human granulosa cells upon stimulation with, e.g. FSH [39], [40], [41]. Therefore, this cell line is an excellent and applicable in vitro model to study effects on human granulosa cell functioning. Here, the action of the selected phytoestrogens on ovarian steroidogenic enzymes such as StAR and CYP19A1 and its promoter-specific expression was investigated. Phytoestrogens have also been shown to affect a wide array of intracellular signaling mechanisms that are important for regulating cell cycle progression. Therefore, we investigated to which extent phytoestrogens influence the metastatic properties of KGN cells by performing a wound healing assay. Finally, expression of several important genes involved in cell progression and/or death, were studied. We chose to study gene expression of VEGF, a critical inducer of tumor angiogenesis and SIRT1, regulator of cellular lifespan and tumor promoter in mammary epithelial cells [32], [42]. Moreover, gene expression of MMP9, a prerequisite for enhanced cell migration, and CADHERIN E, adhesion-activated signaling receptor, were evaluated. The data from these in vitro studies were compared with reported human plasma levels to assess the potential risk for ovarian dysfunction in humans upon high intake levels of phytoestrogens.
Supplementary Fig. S1 related to this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.06.006.
2. Material and methods
2.1. Cell culture
The KGN granulosa-like tumor cells (kindly provided by Riken Biosource Center, Tsukuba, Japan) were cultured in supplemented with 10% FBS (Invitrogen-Gibco) phenol red-free DMEM/F12 (Invitrogen-Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen-Gibco) in an atmosphere of 5% CO2/95% air at 37 °C. KGN cells were subcultured 1:2 once a week. For all experimental conditions, media were replaced with 5% steroid free Serum dextran/charcoal (Hyclone; Invitrogen-Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen-Gibco) and KGN cells were grown for next 4 days before treatments.
2.2. Chemicals and reagents
Estradiol (E2), NAR, 8-PN and dexamethasone (DEX) were dissolved in ethanol. GEN, COU, QUE, RSV, ICI 182,780 and Prostaglandin E2 (PGE2) were dissolved in dimethyl sulfoxide (DMSO). 5-Bromo-2-deoxyuridine (BrdU) was dissolved in Phosphate buffered saline (PBS). All compounds were purchased from Sigma–Aldrich Co. (Zwijndrecht, The Netherlands), unless indicated otherwise. BrdU was purchased from Euro-diagnostics bv, Apeldoorn, the Netherlands. The concentration of each solvent was set as 0.1% of the culture medium.
2.3. Cytotoxicity assay
Cell viability was determined according to Denizot and Lang [43] by measuring the capacity of KGN cells to reduce MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to formazan by the mitochondrial enzyme succinate dehydrogenase. Briefly, KGN cells (0.1 × 106) were plated in 24 well plates in assay medium and then incubated for 1.5 h with MTT at 37 °C. After that time, the formed blue colored formazan was extracted by adding 1 mL of isopropanol at room temperature (RT). Absorbance was measured spectrophotometrically at an absorbance wavelength of 595 nm (POLARstar Galaxy, BMG Labtech GmbH, Ortenberg, Germany).
2.4. Quantitative real time polymerase chain reaction (RT-PCR)
Gene expression studies were performed in KGN cells. Briefly, KGN cells (2.5 × 105 cells) were seeded in 12-well plates in assay phenol red-free DMEM/F12 medium. Cells were exposed to NAR and 8-PN at 3 μM; GEN, COU, QUE and RSV at 10 μM for 24 h. PGE2 (0.1 μM), a potent stimulator of steroidogenic genes such as StAR and CYP19A1 of ovarian-related aromatase [44] was used as a positive control for aromatase induction. Total RNA was harvested from the KGN cells by phenol–chloroform extraction using RNA Instapure (Eurogenetic, Liege, Belgium). Purity and concentration of the RNA samples were determined spectrophotometrically at an absorbance wavelength of 260/280 nm and 230/260 nm. Complementary DNA (cDNA) was synthesized using iScript cDNA Synthesis Kit, according to the manufacturer's instructions (Biorad, Veenendaal, the Netherlands). Obtained cDNA was diluted 10 times and stored at −20 °C until further analysis. The qRT-PCR was performed using CFX Manager (Biorad, Veenendaal, the Netherlands). PCR reaction was initiated by heating at 95 °C for 3 min, then followed by 40 cycles with denaturation at 95 °C for 15 s, annealing/extension at 60 °C for 45 s. After each run a melt curve was performed to ensure that primer-dimers and other non-specific products were omitted. A negative control sample (non-RT) was included in each run. The primers for β-Actin, I.3, PII, I.4, Vascular Endothelial Growth Factor (VEGF) have been described previously [45], [46]. All primer pairs used in the qRT-PCRs are listed in Table 1. The primers were designed using Primer-BLAST of National Center for Biotechnology Information (NCBI) and then checked with BLAST (nucleotide nonredundant database) to confirm specificity. The β-Actin gene expression was not affected by tested phytoestrogens, thus it was used as housekeeping gene in the present study.
Table 1.
Primers used for qRT-PCR.
| Gene name | Forward primer | Reverse primer | PCR product [bp] |
|---|---|---|---|
| β-Actina | AAACTACCTTCAACTCCATC | ATGATCTTGATCTTCATTGT | 163 |
| I.3 | GCTGCAATTCAAGCCAAAAG | GCACGATGCTGGTGATGTTATA | 187 |
| PI | TCTGTCCCTTTGATTTCCACAG | GCACGATGCTGGTGATGTTATA | 112 |
| I.4 | GGCTCCAAGTAGAACGTGACCAACTG | CAGCCCAAGTTTGCTGCCGAA | 475 |
| CYP19A1 | TTGGGCTGCAGTGCATCGGT | CCGGGGCCTGACAGAGCTTTCATA | 109 |
| StAR | TGGCAGTACATGTGCACAAAGCAG | CTGCTTGTTCTGTGGTGTTGCTGT | 94 |
| SIRT1 | TCTGGCATGTCCCACTATCA | GCAGATTAGTAGGCGGCTTG | 152 |
| CADHERIN E | TGGACCGAGAGAGTTTCCCT | CCCTTGTACGTGGTGGGATT | 148 |
| MMP9 | GGCTCCTGGCACACGCCTTT | TGGAACCACGACGCCCTTGC | 101 |
| VGEF | ATCACGAAGTGGTGAAGTTC | TGCTGTAGGAAGCTCATCTC | 265 |
Gene used as reference normalizer gene.
2.5. Aromatase (CYP19A1) activity
CYP19A1 (aromatase) activity was determined in KGN cells after a 24-h exposure using the tritiated water-release method of Lephart and Simpson [47] with minor modification by Sanderson et al. [48]. Briefly, KGN cells (0.1 × 106) were seeded onto 24 well plates in assay medium. CYP19A1 activity was measured as the amount of tritiated water formed after the conversion of the CYP19A1 enzyme's substrate [1β−3H)-androstenedione. PGE2 (0.1 μM), was used as a positive control for aromatase catalytic activation.
2.6. Protein extraction and western blot analysis
KGN cells (6 × 106) were washed once with warm PBS, then trypsinized. Cells were centrifuged and 250 μL of PBS was added to each cell pellet. Cells were lysed using adopted freeze-thaw lysis method using liquid-Nitrogen and 37 °C water bath by alternatively incubating at least 3 times The total protein concentration of lysates was quantified using he Lowry assay [49]. 30 μg of cell protein and protein standards were fractionated by SDS-PAGE (10%), electrophoretically transferred onto polyvinylidene fluoride (pvdf) membrane and probed for 1 h at RT with a primary rabbit monoclonal antibody recommended for detection of human CYP19A1, dilution factor 1:1000 (Abcam). After washing, the membranes were incubated for another 1 h at RT with a secondary polyclonal goat anti-rabbit antibody conjugated to the enzyme horseradish peroxidase (HRP), dilution factor 1:1000 (Abcam). Protein bands were visualized by chemiluminescence using Bio-Rad Clarity™ western ECL substrate kit. Precision Plus Protein™ Standards were used at the 10–250 kDa range as the markers (Bio-Rad Laboratories, Inc.).
2.7. Wound healing assay
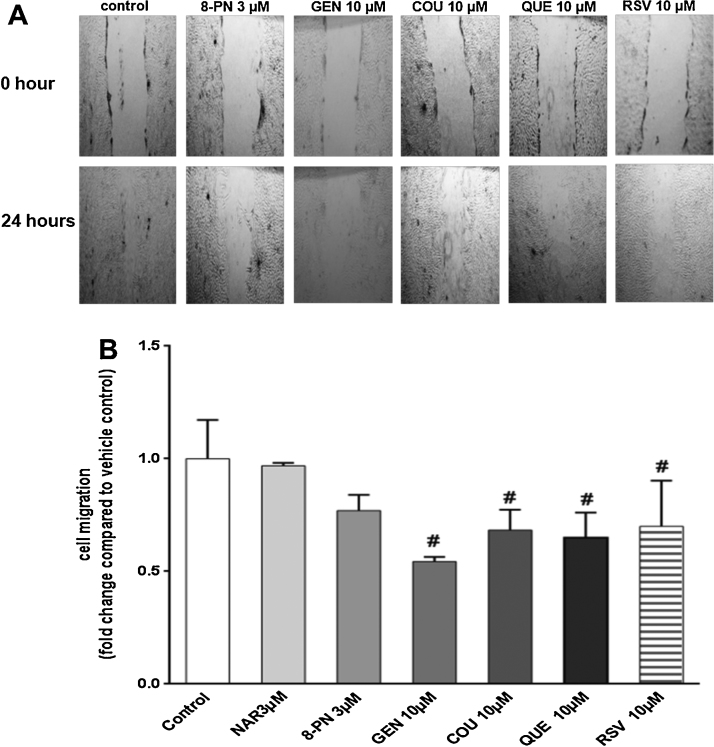
The wound-healing assay was described previously [50] and was used to determine whether KGN cell motility could be affected by diverse phytoestrogens. KGN cells (0.7 × 106) were cultured in 12-well cell plates until confluency. Wounds were made by scratching the cellular layer with a 100 μL pipette tip in the middle of the well. After washing away the cell debris, assay medium with tested phytoestrogens was added to the culture. Zero hour pictures (0 h-control) were taken for each wound with an Olympus U-CMAD3 camera. Cells were incubated for 8 and 24 h and then another picture for each wound was taken. The wound area was measured with an Image J 1.47c software (National Institutes of Health, USA).
2.8. BRdU staining
KGN cells (0.7 × 106) were cultured in 12-well cell plates until confluent. BrdU (10 μM) was added to culture medium for 1 h. When added to culture medium, BrdU (10 μM) is incorporated into the DNA of cells that are in the S-phase of the cell cycle [51]. Then, KGN cells were fixed in ice-cold methanol for 20 min at 37 °C. Immunocytochemical detection of BrDU labled DNA was performed using undiluted anti-BrDU and peroxidase-conjugated Rabbit-anti-mouse Ig (RAM-PO, dilution factor: 1:40) for 90 and 60 min, respectively.
2.9. Cell cycle analysis
Cell cycle analysis was determined according to protocol described by Sangjun et al. [52]. KGN cells (0.25 × 106) were plated onto 12-well plates and further cultured with phytoestrogens for 24 h. Cells were then trypsinized, fixed and permeabilized with 70% ethanol for at least 30 min at 4 °C. KGN cells were then labeled with propidium iodide for 10 min at room temperature (RT). The samples were used for flow cytometry (FACS, Calibur, Becton Dickinson) to determine the cell cycle distribution of the KGN cells. The flow cytometry data was analyzed using Flowjoj software (Tree Star, Inc., USA).
2.10. Data analysis and statistics
All experiments were performed at least three times and within each independent experiment each concentration was tested in duplicate (aromatase activity) or triplicate. The results are displayed as the mean of replicates of each experiment with standard deviation (SD). Statistical analysis of difference of the means between vehicle-treated control (either Ethanol (0.1%) or DMSO (0.1%) or combination of both)-treated cells was determined using a two-tailed unpaired Students’ t-test or a one-way ANOVA and post hoc Dunnett's test. Calculations are performed using GraphPad Prism 6.01 (GraphPad Software Inc., San Diego, USA). Differences were considered statistically significant with P < 0.05.
3. Results
3.1. MTT cytotoxicity assay
None of the tested compounds was cytotoxic at the highest concentration tested, except for GEN (30 μM) and COU (30 μM) (supplementary data Fig. S2B). At the highest concentrations tested, GEN and COU reduced cell viability by 20 and 15%, respectively when compared to vehicle-treated control cells. In the subsequent experiments with KGN cells, non-cytotoxic concentrations of phytoestrogens were used.
Supplementary Fig. S2 related to this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.06.006.
3.2. Modulation of aromatase in KGN cells
3.2.1. Aromatase (CYP19A1) gene expression
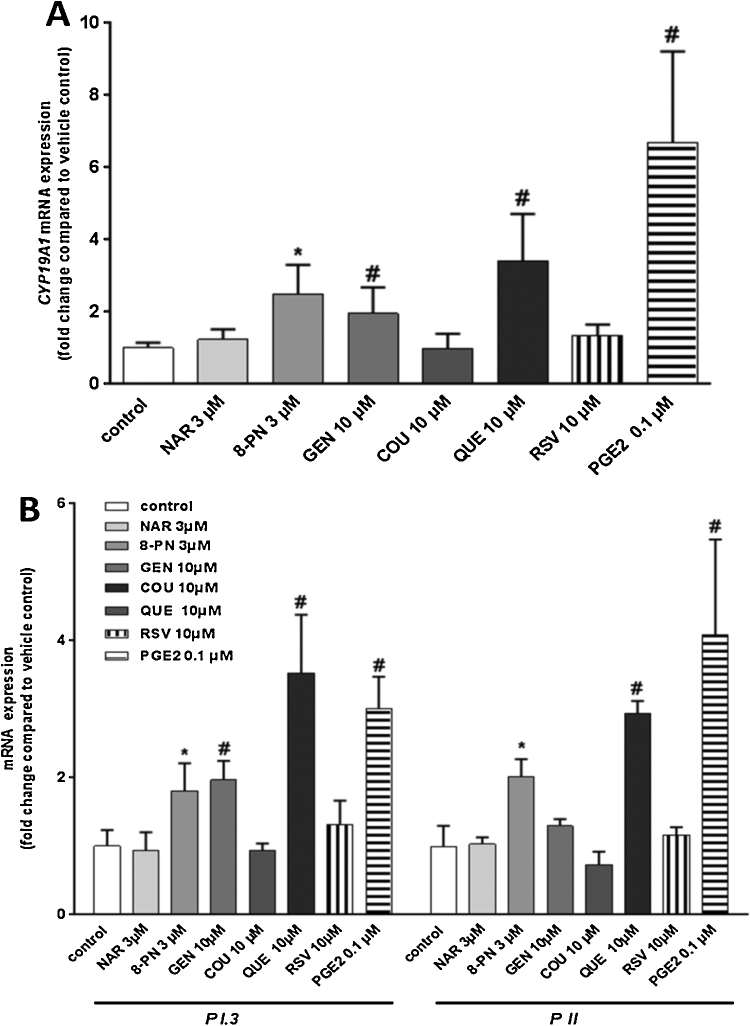
Aromatase (CYP19A1) expressed in granulosa cells surrounding the developing oocyte in the ovary, is responsible for conversion of androgens into estrogens. CYP19A1 mRNA levels in KGN cells statistically significantly increased by 2.3, 1.9 and 3.2-fold after a 24-h exposure to 8-PN (3 μM), GEN (10 μM) and QUE (10 μM). No changes in CYP19A1 mRNA levels were seen in NAR (3 μM), COU (10 μM) and RSV (10 μM) treated-KGN cells (Fig. 1A). A 24-h exposure to PGE2 (0.1 μM) increased CYP19A1 mRNA by approximately 7-fold compared with vehicle-treated control cells (Fig. 1A). Using qRT-PCR, we showed that aromatase expression in KGN cells was promoter II (PII) and I.3-driven, as expected based on its ovarian origin. Expression of both PII- and I.3-driven CYP19A1 was statistically significantly up-regulated by PGE2 (0.1 μM) after 24 h and increased 3.5- and 4-fold for I.3 and PII, respectively (Fig. 1B). Neither promoter I.4 nor I.1-driven CYP19A1 expression was detected in KGN cells (data not shown). Also, DEX (0.1 μM), a potent inducer of promoter I.4-driven transcription of CYP19A1 did not induce aromatase gene expression and activity (data not shown). Based on these data, we tested the effects of phytoestrogens only on PII/1.3-driven CYP19A1 gene expression. Among the phytoestrogens tested, QUE (10 μM) was the most potent activator of PI.3 and PII-driven CYP19A1 (3.5- and 3-fold induction, respectively). Also 8-PN and GEN statistically significantly up-regulated PII/PI.3-driven CYP19A1 expression. 8-PN (3 μM) significantly up-regulated CYP19A1 via promoters 1.3 and II by 1.8- and 2-fold, respectively. GEN (10 μM) had more potent effects toward activation of PI.3 promoter than PII, with a statistically significantly increase of I.3 promoter-driven CYP19A1 mRNA to about 2-fold compared with vehicle-treated control cells. Neither NAR nor COU and RSV affected promoter-specific mRNA expression of CYP19A1. Taken together, the results demonstrated stimulatory effects of diverse phytoestrogens on promoter I.3 and II-driven mRNA of CYP19A1 in KGN cells.
Fig. 1.
Gene expression in KGN cells after a 24-h exposure to various phytoestrogens. (A) CYP19A1 mRNA levels. Significant up-regulation of CYP19A1 mRNA after exposure to 8-PN (3 μM), GEN (10 μM) and QUE (10 μM). PGE2 (0.1 μM) was used as a positive control. (B) Promoter P1.3- and PII-specific expression of CYP19A1 mRNA. Significant up-regulation of PI.3 and PII – driven CYP19A1 mRNA after exposure to 8-PN (3 μM), GEN (10 μM) and QUE (10 μM). PGE2 (0.1 μM) was used as a positive control. Data are expressed as fold-change compared with expression in vehicle-control treated cells. Bars represent mean + SD of three independent experiments that were performed in triplicate (N = 3). * and # significantly from relevant solvent vehicle control Ethanol (0.1%) or DMSO (0.1%)-treated cells, respectively. P < 0.05.
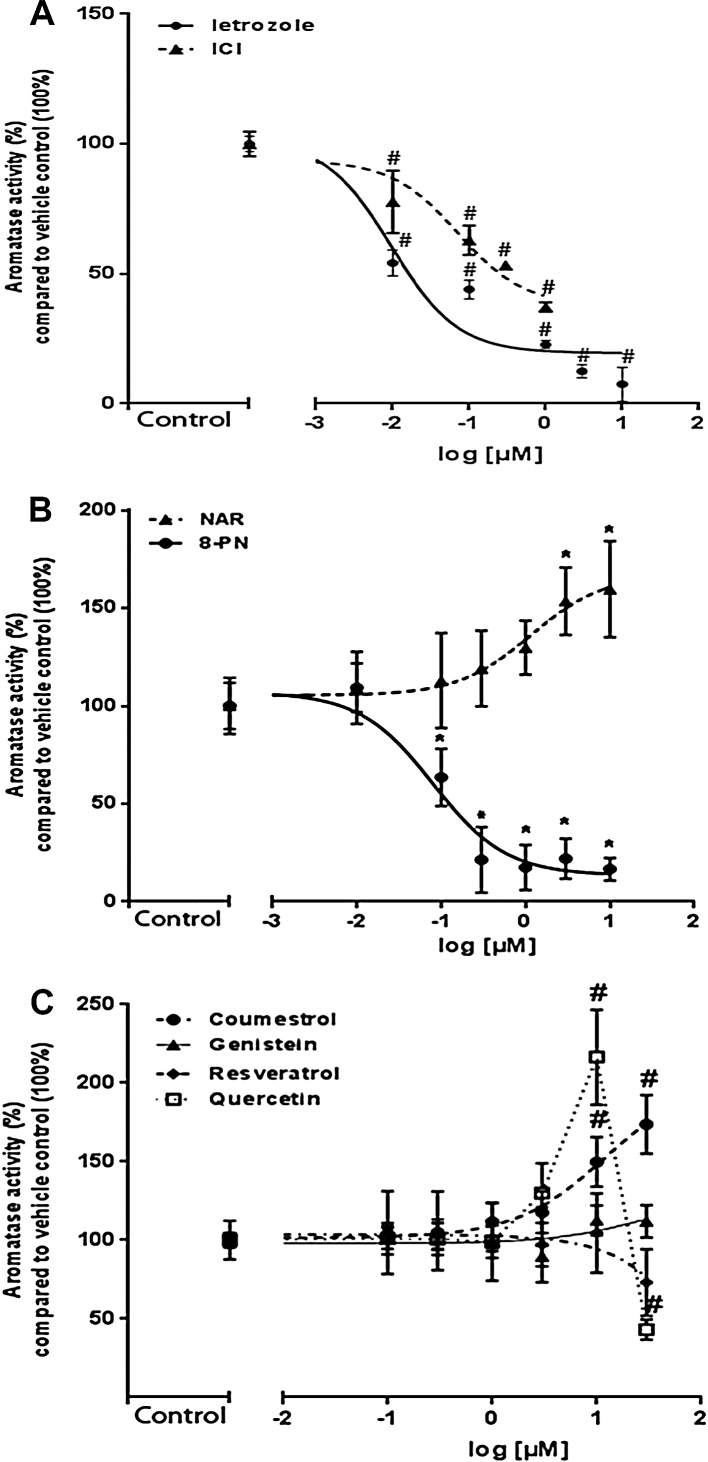
3.2.2. Aromatase CYP19A1 enzyme activity and expression
The known inducer of ovarian-type aromatase PGE2 (0.1 μM) statistically significantly induced aromatase activity up to 315% compared with vehicle-treated control cells (data not shown). In contrast, the known specific CYP19A1 enzyme inhibitor letrozole concentration-dependently inhibited CYP19A1 activity in KGN cells (IC50 value of 10 nM; Fig. 2A). Of all tested phytoestrogens, only 8-PN inhibited aromatase activity (IC50 value of 8 nM; Fig. 2B). COU and NAR concentration-dependently increased aromatase activity to approximately 155% at non-cytotoxic concentrations of 10 μM when compared to vehicle-treated control cells (Fig. 2B and C). Apparent EC50 values for induction of aromatase activity were 10 and 1.3 μM for COU and NAR, respectively. QUE was the most potent CYP19A1 activator with an EC50 value of 4.7 μM and a maximum induction of 210% at 10 μM. Notably, there was a sharp and significant decline in aromatase activity after QUE exposure at 30 μM (Fig. 2C). This decline was not due to cytotoxicity (supplementary data Fig. S2B). The phytoestrogens GEN and RSV did not affect CYP19A1 activity at any of the tested concentrations (Fig. 2C). Because of the differential effects of phytoestrogens on CYP19A1 gene expression and aromatase activity, CYP19A1 protein expression was determined by western blot. A slight increase in protein level of CYP19A1 was observed by PGE2 (0.1 μM) and COU (10 μM)-treated KGN cells after 24 h. However, no changes at CYP19A1 protein level were observed after 24-h exposure to 8-PN (3 μM), GEN (10 μM) and QUE (10 μM) (supplementary data Fig. S3).
Fig. 2.
Aromatase activity in KGN cells. (A) Concentration-dependent inhibition curve of Letrozole and ICI 182,780. (B) 24 h-exposure to different concentrations of NAR and 8-PN. (C) Effects of COU, GEN, RSV and QUE at diverse concentrations on aromatase activity. Data are expressed as % of aromatase activity compared with 100% of aromatase activity in vehicle control treated cells. The experiments were performed in duplicates and repeated three or four times (N = 3; N = 4). * and/or # significantly different from relevant solvent vehicle control Ethanol (0.1%) or DMSO (0.1%)-treated cells, respectively. P < 0.05.
Supplementary Fig. S3 related to this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.06.006.
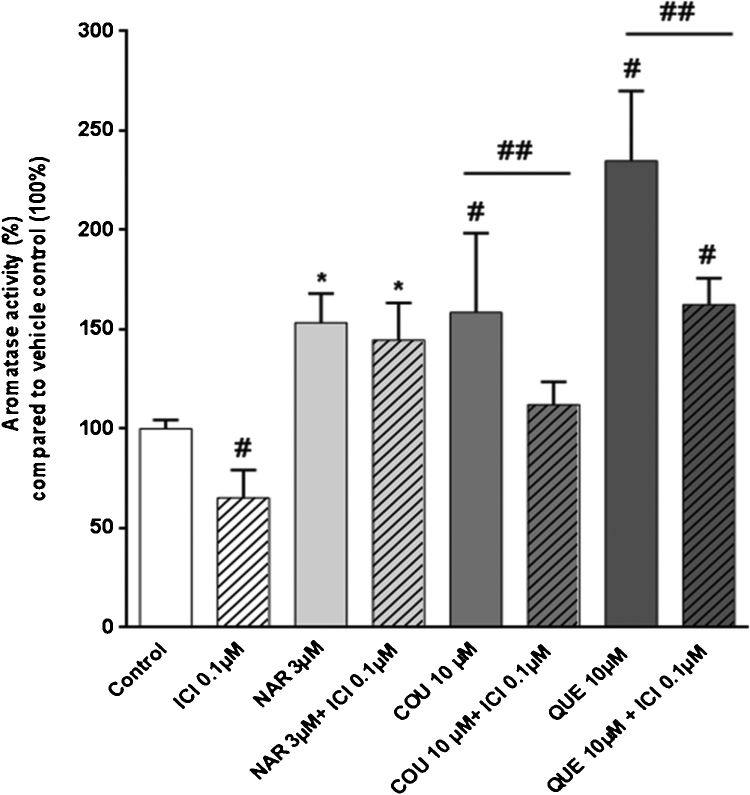
Phytoestrogens are suggested to exert their effects mainly via estrogen receptors. Therefore, we studied the potential involvement of estrogen receptor α (ERα) in the activation of aromatase by non-cytotoxic concentrations of NAR, COU and QUE. Exposure to E2 (0.001–0.1 μM) did not have a statistically significant effect on CYP19A1 activity (data not shown). However, the known pure ERα antagonist ICI 182,780 concentration-dependently reduced aromatase activity (IC50 value of 72 nM; Fig. 2A). Moreover, co-exposure with ICI 182,780 (0.1 μM) and COU (10 μM) or QUE (10 μM), but not NAR (3 μM), statistically significantly decreased aromatase activity in KGN cells when compared to COU or QUE exposed cells alone (Fig. 3).
Fig. 3.
Aromatase activity in KGN cells exposed to ICI (0.1 μM), NAR (3 μM), COU (10 μM) and QUE (10 μM). Striped bars present a co-exposure of ICI (0.1 μM) with tested phytoestrogens. Data are expressed as % of aromatase activity compared with 100% of aromatase activity in vehicle-control treated cells. The experiments were performed in duplicates and repeated three or four times (N = 3; N = 4). * or # significantly different from relevant solvent vehicle control Ethanol (0.1%) and/or DMSO (0.1%)-treated cells, respectively. ## significantly different from COU (10 μM) or QUE (10 μM)-exposed cells. P < 0.05.
3.3. Steroidogenic acute regulatory protein mRNA levels are up-regulated by diverse phytoestrogens
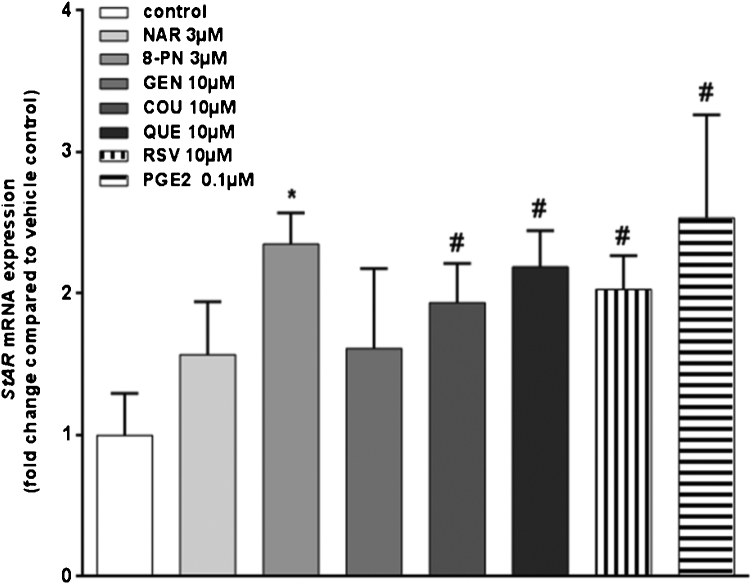
Phytoestrogen-mediated effects on StAR mRNA expression were determined in KGN cells. Results showed that StAR mRNA levels were statistically significantly up-regulated after a 24 h-exposure to 8-PN (2.3-fold), 10 μM COU (1.9 fold), 10 μM QUE (2.2-fold), and 10 μM RSV (2-fold) (Fig. 4). Also 0.1 μM PGE2 up-regulated StAR mRNA by approximately 2.5-fold (Fig. 4).
Fig. 4.
StAR mRNA expression in KGN cells after a 24-h exposure to diverse phytoestrogens. Data are expressed as fold-change compared with expression in vehicle-control treated cells. Bars represent mean + SD of three independent experiments that were performed in triplicate (N = 3). * and # significantly different from relevant solvent vehicle control Ethanol (0.1%) or DMSO (0.1%)-treated cells, respectively. P < 0.05.
3.4. Phytoestrogens do not impair cell cycle but affect cell migration
A wound-healing assay was performed to determine the effect of phytoestrogens on KGN cell migration. To determine the migratory properties of KGN cells, wound healing was assessed at 0, 8 and 24 h after inflicting the wound. In vehicle-treated KGN cells, the wound area was 89% ± 6 and 40% ± 2 after 8 and 24 h, respectively, compared with the initial wound area. Based on this, a 24 h-time point was chosen to evaluate possible inhibition or induction of phytoestrogens on KGN cell migration. The phytoestrogens tested decreased migration of KGN cells after a 24 h-exposure (Fig. 5A and B). GEN (10 μM) exerted the most pronounced effect on cell mobility and statistically significantly inhibited migration of KGN cells to 46% compared with vehicle-treated control cells. The phytoestrogens COU (10 μM), QUE (10 μM) and RSV (10 μM), but not NAR (3 μM) and 8-PN (3 μM), decreased cell migration to about 25–30% compared to vehicle-treated control cells after 24 h (Fig. 5A and B). To ensure that the observed changes on migration were not due to the proliferative effects of phytoestrogens, routine BrdU labeling of proliferating cells was employed and KGN cells were analyzed after a 24-h treatment with the tested phytoestrogens. No changes in cell proliferation of KGN cells were detected upon exposure to the tested phytoestrogens (data not shown). Moreover, cell cycle analysis by flow cytometry did not reveal any significant changes in distribution in phase S, G1 and G2 of KGN cells (supplementary data Fig. S4). These results clearly indicate that phytoestrogens did not affect KGN cell proliferation nor cell cycle distribution after 24 h.
Fig. 5.
Effects of phytoestrogens on KGN cell migration. (A) The examples of pictures of wound-healing assay at time 0 h and after 24 h-exposure of KGN cells to different phytoestrogens. Magnification 4×. (B) Graphical presentation of wound-healing assay. Data are shown as fold-change compared with vehicle-control treated cells. Bars represent mean + SD of three independent experiments that were performed in triplicate (N = 3). * or # significantly different from relevant solvent vehicle control Ethanol (0.1%) or DMSO (0.1%)-treated cells, respectively. P < 0.05.
Supplementary Fig. S4 related to this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.06.006.
3.5. Marginal effects of phytoestrogens on mRNA levels of genes implicated with tumor growth and progression
To investigate potential molecular mechanisms behind the inhibition of migration of KGN cells, expression of several genes that are important in tumor progression was determined. Only GEN (10 μM) statistically significantly up-regulated VEGF gene expression up to 1.5-fold compared with vehicle-treated control cells (supplementary data Fig. S5A). 8-PN (3 μM) caused a statistically significant 1.9-fold induction of SIRT1 gene expression (supplementary data Fig. S5A). None of the tested phytoestrogens statistically significantly affected MMP9 and CADHERIN E mRNA levels (supplementary data Fig. S5B).
Supplementary Fig. S5 related to this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.06.006.
4. Discussion
Phytoestrogens are plant-derived estrogen-like compounds that are increasingly used for their suggested health promoting properties, even by healthy, young women. However, scientific concerns exist regarding potential adverse effects on female reproduction. Here, we show that some phytoestrogens can modulate ovarian-specific aromatase expression and activity. Also, some phytoestrogens affected migration of human KGN granulosa-like tumor cells.
This study is the first to describe the presence and activation of the ovary-specific promoters II and I.3 in KGN cells. Within the ovary, aromatase expression is mediated primarily by gonadotropin receptors and the cyclic-AMP dependent signaling pathway, which finally contributes to an interaction and activation of the cyclic AMP response element binding protein (CREB) and steroidogenic factor 1 (SF-1) with proximal promoter II and I.3 [53]. Interestingly, it is well-known that the activation of promoters I.3 and II is a critical step for abnormal expression of aromatase levels and local estrogen biosynthesis in tumor-bearing breast tissues [54]. Both promoters I.3 and II are located closely to each other (215 base pair distance length) and are uniformly up-regulated by PGE2 via a cAMP-PKA-dependent pathway [55]. It has been well documented that in ovarian granulosa cells, CYP19A1 expression is regulated primarily by promoter II [56], [57]. We showed that PGE2 (0.1 μM) significantly up-regulated mRNA of both I.3 and II promoter-driven CYP19A1 and increased aromatase activity up to 350% when compared to vehicle-treated control cells. Similarly to PGE2, QUE (10 μM) induced CYP19A1 expression via I.3 and II promoters in KGN cells and increased CYP19A1 activity up to 3 fold after a 24-h exposure. Similarly, QUE (10 and 30 μM) was found to increase I.3 and II-specific aromatase transcripts to approximately 2.6- and 2-fold after 24 h exposure in human adrenal H295R cells [31]. However, in a primary culture of human granulosa-luteal cells, QUE (10 μM) reduced CYP19A1 mRNA expression in a concentration-dependent manner after an exposure period of 48 h [28]. The same inhibitory effect of QUE upon aromatase activity was observed in human placental microsomes [58]. Interestingly, in the present study, QUE appeared to exert dual effects on aromatase at non-cytotoxic concentrations, where it stimulated aromatase activity up to 10 μM and inhibited its activity at 30 μM. This type of non-monotonic aromatase activity curve after exposure to QUE was also observed in human adrenal H295R cells [31]. In the study of Sanderson et al., the concentration of 30 μM QUE increased aromatase activity to approximately 4-fold, where after there was a sharp decline in aromatase activity. However, in that study the decline in aromatase activity was concomitant with an increase in cytotoxicity of QUE at 100 μM [31]. QUE did not cause a significant effect on estrogen production in primary cultures of human granulosa-luteal cells [59]. Also differential effects of GEN on aromatase regulation have been reported in gonadal cells. In our study, aromatase activity and protein expression remained unaltered in the presence of 10 μM of GEN. Edmunds et al. and Myllymaki et al. reported an increase of aromatase activity in human endometrial stromal cells and immature rat ovarian follicles after exposure to GEN [60], [61]. In contrast, Rice et al. demonstrated that the CYP19A1 mRNA transcripts and aromatase activity were reduced in human granulosa-luteal cells after exposure to 10 and 50 μM GEN [28]. In the present study, GEN (10 μM) significantly up-regulated CYP19A1 expression, which was mostly PI.3-driven. Ye et al. showed a concentration-dependent induction of mRNA expression of both promoters I.3 and II CYP19A1 transcripts in human HepG2 cells after exposure to 10 μM GEN [62]. In human adrenal H295R cells, PII and to a lesser extent the I.3 promoter-driven CYP19A1 transcripts were increased by GEN at 10 μM [31].
Some dietary flavanones have previously been shown to possess strong aromatase-inhibitory effects [29], nonetheless, their effects on the transcriptional regulation of CYP19A1 and its promoter regions at mRNA level, remain unknown. In our study, 8-PN (3 μM) but not NAR (3 μM) increased mRNA levels of both PII and I.3-driven CYP19A1 in KGN cells up to 1.8- and 2-fold, respectively. Consistently, 8-PN up-regulated CYP19A1 mRNA transcripts, although CYP19A1 activity was concentration-dependently inhibited (IC50 value of 8 nM). Yet, no change at protein level of CYP19A1 was detected after a 24-h exposure to 8-PN. This implies that 8-PN acts as a catalytic inhibitor of aromatase activity and that the 8-prenyl group is involved in inhibition of aromatase activity. It is interesting to note that the IC50 values for inhibition of aromatase activity by 8-PN and letrozole were in the same range. Similarly, the strong inhibitory effects of 8-PN (IC50 value of 100 nM) on aromatase activity was previously demonstrated by Duursen et al. in human adrenal H295R cells [63]. Further studies will need to be performed to show the nature of the aromatase inhibitory actions of 8-PN. Interestingly, our study demonstrated, that in contrast to 8-PN (3 μM), NAR (3 μM) stimulated CYP19A1 activity but not at mRNA level. Also COU (10 μM) and RSV (10 μM) did not affect CYP19A1 mRNA levels. COU has previously been shown to be a weak competitive inhibitor of aromatase enzyme activity in human preadipocytes [24]. In addition, the previous studies showed that RSV (50 μM) inhibited the transactivation of aromatase promoters I.3 and II in SK-BR-3 cells and it inhibited (IC50 value of 25 μM) aromatase activity in MCF-7 cells [64]. Further research on phytoestrogen QUE, NAR, COU-induced aromatase activity is needed to confirm a direct involvement of CREB1 on CYP191A modulation. The seemingly contradictory results regarding modulation of aromatase activity upon exposure to phytoestrogens could be due the use of different in vitro models. As extensively described above, human aromatase is regulated in a highly tissue-specific manner. This implies that cells from different origins might respond differently to phytoestrogens. Our studies were performed using the human KGN cell line, which has previously been shown to be an excellent and applicable in vitro model to study effects on human granulosa cell functioning. Further studies should be performed to confirm the effects on human granulosa cells and could include primary human granulosa cells, however, be believe this is beyond the scope of our study. Also, different experimental set-up, i.e. medium types, solvent used and exposure times, can contribute greatly to the observed effects. The enzymes of P450 family are highly stable proteins with half-life of 24–42 h [65]. In contrast, the half-life of mRNA has been reported to range from 10 to 30 h depending on tissue investigated [66], [67]. The lack of correlation between mRNA and protein levels of CYP19A1 after 24 h in our study most likely reflect the different half-lives of mRNA and the aromatase protein. Moreover, study of Shozu et al. provided evidence of other non-genomic modes of post-transcriptional regulation of aromatase via modulation of mitogen-activated protein kinase (MAPK) pathways [68]. Bearing in mind that phytoestrogens were shown to modulate several kinase signaling pathways including MAPK and/or Akt/protein kinase B (PKB) [69], [70], direct regulation of aromatase activity by phytoestrogens without detectable changes at mRNA or protein levels may occur. Further research is needed to elucidate the modulation of aromatase activity via these mechanisms by plant-derived compounds. In the present study, we also demonstrated that phytoestrogens can induce Steroidogenic acute regulatory protein (StAR) mRNA levels in ganulosa KGN cells. StAR plays a crucial role in regulation of steroidogenesis by transporting steroid from cytosol into the mitochondria [16], [17]. All tested phytoestrogens, except NAR and GEN, statistically significantly increased StAR mRNA levels up to 2-fold. To date, only several studies reported phytoestrogen-mediated effects on StAR gene and protein expression in granulosa cells. Chen et al. reported that RSV and GEN inhibited mouse Star mRNA, whereas QUE induced Star mRNA levels in murine MA-10 Leydig cells [71]. 8-PN has been shown to exert age-dependent effects on steroidogenesis by up-regulating mRNA levels of Star in progenitor and immature but not adult types of rat Leydig cells [72]. Similarly to our results, StAR mRNA levels were significantly up-regulated by 100 μM RSV in rat granulosa cells after 24 h [32].
Many phytoestrogens, including RSV, GEN and QUE, have been shown to bind to both ERα and/or ERβ and induce the transcription of estrogen-responsive target genes [73], [12], [74]. Yet, despite of the structural similarity with E2, relative binding affinities of phytoestrogens for the ERα are at least 1000–10000 times lower compared to E2 and generally show stronger binding affinity for the ERβ [12]. An exception here is 8-PN. It is well-known, that NAR possess a higher relative estrogenic potency (REP) toward ERβ than ERα [75], [76]. Prenylation at the 8-position of NAR increases estrogenicity [75] and 8-PN has been described to possess a higher relative estrogenic potency (REP) in activation of ERα (10−2) than ERβ (3.9 × 103) [76]. Because of the known interaction of phytoestrogens with ERs and the observed effects on aromatase, we investigated whether phytoestrogens can alter aromatase expression via ER-mediated pathways. We investigated whether the known antiestrogen ICI 182,780 possess modulatory effects upon aromatase. ICI 182,780 has been shown to disrupt the nucleocytoplasmic shuttling of ERα that results in nuclear exclusion of the ER and the increase of its turnover and protein degradation [77]. The present study shows that exposure to ICI 182,780 resulted in a concentration-dependent inhibition of aromatase activity in KGN cells. Our results are in agreement with others, who also observed a concentration-dependent decrease of aromatase activity after ICI 182,780 treatment in MCF-7 cells, human fibroblasts and trophoblast cells [78], [79], [80]. The inhibitory mechanism of ICI 182, 780 upon aromatase remains unknown. However, it was demonstrated that inhibition of aromatase activity by ICI 182,780 is not via interaction with the ER, since the aromatase activity was also suppressed in ER-negative cells after exposure to ICI 182,780 [78]. This concurs with our findings that E2 alone did not affect aromatase activity in KGN cells. In our study, co-exposure of KGN cells to ICI 182,780 (0.1 μM) together with COU (10 μM), QUE (10 μM) statistically significantly decreased aromatase activity by 46% and 73%, respectively, when compared to COU and QUE alone. Interestingly, NAR (3 μM)-mediated stimulation of aromatase activity was not attenuated by ICI 182,780 co-treatment, suggesting that the effects of NAR on aromatase is not mediated by ERα signaling pathways. It would be toxicologically relevant to further explore the mechanism behind the role of ERs in granulosa cells and modulation of aromatase activity and gene expression by phytoestrogens. KGN cells have been shown to express ERα, ERβ but also estrogen-related receptor GPER1 [81]. Recent research has highlighted the cross-talk of both genomic and non-genomic activities of ERs. Here, we have only performed studies with a selective ERα inhibitor, but future studies could include the roles of ERβ and estrogen-related receptor GPER1 as well. However, this was beyond the scope of our study.
Inhibition of E2 biosynthesis by selective blockade of the aromatase enzyme has become an established method of hormonal treatment in estrogen-dependent malignant conditions of ER-positive breast cancers. Granulosa cell tumors (GCT) are hormonally sensitive and characterize with excretion of high levels of aromatase activity and frequently secrete estrogens [82]. Treatment of women with GCT has been positively correlated with the responses to hormonal manipulations with aromatase inhibitors [83]. The interest in the potential benefits of diets high in phytoestrogens has increased, especially with regard to cancer chemoprevention. Yet, despite intense investigation, many studies give inconclusive answers whether phytoestrogens are suitable as cancer chemopreventive agents. In this study, we show that the tested phytoestrogens can affect migration of human KGN granulosa-like tumor cells as assessed by a wound healing assay. Similarly, another study described that RSV (IC50 of 18.8 μM) and QUE (IC50 of 37.5 μM) inhibited the migration of vascular endothelial cells in a concentration-dependent manner [84]. It is well known that neovascularization is a crucial factor in tumor growth, invasion, and metastasis [85], [86]. In line with this, QUE was shown to decrease VEGF secretion by myeloblastic leukemia cells NB4 [87] and possess inhibitory effects on proliferation, migration and tube formation of endothelial cells in vitro [84]. Also GEN was shown to potently inhibit VEGF production and suppress ovarian cancer cell metastasis in vitro [88]. Yet, in our study, only 10 μM GEN caused an up-regulation of VEGF mRNA expression. Data from previous studies demonstrated that GEN can also reduce expression of SIRT1. In the present study, SIRT1 gene expression was only significantly up-regulated by 8-PN. Also, gene expression of CADHERIN E and MMP9 were not affected in KGN cells exposed to tested phytoestrogens. These data show that the tested phytoestrogens reduced KGN cell migration, which is in line with the proposed cancer preventive actions of phytoestrogens. However, found no molecular pathways that could explain the inhibition of migration of KGN cells in this study and cell cycle status and proliferation were unaltered in our experimental set-up.
Upon normal dietary intake of isoflavones, the predominant group of phytoestrogens in Western countries, typically reach blood levels that are in the nanomolar range [89]. However, with vegan and vegetarian diets that are rich in isoflavones, plasma isoflavone concentrations are reported to easily reach micromolar concentrations, depending on the phytoestrogen nature and the food source [90].
Several studies have described high peak plasma levels of phytoestrogen in humans upon dietary intake of phytoestrogens. For example, mean peak plasma levels of QUE were 0.30 and 0.74 μM after consumption of apples (325 μM) or onions (225 μM of QUE), respectively [91]. Similarly, 6 h after ingestion of 60 g of baked soybean powder containing 112 μmol GEN, a mean peak plasma level of 2.44 ± 0.65 μmol GEN/L was detected [92]. With regard to RSV, total plasma levels of RSV were found to reach 1.33 ± 0.3 μmol/L and 1.72 ± 0.1 μmol/L after white and red wine intake, respectively [93]. Also, peak plasma concentrations of NAR of 6.0 ± 5.4 μmol/L were found in humans upon an ingestion of grapefruit juice (8 ml/kg body weight) [94]. Beside dietary intake, many phytoestrogens are also readily available as dietary supplements that can contain daily dosages up to 1200 mg of QUE, GEN or 8-PN for example [63], [95], which are several orders of magnitude higher than the dietary intake described above. It has been demonstrated that the bioavailability of soy isoflavones is higher after ingestion of soy-based supplements than after soy-rich food [96]. Several studies described peak plasma levels upon a high single oral dose of phytoestrogens. For example, a kinetic study with healthy women showed 8-PN peak plasma concentrations up to 220 nM after a single oral dose of 750 mg 8-PN [97]. Here, we described that 8-PN inhibited aromatase activity with an IC50 value of 8 nM, suggesting that our effect concentration is 1–2 orders of magnitude lower than reported human plasma levels for 8-PN. Still, a key obstacle in human risk assessment of phytoestrogen-containing supplements lies in the lack of human relevant pharmacokinetic data. Individual differences in gut microflora, (renal) clearance, genetic polymorphisms in metabolizing enzymes, differences in supplementation dose and type of phytoestrogen source all contribute to disparate plasma levels of phytoestrogens that have been reported in humans. Furthermore, the lack of the correlation of plasma levels with tissue concentrations, e.g. in the ovary hampers proper human risk assessment of phytoestrogens. In the present study, the final concentrations of tested phytoestrogens were in the micromolar range, which is in the same order of magnitude as generally reported plasma levels upon intake of these phytoestrogens with dietary supplements. However, supplements are typically taken on a daily basis, which leads to prolonged high systemic exposure to these bioactive compounds. The complexity of phytoestrogen actions is increased by the fact that these compounds are frequently present as mixtures of several dietary components that can affect multiple signaling pathways or the same pathways resulting in additive, synergistic or opposing effects. Based on the effect concentrations from our study and the reported human plasma levels, we conclude that it is not unlikely that adverse effects on ovarian function can occur after intake of high doses of phytoestrogens via dietary supplements. Yet, for better human risk assessment of these compounds, understanding the pharmacokinetics and the combined effects of phytoestrogens on steroidogenic processes is crucial and needs to be addressed further.
5. Conclusion
Our study shows that some phytoestrogens can modulate promoter II and I.3-driven aromatase in KGN granulosa-like tumor cells, which could lead to altered ovarian estrogen production. Most strikingly were the effects of 8-PN that displayed even a more potent inhibition of aromatase activity than the therapeutic compound letrozole, while simultaneously it induced CYP19 mRNA levels. We also showed that most phytoestrogens reduced KGN cell migration. Our study indicates that the use of dietary supplements with high contents of phytoestrogens may interfere with normal female ovarian function and that caution is in place when taking these supplements.
Conflict of interest
The authors declare that there are no conflicts of interest.
Transparency document
Acknowledgements
This work was financially supported by the Doerenkamp-Zbinden Foundation.
Footnotes
Available online 8 July 2014
References
- 1.Setchell K.D. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998;68(6 Suppl.):1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 2.Knight D.C., Eden J.A. A review of the clinical effects of phytoestrogens. Obstet. Gynecol. 1996;87(5 Pt 2):897–904. [PubMed] [Google Scholar]
- 3.Cassidy A., Milligan S. How significant are environmental estrogens to women? Climacteric. 1998;1(3):229–242. doi: 10.3109/13697139809085545. [DOI] [PubMed] [Google Scholar]
- 4.Kouki T., Kishitake M., Okamoto M., Oosuka I., Takebe M., Yamanouchi K. Effects of neonatal treatment with phytoestrogens, genistein and daidzein, on sex difference in female rat brain function: estrous cycle and lordosis. Horm. Behav. 2003;44(2):140–145. doi: 10.1016/s0018-506x(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 5.Nikaido Y., Yoshizawa K., Danbara N., Tsujita-Kyutoku M., Yuri T., Uehara N., Tsubura A. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod. Toxicol. 2004;18(6):803–811. doi: 10.1016/j.reprotox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Delclos K.B., Weis C.C., Bucci T.J., Olson G., Mellick P., Sadovova N., Latendresse J.R., Thorn B., Newbold R.R. Overlapping but distinct effects of genistein and ethinyl estradiol (EE(2)) in female Sprague-Dawley rats in multigenerational reproductive and chronic toxicity studies. Reprod. Toxicol. 2009;27(2):117–132. doi: 10.1016/j.reprotox.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennetts H.W., Underwood E.J., Shier F.L. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust. Vet. J. 1946;22:2–12. doi: 10.1111/j.1751-0813.1946.tb15473.x. [DOI] [PubMed] [Google Scholar]
- 8.Kallela K., Heinonen K., Saloniemi H. Plant oestrogens; the cause of decreased fertility in cows. A case report. Nord. Vet. Med. 1984;36(3–4):124–129. [PubMed] [Google Scholar]
- 9.Amsterdam A., Abu-Rustum N., Carter J., Krychman M. Persistent sexual arousal syndrome associated with increased soy intake. J. Sex. Med. 2005;2(3):338–340. doi: 10.1111/j.1743-6109.2005.20358.x. [DOI] [PubMed] [Google Scholar]
- 10.Chandrareddy A., Muneyyirci-Delale O., McFarlane S.I., Murad O.M. Adverse effects of phytoestrogens on reproductive health: a report of three cases. Complement. Ther. Clin. Pract. 2008;14(2):132–135. doi: 10.1016/j.ctcp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Kim J., Kim S., Huh K., Kim Y., Joung H., Park M. High serum isoflavone concentrations are associated with the risk of precocious puberty in Korean girls. Clin. Endocrinol. (Oxf.) 2011;75(6):831–835. doi: 10.1111/j.1365-2265.2011.04127.x. [DOI] [PubMed] [Google Scholar]
- 12.Kuiper G.G., Lemmen J.G., Carlsson B., Corton J.C., Safe S.H., van der Saag P.T., van der Burg B., Gustafsson J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S.C., Clemens J.W., Pisarska M.D., Richards J.S. Expression and function of estrogen receptor subtypes in granulosa cells: regulation by estradiol and forskolin. Endocrinology. 1999;140(9):4320–4334. doi: 10.1210/endo.140.9.6965. [DOI] [PubMed] [Google Scholar]
- 14.Lorand T., Vigh E., Garai J. Hormonal action of plant derived and anthropogenic non-steroidal estrogenic compounds: phytoestrogens and xenoestrogens. Curr. Med. Chem. 2010;17(30):3542–3574. doi: 10.2174/092986710792927813. [DOI] [PubMed] [Google Scholar]
- 15.Prossnitz E.R., Barton M. Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat. 2009;89(3–4):89–97. doi: 10.1016/j.prostaglandins.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark B.J., Wells J., King S.R., Stocco D.M. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J. Biol. Chem. 1994;269(45):28314–28322. [PubMed] [Google Scholar]
- 17.Clark B.J., Stocco D.M. Steroidogenic acute regulatory protein: the StAR still shines brightly. Mol. Cell. Endocrinol. 1997;134(1):1–8. doi: 10.1016/s0303-7207(97)00166-4. [DOI] [PubMed] [Google Scholar]
- 18.Simpson E.R., Zhao Y., Agarwal V.R., Michael M.D., Bulun S.E., Hinshelwood M.M., Graham-Lorence S., Sun T., Fisher C.R., Qin K., Mendelson C.R. Aromatase expression in health and disease. Recent Prog. Horm. Res. 1997;52:185–213. discussion 213-4. [PubMed] [Google Scholar]
- 19.Simpson E.R., Davis S.R. Minireview: aromatase and the regulation of estrogen biosynthesis – some new perspectives. Endocrinology. 2001;142(11):4589–4594. doi: 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- 20.Naftolin F., Ryan K.J., Petro Z. Aromatization of androstenedione by the anterior hypothalamus of adult male and female rats. Endocrinology. 1972;90(1):295–298. doi: 10.1210/endo-90-1-295. [DOI] [PubMed] [Google Scholar]
- 21.Bulun S.E., Sebastian S., Takayama K., Suzuki T., Sasano H., Shozu M. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J. Steroid Biochem. Mol. Biol. 2003;86(3–5):219–222. doi: 10.1016/s0960-0760(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 22.Simpson E.R. Aromatase: biologic relevance of tissue-specific expression. Semin. Reprod. Med. 2004;22(1):11–23. doi: 10.1055/s-2004-823023. [DOI] [PubMed] [Google Scholar]
- 23.Ohno S., Yukinawa F., Noda M., Nakajin S. Mono-(2-ethylhexyl) phthalate induces NR4A subfamily and GIOT-1 gene expression, and suppresses CYP19 expression in human granulosa-like tumor cell line KGN. Toxicol. Lett. 2009;191(2–3):353–359. doi: 10.1016/j.toxlet.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Wang C., Makela T., Hase T., Adlercreutz H., Kurzer M.S. Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes. J. Steroid Biochem. Mol. Biol. 1994;50(3–4):205–212. doi: 10.1016/0960-0760(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Man Gho W., Chan F.L., Chen S., Leung L.K. The red clover (Trifolium pratense) isoflavone biochanin A inhibits aromatase activity and expression. Br. J. Nutr. 2008;99(2):303–310. doi: 10.1017/S0007114507811974. [DOI] [PubMed] [Google Scholar]
- 26.Campbell D.R., Kurzer M.S. Flavonoid inhibition of aromatase enzyme activity in human preadipocytes. J. Steroid Biochem. Mol. Biol. 1993;46(3):381–388. doi: 10.1016/0960-0760(93)90228-o. [DOI] [PubMed] [Google Scholar]
- 27.Adlercreutz H., Bannwart C., Wahala K., Makela T., Brunow G., Hase T., Arosemena P.J., Kellis J.T., Jr., Vickery L.E. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J. Steroid Biochem. Mol. Biol. 1993;44(2):147–153. doi: 10.1016/0960-0760(93)90022-o. [DOI] [PubMed] [Google Scholar]
- 28.Rice S., Mason H.D., Whitehead S.A. Phytoestrogens and their low dose combinations inhibit mRNA expression and activity of aromatase in human granulosa-luteal cells. J. Steroid Biochem. Mol. Biol. 2006;101(4–5):216–225. doi: 10.1016/j.jsbmb.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 29.van Meeuwen J.A., Nijmeijer S., Mutarapat T., Ruchirawat S., de Jong P.C., Piersma A.H., van den Berg M. Aromatase inhibition by synthetic lactones and flavonoids in human placental microsomes and breast fibroblasts – a comparative study. Toxicol. Appl. Pharmacol. 2008;228(3):269–276. doi: 10.1016/j.taap.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 30.van Duursen M.B., Nijmeijer S.M., de Morree E.S., de Jong P.C., van den Berg M. Genistein induces breast cancer-associated aromatase and stimulates estrogen-dependent tumor cell growth in in vitro breast cancer model. Toxicology. 2011;289(2–3):67–73. doi: 10.1016/j.tox.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Sanderson J.T., Hordijk J., Denison M.S., Springsteel M.F., Nantz M.H., van den Berg M. Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. Toxicol. Sci. 2004;82(1):70–79. doi: 10.1093/toxsci/kfh257. [DOI] [PubMed] [Google Scholar]
- 32.Morita Y., Wada-Hiraike O., Yano T., Shirane A., Hirano M., Hiraike H., Koyama S., Oishi H., Yoshino O., Miyamoto Y., Sone K., Oda K., Nakagawa S., Tsutsui K., Taketani Y. Resveratrol promotes expression of SIRT1 and StAR in rat ovarian granulosa cells: an implicative role of SIRT1 in the ovary. Reprod. Biol. Endocrinol. 2012;10 doi: 10.1186/1477-7827-10-14. 14-7827-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S.S., Michael A., Butler-Manuel S.A. Advances in the treatment of ovarian cancer: a potential role of antiinflammatory phytochemicals. Discov. Med. 2012;13(68):7–17. [PubMed] [Google Scholar]
- 34.Hedelin M., Lof M., Andersson T.M., Adlercreutz H., Weiderpass E. Dietary phytoestrogens and the risk of ovarian cancer in the women's lifestyle and health cohort study. Cancer Epidemiol. Biomarkers Prev. 2011;20(2):308–317. doi: 10.1158/1055-9965.EPI-10-0752. [DOI] [PubMed] [Google Scholar]
- 35.Nishi Y., Yanase T., Mu Y., Oba K., Ichino I., Saito M., Nomura M., Mukasa C., Okabe T., Goto K., Takayanagi R., Kashimura Y., Haji M., Nawata H. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142(1):437–445. doi: 10.1210/endo.142.1.7862. [DOI] [PubMed] [Google Scholar]
- 36.Deura I., Harada T., Taniguchi F., Iwabe T., Izawa M., Terakawa N. Reduction of estrogen production by interleukin-6 in a human granulosa tumor cell line may have implications for endometriosis-associated infertility. Fertil. Steril. 2005;83(Suppl. 1):1086–1092. doi: 10.1016/j.fertnstert.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Horling K., Santos A.N., Fischer B. The AhR is constitutively activated and affects granulosa cell features in the human cell line KGN. Mol. Hum. Reprod. 2011;17(2):104–114. doi: 10.1093/molehr/gaq074. [DOI] [PubMed] [Google Scholar]
- 38.Alexiadis M., Eriksson N., Jamieson S., Davis M., Drummond A.E., Chu S., Clyne C.D., Muscat G.E., Fuller P.J. Nuclear receptor profiling of ovarian granulosa cell tumors. Horm. Cancer. 2011;2(3):157–169. doi: 10.1007/s12672-011-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reverchon M., Cornuau M., Rame C., Guerif F., Royere D., Dupont J. Chemerin inhibits IGF-1-induced progesterone and estradiol secretion in human granulosa cells. Hum. Reprod. 2012;27(6):1790–1800. doi: 10.1093/humrep/des089. [DOI] [PubMed] [Google Scholar]
- 40.Kwintkiewicz J., Nishi Y., Yanase T., Giudice L.C. Peroxisome proliferator-activated receptor-gamma mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ. Health Perspect. 2010;118(3):400–406. doi: 10.1289/ehp.0901161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reverchon M., Cornuau M., Cloix L., Rame C., Guerif F., Royere D., Dupont J. Visfatin is expressed in human granulosa cells: regulation by metformin through AMPK/SIRT1 pathways and its role in steroidogenesis. Mol. Hum. Reprod. 2013;19(5):313–326. doi: 10.1093/molehr/gat002. [DOI] [PubMed] [Google Scholar]
- 42.Elangovan S., Ramachandran S., Venkatesan N., Ananth S., Gnana-Prakasam J.P., Martin P.M., Browning D.D., Schoenlein P.V., Prasad P.D., Ganapathy V., Thangaraju M. SIRT1 is essential for oncogenic signaling by estrogen/estrogen receptor alpha in breast cancer. Cancer Res. 2011;71(21):6654–6664. doi: 10.1158/0008-5472.CAN-11-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 1986;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 44.Attar E., Bulun S.E. Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum. Reprod. Update. 2006;12(1):49–56. doi: 10.1093/humupd/dmi034. [DOI] [PubMed] [Google Scholar]
- 45.Heneweer M., van den Berg M., Sanderson J.T. A comparison of human H295R and rat R2C cell lines as in vitro screening tools for effects on aromatase. Toxicol. Lett. 2004;146(2):183–194. doi: 10.1016/j.toxlet.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y.H., Tan F., Hess K.R., Yung W.K. The expression of PAX6, PTEN, vascular endothelial growth factor, and epidermal growth factor receptor in gliomas: relationship to tumor grade and survival. Clin. Cancer Res. 2003;9(9):3369–3375. [PubMed] [Google Scholar]
- 47.Lephart E.D., Simpson E.R. Assay of aromatase activity. Methods Enzymol. 1991;206:477–483. doi: 10.1016/0076-6879(91)06116-k. [DOI] [PubMed] [Google Scholar]
- 48.Sanderson J.T., Letcher R.J., Heneweer M., Giesy J.P., van den Berg M. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ. Health Perspect. 2001;109(10):1027–1031. doi: 10.1289/ehp.011091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 50.Wang C., Lv X., Jiang C., Cordes C.M., Fu L., Lele S.M., Davis J.S. Transforming growth factor alpha (TGFalpha) regulates granulosa cell tumor (GCT) cell proliferation and migration through activation of multiple pathways. PLOS ONE. 2012;7(11):e48299. doi: 10.1371/journal.pone.0048299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson D., Cook P.R. Analyzing DNA replication I: labeling animals, tissues, and cells with bromodeoxyuridine (BrdU) CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot5031. [DOI] [PubMed] [Google Scholar]
- 52.Sangjun S., de Jong E., Nijmeijer S., Mutarapat T., Ruchirawat S., van den Berg M., van Duursen M.B. Induction of cell cycle arrest in human MCF-7 breast cancer cells by cis-stilbene derivatives related to VIOXX. Toxicol. Lett. 2009;186(2):115–122. doi: 10.1016/j.toxlet.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 53.Bulun S.E., Zeitoun K.M., Takayama K., Sasano H. Estrogen biosynthesis in endometriosis: molecular basis and clinical relevance. J. Mol. Endocrinol. 2000;25(1):35–42. doi: 10.1677/jme.0.0250035. [DOI] [PubMed] [Google Scholar]
- 54.Bulun S.E., Lin Z., Imir G., Amin S., Demura M., Yilmaz B., Martin R., Utsunomiya H., Thung S., Gurates B., Tamura M., Langoi D., Deb S. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol. Rev. 2005;57(3):359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 55.Bulun S.E., Chen D., Lu M., Zhao H., Cheng Y., Demura M., Yilmaz B., Martin R., Utsunomiya H., Thung S., Su E., Marsh E., Hakim A., Yin P., Ishikawa H., Amin S., Imir G., Gurates B., Attar E., Reierstad S., Innes J., Lin Z. Aromatase excess in cancers of breast, endometrium and ovary. J. Steroid Biochem. Mol. Biol. 2007;106(1–5):81–96. doi: 10.1016/j.jsbmb.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Means G.D., Kilgore M.W., Mahendroo M.S., Mendelson C.R., Simpson E.R. Tissue-specific promoters regulate aromatase cytochrome P450 gene expression in human ovary and fetal tissues. Mol. Endocrinol. 1991;5(12):2005–2013. doi: 10.1210/mend-5-12-2005. [DOI] [PubMed] [Google Scholar]
- 57.Jenkins C., Michael D., Mahendroo M., Simpson E. Exon-specific northern analysis and rapid amplification of cDNA ends (RACE) reveal that the proximal promoter II (PII) is responsible for aromatase cytochrome P450 (CYP19) expression in human ovary. Mol. Cell. Endocrinol. 1993;97(1–2):R1–R6. doi: 10.1016/0303-7207(93)90227-b. [DOI] [PubMed] [Google Scholar]
- 58.Kellis J.T., Jr., Vickery L.E. Inhibition of human estrogen synthetase (aromatase) by flavones. Science. 1984;225(4666):1032–1034. doi: 10.1126/science.6474163. [DOI] [PubMed] [Google Scholar]
- 59.Whitehead S.A., Lacey M. Phytoestrogens inhibit aromatase but not 17beta-hydroxysteroid dehydrogenase (HSD) type 1 in human granulosa-luteal cells: evidence for FSH induction of 17beta-HSD. Hum. Reprod. 2003;18(3):487–494. doi: 10.1093/humrep/deg125. [DOI] [PubMed] [Google Scholar]
- 60.Edmunds K.M., Holloway A.C., Crankshaw D.J., Agarwal S.K., Foster W.G. The effects of dietary phytoestrogens on aromatase activity in human endometrial stromal cells. Reprod. Nutr. Dev. 2005;6:709–720. doi: 10.1051/rnd:2005055. [DOI] [PubMed] [Google Scholar]
- 61.Myllymaki S., Haavisto T., Vainio M., Toppari J., Paranko J. In vitro effects of diethylstilbestrol, genistein, 4-tert-butylphenol, and 4-tert-octylphenol on steroidogenic activity of isolated immature rat ovarian follicles. Toxicol. Appl. Pharmacol. 2005;204(1):69–80. doi: 10.1016/j.taap.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Ye L., Chan M.Y., Leung L.K. The soy isoflavone genistein induces estrogen synthesis in the extragonadal pathway. Mol. Cell. Endocrionol. 2009:73–80. doi: 10.1016/j.mce.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 63.van Duursen M.B., Smeets E.E., Rijk J.C., Nijmeijer S.M., van den Berg M. Phytoestrogens in menopausal supplements induce ER-dependent cell proliferation and overcome breast cancer treatment in an in vitro breast cancer model. Toxicol. Appl. Pharmacol. 2013;269(2):132–140. doi: 10.1016/j.taap.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y., Lee K.W., Chan F.L., Chen S., Leung L.K. The red wine polyphenol resveratrol displays bilevel inhibition on aromatase in breast cancer cells. Toxicol. Sci. 2006;92(1):71–77. doi: 10.1093/toxsci/kfj190. [DOI] [PubMed] [Google Scholar]
- 65.Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J. Steroid Biochem. Mol. Biol. 1992;43(8):779–804. doi: 10.1016/0960-0760(92)90307-5. [DOI] [PubMed] [Google Scholar]
- 66.Genissel C., Levallet J., Carreau S. Regulation of cytochrome P450 aromatase gene expression in adult rat Leydig cells: comparison with estradiol production. J. Endocrinol. 2001;168(1):95–105. doi: 10.1677/joe.0.1680095. [DOI] [PubMed] [Google Scholar]
- 67.Mu Y.M., Yanase T., Nishi Y., Takayanagi R., Goto K., Nawata H. Combined treatment with specific ligands for PPARgamma:RXR nuclear receptor system markedly inhibits the expression of cytochrome P450arom in human granulosa cancer cells. Mol. Cell. Endocrinol. 2001;181(1–2):239–248. doi: 10.1016/s0303-7207(00)00457-3. [DOI] [PubMed] [Google Scholar]
- 68.Shozu M., Sumitani H., Murakami K., Segawa T., Yang H.J., Inoue M. Regulation of aromatase activity in bone-derived cells: possible role of mitogen-activated protein kinase. J. Steroid Biochem. Mol. Biol. 2001;79(1–5):61–65. doi: 10.1016/s0960-0760(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez Y., Amran D., de Blas E., Aller P. Regulation of genistein-induced differentiation in human acute myeloid leukaemia cells (HL60, NB4) protein kinase modulation and reactive oxygen species generation. Biochem. Pharmacol. 2009;77(3):384–396. doi: 10.1016/j.bcp.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 70.Spencer J.P., Rice-Evans C., Williams R.J. Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J. Biol. Chem. 2003;278(37):34783–34793. doi: 10.1074/jbc.M305063200. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y.C., Nagpal M.L., Stocco D.M., Lin T. Effects of genistein, resveratrol, and quercetin on steroidogenesis and proliferation of MA-10 mouse Leydig tumor cells. J. Endocrinol. 2007;192(3):527–537. doi: 10.1677/JOE-06-0087. [DOI] [PubMed] [Google Scholar]
- 72.Izzo G., Soder O., Svechnikov K. The prenylflavonoid phytoestrogens 8-prenylnaringenin and isoxanthohumol differentially suppress steroidogenesis in rat Leydig cells in ontogenesis. J. Appl. Toxicol. 2011;31(6):589–594. doi: 10.1002/jat.1602. [DOI] [PubMed] [Google Scholar]
- 73.Bowers J.L., Tyulmenkov V.V., Jernigan S.C., Klinge C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141(10):3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- 74.Maggiolini M., Bonofiglio D., Marsico S., Panno M.L., Cenni B., Picard D., Ando S. Estrogen receptor alpha mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells. Mol. Pharmacol. 2001;60:595–602. [PubMed] [Google Scholar]
- 75.Kretzschmar G., Zierau O., Wober J., Tischer S., Metz P., Vollmer G. Prenylation has a compound specific effect on the estrogenicity of naringenin and genistein. J. Steroid Biochem. Mol. Biol. 2010;118(1–2):1–6. doi: 10.1016/j.jsbmb.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 76.Bovee T.F., Helsdingen R.J., Rietjens I.M., Keijer J., Hoogenboom R.L. Rapid yeast estrogen bioassays stably expressing human estrogen receptors alpha and beta, and green fluorescent protein: a comparison of different compounds with both receptor types. J. Steroid Biochem. Mol. Biol. 2004;91(3):99–109. doi: 10.1016/j.jsbmb.2004.03.118. [DOI] [PubMed] [Google Scholar]
- 77.Dauvois S., White R., Parker M.G. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J. Cell. Sci. 1993;106(Pt 4):1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- 78.Chen S., Zhou D., Yang C., Okubo T., Kinoshita Y., Yu B., Kao Y.C., Itoh T. Modulation of aromatase expression in human breast tissue. J. Steroid Biochem. Mol. Biol. 2001;79(1–5):35–40. doi: 10.1016/s0960-0760(01)00132-7. [DOI] [PubMed] [Google Scholar]
- 79.Long B.J., Tilghman S.L., Yue W., Thiantanawat A., Grigoryev D.N., Brodie A.M. The steroidal antiestrogen ICI 182,780 is an inhibitor of cellular aromatase activity. J. Steroid Biochem. Mol. Biol. 1998;67(4):293–304. doi: 10.1016/s0960-0760(98)00122-8. [DOI] [PubMed] [Google Scholar]
- 80.Kumar P., Kamat A., Mendelson C.R. Estrogen receptor alpha (ERalpha) mediates stimulatory effects of estrogen on aromatase (CYP19) gene expression in human placenta. Mol. Endocrinol. 2009;23(6):784–793. doi: 10.1210/me.2008-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang C., Lv X., Jiang C., Davis J.S. The putative G-protein coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian and breast cancer cells in a GPER-independent manner. Am. J. Transl. Res. 2012;4(4):390–402. [PMC free article] [PubMed] [Google Scholar]
- 82.Schumer S.T., Cannistra S.A. Granulosa cell tumor of the ovary. J. Clin. Oncol. 2003;21(6):1180–1189. doi: 10.1200/JCO.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 83.Freeman S.A., Modesitt S.C. Anastrozole therapy in recurrent ovarian adult granulosa cell tumors: a report of 2 cases. Gynecol. Oncol. 2006;103(2):755–758. doi: 10.1016/j.ygyno.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 84.Igura K., Ohta T., Kuroda Y., Kaji K. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett. 2001;171(1):11–16. doi: 10.1016/s0304-3835(01)00443-8. [DOI] [PubMed] [Google Scholar]
- 85.Folkman J., Watson K., Ingber D., Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 86.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 87.Zhong L., Chen F.Y., Wang H.R., Ten Y., Wang C., Ouyang R.R. Effects of quercetin on morphology and VEGF secretion of leukemia cells NB4 in vitro. Zhonghua Zhong Liu Za Zhi. 2006;28(1):25–27. [PubMed] [Google Scholar]
- 88.Luo H., Jiang B.H., King S.M., Chen Y.C. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr. Cancer. 2008;60(6):800–809. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]
- 89.Morton M.S., Wilcox G., Wahlqvist M.L., Griffiths K. Determination of lignans and isoflavonoids in human female plasma following dietary supplementation. J. Endocrinol. 1994;142(2):251–259. doi: 10.1677/joe.0.1420251. [DOI] [PubMed] [Google Scholar]
- 90.Bhathena S.J., Velasquez M.T. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am. J. Clin. Nutr. 2002;76(6):1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 91.Hollman P.C., van Trijp J.M., Buysman M.N., van der Gaag M.S., Mengelers M.J., de Vries J.H., Katan M.B. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418(1–2):152–156. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- 92.Watanabe S., Yamaguchi M., Sobue T., Takahashi T., Miura T., Arai Y., Mazur W., Wahala K., Adlercreutz H. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako) J. Nutr. 1998;128(10):1710–1715. doi: 10.1093/jn/128.10.1710. [DOI] [PubMed] [Google Scholar]
- 93.Pignatelli P., Ghiselli A., Buchetti B., Carnevale R., Natella F., Germanò G., Fimognari F., Di Santo S., Lenti L., Violi F. Polyphenols synergistically inhibit oxidative stress in subjects given red and white wine. Atherosclerosis. 2006;1:77–83. doi: 10.1016/j.atherosclerosis.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 94.Erlund I., Meririnne E., Alfthan G., Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 2001;131(2):235–241. doi: 10.1093/jn/131.2.235. [DOI] [PubMed] [Google Scholar]
- 95.Egert S., Wolffram S., Bosy-Westphal A., Boesch-Saadatmandi C., Wagner A.E., Frank J., Rimbach G., Mueller M.J. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008;138(9):1615–1621. doi: 10.1093/jn/138.9.1615. [DOI] [PubMed] [Google Scholar]
- 96.Vergne S., Bennetau-Pelissero C., Lamothe V., Chantre P., Potier M., Asselineau J., Perez P., Durand M., Moore N., Sauvant P. Higher bioavailability of isoflavones after a single ingestion of a soya-based supplement than a soya-based food in young healthy males. Br. J. Nutr. 2008;99(2):333–344. doi: 10.1017/S0007114507803953. [DOI] [PubMed] [Google Scholar]
- 97.Rad M., Humpel M., Schaefer O., Schoemaker R.C., Schleuning W.D., Cohen A.F., Burggraaf J. Pharmacokinetics and systemic endocrine effects of the phyto-oestrogen 8-prenylnaringenin after single oral doses to postmenopausal women. Br. J. Clin. Pharmacol. 2006;62(3):288–296. doi: 10.1111/j.1365-2125.2006.02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.