Abstract
The effect of two zeolites, HUSY, NaY and a mesoporous synthesized Al-MCM-41 material on the smoke composition of ten commercial cigarettes brands has been studied. Cigarettes were prepared by mixing the tobacco with the three powdered materials, and the smoke obtained under the ISO conditions was analyzed. Up to 32 compounds were identified and quantified in the gas fraction and 80 in the total particulate matter (TPM) condensed in the cigarettes filters and in the traps located after the mouth end of the cigarettes. Al-MCM-41 is by far the best additive, providing the highest reductions of the yield for most compounds and brands analyzed. A positive correlation was observed among the TPM and nicotine yields with the reduction obtained in nicotine, CO, and most compounds with the three additives. The amount of ashes in additive free basis increases due to the coke deposited on the solids, especially with Al-MCM-41. Nicotine is reduced with Al-MCM-41 by an average of 34.4% for the brands studied (49.5% for the brand where the major reduction was obtained and 18.5 for the brand behaving the worst). CO is reduced by an average of 18.6% (ranging from 10.3 to 35.2% in the different brands).
Keywords: Tobacco smoke composition, Reduction of smoke components yield, Zeolite applications, Al-MCM-41 applications
1. Introduction
Tobacco smoking is a dangerous and extended practice in modern society. Tobacco smoke is a complex mixture formed by more than 4000 compounds, where at least 70 are severely toxic and carcinogenic for humans [10], [13]. It is compulsory for information about the maximum nicotine, tar and carbon monoxide content in cigarette smoke to be shown in the labelling of tobacco cigarettes in Europe as well as warnings regarding the adverse health effects of smoking. In addition, measures concerning the ingredients and description of tobacco products are also being adopted. The regulation of tobacco products and the adoption of standards to reduce the yield of smoke constituents, and hence human exposure, are also being studied in an attempt to reduce the risks related to cigarette smoking. For example, in 2008 the WHO Study Group on Tobacco Regulations established a regulatory strategy to reduce the level of toxic compounds in tobacco smoke measured under standardized conditions (WHO technical report series 951). The selection of toxicants was made according to the Health Canadian list and yield data were based on the market survey carried out by Counts et al. [6] on 48 commercial cigarette brands. These authors analyzed a considerable number of smoke constituents and established some predicting relationships between tar yield and the smoke constituents for three smoking regimes.
It is well known that general lowering of smoke yields can be achieved by a combination of various design parameters including increased ventilation into the paper wrapping the tobacco rod, filter components, faster paper burn rate, paper permeability and lower tobacco density [1], [24], [8], [27]. Branton et al. [4] described the modification of filters by activated carbon to adsorb the constituents of the mainstream tobacco smoke (MSS). Deng et al. [9] studied the effect of titanate nanosheets and nanotubes and reported significant reductions of harmful compounds in tobacco smoke, and Chen et al. [5] studied the effect of oxidized carbon nanotubes on the composition of the MSS smoke. All these studies were carried out on reference cigarettes, on specially prepared cigarettes, or sometimes on a non-specified commercial brand.
The use of zeolites and other aluminosilicates in the filter or directly mixed with tobacco to reduce nitrosamines and polycyclic aromatics in the main MSS has been described by several authors [7], [30], [31], [11], who employed NaA, NaY, KA and NaZSM-5, Cu-ZSM-5, SBA-15, MCM-48, Cerium-containing MCM-48 and other calco-silicates. Our research group has studied the synthesis of MCM-41 catalyst for different purposes [17]. For example, it was demonstrated that removing the template by solvent extraction prior to calcination [19], employing the adequate solvents [18] or varying the aluminium content [20], catalysts with the adequate properties to be used as tobacco additives were obtained. Other zeolitic materials (HUSY, HZSM-5 and Hβ) together with Al-MCM-41 [21] were also studied, obtaining a remarkable ability for Al-MCM-41 to reduce the yields of some known carcinogenic compounds (up to 40% reduction).
Due to the high interest in the subject and to the promising results obtained, in the last few months new papers have appeared on the topic of reducing tobacco smoke toxicity by zeolites and aluminosilicates. Lin et al. [15] studied the effect of different molecular sieve materials on the elimination of specific tobacco nitrosamines. They tested A, ZSM5 and USY type zeolites as well as mesoporous materials such as MCM and SBA-15. They also studied the effect of the morphology of the materials and the acidity and concluded that the mesoporous materials were the more effective in reducing such compounds. The effect of ferric zeolites in reducing specific tobacco nitrosamines in tobacco smoke was also studied [16]. They concluded that the iron cations exchanged in the zeolite were more efficient than iron oxide particles deposited on the catalyst by impregnation. These studies on reducing toxic compounds by zeolites or aluminosilicate materials were carried out on reference cigarettes or on a single commercial brand, and the results have to be understood as specific to the tobacco blends or cigarette configurations investigated in each work.
Some interesting studies comparing the yield of smoke components among a large number of commercial brands under different smoking conditions and cigarettes design characteristics have been published. Kalaitzoglou and Samara [14] studied the content of PAH in MSS of 59 commercial cigarettes brands from Greece. The dioxin and dioxin-like compounds content in MSS of commercial US brands was studied by Wilson et al. [28]. Marcilla et al. [22] compared the smoke yields of 10 commercial brands sold in Spain, and more recently these authors [23] compared the smoke composition of 11 roll your own (RYO) commercial brands with a reference tobacco. In general, it can be said that the relative yield (both, on per cigarette or amount of smoked tobacco basis) of individual compounds varies considerably among the different brands. The differences in the tobacco type, design configuration and smoking regime may affect differently the yield of any particular toxic compound evolved.
The objective of the present paper is to study the effect of the porous structure and acidity of three additives on the smoke composition when smoking a series of commercial cigarette brands, in order to obtain valuable data of practical potential utility of these solids for reducing the toxicity of tobacco smoke. For this purpose, the materials employed were two microporous zeolites with similar porous texture but different acidity, i.e. a USY zeolite as received from the supplier in its acid form (HUSY) and another one Na exchanged (NaY), as well as one of the mesoporous Al-MCM-41 synthesized in our laboratory. The commercial brands of cigarettes studied were ten top brands by market share in Spain, which were analyzed in a previous paper [22].
2. Materials and experimental methods
2.1. Additives
Commercial HUSY and NaY zeolites were supplied by GRACE Davison and Wako. Al-MCM-41 was synthesized according to the reported method [12]. The N2 adsorption isotherms were measured at 77 K in an AUTOSORB-6 supplied by Quantachrome. The Si/Al ratio was measured by X-ray fluorescence (XRF) in a PHILIPS MAGIX PRO, model PW2400 sequential X-ray spectrometer. The acidity of the materials was measured by temperature programmed desorption (TPD) of ammonia, performed in a Netzsch TG 209 thermobalance. All materials were sieved to sizes lower than 70 μm prior to its usage.
The physicochemical properties of the three materials are shown in Table 1. HUSY has the higher aluminium content (lower Si/Al ratio as seen in Table 1) and lower pore size (0.74 nm of diameter). Al-MCM-41 is a mesoporous material with a pore size of 2.7 nm and a very high BET surface area. The acidity of these materials increases in the order NaY < Al-MCM-41 < HUSY, which, as expected, is in accordance with the aluminium content for Al-MCM-41 and HUSY.
Table 1.
Properties of the materials added to the tobacco as additives.
| Property | HUSY | NaY | Al-MCM-41 |
|---|---|---|---|
| Pore size (nm)a | 0.74 | 0.95 | 2.73 |
| Surface area (m2/g)b | 614 | 827.2 | 1007 |
| Pore volume (cm3/g)c | 0.278 | 0.329 | 0.722 |
| Si/Al ratiod | 4.8 | 25 | 119 |
| Acidity (mmol/g)e | 2.12 | 0 | 0.31 |
Nitrogen adsorption isotherm, BJH model.
Nitrogen adsorption isotherm, BET method considering values in the 0.04–0.25 range of relative pressure.
Nitrogen adsorption isotherm at P/P0 = 0.8.
XRF.
Thermal desorption of NH3 according to the method previously published [17].
2.2. Cigarettes
Ten commercial cigarettes brands were chosen among the best-selling brands in Spain in 2013. They were: Marlboro, Winston, Fortuna, Chesterfield, Ducados Rubio, Camel, L&M, Nobel, Lucky Strike and John Player SP. For privacy reasons in the following Figures and Tables, brands have been named with letters from A to J. As mentioned above, these brands were the object of a previous study comparing the yields of the Spanish commercial cigarettes. More details can be found in the paper published elsewhere [22]. Table 2 shows the more important design characteristics available of these cigarettes. All the filters were cellulose acetate tips.
Table 2.
Design data of the commercial cigarette brands used.
| Cigarette brand | Type of blend | Weight of tobacco per cigarette (mg/cigarette) | Filter length (mm) | Filter weight (mg/cigarette) | Paper length (mm) | Paper weight (mg/cigarette) |
|---|---|---|---|---|---|---|
| A | American Blend | 660 | 19.9 | 120 | 57.3 | 41 |
| B | 605 | 26.7 | 157.8 | 50.3 | 35.6 | |
| C | American Blend | 605 | 20.8 | 114.1 | 57.4 | 39.4 |
| D | American Blend | 684 | 21.2 | 126.9 | 58.7 | 43.8 |
| E | 673 | 21.1 | 120.8 | 58.7 | 40.3 | |
| F | American Blend | 677 | 21 | 117.7 | 59.7 | 42.4 |
| G | American Blend | 698 | 21.1 | 114 | 59.2 | 41.7 |
| H | European Blend | 718 | 22.2 | 116.7 | 56.6 | 35.5 |
| I | Turkish and Virginia | 696 | 21.1 | 120.4 | 59.3 | 42.9 |
| J | American Blend | 690 | 21 | 122.4 | 59.6 | 40.7 |
In order to allow the adequate comparison, 200 cigarettes of each of the ten brands considered were emptied and disassembled, and filters and papers were weighed separately. The mixtures tobacco + additive were prepared by manually mixing the required amount of powdered additive with the amount of tobacco contained in each cigarette to make a mixture of 4% mass of additive. 0.1 g of ethanol (99.9%. AnalaR NORMAPUR, from Prolabo) were added to wet the tobacco and assist in mixing the tobacco with the additive. Ethanol was evaporated prior to the refilling of the cigarettes. All the experiments were triplicated and Table 3 shows the average mass fraction of additive in the mixtures studied among other parameters. The refilled cigarettes were kept at 23 °C and 60% relative humidity for at least 48 h.
Table 3.
CL(%), WTS, CO, TPM(F + T), TPN. N(F + T), N, ASH, Liq(F + T) and TG in all the smoking experiments carried out. All the parameters, except CL are expressed in mg/cigarette.
| Sample | CL | WTS | CO | TPM(F + T) | TPM | N(F + T) | N | ASH | TG | Liq(F + T) |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.0 | 529.6 | 8.5 | 10.0 | 6.34 | 0.96 | 0.61 | 90.70 | 1.96 | 1.55 |
| B | 0.0 | 488.7 | 5.4 | 10.9 | 5.00 | 0.58 | 0.28 | 84.50 | 1.61 | 1.00 |
| C | 0.0 | 579.9 | 10.8 | 22.5 | 11.16 | 1.27 | 0.61 | 101.60 | 3.23 | 2.30 |
| D | 0.0 | 486.7 | 7.8 | 9.2 | 5.51 | 0.77 | 0.42 | 80.50 | 2.25 | 1.33 |
| E | 0.0 | 495.7 | 8.3 | 9.0 | 5.84 | 0.92 | 0.48 | 83.30 | 2.55 | 1.56 |
| F | 0.0 | 499.0 | 6.9 | 8.0 | 5.18 | 0.98 | 0.56 | 85.00 | 2.10 | 1.57 |
| G | 0.0 | 466.0 | 6.5 | 8.4 | 3.90 | 0.58 | 0.35 | 75.00 | 1.81 | 0.93 |
| H | 0.0 | 480.7 | 9.0 | 8.6 | 4.53 | 0.72 | 0.39 | 79.40 | 1.89 | 1.14 |
| I | 0.0 | 506.4 | 6.5 | 6.8 | 4.06 | 0.70 | 0.41 | 80.00 | 2.12 | 1.10 |
| J | 0.0 | 491.1 | 7.4 | 9.3 | 5.50 | 0.87 | 0.48 | 82.30 | 2.11 | 1.40 |
| Av | 0.0 | 502.4 | 7.6 | 10.3 | 5.70 | 0.84 | 0.46 | 84.22 | 2.16 | 1.39 |
| Min | 0.0 | 465.8 | 5.0 | 6.8 | 3.90 | 0.58 | 0.28 | 75.00 | 1.61 | 0.93 |
| Max | 0.0 | 579.9 | 10.8 | 22.5 | 11.16 | 1.27 | 0.61 | 101.60 | 3.23 | 2.30 |
| A + HUSY | 3.8 | 541.6 | 9.4 | 12.9 | 7.60 | 1.00 | 0.60 | 123.80 | 2.71 | 1.84 |
| B + HUSY | 3.8 | 455.8 | 6.2 | 10.4 | 5.18 | 0.58 | 0.31 | 107.30 | 1.66 | 1.03 |
| C + HUSY | 3.7 | 542.2 | 10.0 | 14.2 | 7.37 | 0.85 | 0.46 | 125.30 | 2.98 | 1.66 |
| D + HUSY | 3.9 | 506.6 | 8.1 | 9.2 | 5.55 | 0.74 | 0.39 | 111.30 | 1.98 | 1.34 |
| E + HUSY | 4.0 | 470.8 | 6.3 | 7.2 | 4.45 | 0.72 | 0.39 | 99.80 | 2.34 | 1.24 |
| F + HUSY | 3.5 | 469.6 | 7.2 | 6.8 | 4.10 | 0.74 | 0.41 | 94.20 | 2.72 | 1.27 |
| G + HUSY | 3.9 | 508.2 | 5.4 | 6.8 | 4.87 | 0.48 | 0.29 | 99.80 | 1.68 | 0.82 |
| H + HUSY | 3.9 | 415.7 | 9.4 | 8.2 | 3.76 | 0.58 | 0.31 | 86.80 | 1.67 | 0.96 |
| I + HUSY | 3.8 | 461.0 | 6.8 | 6.8 | 3.83 | 0.59 | 0.34 | 96.10 | 1.87 | 1.00 |
| J + HUSY | 3.9 | 497.1 | 8.2 | 10.4 | 5.06 | 0.75 | 0.43 | 108.40 | 2.52 | 1.24 |
| Av (HUSY) | 486.9 | 7.7 | 9.3 | 5.18 | 0.70 | 0.39 | 105.28 | 2.21 | 1.24 | |
| Min (HUSY) | 415.7 | 5.4 | 6.8 | 3.76 | 0.48 | 0.29 | 86.81 | 1.66 | 0.82 | |
| Max (HUSY) | 542.2 | 10.0 | 14.2 | 7.60 | 1.00 | 0.60 | 125.26 | 2.98 | 1.84 | |
| A + NaY | 3.8 | 565.8 | 10.4 | 12.0 | 4.10 | 1.13 | 0.64 | 128.30 | 3.56 | 1.98 |
| B + NaY | 4.0 | 473.6 | 7.4 | 11.1 | 5.92 | 0.80 | 0.42 | 109.70 | 2.04 | 1.41 |
| C + NaY | 3.8 | 526.8 | 7.9 | 12.8 | 6.24 | 0.80 | 0.39 | 110.80 | 2.72 | 1.44 |
| D + NaY | 4.0 | 476.7 | 8.2 | 9.9 | 5.76 | 0.85 | 0.48 | 108.60 | 2.20 | 1.48 |
| E + NaY | 4.0 | 500.5 | 8.5 | 10.9 | 5.44 | 0.94 | 0.50 | 108.10 | 2.48 | 1.54 |
| F + NaY | 3.5 | 482.4 | 7.1 | 8.6 | 4.90 | 0.86 | 0.48 | 99.80 | 2.05 | 1.40 |
| G + NaY | 3.8 | 471.3 | 4.9 | 8.8 | 5.74 | 0.53 | 0.34 | 101.90 | 1.77 | 0.91 |
| H + NaY | 3.7 | 452.2 | 10.4 | 8.3 | 3.58 | 0.60 | 0.31 | 95.30 | 2.04 | 0.97 |
| I + NaY | 3.9 | 499.4 | 6.8 | 8.2 | 3.93 | 0.72 | 0.38 | 103.90 | 2.16 | 1.12 |
| H + NaY | 3.7 | 452.2 | 10.4 | 8.3 | 3.58 | 0.60 | 0.31 | 95.30 | 2.16 | 1.40 |
| Av (NaY) | 494.8 | 8.0 | 10.2 | 5.11 | 0.81 | 0.44 | 107.88 | 2.32 | 1.37 | |
| Min (NaY) | 452.2 | 4.9 | 8.2 | 3.58 | 0.53 | 0.31 | 95.33 | 1.77 | 0.91 | |
| Max (NaY) | 565.8 | 10.4 | 12.8 | 6.24 | 1.13 | 0.64 | 128.30 | 3.56 | 1.98 | |
| A + Al-MCM-41 | 3.7 | 487.4 | 7.2 | 8.9 | 5.81 | 0.38 | 0.19 | 108.90 | 1.78 | 1.26 |
| B + Al-MCM-41 | 4.0 | 465.4 | 4.8 | 9.7 | 4.97 | 0.52 | 0.21 | 110.00 | 1.60 | 0.76 |
| C + Al-MCM-41 | 3.8 | 487.4 | 7.0 | 16.0 | 6.96 | 0.88 | 0.37 | 129.00 | 2.88 | 1.62 |
| D + Al-MCM-41 | 3.8 | 441.1 | 7.0 | 7.1 | 4.09 | 0.52 | 0.24 | 106.20 | 1.94 | 0.87 |
| E + Al-MCM-41 | 3.8 | 507.6 | 7.2 | 8.2 | 5.31 | 0.75 | 0.40 | 114.70 | 2.96 | 1.43 |
| F + Al-MCM-41 | 3.8 | 462.7 | 5.5 | 5.0 | 3.19 | 0.50 | 0.26 | 109.70 | 1.41 | 0.89 |
| G + Al-MCM-41 | 3.8 | 446.7 | 4.1 | 5.3 | 3.91 | 0.42 | 0.25 | 97.50 | 1.48 | 0.71 |
| H + Al-MCM-41 | 3.7 | 430.9 | 7.0 | 6.3 | 3.01 | 0.37 | 0.20 | 91.90 | 1.73 | 0.59 |
| I + Al-MCM-41 | 3.8 | 465.7 | 5.4 | 5.8 | 3.16 | 0.43 | 0.25 | 94.30 | 1.93 | 0.76 |
| J + Al-MCM-41 | 3.8 | 460.1 | 6.0 | 7.9 | 4.53 | 0.55 | 0.26 | 98.00 | 2.05 | 0.96 |
| Av (Al-MCM-41) | 465.5 | 6.1 | 8.0 | 4.49 | 0.53 | 0.26 | 106.02 | 1.98 | 0.99 | |
| Min(Al-MCM-41) | 430.9 | 4.1 | 5.0 | 3.01 | 0.37 | 0.19 | 91.94 | 1.41 | 0.59 | |
| Max(Al-MCM-41) | 507.6 | 7.2 | 16.0 | 6.96 | 0.88 | 0.40 | 129.00 | 2.96 | 1.62 | |
2.3. Smoking experiments
Five cigarettes were simultaneously smoked in each run and at least three runs were carried out for each cigarette brand. The smoking regimen was selected according to the specifications of the ISO 3308 standard, with the difference that, as in the previous study and for the same reasons commented therein, 8 puffs were always taken. Condensed products from the MSS were collected in a 44 mm Cambridge Filter Pad (CFP) located between the mouth end of the cigarettes and the smoking machine. The CFP and the filters of the cigarettes were extracted separately with isopropanol (99.9% purity from Fluka) and analyzed by GC/MS. After passing through the filter and the CFP, the smoke was collected in a Tedlar bag and appears throughout the text as “gas fraction”.
According to the ISO 4387, total particulate matter (TPM) and nicotine (N) refer to that collected in the CFP traps. In this work, in order to properly evaluate the additives effect, the particulate matter condensed in the filters of the cigarettes has also been quantified and analyzed. Results are presented as TPM for the particulate matter condensed in the CFP traps and TPM(F + T) which indicates the total amount of TPM contained in the smoke, i.e., that condensed in the filters of the cigarettes plus that condensed in the CFP traps located after the filters. TPM(F + T) is not commonly reported since it is partially retained in the filters, but it is interesting to analyze it to better evaluate the effect of the additive. Nicotine and other components of the particulate matter are also presented maintaining the same nomenclature; N(F + T), corresponds to the amount of nicotine collected in the filters of the cigarettes plus that in the traps. The weight of tobacco smoked (WTS) was calculated as the difference between the weight of tobacco per cigarette (WTC) before and after smoking. The amount ASH corresponds to the total mass of ash collected and expressed on an additive free basis (taking into account the WTS, the initial WTC and the weight fraction of additive per cigarette).
2.4. Analytical procedure
In this work 80 compounds are reported in the case of the TPM and 32 in the case of the gas fraction. The analytical procedure was explained elsewhere [22]. As explained there in, response factors for nicotine in the TPM and CO, 1,3-butadiene, HCN, isoprene, acrolein, propionaldehyde, crotonaldehyde, benzene, toluene and acetaldehyde in the gas fraction were obtained. Consequently, results are semi-quantitative. Standard deviations in the three replicate runs lower than 25% for all the compounds analyzed were obtained. The results of the analysis of the gas fraction by FID for one of the brands are shown in Table 4, while those of the particulate matter carried out by GC/MS are in Table 5. The sum of all the compounds identified and quantified in the gas fraction by FID has been named as TG (in Table 3) and that of the compounds from the TPM(F + T) analyzed by GC/MS appears in the following as Liq(F + T) (Table 3).
Table 4.
Yield of the compounds analyzed in brand F in the gas fraction with and without the additives (μg/cigarette) and adscription of the compounds to families.
| Gases | |||||
|---|---|---|---|---|---|
| Asignation | Families | F | F-HUSY | F-NaY | F-Al-MCM-41 |
| Methane | AL | 491.92 | 492.73 | 484.64 | 341.81 |
| Ethane | AL | 201.71 | 193.16 | 192.82 | 136.59 |
| Ethylene | AL | 114.75 | 109.48 | 107.77 | 76.18 |
| ethine | AL | 16.43 | 14.39 | 14.13 | 10.03 |
| Propane | AL | 93.39 | 86.92 | 88.87 | 61.47 |
| Propeylene | AL | 107.73 | 104.45 | 103.09 | 73.02 |
| Iso-butane | AL | 8.47 | 7.58 | 7.92 | 5.02 |
| Chloromethane | OT | 21.96 | 19.58 | 20.01 | 14.86 |
| Butane | AL | 28.56 | 26.30 | 27.07 | 17.79 |
| 1-Butene | AL | 26.31 | 23.84 | 24.18 | 16.49 |
| 1,2-Propadiene | AL | 7.02 | 5.70 | 5.11 | 4.16 |
| 1,3-Butadiene | AL | 15.15 | 14.83 | 14.68 | 10.32 |
| Isobutene | AL | 24.39 | 23.50 | 23.84 | 17.33 |
| cis-2-Butene | AL | 13.45 | 18.05 | 18.47 | 11.24 |
| Pentane | AL | 8.39 | 7.24 | 7.75 | 5.43 |
| Methanethiol | OT | 10.73 | 9.87 | 10.81 | 4.62 |
| HCN | OT | 5.53 | 5.36 | 5.28 | 3.92 |
| 1-Pentene | AL | 6.98 | 6.30 | 7.49 | 4.26 |
| Furane | AR | 9.82 | 9.24 | 8.85 | 9.30 |
| Isohexane | AL | 20.73 | 19.32 | 20.52 | 12.34 |
| Isoprene | AL | 107.35 | 120.97 | 102.24 | 85.71 |
| Hexane | AL | 4.85 | 7.32 | 6.21 | 5.31 |
| 1-Hexene | AL | 2.00 | 1.70 | 1.53 | 0.00 |
| Benzene | AR | 68.95 | 62.08 | 64.34 | 46.03 |
| Acetaldehyde | CA | 314.53 | 300.07 | 304.59 | 194.02 |
| Acroleine | CA | 18.30 | 14.64 | 10.30 | 16.49 |
| Propionaldehyde | CA | 14.94 | 13.96 | 8.26 | 12.28 |
| Acetonitrile | OT | 55.78 | 48.68 | 42.26 | 43.83 |
| Toluene | AR | 14.30 | 14.00 | 11.36 | 8.65 |
| 2,5-Dimethylfurane | AR | 5.36 | 4.60 | 3.66 | 3.35 |
| Crotonaldehyde | CA | 2.98 | 2.30 | 2.30 | 1.96 |
| Isobutiraldehyde | CA | 4.60 | 6.55 | 3.41 | 4.15 |
Table 5.
Yield of the compounds analyzed in brand F in the filters and CFP with and without the additives (μg/cigarette) and adscription of the compounds to families.
| CFP |
FILTERS |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Asignation | Families | F | F-HUSY | F-NaY | F-Al-MCM-41 | F | F-HUSY | F-NaY | F-Al-MCM-41 |
| Pyridine, 4-methyl- | NI | 0.00 | 0.00 | 0.56 | 0.00 | 3.05 | 2.77 | 2.15 | 0.73 |
| Pyrazine, methyl- | NI | 0.36 | 0.36 | 0.56 | 0.06 | 2.77 | 2.47 | 2.28 | 0.85 |
| Furfural | CA | 1.76 | 1.71 | 3.32 | 1.06 | 24.43 | 19.53 | 17.32 | 7.63 |
| 2-Pentanone, 4-hydroxy-4-methyl- | CA | 1.12 | 1.05 | 1.22 | 1.00 | 1.86 | 2.45 | 2.09 | 1.60 |
| Ethanol, 2-(1-methylethoxy)- | OT | 5.09 | 3.64 | 3.66 | 2.49 | 4.57 | 2.99 | 3.77 | 3.60 |
| 2-Furanmethanol | EP | 0.00 | 0.00 | 0.00 | 0.00 | 6.49 | 6.70 | 5.58 | 2.55 |
| Pyridine, 3-methyl- | NI | 0.23 | 0.30 | 0.61 | 0.00 | 6.62 | 9.65 | 5.21 | 2.50 |
| 2-Propanone, 1-(acetyloxy)- | CA | 0.48 | 0.32 | 0.73 | 0.26 | 10.47 | 7.76 | 7.74 | 3.74 |
| 4-Cyclopentene-1,3-dione | CA | 1.27 | 0.96 | 1.68 | 1.12 | 9.47 | 7.46 | 7.42 | 8.48 |
| Styrene | AR | 1.03 | 0.48 | 0.92 | 0.19 | 2.36 | 1.94 | 1.49 | 0.00 |
| 2-Cyclopenten-1-one, 2-methyl- | CA | 0.70 | 0.68 | 1.13 | 0.56 | 8.95 | 7.98 | 6.71 | 3.32 |
| 2-Acetylfuran | CA | 0.40 | 0.23 | 0.63 | 0.50 | 4.74 | 4.98 | 4.19 | 1.36 |
| 2(5H)-furanone | CA | 0.69 | 1.23 | 1.14 | 0.58 | 4.97 | 5.43 | 4.27 | 2.09 |
| Pyrazine, 2,3-dimethyl- | NI | 0.00 | 0.00 | 0.00 | 0.00 | 1.33 | 1.07 | 0.97 | 0.64 |
| 2-Hydroxycyclopent-2-en-1-one | CA | 0.98 | 0.83 | 0.71 | 0.50 | 5.02 | 3.99 | 4.02 | 2.23 |
| Pyridine, 3,5-dimethyl- | NI | 0.00 | 0.00 | 0.00 | 0.00 | 0.96 | 1.15 | 0.66 | 0.00 |
| 2,5-Dimethyl-2-cyclopentenone | CA | 0.00 | 0.00 | 0.00 | 0.00 | 0.61 | 1.20 | 0.79 | 0.25 |
| 2(3H)-furanone, 5-methyl- | CA | 0.00 | 0.00 | 0.00 | 0.00 | 0.98 | 0.65 | 0.88 | 0.40 |
| Butanoic acid, 3-methyl- | OT | 0.90 | 0.60 | 0.56 | 0.41 | 0.36 | 0.86 | 0.53 | 0.19 |
| Ethanol, 2-butoxy- | OT | 1.56 | 1.19 | 1.39 | 0.80 | 2.43 | 1.75 | 2.15 | 0.82 |
| Benzaldehyde | CA | 0.55 | 0.45 | 0.64 | 0.36 | 2.59 | 2.22 | 2.46 | 1.36 |
| Furfural, 5-methyl- | CA | 0.82 | 0.81 | 1.15 | 0.60 | 14.01 | 11.89 | 11.35 | 6.06 |
| Pyridine, 3-ethenyl- | NI | 0.00 | 0.00 | 0.00 | 0.00 | 3.30 | 4.16 | 2.64 | 3.58 |
| 2(5H)-Furanone, 3-methyl- | CA | 0.00 | 0.00 | 0.00 | 0.00 | 3.48 | 2.64 | 2.82 | 2.18 |
| Phenol | PHE | 5.28 | 4.44 | 5.06 | 3.04 | 23.57 | 21.38 | 21.64 | 11.76 |
| 2-isopropylfuran | EP | 0.00 | 0.00 | 0.00 | 0.00 | 3.01 | 2.21 | 1.60 | 0.79 |
| 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | CA | 1.77 | 1.26 | 1.51 | 0.71 | 9.10 | 7.69 | 7.01 | 3.56 |
| Limonene | OT | 0.89 | 0.82 | 0.51 | 0.38 | 4.08 | 4.37 | 2.75 | 0.86 |
| 2,3-Dimethyl-2-cyclopenten-1-one | CA | 0.88 | 1.00 | 0.74 | 0.00 | 5.25 | 4.70 | 4.16 | 2.16 |
| Indeno | PAH | 0.42 | 0.45 | 0.43 | 0.30 | 3.89 | 2.50 | 2.30 | 1.84 |
| o-Cresol | PHE | 2.11 | 1.53 | 2.03 | 0.99 | 7.86 | 7.03 | 7.56 | 3.70 |
| 2-Acetylpyrrole | NI | 0.00 | 0.00 | 0.00 | 0.00 | 2.30 | 1.41 | 1.65 | 1.58 |
| Phenol, 4-methoxy- | PHE | 0.00 | 0.00 | 0.00 | 0.00 | 1.92 | 1.48 | 1.66 | 0.92 |
| Ethanone, 1-phenyl- | CA | 0.00 | 0.00 | 0.00 | 0.00 | 1.73 | 1.28 | 1.15 | 0.90 |
| p-Cresol | PHE | 5.30 | 4.06 | 4.70 | 2.43 | 15.46 | 13.00 | 14.03 | 8.35 |
| 2 ethyl tiophene | OT | 1.52 | 1.25 | 1.49 | 0.69 | 1.46 | 1.54 | 1.58 | 0.95 |
| Phenol, 2-methoxy- | PHE | 1.27 | 0.96 | 1.25 | 0.49 | 7.73 | 6.42 | 5.92 | 3.39 |
| 2-Propanamine | NI | 3.72 | 2.52 | 2.76 | 1.73 | 5.75 | 4.97 | 5.12 | 4.00 |
| 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | CA | 1.40 | 0.83 | 0.84 | 0.85 | 3.51 | 2.60 | 3.10 | 2.59 |
| Benzeneacetonitrile | NI | 0.00 | 0.00 | 0.00 | 0.00 | 2.89 | 2.70 | 2.93 | 1.86 |
| 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | CA | 7.04 | 4.89 | 5.40 | 3.14 | 7.73 | 6.59 | 7.06 | 5.05 |
| Phenol, 2,4-dimethyl- | PHE | 1.81 | 0.87 | 3.24 | 0.95 | 4.71 | 4.94 | 5.19 | 2.40 |
| Phenol, 4-ethyl- | PHE | 2.62 | 1.60 | 1.79 | 1.53 | 3.30 | 3.28 | 2.95 | 3.94 |
| Naphthalene | PAH | 0.00 | 0.00 | 0.00 | 0.00 | 1.46 | 1.21 | 1.04 | 0.83 |
| Ethanone, 1-(3-methylphenyl)- | CA | 0.00 | 0.00 | 0.00 | 0.00 | 2.94 | 2.46 | 2.68 | 2.22 |
| p-cresol 2 methoxy | PHE | 0.00 | 0.00 | 0.00 | 0.00 | 3.91 | 3.77 | 3.36 | 2.72 |
| 2,3-Dihydro-benzofuran | EP | 3.08 | 2.82 | 2.61 | 2.00 | 3.57 | 3.26 | 2.92 | 3.89 |
| 2-furancarboxaldehyde, 5-(hydroxymethyl)- | CA | 4.86 | 3.59 | 3.11 | 1.66 | 4.69 | 3.99 | 4.07 | 2.83 |
| 1H-Inden-1-one, 2,3-dihydro- | CA | 0.85 | 0.53 | 0.88 | 0.00 | 2.24 | 1.97 | 1.77 | 0.00 |
| Hydroquinone | PHE | 10.85 | 9.11 | 10.91 | 5.99 | 3.80 | 5.58 | 5.86 | 1.95 |
| 1H-Indole | NI | 4.11 | 4.03 | 4.21 | 2.12 | 8.79 | 8.55 | 9.80 | 4.35 |
| 4-vinyl-2-methoxy-phenol | PHE | 3.51 | 1.93 | 1.99 | 1.58 | 4.45 | 4.51 | 3.36 | 2.56 |
| Nicotine | NI | 557.00 | 412.14 | 478.76 | 258.16 | 418.10 | 326.72 | 382.64 | 242.78 |
| 1H-Indole, 3-methyl- | NI | 2.62 | 2.50 | 1.97 | 1.17 | 2.78 | 2.78 | 3.02 | 2.18 |
| Myosmine | NI | 4.66 | 4.20 | 4.39 | 3.46 | 4.87 | 4.36 | 4.55 | 3.40 |
| Phenol, 2-methoxy-4-(2-propenyl)- | PHE | 2.78 | 2.75 | 2.17 | 1.03 | 1.79 | 1.68 | 1.31 | 0.68 |
| Nicotyrine | NI | 1.66 | 1.17 | 1.58 | 2.23 | 2.15 | 2.14 | 1.73 | 1.68 |
| Norsolanadiona | CA | 2.14 | 1.63 | 1.87 | 1.64 | 2.45 | 1.69 | 1.76 | 2.97 |
| 2,3′-Bipyridine | NI | 3.59 | 3.32 | 3.19 | 2.22 | 3.29 | 2.74 | 3.13 | 2.58 |
| 1,4-dihydrophenantrhene | PAH | 3.56 | 3.12 | 2.96 | 2.93 | 0.73 | 2.08 | 1.19 | 0.42 |
| Megastigmatrienone | CA | 1.48 | 0.90 | 2.82 | 0.00 | 1.72 | 1.93 | 1.14 | 1.16 |
| N-propyl-nornicotine | NI | 2.58 | 1.41 | 1.91 | 2.00 | 1.01 | 1.26 | 1.08 | 0.85 |
| Cotinine | NI | 6.24 | 5.11 | 5.49 | 2.99 | 4.07 | 4.25 | 3.99 | 2.04 |
| 1H-Indene, 2,3-dihydro-1,1,3-trimethyl-3-phenyl- | AR | 2.99 | 2.56 | 2.90 | 3.49 | 0.00 | 0.00 | 0.00 | 0.00 |
| 5-Tetradecene | AL | 1.35 | 1.11 | 1.36 | 0.78 | 1.68 | 1.38 | 1.80 | 1.11 |
| N(b)-formylnornicotine | NI | 5.31 | 3.55 | 4.25 | 3.87 | 1.80 | 1.36 | 1.50 | 0.63 |
| 2,4-Diphenyl-4-methyl-penten-1ene | AR | 5.31 | 5.43 | 5.66 | 3.19 | 0.00 | 0.00 | 0.00 | 0.00 |
| NEOPHYTADIENE (pentadeceno…) | AL | 27.91 | 25.12 | 26.29 | 15.16 | 31.38 | 29.62 | 28.73 | 22.18 |
| Farnesol | OT | 2.90 | 2.43 | 4.04 | 1.99 | 4.12 | 3.74 | 3.42 | 2.90 |
| 8-Quinolinemethanol | NI | 0.72 | 0.89 | 0.86 | 0.51 | 2.07 | 3.25 | 4.36 | 9.08 |
| Hexadecanoic acid, ethyl ester | OT | 2.82 | 2.05 | 2.62 | 1.94 | 1.52 | 2.50 | 2.94 | 1.15 |
| Eicosane | AL | 1.70 | 1.54 | 1.38 | 1.18 | 0.00 | 1.21 | 1.64 | 0.00 |
| pentadecane | AL | 1.09 | 0.86 | 1.28 | 0.90 | 1.28 | 1.50 | 1.29 | 0.90 |
| Docosano | AL | 3.38 | 3.75 | 3.30 | 4.20 | 4.78 | 3.43 | 4.34 | 4.66 |
| Tricosane | AL | 8.16 | 7.33 | 5.98 | 4.48 | 4.50 | 7.22 | 4.62 | 4.05 |
| 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl- | AL | 4.47 | 2.75 | 2.73 | 30.53 | 2.76 | 5.63 | 2.50 | 13.07 |
| Heptacosane | AL | 6.83 | 5.30 | 6.45 | 3.91 | 4.78 | 5.32 | 4.29 | 3.55 |
| Triacontane | AL | 6.43 | 4.81 | 5.66 | 3.78 | 4.71 | 3.43 | 6.43 | 3.30 |
| Octadecane | AL | 15.29 | 13.03 | 13.59 | 10.23 | 9.70 | 9.58 | 9.52 | 6.99 |
| Tocopherol | PHE | 7.12 | 4.77 | 4.63 | 5.62 | 3.26 | 3.08 | 4.10 | 2.32 |
3. Results
3.1. Analysis of the general trends
Table 3 shows the results obtained for the average mass fraction of additive loaded (CL), the WTS, TPM(F + T), TPM, N(F + T), Liq(F + T), TG, and ASH, for the ten commercial brands when no additive was used, and when the three additives were included. The average (Av), minimum (min) and maximum (Max) values of the variables for each set of experiments has also been included in order to facilitate comparisons. The difference between the amounts TPM(F + T) and TPM, and N(F + T) and N is the amount of compounds condensed in the filter of the cigarettes, which varies depending mainly on the length and characteristics of the filters, but also on the amount of TPM(F + T). In all cases approximately half of nicotine and particulate matter reaching the filters is retained on them. The importance of the filters in reducing the harm effects of tobacco smoke on primary smokers is once more highlighted.
A relatively wide dispersion among brands of the studied parameters (Table 3) can be observed in spite of, as commented [22], comparing the results of these cigarettes brands with those for international brands from other markets, nicotine is in the low to medium range, and CO in the medium to high range.
When the additives are introduced, WTS for a fixed number of puffs tends to be reduced as a consequence of the different packing. If the WTS is reduced, the yield of the compounds analyzed is also expected to be reduced, but there is also an important reduction due to the additive action itself, as shown below.
The effect of the additives can be clearly observed if the reduction percentages (xr) are analyzed. Reduction percentages have been calculated as follows:
where xcat is the yield of a given compound, group of compounds obtained in presence of an additive, and x is the yield of the same when no additive was added. Consequently, negative values represent an increase of yields with respect to those obtained when smoking the cigarettes without additive.
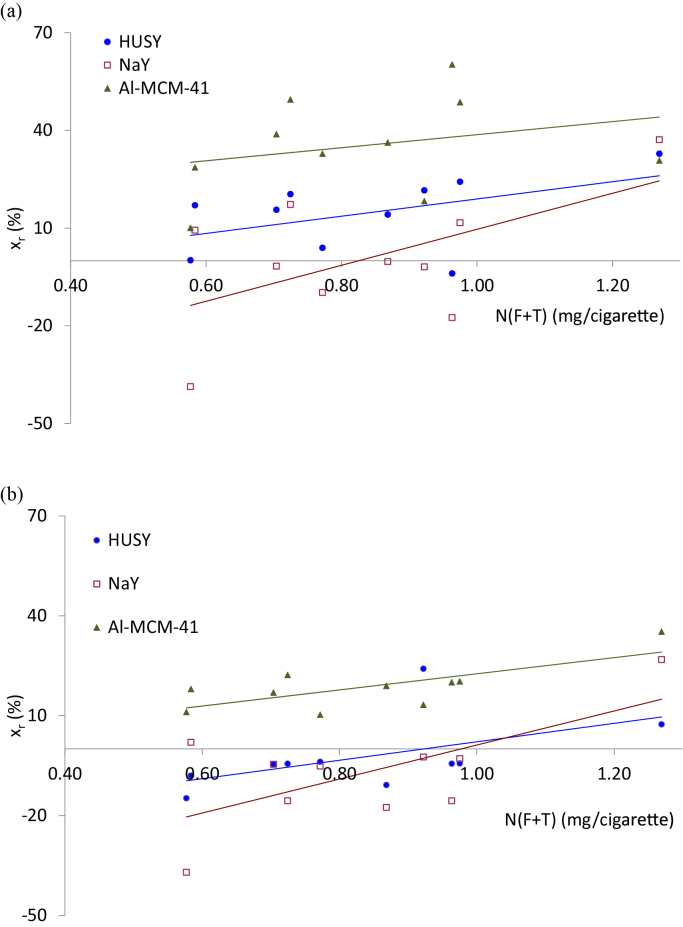
Fig. 1a and b shows the reduction percentage in N(F + T) and CO for all the cigarettes with the three additives, as a function of the N(F + T) yield when smoking each cigarette without additive. It can be observed that the data correspond to three lines of positive slope, showing the positive correlation among the greater ability of the additives in reducing nicotine and CO yields when tobacco yields higher amounts of nicotine (within the range studied). For all the variables studied, except for ASH, a positive correlation has been obtained for the reduction of the variable with the nicotine and TPM yields, similar to that observed in Fig. 1a and b. In general, the larger the nicotine or tar yields, the larger the reductions attained of any particular smoke constituent when the additives were introduced. It is relatively frequent to assume that if a cigarette yields more tar than other it is more toxic, and many authors support this hypothesis [2]. In fact, most of the current Regulations on tobacco smoke are still limiting CO, nicotine and tar content in the smoke evolved per cigarette. CO because is a well-known poison and the more abundant toxicant component, nicotine because is responsible for addiction and the more abundant toxic component present in the TPM and tar, which includes all the components of the TPM excluding water and nicotine. According to the above mention results, the addition of these catalysts to the tobacco would aid to reduce CO, tar and nicotine, and the greater the nicotine or tar yield the greater the reduction attained. In this sense, they could be considered as toxic reducers. When comparing the results for the three additives in Fig. 1a and b, Al-MCM-41 is by far the best one, showing always positive reductions, and followed by HUSY and NaY. With the latter, the reductions observed are negative in most cases (increase of the yield) but it behaves better than HUSY as the N(F + T) yield increases. The main ability of these mesoporous materials, such as Al-MCM-41, to reduce the yield of most compounds in MSS is demonstrated.
Fig. 1.
Reductions obtained in (a) N(F + T) and (b) CO versus N(F + T) yields.
By the other hand, the last recommendations on Tobacco Regulations proposed by the WHO [29] were trying to promote laws limiting the content in smoke of some specific toxics, especially the tobacco specific nitrosamines (TSNA), which are well-known strong carcinogens. For different reasons these recommendations are still not being applied in the different Regulations on tobacco smoke. In this mean, Lin et al. [15], [16] showed the ability of NaY and specially MCM-41, among other catalysts, to reduce TSNA in tobacco smoke.
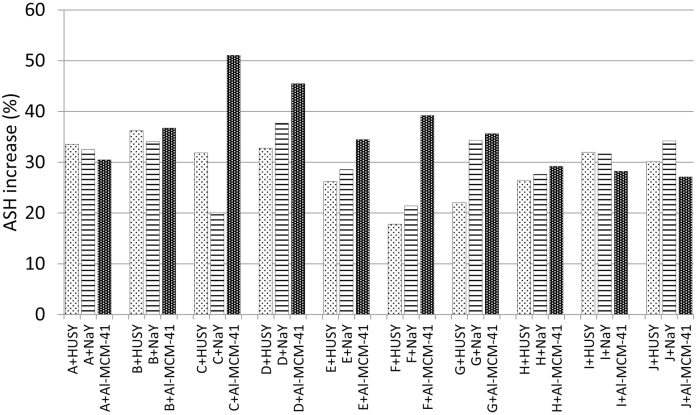
As shown in Table 3, ASH is the only single parameter with an increasing trend when the additives are added to the cigarette rod. Fig. 2 shows the increase in ASH calculated as the difference between the following ratios; ASH in the smoking experiment with additive to the WTS and ASH when there was no additive to the corresponding WTS and expressed in mass percentage. It can be observed that the Al-MCM-41 is the one showing the largest increases of such solid residue with almost all brands, followed by the NaY and the HUSY. This increase is due to coke deposited on the material and must be related to the reduction observed in the yields of some compounds, as was proved in a previous paper [19]. Nevertheless, this correlation is not straightforward due to the large number of factors influencing the behaviour of the different systems in the pyrolysis and oxidation reactions. Factors such as the type of paper, its permeability, the number of ventilation holes in the filter, the type of tobacco, the type of material, the temperatures of the processes, etc. are affecting the final results [1], [3].
Fig. 2.
ASH increase for the different brands with the additives.
The effect of these additives on the brands studied is quite different. In order to simplify the analysis, the reductions calculated are shown with more detail only for the best material, Al-MCM-41, and the 10 brands (Table 6). The addition of the catalysts may affect packing of the tobacco into the cigarettes rod, and consequently, to the oxygen permeability, to the temperature profiles during smoking [25] and to the yield of most compounds [22]. As commented above, if the amount of tobacco smoked is less, the yield of any compound is expected to be reduced accordingly. Nevertheless, as a consequence of the presence of Al-MCM-41, some compounds show important changes, not only due to the lower WTS. The WTS is reduced by an average of 7.2% through the introduction of powdered Al-MCM-41, while the other variables shown in Table 6 are reduced in larger proportions. For example, Liq(F + T) is reduced by an average of 29.2% by Al-MCM-41. The reductions in the gas fraction are lower than those in the liquid fraction, but are still higher than the reduction in WTS. The larger reduction of the compounds which form the condensed fraction of the smoke can be attributed to some extent to the catalytic action, as described by Lin et al. [15], [16] and Marcilla et al. [19], [20]. The compounds contained in the particulate matter of the smoke could eventually collide with the catalyst surface spread on the tobacco. These compounds may be retained by the material or rebound or remain in the TPM which, any case, would give an important reduction in the amount of compounds in the TPM. Those compounds forming the gas fraction would not collide with the material in the same way, and would undergo lower reductions, mainly due to the reduction in WTS.
Table 6.
Reduction percentage obtained for the main variables considered by all the brands and Al-MCM-41.
| Sample | WTS | TPM(F + T) | Liq(F + T) | N(F + T) | CO | TG | ASH |
|---|---|---|---|---|---|---|---|
| A + Al-MCM-41 | 8.0 | 11.0 | 18.9 | 18.5 | 20.0 | 9.6 | 5.2 |
| B + Al-MCM-41 | 4.8 | 10.9 | 23.5 | 29.5 | 11.1 | 2.1 | 6.3 |
| C + Al-MCM-41 | 15.9 | 28.8 | 29.6 | 36.1 | 35.2 | 10.5 | 8.9 |
| D + Al-MCM-41 | 9.4 | 22.9 | 34.4 | 40.0 | 10.3 | 12.8 | 7.5 |
| E + Al-MCM-41 | −2.4 | 8.9 | 8.3 | 18.2 | 13.3 | 7.2 | 5.8 |
| F + Al-MCM-41 | 7.3 | 37.8 | 43.4 | 48.6 | 20.3 | 33.4 | 6.7 |
| G + Al-MCM-41 | 4.1 | 36.8 | 23.3 | 28.6 | 18.0 | 17.7 | 5.7 |
| H + Al-MCM-41 | 10.4 | 27.1 | 48.2 | 49.5 | 22.2 | 8.0 | 4.8 |
| I + Al-MCM-41 | 8.0 | 14.9 | 31.5 | 38.8 | 16.9 | 9.2 | 4.5 |
| J + Al-MCM-41 | 6.3 | 14.6 | 31.1 | 36.2 | 18.9 | 4.4 | 4.5 |
| Average | 7.2 | 21.4 | 29.2 | 34.4 | 18.6 | 11.5 | 6.0 |
By brands, brand C, which is the one yielding the major TPM(F + T), shows the main reduction of WTS (Table 6) with Al-MCM-41, while brand E shows a small increase of the WTS. On average, TPM(F + T) is reduced by 21.4% for all the brands. Brands F and G show the major reductions of TPM(F + T) (37.8 and 36.7%) while brands E, B and A show the lower reductions (8.9, 10.9, 11.0%, respectively). As can be seen, Liq(F + T) is on average more reduced (29.2%) than TPM(F + T) (21.4%). By brands, H and F are those showing the highest reductions (48.2 and 43.4%) and E and A the lowest (8.3 and 18.9%). Nicotine represents around 70% of the Liq(F + T) and by brands reductions attained in nicotine are very large; brands F and H (44.6 and 49.5%) are the main brands reducing nicotine and A and E the least (18.5 and 18.2%).
As mentioned before, the non-condensed fraction is less reduced than the compounds in the condensed fraction. The TG was reduced by an average of 11.5%, where the higher reduction is once more achieved for brand F (33.4%), while very low reductions are attained by B and J (2.1 and 4.4%, respectively). The reductions of CO for most of the brands are close to the average (18.6%), except for brand C which is the one showing the higher reduction. As commented above, CO is one of the most toxic compounds present in tobacco smoke and together with nicotine, its sealing content in tobacco smoke is regulated by law in most of countries. Summarizing, brands H and F are those showing the most important reductions in nicotine and other compounds which form the condensed fraction, and for CO it is brand C. The lowest reductions are for brands A and E in the condensed fraction and B in the non-condensed fraction.
According to the design characteristics of cigarettes provided in Table 2, brand B is the one with the shortest paper length and weight, while brand E has a very large paper length, and they both present a similar reduction of TPM(F + T) and nicotine. On the other hand, brand E is very similar to brand A in these features, and they both present extreme behaviour in the presence of the additives. Consequently, other important characteristics of the cigarettes, such as the tobacco type and composition, additives included during manufacturing, the paper additives and permeability, which are not specified by the tobacco companies, may affect their behaviour. In a previous paper [22] the composition of the smoke evolved from these tobacco cigarettes brands was studied and multivariant analysis was applied to establish relationships among the main features of the cigarette design and the smoke composition. It was shown as some of the variables considered, especially the WTC and also filter and paper length, play an important role in the smoking process. By brands the classification of the studied brands based on the chemical composition of the gas phase and the TPM revealed that brand C always appeared separated from the other brands, while brands G, H and I form a homogeneous group. Nevertheless, in this work, with the inclusion of the catalyst in the tobacco, the scene is much more complex and such relationships have not been found.
3.2. Analysis of individual components and by chemical families
Table 4 shows, as an example, the results of the gas fraction analyzed by GC/FID in the case of tobacco F, which is the one where the largest reductions were observed, while Table 5 shows the results for the compounds condensed in the filters and in the CFP, analyzed by GC/MS. The results obtained for the other brands are annexed as supplementary data. The distribution of the different compounds retained in the filters and in the CFP reveals that the filters seem to preferably retain the lighter components, whereas the heaviest are preferably retained in the CFP located thereafter. This trend was also observed in previous works [21], [22] and may be related to the vapour pressure of the different compounds, their affinity for the filter and the traps and their relative concentrations in addition to the pressure fluctuations during and between the puffs [4], [14]. In the following, the analysis of liquids is carried out on the sum of the yields obtained in filters plus traps, in order to better represent the additives action.
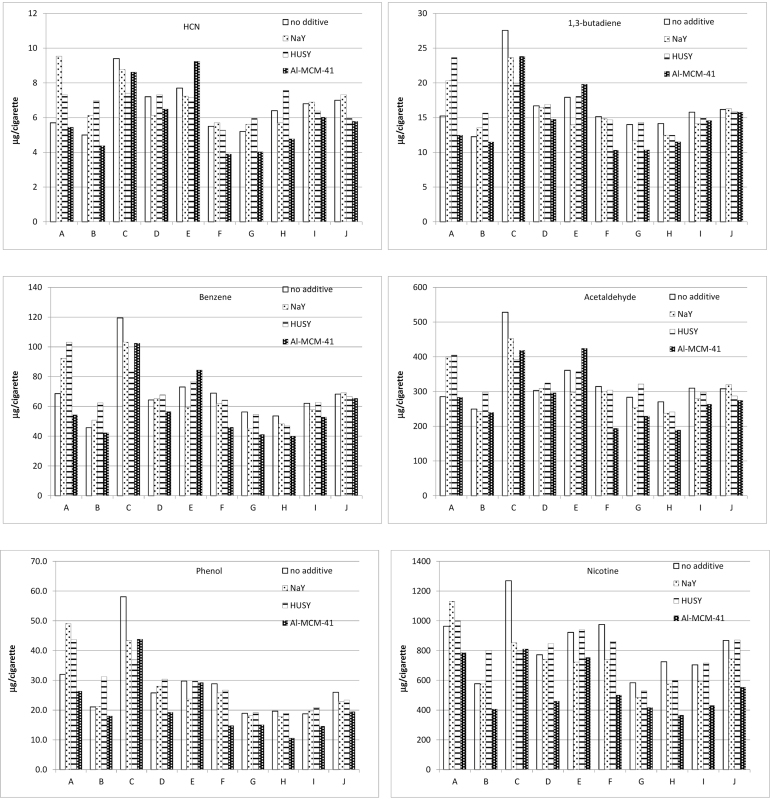
Fig. 3 shows the total yields obtained for HCN, 1,3-butadiene, benzene, acetaldehyde from the gas fraction and phenol and nicotine from the liquid fraction. These compounds have been selected because of their high toxicity, since all of them are included in the Hoffman and in the Canadian lists [13], [3], [29]. According to Fowles and Dybing [10], HCN is the smoke component presenting the highest index of cardiovascular effects, while 1,3-butadiene is the one showing the highest cancer risk index (CRI). Acetaldehyde and other small aldehyde molecules presents in the vapour phase are especially harmful, and they present both, high CRI and respiratory effects. Similarly benzene presents a high CRI and cardiovascular effects. Phenols are known to affect the respiratory and immune system. Nicotine apart from the high addictive effect has potential influence on cardiovascular diseases and reproductive system. The major effect of the catalysts on the compounds forming the condensed fraction can be observed once more. On the other hand, the reduction obtained depends strongly on the brand of cigarettes. The highest reductions are obtained for brands F and H. Al-MCM-41 is always the best, and the only one providing reductions of all the compounds and brands, to such extent that the yields obtained for some compounds in the CFP (the fraction that would be inhaled by the smoker) are below the detection limits (Table 5). NaY and HUSY do not reduce most of the compounds of brands A and B. Nevertheless, these materials work reasonably well with, for example, brand E.
Fig. 3.
Yield of some selected compounds in the different brands with and without the additives.
In order to consider all compounds analyzed in a more concise way, they were classified in different families of compounds as in a previous paper [21]. The families considered were aliphatics (AL), aromatics (AR), carbonyls (CA) and others (OT), in the case of the gas fraction, and in the liquid fraction in addition, nitrogenous (NI), polycyclic aromatics (PAH), epoxies (EP) and phenolics (PHE).
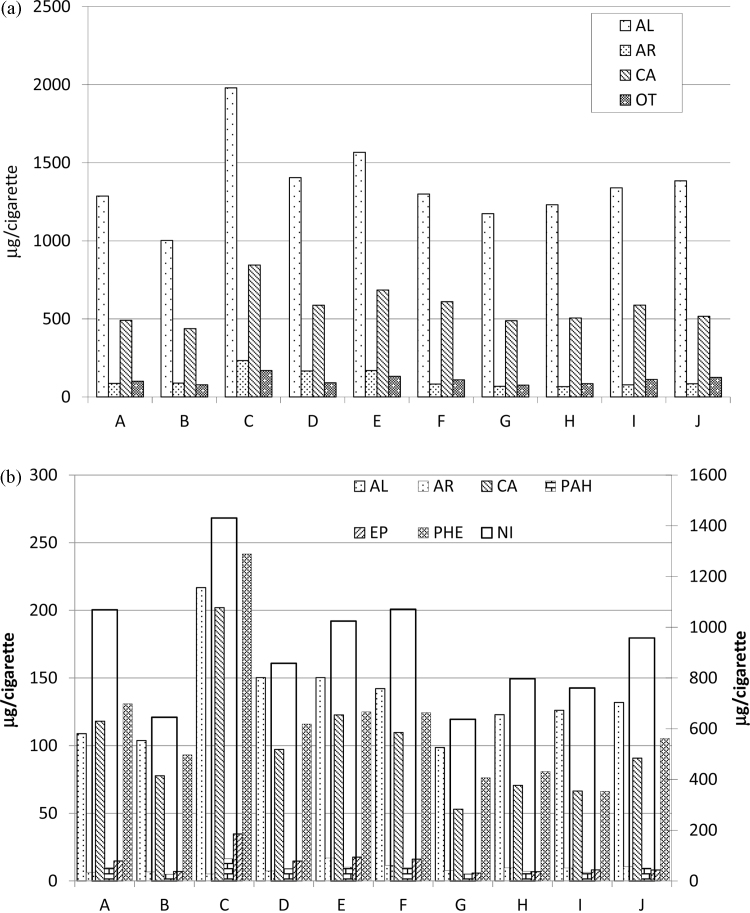
The yield of the families considered in the gas and Liq(F + T) fractions is shown in Fig. 4a and b, respectively. In the gas fraction, the AL family is the most important followed by CA. The family of CA in the gases is mainly formed by very harmful aldehydes, as seen in Table 4. In the liquid fraction, the NI compounds (referred to a secondary axis) are by far the more numerous due to the high yield in nicotine. PHE, CA and AL present similar yields, despite their order varying from brand to brand, while the less significant compounds are EP, PAH, AR. The average reductions of all the brands for the families of compounds considered are shown in Table 7. In general the reductions in liquids are larger than in the gas fraction. It can be observed that Al-MCM-41 reduces the yield of all families of compounds, especially the group of OT and AR in the gas fraction and NI and AR in the liquid fraction. The lowest reductions are for the families of AL and PAH, but even so, reductions are close to 15% in these families. NaY seems to be only capable of slightly reducing the AR in gases and NI and EP in liquids, while the HUSY behaviour is in between Al-MCM-41 and NaY. Reductions in PAH compounds were studied by Radojičić et al. [26] using a CuZSM5 zeolite, and they reported average reductions in PAH of around 40%. In our case the reduction found in the CFP traps for PAH with Al-MCM-41 was 22%.
Fig. 4.
Yield of the families considered in the (a) gas and (b) liquid fractions (F + T). NI is referred to the secondary y-axis.
Table 7.
Average reductions of all the brands for the families of compounds considered and the three additives studied.
| NI | CA | AL | AR | PHE | EP | PAH | OT | |
|---|---|---|---|---|---|---|---|---|
| Gases | ||||||||
| NaY | – | −5.1 | −4.5 | 3.9 | – | – | – | 0.6 |
| HUSY | – | 6.2 | −0.2 | 5.5 | – | – | – | 4.8 |
| Al-MCM-41 | – | 9.2 | 10.6 | 15.2 | – | – | – | 19.7 |
| Liquids(F + T) | ||||||||
| NaY | 12.7 | 3.5 | 0.9 | 3.0 | 3.8 | 6.1 | −11.0 | – |
| HUSY | 3.9 | 0.1 | −1.2 | 1.6 | −0.4 | 5.7 | −1.2 | |
| Al-MCM-41 | 32.7 | 25.0 | 14.8 | 34.4 | 24.0 | 28.3 | 15.5 | |
4. Discussion
The number of results obtained is very large and has been discussed from different points of view, considering individual compounds, families of compounds, differences among brands, the compounds collected in the gas, in the filter and trap, the effect of the additives, etc. More aspects could have been considered but the discussion seems to be clear from the results presented. The intrinsic complexity of the smoking process has been pointed out, where the pyrolysis and oxidation reactions under different dynamic conditions are present in all the experiments, depending on a large number of variables, especially when working with added materials. Thus, and consequently, the dispersion of the results is typically large and the results must be handled with care as well as the conclusions stated.
During a puff, the compounds contained in the TPM and in the gas fraction may collide with the additive particles and with the tobacco threads where the additive is spread out. Some compounds in TPM would condense on the threads or the additive surface, while the rest would move with the gas to the filters. Other compounds of the smoke may diffuse out from the cigarette paper wrapping the tobacco rod during puffing and smouldering [24]. As the hot zone during smouldering approaches the compounds condensed on the tobacco threads or the additive, they would, in part, evaporate and condense again on the tobacco plus additive system found thereafter, or would remain on the additive, which due to the high temperatures may be partially destroyed, and become part of the ash [15], [16]. In a previous work [19] it was shown that the amount of ash increases in those cigarettes where these mesoporous materials were added as a consequence of the coke deposition. This combined mechanism would explain the high reduction attained for compounds in the TPM, and especially for those which are present in a higher amount, and also the lower reduction obtained on the gas fraction. On the other hand some catalytic effect may also accompany the described filtering mechanism and is likely to be responsible for the coke generation. The selectivity to the harmful aromatics of Al-MCM-41 despite the low yield of the AR family, or the relatively low reduction attained by the non-polar AL compounds, regardless of their relatively high yield (Fig. 4 and Table 7), in addition to the highest coke yields, are the results of its catalytic activity. Nonetheless, it remains very difficult to explain the different reductions observed in the individual compounds or even in the families considered for the different tobacco brands.
Nevertheless, it seems clear that the use of porous solids of the type used in the present study have an effect on these reactions. Such effects depend on the nature of the solid, the porous texture and the acidity of its active centres. Considering the effect on the different parameters analyzed, it can be stated that Al-MCM-41 is an effective and promising material to reduce the amount of the different harmful compounds in tobacco smoke. These reductions are different from brand to brand, and may depend on different variables or combinations among them, such as the tobacco type and composition, the additives added during manufacturing or the paper porosity and additives. Even though, it is clear that the brands yielding the largest reductions in TPM are also those yielding the largest reduction in the individual components and also in those where the amount of coke deposited was the highest. The HUSY zeolite is less effective on average for all the brands, and the Na exchanged zeolite is the one showing the poorest results (once more exceptions can be found to this statement). Also, this zeolite is the one having the highest microporous character, showing a 77 K nitrogen adsorption isotherm with a very flat plateau. The amount of pores in the 0.3–0.8 relative pressure range is the lowest one (0.019 cm3/g). In addition this zeolite has a neutral character, and consequently is the one showing the poorest activity. The HUSY N2 isotherm is not as flat and has a larger external surface area and is the one with the largest acidity. It can be concluded that, in spite the complexity of the reactions, reactants and parameters involved, a certain correlation can be observed with the characteristics of the materials used. The pore volume and mesoporous character are the most important factors, making Al-MCM-41 to be the most effective catalyst of the three considered. Considering the nature of the materials used, the mesoporous solids of a certain level of acidity are the most promising for reducing the amount of the different compounds analyzed in the smoke of the ten brands studied.
5. Conclusions
The effect of three potential additives for reducing the amount of toxic compounds in the tobacco smoke has been studied on ten commercial cigarette brands sold in Spain.
NaY zeolite is the material showing the poorest behaviour, whereas Al-MCM-41 is the more effective in reducing the amount of all the compounds and families of compounds in the gas and liquid fractions. The pore size, acidity and dispersion degree of this catalyst play an important role on reducing the amount of compounds in the tobacco smoke.
Linear positive correlations have been obtained between the TPM and nicotine yields with the reduction of most compounds when the additives were employed, while the solid residue generated (ash and coke generated and deposited on the catalyst) increases. When 4% of Al-MCM-41 was employed, nicotine was reduced from 49.5% to 18.2% depending on the brand, while reductions in CO were between 35.2 and 10.3%. By families of compounds, the most important reductions by far are attained for the nitrogenous compounds followed by aromatics.
Regarding the behaviour of the tobacco brands, no clear correlation were found between the cigarettes design features and the ability of the additives considered, but it has been observed that they seem to be more effective when the smoke is more concentrated.
Conflict of interest
None declared.
Transparency document
Acknowledgements
Financial support for this investigation has been provided by the Spanish “Secretaría de Estado de Investigación” del Ministerio de Economía y Competitividad (MAT2011-24991) and by the Generalitat valenciana (PROMETEO/2012/015).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.11.014.
Appendix B. [{(Appendix A)}]Supplementary data
References
- 1.Adam T., McAughey J., Mocker C., McGrath C., Zimmermann R. Simultaneous on-line size and chemical analysis of gas phase and particulate phase of cigarette mainstream smoke. Anal. Chim. Acta. 2010;657(1):36–44. doi: 10.1016/j.aca.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Baker R.R. Smoke generation inside a burning cigarette: modifying combustion to develop cigarettes that may be less hazardous to health. Prog. Energy Comb. Sci. 2006;32:373–385. [Google Scholar]
- 3.Borgerding M.F., Bodnar J.A., Chung H.L., Mangan P.P., Morrison C.C., Risner J.C., Rogers C.H., Simmons D.F., Uhrig M.S., Wendelboe F.N., Wingate D.E., Winkler L.S. Chemical and biological studies of a new cigarette that primarily heats tobacco. Part 1. Chemical composition of mainstream smoke. Food Chem. Toxicol. 1997;36:169–182. [PubMed] [Google Scholar]
- 4.Branton P., Lu A.H., Schüth F. The effect of carbon pore structure on the adsorption of cigarette smoke vapour phase compounds. Carbon. 2009;47(4):1005–1011. [Google Scholar]
- 5.Chen Z., Zhang L., Tang Y., Jia Z. Adsorption of nicotine and tar from the mainstream smoke of cigarettes by oxidized carbon nanotubes. Appl. Surf. Sci. 2006;252:2933–2937. [Google Scholar]
- 6.Counts M.E., Morton M.J., Laffoon S.W., Cox R.H., Lipowicz P.J. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine smoking conditions. Regul. Toxicol. Pharm. 2005;41:185–227. doi: 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Cvetkovic N., Adnadjevich B., Nikolic M. Catalytic reduction of NO and NOx content in tobacco smoke. Beitr. Tabakforsch. 2002;20(1):43–48. [Google Scholar]
- 8.Dagnon S., Stoilova A., Ikanov I., Nikolova S. The effect of cigarette design on the content of phenols in mainstream smoke. Beitr. Tabakforsch. 2011;24(4):187–193. [Google Scholar]
- 9.Deng Q., Huang C., Xie W., Zhang J., Zhao Y., Hong Z., Pang A., Wei M. Significant reduction of harmful compounds in tobacco smoke by the use of titanate nanosheets and nanotubes. Chem. Commun. 2011;47:6153–6155. doi: 10.1039/c1cc10794a. [DOI] [PubMed] [Google Scholar]
- 10.Fowles J., Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob. Control. 2003;12(4):424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L., Cao Y., Zhou S.L., Zhuang T.T., Wang Y., Zhu J.H. Eliminating carcinogenic pollutants in environment: reducing the tobacco specific nitrosamines level of smoke by zeolite-like calcosilicate. J. Hazard. Mater. 2009;169:1034–1039. doi: 10.1016/j.jhazmat.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 12.Gaydhankar T.R., Samuel V., Jha R.K., Kumar R., Joshi P.N. Room temperature synthesis of Si-MCM-41 using polymeric version of ethyl silicate as a source of silica. Mater. Res. Bull. 2007;42(8):1473–1484. [Google Scholar]
- 13.Hoffmann D., Hoffmann I. The changing cigarette 1950–1995. J. Toxicol. Environ. Health. 1997;50:307–364. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 14.Kalaitzoglou M., Samara C. Gas/particle partitioning and yield levels of polycyclic aromatic hydrocarbons and n-alkanes in the mainstream cigarette smoke of commercial cigarette brands. Food Chem. Toxicol. 2006;44:1432–1442. doi: 10.1016/j.fct.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Lin W.G., Zhou Y., Gu F.N., Zhou S.L., Zhu J.H. Catalytic degradation of tobacco-specific nitrosamines by ferric zeolite. Appl. Catal. B – Environ. 2013;129:301–308. [Google Scholar]
- 16.Lin W.G., Zhou Y., Cao Y., Zhou S.L., Wan M.M., Wang Y., Zhu J.H. Applying heterogeneous catalysis to health care: in situ elimination of tobacco-specific nitrosamines (TSNAs) in smoke by molecular sieves. Catal. Today. 2013;212:52–61. [Google Scholar]
- 17.Marcilla A., Gómez-Siurana A., Berenguer D. Study of the influence of the characteristics of different acid solids in the catalytic pyrolysis of different polymers. Appl. Catal. A – Gen. 2006;301(2):222–231. [Google Scholar]
- 18.Marcilla A., Beltrán M., Gómez-Siurana A., Martínez I., Berenguer D. Catalytic effect of MCM-41 on the pyrolysis and combustion process of tobacco. Effect of the aluminium content. Appl. Catal. A – Gen. 2010;378:107–113. [Google Scholar]
- 19.Marcilla A., Beltrán M., Gómez-Siurana A., Martínez I., Berenguer D. Template removal in MCM-41 type materials by solvent extraction. Influence of the treatment on the textural properties of the material and the effect on its behaviour as catalyst for reducing tobacco smoking toxicity. Chem. Eng. Res. Des. 2011;89:2330–2343. [Google Scholar]
- 20.Marcilla A., Gómez-Siurana A., Beltrán M.I., Martínez I., Berenguer D. Catalytic effect of MCM-41 on the pyrolysis and combustion process of tobacco. Effect of the aluminium content. Thermochim. Acta. 2011;518(1–2):47–52. [Google Scholar]
- 21.Marcilla A., Gómez-Siurana A., Berenguer D., Martínez-Castellanos I., Beltrán M.I. Reduction of tobacco smoke components yields by zeolites abd synthesized Al-MCM-41. Micropor. Mesopor. Mater. 2012;161:14–24. [Google Scholar]
- 22.Marcilla A., Martínez I., Berenguer D., Gómez-Siurana A., Beltrán M.I. Comparative study of the main characteristics and composition of the mainstream smoke of ten cigarette brands sold in Spain. Food Chem. Toxicol. 2012;50(5):1317–1333. doi: 10.1016/j.fct.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 23.Marcilla A., Beltrán M.I., Gómez-Siurana A., Berenguer D., Martínez-Castellanos I. Comparison between the mainstream smoke of eleven RYO tobacco brands and the reference tobacco 3R4F. Toxicol. Rep. 2014;1:122–136. doi: 10.1016/j.toxrep.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purkis S.W., Mueller C., Intorp M., Seidel H. The influence of cigarette designs and smoking regimes on vapour phase yields. Beitr. Tabakforsch. 2010;24(1):33–46. [Google Scholar]
- 25.Radojičić V., Nikolić M., Adnadjevic B. The influence of zeolite type added to cigarette blend on the changes in pyrolytic temperatures. Hem. Ind. 2009;63(5a):579–583. [Google Scholar]
- 26.Radojičić V., Alagic S., Adnadjevic B., Maktouf A.M. Effect of varied quantities of zeolite on the reduction of polycyclic aromatic hydrocarbons in tobacco smoke. Afr. J. Biotechnol. 2012;11(42):10041–10047. [Google Scholar]
- 27.Shen J., Li J., Qian X., Ren W., Fatehi P. A review on engineering of cellulosic cigarette paper to reduce carbon monoxide delivery of cigarettes. Carbohydr. Polym. 2014;101:769–775. doi: 10.1016/j.carbpol.2013.09.101. [DOI] [PubMed] [Google Scholar]
- 28.Wilson C.L., Bodnar J.A., Brown B.G., Morgan W.T., Potts R.J., Borgerding M.F. Assessment of dioxin and dioxin-like compounds in mainstream smoke from selected US cigarette brands and reference cigarettes. Food Chem. Toxicol. 2008;46:1721–1733. doi: 10.1016/j.fct.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 29.WHO technical report series 951. World Health Organization Framework Convention on Tobacco Control. The scientific basis of tobacco product regulation. ISBN: 978 92 4 120951 9. http://www.who.int/tobacco/global_interaction/tobreg/publications/tsr_951/en/.
- 30.Xu Y., Zhu J.H., Ma L.L., Ji A., Wei Y.L., Shang X.Y. Removing nitrosamines from mainstream smoke of cigarettes by zeolites. Micropor. Mesopor. Mater. 2003;60:125–138. [Google Scholar]
- 31.Yong G., Jin Z., Tong H., Yan X., Li G., Liu S. Selective reduction of bulky polycyclic aromatic hydrocarbons from mainstream smoke of cigarettes by mesoporous materials. Micropor. Mesopor. Mater. 2006;91(2006):238–243. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.