Abstract
In present study two formulations of Koflet (syrup and lozenges) were evaluated against pyridine-induced pharyngitis in rats. Topical application of 10% pyridine showed extravasation of Evans blue stain as a characteristic feature of on-going inflammation. In addition, the levels of TNF-α (p < 0.01) and IL-6 (p < 0.01) were significantly increased compared to control. Further, histopathology of the pharyngeal tissue showed submucosal gland hypertrophy, severe mucosal inflammation characterized by presence of mononuclear cells and neutrophils along with haemorrhages and congestion; however, saline applied animals (normal control) showed normal cytoarchitecture of the pharynx. Interestingly, pre-treatment with dexamethasone (1 mg/kg, p.o.), Koflet lozenges (KL) (500 and 1000 mg/kg, p.o.) and Koflet syrup (KS) (2 and 4 ml/kg, p.o.) for 7 days showed significant and dose dependent protection by decreasing the EB dye extravasation, and serum levels of TNF-α and IL-6. In addition, histopathological findings have further supported the protective effect of Koflet formulations. These findings suggest that, both Koflet syrup and Koflet lozenges are highly effective in treating non-infectious type of pharyngitis. Among the two formulations KS was found to be more potent than KL, and possible mechanism of action thought to be mediating through inhibition of TNF-α and/or phospholipids–arachidonic acid pathway.
Keywords: Pharyngitis, Pyridine, Novel animal model, Koflet, Non-infectious pharyngitis
1. Introduction
The inflammation of the mucus membrane of pharynx is termed as pharyngitis, commonly known as sore throat [1], it is the most common and frequent among the upper respiratory tract diseases, which is accompanied by fever and/or cough [2]. In United states, acute pharyngitis accounts for about 1–2% of overall visits to the outpatient departments (OPD) and emergency departments [3]. Pharyngitis is known to be commonly associated with symptoms such as hoarseness, sore throat, cough, pain, difficulty in swallowing, airway obstruction, due to pathologic features like mucosal inflammation and submucosal oedema [4]. The frequent causes of pharyngitis is mainly due to infections associated with virus, bacteria and rarely due to candidal, fungal and parasites (infectious pharyngitis) [5], apart from infectious causes tracheal intubation during medical procedures, smoking, snoring, shouting, drugs such as ACE inhibitors, chemotherapy, corticosteroids, exposure to pesticides and environmental factors such as pollution, temperature, humidity/air conditioning are the non-infectious causative factors (non-infectious pharyngitis) [6], [7]. Additionally, the diseases such as GERD (gastroesophasengeal reflux disease), thyroiditis are well known to cause non-infectious type of pharyngitis [8], [9].
In spite of many available treatment strategies for pharyngitis, the side/adverse effects associated with them always made the scientists to think about the better, safe medicine. However, currently there is a lack of rigorous trials (both preclinical and clinical) for the treatment of non-infectious pharyngitis, one of the important factor hampering the efforts in identifying the effective new treatments is the lack of a suitable animal model for non-infectious pharyngitis [5]. In this context, we have developed a novel animal model for non-infectious pharyngitis in rats using pyridine as an inducer [10], it was found to be useful in screening the beneficial effect of synthetic, plant based medicines in treating non-infectious pharyngitis. In continuation, the present study was aimed to evaluate Koflet syrup, Koflet lozenges against pyridine-induced pharyngitis in rats.
2. Materials and methods
2.1. Drugs and chemicals
Pyridine (SD Fine chemicals, Bangalore), Dexamethasone (Zydus Cadila Healthcare Ltd., Mumbai), Koflet syrup (The Himalaya Drug Company, Bangalore), Koflet lozenges (The Himalaya Drug Company, Bangalore), TNF-α and IL-6 ELISA Kits (Krishgen Biosystems, Mumbai) were used for the study, other solvents and chemicals used were highly pure and of analytical grade purchased from HiMedia Laborateries Pvt. Limited, India.
2.2. Experimental animals
Inbred Wistar rats (250–300 g) were used for the study. The animals were maintained in polypropylene cages at a temperature of 25 °C ± 1 °C and relative humidity of 45–55% in a clean environment under 12:12 h light–dark cycle. The animals had free access to food pellets (Pranav Agro Industry, Bangalore, India) and purified water.
All the experimental protocols were approved by Institutional Animal Ethics Committee (IAEC) of The Himalaya Drug Company and were conducted according to the guidelines of Committee for the Purpose of Control and Supervision of Experimentation on Animals (CPCSEA), India.
2.3. Experimental protocol
2.3.1. Grouping and treatment schedule
Wistar rats (250–300 g) were divided into seven groups (G-I to G-VII, n = 10), G-I and G-II served as normal control and positive control; G-III served as standard and received dexamethasone (1 mg/kg, p.o.), G-IV and V have received 2 and 4 ml/kg, p.o., doses of Koflet syrup, while G-VI and VII have received Koflet lozenges at 500 and 1000 mg/kg, p.o. doses respectively for 7 days.
2.3.2. Induction of pharyngitis
On seventh day after administration of last dose of assigned treatments, EB dye (30 mg/kg, i.v.) was administered to all the animals via lateral tile vein. Ten minutes after the administration of EB dye, 10% pyridine was applied to the pharyngeal mucosa. In short, The tongue was slightly pulled out and pharynx area was opened deep into the oral cavity with the help of blunt forceps and the pyridine was applied with the help of cotton swab, gently for 5 s at each time point, for three times (approximately 50 μl). For G-I saline solution was applied similarly, since the pyridine solution was prepared in saline [10].
2.3.3. Induction of pharyngitis
After 60 min of pyridine/saline application, all the animals were sacrificed by exsanguination and the head portion was perfused with heparinised saline (40 IU/ml) to expel the intravascular EB dye. Then, the bilateral musculus masseter of the rat was incised and the lower jaw was removed to enable the extirpation of the pharynx. The portion of pharynx ranging from the caudal end of the soft palate to the epiglottis was isolated and weighed (approximately 40–50 mg).
The EB dye in the tissue was extracted in formamide at 55 °C for 24 h and determined spectrophotometrically at 620 nm, the tissue dye content was expressed as microgram of dye per gram of wet weight of the tissue (μg/g). Parallel to these experiments another set of experiments were run without administration of EB dye and the tissue samples collected were subjected to histopathological evaluation.
The blood samples were collected and serum was separated for the estimation of proinflammatory cytokines such as tumour necrosis factor alpha (TNF-α) and interleukin-6 (IL-6). These estimations were performed as per the user manual provided along with the respective ELISA Kits (Krishgen Biosystems, Mumbai, India).
3. Results
The polyherbal formulations, Koflet syrup and Koflet lozenges manufactured by Ms. The Himalaya Drug Company, Bangalore are well known for their beneficial effect in the treatment of pharyngitis and other upper respiratory tract diseases. The test formulations used in the present study (Koflet syrup and Koflet lozenges) are well known for the treatment of both infectious and non-infectious types of pharyngitis. However, there is a lack of scientific evidence related to their beneficial effect with respect to non-infectious type of pharyngitis. Incidentally, there is a paucity of scientific literature and reports related to screening models for non-infectious type of pharyngitis, hence in our previous study we have standardized a novel experimental animal model for non-infectious type of pharyngitis in rat using pyridine as a inducer [10]. In the present study, we have evaluated KS (2 and 4 ml/kg, p.o.) and KL (500 and 1000 mg/kg, p.o.) against pyridine-induced pharyngitis in rats using dexamethasone (1 mg/kg, p.o.) as a reference standard. The doses of KS and KL were selected by extrapolating human dose to animals dose, while dexamethasone dose was selected based on our previous study.
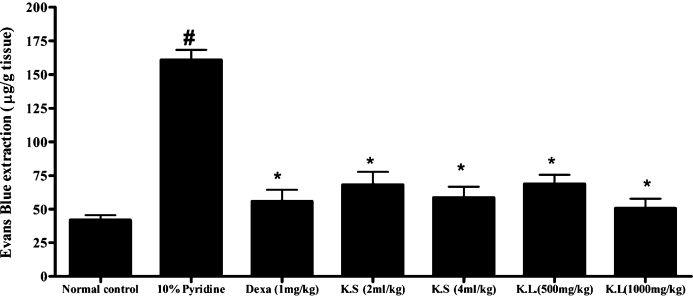
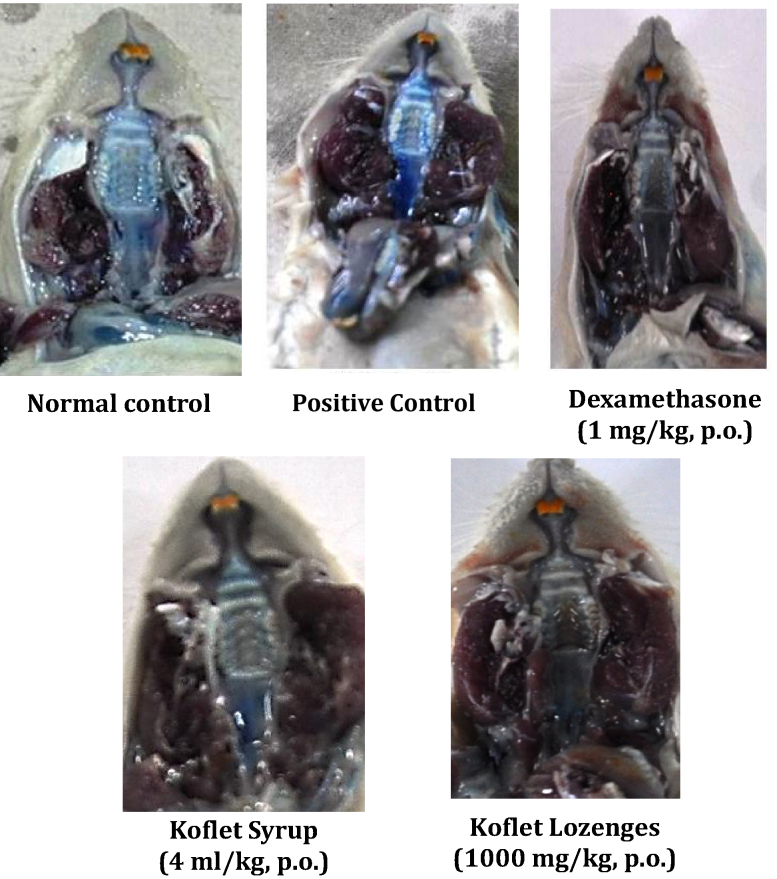
All the treatments were given for 7 days, on day-7 after administration of EB dye (30 mg/kg, i.v.) the pharyngeal tissue was separated and the quantity of EB dye present in the pharyngeal tissue was quantified by using standard curve for EB dye (Fig. 1). In 10% pyridine per se applied animals severe extravasation of EB dye was observed which is due to inflammation of pharynx, however normal control animals applied with saline showed very minimal/no extravasation of EB dye. Interestingly, dexamethasone (1 mg/kg, p.o.), KS (2 and 4 ml/kg, p.o.) and KL (500 and 1000 mg/kg, p.o.) treated animals showed negligible/no blue ting, as an indication of their protective effect against pyridine-induced damage and the morphology of pharyngeal tissues were comparable with that of normal control (Fig. 2, Fig. 3, Fig. 4).
Fig. 1.
Standard curve for Evans Blue.
Fig. 2.
Effect of Koflet formulations on pyridine-induced pharyngitis in rats. Note: Dexa – dexamethasone, KS – Koflet syrup, KL – Koflet lozenges. All the values are expressed as mean ± SEM (n = 6); all the groups were statistically compared by ANOVA followed by Tukey's multiple comparison. #p < 0.001 compare to control, *p < 0.01 compare to positive control (10% pyridine).
Fig. 3.
Percentage inhibition graph of Koflet formulations on pyridine-induced pharyngitis. Note: Dexa – dexamethasone, KS – Koflet syrup, KL – Koflet lozenges
Fig. 4.
Effect of Koflet formulations on pyridine-induced morphological damage of rat pharynx. The images of rat oral cavity demonstrates the effect of pyridine application on morphology of pharynx. The induction of pharyngitis was confirmed by administering Evans Blue dye and the intensity of blue colour in the target area (pharynx) is considered as a direct measure of inflammation (pharyngitis). The normal control shows negligible or no blue tinge, positive control with intense blue tinge is a sign of severe induction of pharyngitis. The reference drugs dexamethasone (1 mg/kg, i.v.), diclofenac (5 mg/kg, i.v.) and test drugs Koflet syrup (4 ml/kg, p.o.) and Koflet lozenges (1000 mg/kg, p.o.) treated groups with very minimal blue tinge indicates their protective effect against pyridine-induced pharyngitis.
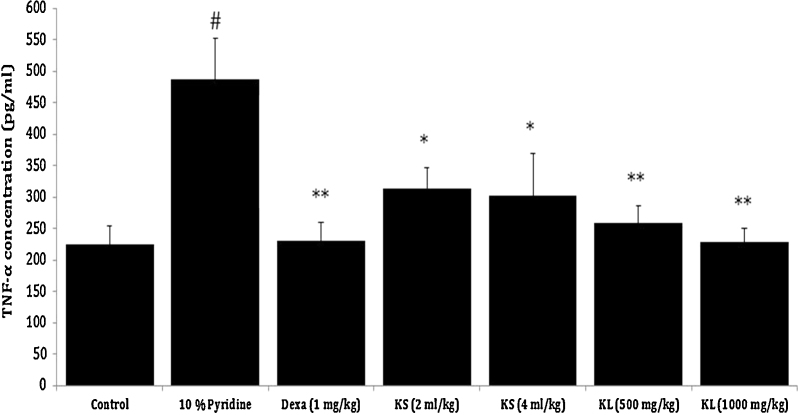
Besides EB dye test, serum levels of proinflammatory cytokines (TNF-α and IL-6) and histopathological evaluations were carried out for further evidences. The outcomes were in line with the EB dye test, the serum levels of TNF-α (p < 0.01) and IL-6 (p < 0.01) were found to be significantly increased in 10% pyridine applied group when compared to normal control. Exceptionally, dexamethasone (1 mg/kg, p.o.) (p < 0.01), Koflet lozenges (KL) (500 and 1000 mg/kg, p.o.) (p < 0.01) and Koflet syrup (KS) (2 and 4 ml/kg, p.o.) (p < 0.01) treatments for 7 days has brought down the serum levels of TNF-α and IL-6 near to normal control and thus showed significant protection against 10% pyridine-induced elevation of proinflammatory cytokines (Fig. 5, Fig. 6).
Fig. 5.
Effect of Koflet formulations on pyridine-induced elevated serum TNF-α levels in rats. Note: Dexa – dexamethasone. Values are expressed as mean ± SEM (n = 6); all the groups were statistically compared by ANOVA followed by Tukey's multiple comparison. #p < 0.001 compare to control, * p < 0.05, ** p < 0.01 compare to positive control (10% pyridine).
Fig. 6.
Effect of Koflet formulations on pyridine-induced elevated serum IL-6 levels in rats. Note: Dexa – dexamethasone. Values are expressed as mean ± SEM (n = 6); all the groups were statistically compared by ANOVA followed by Tukey's multiple comparison. #p < 0.001 compare to control, *p < 0.05, **p < 0.01 compare to positive control (10% pyridine).
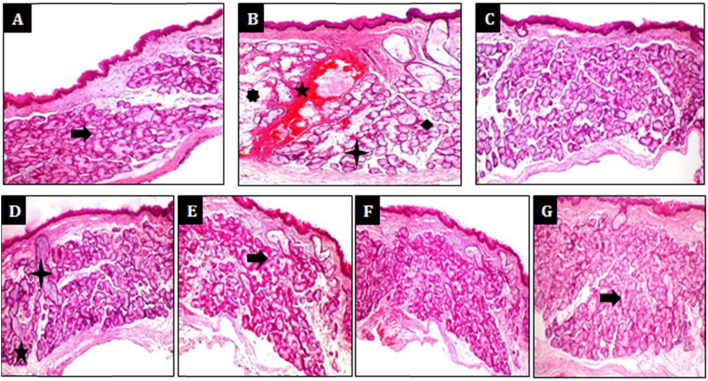
Furthermore, histopathology of the pharynx showed hypertrophy of submucosal glands, severe inflammation of the mucosa characterized by the presence of mononuclear cells, neutrophils, ruptured mucosal glands along with haemorrages and congestion in pyridine per se applied animals, however normal control animals applied with saline showed normal cytoarchitecture of the pharynx. Interestingly, dexamethasone (1 mg/kg, p.o.), KS (4 ml/kg, p.o.) and KL (1000 mg/kg, p.o.) treated animals showed only mild haemorrhages with mild hypertrophy of mucus glands. Exceptionally, mucosal gland rupture, haemorrhages and congestions were found to be completely absent in dexamethasone (1 mg/kg, p.o.), (4 ml/kg, p.o.) and KL (1000 mg/kg, p.o.) treated animals (Fig. 7).
Fig. 7.
Protective effect of Koflet formulations on against pyridine-induced histological changes in rat pharynx. Histopathology of Pharynx after pyridine application in rats. (A) Normal control – normal cytoarchitecture of pharynx, (B) positive control – showing hypertrophy of mucosal glands, haemorrhages, mass of inflammatory cells, ruptured mucosal glands as signs of severe inflammation, (C) dexamethasone (1 mg/kg, p.o.) – negligible signs of inflammation, (D) Koflet syrup (2 ml/kg, p.o.) – mild hypertrophy of mucosal glands and hemmorrhages, (E) Koflet syrup (4 ml/kg, p.o.) – very negligible inflammatory changes, (F) Koflet lozenges (500 mg/kg, p.o.) – mild hypertrophy of mucosal glands and haemorrhages, (G) Koflet lozenges (1000 mg/kg, p.o.) – very negligible inflammatory changes.
Noteworthy, KS (2 and 4 ml/kg, p.o.) and KL (500 and 1000 mg/kg, p.o.) were found to be equally potent and comparable with reference standard dexamethasone (1 mg/kg, p.o.).
The findings have revealed that, pre-treatment with dexamethasone (1 mg/kg, p.o.), KS (2 and 4 ml/kg, p.o.) and KL (500 and 1000 mg/kg, p.o.) for 7 days could be highly beneficial in preventing the pyridine-induced pharyngitis in rats.
4. Discussion
Recently a paper published by Bertold et al., stated that currently there is a lack rigorous trials (both preclinical and clinical) relevant to non-infectious pharyngitis and it is mainly due to lack of suitable preclinical animal model for non-infectious pharyngitis [5]. In this context, in our previous studies we have developed a novel experimental animal for non-infectious pharyngitis using various concentrations of pyridine in rats [10].
Pyridine is one of the commonly used reagent and mainly used as a precursor for the synthesis of various pharmaceuticals (sulfapyridine, tripelennamine, mepyramine) and agrochemicals (herbicides, pesticides), also in in vitro DNA synthesis [11]. Pyridine is known to be absorbed through the skin and mucus membrane, also irritates eyes, nose and respiratory tract. Acute exposure to pyridine can lead to headaches, dizziness, nausea, anorexia, dermatitis. While chronic exposure to pyridine results in health hazards such as CNS depression, hepatotoxicity, nephrotoxicity, neurotoxicity, genotoxicity and GI tract damage [12].
While standardizing the model we have used both dexamethasone (corticosteroid) and diclofenac (NSAID) as reference standards against pyridine-induced pharyngitis and we found that dexamethasone was more potent and reliable, and hence in the present study we have used dexamethasone as a reference standard [10].
Noteworthy, dexamethasone is commonly used for treating the non-infectious type of pharyngitis, by considering the potency and therapeutic application in treating non-infectious pharyngitis; in the present study we have chosen dexamethasone as reference standard.
Pyridine in known to cause mucus membrane damage and irritation of respiratory tract upon exposure [12], [13], [14], in the present study after 10% pyridine application to the pharyngeal region various parameters were evaluated to confirm and quantify the extent of inflammation. It is well known that, inflammation is the response of the tissue to injury which is characterized by increased blood flow and vascular permeability along with accumulation of fluid (extravasation), leukocytes and inflammatory mediators such as cytokines [15]. Extravasation is one of the important hall marks of inflammation and in literature it was commonly evaluated by means of EB dye test, the quantity of EB dye present in the pharyngeal tissue is considered to be a direct measure to rate the severity of inflammation [16]. Also, the acute phase pro-inflammatory cytokines such as TNF-α and IL-6 were estimated to see the extent of inflammation.
The cytokines are the groups of cell derived polypeptides which play a pivotal role in orchestrating the inflammatory response by increasing the cellular infiltration (leucocyte recruitment), cellular activation (mast cells, endothelial cells, tissue macrophages, etc.) and systemic response to inflammation (fever, hypotension, cachexia, leucocytosis, etc.) [15], TNF-α and IL-6 are considered to be the most potent proinflammatory cytokines and they are well proved to play an important role in the acute phase inflammation and hence in present study serum levels of TNF-α and IL-6 were estimated along with histopathological evaluation of pharyngeal tissue after pyridine application.
In experimental findings, 10% pyridine per se applied animals showed severe extravasation of EB dye, also the serum levels of TNF-α (p < 0.01) and IL-6 (p < 0.01) were found to be significantly increased in 10% pyridine applied group when compared to normal control. Additionally, histopathology of the pharynx showed hypertrophy of submucosal glands, severe inflammation of the mucosa characterized by the presence of mononuclear cells, neutrophils, ruptured mucosal glands along with haemorrhages and congestion in 10% pyridine applied group when compared to normal control.
Interestingly, one week pre-treatment with KS (2 and 4 ml/kg, p.o.), KL (500 and 1000 mg/kg, p.o.) and dexamethasone (1 mg/kg, p.o.) showed significant and dose dependent decrease in EB dye extravasation, and also showed significant decrease in serum levels of TNF-α and IL-6 compared to pyridine per se applied animals.
In similar lines, histopathological findings have showed only mild haemorrhages with mild hypertrophy of mucus glands in KS (2 ml/kg, p.o.) and KL (500 mg/kg, p.o.) treated animals. Exceptionally, the pathological changes were found to completely absent in dexamethasone (1 mg/kg, p.o.), KS (4 ml/kg, p.o.) and KL (1000 mg/kg, p.o.) treated animals. Furthermore, we thought pyridine induced pharyngitis involves multiple mechanisms such as enhanced expression of TNF-α (which further increases IL-6 levels), stimulation of phospholipid–arachidonic acid pathway through activation of phospholipase A2 and cyclooxygenases (COX's) and hence both dexamethasone and diclofenac have showed protective effect against pyridine-induced pharyngitis. In line with the above statement, in the present study KS and KL have showed significant protection against pyridine-induced pharyngitis and possible mechanism behind the protective effect of KS and KL were thought to be associated with multiple mechanisms such as inhibition of TNF-α and/or phospholipid–arachidonic acid pathway.
5. Conclusion
These findings suggest that, both Koflet syrup and Koflet lozenges are highly effective in treating non-infectious type of pharyngitis. Furthermore, KS was found to be more potent than KL in tested doses and possible mechanism of action thought to be mediating through inhibition of TNF-α and/or phospholipids–arachidonic acid pathway.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgment
The authors are thankful to Ms. The Himalaya Drug Company, Makali, Bangalore for providing all the necessary facilities to carry out the research work.
Footnotes
Available online 3 June 2014
Contributor Information
G.L. Viswanatha, Email: glv_000@yahoo.com.
Mohamed Rafiq, Email: dr.rafiq@himalayahealthcare.com.
References
- 1.Mutsumi Y., Tomokazu H., Teruro T., Miwa M. New pharyngitis model using capsaicin in rats. Gen. Pharmac. 1998;30(1):109–114. doi: 10.1016/S0306-3623(97)00084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alan L., Bisno M.D. Acute pharyngitis. N. Engl. J. Med. 2001;344:205–211. doi: 10.1056/NEJM200101183440308. [DOI] [PubMed] [Google Scholar]
- 3.Tan T.Q. The appropriate management of pharyngitis in children and adults. Expert Rev. AntiInfect. Ther. 2005;3(5):751–756. doi: 10.1586/14787210.3.5.751. [DOI] [PubMed] [Google Scholar]
- 4.McGuirt W.F. Gastroesophageal reflux and the upper airway. Pediat. Clin. North Am. 2003;50:487–502. doi: 10.1016/s0031-3955(03)00033-6. [DOI] [PubMed] [Google Scholar]
- 5.Bertold R., Christian A.M., Adrian S. Environmental and non-infectious factors in the aetiology of pharyngitis (sore throat) Inflamm. Res. 2012 doi: 10.1007/s00011-012-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnavita N. Cacosmia in healthy workers. Br. J. Med. Psychol. 2001;74(1):121–127. [PubMed] [Google Scholar]
- 7.Lyons R.A., Temple J.M., Evans D., Fone D.L., Palmer S.R. Acute health effects of the Sea Empress oil spill. J. Epidemiol. Community Health. 1999;53(5):306–310. doi: 10.1136/jech.53.5.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barry D.W., Vaezi M.F. Laryngopharyngeal reflux: more questions than answers. Cleve. Clin. J. Med. 2010;77(5):327–334. doi: 10.3949/ccjm.77a.09121. [DOI] [PubMed] [Google Scholar]
- 9.Rotman-Pikielny P., Borodin O., Zissin R., Ness A.R., Levy Y. Newly diagnosed thyrotoxicosis in hospitalized patients: clinical characteristics. QJM. 2008;101(11):871–874. doi: 10.1093/qjmed/hcn107. [DOI] [PubMed] [Google Scholar]
- 10.Viswanatha G.L., Thippeswamy A.H.M., Rafiq M., Jagadeesh M., Baig M.R., Suryakanth D.A., Azeemuddin M., Patki P.S. Novel experimental model of non-infectious pharyngitis in rats. J. Pharmacol. Toxicol. Methods. 2013 doi: 10.1016/j.vascn.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Pearson R.G., Williams F.V. Rates of ionization of pseudo acids. 1 V. Steric effects in the base-catalyzed ionization of nitroethane. J. Am. Chem. Soc. 1953;75(13):3073–3075. [Google Scholar]
- 12.Buron G., Hacquemand G., Pourié L., Jacquot G. Effects of pyridine inhalation exposure on olfactory epithelium in mice. Exp. Toxicol. Pathol. 2011 doi: 10.1016/j.etp.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Goppelt S.M., Wolter D., Resch K. Glucocorticoids inhibit prostaglandin synthesis not only at the level of phospholipase A2 but also at the level of cyclo-oxygenase/PGE isomerase. Br. J. Pharmacol. 1989;98(4):1287–1295. doi: 10.1111/j.1476-5381.1989.tb12676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishiki K., Nishinaga K., Kudoh D., Iwai K. Croton oil-induced hemorrhoid model in rat: comparison of anti-inflammatory activity of diflucortolone valerate with other glucocorticoids. Nihon Yakurigaku Zasshi. 1989;92(4):215–225. doi: 10.1254/fpj.92.215. [DOI] [PubMed] [Google Scholar]
- 15.Carol A.F., Timothy M.W. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:12–26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- 16.Yasmina M., Carlos A., Maria J.P., Agnieszka K. Evaluation of Evans Blue extravasation as a measure of peripheral inflammation. Protocol Exchange. 2010 [Google Scholar]