Abstract
This study was designed to evaluate the protective activity of gallic acid (GA) against glyoxal (GO) an advanced glycation intermediate-induced renal fibrosis in experimental rats. Glyoxal (i.p) at a dose of 15 mg/Kg body weight/day for 4 weeks induces renal fibrosis. GA was administered orally (100 mg/Kg body weight/day) along with GO for 4 weeks. The anti-fibrotic activity of GA was analyzed by measuring the collagen synthesis and deposition in renal tissues using mRNA expression analysis and Masson trichrome staining (MTS), respectively. The nephroprotective potential of GA was assessed by quantifying the markers of kidney damage such as serum blood-urea-nitrogen (BUN), creatinine (CR) and alkaline phosphatase (AP). Moreover, basement membrane damage in renal tissues was analysed by periodic acid Schiff’s (PAS) staining. GA co-treatment markedly suppressed the GO-induced elevation in mRNA expression of collagenIand III, MMP-2, MMP-9 and NOX (p < 0.05, respectively) genes as compared with GO alone infused rats. In addition, GA co-treatment significantly attenuated the GO -induced elevation in serum markers such as BUN, CR and AP levels (p < 0.05, respectively). Furthermore, GA co-treatment restored back the decreased renal super oxide dismutase (SOD) activity (p < 0.05) thereby assuage the reactive oxygen species (ROS) generation, and maintained the normal architecture of glomerulus. The present study clearly indicates that GO -induces renal fibrosis by enhancing GO/receptor of advanced glycation end product (RAGE) induced ROS generation and GA effectively counteracted GO-induced renal fibrosis by its ROS quenching and anti-glycation activity.
Keywords: Glycation, Glyoxal (GO), AGE intermediate, Gallic acid (GA), Renal hypertrophy, Fibrosis
1. Introduction
Glyoxal (GO) is a well known physiological α-oxoaldehyde, and is involved in the formation of advanced glycation end products (AGE) [24]. α-oxoaldehydes are implicated as an important causative factor of renal dysfunction [51], [66]. Studies have shown that the α-oxoaldehydes are capable of interacting with membrane proteins, nucleotides, and phospholipids [56]. Besides being a natural metabolite of glucose, GO can be formed by lipid peroxidation, nucleic acid oxidation and degradation of glycated proteins [10], [59]. GO is also present in foods products, beverages and widely used in industrial chemicals [1]. The reactive carbonyl group of GO can react with amino group of proteins to form covalent adducts known as AGEs [45]. It has been reported that the circulatory levels of α-oxoaldehydes are increased in hyperglycemic patients due to an imbalance in the intracellular glucose metabolism [36].
Irreversible forms of AGEs are capable of altering the structure and functions of membrane proteins as well as contractile proteins [47]. An increased circulatory and tissues levels of AGEs have been attributed to the secondary complication of diabetes [39]. We have previously demonstrated that infusion of GO induces renal damage and modulates the redox potential in experimental rats, most likely via receptor for AGEs (RAGE) mediated pathway [53]. In vitro studies have shown that, AGE-RAGE-ROS signaling contribute to the release of proinflammatory cytokines and growth factors that are implicated in the pathogenesis of diabetic complications [27].
Recently studies have been initiated to identify molecules that are capable of suppressing AGEs formation and its secondary complication in diabetic patients [57]. Aminoguanidine (AG) is a well-known synthetic inhibitor of AGEs formation, and it was reported to confer protection against AGE-mediated diabetic nephropathy [41]. However, AG exhibits deleterious side-effects such as gastrointestinal disturbances and abnormalities in liver function besides its beneficial counteractions against AGEs [57]. At this juncture, natural products have generally been proven to be relatively safe for human consumption, as compared to synthetic compounds. There, has been an increasing interest in the use of natural plant compounds, as anti-glycating agents [14].
Experimental studies on natural polyphenols such as, naringenin and its glycosides, quercetin and epicatechin exhibit a significant anti-glycation property [65]. Gallic acid (GA) (3,4,5-trihydroxybenzoic acid), a natural antioxidant, has been reported to confers free radical scavenging activity [50], anti- inflammatory [23] anti-hyperglycemic properties [3]. Gallic acid was reported to contain three hydroxyl groups bonded to the aromatic ring in an ortho position exhibits the strongest free radical, scavenging activity [49]. The ortho substitution of hydroxyl groups to the aromatic ring is good for anti-oxidant and free radical scavenging activity of phenolic acids [6]. We have previously reported that GA effectively counteracted AGE-induced cell proliferation and oxidative stress in H9C2 (2-1) rat cardiomyocyte cells [52]. Hence, in the present study, we made an attempt to study the beneficial counter actions of GA against GO, an intermediate metabolite of AGEs induced renal fibrosis in experimental rats.
2. Materials and methods
2.1. Animals
Experimental protocol pertaining the use of adult male Wistar rats weighing about 120–150 g used in this study and has been approved by the Institutional Animal Ethics Committee. Animals were housed in plastic cages and kept at 25 °C with 12:12 h light: dark schedule. All animals were fed standard pellet diet (M/s. Hindustan Lever Ltd., Mumbai) and water ad libitum.
2.2. Drugs and chemicals
Glyoxal, Bradford’s reagent and GA was purchased from Sigma Aldrich (MO, USA). All the other chemicals used were of analytical grade.
2.3. Experimental design
Animals are divided into four groups of 6 animals each. Group 1: Control group animals received a daily injection of saline during experimental period. Group 2: Animals received intraperitoneal injections of glyoxal (15 mg/kg BW/day) for 4 weeks (GO). Group 3: Animals receive an intraperitoneal injection of GO (15 mg/kg BW/day) along with oral administration of Gallic acid (100 mg/Kg BW/day) (GO + GA). Group 4: Animals received GA alone orally (100 mg/Kg BW/day) during the experimental period.
2.4. Biochemical analysis
After the experimental period the blood sample was collected and centrifuged at 1500 × g for 10 min at 4 °C. The clear supernatant was used for further serum biochemical investigations, Blood urea nitrogen (BUN) [40], serum creatinine (CR) [58] and serum alkaline phosphatase (AP) [62]. Kidney tissues were homogenized in Tris–HCl buffer (100 mM, pH 7.4) using Teflon homogenizer and centrifuged at 12,000 × g for 30 min at 4 °C. The supernatant obtained was used for further studies. Protein content of renal tissue extract was assessed by standard Bradford’s method [7]. The total collagen content was quantified by modified protocols of Bergman and Loxley [5]. Thiobarbituric acid reactive substance, an index of lipid peroxidation (LPO) was performed using protocols of Ohkawa et al. [37]. Superoxide dismutase (SOD) activity was determined using the methods of Kakkar et al. [20].
2.5. Histological examinations
Kidney tissue was fixed in 4% paraformaldehyde solution and embedded in paraffin wax. The sections obtained from kidney tissues were stained using hematoxylin and eosin (H&E) staining [29], Periodic acid Schiffs (PAS) staining [55] and Masson’s trichrome staining [29] to observe the histological changes.
2.6. PCR analysis
Total cellular RNA was isolated by Trizol method based upon the method developed by Chomczynski and Sacchi [11]. To remove genomic DNA contamination, RNA samples were treated with RNase-free DNase I at 37 °C for 30 min. The integrity of RNA was visualized by distinct 28 S and 18 S bands in 1.5% agarose gel electrophoresis. PCR was performed using GeNei M-MuLV RT-PCR kit along with the primers listed in Table 1.
Table 1.
RT-PCR oligonucleotide gene-specific primers.
| Gene | Primer sequences | Size (bp) | An. Tm (°C) |
|---|---|---|---|
| Collagen-I | Sense 5′-TGCTGCTTGCAG TAACGTCG-3′ | 136 | 60.8 |
| Antisense 5′-TCAACACCATCTCTGCCTCG-3′ | |||
| Collagen III | Sense 5′- AAAGGTGAAACTGGTGAACGTGGC-3′ | 578 | 61.3 |
| Antisense 5′- TCCATCTTGCAGCCTTGGTTAGGA- 3′ | |||
| MMP-2 | Sense 5′- CTATTC TGTCAGCACTTTGG- 3′ | 309 | 53 |
| Antisense 5′-CAGACTTTGGTTCTCCAACTT-3′ | |||
| MMP-9 | Sense 5′- AGTTTGGTGTCGCGGAGCAC-3′ | 754 | 57 |
| Antisense 5′- TACATGAGCGCTTCCGGCAC-3′ | |||
| NOX | Sense 5′- GGACCCCGATCCCAACTACGC-3′ | 298 | 59 |
| Antisense 5′- GCGCTTCCGAGAACGCTGGT-3′ | |||
| GAPDH | Sense 5′-TCCACCACCCTGTTGCTGTAGC-3′ | 401 | 58 |
| Antisense 5′-TGGAAAGCTGTGGCGTGATG-3′ |
2.7. Gel activity assays
The activity of MMPs was detected using gelatin zymography [33]. MMPs activity was visualized as clear distinct bands against blue background and was quantified by scanning densitometry. The antioxidant gel activity of SOD was determined by recommended protocols. Tissue extracts were separated in 10% native PAGE and the gel was stained in Riboflavin-NBT solution gives clear SOD bands against the blue–purple background of the gel [67].
2.8. Immunostaining
The tissue sections were dewaxed; rehydrated using series of alcohol and were incubated for antigen retrieval in citrate buffer (pH 6.0). Following antigen retrieval process, the sections were incubated with Goat polyclonal IgG RAGE, Rabbit polyclonal IgG MMP-2, MMP-9, and Mn-SOD (1:100 each) primary antibodies diluted in 1% BSA in PBS for 2 h at room temperature. The sections were washed in PBS and incubated with its specific secondary HRP conjugated antibody at a dilution 1:250 for 1 h at room temperature. The peroxidase activity was visualized by treating the slides with DAB and was counterstained using Meyer’s hematoxylin.
2.9. Immunoblot
Proteins were separated using SDS-PAGE and transferred to PVDF membrane, the membrane was processed with Goat polyclonal IgG RAGE (1:1000), Rabbit polyclonal IgG MMP-2 (1:500), MMP-9 (1:500) primary antibodies followed by HRP-conjugated antibody (1:2500) and developed using DAB solution.
2.10. Reactive oxygen species
Kidney tissue samples were prepared for dichlorodihydrofluorescein (DCF) fluorescence according to previously described method [32]. Formation of dichlorodihydrofluorescein (DCF) was measured using a fluorometer with excitation and emission wavelengths of 488 nm and 525 nm respectively.
2.11. Statistical analysis
Statistical analysis was performed using GraphPad Software (San Diego, CA). The results are presented as mean ± SEM. Statistical differences between groups were determined by one-way analysis of variance (ANOVA) with Students t test.
3. Results
3.1. Physiological parameters
Table 2 depicts the KW/BW ratio, which is an indicative of hypertrophic growth was increased (36%) significantly in GO infused rats as compared with control. Whereas, animals co-treated with gallic acid exhibits protection against GO- induced hypertrophy.
Table 2.
Effects of glyoxal, an AGE intermediate on body weight, kidney weight and KW/BW ratio in control and experimental group of rats.
| Parameters | Control | GO | GO + GA | GA |
|---|---|---|---|---|
| Body Weight (gm) | 135 ± 5.8 | 110 ± 7.1a, * | 125 ± 5.31b, * | 130 ± 6.3 NS |
| Kidney Weight (gm) | 0.7 ± 0.01 | 0.9 ± 0.02a, * | 0.79 ± 0.03b, * | 0.6 ± 0.02 NS |
| KW/BW ratio | 0.52 ± 0.02 | 0.81 ± 0.02a, * | 0.63 ± 0.01b, * | 0.46 ± 0.01 NS |
Values in the table are expressed as mean ± SEM, n = 6. No significant NS-Control Vs GA.
GO Vs. Control.
GO + GA Vs. GO.
p < 0.05.
3.2. Biochemical parameters
Table 3 shows the levels of Blood Urea Nitrogen, serum creatinine and serum alkaline phosphatase in control and experimental group of animals. Animals co-treated with gallic acid showed a significant decline (p < 0.05) in the levels of serum biochemical markers as compared with GO alone treated rats. Animals received GA alone did not show any significant changes in the serum biochemical parameters as compared with that of the control animals.
Table 3.
Effects of glyoxal, an AGE intermediate on serum BUN, Creatinine and alkaline phosphatase in control and experimental group of rats.
| Parameters | Control | GO | GO + GA | GA |
|---|---|---|---|---|
| BUN (mg/dL) | 14 ± 0.2 | 20 ± 1.4a,* | 16 ± 0.8b,* | 13 ± 0.2 NS |
| Creatinine (mg/dL) | 0.15 ± 0.01 | 0.25 ± 0.02a,* | 0.2 ± 0.02b,* | 0.13 ± 0.01 NS |
| Alkaline phosphatase (U/L) | 187 ± 9.8 | 239 ± 21.1a,* | 197 ± 11.9b,* | 185 ± 8.2 NS |
Values in the table are expressed as mean ± SEM, n = 6. No significant NS-Control Vs GA.
aGO Vs. Control.
bGO + GA Vs. GO.
*p < 0.05.
3.3. Morphology
Fig. 1. depicts the renal sections from control and experimental group of animals. Degenerating glomerulus and tubular epithelial cells were seen in GO treated animals indicated by arrows (panel b) However, animals co-treated with GA (panel c) showed a protection against glomerular damage as compared with the animals treated with GO alone. No significant change was observed in the gallic acid alone treated animals (plate d).
Fig. 1.
Kidney sections from the control and experimental group animals were stained with H & E; Masson trichrome and Periodic Schiff’s stain (n = 6/group). H&E stain (a–d). Panel (a) normal control; Panel (b) GO infused rats; Panel (c) GO + GA treated rats; Panel (d) GA control animals. Masson’s trichrome to visualize collagen deposition (e–h). Panel (e) normal control; Panel (f) GO infused rats shows increase blue staining reflects interstitial fibrosis, panel (g) gallic acid treated renal sections GO + GA shows mild increase in the blue stain in the tubules. Panel (h) GA control rats. PAS staining (i–l) visualize basement membrane damage. Panel (i) normal control; Panel (j) GO alone infused rats; Panel (k) GO + GA group animals; Panel (l) GA control animals, renal sections were shown at 40X magnification. Scale bar- 50 μm.
Collagen content was assessed by Masson’s trichrome staining was shown in Fig.1(f) depicts the collagen deposition in the kidney tissues of GO received animals. However, animals co-treated with GA (panel g) showed decrease in the collagen content in the renal tissue as compared with GO treated animals.
Periodic Acid Schiff’s staining of the kidney tissue shows the thickening of the basement membrane was evidenced by an increase in the PAS stain in the tubular basement membrane, and in the glomerulus in GO group animals (panel j). However, a decrease in the PAS stain was observed in the GO + GA group of animal (panel k) in the degenerating tubules. No significant changes in the glomerulus and tubules were observed in control and GA control group animals panel (i) and (l).
3.4. Collagen content
Fig. 2(A) depicts total collagen deposition in the kidney tissues of control and experimental group of animals. An increase in the collagen accumulation in the kidney tissues of GO group animals (p < 0.01) was observed as compared with control group animals. On the other hand, a significant decrease in the total collagen content was evidenced in the kidney tissues of GO + GA group animals (p < 0.05) as compared with GO treated animals. No significant change was observed in the animals treated with GA alone.
Fig. 2.
(A) Shows the total collagen content in the kidney tissue of experimental animals. Increased in the collagen content in GO group animals compared with the control group and there was a significant decrease in the collagen levels of GO + GA group animals as compared with the GO group. No significant change was observed in the GA group compared with the control group animals. (B and C) represents an increased mRNA expression of Collagen I and Collagen III in GO group animals and there was a significant decrease in the mRNA expression levels of GO + GA group animals. GAPDH was used as internal control. Results were expressed as Mean ± SEM (n = 6/group). Significance is indicated as *p < 0.05; **p < 0.01 and NS- non significant.
Fig. 2(B) explains the mRNA expression profile of collagen I and collagen III gene in the kidney tissues of control and experimental group of animals. It was evidenced that an increase in the expression of collagen I (p < 0.01) and collagen III (p < 0.05) gene in the GO group animals was observed as compared with control group animals. Animals co-treated with GA showed a marked reduction in the expression profile of collagen I and III genes. No significant change was observed in GA alone treated animals.
3.5. Metalloproteinase accumulation
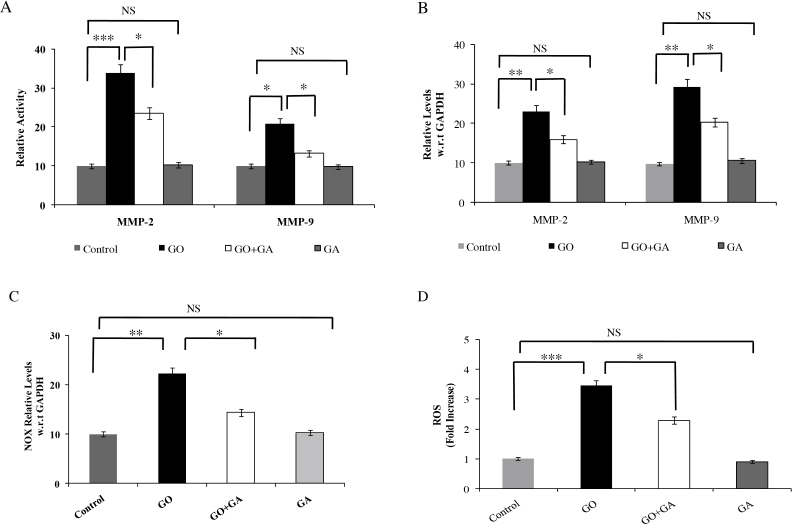
Fig. 3(A) exhibits the gelatinase activity in the kidney tissues of control and experimental group animals. MMP-2 and -9 activity was observed to be increased in the GO treated animals (p < 0.001 and p < 0.05). However, animals co-treated with GA showed a significant reduction in the gelatinase activity in the renal tissue as compared with GO treated animals. No significant change was observed in the animals treated with GA alone.
Fig. 3.
Zymogram analysis of MMP-2 and -9 activity showing activity of MMP-2 and MMP-9 in the tissue extracts. Gels were stained with 0.25% Coommassie brilliant blue and destained until the clear lytic bands were visible. Results were expressed as Mean ± SEM (n = 6/group). Significance is indicated as *p < 0.05; **p < 0.01; ***p < 0.01 and NS- non significant. (B) mRNA expression of MMP-2 and -9 in control and experimental group animals. GAPDH was used as internal control. Results were expressed as Mean ± SEM (n = 6/group). Significance is indicated as *p < 0.05; **p < 0.01 and NS- non significant. (C) mRNA expression levels of NOX-4 in control and experimental group animals. GAPDH was used as internal control. Results were expressed as Mean ± SEM (n = 6/group). Significance is indicated as *p < 0.05; **p < 0.01 and NS- non significant. (D) The figure shows the spectroflourimetric analysis of ROS generation in control, GO, GO + GA and GA treated tissue extract. Results were expressed as Mean ± SEM (n = 6/group). Significance is indicated as *p < 0.05; ***p < 0.001 and NS- non significant.
Fig. 3(B) depicts the mRNA expression profile of MMP-2 and -9 genes in the kidney tissue of control and experimental group animals. GO treated animals showed increased mRNA expression of MMP-2 and -9 (p < 0.01, respectively) as compared with control animals. Conversely, animals co-treated with GA showed a significant decrease in the expression levels of the MMP-2 and -9 (p < 0.05, respectively) in the kidney tissue as compared with GO treated animals.
3.6. NADPH oxidase and ROS levels
Fig. 3(C) depicts the mRNA expression levels of NOX in the kidney tissues of the experimental group of animals. GO group animals showed an increase in the expression levels of NOX (p < 0.01) as compared with control animals. Animals co-treated with GA exhibited a significant reversion in the levels of NOX as compared with GO treated animals.
Fig. 3(D) depicts the ROS generation in control and experimental group animals. GO treated animals showed an increase in the ROS generation in the renal tissue extract (p < 0.001) as compared with control group animals. However, GA co-treated rats showed a significant reduction in the ROS generation as compared with GO alone treated rats.
3.7. Immunostaining
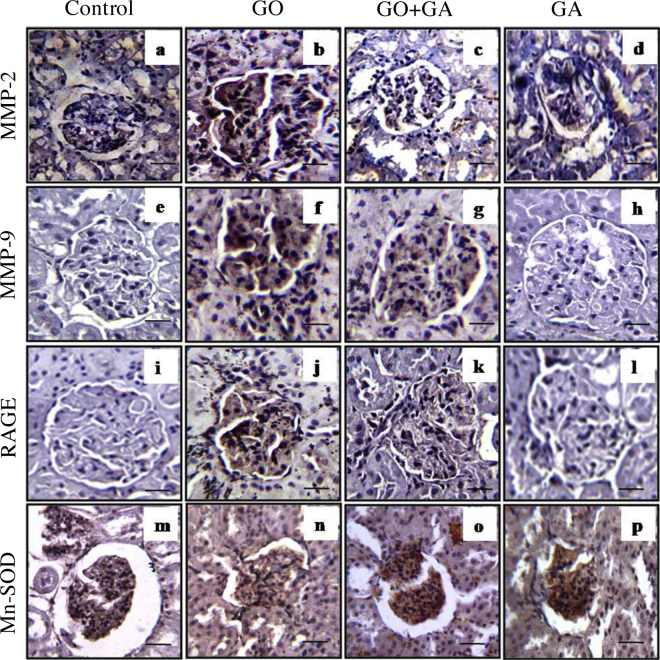
Fig. 4 shows MMP-2 and MMP-9, RAGE and Mn SOD protein expression in the renal tissue sections of control and experimental group of rats. An increased expression of MMP-2 and MMP-9 in the glolmerulus (panel b and f) was observed in the animals treated with GO alone. However, there was comparatively decreased in the levels of MMP-2 and MMP-9 in the glomerulus (panel c and g) of the GO + GA group animals. The photograph of glomerulus from the control and GA control group animals showed no significant changes. RAGE serves as a gateway for AGEs to induce cellular damage [61] was found to be increased in the GO administered group of animals Fig. 4 (Panel j). The expression of RAGE was reduced in the GA co-treatment group of animals (Panel k) which shows that GA may possess anti-AGEs property in renal injury. No change in the glomerulus was observed in the control and GA group animals. The immune expression of antioxidant enzymes superoxide dismutase was measured using immunostaining Fig. 4 shows the detection of Mn-SOD activity in experimental group animals and it was observed that there was a decrease in the expression of MnSOD levels in GO alone administered group (Panel n) as compared with control group animals (Panel m) and animals receives GO along with GA reveals decreased levels of MnSOD in the kidney tissue (Panel o) which explains that natural bioflavanoid GA might be an alternate source in delaying renal injury caused by food derived glycotoxins.
Fig. 4.
Immunostaining analysis of kidney sections from the control and experimental group animals (n = 6/group). Panel (a–d) shows MMP-2, Panel (e–h) shows MMP-9, Panel (i–l) shows RAGE, Panel (m–p) shows SOD expressions in the kidney sections of control and experimental group of rats respectively. An increased MMP-2 and -9 expression is evidenced in (panel b and f, respectively) in GO treated rat renal sections. An increased RAGE and decreased SOD expression is evidenced in (panel j and n, respectively) in GO treated rat renal sections. GA treatment neutralizes GO induced MMPs (panel c and g, respectively) RAGE (panel k) and SOD (panel n) expressions in the renal tissue sections. Kidney tissue sections were observed at 40X Magnification.
3.8. Immunoblotting
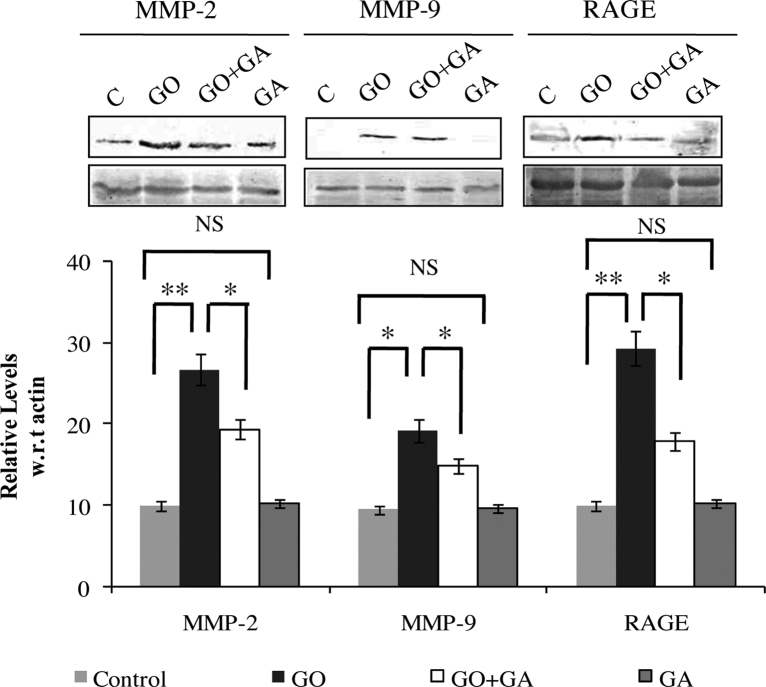
Fig. 5 depicts the protein expression of MMP-2, MMP-9 and RAGE in control and experimental group animals. An increase in the levels of MMP-2 (p < 0.01), MMP-9 (p < 0.05) and RAGE (p < 0.01) was observed in the renal tissues of GO treated animals, on the other hand animals receives GA along with GO showed a significant reversion of the MMP-2, MMP-9 and RAGE in the kidney tissues of experimental group animals. Animals receive GA alone showed no significant change as compared with the control group.
Fig. 5.
Immunoblotting analysis of MMP-2, MMP-9 and RAGE. β-actin was used as internal control. Where, Lane C-control; Lane GO – Glyoxal (GO) alone infused rats; Lane GO + GA - Gallic acid (GA) along with GO group animals; Lane GA - GA control animals. Results were expressed as Mean ± SEM (n = 6/group). Significance is indicated as *p < 0.05; **p < 0.01 and NS- non significant.
3.9. Antioxidant status
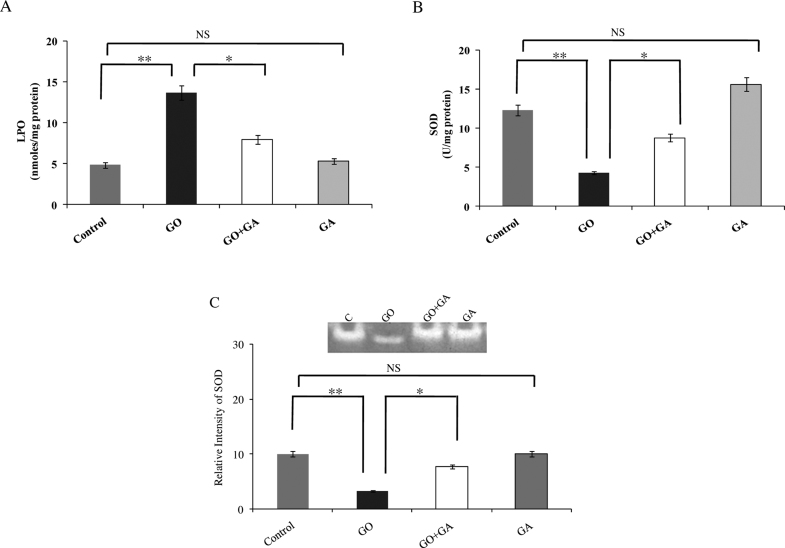
The results from the oxidative stress markers and the levels of antioxidant enzymes in the kidney tissue of experimental animals are represented in Fig. 6. GO received animals induced an imbalance in the redox status, with increased levels of thiobarbituric acid reactive substances (TBARS) (p < 0.01) and declining activities of SOD (p < 0.01) was found as compared to the control animals; whereas, animals received GA express a reversion in the LPO and SOD levels as compared with the GO group. Fig. 6(C) shows the detection of Mn-SOD gel activity in renal tissue homogenate of the experimental animals. SOD activity was significantly decreased in GO infused animal kidney tissue (p < 0.01). However, animals receive GO along with GA has a remarkable increase in the levels of SOD as compared with that of the GO group (p < 0.05).
Fig. 6.
(A) indicates the released Malondialdehyde-an indicator of LPO is expressed as nmoles/mg protein. (B) Activity is expressed as 50% inhibition of epinephrine auto-oxidation for SOD. (C) Native polyacrylamide gel activity assay for SOD activity in kidney tissue extract of experimental group animals. Where, Lane C-control; Lane GO – Glyoxal (GO) alone infused rats; Lane GO + GA - Gallic acid (GA) along with GO group animals; Lane GA - GA control animals. Results were expressed as Mean ± SEM (n = 6/group). Significance is indicated as *p < 0.05; **p < 0.01 and NS- non significant.
4. Discussion
The results of the present study demonstrated that GO an intermediate metabolite of AGEs induces renal fibrosis and that the natural antioxidant GA effectively attenuated the GO- induced renal fibrosis probably by attenuating the GO-induced ROS generation in the kidney tissues of experimental rats.
The kidneys are the vital organs of circulatory system that are responsible for maintaining the electrolyte balance in the blood and removing the toxic materials from the blood. In the present study, glyoxal treated animals showed a loss of body weight (Table 2) and renal hypertrophy (36%). AGEs and its intermediate glyoxal are considered as uremic toxins. These reactive molecules reported to accumulate in the tubular epithelial cells and mesangium of the kidneys, and induce glomerular damage [43], [17], [34]. It has been shown previously that, morphological changes in the kidney may reflect the renal hypertrophy and chronic progressive nephropathy [16]. Hence, the observed loss of body weight and increase in the kidney weight/body weight ratio in the glyoxal treated rats was probably due to glyoxal-induced renal injury.
Serum creatinine, and to a lesser extent blood urea, detects renal dysfunction in many circumstances [2]. Creatinine is found to be a reliable marker in renal injury. The kidneys maintain the levels of creatinine by clearing it out from the body and elevation in the levels of serum creatinine shows malfunction of kidneys. Similarly urea is also a metabolic byproduct which can build up in the plasma during renal insult. The BUN-to-creatinine ratio generally provides more information about kidney function [26]. In the present study, GO-induced animals showed elevated levels of serum creatinine and BUN (Table 3) (p < 0.05). In contrast, animals received GA along with GO showed significantly decreased (p < 0.05) levels of serum renal markers as compared to the GO treated animals. In addition, Alkaline phosphatase (ALP) was also elevated in the GO treated animals while GA treatment reverted back the elevated levels to near normal. ALP is a hydrolyze enzyme that dephosphorylates various molecules, most effectively operating in an alkaline environment. Pathologic conditions are most commonly associated with elevations in ALP [13]. In the present study, the elevated levels of ALP might be due to the poor detoxification of glyoxal leads to liver discomfort and thereby results in the elevation in the ALP. Combined together, these results clearly indicated the nephroprotective role of GA against the GO induced changes in kidney.
Diabetic nephropathy (DN) is characterized by enlargement of the glomerulus and tubules [54]. Previous studies have shown that, AGEs were found to be deposited in tubular epithelial cells and in the glomerular mesangium of kidneys [60]. It has been suggested that AGEs and its intermediates such as GO have the ability to cause cytotoxicity [46], altered cell morphology, [54], [44] and finally loss of cellular architecture in kidney tissues [53]. The renal tissue sections from the glyoxal treated animals showed an increase in glomerular damage and basement membrane thickening. It explains the reason for the renal impairment in these rats. An increase in the accumulation of polysaccharides in the glomerulus which are indicated by arrows (Fig. 1) eventually affects the filtration capabilities of glomerulus as evidenced by an increased in PAS staining [18]. The gallic acid co-treated animals have shown a decreased PAS staining in the basement membrane, and thereby minimize the severity of basement damage as compared with glyoxal received animals.
Matrix metalloproteinase (MMPs) are the family of endopeptidases involved in the collagen degradation. It has been shown that degraded products of MMPs are the catalyst for collagen synthesis [38], [28]. Moreover, an increase in the expression of MMPs activity ultimately leads to fibrosis; whereas suppression of MMPs activity is eventually results in decreased accumulation of ECM proteins in tissues [25]. Studies have shown that engagement of AGE-RAGE induces ROS generation, thereby accelerates matrix metalloproteinase activity in experimental animals [9]. In the present study, an increased MMP-2 and MMP-9 expression was observed in GO received animals suggested that GO-RAGE-mediated ROS generation might induce MMP-2 and -9 activation. Conversely, a significant decrease in the expression of MMP-2 and -9 (p < 0.05) was observed in GO + GA treated group of animals. Thus, GA being a potential antioxidant might have scavenged the ROS thereby minimizing the production and accumulation of collagen in the GO treated renal tissues.
Receptor for advanced glycation end products (RAGE) serves as the surface receptor for the AGEs, and it is responsible for the downstream signaling of various pathological events. [12]. It was reported that blockade or down-regulation of RAGE downstream signaling could be a therapeutic target for various diseases [64]. Studies have showed that RAGE was minimally expressed during normal physiological conditions [48]. Studies have shown that interactions of AGE/RAGE stimulate ROS generation, which could promote RAGE expression, which warns to a vicious cycle between RAGE-downstream signaling pathways [48]. Result of the present study has shown that GO treated animals exhibited an increased expression of RAGE. However, animals co-treated with GA showed decreased expression of RAGE, suggesting that GA counteracts GO, and thereby minimizing the GO-induced RAGE activation and further downstream signaling thus exhibited that it may act as a natural anti-glycation agent.
A subunit of NADPH oxidase (NOX) plays a key role in production of superoxide (O2−) by transferring electrons across the membrane from NAD(P)H to molecular oxygen [4]. Activation of NOX results in mitochondrial dysfunction and impaired antioxidant status and ultimately leads to oxidative stress [8]. Studies have suggested that the activation of RAGE induces NADPH oxidase and cellular ROS production in endothelial cells [63]. Our studies support the hypothesis that AGE-RAGE interaction, play a key role in amplifying NOX and induces ROS generation in the renal tissue [35]. Interactions of GO and RAGE stimulate the NADPH oxidase and cytosolic production of ROS within the renal tissues and this is inhibited by the natural anti-oxidant GA. Furthermore, our own recent study demonstrated the effectiveness of GA as an anti-glycating agent for AGE mediated macro vascular complications [53]. In the present study, natural dietary flavonoid GA attenuates the expression of NOX in the kidney tissues by demonstrating its capability in GO/RAGE mediated NOX activation during micro vascular complications (Fig. 3).
Interaction of AGE/RAGE mediated oxidative stress alters the antioxidant status of the renal system in diabetic nephropathy [42], and this would alter the activity of enzymatic defense mechanisms, ultimately contributing to excess cellular ROS accumulation [22]. Studies have shown that decrease in the activity of super oxide dismutase (SOD) leads to the accumulation of ROS generation and thereby leads to the amplification of lipid peroxidation (LPO) [21]. Previous studies by Huang et al., showed that flavanoids has the ability to suppress AGEs mediated oxidative stress via RAGE [19]. Recent studies showed that increased collagen synthesis and MMPs activity are related to SOD status and ROS formation [15]. In the present study we observed that more toxic role of GO in the induction of oxidative species with depletion of antioxidant enzyme (Fig. 6) and increase in the ROS levels (Fig. 3). Animals exhibited a significant (p < 0.01) increase in the level of lipid peroxides (LPO) with declining activities (p < 0.01) of antioxidant enzymes such as superoxide dismutase (Mn-SOD). On the other hand, animals co-treated with GA reduces the LPO activity probably due to its metal chelating properties [30] and thereby restoring the levels of SOD to near normal levels might be due to the action of GA on superoxide, hydroxyl and alkoxyl radical coupled to attenuate the oxidative stress which eventually reduces cellular damage [31]. The experimental evidence suggests that AGEs formed from GO and methylglyoxal contribute to collagen cross linking. In addition, AGE/RAGE downstream signaling stimulates ROS and negatively regulates the antioxidant status, which collectively induced the collagen synthesis and MMP mediated ECM rearrangement and subsequently fibrosis. However, GA co-treatment along with GO inhibited these changes and improved the GO-induced fibrotic complications in experimental Wistar rats.
Acknowledgements
Dr. EV wishes to thank DBT for the financial assistance in the form of research project (D.O.BT/PR6259/FNS/20/587/2012). Md greatly acknowledges University Grants Commission (UGC) for providing financial assistance in the form of fellowship.
Contributor Information
Mohammed Jainuddin Yousuf, Email: jainuddin.y@gmail.com.
Elangovan Vellaichamy, Email: vellaie@gmail.com, vellaie@unom.ac.in.
References
- 1.Arribas-Lorenzo G., Morales F.J. Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. J. Agric. Food Chem. 2010;58(5):2966–2972. doi: 10.1021/jf902815p. [DOI] [PubMed] [Google Scholar]
- 2.Baek S.M., Makabali G.G., Brown R.S., Shoemaker W.C. Free-water clearance patterns as predictors and therapeutic guides in acute renal failure. Surgery. 1975;77:632–640. [PubMed] [Google Scholar]
- 3.Bak E.J., Kim J., Jang S., Woo G.H., Yoon H.G., Yoo Y.J., Cha J.H. Gallic acid improves glucose tolerance and triglyceride concentration in diet-induced obesity mice. Scand. J. Clin. Lab. Invest. 2013;73:607–614. doi: 10.3109/00365513.2013.831470. [DOI] [PubMed] [Google Scholar]
- 4.Bedard K., Krause K.H., The N.O.X. family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 5.Bergman I., Loxley R. New spectrophotometric method for the determination of proline in tissue hydrolyzates. Anal. Chem. 1970;42:702–706. doi: 10.1021/ac60289a036. [DOI] [PubMed] [Google Scholar]
- 6.Borde V.U., Pangrikar P.P., Tekale S.U. Gallic acid in ayurvedis herbs and formulations. Resent Res. Sci. Technol. 2011;3:51–54. [Google Scholar]
- 7.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Cai L., Wang Y., Zhou G., Chen T., Song Y., Li X., Kang Y.J. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2006;48(8):1688–1697. doi: 10.1016/j.jacc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Cau S.B., Guimaraes D.A., Rizzi E., Ceron C.S., Souza L.L., Tirapelli C.R., Gerlach R.F., Tanus-Santos J.E. Pyrrolidine dithiocarbamate down-regulates vascular matrix metalloproteinases and ameliorates vascular dysfunction and remodelling in renovascular hypertension. Br. J. Pharmacol. 2011;164(2):372–381. doi: 10.1111/j.1476-5381.2011.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chetyrkin S., Mathis M., Pedchenko V., Sanchez O.A., McDonald W.H., Hachey D.L., Madu H., Stec D., Hudson B., Voziyan P. Glucose autoxidation induces functional damage to proteins via modification of critical arginine residues. Biochemistry. 2011;50:6102–6112. doi: 10.1021/bi200757d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomczynski P., Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat. Protoc. 2006;1:581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 12.D’Agati V., Schmidt A.M. RAGE and the pathogenesis of chronic kidney disease. Nat. Rev. Nephrol. 2010;6:352–360. doi: 10.1038/nrneph.2010.54. [DOI] [PubMed] [Google Scholar]
- 13.Eknoyan G., Hostetter T., Bakris G.L., Hebert L., Levey A.S., Parving H.H., Steffes M.W., Toto R. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK) Am. J. Kidney Dis. 2003;42:617–622. doi: 10.1016/s0272-6386(03)00826-6. [DOI] [PubMed] [Google Scholar]
- 14.Elosta A., Ghous T., Ahmed N. Natural products as anti-glycation agents: possible therapeutic potential for diabetic complications. Curr. Diabetes Rev. 2012;8(2):92–108. doi: 10.2174/157339912799424528. [DOI] [PubMed] [Google Scholar]
- 15.Giftson J.S., Jayanthi S., Nalini N. Chemopreventive efficacy of gallic acid, an antioxidant and anticarcinogenic polyphenol, against 1,2-dimethyl hydrazine induced rat colon carcinogenesis. Invest. New Drugs. 2010;28:251–259. doi: 10.1007/s10637-009-9241-9. [DOI] [PubMed] [Google Scholar]
- 16.Greaves P. 2nd ed. Elsevier Science; Amsterdam: 2000. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation. [Google Scholar]
- 17.Greven W.L., Waanders F., Nagai R., van den Heuvel M.C., Navis G., van Goor H. Mesangial accumulation of GA-pyridine, a novel glycolaldehyde-derived AGE, in human renal disease. Kidney Int. 2005;68:595–602. doi: 10.1111/j.1523-1755.2005.00437.x. [DOI] [PubMed] [Google Scholar]
- 18.Horobin R.W., Kiernan J.A. 10th ed. BIOS Scientific Publishers; Oxford, UK: 2002. Conn’s Biological Stains: A Handbook of Dyes, Stains and Fluorochromes for Use in Biology and Medicine. [Google Scholar]
- 19.Huang S.M., Wu C.H., Yen G.C. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol. Nutr. Food Res. 2006;50:1129–1139. doi: 10.1002/mnfr.200600075. [DOI] [PubMed] [Google Scholar]
- 20.Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 21.Kakkar R., Kalra J., Mantha S.V., Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol. Cell. Biochem. 1995;151:113–119. doi: 10.1007/BF01322333. [DOI] [PubMed] [Google Scholar]
- 22.Kalim M.D., Bhattacharyya D., Banerjee A., Chattopadhyay S., Oxidative D.N.A. damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complement Altern. Med. 2010;10:77. doi: 10.1186/1472-6882-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroes B.H., van den Berg A.J., Quarles van Ufford H.C., van Dijk H., Labadie R.P. Anti-inflammatory activity of gallic acid. Planta Med. 1992;58(6):499–504. doi: 10.1055/s-2006-961535. [DOI] [PubMed] [Google Scholar]
- 24.Lange J.N., Wood K.D., Knight J., Assimos D.G., Holmes R.P. Glyoxal formation and its role in endogenous oxalate synthesis. Adv. Urol. 2012;2012:819202. doi: 10.1155/2012/819202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y.Y., Feng Y.Q., Kadokami T., McTiernan C.F., Draviam R., Watkins S.C. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12746–12751. doi: 10.1073/pnas.97.23.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lip H., Yang K., MacAllister S.L., O'Brien P.J. Glyoxal and methylglyoxal: autoxidation from dihydroxyacetone and polyphenol cytoprotective antioxidant mechanisms. Chem. Biol. Interact. 2013;202:267–274. doi: 10.1016/j.cbi.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Liu J., Zhao S., Tang J., Li Z., Zhong T., Liu Y., Chen D., Zhao M., Li Y., Gong X., Deng P., Wang J.H., Jiang Y. Advanced glycation end products and lipopolysaccharide synergistically stimulate proinflammatory cytokine/chemokine production in endothelial cells via activation of both mitogen-activated protein kinases and nuclear factor-kappaB. FEBS J. 2009;276(16):4598–4606. doi: 10.1111/j.1742-4658.2009.07165.x. [DOI] [PubMed] [Google Scholar]
- 28.Löffek S., Schilling O., Franzke C.W. Series matrix metalloproteinases in lung health and disease: biological role of matrix metalloproteinases: a critical balance. Eur. Respir. J. 2011;38:191–208. doi: 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- 29.Luna L. 3rd ed. Mcgraw hill book co.; 1968. Manual of Histological Staining Methods of the Armed Forces Institute of Pathology. [Google Scholar]
- 30.Andjelković Mirjana, Van Camp John, De Meulenaer Bruno, Depaemelaere Griet, Socaciu Carmen, Verloo Marc, Verhe Roland. Iron-chelation properties of phenolic acids bearing catechol and galloyl groupsl. Food Chem. 2006;98:23–31. [Google Scholar]
- 31.Mitić M.N., Obradović M.V., Grahovac Z.B., Pavlović A.N. Antioxidant capacities and phenolic levels of different varieties of Serbian white wines. Molecules. 2010;15:2016–2027. doi: 10.3390/molecules15032016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montoliu C., Valles S., Renau-Piqueras J., Guerri C. Ethanol-induced oxygen radical formation and lipid peroxidation in rat brain: effect of chronic alcohol consumption. J. Neurochem. 1994;63:1855–1862. doi: 10.1046/j.1471-4159.1994.63051855.x. [DOI] [PubMed] [Google Scholar]
- 33.Neely A.N., Brown R.L., Clendening C.E., Orloff M.M., Gardner J., Greenhalgh D.G. Proteolytic activity in human burn wounds. Wound Repair Regener. 1997;5:302–309. doi: 10.1046/j.1524-475X.1997.50404.x. [DOI] [PubMed] [Google Scholar]
- 34.Nematbakhsh M., Ashrafi F., Pezeshki Z., Fatahi Z., Kianpoor F., Sanei M.H., Talebi A. A histopathological study of nephrotoxicity, hepatoxicity or testicular toxicity: Which one is the first observation as side effect of Cisplatin-induced toxicity in animal model. J. Nephropathol. 2012;1:190–193. doi: 10.5812/nephropathol.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishikawa T., Edelstein D., Du X.L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M.A., Beebe D., Oates P.J., Hammes H.P., Giardino I., Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa S., Nakayama K., Nakayama M., Mori T., Matsushima M., Okamura M., Senda M., Nako K., Miyata T., Ito S. Methylglyoxal is a predictor in type 2 diabetic patients of intima-media thickening and elevation of blood pressure. Hypertension. 2010;56(3):471–476. doi: 10.1161/HYPERTENSIONAHA.110.156786. [DOI] [PubMed] [Google Scholar]
- 37.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 38.Parthasarathy A., Gopi V., Umadevi S., Simna A., Sheik M.J., Divya H. Suppression of atrial natriuretic peptide/natriuretic peptide receptor-A-mediated signaling upregulates angiotensin-II-induced collagen synthesis in adult cardiac fibroblasts. Mol. Cell. Biochem. 2013;378:217–228. doi: 10.1007/s11010-013-1612-z. [DOI] [PubMed] [Google Scholar]
- 39.Peppa M., Uribarri J., Vlassara H. Glucose advanced glycation end products, and diabetes complications: what is new and what works. Clin. Diabetes. 2003;21:186–187. [Google Scholar]
- 40.Rahmatullah M., Boyde T.R. Improvements in the determination of urea using diacetyl monoxime; methods with and without deproteinisation. Clin. Chim. Acta. 1980;107:3–9. doi: 10.1016/0009-8981(80)90407-6. [DOI] [PubMed] [Google Scholar]
- 41.Ravelojaona V., Péterszegi G., Molinari J., Gesztesi J.L., Robert L. Demonstration of the cytotoxic effect of advanced glycation endproducts (AGE-s) J. Soc. Biol. 2007;201:185–188. doi: 10.1051/jbio:2007023. [DOI] [PubMed] [Google Scholar]
- 42.Rosca M.G., Mustata T.G., Kinter M.T., Ozdemir A.M., Kern T.S., Szweda L.I., Brownlee M., Monnier V.M., Weiss M.F. Glycation of mitochondrial proteins from diabetic rat kidney is associated with excess superoxide formation. Am. J. Physiol. Renal. Physiol. 2005;289:F420–F430. doi: 10.1152/ajprenal.00415.2004. [DOI] [PubMed] [Google Scholar]
- 43.Sebeková K., Blazícek P., Syrová D., Krivosíková Z., Spustová V., Heidland A., Schinzel R. Circulating advanced glycation end product levels in rats rapidly increase with acute renal failure. Kidney Int. Suppl. 2001:S58–62. doi: 10.1046/j.1523-1755.2001.59780058.x. [DOI] [PubMed] [Google Scholar]
- 44.Shangari N., O'Brien P.J. The cytotoxic mechanism of glyoxal involves oxidative stress. Biochem. Pharmacol. 2004;68:1433–1442. doi: 10.1016/j.bcp.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Singh V.P., Bali A., Singh N., Jaggi A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014;18(1):1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sliman S.M., Eubank T.D., Kotha S.R., Kuppusamy M.L., Sherwani S.I., Butler E.S., Kuppusamy P., Roy S., Marsh C.B., Stern D.M., Parinandi N.L. Hyperglycemic oxoaldehyde, glyoxal, causes barrier dysfunction, cytoskeletal alterations, and inhibition of angiogenesis in vascular endothelial cells: aminoguanidine protection. Mol. Cell. Biochem. 2010;333:9–26. doi: 10.1007/s11010-009-0199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snow L.M., Fugere N.A., Thompson L.V. Advanced glycation end-product accumulation and associated protein modification in type II skeletal muscle with aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2007;62(11):1204–1210. doi: 10.1093/gerona/62.11.1204. [DOI] [PubMed] [Google Scholar]
- 48.Soro-Paavonen A., Watson A.M., Li J., Paavonen K., Koitka A., Calkin A.C., Barit D., Coughlan M.T., Drew B.G., Lancaster G.I., Thomas M., Forbes J.M., Nawroth P.P., Bierhaus A., Cooper M.E., Jandeleit-Dahm K.A. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sroka Z., Cisowski W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003;41:753–758. doi: 10.1016/s0278-6915(02)00329-0. [DOI] [PubMed] [Google Scholar]
- 50.Stanely P., Prince M., Priscilla H., Devika P.T. Gallic acid prevents lysosomal damage in isoproterenol induced cardiotoxicity in Wistar rats. Eur. J. Pharmacol. 2009;615:139–143. doi: 10.1016/j.ejphar.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Subramanian U., Sharma A., Venkatachalam G., Anoop S., Arumugam P., Sheik M.J.Y., Vellaichamy E. Induction of renal damage and modulation of redox potential in rats infused with glyoxal. Biomed. Preventive Nutr. 2012;2:119–124. [Google Scholar]
- 52.Subramanian U., Venkatachalam G., Anoop S., Arumugam P., Sheik M.J.Y., Vellaichamy E. Studies on the cardio protective role of gallic acid against AGE-induced cell proliferation and oxidative stress in H9C2 (2-1) cells. Cardiovasc. Toxicol. 2012;12:304–311. doi: 10.1007/s12012-012-9170-2. [DOI] [PubMed] [Google Scholar]
- 53.Subramanian U., Venkatachalam G., Vellaichamy E. Inhibitory effect of gallic acid on advanced glycation end products induced up-regulation of inflammatory cytokines and matrix proteins in H9C2 (2-1) cells. Cardiovasc. Toxicol. 2013;13(4):396–405. doi: 10.1007/s12012-013-9222-2. [DOI] [PubMed] [Google Scholar]
- 54.Thomas M.C., Burns W.C., Cooper M.E. Tubular changes in early diabetic nephropathy. Adv. Chronic Kidney Dis. 2005;12:177–186. doi: 10.1053/j.ackd.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Thompson Samuel W. In: Selected Histochemical And Histopathological Methods. Charles Thomas C., editor. Springfield; IL: 1966. [Google Scholar]
- 56.Thornalley P.J. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems–role in ageing and disease. Drug Metabol. Drug Interact. 2008;23:125–150. doi: 10.1515/dmdi.2008.23.1-2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thornalley P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003;419:31–40. doi: 10.1016/j.abb.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 58.Tietz N.W. 3rd ed. W.B. Saunders; Philadelphia: 1987. Fundamentals of Clinical Chemistry. [Google Scholar]
- 59.Turk Z. Glycotoxines, carbonyl stress and relevance to diabetes and its complications. Physiol. Res. Acad. Sci. Bohemoslov. 2010;59:147–156. doi: 10.33549/physiolres.931585. [DOI] [PubMed] [Google Scholar]
- 60.Vlassara H., Striker L.J., Teichberg S., Fuh H., Li Y.M., Steffes M. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc. Natl. Acad. Sci. U. S. A. 1994;91:11704–11708. doi: 10.1073/pnas.91.24.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wada R., Yagihashi S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann. N. Y. Acad. Sci. 2005;1043:598–604. doi: 10.1196/annals.1338.067. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe K., Tsuda T., Kitamura M. Measurement of serum alkaline phosphatase–improvement on the Kind-King test. Rinsho Byori. 1967;15(10):708–712. [PubMed] [Google Scholar]
- 63.Wautier M.P., Chappey O., Corda S., Stern D.M., Schmidt A.M., Wautier J.L. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 64.Yamagishi S., Takeuchi M. Nifedipine inhibits gene expression of receptor for advanced glycation end products (RAGE) in endothelial cells by suppressing reactive oxygen species generation. Drugs Exp. Clin. Res. 2004;30:169–175. [PubMed] [Google Scholar]
- 65.Zhang X., Chen F., Wang M. Antioxidant and antiglycation activity of selected dietary polyphenols in a cookie model. J. Agric. Food Chem. 2014;62:1643–1648. doi: 10.1021/jf4045827. [DOI] [PubMed] [Google Scholar]
- 66.Matsumura Y., Iwasawa A., Kobayashi T., Kamachi T., Ozawa T., Kohno M. The reactivity of α-oxoaldehyde with reactive oxygen species in diabetes complications. J. Clin. Biochem. Nutr. 2013;52:128–132. doi: 10.3164/jcbn.12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]