Abstract
Artesunate is a potent and rapidly acting blood schizontocide used to treat chloroquine resistant malaria. Artesunate has been reported to cause embryo, reduced reproductive capacity, hepatotoxicity, neurotoxicity and hematological abnormalities. Previously toxicity studies on artesunate have been done in 2–10 mg/kg dose range mostly for 7 days, scientific studies on sub-chronic exposure of artesunate is not been reported so for. The present study evaluates sub-chronic safety profile of artesunate on 45 days oral administration at 2, 4 and 8 mg/kg/day. Authentication of artesunate has been done by color test, pH, melting point, loss on drying, UVmax, TLC and HPLC study. Artesunate has non-significant effect on liver and kidney weight. Serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), serum alkaline phosphate (ALP), cholesterol (TC), triglyceride (TG), total protein, albumin, bilirubin, creatinine, urea and glucose content were estimated after 45 days treatment along with hematological screening. Artesunate treatment for 45 days significantly increased (p < 0.05–0.001) SGOT, SGPT, ALP, TC, TG, total bilirubin, glucose level at 8 mg/kg/day dose. It has non-significant effect on serum total protein, albumin, creatinine and urea. Hemoglobin, total RBC, platelet, lymphocytes, basophil, mean cell volume and mean corpuscular hemoglobin concentration have not changed but total WBC, neutrophil, eosinophil, packed cell volume and mean cell hemoglobin were increased significantly (p < 0.01) at 8 mg/kg/day dose. Artesunate treatment at 4 and 8 mg/kg/day showed sinusoidal dilation, cytoplasmic vaculation, focal necrosis, sinusoidal congestion and extensive inflammatory changes, whereas kidney was free of any deleterious effect.
Conclusion
Sub-chronic exposure of artesunate at 8 mg/kg/day dose for 45 days period cause hepatic damage along with hematological abnormalities signifying safety concern.
Keywords: Artesunate, Hematology, Histology, Sub-chronic, Toxicity
1. Introduction
Artemisia annua commonly known as sweet wormwood or sweet annie is a annual herb used in treatment of malaria over 2000 years in China. The active ingredient artemisinin was isolated in 1972 and subsequently other semi-synthetic antimalarial drugs were produced. The low water solubility of artemisinin made it marginally effective against malaria. Derivatives like artemether, arteether, artesunate, artenilic acid are effective anti-malarial against the asexual forms of the erythrocytic stage of Plasmodium falciparum and Plasmodium vivax malaria [1]. Artesunate is a semi-synthetic water soluble derivative of artemisinin [2]. Artesunate is always given with another antimalarial such as mefloquine [3] or amodiaquine [4] to avoid the development of resistance. The combination of artesunate and amodiaquine has been found to be equivalent to co-artemether [5].
Artesunate is used especially to treat chloroquine resistant malaria. Artesunate is a potent and rapidly acting blood schizontocide concentrated in parasitized erythrocytes but had no hypozoiticidal activity. Artesunate exerts its anti-malarial activity by generation of reactive oxygen species (ROS) by iron-catalyzed cleavage of the endoperoxide bridge through endoperoxide bond [6], [7]. It is converted to active metabolite dihydroartemesinin that binds tightly to parasite infected erythrocyte membrane then inhibits the sarcoplasmic/endoplasmic reticulum calcium ATPase encoded by P. falciparum [8], [9], [10]. Artesunate has demonstrated the fastest clearance of all antimalarial currently used and acts primarily on the trophozite phase, thus preventing progression of the malaria disease.
Literature citation showed that reported toxicity studies on artesunate have been mostly done following 7 days administration in a dose range of 2–10 mg/kg/day (oral or IP). Artesunate has been reported to cause embryo lethality and malformations when administered orally to rats during organogenesis [11], [12]. Long term administration for 6 weeks at 2.9 mg/kg dose reduces reproductive efficiency of male rat and toxicity on development of fetus in female rats [13]. Artesunate induced hepatotoxicity when given at 4–8 mg/kg/day dose for 5–7 days along with adverse action on kidney, biochemical parameters of serum and hematological induces in rat [14], [15]. Artesunate at 16 mg/kg/day dose causes hepatotoxicity in guinea pig when administered for 7 days [16]. Artesunate at 4–8 mg/kg/day dose given into 7 day had no effect on the histology of the heart [17]. The studies showed hepatotoxicity and hemolytic effects were clearly associated with artesunate [18]. The therapeutic dose of artesunate in adult human is minimum 50–200 mg/day. Scientific studies on sub-chronic toxicity in this dose range have not been reported so far.
Continued research is needed to establish safety and efficacy of the anti-malarial regimen of artesunate in patients receiving the therapy. The present study aims at evaluation of complete safety profile of artesunate considering effect on liver, kidney and blood parameters of rat following sub-chronic 45 days oral administration of artesunate in three graded dose that is 2, 4 and 8 mg/kg/day. The dose 8 mg/kg/day is twice the recommended daily dose, selected for this study to explore maximum possible adverse effect of artesunate on long term dosing. The study outcome will provide data regarding sub-chronic safety profile of artesunate.
2. Materials and methods
2.1. Procurement of artesunate
Artesunate was obtained as gift sample from IPCA Pharmaceutical Ltd., Ratlam, MP, India.
2.2. Authentication and standardization of artesunate
Identification test: Artesunate 100 mg was dissolved in 40 ml of anhydrous ethanol, shaken and filtered through Whatman filter paper. Hydroxylamine hydrochloride 0.5 ml and 8% NaOH 0.25 ml was added and heated to boiling followed by cooling. Few drops of dilute HCl and then few drops 5% ferric chloride was added to observe color development [19].pH determination: Artesunate 10 mg was dissolved 10 ml of distilled water. pH of this solution was measured with a digital pH meter (MS Electronics India Pvt. Ltd., Panchkula, India) and recorded [20].
Melting point determination: A thin walled capillary was closed at one end than sufficient quantity of artesunate was introduced in the capillary. The temperature of melting point apparatus (Jyoti Scientific Industry, Gwalior, India) was maintained initially at 100 °C, the capillary was placed in apparatus and melting point was recorded [20].
Loss on drying: The loss on drying test is designed to measure the amount of water and volatile matters in a sample when the sample is dried under specified conditions. The 500 mg artesunate sample was weighted, heated in an oven for appropriate period, cooled in the dry atmosphere of a desiccator and then reweighted [20].
2.3. UV spectroscopy
A simple, rapid, precise and accurate UV–vis spectrophotometric method developed by Kalyankar et al. [21] has been used for screening UVmax of artesunate. Accurately weighed quantity of artesunate was dissolved in methanol to get the concentration of 50 mg/ml. Appropriate dilutions were prepared from the standard stock solution and scanned in the spectrum range of 400–200 nm.
2.4. Thin layer chromatography (TLC)
On the basis of literature, TLC of artesunate was performed on 10 cm × 10 cm precoated Merck silica gel 60 F254 plates using ethyl acetate:toluene (5:95) as a solvent system [20]. Artesunate was dissolved in dehydrated ethanol and filtered through Whatman filter paper. The filtrate was evaporated, the residue dissolved in toluene (0.1 mg/ml) and spotted. The developed plates were air-dried, sprayed with anisaldehyde methanol solution and heated to 120 °C for 5 min. The plates were visualized and Rf value calculated.
2.5. High performance liquid chromatography (HPLC)
Waters HPLC instrument (Alliance 510) with UV-484 Data Ace software (I.D.: AL-011) was used for analysis. Separation was carried out on Thermo C18 (250 × 4.6) column using acetonitrile and 25 mM potassium dihydrogen phosphate buffer in ratio of 70:30 (v/v) filtered through 0.2 μm nylon membrane filter and degassed prior to use. Mobile phase flow rate was maintained at 1.0 ml/min in a run time of 10 min. Sample solution of artesunate was prepared in mobile phase, sonicated to dissolve and get a working standard concentration of 100 μg/ml. Samples were injected using Rheodyne injector with 20 μl loop and detection was carried out at 220–240 nm [22].
2.6. Sub-chronic toxicity study
Experimental animals: Wistar albino rats of either sex weighing between 150 and 200 g were obtained from the animal house of the Radharaman College of Pharmacy, Bhopal. The animals were allowed to free access of water and standard palette diet (Hindustan Lever Ltd.). Animals were housed in polycarbonate cages, paddy husk bedding with a controlled ambient temperature (22 ± 2 °C), humidity (60 ± 5%) and a 12 h light/dark cycle. All the experimental procedures and protocols used in the study were reviewed and approved by the Institutional Animal Ethical Committee (Approval no. IAEC/RCP/Oct 2012/05).
Study protocol: Wistar rats were randomly divided into 4 groups of 6 rats each. Group A animals were the vehicle control, treated with 0.9% sodium chloride in 2% carboxy methyl cellulose (CMC). Group B, C and D animals were administered with oral artesunate at 2, 4 and 8 mg/kg/day, p.o. in 2% CMC continuously for 45 days.
Starting from the 1st day of the drug administration body weight was measured on each 7th day and percent increase in body weight was calculated. After the experimental period the animals were sacrificed 2 h after last dose administration by cervical dislocation, the thoracic region was opened to expose the heart. Blood was collected by cardiac puncture in dry heparinised tubes and used for estimation of hematological indices. Another aliquot of blood was placed in plain centrifuge tubes allowed to coagulate for 30 min in room temperature and centrifuged at 3000 rpm for 10 min. The supernatant serum was collected and used for estimation of biochemical parameters. Immediately following blood collection, the liver and kidney were removed, washed in cold saline, pressed between filter paper pads and carefully weighed using a digital weighing balance. Relative organ weights for 100 g body weight was calculated and recorded. The liver and kidney tissues were immediately preserved in 10% formalin for histopathology.
Estimation of blood marker enzymes: Assay of serum glutamate oxaloacetate transaminase (SGOT) [23] and serum glutamate pyruvate transaminase (SGPT) [23], cholesterol (TC) [24], triglyceride (TG) [25], bilirubin [26], creatinine [27], urea [28] and glucose [29] was done in Star 21 Plus, Autoanalyzer by using the commercially available standard kit (ERBA Diagnostic Mannheim GmbH, Mannheim, Germany). Total protein [30] content of serum was estimated following modified biuret method using a standard kit of Span Diagnostics Ltd., Surat, India. Serum alkaline phosphate (ALP) [31] and albumin [32] were estimated using kit obtained from Lab-Care Diagnostic Pvt. Ltd., Mumbai, India.
Hematological parameters: Estimation of hemoglobin (Hb) content (Sahli's hemoglobinometer), total WBC, RBC and platelet count (Neubauer hemocytometer; Feinoptik, Germany) was done using standard technique and differential WBC count (neutrophil, eosinophil, basophil, lymphocyte and monocyte) was done by Leishman's staining method. Packed cell volume (PCV) is the percentage of RBC in the blood. PCV was estimated centrifuging blood in hematocrit tube and reading % RBC in a hematocrit reader [33].
Mean cell volume (MCV) indicates the volume of the average red cell in a sample expressed in femtoliters (fl) and calculated by using the formula: MCV = PCV/RBC × 10.
Mean cell hemoglobin (MCH) represents the absolute amount of hemoglobin in the average red cell in a sample in units of picogram (pg) per cell. The MCH is calculated from the hemoglobin and the RBC using the following formula: MCH = (Hb × 10)/RBC.
Mean corpuscular hemoglobin concentration (MCHC) is the average hemoglobin concentration in the red blood cells. The MCHC expressed as the amount of hemoglobin per deciliter of red cells (g/dl) and calculated as follows: MCHC = Hb/PCV.
Histology of liver and kidney: Paraffin sections of liver and kidney were prepared, stained with hematoxylin and eosin and processed for light microscope following the technique of Nanji et al. [34].
2.7. Statistical analysis
All data are presented as mean ± SEM. Experimental data was analyzed using one-way ANOVA followed by Student's t-test to compare the difference between the control and treated values. p value < 0.05 was considered significant. Graph Pad Prism Version 3.02 was used for statistical calculations.
3. Result
3.1. Authentication and standardization of artesunate
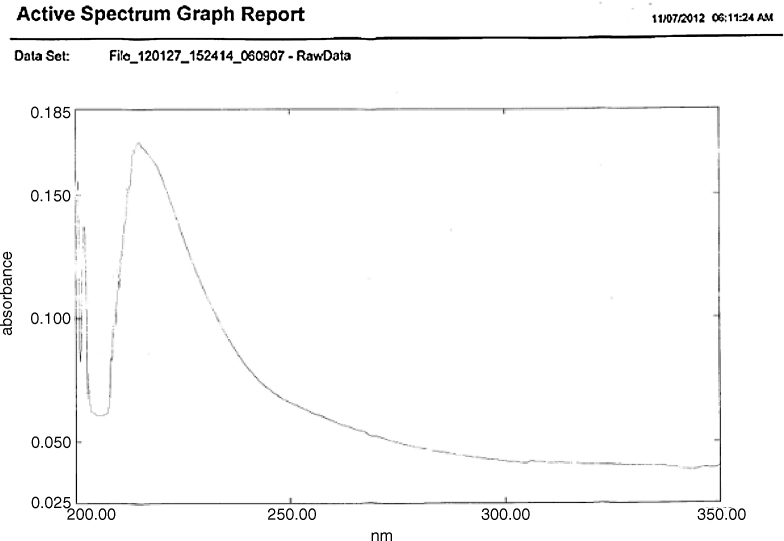
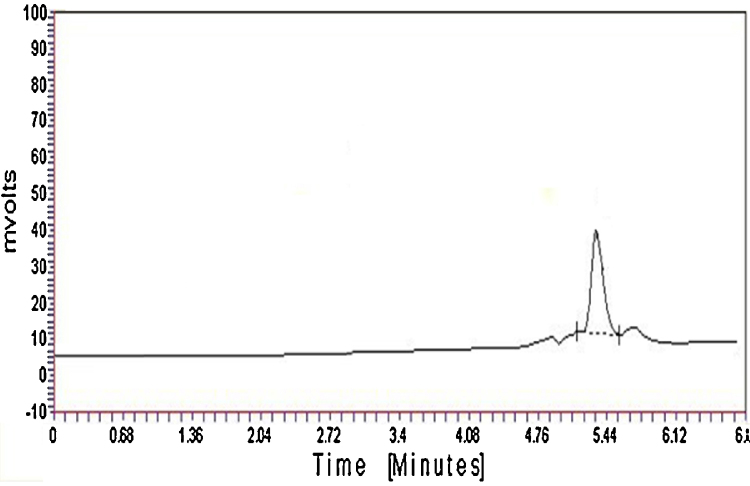
Emergence of light red violet color confirmed presence of artesunate as described in International Pharmacopoeia [20]. The pH of artesunate was obtained 5.38. The melting point of artesunate was obtained at 134.6 °C. The loss on drying of artesunate was obtained as 0.25%. UV spectrum of artesunate in methanol at 50 μg/ml concentration showed UVmax at 225 nm as compared to reported 240 nm (Fig. 1) in Kalyankar et al. [21]. In Fig. 2 artesunate showed Rf value of 0.43 in solvent system ethyl acetate:toluene (5:95). HPLC spectrum of artesunate in acetonitrile and 25 mM potassium dihydrogen phosphate buffer (70:30) showed at retention time 2.904 min (Fig. 3).
Fig. 1.
UV spectrum of artesunate sample in methanol at 50 μg/ml concentration screened at 220–240 nm showed UVmax 225 nm as compared to 240 nm reported in Kalyankar et al. [21].
Fig. 2.
TLC of artesunate sample in solvent system ethyl acetate:toluene (5:95) showed Rf at 0.43.
Fig. 3.
HPLC spectrum of artesunate sample in acetonitrile and 25 mM potassium dihydrogen phosphate buffer (70:30, v/v) showed retention time at 5.324 min compared to 5.202 min reported by Gandhi et al. [22].
3.2. Sub-chronic toxicity of artesunate
Effect on body weight: Body weight was determined on day 0, 7th, 14th, 21st, 28th, 35th and 42nd days. The body weight of rat in control group showed gradual increase during the 42 days treatment period up to 27.68% of weight gain. In contrast administration of artesunate at 2, 4 and 8 mg/kg/day doses showed respectively 18.35, 11.65 and 10.22% increase in body weight (Table 1).
Table 1.
Effect of artesunate 45 days treatment on % change in body weight.
| Treatment group (mg/kg/day, p.o.) | On 7th day | On 14th day | On 21st day | On 28th day | On 35th day | On 42nd day |
|---|---|---|---|---|---|---|
| Vehicle control | 5.54 | 6.78 | 8.46 | 13.12 | 22.39 | 27.68 |
| Artesunate (2) | 5.60 | 6.25 | 6.70 | 8.41 | 15.42 | 18.35 |
| Artesunate (4) | 4.25 | 5.95 | 6.72 | 7.70 | 9.52 | 11.65 |
| Artesunate (8) | 4.46 | 3.90 | 4.25 | 5.78 | 8.32 | 10.22 |
Effect on relative weight of liver and kidney: The intact weight of liver and kidney was converted to relative weight of 100 g body weight as shown in Table 2. The result showed that artesunate in different doses (2, 4 and 8 mg/kg/day) administered for 45 days has non-significant effect on liver and kidney weight compared to vehicle control.
Table 2.
Effect of artesunate 45 days treatment on relative weight of liver and kidney.
| Treatment group (mg/kg/day, p.o) | Liver weight (g/100 g body weight) | Liver weight (g/100 g body weight) |
|---|---|---|
| Vehicle control | 3.95 ± 0.83 | 0.89 ± 0.07 |
| Artesunate (2) | 3.46 ± 0.45ns | 0.84 ± 0.03ns |
| Artesunate (4) | 3.24 ± 0.73ns | 0.86 ± 0.04ns |
| Artesunate (8) | 2.94 ± 0.36ns | 0.76 ± 0.02ns |
n = 6. All values were ns = not significant when compared to vehicle control group.
Effect on serum biochemical parameters: The effects of artesunate on plasma lipid levels were examined at the end of treatment period (Table 3). Artesunate treatment for 45 days showed significant (p < 0.05–0.001) increase in SGOT, SGPT, ALP, TC, TG, at 4 and 8 mg/kg/day doses. Total bilirubin level was increased only at 8 mg/kg/day dose (p < 0.05). The level of total protein, albumin, creatinine and urea has non-significant effect but glucose level was elevated significant (p < 0.01) only 8 mg/kg/day dose.
Table 3.
Effect of artesunate 45 days treatment on biochemical parameter of rats.
| Treatment group (mg/kg/day, p.o) | SGOT (IU/L) | SGPT (IU/L) | ALP (IU/L) | TC (mg/dl) | TG (mg/dl) | TP (g/dl) | Albumin (g/dl) | Total bilirubin (mg/dl) | Creatinine (mg/dl) | Urea (mg/dl) | Glucose (g/dl) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle control | 27.70 ± 2.20 | 14.91 ± 1.31 | 110.30 ± 4.03 | 175.78 ± 10.33 | 105.35 ± 7.36 | 7.70 ± 1.02 | 3.90 ± 0.65 | 0.99 ± 0.06 | 0.68 ± 0.02 | 4.35 ± 0.93 | 110.93 ± 5.63 |
| Artesunate (2) | 28.66 ± 2.49ns | 26.62 ± 2.76a | 213.15 ± 9.65c | 255.64 ± 13.10b | 168.17 ± 9.82b | 8.15 ± 1.96ns | 4.30 ± 0.96ns | 1.76 ± 0.21ns | 0.85 ± 0.01ns | 4.49 ± 0.33ns | 105.32 ± 4.96ns |
| Artesunate (4) | 41.39 ± 2.78a | 37.58 ± 2.86c | 285.82 ± 8.66c | 277.99 ± 12.75c | 178.95 ± 10.05c | 9.11 ± 1.14ns | 2.77 ± 0.26ns | 2.13 ± 0.42ns | 1.08 ± 0.21ns | 4.27 ± 0.67ns | 125.75 ± 6.33ns |
| Artesunate (8) | 62.64 ± 3.75c | 51.62 ± 2.77c | 362.31 ± 1.19c | 285.18 ± 15.06c | 245.36 ± 12.09c | 9.27 ± 1.06ns | 1.80 ± 0.63ns | 3.73 ± 0.96a | 1.14 ± 0.19ns | 4.39 ± 0.85ns | 144.97 ± 8.42b |
n = 6. ns = not significant when compared to vehicle control group, SGOT = serum glutamate oxaloacetate transminase, SGPT = serum glutamate pyruvate transminase, ALP = alkaline phosphate, TC = total cholesterol, TG = triglyceride and TP = total protein.
p < 0.05.
p < 0.01.
p < 0.001.
Effect on hematological parameters: The effect of artesunate on hematological indices was examined at the end of treatment (Table 4). Treatment for 45 days has non-significant effect on hemoglobin, total RBC, MCH, MCHC level, differential count of lymphocytes and basophil and total platelet count. At 8 mg/kg/day dose PCV, MCH concentration was increased significantly (p < 0.01) along with increase in total WBC count, neutrophil and eosinophil percentage.
Table 4.
Effect of artesunate 45 days treatment on hematological parameters of rats.
| Treatment group (mg/kg/day, p.o) | Hb (g/dl) | RBC (×106/cm3) | PCV (%) | MCV (fl) | MCH (pg) | MCHC (g/dl) | WBC (×103/cm3) | NEU (%) | LYM (%) | MON (%) | EOS (%) | BAS (%) | Platelet (× 103/cm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle control | 13.90 ± 1.25 | 5.19 ± 0.36 | 51.05 ± 2.31 | 86.12 ± 2.16 | 31.97 ± 2.36 | 34.90 ± 2.46 | 43.18 ± 2.07 | 40.65 ± 1.63 | 45.13 ± 2.09 | 11.05 ± 1.03 | 2.15 ± 0.04 | 1.02 ± 0.02 | 4.17 ± 0.93 |
| Artesunate (2) | 15.62 ± 1.07ns | 5.63 ± 0.79ns | 46.92 ± 2.15ns | 95.75 ± 3.77ns | 31.15 ± 1.96ns | 33.23 ± 1.66ns | 44.95 ± 2.18ns | 44.35 ± 2.17ns | 42.35 ± 2.13ns | 10.16 ± 1.20ns | 2.06 ± 0.12ns | 0.98 ± 0.01ns | 4.42 ± 0.72ns |
| Artesunate (4) | 11.52 ± 1.33ns | 5.15 ± 0.86ns | 42.85 ± 2.17ns | 99.25 ± 3.15ns | 32.43 ± 1.78ns | 36.73 ± 2.17ns | 57.63 ± 3.02b | 45.09 ± 2.44ns | 43.19 ± 2.33ns | 7.07 ± 1.09ns | 2.75 ± 0.11ns | 1.90 ± 0.04c | 3.5 ± 0.25ns |
| Artesunate (8) | 10.46 ± 1.28ns | 4.65 ± 0.97ns | 38.15 ± 2.63b | 109.23 ± 4,75b | 34.16 ± 1.09ns | 39.12 ± 2.05ns | 59.25 ± 3.11b | 50.60 ± 3.01a | 36.60 ± 2.08ns | 7.25 ± 1.55ns | 4.45 ± 0.32c | 1.10 ± 0.03ns | 3.3 ± 0.36ns |
n = 6. ns = not significant when compared to vehicle control group, Hb = hemoglobin, RBC = red blood cell, PCV = packed cell volume, MCV = mean cell volume, MCH = mean cell hemoglobin, MCHC = mean cell hemoglobin concentration, WBC = white blood cell, NEU = neutrophil, LYM = lymphocyte, MON = monocyte, EOS = eosinophil and BAS = basophil.
p < 0.05.
p < 0.01.
p < 0.001.
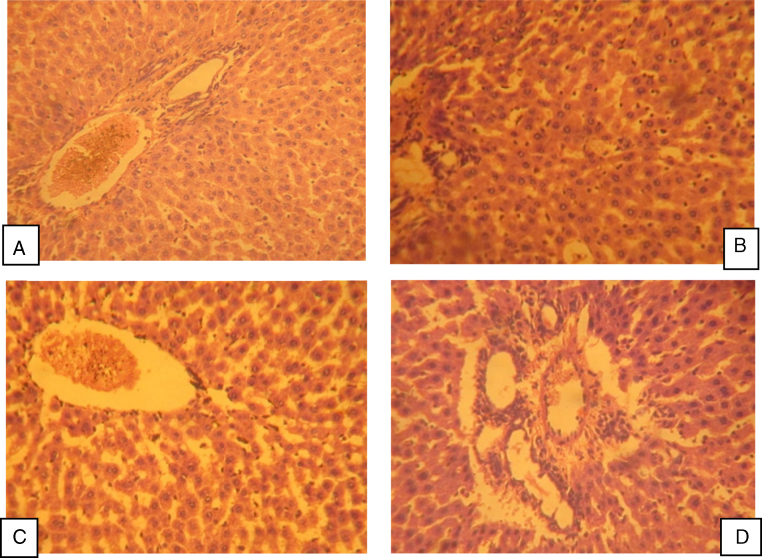
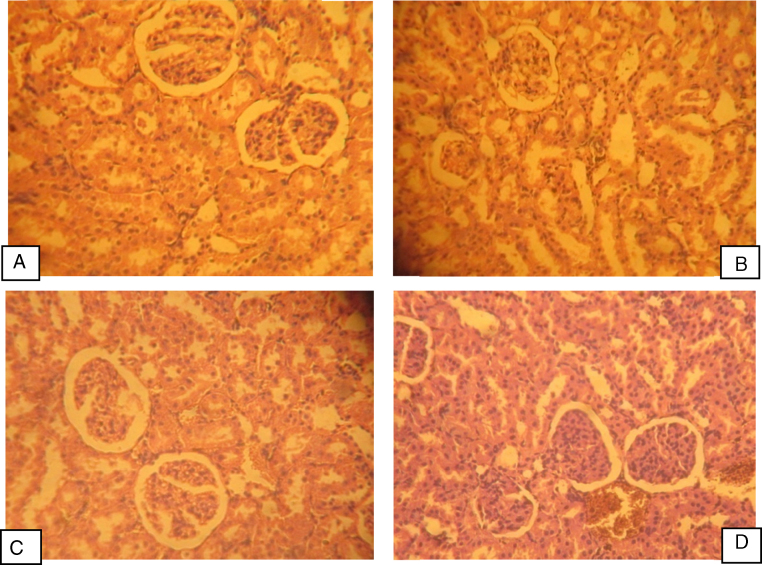
Histology of liver and kidney: Light microscopic examination of liver and kidney sections of the control group showed a normal histology of liver and kidney. Artesunate (2 mg/kg/day) treatment showed normal hepatocytes and sinusoids with mild inflammatory infiltration. Treatment artesunate at 4 mg/kg/day showed sinusoidal dilation and cytoplasmic vaculation whereas 8 mg/kg/day showed focal necrosis, sinusoidal congestion and extensive inflammatory changes (Fig. 4). Treatment of artesunate at all three doses does not have any deleterious effect on histological features of rat kidney (Fig. 5).
Fig. 4.
Photomicrograph of rat liver treated with different doses of artesunate for 45 days. (A) Control group showing normal hepatocytes. (B) Artesunate (2 mg/kg/day) group showing normal hepatocytes and sinusoids with mild inflammatory infiltration. (C) Artesunate (4 mg/kg/day) group showing sinusoidal dilation and cytoplasmic vaculation. (D) Artesunate (8 mg/kg/day) group showing diffuse focal necrosis, sinusoidal congestion, cytoplasmic vaculation and some areas with extensive inflammatory changes.
Fig. 5.
Photomicrograph of rat kidney treated with different doses of artesunate for 45 days. (A) Control group, (B) artesunate (2 mg/kg/day) group, (C) artesunate (4 mg/kg/day) group and (D) artesunate (8 mg/kg/day) group showing normal kidney histology.
4. Discussion
Artesunate introduced by World Health Organization (WHO) to combat the problems of multi-drug resistant P. falciparum malaria [35]. Artesunate is the most widely available artemisinin-related compounds, which is a semi-synthetic hemisuccinate derivative of dihydroartemisinin [36]. The artemisinin is potent antimalarial drugs that are remarkably well tolerated [37]. Artesunate can be given parentally, intravenously, intramuscularly, orally or rectally [38].
Generally, artesunate exert its anti-malarial activity by the generation of reactive oxygen species from its endoperoxide bond leading to lipid peroxidation. The reactive oxygen species cause macromolecular damage by alkylating heme and several other proteins. The recommended dose of artesunate is 2–10 mg/kg/day or maximum up to 200 mg/day in adult human body. Large clinical studies with malaria patients have shown that artesunate is well tolerated, with a few and insignificant side effects. However, several studies have showed evidence of toxicity on the brainstem, superior colliculus, stomach, testis and liver in artesunate treated rats. Moreover, artesunate has been reported to destroy cancer cells and also reduces proliferation, interferes in DNA replication and cell cycle and enhance apoptosis through the intrinsic death pathway by ROS generation. It has been reported that artesunate is toxic to malaria parasites at nanomolar concentration, whereas micromolar concentration is required for toxicity in mammalian cells [39]. Evidences showed the neurotoxicity of artesunate at high doses (50–100 mg/kg/day oral and IM) in laboratory animals [40] including the cytotoxicity of artesunate on tumor cell lines have been reported [41].
Standard sample of artesunate has been obtained as a gift sample following which identification and authentification was done by measurement of melting point, UV spectroscopy, TLC and HPLC. The objective of this study is to investigate the effect of sub-chronic oral administration of artesunate for 45 days at the dose of 2 mg/kg/day (equivalent to half of minimum recommend therapeutic dose), 4 mg/kg/day (equivalent to therapeutic dose) and 8 mg/kg/day (double the therapeutic dose) on biochemical, hematological and histological parameters of liver and kidney. These parameters have been selected to assess the biochemical integrity of blood cells (erythrocytes the primary site of action), liver (the site of metabolism) and kidney (the site of excretion) due to their role in parasiticidal action, detoxification and eventual elimination of artesunate in the host. The parameter assessed for hepatotoxicity and nephrotoxicity is serum SGOT, SGPT, ALP, TG, TC, total protein, bilirubin, albumin, creatinine, urea and glucose level. The hematological parameters assessed were hemoglobin, total RBC, total WBC, PCV, MCV, MCH, MCHC, differential WBC and platelet count along with body weight and relative organ weight.
In the present study artesunate treatment for 45 days at 4 and 8 mg/kg/day treatment showed nearly 50% less body weight gain in rats compared to vehicle control animals. Artesunate showed significant increase in SGOT, SGPT, ALP, TC and TG at 4 and 8 mg/kg/day doses. Total bilirubin level was increased only at 8 mg/kg/day dose and has no effect on total protein, albumin, creatinine and urea but glucose level was elevated significantly only 8 mg/kg/day dose. The liver is the second largest organ in the body involved in a host of functions including synthesis of clotting factors, detoxification and metabolism of lipids and carbohydrates. Substantial disruption in its anatomy or function may result in severe alteration in its metabolic roles, and this may adversely affect physiological functions. Release of active oxygen species from artesunate bonded with hemoglobin kills the malaria parasite accumulated in the erythrocytic cells. Artesunate may indirectly through generation of ROS or directly as toxin to the cells of the liver, affect their cellular integrity and cause defect in membrane permeability and cell volume homeostasis. In humans hepatotoxicity can be particularly severe if artesunate is used in combination with HIV antiretroviral drugs [42].
Izunya et al. [17] reported that artesunate treatment at 4–8 mg/kg for 7 days has no effects on the histology of the heart in rats. Artesunate given at 16 mg/kg, orally for 7 days caused disturbance in liver function with damage to hepatocytes of guinea pig [16]. Hepatotoxicity and hemolytic effects of artesunate on rats at 4 mg/kg, oral for 5 days treatment are reported by Omotuyi et al. [18]. Potential hepatotoxicity of artesunate is also reported by Izunya et al. [14] at 4–8 mg/kg, orally administered for 7 days on rats. Several studies on artesunate showed evidence of toxicity on the brain stem [6], [7], superior colliculus [43], stomach [44] and testis [45]. Artemisinin derivatives showed male reproductive dysfunction and hematological abnormalities effecting liver blood cells and testis of guinea pigs at 2–8 mg/kg oral dosing for 7 days. Nduka and Olufunso [15] reported alteration in biochemical parameters of liver and kidney of rats by artesunate at 2–5 mg/kg, oral dosing for 7 days.
Artesunate has non-significant effect on hemoglobin, total RBC, MCH, MCHC level, differential count of lymphocytes and basophil and total platelet count. Packed cell volume and mean cell hemoglobin concentration showed significant rise amongst the hematological indices measured at 8 mg/kg/day dose. This may not be connected with artesunate's ability to accumulate in the red blood cells being the primary site of action. The RBC counts showed statistically insignificant difference, thus the increase in PCV may have occurred due to decrease in plasma volume or alteration of the size and shape of the erythrocytes [46]. Since the hemoglobin concentration does not alter in the same pattern as the PCV, the most likely alteration may have occurred in the size and shape of the erythrocytes. This could be as a result of artesunate binding to the plasma membrane of the erythrocytes, as it is the target site of action. The under-lying mechanism for the parasiticidal activity of artesunate is a free radical reaction [47]. This reaction is initiated with the reaction of artesunate with iron present in the heme prosthetic group of hemoglobin, leading to the generation of activated oxygen species, such as oxygen radicals and superoxide radicals. These radicals then propagate free radical reaction capable of inducing oxidative damage in the parasite [48]. The mechanism of action of these drugs and their ability to accumulate in the red blood cell being its primary site of action is associated with hemotoxicity. The hemolytic effect of artesunate at 2, 4 and 8 mg/kg/day, oral for 7 days was monitored by changes in the packed cell volume (PCV), total bilirubin, conjugated bilirubin and MDA levels of erythrocyte [18]. An increased hepatocyte and erythrocyte malondialdehyde level was observed in 4 mg/kg/day group. The result also showed increased activities for the serum enzymes in 2 and 4 mg/kg/day treated groups. There is a clear indication that hepatotoxicity and hemotoxicity are associated with artesunate administration at both required and overdose conditions however these effects are magnified in overdose conditions.
5. Conclusion
Artesunate at 4 mg/kg/day caused sinusoidal dilation and cytoplasmic vaculation and at 8 mg/kg/day dose showed focal diffuse necrosis, sinusoidal congestion, cytoplasmic vaculation and extensive inflammation. Rats were orally administration with 2, 4 and 8 mg/kg/day of artesunate daily for 7 days. The histological findings showed sinusoidal congestion with cytoplasmic vaculation (hepatocyte edema) and mild inflammation of the portal tracts though it does not seem to induce change in relative weight of liver. Artesunate treatment has not showed any damage to kidney structure. This study suggests that artesunate at normal dose has a toxic effect on the liver cells and could be a potential hepatotoxic drug [14]. Thus, sub-chronic exposure of artesunate at 8 mg/kg/day dose for 45 days period may cause hepatic damage along with hematological abnormalities. Future studies are also required to be done to assess the safety of this group of antimalarial drugs in vulnerable populations, therefore, be considered in order to generate a useful safety information and database to improve the quality of health care offered to the patient.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Transparency document
Acknowledgements
The authors are thankful to the Board of Trustees of Radharaman Group of Institutes, Radharaman College of Pharmacy, Bhopal, Madhya Pradesh, India for providing research facilities to carry out present work.
Contributor Information
Papiya Bigoniya, Email: p_bigoniya2@hotmail.com.
Taranginee Sahu, Email: taranginee.sahu14@gmail.com.
Vikalp Tiwari, Email: vikalp.tiwari.mgm@gmail.com.
References
- 1.Chen P.K., Leather G.R., Klayman D.L. Allelopathic effect of artemisinin and its related compounds from Artemisia annua. Plant Physiol. 1987;83S:406. [Google Scholar]
- 2.Ellman A. Cultivation of Artemisia annua in Africa and Asia. Outlooks Pest Manag. 2010 [Google Scholar]
- 3.Dondorp A., Nosten F., Stepniewska K., Day N., White N., South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) Group Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–725. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 4.Adjuik M., Babiker A., Garner P., Olliaro P., Taylor W., White N., International Artemisinin Study Group Artesunate combinations for treatment of malaria: meta-analysis. Lancet. 2004;363:9–17. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- 5.Oyakhirome S., Potschke M., Schwarz N.G., Dornemann J., Laengin M., Salazar C.O., Lell B., Kun V., Kremsner P.G., Grobusch M.P. Artesunate – amodiaquine combination therapy for falciparum malaria in young Gabonese children. Malaria J. 2007;6 doi: 10.1186/1475-2875-6-29. Available at: http://www.malariajournal.com/content/6/1/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nontprasert A., Nosten-Bertrand M., Pukrittayakamee S., Vanijanonta S., Angus B.J., White N.J. Assessment of the neurotoxicity of parenteral artemisinin derivatives in mice. Am. Soc. Trop. Med. Hyg. 1998;59:519–522. doi: 10.4269/ajtmh.1998.59.519. [DOI] [PubMed] [Google Scholar]
- 7.Nontprasert A., Pukrittayakamee S., Dondorp A.M., Clemens R., Looareesuwan S., White N.J. Neuropathologic toxicity of artemisinin derivatives in a mouse model. Am. Soc. Trop. Med. Hyg. 2002;67:423–429. doi: 10.4269/ajtmh.2002.67.423. [DOI] [PubMed] [Google Scholar]
- 8.McGready R., Stepniewska K., Ward S.A., Cho T., Gilveray G., Looareesuwan S., White N.J., Nosten F. Pharmacokinetics of dihydroartemisinin following oral artesunate treatment of pregnant women with acute uncomplicated falciparum malaria. Eur. J. Clin. Pharmacol. 2006;62:367–371. doi: 10.1007/s00228-006-0118-y. [DOI] [PubMed] [Google Scholar]
- 9.Adebisi S.S. Artesunate, a promising anti-malarial drug: a review. Ebonyi Med. J. 2007;6:100–105. [Google Scholar]
- 10.Nosten F., White N.J. Artemisinin-based combination treatment of falciparum malaria. Am. Soc. Trop. Med. Hyg. 2007;77:181–191. [PubMed] [Google Scholar]
- 11.White T.E., Bushdid P.B., Ritter S., Laffan S.B., Clark R.L. Artesunate-induced depletion of embryonic erythroblasts precedes embryolethality and teratogenicity in vivo. Birth Defects Res. B: Dev. Reprod. Toxicol. 2006;77:413–429. doi: 10.1002/bdrb.20092. [DOI] [PubMed] [Google Scholar]
- 12.Li Q., Si Y., Smith K.S., Zeng Q., Weina P.J. Embryotoxicity of artesunate in animal species related to drug tissue distribution and toxicokinetic profiles. Birth Defects Res. B: Dev. Reprod. Toxicol. 2008;83:435–445. doi: 10.1002/bdrb.20164. [DOI] [PubMed] [Google Scholar]
- 13.Clark R.L., White T.E.A., Clode S., Gaunt I., Winstanley P., Ward S.A. Developmental toxicity of artesunate and an artesunate combination in the rat and rabbit. Birth Defects Res. B: Dev. Reprod. Toxicol. 2004;71:380–394. doi: 10.1002/bdrb.20027. [DOI] [PubMed] [Google Scholar]
- 14.Izunya A.M., Nwaopara A.O., Aigbiremolen A., Odike M.A.C., Oaikhena G.A., Bankole J.K. Histological effects of oral administration of artesunate on the liver in Wistar rats. Res. J. Appl. Sci. 2010;2:314–318. [Google Scholar]
- 15.Nduka A.G., Olufunso O.O. Evaluation of selected biochemical parameters in renal and hepatic functions following oral administration of artesunate to albino rats. Researcher. 2011;3 Available at: http://www.sciencepub.net/researcher (accessed 10.02.14) [Google Scholar]
- 16.Nwanjo H., Oze G. Acute hepatotocixity following administration of artesunate in guinea pigs. Internet J. Toxicol. 2006;4 Available at: http://ispub.com/IJTO/4/1/8628. [Google Scholar]
- 17.Izunya A.M., Nwaopara A.O., Ayanwu L.C., Odike M.A.C., Oaikhena G.A., Bankole J.K., Okhiai O. Histological studies of the cardiotoxicity of artesunate in Wistar rats. Arch. Appl. Sci. Res. 2011;3:1–6. [Google Scholar]
- 18.Omotuyi I.O., Nwangwu S.C., Okugbo O.T., Okoye O.T., Ojieh G.C., Wogu D.M. Hepatotoxic and hemolytic effects of acute exposure of rats to artesunate overdose. Afr. J. Biochem. Res. 2008;2:107–110. [Google Scholar]
- 19.3rd ed. vols. 1–5. WHO; Geneva: 2009. International Pharmacopoeia; p. 228. (Quality Specifications for Pharmaceutical Substances). [Google Scholar]
- 20.vol. 1. The Indian Pharmacopoeia Commission; Ghaziabad: 2007. p. 144. (Indian Pharmacopoeia). 741–742. [Google Scholar]
- 21.Kalyankar T.M., Kakde R.B., Attar M.S., Kamble A.R. Simultaneous spectrophotometric estimation of artesunate and mefloquine. J. Chem. 2013;2013:5. Available at: http://dx.doi.org/10.1155/2013/679857 (article ID 679857) [Google Scholar]
- 22.Gandhi S., Deshpande P., Jagdale P., Godbole V. A simple and sensitive RP-HPLC method for simultaneous estimation of Artesunate and Amodiaquine in combined tablet dosage form. J. Chem. Pharm. Res. 2010;2:429–434. [Google Scholar]
- 23.Reitman S., Frankel S. A colorimetric method for determination of serum glutamic-oxaloacetic and glutamic-pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Allain C.C., Poon L.S., Chan C.S., Richmond W., Fu P.C. Enzymatic determination of total serum cholesterol. Adv. Clin. Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 25.McGowan M.W., Artiss J.D., Strandbergh D.R., Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Adv. Clin. Chem. 1983;29:538–542. [PubMed] [Google Scholar]
- 26.Jendrassik L., Gorf P. Verainfachte photometrische Methoden zur Bestimmung des Blutbilirrubins. Biochem. Zeitsch. 1938;297:81–89. [Google Scholar]
- 27.Husdan H., Rapoport A. Estimation of creatinine by the Jaffe reaction. A comparison of three methods. Clin. Chem. 1968;14:222–238. [PubMed] [Google Scholar]
- 28.Tietz N.W. 3rd ed. W.B. Saunders Company; London: 1987. Fundamentals of Clinical Chemistry; pp. 577–678. [Google Scholar]
- 29.Trinder P. Determination of glucose in blood glucose oxidase with an alternative oxygen receptor. Ann. Clin. Biochem. 1969;6:24–27. [Google Scholar]
- 30.Lowry O.H., Rosenbrough N.J., Farr A.L., Randall R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Kind P.R.N., King E.J. In: Varley H., Gowenlock A.H., Bell M., editors. Heinemann; London: 1980. pp. 899–900. (Method of Practical Clinical Biochemistry). [Google Scholar]
- 32.Keyser J.W. Rapid estimation of albumin and total protein in small amounts of blood serum. Clin. Chim. Acta. 1962;7:299–300. doi: 10.1016/0009-8981(62)90026-8. [DOI] [PubMed] [Google Scholar]
- 33.Ghai C.L. 5th ed. Jaypee Brothers; New Delhi: 1999. A Textbook of Practical Physiology; pp. 47–69. [Google Scholar]
- 34.Nanji A.A., Jakelainen K., Fotouhinia M., Rahemtulla A., Thomas P., Tipoe L.G., Su G.L., Dannenberg A.J. Increased severity of alcoholic liver injury in female rats: role of oxidative stress, endotoxin and chemotoxin. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;281:G1348–G1356. doi: 10.1152/ajpgi.2001.281.6.G1348. [DOI] [PubMed] [Google Scholar]
- 35.Turschner S., Efferth T. T. Drug resistance in plasmodium: natural products in the fight against malaria. Mini Rev. Med. Chem. 2009;9:206–214. doi: 10.2174/138955709787316074. [DOI] [PubMed] [Google Scholar]
- 36.Ittarat W W., Udomsangpeth R., Chotivanich K.T., Looareesuwan S. The effects of quinine and artesunate treatment on plasma tumor necrosis factor levels in malaria infected patients. Southeast Asian J. Trop. Med. Public Health. 1999;30:7–10. [PubMed] [Google Scholar]
- 37.Maude R.J., Plewes K., Faiz M.A., Hanson J., Charunwatthana P., Lee S.J., Tarning J., Yunus E.B., Hoque M.G., Hasan M.U., Hossain A., Lindegardh N., Day N.P., White N.J., Dondorp A.M. Does artesunate prolong the electrocardiograph QT interval in patients with severe malaria. Am. J. Trop. Med. Hyg. 2009;80:126–132. doi: 10.4269/ajtmh.2009.08-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newton P., Suputtamongkol Y., Teja-Isavadharm P., Pukrittayakamee S., Navaratnam V., Bates I., White N. Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob. Agents Chemother. 2002;44:972–977. doi: 10.1128/aac.44.4.972-977.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meshnick S.R. Artemisinin: mechanisms of action, resistance and toxicity. Int. J. Parasitol. 2002;32:1655–1660. doi: 10.1016/s0020-7519(02)00194-7. [DOI] [PubMed] [Google Scholar]
- 40.Brewer T.G., Peggins J.O., Grate S.J., Petras J.M., Levine B.S., Weina P.J., Swearengen J., Heiffer M.H., Schuster B.G. Neurotoxicity in animals due to arteether and artemether. Trans. R. Soc. Trop. Med. Hyg. 1994;88:33–36. doi: 10.1016/0035-9203(94)90469-3. [DOI] [PubMed] [Google Scholar]
- 41.Effert T., Dunston H., Sauerbrey H. The antimalarial artesunate is also active against cancer. Int. J. Oncol. 2001;18:767–773. doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- 42.German P., Greenhouse B., Coates C., Dorsey G., Rosenthal P.J. Hepatotoxicity due to a drug interaction between amodiaquine plus artesunate and efavirenz. Clin. Infect. Dis. 2007;44:889–891. doi: 10.1086/511882. [DOI] [PubMed] [Google Scholar]
- 43.Eweka A.O., Adjene J.O. Histological studies of the effects of oral administration of artesunate on the superior colliculus of adult Wistar rats. Internet J. Trop. Med. 2008;4:1–9. [Google Scholar]
- 44.Eweka A.O., Adjene J.O. Histological studies of the effects of oral administration of artesunate on the stomach of adult Wistar rats. Internet J. Health. 2008;7:1–7. [Google Scholar]
- 45.Izunya A.M., Nwaopara A.O., Aigbiremolen A.E., Odike M.A.C., Oaikhena G.A., Bankole J.K. Histological studies of the toxicity of artesunate on the testes in Wistar rats. Biol. Med. 2010;2:49–56. [Google Scholar]
- 46.Widman F.K. 9th ed. American Association of Publishers; Washington, DC: 1980. Clinical Interpretation of Laboratory Test; pp. 293–295. [Google Scholar]
- 47.Olliaro P., Haynes R.K., Meunier B., Yuthavong Y. Possible modes of action of the artemisinin type compounds. Trends Parasitol. 2001;17:122–126. doi: 10.1016/s1471-4922(00)01838-9. [DOI] [PubMed] [Google Scholar]
- 48.Meshnick S.R., Taylor T.E., Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol. Rev. 1996;60:301–315. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.