Highlights
-
•
Distinct concentrations of Ketorolac/Gentamicin are toxic to mesangial (MES 13) cells.
-
•
Omega-3 fatty acids protect intraglomerular mesangial cells against nephrotoxic drugs.
-
•
Omega-6 fatty acids do not protect MES 13 cells against nephrotoxic drugs.
-
•
Cytoprotection by n-3 PUFA may be due to increased intracellular redox state.
Keywords: Omega-3 fatty acids, Cytoprotection, Nephrotoxic drugs, Ketorolac, Gentamicin, Intraglomerular mesangial cells
Abstract
During therapeutic interventions, blood concentrations of intravenously applied drugs are higher, and their onset of pharmacological action is faster than with other routes of drug administration. However, acute drug therapy often produces nephrotoxic side effects, as commonly seen after treatment with Ketorolac or Gentamicin leading to questions about their use, especially for patients at risk for acute renal failure. Omega-6(n-6) and omega-3(n-3) polyunsaturated fatty acids (PUFA) affect eicosanoid metabolism, which plays a role in the regulation of inflammation. Eicosanoids derived from n-6 FA have proinflammatory and immunoactive functions, whereas eicosanoids derived from n-3 PUFA have anti-inflammatory and cytoprotective properties. We hypothesized that providing such injectable drugs with nephrotoxic potential in combination with n3-PUFAs from the outset, might afford rapid cytoprotection of renal cells, given the recent evidence that intravenously administered n3-PUFAs are rapidly incorporated into cell membranes. We used intraglomerular mesangial cells (MES13) that are sensitive to treatment with Ketorolac or Gentamicin instead of proximal tubular cells which do not respond to Ketorolac. We found a significant inhibition of Ketorolac (0.25, 0.5, 1 mM) or Gentamicin (2.5, 5 mM) induced cytotoxicity after pretreatment of MES13 cells with 0.01% of 20%w/v LipOmega-3 Emulsion 9/1, containing 90:10 wt/wt mixture of fish oil derived triglycerides to medium chain triglycerides.
1. Introduction
The underlying toxicity of many drugs to vital organs of the body is often a result of localized ischemia which triggers a series of complex biochemical events related to hypoxia, inflammation, and with the potential for subsequent oxidative stress with reperfusion, all of which induce cellular damage. This is particularly true in the critically ill patient in the intensive care unit with an ineffective circulating blood volume (e.g., mean arterial blood pressure < 60 mmHg) causing moderate to severe hypoperfusion to vital organs such as the kidney, producing acute tubular necrosis. In this setting, the likelihood or severity of acute kidney injury (AKI) can be aggravated by the concurrent administration of injectable nephrotoxic drugs, with mortality rates from acute renal failure ranging from 25 to 64% [21].
Thus, the combined impact of ischemic insult to aerobic tissues, from both disease(s) and drug(s), greatly accentuates the potential for damage to vital organs in the critically ill. During ischemia, oxygen, as well as other vital nutrients become limiting to dependent tissues such as the kidneys, causing mitochondrial dysfunction [4]. Blood flow to the kidneys as a fraction of the total cardiac output is approximately 20% [11]. Thus, even limited reductions in normal blood flow to the kidneys resulting from a compromised circulating blood volume as described above, can have devastating consequences for renal tissues, especially during the concomitant intravenous administration of nephrotoxic drugs in full therapeutic doses.
Polyunsaturated omega-3 or n-3 fatty acids (n3-PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) from fish oil triglycerides, have been shown to mitigate the nephrotoxic effects of cyclosporine A in an experimental animal model [9]. Animals were pre-fed via gastric lavage for 14 days prior to nephrotoxic drug exposure in order to obtain sufficient incorporation of n3-PUFAs into plasma cell membranes to exert their cytoprotective effects. Although this approach would seem viable in patients receiving such therapy in the long-term setting (e.g., lifelong immunosuppression with cyclosporine A for organ transplantation), the requisite time course for pre-treatment with n3-PUFAs in the acute care setting in patients with, for example, life-threatening blood infections, or, for acute postoperative pain, is not achievable. That is, acute drug therapy is often vital with a potential nephrotoxin given intravenously to achieve higher concentrations more rapidly and completely than achievable with oral administration in order to improve therapeutic efficacy, which also increases the potential for adverse renal effects that are generally related to drug levels. However, we hypothesize that providing such injectable drugs in combination with n3-PUFAs from the outset, may afford rapid cytoprotection of renal cells, especially in light of recent evidence that intravenously administered n3-PUFAs are rapidly incorporated into plasma cell membranes within 1 h of infusion after a single dose [6]. Once incorporated into cell membranes, a key step to exert cytoprotection, n3-FAs dramatically modulate the body's response to inflammation, oxidative stress, ischemia and immune function through several downstream bioactive mediators, (e.g., cytokines, prostaglandins, thromboxanes, leukotrienes, resolvins, protectins, etc.) [2].
The purpose of this investigation was to study the potential cytoprotective effects of omega-3 fatty acids against known nephrotoxic drugs in an in vitro murine intraglomerular mesangial cell model to establish proof of concept. The combination of “cytoprotective excipients” with selected nephrotoxic drugs constitutes a unique opportunity for potentially safer intravenous therapy in the acute care setting, where the inclusion of omega-3 fatty acids could also serve as a secondary supportive, but important, active pharmaceutical ingredient, to form a novel therapeutic drug vehicle.
2. Material and methods
At the outset, SV 40-transformed mouse mesangial cells, MES 13 (CRL-1927™; purchased from the American Type Culture Collection [ATCC-LGC Standards GmbH], Wesel, Germany) were used.
The selection of these cells, as opposed to proximal tubule cells typically associated with renal tubular damage, was based on observations that nephrotoxicity from drugs such as Gentamicin is related to their direct effect (proliferation and apoptosis) on cultured mesangial cells [20] In contrast to MES13, the HK-2, an immortalized proximal tubule epithelial cell line from normal adult human kidney have not shown any response after treatment with Ketorolac (not shown). Thus, we chose to study MES13 and two nephrotoxins typically given by intravenous administration in the ICU setting: The potent analgesic, Ketorolac, and the aminoglycoside antibiotic, Gentamicin.
2.1. Cell lines and culture conditions
The MES 13 cells were cultured in ATCC complete growth medium: the basic medium for these cell lines is a 3:1 mixture of ATCC-formulated Dulbecco's Modified Eagle's Medium [ATCC (DMEM)], and Ham's F12 medium (PAA Laboratories GmbH, Cölbe, Germany) with 14 mM HEPES, supplemented with 5% fetal bovine serum (FBS), 100 U/ml penicillin, 0.1 mg/ml streptomycin. Under these culture conditions the MES 13 retained many of the differentiated characteristics of mesangial cells. Cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air; the medium was changed every 48 h.
2.2. Substances under test
2.2.1. The control emulsions
20%w/v Lipofundin N, containing 20% soybean oil only triglycerides, Lot No 111258082.
2.2.2. The test emulsions
20%w/v LipOmega-3 Emulsion 5/5, containing 50:50wt/wt mixtures of fish oil (FO) derived triglycerides and (MCTs), solution number 356. Lot No 11182049, 20%w/v LipOmega-3 Emulsion 7/3, containing 70:30wt/wt mixtures of fish oil (FO) derived triglycerides and medium chain triglycerides (MCTs), respectively, solution number 358. Lot No 11182050; 20%w/v LipOmega-3 Emulsion 9/1, containing 90:10wt/wt mixtures of fish oil (FO) derived triglycerides and medium chain triglycerides (MCTs), respectively, solution number 359. Lot No 11202049. All lipid emulsions by B. Braun Melsungen AG, Melsungen, Germany.
The fish oil used in all 3 fish oil-containing emulsions tested is highly enriched, and we have data on this oil from the manufacturer citing the following minimum omega-3 concentrations: Total—60%; EPA—33%; DHA—22%, but we have measured (GC/MS) the total at nearly 70% and EPA + DHA of 62% (1.5:1 ratio). So given this information, we estimate the average EPA + DHA in the oil to be 60% enriched. From this, the test emulsions should contain the approximate amounts of FO, EPA and DHA (in g/dl) as follows: 9/1 (18 FO, 10.8 ΣEPA + DHA, 6.5 EPA, 4.3 DHA), 7/3 (14 FO, 8.4 ΣEPA + DHA, 5.1 EPA, 3.3 DHA), 5/5 (10 FO, 6 ΣEPA + DHA, 3.6 EPA, 2.4 DHA).
2.2.3. The nephrotoxic drugs
Ketorolac Tromethamine Injection, USP, I.V./I.M. 30 mg/ml (Hospira, Inc., Lake Forest USA.); Gentamicin solution, 50 mg/ml, (Sigma-Aldrich, Taufkirchen, Germany).
2.3. Determination of cytotoxic concentrations of Ketorolac and Gentamicin
4 × 103 (for Gentamicin test) or 8 × 103 (for Ketorolac test) MES 13 100 μl medium/well was seeded in 96-well plates (BD Falcon™, Becton Dickinson GmbH, Heidelberg, Germany). After 48 h, the medium was changed and different concentrations of Ketorolac (0.1–1 mM) and Gentamicin (0.5–5 mM) were tested after 24 h treatment, and cytotoxicity was measured as described below (see Section 2.5). As a control (=0% cytotoxicity), we used cells cultured with medium alone, i.e., without Ketorolac or Gentamicin treatment.
2.4. Determination of the protective effects of the emulsions against cytotoxicity from Ketorolac or Gentamicin
4 × 103 or 8 × 103 MES 13 was seeded in 100 μl medium/well in 96-well plates (BD Falcon™, Becton Dickinson GmbH, Heidelberg, Germany). After 48 h, the medium was changed and 50 μl/well medium was added. Immediately the emulsions were dispersed in medium at 50 μl/well at concentrations of 0.01%, 0.02% or 0.04% (end concentration: 0.005%, 0.01% or 0.02%) added to the cells. As a negative control, we used cells which received 100 μl medium without any emulsions. After 24 h pre-treatment or pre-incubation with the above mentioned emulsions, the following concentrations of Ketorolac were added to the cells for the next 24 h together with the emulsions: 0.1 mM, 0.25 mM, 0.5 mM or 1 mM, whereas Gentamicin was added at 0.5 mM, 1 mM, 2.5 mM or 5 mM. The different concentrations of Ketorolac or Gentamicin were added to the cells either pre-treated (24 h) with emulsions (potential protective effect) or without emulsion (cytotoxicity of Ketorolac or Gentamicin [control]). Additional controls were performed: cells pre-incubated with emulsions, however, without Ketorolac or Gentamicin (control of the emulsion effect) as well as cells without emulsion pre-treatment, and without Ketorolac or Gentamicin (control = 0% cytotoxicity).
Finally, co-incubation or co-treatment with the test emulsions plus Ketorolac or Gentamicin was performed. Briefly, different concentrations of Ketorolac (0.5 mM or 1 mM) and Gentamicin (2.5 or 5 mM), were added to the cells at the same time as the emulsions (0.005%, 0.01% or 0.02%) and incubated for 24 h or 48 h. Cytotoxicity was measured as described below (see Section 2.5).
2.5. Determination of cell death or cytotoxicity
Cell cytotoxicity was assessed using PrestoBlue™ [3] reagent (Invitrogen-Life Technologies GmbH, Darmstadt, Germany). PrestoBlue™ reagent is a resazurin-based solution that functions as a cell viability indicator by using the reducing power of living cells to quantitatively measure the viability. When added to cells, the PrestoBlue™ reagent – containing a non-fluorescent, cell-permeant compound – is modified by the reducing environment of the viable cells, becoming highly fluorescent, which can be detected using fluorescence or absorbance measurements. PrestoBlue™ reagent is more sensitive than AlamarBlue®, which is a redox indicator of enzyme activity widely used in whole organism screening [18] and is extensively used in screening tests of viability and cytotoxicity [18], [22], [12], [29], [1], [27]. PrestoBlue™ was directly added to the cells in the culture medium at a final concentration of 10%. Thereafter the plates were returned to the incubator. 30 min, 1 h, 2 h, 3 h and 4 h after addition of PrestoBlue™ the optical density (OD) was measured at 570 nm and 600 nm (as reference) with a SUNRISE ELISA-reader (Tecan, Salzburg, Austria). Results are expressed in % of cytotoxicity [100 − (OD570/600 of samples × 100/OD570/600 of control without substances)].
2.6. RNA-preparation and reverse transcription polymerase chain reaction (RT-PCR)
RNA was extracted from 1 × 106 cells using peqGOLD Isolation Systems TriFast™ (PEQLAB Biotechnologie GmbH Erlangen Germany) according to the manufacturer's specifications. RNA concentration and purity were determined by A260 and A280 (A260/A280 = 1.7 to 2.0) measurements using a NanoDrop 2000c Spectrophotometer (Thermo Scientific, Schwerte, Germany). Total RNA integrity was confirmed by lab-on-a-chip technology, using an RNA 6000 NanoChip kit on an Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). An aliquot of 1 μg total RNA was treated with 1 unit of RNAse (Thermo Scientific, St. Leon-Rot, Germany) for 30 min at 37 °C. Reverse transcription of RNA (1 μg) was performed with 500 ng oligo (dT)12–18 primer, 20 unit of the Affinity Script multiple temperature cDNA synthesis (Agilent) and 24 unit of Ribo LockTM RNAse inhibitor (Fermentas) for 1 h at 42 °C. The cDNA was used for quantitative real-time polymerase chain reaction (qRT-PCR) using the QuantiTect/primerAssays from QIAGEN GmbH (Hilden, Germany). The mRNA expression was analyzed for: BCL2-associated X protein, NM_007527 (BAX), amplicon length 78 bp; B-cell CLL/lymphoma 2, NM_009741 (BCL2), amplicon length 104 bp; caspase 3, apoptosis-related cysteine peptidase, NM_009810 (Casp3), amplicon length 150 bp; prostaglandin-endoperoxide synthase 2, NM_011198 (PTGS2, cyclooxygenase-2, COX-2), amplicon length 95 bp; glutathione reductase, NM_010344 (GSR), amplicon length 92 bp; glutathione synthetase, NM_008180 (GSS), amplicon length 95 bp; proliferating cell nuclear antigen NM_011045 (PCNA), amplicon length 104 bp, thromboxane A synthase 1; NM_011539 (TBXAS), amplicon length 179 bp. The following reference gene was used: Ribosomal protein L32, NM_172086 (RPLP32), amplicon length 88 bp. cDNAs were amplified with Brilliant III Ultra-Fast SYBR Green QRT-PCR Master Mix (Stratagene-Agilent Technologies, Waldbronn, Germany). The thermal profile consisted of 1 cycle at 95 °C for 3 min followed, 40 cycles at 95 °C (10 s) and 60 °C (20 s). Amplification and data analysis were performed using the Mx3005PTM QPCR System (Stratagene). The data were analyzed using the relative standard curve method. For each unknown sample, the relative amount was calculated using linear regression analysis from their respective standard curves. For relative quantification, a standard curve was generated from a pool of cDNA. Specificity of the amplified product was confirmed by melting curve analysis (55–95 °C), and additionally, by using 2% agarose gel electrophoresis to verify the amplicon size in conjunction with melting curve data. The NormFinder software program was used to ascertain the most suitable reference gene to normalize the RNA input as described earlier [26].
2.7. Biochemical determination of total glutathione (tGSH), glutathione disulfide (GSSG), and reduced glutathione (rGSH)
The MES 13 cells were washed three times with 10 mM phosphate-buffered saline (pH 7.4), mixed with 2.5% sulfosalicylic acid (SSA), and subjected to sonication and centrifugation. The clear supernatants were finally used for determining tGSH and GSSG (oxidized GSH) by the method of [28], as described previously [15]. rGSH was computed by subtraction (rGSH tGSH—GSSG). The pellets were subjected to protein determination [23] and used to normalize the values of tGSH and GSSG. The intracellular REDST of MES 13 cells was defined as rGSH2 GSSG−1.
2.8. TNF-α, Thromboxane B2 and nitrite determination
The release of TNF-α and Thromboxane B2 (Abnova GmbH, Heidelberg, Germany) were determined using an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions. Total nitrite formed from NO release into the culture medium was spectrophotometrically determined by using the Griess reagent (Sigma-Aldrich, St. Louis, USA). The optical densities were measured at 540 nm with the Sunrise ELISA Reader (Tecan, Salzburg, Austria).
2.9. Apoptosis assay
MES 13 cells were seeded in 24-well plates at a density of 5 × 104 cells per well and the substances under test were added at various concentrations as indicated. Apoptotic cells were identified by YO-PRO-1 (1 μM) staining [13] in combination with the Hoechst 33342 dye (5 μg/ml) (Mobitec Company, Göttingen, Germany) as previously described [7]. The apoptotic cells were counted and their percentage determined as described earlier, using an inverse fluorescence microscope Eclipse TS100 (Nikon GmbH, Dűsseldorf, Germany) provided with a camera AxioCam MRc (Carl Zeiss Microscopy GmbH, Göttingen, Germany) and a computer-assisted morphometry system AxioVision 4 (Carl Zeiss Microscopy GmbH).
2.10. SDS-PAGE and Western blot
MES 13 cells were scraped off in RIPA buffer pH 7.5 (Cell Signaling Technology Europa, Leiden, The Netherlands). An aliquot was used for protein quantification using Pierce™ BCA Protein Assay Kit (Pierce Biotechnology, Rockford, USA), and after addition of sample buffer, pH 8.3, boiled at 95 °C (10 min). Samples were stored at −20 °C. Afterwards, electrophoresis was done using NuPAGE® Novex® 4–12% Bis–Tris Gels, pre-cast polyacrylamide gels (Life Technologies GmbH, Darmstadt Germany) and a loading of 30 μg total protein per lane. Additionally, prestained peqGOLD Protein-Marker VI (PEQLAB Biotechnologie GmbH, Erlangen Germany) was used. Blotting was performed with wet/tank blotting systems (Bio-Rad Laboratories GmbH, München Germany) and nitrocellulose Amersham Hybond™-ECL membrane (GE Healthcare Europe GmbH, Glattbrugg, Switzerland) for enhanced chemiluminescence (ECL). Protein transfer was done at 0.6 mA/cm2 (overnight, rt) with transfer buffer containing 10% methanol. Nonspecific sites were blocked with TTBS 5% milk (150 mM NaCl, 10 mM Tris/HCl, pH 7.6; Tween-20 0.1%; 5% low-fat dried milk; 1 h, rt). Rabbit polyclonal antibodies against active caspase-3 (ab2302, 1:200, Abcam, Cambridge, UK.); Atg5 (A0731, 1:1000, Sigma-Aldrich); BAX (Ab7977, 1:1000, Abcam); glutathione synthetase (GSS) (ab91591, 1:200, Abcam); PCNA (13110, 1:1000, New England Biolabs, UK); p62/SQSTM1 (P0068, 1:500, Sigma-Aldrich) PTGS2 (Ab15191, 1:200, Abcam) or α-tubulin (ab4074, 1:1000, Abcam) were used diluted in blocking buffer, overnight, 4 °C. An anti-rabbit IgG peroxidase conjugate (1:3000, GE Healthcare Europe GmbH, Germany) in blocking buffer; 1 h, rt) and the chemiluminescence ECL detection kit, AceGlow™ (PEQLAB Biotechnologie GmbH) were used for detection. A FUSION-FX7 system (PEQLAB Biotechnologie GmbH) served for documentation. Washing steps after every incubation period were performed using TTBS. Signals were quantified by computer software (Scion ImageJ, National Institutes of Health, Bethesda, USA).
2.11. Statistical analyses
The SigmaPlot® 12 software was used to carry out statistical analyses. After testing for Normality (by Shapiro–Wilk), the unpaired Student's t-test or Mann–Whitney U-test were used, as well as Pearson's product moment correlation test of gene expression. Data are shown as mean + SEM, with p < 0.05 considered to be statistically significant.
3. Results
3.1. Determination of cytotoxic concentrations of Ketorolac and Gentamicin
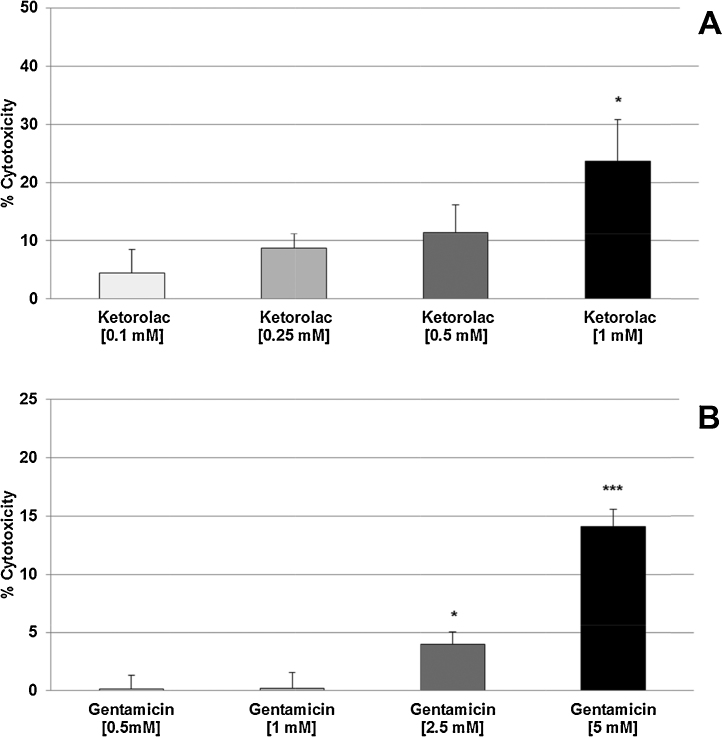
In vitro experiments were performed using the intraglomerular SV40-transformed mouse mesangial cell line (MES 13). These cells show characteristics of intraglomerular mesangial cells, e.g., expressing cytoskeletal α-SM Actin and α-Tubulin, as well as phagocytic properties (not shown). Fig. 1A shows the cytotoxic effects of Ketorolac on MES 13 cells. MES 13 cells depicted as a plot of percent of cytotoxicity, or cell death rate, after 24 h of drug exposure versus drug concentration. Four different Ketorolac concentrations spanning a log-range (0.1 mM to 1 mM) were tested, showing progressive escalation in the degree of cytotoxicity commensurate with increasing Ketorolac concentration. At the highest Ketorolac concentration (1 mM) tested, cytotoxicity was significantly (p < 0.05) 5.3-fold increased compared with the negative control. Fig. 1B shows the cytotoxic effects of Gentamicin concentration over a log-range beginning at 0.5 mM and going up to 5 mM. Significant degrees of cytotoxicity were first noted at a Gentamicin concentration of 2.5 mM (p < 0.05), and was highly significant at 5 mM (p < 0.001). The cell culture model utilizing mouse MES 13 cells correlated nephrotoxic drug concentration and cytotoxic potential for both compounds. When assessed by linear regression analysis, Ketorolac had the highest correlation coefficient (r-values: 0.991 vs. 0.984) and consequently, the greatest level of statistical significance (p-values: 0.009 vs. 0.016) compared to gentamicin. Hence, Ketorolac was selected as the prototypic nephrotoxin in our cell culture model for testing with all 3 lipid emulsions containing n3-PUFAs.
Fig. 1.
Cytotoxic effects of 24 h treatment with Ketorolac (A) or Gentamicin (B) on MES 13 cells. Values [in % cytotoxicity of cells without treatment (=control, =0% cytotoxicity)] are given as mean + SEM; *p ≤ 0.05, **p ≤ 0.001 (by t-test) significance vs. cells without treatment (=control); n = 6 independent experiments.
3.2. Influence of lipid emulsions on cytotoxic effects of Ketorolac or Gentamicin
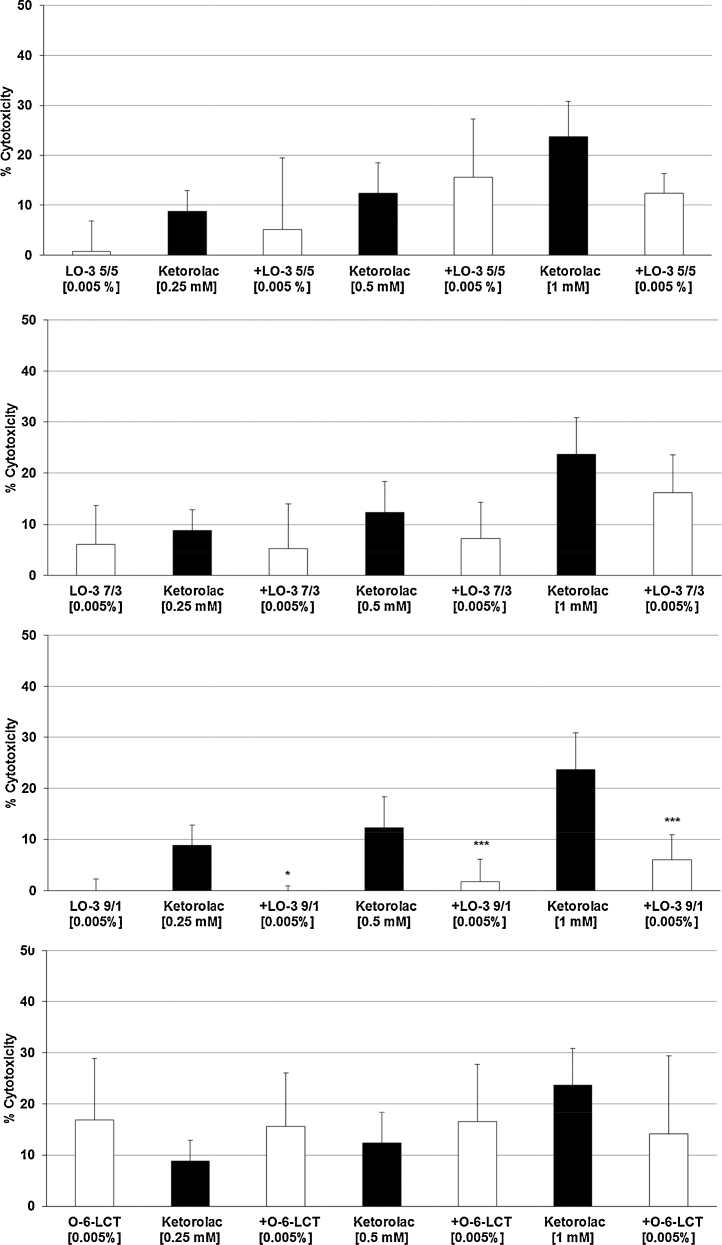
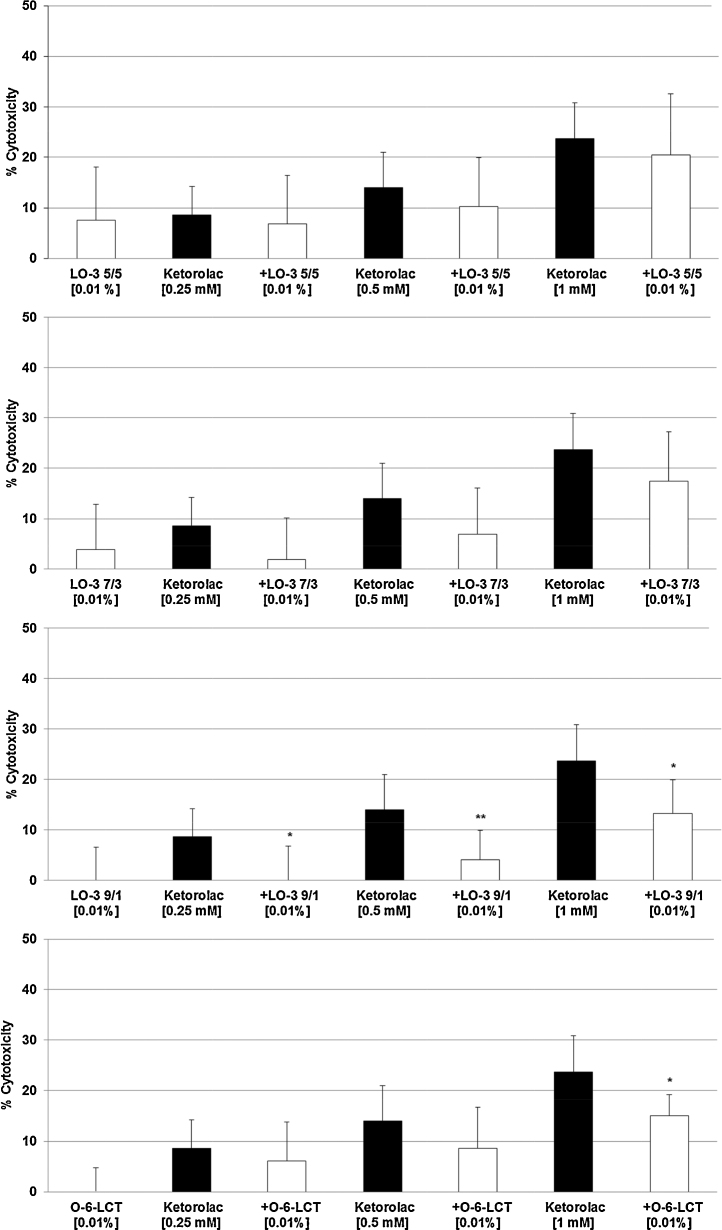
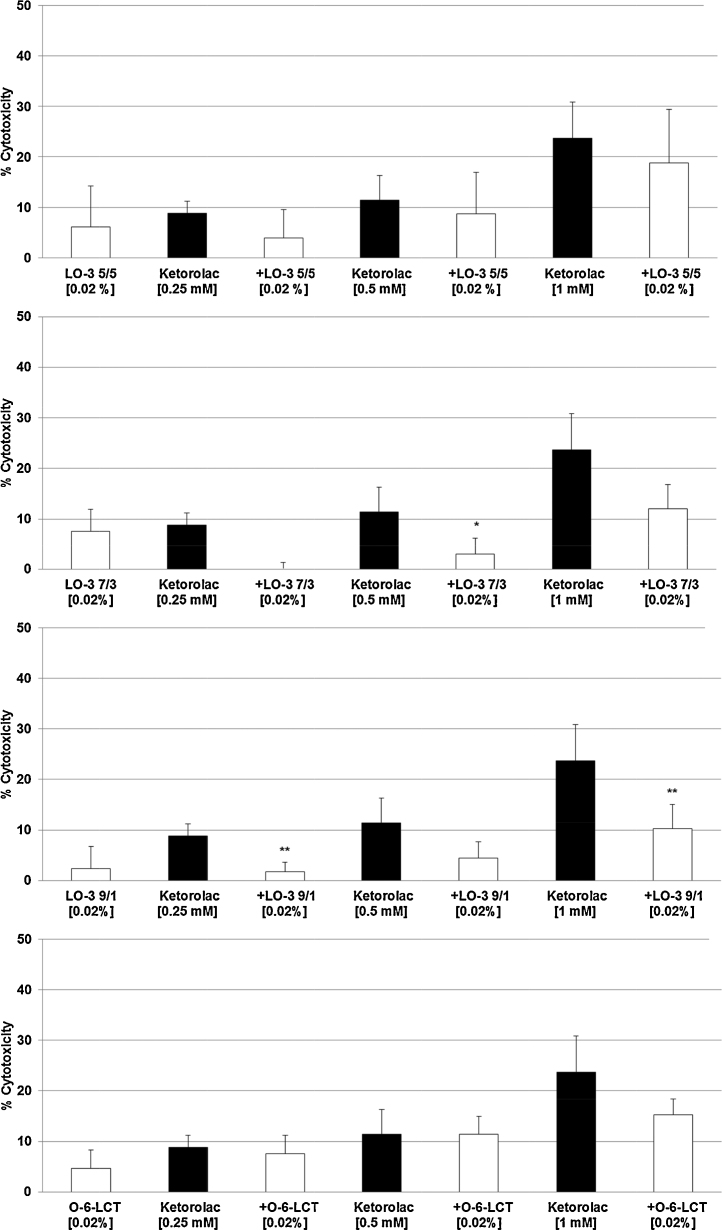
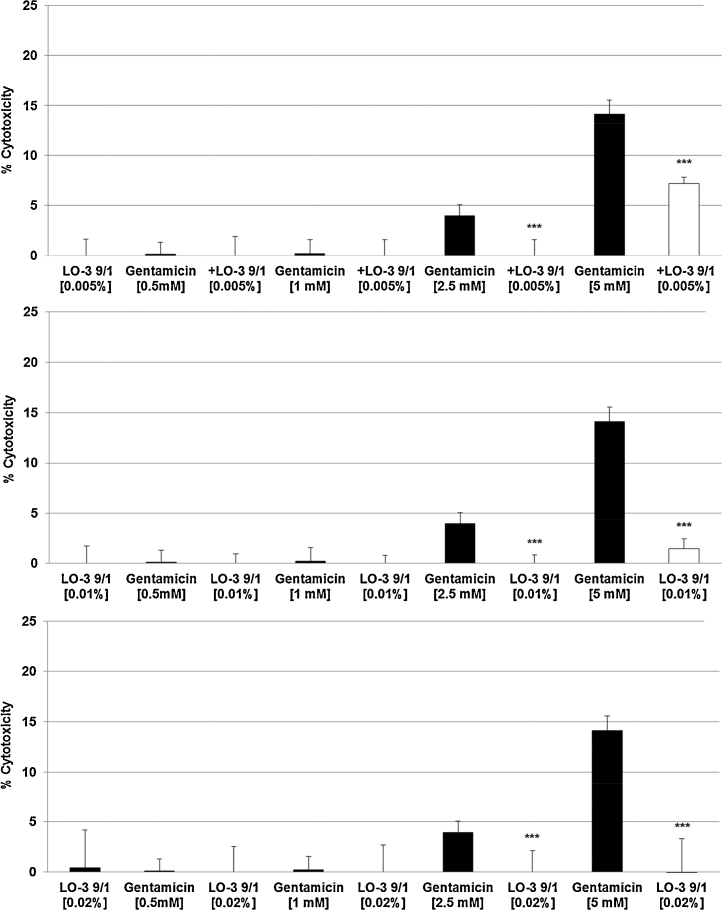
The three highest concentrations of Ketorolac (0.25 mM, 0.5 mM, 1 mM) – with 9–23% cytotoxicity (Fig. 1A) – were tested with all three LipOmega-3 emulsions with Fish Oil (FO) derived Triglyceride and Medium Chain Triglyceride (MCT) ratios or FO/MCTs of “9/1, 7/3, 5/5” against the control emulsion Lipofundin N at final lipid concentrations in cell cultures at 0.005% (Fig. 2A–D), 0.010% (Fig. 3A–D) and 0.020% (Fig. 4A–D). Clearly, of the multiple FO/MCT ratios and lipid concentrations in cell culture, LipOmega-3 “9/1” in cell culture provided superior levels of cytoprotection, as only this formulation was protective against demonstrable toxicity at all doses of Ketorolac and significantly so for all but one dose as shown in Fig. 4C. Thus, this FO/MCT combination was used in all subsequent studies of Gentamicin toxicity (Fig. 5A–C) and, for all markers of e.g., cell death. LipOmega-3 “9/1” was significantly protective at both toxic levels of gentamicin, 2.5 mM Gentamicin and 5 mM Gentamicin (Fig. 5A–C).
Fig. 2.
Effects in MES 13 cells of 24 h pre-treatment with 0.005% LipOmega-3 “5/5” (A), LipOmega-3 “7/3” (B), LipOmega-3 “9/1” (C) or Lipofundin N (O-6 LCT) (D) on cytotoxic effect of 24 h treatment with Keterolac (after treatment with the emulsions). Values (in% cytotoxicity of cells without treatment [=control, =0% cytotoxicity]) are given as mean + SEM; p, (by t-test) significance vs. Ketorolac treatment, using 4–6 wells per treatment and experiment; n = 6 independent experiments.
Fig. 3.
Effects in MES 13 cells of 24 h pre-treatment with 0.01% LipOmega-3 “5/5” (A), LipOmega-3 “7/3” (B), LipOmega-3 “9/1” (C) or Lipofundin N (O-6 LCT) (D) on cytotoxic effect of 24 h treatment with Keterolac (after treatment with the emulsions). Values (in % cytotoxicity of cells without treatment [=control, =0% cytotoxicity]) are given as mean + SEM; *p ≤ 0.05, **p ≤ 0.01 (by t-test) significance vs. Ketorolac treatment; n = 6 independent experiments.
Fig. 4.
Effects in MES 13 cells of 24 h pre-treatment with 0.02% LipOmega-3 “5/5” (A), LipOmega-3 “7/3” (B), LipOmega-3 “9/1” (C) or Lipofundin N (O-6 LCT) (D) on cytotoxic effect of 24 h treatment with Keterolac (after treatment with the emulsions). Values (in % cytotoxicity of cells without treatment [=control, =0% cytotoxicity]) are given as mean + SEM; p, significance vs. Ketorolac treatment, using 4–6 wells per treatment and experiment. *p ≤ 0.05, **p ≤ 0.01 (by t-test) significance vs. Ketorolac treatment; n = 6 independent experiments.
Fig. 5.
Effects in MES 13 cells of 24 h pre-treatment with 0.005% (A), 0.01% (B) or 0.02% (C) on cytotoxic effects of 24 h treatment with Gentamicin (after treatment with the emulsions). Values [in % cytotoxicity of cells without treatment (=control, =0% cytotoxicity)] are given as mean + SEM; p, significance vs. Gentamicin treatment; n = 6 independent experiments, using 4–6 wells per treatment and experiment. ***p ≤ 0.001 (by t-test) significance vs. Gentamicin treatment; n = 6 independent experiments.
Although there was some suggestion of relief of cytotoxicity with the lower concentrations of fish oil of the three FO/MCT ratios, with an isolated case of significant reduction, this was limited (i.e., LipOmega-3 “5/5”: 0 of 9 cell culture conditions as shown in Fig. 2, Fig. 3, Fig. 4; LipOmega-3 “7/3”: 1 of 9 cell culture conditions as shown in Fig. 2, Fig. 3, Fig. 4. Similarly, in the Lipofundin, soybean oil control: 1 of 9 cell culture conditions as shown in 2D, 3D and 4D. However, in the emulsion containing the highest level of omega-3 enriched fish, i.e., LipOmega-3 “9/1”, 8 of 9 cell culture conditions as shown in Fig. 2, Fig. 3, Fig. 4 were cytoprotective. Consequently, further analyses (i.e., inflammation-related or apoptosis-related measures of cell death) of the effects of n3-PUFAs on key biochemical processes were conducted on this formulation, in combination with the highest concentration of the nephrotoxins studied, to help further understand the mechanism(s) responsible for the observed cytoprotection against the nephrotoxins investigated.
When the lipid emulsions in cell culture were added at the same time as the nephrotoxin (co-incubation vs. pre-incubation of cells), the cytoprotective effects of the LipOmega-3 “9/1” were lost (data not shown). As suspected, the nephrotoxins tested were as water-soluble salts, which would therefore reside in the aqueous phase with immediate and toxic exposure to the mesangial cells in culture.
3.3. Effect of LipOmega-3 “9/1” on Ketorolac-/Gentamicin-mediated inflammation-relevant parameters
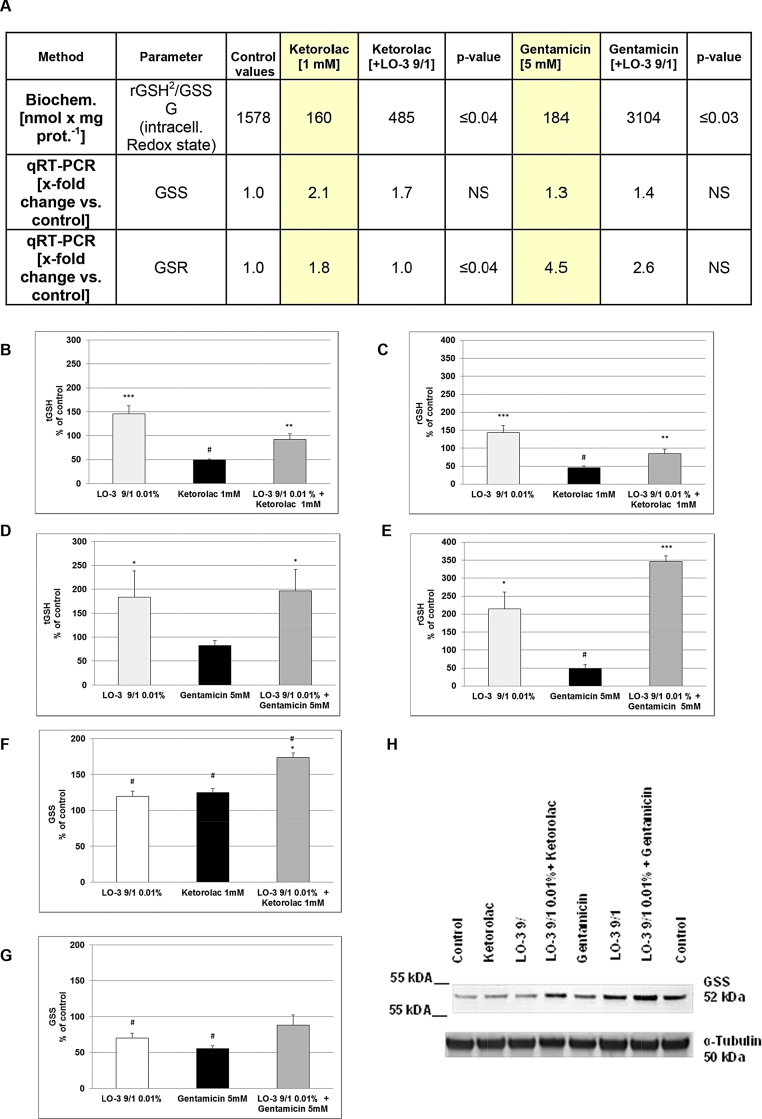
For all studies of proliferation- and inflammation-relevant parameters, only the highest concentration of each nephrotoxin was assessed. Supplementary Table 1 provides a summary of several proliferation- and inflammation-relevant parameters taken from both the Gentamicin and Ketorolac studies. With respect to Gentamicin, there were significant (p ≤ 0.01) elevations in the release of TNFα, NO and TXB2 when cells were pre-incubated with LipOmega-3 “9/1” and Gentamicin compared with Gentamicin alone, and this was associated with a significant (p < 0.04 and p < 0.03) heightened intracellular Redox state to withstand oxidative stress in cells pre-treated with LipOmega-3 “9/1” and Ketorolac or Gentamicin, respectively (Fig. 6A) compared with the nephrotoxins alone. Fig. 6B shows the intracellular concentration of total glutathione (tGSH) in % of the untreated control (=100%). Pre-treatment of MES 13 with 0.01% LipOmega-3 “9/1” resulted in a significant (p ≤ 0.001) about 50% increase in tGSH, whereas 1 mM Ketorolac significantly (p ≤ 0.05) reduces the tGSH about 50% (Fig. 6B); however, pre-treatment with LipOmega-3 “9/1”, significantly (p ≤ 0.01) improved/restored the Ketorolac-mediated decreased tGSH levels almost to control levels (Fig. 6B). Additionally, pre-treatment of MES 13 with 0.01% LipOmega-3 “9/1” also resulted in an about 50% increase in rGSH, whereas 1 mM Ketorolac significantly (p ≤ 0.05) reduces the rGSH about 50% (Fig. 6C); however, pre-treatment with LipOmega-3 “9/1”, significantly (p ≤ 0.01) improved/restored the Ketorolac-mediated decreased rGSH levels almost to control levels (Fig. 6C).
Fig. 6.
Effects in MES 13 cells of 24 h pre-treatment with 0.01% LipOmega-3 “9/1” on Gentamicin/Ketorolac induced cytotoxicity: Changes in glutathione metabolism. (A) mRNA expression of glutathione synthetase (GSS), glutathione reductase (GSR) [measured by qRT-PCR] and biochemically quantified intracellular glutathione (GSH) Redox state (=rGSH2/GSSG); p, significance (by t-test) nephrotoxin (Ketorolac or Gentamicin) plus 0.01% LipOmega “9/1” pre-treatment vs. nephrotoxin alone; values are given as mean of 3–4 independent experiments. NS, not significant. (B) and (D), intracellular total GSH (tGSH); (C) and (E), intracellular reduced GSH (rGSH). (F) and (G) Western blot of GSS protein expression, quantified by densitometric analysis using ImageJ software; (H) representative Western blot of GSS expression. (B)–(G). Values expressed in % of control (100%) are given as mean + SEM. #p ≤ 0.05, (by t-test) significance vs. control; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 (by t-test) significance vs. nephrotoxin treatment; n = 4 independent measurements.
As already shown for Ketorolac, also Gentamicin decreases the tGSH content by about 20% (Fig. 6D) and significantly (p < 0.05) about 50% the rGSH content in comparison with the control (Fig. 6E). Once again, pre-treatment with LipOmega-3 “9/1”, significantly (p ≤ 0.01; p ≤ 0.001) improved/restored the Gentamicin-mediated reduced tGSH and rGSH levels to higher than control levels (Fig. 6D and E). GSS is an enzyme essential for glutathione synthesis. Thus we tested GSS expression by qRT-PCR and Western blotting. We found that, Ketorolac and Gentamicin alone significantly (p ≤ 0.05) affect protein expression of GSS (Fig. 6F and G). However, pre-treatment with LipOmega-3 “9/1” improved/restored the nephrotoxin-mediated GSS effects up to (in case of Gentamicin) or about 70% higher (in case of Ketorolac) than control levels (Fig. 6F and G).
Furthermore, in the Ketorolac studies, pre-incubation with LipOmega-3 “9/1” resulted in significant (p ≤ 0.001 and p ≤ 0.05) reductions of nephrotoxin-mediated increase in PCNA-mRNA expression and TXA Synthetase levels, respectively (Supplementary Table 1). In both cases, the overall cytoprotective effects of LipOmega-3 were significant. Western blot analyses of PCNA protein expression could partly confirm the qRT-PCR data (Supplementary Fig. 1A–C). Moreover, Ketorolac treatment (1 mM) leads to an induction of PTGS2 (prostaglandin-endoperoxide synthase 2/prostaglandin G/H synthase/cyclooxygenase) on RNA and protein level (Supplementary Table 1 and Supplementary Fig. 1D and F), which could not be decreased to control levels by pre-incubation with LipOmega-3 “9/1” (Supplementary Table 1 and Supplementary Fig. 1D and F). Treatment of MES cells with Gentamicin w/o LipOmega-3 “9/1” did not alter PTGS2 expression (mRNA & protein) (Supplementary Table 1 and Supplementary Fig. 1E and F).
3.4. Effect of LipOmega-3 9/1 on Ketorolac/Gentamicin mediated apoptosis-relevant parameters
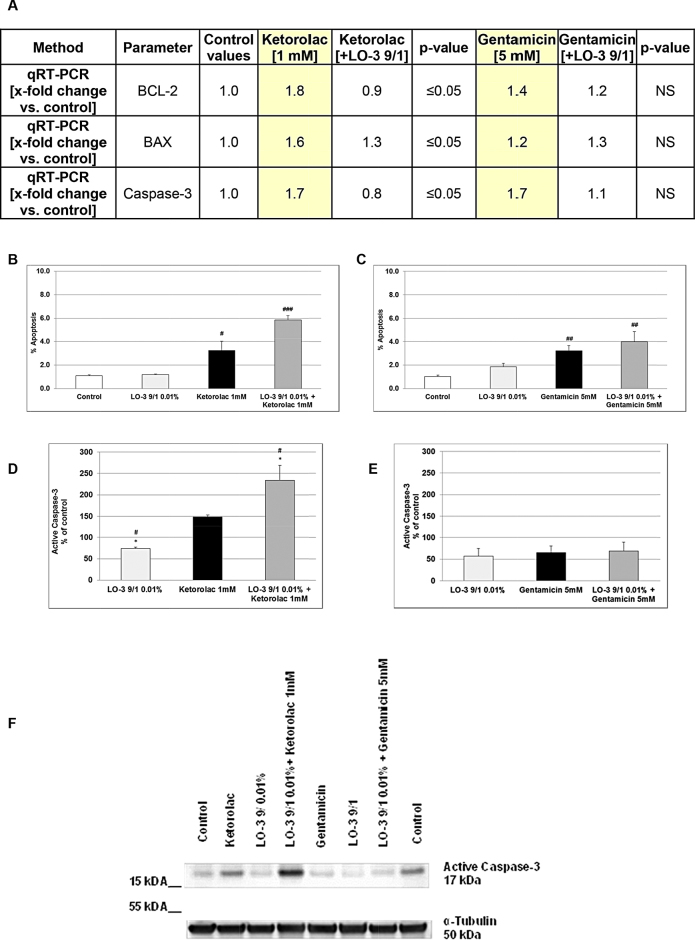
Fig. 7A provides a summary of the apoptosis-relevant parameters taken for both, the Ketorolac and Gentamicin studies. Ketorolac is associated with significant increases in apoptosis-related gene expression for each of the two regulator proteins BCL2, and the enzyme caspase-3 that was significantly (p ≤ 0.05) about 50% diminished when the cells were pre-treated with LipOmega-3 despite no significantly relevant change of apoptosis between Gentamicin alone and with LipOmega-3 “9/1” (Fig. 7A). For all studies of apoptosis-relevant parameters, only the highest concentration of each nephrotoxin was assessed. Incubation of MES13 cells with 1 mM Ketorolac (Fig. 7B) or Gentamicin (5 mM; Fig. 7C) alone leads to a significant about 3-fold induction of apoptosis compared with untreated control. Although not significant, Fig. 7B and C shows a net about 6-fold or 4-fold increase in apoptosis (% apoptosis) from the nephrotoxins when the cells are pre-incubated with LipOmega-3 “9/1” and afterwards with nephrotoxins. Activated caspase-3 plays a central role during apoptosis execution. Thus, we performed Western blotting to measure protein expression of active caspase-3. We found pre-treatment of MES 13 cells with LipOmega-3 “9/1” resulted in an about 20% inhibition of protein expression of active caspase-3 in comparison with the control (Fig. 7D–F). Moreover, Ketorolac—in contrast to Gentamicin, increased the protein expression of active caspase-3 about 50% (Fig. 7D–F). Furthermore, pre-treatment of MES 13 cells with LipOmega-3 “9/1” and afterwards with 1 mM Ketorolac resulted in an about 140% increase in protein expression of active caspase-3 in comparison with the untreated control and an about 90% increase in comparison with Ketorolac treated MES 13 cells (Fig. 7D and F). No significant differences were observed when the MES 13 were treated with Gentamicin alone or after pretreatment with LipOmega-3 “9/1” and afterwards with Gentamicin (Fig. 7E and F).
Fig. 7.
Effects in MES 13 cells of 24 h pre-treatment of with 0.01% LipOmega-3 “9/1” on Gentamicin/Ketorolac induced cytotoxicity: Changes in apoptosis; (A) mRNA expression of apoptosis-relevant parameters BCL-2, BAX and active Caspase-3; p, significance (by t-test) nephrotoxin (Ketorolac or Gentamicin) plus 0.01% LipOmega “9/1” pre-treatment vs. nephrotoxin alone. Values are given as mean of 3–4 independent experiments. NS, not significant. (B) and (C), morphologically measured apoptosis rate. Values expressed (in % of apoptosis) are given as mean + SEM. #p ≤ 0.05, ###p ≤ 0.001 (by t-test) significance vs. control; n = 3 independent experiments. (D) and (E) Western blot of active caspase-3 protein expression, quantified by densitometric analysis using ImageJ software; values expressed in % of control (100%) are given as mean + SEM. #p ≤ 0.05, (by t-test) significance vs. control (=100%). *p ≤ 0.05, (by t-test) significance vs. nephrotoxin; (F) representative Western blot of active caspase-3. n = 4 independent measurements.
Despite a significant decrease (p ≤ 0.05) of the BAX mRNA expression (Fig. 7A) after pre-treatment with LipOmega-3 “9/1” and Ketorolac, no significant difference in the protein expression of the pro-apoptotic BAX was observed neither after pre-treatment with LipOmega-3 “9/1”, Ketorolac or Gentamicin alone nor with nephrotoxins together with LipOmega-3 (Supplementary Fig. 1G–I). Additional investigations of the expression (protein level) of the ubiquitin-binding autophagic adaptor p62/SQSMT1 (hereafter p62) showed a significant (p < 0.001) inhibition by 70% after pre-treatment with LipOmega-3 “9/1” and afterwards with Ketorolac when compared with the control or Ketorolac (p < 0.05) (Supplementary Fig. 2A and C). Pre-treatment with LipOmega-3 “9/1” and afterwards Gentamicin exhibited a significant (p < 0.001) 100% increase in the protein-expression of p62 when compared with the control or Gentamicin (p < 0.05) (Supplementary Fig. 2B and C). Because there are 38 known autophagy-related (Atg) genes regulating the steps of autophagosome formation and breakdown [16] we have just investigated in our system the ubiquitin-like conjugation system Atg5-Atg12-Atg16, that is responsible for vesicle expansion (Lippai and Löw, 2014). Atg5 (also known as Apg5) forms a covalent complex with Atg12. We found that after pre-treatment with LipOmega-3 “9/1” and afterwards Ketorolac the Atg5-Atg12 protein-expression was significantly (p < 0.05) inhibited when compared with Ketorolac (Supplementary Fig. 2D and F). However, no significant difference in the protein expression of Atg5-Atg12 was observed neither after pre-treatment with LipOmega-3 “9/1” and afterwards Gentamicin nor with Gentamicin alone (Supplementary Fig. 2E and F).
4. Discussion
It is important to distinguish the biochemical effects elicited by the different nephrotoxins, as well as those resulting from the different fatty acid profiles among the lipid-based culture media studied. Ketorolac is a non-steroidal anti-inflammatory drug (NSAID) frequently prescribed in its intravenous form for postoperative pain management that provides opiate-like analgesia without the accompanying adverse reactions. All NSAIDs share a common mechanism of pharmacological action that includes blockade or inhibition of the critical cyclooxygenase isoenzymes, COX-1 and COX-2 that affect eicosanoid metabolism. COX enzymes play a crucial role in the generation of prostaglandins, prostacyclins and thromboxanes formed from the FA precursors (e.g., arachidonic acid and eicosapentaenoic acid, which have direct effects on the body's metabolic response to inflammation, both locally and systemically [5]. In the kidney, Ketorolac can alter the balance of these vasoactive eicosanoids and may disrupt normal renal hemodynamics with devastating clinical consequences. With respect to renal toxicity, there is no significant difference between non-selective COX-1 + COX-2 inhibitors (e.g., ibuprofen, acetominophen) and selective COX-1 (e.g., ketorolac, indomethacin) or COX-2 (e.g., etoricoxib, lumitacoxib) inhibitors [10]. Consequently, despite the important potential role of non-opiate parenteral analgesics in medicine, the use of Ketorolac in the critical care setting is severely restricted.
Gentamicin belongs to an important class of parenteral antimicrobials known as the aminoglycosides. In contrast to Ketorolac, Gentamicin is widely used around the world in the clinical setting. Nephrotoxicity from this drug has been mainly attributed to proximal tubule damage and obstruction, and a reduction in the glomerular filtration rate, or GFR. We focused on the latter mechanism of injury given the effects of Gentamicin to: (1) cause mesangial cell contraction, (2) decrease renal blood flow, (3) induce electrostatic binding between its cationic amino acid groups with negatively charged phospholipids in cell membranes; and, (4) promote vasoconstrictive prostanoids (e.g., thromboxane A2) [17].
According to the literature tubular effects cannot solely explain the reduced glomerular filtration rate [20], [17], the injury must also include biochemical consequences that accentuates renal damage (i.e., exaggerated thromboxane A-2 production causing pronounced vasoconstriction and ischemia. Which mechanism (mechanical and/or biochemical) is most responsible is difficult to explain. When we repeated these studies in the proximal tubule cell cultures, we did not observe cytoprotection, but only in MES cells, which might suggest an important role of these cells in mitigating damage. We used a cell culture model, and hence, this investigation is a first approximation to help establish proof-of-concept but we cannot assert the clinical relevance of the data. Our next investigations will be in an intact animal model to prove or disprove the cytoprotective role of the MES cells when exposed to a metabolically active substrate substrate(s), i.e., EPA and DHA, that reduces inflammation and vasoconstriction from proximal tubule damage. An intact animal model would be most appropriate to observe the effects in both proximal tubular cells and MES cells and to argue for a possible clinical applicability. In each phase of these investigations, the central issue is cytoprotection from known nephroxicants. Thus, dose equivalency to humans is a secondary issue to be dealt with if the current in vitro and subsequent in vivo pre-human investigations continue to show protection against renal injury via omega-3 fatty acids. For example, from current clinical experience, we know the incidence of nephrotoxicity from standard intravenous doses of aminoglycosides, and thus these studies will be designed accordingly to include omega-3 fatty acids with each dose of gentamicin to reduce this adverse effect from conventional therapy.
Glomerular apoptosis and proliferation have been shown in several glomerulosclerotic lesions that either proliferation or apoptosis (or both) might be (in some pathophysiological conditions) the consequence of tissue homeostasis as a response to an insult [20]. It might be speculated that growing doses of nephrotoxins as gentamicin or Ketorolac would induce further cytotoxicity (i.e., apoptosis). Apoptotic cells are metabolic active and we performed viability test with the reagent Presto Blue that detects active cells. Therefore, despite an increased viability, a pre-incubation with LO-3 might also prolongate the survival of apoptotic cells and thus, an increased apoptosis rate (detected by microscopical methods [YO-PRO-1] or Western blot [active caspase-3]) is found. An alternative outcome of apoptosis is secondary necrosis termed also late apoptosis, an autolytic process of cell disintegration. The early apoptotic cells preserve their plasma membrane integrity to retain the potentially harmful cellular contents inside. If not successfully taken up by phagocytes, apoptotic cells proceed to the phase of late apoptosis when the plasma membrane becomes permeable for macromolecules [25]. The leakage of intracellular molecules during secondary necrosis provokes an inflammatory response [8], but the pre-treatment with LO-3 probably delay secondary necrosis triggered by the treatment with the nephrotoxic drugs and consequently inhibit the pro-inflammatory response. Associated with cell death/apoptosis, autophagy is a constitutive cellular event and is enhanced under certain conditions such drug treatments [14]. We found an inhibition of the two autophagy markers, Atg5 and p62 after pretreatment with LipOmega-3 “9/1” and subsequent treatment with Ketorolac Adaptive mechanisms as autophagy are initiated when the intensity or duration of stress reaches the limit of the cell and suicidal cell death programs are activated [19]. Apoptosis usually is accompanied by a high degree of caspase activation, as also observed in our results and may lead to the cleavage of multiple proteins which promotes an apoptotic morphology [19]. In this context caspases can digest several essential autophagy proteins, resulting in the inactivation of the autophagic program perhaps with the goal to abort its cytoprotective function and to accelerate cellular demise [19] induced e. g. by nephrotoxins treatment. On the other hand the pre-treatment with LipOmega-3 “9/1” may delay the final destination of the cells with the consequence that these cells remain in an “apoptotic status” and retard the pro-inflammatory pathway activation.
In the kidney, there are two distinct mechanisms of cell death, namely by oncosis (necrotic cell death) or by apoptosis, and each process has a unique cellular presentation depending upon the concentration of the nephrotoxicant, with apoptosis generally occurring with low-concentration toxicities, and oncosis being the general mechanism of cell death at high-concentration toxicities [24]. Oncosis often involves adjoining cells, and causes an increase in cell volume and eventual rupture, causing an inflammatory response; whereas apoptosis has an opposite reaction involving individual cells and a decrease in cell volume without inflammation.
The lipid-based emulsions used in cell culture also included an omega-6 acid-enriched triglyceride (20% soybean only oil-in-water emulsion). This emulsion contains large quantities of the omega-6 fatty acid linoleic acid, a precursor to arachidonic acid, or AA [2], comprising approximately 50% of the fatty acid profile of soybean oil triglycerides. Omega-6 fatty acids are pro-inflammatory by virtue of the metabolic conversion (via the COX pathway) of AA-derived lipid mediators, including the vasoactive eicosanoids of the 2-series, prostaglandin E2, prostacyclin I2 and thromboxane A2. In contrast, the omega-3 acid enriched triglycerides from the 3 test emulsions contain increasing concentrations of EPA + DHA, wherein EPA is the preferential substrate over AA for the COX enzymes [2]. This results in the formation of EPA-derived lipid mediators, but of the less vasoactive, and less pro-inflammatory 3-series of the above eicosanoids. Hence, depending on the fatty acid substrate, the inflammatory response can be exaggerated or up-regulated (i.e., AA substrate), or alternatively, it may be down-regulated or modulated (i.e., EPA-substrate).
Thus, multiple mechanisms of injury and repair (e.g., structural alteration of the integrity of cell membranes, inhibition of key metabolic enzymes, modified response based on lipid substrate) of cells are facilitated by variable alterations in phospholipid metabolism caused by both the nephrotoxins and the lipid-based emulsions studied in cell culture. Recognizing the importance of phospholipid mediators involved: (1) in terms of membrane stability; (2) the metabolic response to injury (inflammation, oxidative stress, and ischemia), as well as, (3) the predilection for omega-3 fatty acids as the preferred metabolic substrate over omega-6 fatty acids eicosanoid metabolism [2], assists in interpreting the results from these studies. For example, the provision of pro-inflammatory omega-6 fatty acids, along with the nephrotoxin studied, would be expected to either accentuate the cytotoxic response, or have no beneficial effect on cytoprotection. With the exception of one isolated case at the highest concentration of the nephrotoxin (Fig. 4D), this is precisely what was observed in this study.
In contrast, the provision of less pro-inflammatory omega-3 fatty acids in the presence of nephrotoxins should be cytoprotective. With regards to inflammation-related cell death, there were significant reductions in the overall %-cytotoxicity for both nephrotoxins studied when the mesangial cell cultures were pre-incubated with omega-3 fatty acids. The optimum response was achieved with the LipOmega-3 with a FO/MCT ratio of 9/1, and in a cell culture concentration of 0.010%. The cytoprotective effects occurred at all Ketorolac concentrations studied, whereas such effects were present only at the two highest concentrations of Gentamicin. This difference most likely reflects the dissimilar mechanisms of cell injury between nephrotoxins. For example, Ketorolac blocks the COX enzymes involved in the de novo generation of prostaglandins, among the most responsible for maintaining renal hemodynamics. Evidence of this effect was observed by significant reductions in gene expression of thromboxane synthase, which was accompanied by significant reductions in expression of the PCNA gene involved in the DNA repair pathway. This was also associated with a significantly elevated intracellular redox state generally consistent with an improved energy state and a heightened ability to manage oxidative stress. In this context, the protective effect of LipOmega-3 “9/1” is clearly shown at the level of GSS protein expression. The pre-treatment with LipOmega-3 “9/1” enhances the expression of GSS, enzyme responsible for the glutathione (tGSH) synthesis with well know anti-oxidative function. The protective effect may be corroborated by the increase of intracellular rGSH observed in the MES 13 cells after pre-treatment with LipOmega-3 “9/1” alone or afterwards with nephrotoxins, meaning that most of the produced tGSH probably is converted in rGSH as a result of LipOmega-3 “9/1” effect and consequently improves the intracellular redox state and the anti-oxidative defense against the nephrotoxins Ketorolac and Gentamicin.
On the other hand, Gentamicin, which, in part, disrupts prostaglandin synthesis by altering the integrity of cell membranes, caused significant increases in TNFalpha, nitric oxide production and thromboxane B2 release from mesangial cells suggesting a greater inflammatory response to its nephrotxic effects, as well as alterations in renal hemodynamics that favor ischemia. The increase in redox state in this instance presumably reflected a component of the compensatory response to inflammatory stress. Although apoptosis increased for both nephrotoxins, the effects were not significant except when the nephrotoxin plus lipid LipOmega-3 “9/1” were compared vs the nephrotoxin alone. However, Ketorolac and to a more limited extent Gentamicin were both associated with significant increases in the expression of several apoptosis-related genes and active caspase-3 protein expression. This would suggest limited to no tendency for reduction in apoptosis by the lipid. Therefore inflammation-related death does indeed appear to be the more dominant mechanism for cell death, compared to apoptosis, with the data suggesting that modulation of eicosanoid production (prostaglandin and thromboxane, respectively) via omega-3 fatty acids significantly reduces the cytotoxicity of the nephrotoxins studied, although by distinctly different mechanisms.
5. Conclusions
The findings of greater toxicity, particularly at the higher concentrations of nephrotoxins in this study, as well as the cellular presentations from necrosis and apoptosis observed in the mouse mesangial cells used, are consistent with current expert toxicological views on the important biochemical mechanisms and mediators of renal cell injury [24]. Preincubation of MES 13 cells with omega-3 fatty acids mitigates cell damage from two injectable nephrotoxins with different mechanisms of injury. In the present context, the inclusion of omega-3 fatty acids may play an important therapeutic role to offset the nephrotoxicity of selected drugs used in the clinical setting. This would be a distinctly different therapeutic role for particular lipid components compared to the conventional application of drug vehicles as delivery systems for poorly water-soluble drugs. As a first approximation, we have established proof-of-concept in a cell culture model using intraglomerular mesangial cells. The next phase of research will need to be performed in an intact animal model (e.g., rodent) and with the drugs loaded into the omega-3 fatty acid-containing emulsion to confirm these findings that support the concept of a therapeutic drug vehicle.
Transparency document
Transparency document
Acknowledgements
The authors thank Mrs. Andrea Cordes, Claudia Keppler, Anne Henkeler and Elke Völck-Badouin for the excellent technical assistance, as well as Mrs. Ellen Essen and Gabriella Stauch for preparation of the manuscript.
Footnotes
Available online 22 October 2014
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.10.011.
Appendix B. [{(Appendix A)}]Supplementary data
(Supplementary Figs. 1 and 2).
Supplementary Fig. 1 Western blot of PCNA (A and B); PTGS2 (D and E) and BAX (G and H) quantified by densitometric analysis using ImageJ software; values expressed in % of control (100%) are given as mean + SEM. # p ≤ 0.05, (by T-TEST) significance vs. control (=100%). Representative Western blots of PCNA (C), PTGS2 (F) and BAX (I) are shown; n = 4 independent measurements.
Supplementary Fig. 2 Western blot of autophagy markers p62/SQSTM1 (A and B) and Atg5 (D and E) quantified by densitometric analysis using ImageJ software; values expressed in % of control (100%) are given as mean + SEM. ## p ≤ 0.01 and ### p ≤ 0.001 (by t-test) significance vs. control (=100%). * p ≤ 0.05, (by t-test) significance vs. nephrotoxin. Representative Western blots of p62/SQSTM1 (C), Atg5-Atg12 complex (F) are shown; n = 3 independent measurements.
The following are the supplementary data to this article:
References
- 1.Al-Nasiry S., Geusens N., Hanssens M., Luyten C., Pijnenborg R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum. Reprod. 2007;22(5):1304–1309. doi: 10.1093/humrep/dem011. [DOI] [PubMed] [Google Scholar]
- 2.Bistrian B.R. Clinical aspects of essential fatty acids metabolism: Jonathan Rhoads lecture. J. Parenter. Enter. Nutr. 2003;27:168–175. doi: 10.1177/0148607103027003168. [DOI] [PubMed] [Google Scholar]
- 3.Boncler M., Różalski M., Krajewska U., Podsędek A., Watala C. Comparison of PrestoBlue and MTT assays of cellular viability in the assessment of anti-proliferative effects of plant extracts on human endothelial cells. J. Pharmacol. Toxicol. Methods. 2014;69:9–16. doi: 10.1016/j.vascn.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Brooks C., Wei Q., Cho S.G., Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calder P.C. Fatty acids and inflammation: the cutting edge between food and pharma. Eur. J. Pharmacol. 2011;668:50–58. doi: 10.1016/j.ejphar.2011.05.085. [DOI] [PubMed] [Google Scholar]
- 6.Carpentier Y.A., Hacquebard M., Portois L., Dupont I.E., Deckelbaum R.J., Malaisse W.J. Rapid cellular enrichment of eicosapentaenoate after a single intravenous injection of a novel medium-chain triacylglycerol:fish oil emulsion in humans. Am. J. Clin. Nutr. 2010;91:875–882. doi: 10.3945/ajcn.2009.27951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deigner H.P., Claus R., Bonaterra G.A., Gehrke C., Bibak N., Blaess M., Cantz M., Metz J., Kinscherf R. Ceramide induces aSMase expression: implications for oxLDL-induced apoptosis. FASEB J. 2001;15(3):807–814. doi: 10.1096/fj.15.3.807. [DOI] [PubMed] [Google Scholar]
- 8.Elliott M.R., Ravichandran K.S. Clearance of apoptotic cells: implications in health and disease. J. Cell. Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elzinga L., Kelley V.E., Houghton D.C., Bennett W.M. Modification of experimental nephrotoxicity with fish oil as the vehicle for cyclosporine. Transplantation. 1987;43:271–274. doi: 10.1097/00007890-198702000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Grosser T., Smyth E., Fitzgerald G.A. In: Goodman and Gilman's The Pharmacological Basis of Therapeutics. Brunton L.L., Chabner B.A., Knollmann B.C., editors. McGraw Hill; New York, NY: 2011. Anti-inflammatory, antipyretic, and analgesic agents: pharmacotherapy of gout; pp. 959–1004. (Chapter 34) [Google Scholar]
- 11.Guyton A.C. eighth ed. W.B. Saunders; Jackson, MS: 1991. Formation of urine by the kidney: I. Renal blood flow, glomerular filtration, and their control, Textbook of Medical Physiology; pp. 286–297. [Google Scholar]
- 12.Hamid R., Rotshteyn Y., Rabadi L., Parikh R., Bullock P. Comparison of AlamarBlue and MTT assays for high through-put screening. Toxicol. In Vitro. 2004;18:703–710. doi: 10.1016/j.tiv.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Idziorek T., Estaquier J., De Bels F., Ameisen J.C. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J. Immunol. Methods. 1995;185:249–258. doi: 10.1016/0022-1759(95)00172-7. [DOI] [PubMed] [Google Scholar]
- 14.Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinscherf R., Fischbach T., Mihm S., Roth S., Hohenhaus-Sievert E., Weiss C., Edler L., Bärtsch P., Dröge W. Effect of glutathione depletion and oral N-acetyl-cysteine treatment on CD4+ and CD8+ T cells. FASEB J. 1994;8:448–451. [PubMed] [Google Scholar]
- 16.Lippai M., Lőw P. The role of the selective adaptor p62 and ubiquitin-like proteins in autophagy. Biomed. Res. Int. 2014;2014:832704. doi: 10.1155/2014/832704. (Epub 2014 Jun 12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Novoa J.M., Quiros Y., Vicente L., Morales A.I., Lopez-Hernandez F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79(1):33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- 18.Mansour N.R., Bickle Q.D. Comparison of microscopy and AlamarBlue reduction in a larval based assay for schistosome drug screening. PLoS Negl. Trop. Dis. 2010;4(8):e795. doi: 10.1371/journal.pntd.0000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariño G., Niso-Santano M., Baehrecke E.H., Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell. Biol. 2014;15(2):81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez-Salgado C., López-Hernández F.J., López-Novoa J.M. Glomerular nephrotoxicity of aminoglycosides. Toxicol. Appl. Pharmacol. 2007;223:86–98. doi: 10.1016/j.taap.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Montague J., Abramov K. Irwin and Rippe's Intensive Care Medicine. seventh Edition. Wolters Kluwer|Lippincott Williams and Wilkins Publishers; Philadelphia, PA: 2011. Acute kidney injury in the intensive care unit; pp. 867–893. [Google Scholar]
- 22.Nociari M.M., Shalev A., Benias P., Russo C. A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicity. J. Immunol. Meth. 1998;213:157–167. doi: 10.1016/s0022-1759(98)00028-3. [DOI] [PubMed] [Google Scholar]
- 23.Peterson G.L. A simplification of the protein assay method of Lowry et al. Which is more generally applicable. Anal. Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 24.Schnellmann R.G. In: Casarett and Doull's Toxicology: The Basic Science of Poisons. eighth ed. Klaasen C.D., editor. McGraw-Hill; New York, NY: 2013. Toxic responses of the kidney; pp. 665–690. [Google Scholar]
- 25.Stepanek O., Brdicka T., Angelisova P., Horvath O., Spicka J., Stockbauer P., Man P., Horejsi V. Interaction of late apoptotic and necrotic cells with vitronectin. PLoS One. 2011;6(5):e19243. doi: 10.1371/journal.pone.0019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern-Straeter J., Bonaterra G.A., Hörmann K., Kinscherf R., Goessler U.R. Identification of valid reference genes during the differentiation of human myoblasts. BMC Mol. Biol. 2009;10:66–74. doi: 10.1186/1471-2199-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sykes M.L., Avery V.M. Development of an AlamarBlue viability assay in 384-well format for high throughput whole cell screening of Trypanosoma brucei brucei bloodstream form strain 427. Am. J. Trop. Med. Hyg. 2009;81(4):665–674. doi: 10.4269/ajtmh.2009.09-0015. [DOI] [PubMed] [Google Scholar]
- 28.Tietze F. Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione: application to mammalian blood and other tissues. Anal. Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 29.Van der Harst M.R., Bul l S., Laffont C.M., Klein W.R. Gentamicin neprotoxicyty-a comparison of in vitro finding with in vivo experiments in equines. Vet. Res. Commun. 2005;29(3):247–261. doi: 10.1023/b:verc.0000047492.05882.bb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document