Highlights
-
•
[THg], δ13C, and δ15N were measured in hair segments of pregnant women from Mexico.
-
•
Higher δ15N was related to [THg] and frequency of fish and shellfish consumption.
-
•
THg exposure and assimilation are more complex in higher fish consumption categories.
-
•
At high fish consumption, trophic level remains elevated while [THg] is lower.
Keywords: Mercury, Hair, Piscivory, Pregnancy, Stable isotopes
Abstract
Seafood is a valuable source of nutrients important for fetal development. However, seafood consumption is the main route of exposure to monomethyl mercury (MeHg+) for humans. MeHg+ is highly bioavailable and potentially adversely affects fetal neurodevelopment. MeHg+ exposure from fish consumption varies significantly by age and trophic level of fish consumed as well as the frequency and amount of fish consumed. This study investigates total Hg concentrations ([THg]) in hair segments of pregnant Mexican women in relation to (1) self-reported frequency of fish and shellfish consumption, (2) maternal trophic level and marine diet contributions, determined using hair carbon (C) and nitrogen (N) stable isotopes, and (3) relates [THg] to various hair advisory thresholds. We also examined whether variation in C and N isotope values is explained by self-reported frequency of fish and shellfish consumption. A significant proportion of hair samples had [THg] higher than suggested agency thresholds and, for women within the range of the various advisory thresholds (1–20 μg g−1), the specific statistic used and threshold applied are important considerations for assessing and communicating risk. Individuals enriched in 15N (δ15N values) had higher [THg] as did individuals that reported consuming fish and shellfish more frequently, suggesting that variation in [THg] can be explained by both consumer reported diet and diet as determined by C and N stable isotope assessment. However, at higher reported fish consumption levels the trophic level is maintained while [THg] is paradoxically lower. This suggests that THg exposure and assimilation are more complicated in higher fish frequency consumption categories. [THg] is more variable at the higher concentrations, possibly indicating some exposure to non-dietary Hg, heritable variations affecting Hg toxicodynamics, and BMI and tobacco exposure factors as outlined in our companion paper.
1. Introduction
Finfish, mollusks, and crustaceans are seafood sources high in lean protein, omega-3 fatty acids, zinc, iron and, especially in the case of marine species, provide iodine, selenium, and vitamins A and D [1]. These nutrients, especially the omega-3 fatty acids, are vital to human fetal development with eicosapentaenoic acid (EPA) and docosapentaenoic acid (DPA) contributing to development of the central nervous system [2], [3]. Women consuming more fish during pregnancy have babies with higher IQs and fewer behavioral problems than mothers that ate little or no fish high in omega-3 fatty acids [4]. Additionally, fish consumption during pregnancy has been linked to a decrease in preterm delivery [5], increased likelihood of foveal steroactuity in the child [6], and to development of the child's immune system [7], [8].
Consumption of fish that accumulate monomethyl mercury (MeHg+) in their muscle is the primary pathway of exposure to mercury (Hg) in humans [9]. Inorganic forms of Hg enter ecosystems through natural sources such as volcanism, and anthropogenic sources such as mining, coal combustion, and cement production [10]. Hg is converted to the more toxic MeHg+ by bacteria [10] and in most systems biomagnifies with each trophic transfer. MeHg+ is highly bioavailable in humans [>95% of ingested dose absorbed; Aberg et al. [11]], easily passes the placenta to the fetus, and crosses the blood brain barrier (BBB), potentially adversely affecting the developing nervous system [12], [13], [14]. Nearly 100% of the Hg that bioaccumulates in upper-trophic level fish skeletal muscle is MeHg+ [15].
Effects on brain function associated with prenatal MeHg+ exposure were found to be multi-focal and permanent [16] although these results may have been confounded by prenatal exposure to polychlorinated biphenyls [17]. The amount of Hg exposure to humans from fish consumption varies significantly by the age and trophic level of the fish consumed [18], [19] and the frequency (Gaxiola-Robles et al. companion paper) and amount (mass) of the fish meals. Pouzaud et al. [20] found that a global increase in seafood consumption could lead to Hg exposure above some conservative consumption advisory limits for pregnant women, and examined the balance between Hg exposure risk and the beneficial effects of omega-3 long-chain polyunsaturated fatty acid intake. Pouzaud et al. [20] concluded pregnant women consuming a high proportion of some fatty fish, such as sardines and salmon, meet the omega-3 long-chain polyunsaturated fatty acid intake requirements without exceeding the advisory limits for Hg. In addition, Myers et al. [3] examined neurobehavioral effects in the Republic of Seychelles related to prenatal exposure to Hg resulting from a high fish diet and concluded that the beneficial influence of nutrients from fish may counter adverse effects of Hg on the developing nervous system.
Humans can be exposed to Hg through abiotic non-fish sources. Cigarette smoking and passive exposure, addressed in our companion paper (Gaxiola-Robles et al.), may be a substantial source of Hg not only to the smoker but also, through passive smoking, to nonsmokers [21], and has been shown to result in increased Hg concentrations in breast milk [22]. However, Gaxiola-Robles et al. (companion paper) did not find as strong a link between tobacco exposure and [THg] in hair in the population of women included in this study. Dental amalgam is a potentially significant source of exposure since it can contain up to 50% elemental Hg [23]. The use of Hg-containing beauty creams and other cosmetic products may also result in significant exposure to Hg [23]. Elemental Hg is used in some therapies, religions and other practices (e.g. Santería, Espiritismo) and can result in exposure with subsequent absorption and/or externally contaminated samples [e.g. hair; WHO [23]]. These are important confounders to consider in study designs and interpretation of fish consumption studies that determine [THg] in hair, blood, or both.
The feeding ecology/trophic level of individual mammals can be determined by naturally occurring variations in the ratio of heavy to light isotopes of carbon (13C/12C, δ13C) and nitrogen (15N/14N, δ15N) and can be used to better understand contaminant exposure [24], [25], [26], [27] including Hg bioaccumulation and biomagnification [28], [29], [30]. Enrichment of δ15N can be used to estimate trophic position because δ15N increases predictably with each trophic level transfer [31]. Changes in δ13C can provide information on the location of dietary resources [e.g. terrestrial vs. marine and pelagic vs. benthic; France [32], France and Peters [33], Newsome et al. [34]].
Understanding Hg pathways in human exposure is critical to assess risk and properly manage exposure, specifically in cohorts of concern, such as women of childbearing age. This is the 2nd of two papers examining [THg] in women in Baja California Sur, Mexico. We measured [THg] in the hair segments of pregnant women along with reported frequency of fish and shellfish consumption with the goal of evaluating whether [THg] varied with diet. Second, we measured δ15N and δ13C signatures in hair to determine longer-term dietary exposure to higher trophic level and marine derived protein sources, with the goal of evaluating whether [THg] varied in relation to the trophic level of the mother's diet and whether variation in C and N stable isotopes could be explained by reported frequency of consumption of fish and shellfish.
2. Materials and methods
2.1. Sampling
Samples of occipital scalp hair were collected from women in Baja California Sur, Mexico, following the established sample collection procedure [McDowell et al. [35], see Gaxiola-Robles et al. companion paper]. The study site was chosen after Hg concentration in muscle samples from larger sharks (>200 cm LT) caught by local artisan fisheries in this area were found to exceed the permissible limit (>1 ppm wet weight) for human consumption set by numerous international agencies [36], [37]. Informed consent and hair samples were collected the day of discharge from the hospital postpartum and in a follow-up interview, conducted 7–10 days after delivery, a survey was administered exploring food consumption 30 days prior to hair sample collection (between July and December 2011). No information was obtained about meal portion size, recipes, or preparation methods. Fish, shellfish, and dairy consumption frequency data were grouped into four categories: none consumed; consumed once a month; consumed once every two weeks; and consumed more than twice a week. 114 women contributed hair samples and 78 of these completed the survey. This research (project ID, CONACYT-SALUD 2010-C01-140272) was approved by the Baja California Sur Chapter of the National Mexican Academy for Bioethics.
This population consumes fish on a regular basis, generally sea bass, groupers, red and other snappers, sharks, rays, jacks, and dorados [38]. Beef (grass or corn-fed cattle) is consumed at most twice a week; corn-fed chicken is consumed more often than beef; generally, the population relies on eggs, corn, beans and rice for most meals [39]. Known consumption of corn or corn-fed cattle or chicken can affect the interpretation of C and N stable isotopes.
2.2. Sample preparation
Samples were analyzed for [THg] and stable isotopes of nitrogen (N) and carbon (C) values at the Wildlife Toxicology Laboratory (WTL), University of Alaska Fairbanks (UAF). Samples were provided with no indication of participant identification (de-identified). Samples were immersed in a 1% solution of Triton X-100® for 15–20 min to remove external contamination, then rinsed by an initial 10 min immersion in ultrapure water (NANOpure Model D4751, Barnstead International, Dubuque, Iowa), followed by a 5 min immersion and a further 3 sequential immersions. Cleaned samples were placed in labeled 4 oz polyethylene WhirlPak™ bags and freeze dried for 48 h. Full length hair samples (n = 97) were subsampled into 3 sections (proximal, middle and distal segments) along the length of the hair, with the proximal sample representing the most recent hair growth, in order to assess temporal variability within an individual. Individual segments were analyzed for [THg], and the remaining hair was used for determination of δ15N and δ13C stable isotope values. See Gaxiola-Robles et al. (companion paper) for additional details of the segmental analysis.
2.3. Mercury analysis
Total mercury concentration (μg g−1) was measured in hair segments using a DMA80 Direct Mercury Analyzer [Milestone Inc., Shelton, Connecticut; US EPA method 7473; Knott et al. [40], Castellini et al. [41], Rea et al. [30]; see Gaxiola-Robles et al. companion paper for additional details of the segmental analysis]. Values from the three segments were used to establish the range and variability within each individual and for comparison with established thresholds, but for comparison with the diet surveys, only the value from the proximal (most recent) segment was used. Mean [THg] for each individual (across the three segments) was used in comparison with the carbon and nitrogen stable isotope values.
2.4. Stable isotope analysis
Hair samples (n = 77) were analyzed for stable isotopes of nitrogen (N) and carbon (C). The stable isotope sample was comprised of all the remaining hair after the segmental [THg] analysis was done. Approximately 0.5 mg of clean, dry hair was wrapped in ultrathin foil sheets (Elemental Microanalysis, Cambridge, UK) and analyzed at the Alaska Stable Isotopes Facility at the University of Alaska Fairbanks. An elemental analyzer–isotopic ratio mass spectrometer (Costech Elemental Analyzer [ESC 4010] and Finnigan MAT Conflo III interface with a Delta + XP mass spectrometer) was used [28], [30]. The ratio of stable isotopes is expressed in delta (δ) notation and calculated as:
where X = 15N or 13C and R = 15N/14N or 13C/12C in the sample and standard.
2.5. Statistical Analysis
We generated mean total [THg] and 95% confidence interval for most individuals using 3 segments per individual to examine the percentage of women that had means and/or 95% confidence intervals significantly higher than various published health-related thresholds for women of child bearing age. The selected thresholds are 1 μg g−1 [42], 5 μg g−1 [43], 10 μg g−1 [44], [45], [46], 15.3 μg g−1 [47] and 20 μg g−1 [46], as they represent a range of advisory levels that we are aware of. These advisory levels were generally developed to protect the most sensitive health outcomes of mercury exposure, the neurodevelopmental effects on the fetus of mothers who consume fish, but also young children.
We used general linear models (Proc GLM) to evaluate the relationship between the frequency of self-declared categorical consumption of fish and shellfish (never, once a month, every 2 weeks, or more than twice a week) as reported by the individual for the month prior to sampling, and [THg] in the proximal hair segment in pregnant Mexican women (n = 78) using 4 a priori candidate models. Only 78 women had both hair [THg] measured and completed diet recalls. Frequency of consumption of dairy products was also collected but not included in the model set as 72 individuals consumed dairy more than twice a week, 5 consumed dairy every 2 weeks, and 1 consumed dairy once a month resulting in a distribution not amenable to statistical evaluation. The proximal hair segment was chosen as it best correlates with the one month time frame of the diet data. Models in the candidate set included all combinations of the variables (e.g. Modelfish; Modelshellfish; Modelfish + shellfish; Modelfish × shellfish). [THg] was log transformed to improve normality.
We examined the relationship between [THg] and δ15N and δ13C values using two separate simple linear regressions to test whether diet, as determined by δ15N and δ13C, affects mean [THg] (across segments; Proc REG; n = 73). Seventy three women had hair [THg], δ15N, and δ13C values determined. We log-transformed the data to meet the assumption of homoscedasticity and examined for influential outliers. As we did not account for the negative sign associated with δ13C, a negative β-value indicates that [THg] decreased as δ13C is enriched (i.e. becomes less negative). Additionally, we ranked δ13C from 1 to 73 (from values of −18.52 to −12.19) and ran a regression on the ranks, reducing the influence of an outlying individual (δ13C = −12.19).
Lastly, we used general linear models (GLM) to evaluate the relationship between the frequency of consumption of fish and shellfish as reported by the individual and δ13C and δ15N stable isotopes values (n = 61), using 2 separate a priori candidate model sets, each with 3 models. Sixty one women had δ13C and δ15N measured and completed diet recalls. Models in the two candidate sets included all additive combinations of the variables (e.g. δ15Nfish; δ15Nfish + shellfish; δ13Cfish; δ13Cfish + shellfish). We added a constant (20) and square root-transformed δ13C to improve normality.
Values are reported as means ± SE unless otherwise indicated. Analyses were conducted using SAS (SAS Institute, Cary, NC). We considered results significant at α < 0.05. All statistical analyses were conducted with and without an individual with exceptionally high [THg] to ensure that this individual was not overly influential in our assessment. We used Akaike's information criterion adjusted for small sample size (AICc) to select the best approximating models as it allowed us to evaluate a number of competing nested models without violating the rules of multiple comparisons and error rates [48]. We used Tukey's multiple comparison test to compare means.
3. Results
3.1. Total mercury concentrations
We measured [THg] in the proximal hair segments of 97 women. [THg] averaged 3.26 ± 0.97 μg g−1, ranging from 0.12 to 90.0 μg g−1 (750-fold range). When the individual with [THg] of 90.0 μg g−1 was excluded as an outlier, [THg] averaged 2.35 ± 0.38 μg g−1 and ranged from 0.12 to 24.20 μg g−1 (202-fold range). There was considerable variation in the number of women that had [THg] higher than specific thresholds for pregnant women or women of childbearing age, depending on which agency's threshold is considered (Table 1). However, including a measure of the variation in [THg] for an individual woman did not have a large effect on the number of women exceeding any given threshold (Table 1).
Table 1.
The number (%) of women with a mean and/or upper and lower 95% confidence limits of total mercury concentration ([THg]) significantly greater than various advisory thresholds or guidelines.
| Agency/group | Threshold concentration (μg g−1) | # (%) of participants with mean > threshold | # (%) of participants with upper 95% confidence interval > threshold | # (%) of participants with lower 95% confidence interval > threshold |
|---|---|---|---|---|
| US EPAa | 1 | 64 (69%) | 70 (75%) | 49 (53%) |
| Alaska Statewide Hair Mercury Biomonitoring Programb | 5 | 7 (8%) | 9 (7%) | 7 (8%) |
| Health Canadac | 10 | 4 (4%) | 6 (6%) | 4 (4%) |
| Agency for Toxic Substances and Disease Registryd | 15.3 | 3 (3%) | 4 (4%) | 2 (2%) |
| World Health Organizatione | 20 | 1 (1%) | 3 (3%) | 1 (1%) |
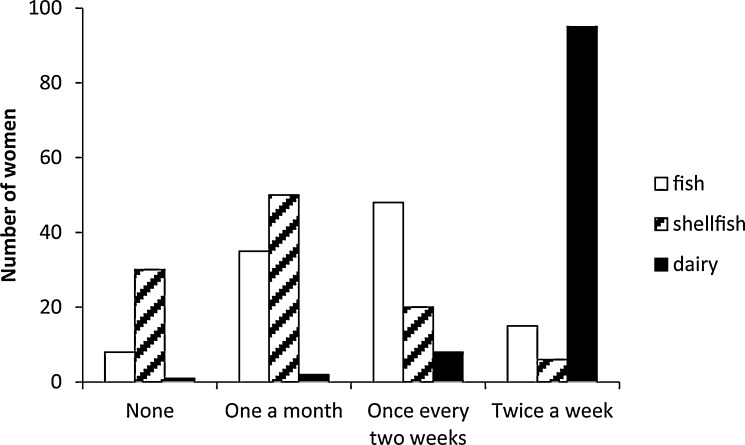
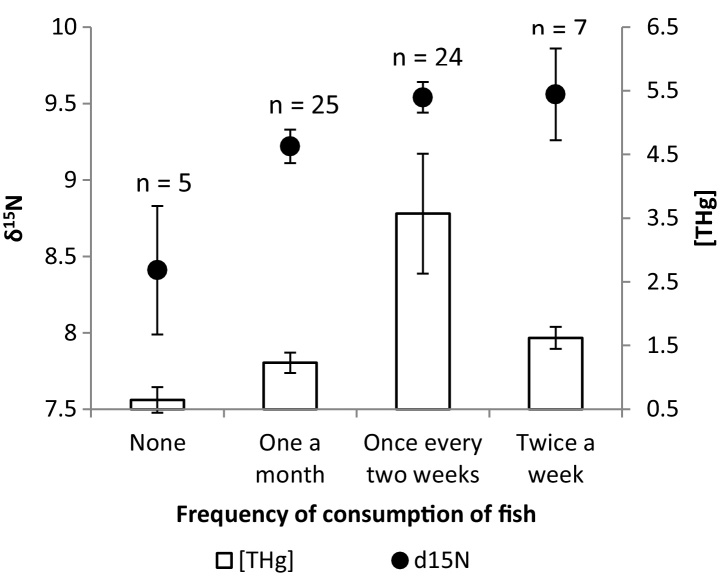
Frequency of self-reported consumption of fish, shellfish and dairy products are shown in Fig. 1. The best approximating a priori model describing [THg] in the proximal segment of hair of these pregnant women included the frequency of consumption of fish (AICc = −25.88, wi = 0.77, K = 5), and was 2.9 AICc units from the next best model, which included an effect of shellfish consumption (AIC = −22.95, wi = 0.18, K = 8). [THg] varied significantly with fish consumption (F = 8.8, p < 0.0001; Fig. 2). Although the 2nd best model included an effect of shellfish consumption, the effect was not significant (F = 0.67, p = 0.58). These findings and results did not change significantly when the 90 ppm outlier was included.
Fig. 1.
Number of women (n = 78) consuming fish, shellfish, and dairy in each consumption category (never, once per month, once every two weeks, or twice a week).
Fig. 2.
Mean (±standard error) total mercury ([THg] concentration, μg g−1) and δ15N in relation to reported frequency of fish consumption.
3.2. Stable isotope associations
The δ15N values ranged from 7.43‰ to 10.70‰ (mean = 9.35 ± 0.08‰) and δ13C ranged from −18.52‰ to −12.19‰ (mean = −16.62 ± 0.09‰). The [THg] increased with δ15N (F = 5.76, p = 0.02, R2 = 0.08), independent of the 90 ppm outlier, while [THg] decreased as δ13C became more enriched or less negative (F = 4.26, p = 0.04, R2 = 0.06), independent of the 90 ppm outlier. However, the relationship between δ13C and [THg] was not significant when δ13C was ranked (F = 0.7, p = 0.41) because the influence of an outlying individual is reduced. This individual had the lowest δ15N (7.43‰) as well as the most enriched δ13C (−12.19‰) and the lowest mean [THg] (0.12 μg/g), and reported consuming no fish or shellfish and dairy only once a month. The individual with the high [THg] (90 μg g−1) had values of δ15N and δ13C that fell near the mean (9.2‰, −16.58‰, respectively) and reported consuming fish once every two weeks, no shellfish, and dairy twice or more per week.
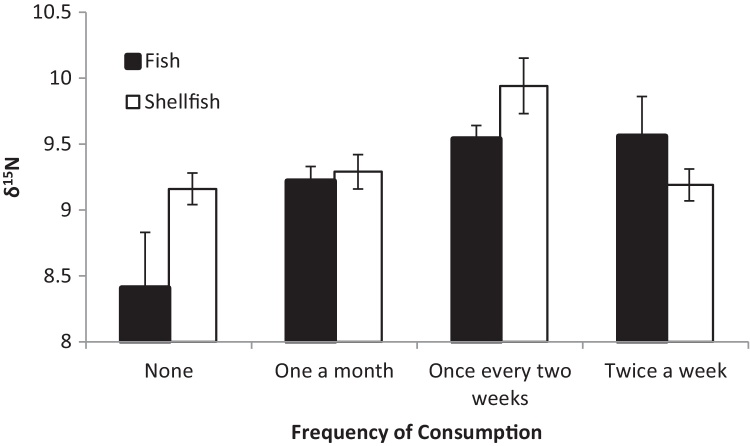
The best approximating a priori model describing variation in δ15N in the hair of these pregnant women in relation to reported diet included the frequency of consumption of fish and shellfish (AICc = −56.26, wi = 0.78, no. of parameters K = 8), and was 2.56 AICc units from the next best model, which did not include the effects of frequency of shellfish consumption (AICc = −53.70, wi = 0.22, K = 5). δ15N varied significantly with fish consumption (F = 5.6, p < 0.01) and shellfish consumption (F = 3.3, p = 0.03; Fig. 3).
Fig. 3.
Mean (±standard error) δ15N in relation to reported frequency of consumption of fish and shellfish.
The best approximating a priori model describing variation in δ13C in the hair in relation to reported diet included the frequency of consumption of fish (AICc = −182.91, wi = 0.93, K = 5), and was 5.96 AICc units from the next best model which included the effect of frequency of shellfish consumption (AICc = −176.94, wi = 0.05, K = 5). δ13C did not vary significantly with either fish or shellfish consumption (F < 1.95, p > 0.13).
4. Discussion
Total mercury concentrations in the hair of pregnant women living in Baja California Sur, Mexico were quite variable with a significant proportion higher than suggested agency thresholds or guidelines [43], [44], [45], [46], [47] and may be of concern that warrant follow up investigations. We evaluated both the total number of women whose mean [THg] was higher than suggested agency thresholds and the number of women whose upper 95% confidence were higher than the thresholds and found minor differences. We note large variability based on the advisory guidelines selected; for example, 1–53% exceeded various guidelines when using the lower 95% confidence limit for individuals and 1–69% when using mean [THg]. Most women with ‘high’ [THg] were considerably higher than the thresholds of 5 and 10 μg g−1 and their [THg] were not variable enough between segments of hair (e.g. 95% confidence limit) to change the outcome. While it did not appear that there was a benefit to including variation around the mean when comparing [THg] to concentrations of concern for this ‘high’ group, it is apparent for women within the range of the various advisory thresholds (1–20 μg g−1) that the specific statistic and consumption threshold used are important considerations.
The variation in [THg] can be partially explained by reported consumption of finfish but not shellfish consumption. An increase in fish consumption from once a month to once every two weeks resulted in [THg] in hair increasing by more than 2 μg g−1, although women in the highest consumption category actually had lower [THg] (Fig. 2) while δ15N remained equivalent. The women in the study are consuming relatively low amounts of fish (Fig. 1); however, some are known to be predatory fish and both high in [THg] and of a high trophic position [4], [36], [38]. Finfish are, in general, of a higher trophic level than shellfish [49] and thus likely have higher [THg], so it is not surprising that there was no obvious link between shellfish consumption and [THg]. Greater variability at higher [THg] may indicate that while diet (e.g. consumption of fish) explains most of an individual's [THg], some of the higher [THg] are attributable to non-dietary or non-fish dietary exposure [e.g. rice; Li et al. [50]] or to individual variation in genetic drivers; as well as BMI and tobacco exposure as indicated in our companion paper (Gaxiola-Robles et al. companion paper). One individual in particular illustrates this; the individual with 90.0 ppm THg had δ15N and δ13C values near the mean values, as was the reported fish and shellfish consumption, suggesting that a gross measure of seafood diet was not the main driver of the relatively high [THg]. The authors recognize the benefits and limitations of dietary recall information and caution that detailed assessments are not warranted in many cases and that our findings will require more detailed follow up [51].
While consumption frequency provides evidence that [THg] is related to fish in the diet, interpretation is complicated by potential differences in meal size, age or size of the fish, and species consumed. Given these caveats, δ15N may be a better predictor of [THg] in hair, or may significantly supplement dietary information. In this study, the strength of conclusion varies by whether we are assessing [THg] in the proximal hair segment or mean [THg] across the hair sample. This is likely due to the fact that the time frame for the proximal hair segment better matches the diet recall survey while the mean hair [THg] time frame better matches the C and N stable isotope kinetics. The stable isotope sample was comprised of all the remaining hair after the segmental [THg] analysis was done.
Individuals that were relatively enriched in δ15N had significantly higher [THg], likely due to higher finfish consumption although δ15N values in this population did not have a wide range (7.43–10.7‰). The relationship between δ15N and [THg] only explained 8% of the variability in [THg], thus we speculate this is likely due to the low protein consumption and multiple protein sources of this population and to additional abiotic Hg exposure. We will address this in future studies where we will include Hg, C and N data from actual food items related to observations in the hair of pregnant women. Women are consuming relatively little fish mass (Fig. 1), but as the fish consumed is generally of a high trophic level and associated high [THg], even at the consumption rates reported there could be link between fish consumption and [THg]. Future studies should collect data on meal size (mass), frequency, species of fish consumed (including fish size/age), and amount of consumption of other protein sources such as beef, chicken and eggs, as well as rice consumption [additional dietary source of Hg, Zhang et al. [52]] including measures of [THg] and C and N stable isotope values.
The variation in δ13C cannot be explained by reported diet and was not clearly related to [THg] possibly due to limitations of the study design (did not chemically characterize food items). In addition, this may be due to this population having a high use of maize, corn-based food additives (e.g. high fructose corn syrup), and marine protein sources [53]. Plants using the C3-photosynthetic pathway (such as rice and beans) are depleted in 13C relative to C4-photosynthetic plants [such as maize; Codron et al. [54]], allowing the determination of the relative contribution of C3 and C4 plants in the terrestrial diet. However, δ13C may help to identify consumers of marine resources if future studies were attempting to focus on that group and wanted to chemically exclude non-fish consumers. Including sulfur stable isotope analysis (δ34S) would strengthen this ability even further [55] and is being considered for future studies.
Individuals that report consuming no fish are depleted in δ15N relative to higher fish consumers, but δ15N values are not significantly different in individuals that report consuming fish once per month. However, we can determine which individuals are consuming little to no marine derived protein using δ15N. The lack of a clearer differentiation may be due to the fact that we have information on frequency of fish consumption rather than mass consumed; mass of the marine based diet is important since changes in C and N isotope signatures are altered based on the proportion of the amount of C and N containing macromolecules that are ingested and assimilated into the consumer based on the total amount of those constituents (proportion marine derived C and N nutrients relative to total intake). This is illustrated by one individual who had the lowest δ15N (7.43‰), as well as the most enriched δ13C (−12.19‰), and the lowest mean [THg] (0.12 μg/g), and reported consuming no fish or shellfish and dairy only once a month. This individual is likely a vegetarian and additionally is consuming very little dairy, and her diet explains her low [THg] fairly well. This individual could be removed if one were attempting to study only fish consumers.
The variation in [THg] can, in part, be explained by both reported diet and diet as determined by C and N stable isotopes. Individuals that were enriched in δ15N had higher [THg] as did individuals that reported consuming fish and shellfish more frequently. However, the link between [THg], fish consumption, and δ15N gets more complicated with higher reported levels of fish consumption. While [THg] and δ15N (trophic level) increase with fish consumption at the lower reported levels of fish consumption, for the higher fish consumption levels, trophic level is maintained but [THg] is lower (Fig. 2). This apparent disconnect could be due to types of fish consumed or meal size (mass consumed vs. frequency). Given that trophic level (δ15N) is maintained (although the values are more variable) at higher fish consumption levels, the decrease in [THg] may be due to types of fish consumed (e.g. [THg] varies with fish species, trophic level, and with age within species) than to a decreased or increased variability in actual mass of fish consumed. It seems unlikely that people reporting more frequent fish consumption would actually be consuming less fish, and δ15N values indicate that mean trophic level remains the same but we cannot account for the age of the fish consumed ([THg] are well known to increase with age of fish independent of trophic level). Lastly, the maintenance of trophic level with decreased [THg] could be due to a combination of more frequent fish consumption, but lower fish mass consumed from younger fish [36], and with increased consumption of beef or chicken protein (e.g., increases the proportion of non-marine N). This could result in maintenance of trophic position, relatively lower [THg], but relatively increased reported frequency of fish consumption.
5. Conclusions
There is a statistically significant relationship between increased [THg] and enriched δ15N (trophic position), and an increase in reported consumption of fish and increased [THg], suggesting that the increase in [THg] is due to fish consumption, at least at lower fish consumption frequencies and low to moderate [THg]. While we cannot completely tease apart the contribution of corn and corn-fed beef versus marine fish using C and N stable isotopes the significant relationship between δ15N values and reported consumption of fish supports the conclusion that fish consumption is an important pathway for Hg exposure in this population. Increased consumption of terrestrial fauna could result in an increase in trophic position but is unlikely to result in increased [THg]. We recommend that caution be used when consuming high trophic level fish during pregnancy based on our assessment of using various statistic measures (mean, lower and upper 95% CI) and a range of advisories based on [THg] in hair (1–20 μg g−1).
Conflict of interest
The authors declare that there are no conflicts of interest.
Transparency document
Acknowledgments
This project was funded by grants from CONACYT – Salud (2010-C01-140272) and CIBNOR (PC2.0, PC0.10, PC0.5). This study would not have been possible without the assistance of some current and former members of the Wildlife Toxicology Laboratory and School of Fisheries and Ocean Sciences at the University of Alaska Fairbanks. University of Alaska personnel were partially supported through the Center for Alaska Native Health Research by Award Number P20RR016430 from the National Center for Research Resources and through the IDeA Network of Biomedical Research Excellence Award Number P20GM103395 from the National Institute of General Medical Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Available online 13 October 2014
Contributor Information
Rebecca Bentzen, Email: rlmcguire@alaska.edu.
J. Margaret Castellini, Email: maggie.c@alaska.edu.
Ramón Gaxiola-Robles, Email: r.gaxiolar@gmail.com.
Tania Zenteno-Savín, Email: tzenteno04@cibnor.mx.
Lía Celina Méndez-Rodríguez, Email: lmendez04@cibnor.mx.
Todd O’Hara, Email: tmohara@alaska.edu.
References
- 1.EFSA Opinion of the scientific panel on contaminants in the food chain [CONTAM] related to the safety assessment of wild and farmed fish. EFSA J. 2005;3:1–118. [Google Scholar]
- 2.Alessandri J.M., Guesnet P., Vancassel S., Astorg P., Denis I., Langelier B. Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reprod. Nutr. Dev. 2004;44:509–538. doi: 10.1051/rnd:2004063. [DOI] [PubMed] [Google Scholar]
- 3.Myers G.J., Davidson P.W., Nutrient Strain J.J. Methyl mercury exposure from consuming fish. J. Nutr. 2007;137:2805–2808. doi: 10.1093/jn/137.12.2805. [DOI] [PubMed] [Google Scholar]
- 4.Hibbeln J.R., Davis J.M., Steer C., Emmett P., Rogers I., Williams C. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 5.Olsen S.F., Secher N.J. Low consumption of seafood in early pregnancy as a risk factor for preterm delivery: prospective cohort study. Br. Med. J. 2002;324:447. doi: 10.1136/bmj.324.7335.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams C., Birch E.E., Emmett P.M., Northstone K., Pregnancy tALSo, Team CS Stereoacuity at age 3.5 y in children born full-term is associated with prenatal and postnatal dietary factors: a report from a population-based cohort study. Am. J. Clin. Nutr. 2001;73:316–322. doi: 10.1093/ajcn/73.2.316. [DOI] [PubMed] [Google Scholar]
- 7.Denberg J.A., Hatfield H.M., Hayes M.C.M.L., Holt P.G., Sehmi R. Fish oil supplementation in pregnancy modifies neonatal progenitors at birth in infants at risk of atopy. Pediatr. Res. 2005;57:276–281. doi: 10.1203/01.PDR.0000148279.72611.1D. [DOI] [PubMed] [Google Scholar]
- 8.Dunstan J.A., Roper J., Mitoulas L., Hartmann P.E., Simmer K., Prescott S.L. The effect of supplementation with fish oil during pregnancy on breast milk immunoglobulin A, soluble CD14, cytokine levels and fatty acid composition. Clin. Exp. Allergy. 2004;34:1237–1242. doi: 10.1111/j.1365-2222.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- 9.Wagemann R., Trebacz E., Hunt R., Boila G. Percent methylmercury and organic mercury in tissues of marine mammals and fish using different experimental and calculation methods. Environ. Toxicol. Chem. 1997;16:1859–1866. [Google Scholar]
- 10.AMAP . Arctic Monitoring and Assessment Programme (AMAP); Oslo, Norway: 2011. AMAP Assessment 2011: Mercury in the Arctic; p. xiv + 193. [Google Scholar]
- 11.Aberg B., Ekman L., Falk R., Greitz U., Persson G., Snihs J-O. Metabolism of methyl mercury (203Hg) compounds in man. Arch. Environ. Health. 1969;19:478–484. doi: 10.1080/00039896.1969.10666872. [DOI] [PubMed] [Google Scholar]
- 12.Kjellström T. Effects on early childhood development of prenatal exposure to methylmercury. Arch. Environ. Health. 1991;46:118. [Google Scholar]
- 13.McKeown-Eyssen G., Ruedy J., Neims A. Methylmercury exposure in northern Quebec. II: neurological findings in children. Am. J. Epidemiol. 1983;118:470–479. doi: 10.1093/oxfordjournals.aje.a113652. [DOI] [PubMed] [Google Scholar]
- 14.Stewart P.W., Reihman J., Lonky E.I., Darvill T.J., Pagano J. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol. Teratol. 2003;25:11–22. doi: 10.1016/s0892-0362(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 15.Borum D., Manibusan M.K., Schoeny R., Winchester E.L. U.S. Environmental Protection Agency; Washington, DC: 2001. Water Quality Criterion for the Protection of Human Health: Methylmercury; p. 303. [Google Scholar]
- 16.Debes F., Budtz-Jørgensen E., Weihe P., White R.F., Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol. Teratol. 2006;28:536–547. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Dietz R., Basu N., Braune B., O’Hara T., Scheuhammer T., Sonne C. What are the toxicological effects of mercury in arctic biota? In: Outridge P., Dietz R., Wilson S., editors. AMAP Assessment 2011: Mercury in the Arctic. Oslo; Norway: 2011. pp. 113–137. [Google Scholar]
- 18.Airey D. Mercury in human hair due to environment and diet: a review. Environ. Health Perspect. 1983;52:303–316. doi: 10.1289/ehp.8352303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.State of Alaska Epidemiology Bulletin . Department of Health and Human Services, Division of Public Health, Epidemiology; Anchorage, AK: 2007. Fish Consumption Advice for Alaskans: A Risk Management Strategy to Optimize The Public's Health. [Google Scholar]
- 20.Pouzaud F., Ibbou A., Blanchemanche S., Grandjean P., Krempf M., Philippe H-J. Use of advanced cluster analysis to characterize fish consumption patterns and methylmercury dietary exposures from fish and other sea foods among pregnant women. J. Expo. Sci. Environ. Epidemiol. 2010;20:54–68. doi: 10.1038/jes.2009.2. [DOI] [PubMed] [Google Scholar]
- 21.Chiba M., Masironi R. Toxic and trace elements in tobacco and tobacco smoke. Bull. World Health Organ. 1992;70:269–275. [PMC free article] [PubMed] [Google Scholar]
- 22.Gaxiola-Robles R., Zenteno-Savín T., Labrada-Martagón V., Celis A., Acosta-Vargas B., Méndez-Rodríguez L.C. Concentraciones de mercurio en leche de mujeres del noroeste de México; posible asociación a la dieta, tabaco y otros factores maternos. Nutr. Hosp. 2013;28:934–942. doi: 10.3305/nh.2013.28.3.6447. [DOI] [PubMed] [Google Scholar]
- 23.WHO . World Health Organization; Geneva: 2007. Exposure to Mercury: A Major Public Health Concern. [Google Scholar]
- 24.Bentzen T.W., Follmann E.H., Amstrup S.C., York G.S., Wooller M.J., Muir D.C.G. Dietary biomagnification of organochlorine contaminants in Alaskan polar bears. Can. J. Zool. 2008;86:177–191. [Google Scholar]
- 25.Hobson K.A., Riget F.F., Outridge P.M., Dietz R., Born E. Baleen as a biomonitor of mercury content and dietary history of North Atlantic Minke Whales (Balaenopetra acutorostrata): combining elemental and stable isotope approaches. Sci. Total Environ. 2004;331:69–82. doi: 10.1016/j.scitotenv.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Hobson K.A., Welsh H.E. Determination of trophic relationships within a high Arctic marine food web using δ13C and δ15N analysis. Mar. Ecol. Prog. Ser. 1992;84:9–18. [Google Scholar]
- 27.Hoekstra P.F., Braune B.M., O’Hara T.M., Elkin B., Solomon K.R., Muir D.C.G. Organochlorine contaminant and stable isotope profiles in Arctic fox (Alopex lagopus) from the Alaskan and Canadian Arctic. Environ. Pollut. 2003;122:423–433. doi: 10.1016/s0269-7491(02)00310-x. [DOI] [PubMed] [Google Scholar]
- 28.Cardona-Marek T., Knott K.K., Meyer B.E., O’Hara T.M. Mercury concentrations in Southern Beaufort Sea polar bears: variation based on stable isotopes of carbon and nitrogen. Environ. Toxicol. Chem. 2009;28:1416–1424. doi: 10.1897/08-557.1. [DOI] [PubMed] [Google Scholar]
- 29.Dehn L-A., Follmann E.H., Thomas D.L., Sheffield G.G., Rosa C., Duffy L.K. Trophic relationships in an Arctic food web and implications for trace metal transfer. Sci. Total Environ. 2006;362:103–123. doi: 10.1016/j.scitotenv.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Rea L.D., Castellini J.M., Correa L., Fadely B.S., O’Hara T.M. Maternal Steller sea lion diets elevate fetal mercury concentrations in an area of population decline. Sci. Total Environ. 2013;45:4–455. doi: 10.1016/j.scitotenv.2013.02.095. 277-282. [DOI] [PubMed] [Google Scholar]
- 31.Post D.M. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology. 2002;83:703–718. [Google Scholar]
- 32.France R. Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Mar. Ecol. Prog. Ser. 1995;124:307–312. [Google Scholar]
- 33.France R.L., Peters R.H. Ecosystem differences in the trophic enrichment of δ13C in aquatic food webs. Can. J. Fish. Aquat. Sci. 1997;54 [Google Scholar]
- 34.Newsome S.D., Clementz M.T., Koch P.L. Using stable isotope biogeochemistry to study marine mammal ecology. Mar. Mammal Sci. 2010;26:509–572. [Google Scholar]
- 35.McDowell M.A., Dillon C.F., Osterloh J., Bolger P.M., Pellizzari E., Fernando R. Hair mercury levels in U.S. children and women of childbearing age: reference range data from NHANES 1999–2000. Environ. Health Perspect. 2004;112:1165–1171. doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrera-García A., O’Hara T., Galván-Magaña F., Méndez-Rodríguez L.C., Castellini J.M., Zenteno-Savín T. Oxidative stress indicators and trace elements in the blue shark (Prionace glauca) off the east coast of the Mexican Pacific Ocean. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2012;156:59–66. doi: 10.1016/j.cbpc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Barrera-García A., O’Hara T., Galván-Magaña F., Méndez-Rodríguez L.C., Castellini J.M., Zenteno-Savín T. Trace elements and oxidative stress indicators in the liver and kidney of the blue shark (Prionace glauca) Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2013;165:483–490. doi: 10.1016/j.cbpa.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 38.Erisman B.E., Paredes G.A., Plomozo-Lugo T., Cota-Nieto J.J., Hastings P.A., Aburto-Oropeza O. Spatial structure of commercial marine fisheries in Northwest Mexico. ICES J. Mar. Sci.: J. Cons. 2011;68:564–571. [Google Scholar]
- 39.Galván-Portillo M., Jiménez-Gutiérrez C., Torres-Sánchez L., López-Carrillo L. Food consumption and adipose tissue DDT levels in Mexican women. Cad. Saúde Pública. 2002;18:447–452. doi: 10.1590/s0102-311x2002000200009. [DOI] [PubMed] [Google Scholar]
- 40.Knott K.K., Boyd D., Ylitalo G.M., O’Hara T.M. Concentrations of mercury and polychlorinated biphenyls in blood of Southern Beaufort Sea polar bears (Ursus maritimus) during spring: variations with lipids and stable isotope (δ15N, δ13C) values. Can. J. Zool. 2011;89:999–1012. [Google Scholar]
- 41.Castellini J.M., Rea L.D., Lieske C.L., Beckmen K.B., Fadely B.S., Maniscalco J.M. Mercury concentrations in hair from neonatal and juvenile steller sea lions (Eumetopias jubatus): implications based on age and region in this northern Pacific marine sentinel piscivore. EcoHealth. 2012;9:267–277. doi: 10.1007/s10393-012-0784-4. [DOI] [PubMed] [Google Scholar]
- 42.U.S. EPA . Office of Air Quality Planning and Standards and Offices of Research and Development. Enviromental Protection Agency, International Programme on Chemical Safety (IPCS); 1997. Mercury Study Report to Congress. [Google Scholar]
- 43.Hamade A.K. Alaska Scientific Advisory Committee for Fish Consumption. Section of Epidemiology. Division of Public Health, Department of Health and Social Services, State of Alaska; 2014. Fish Consumption Advice for Alaskans: A Risk Management Strategy to Optimize the Public's Health. July 21; p. 78. http://www.epi.hss.state.ak.us/bulletins/docs/rr2007_04.pdf. [Google Scholar]
- 44.Feeley M.M., Lo M.-T. Risk assessment for mercury in Health Canada – development of the Provisional Tolerable Daily Intake (pTDI). In: Burgess W.P.N., Giguère M.-F., editors. Proceedings of the Conference on Mercury in Eastern Canada and the Northeast States; September; 1998. pp. 21–23. http://www.eman-rese.ca/eman/reports/publications/98_mercury2/intro.html. [Google Scholar]
- 45.NRC . National Research Council. National Academy Press; Washington, DC: 2000. (Toxicological Effects of Methylmercury). [Google Scholar]
- 46.WHO . World Health Organization, International Programme on Chemical Safety (IPCS); Geneva: 1990. Environmental Health Criteria for Methylmercury: Evaluation of Human Health Risks. Environmental Health Criteria 101. [Google Scholar]
- 47.Risher J., DeWoskin R. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (ATSDR); Atlanta, GA: 1999. Health effects: Relevance to Public Health. Toxicological Profile for Mercury; pp. 220–300. [Google Scholar]
- 48.Burnham K.P., Anderson D.R. Springer-Verlag; New York, NY, USA: 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. [Google Scholar]
- 49.Schober T.M., Molto J.E. Marine resource consumption in ancient California. Pac. Coast Archeol. Soc. Q. 2011;45 [Google Scholar]
- 50.Li P., Feng X., Qiu G. Methylmercury exposure and health effects from rice and fish consumption: a review. Int. J. Environ. Res. Public Health. 2010;7:2666–2691. doi: 10.3390/ijerph7062666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ngo J., Engelen A., Molag M., Roesle J., Garcìa-Segovia P., Serra-Majem L. A review of the use of information and communication technologies for dietary assessment. Br. J. Nutr. 2009;101:S102–S112. doi: 10.1017/S0007114509990638. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H., Feng X., Larssen T., Qiu G., Vogt R.D. In inland China, rice, rather than fish, is the major pathway for methylmercury exposure. Environ. Health Perspect. 2010;118:1183–1188. doi: 10.1289/ehp.1001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nash S.H., Kristal A.R., Bersamin A., Hopkins S.E., Boyer B.B., O’Brien D.M. Carbon and nitrogen stable isotope ratios predict intake of sweeteners in a Yup’ik Study Population. J. Nutr. 2013;143:161–165. doi: 10.3945/jn.112.169425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Codron D., Lee-Thorp J.A., Sponheimer M., de Ruiter D., Codron J. Inter- and intrahabitat dietary variability of chacma baboons (Papio ursinus) in South African savannas based on fecal δ13C, δ15N, and %N. Am. J. Phys. Anthropol. 2006;129:204–214. doi: 10.1002/ajpa.20253. [DOI] [PubMed] [Google Scholar]
- 55.Buchardt B., Bunch V., Helin P. Fingernails and diet. Stable isotope signatures of a marine hunting community from modern Uummannaq, North Greenland. Chem. Geol. 2007;244:316–329. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.