Abstract
Amphimas pterocarpoides Harms (Leguminosae) is widely used traditionally in Central and West Africa for the treatment of various ailments. However, no data regarding its safety have been published until now. Thus, the present study aimed to investigate the potential toxicity of the methanol extract of the stem bark of Amphimas pterocarpoides (AP) in Wistar rats following the OECD guidelines. In acute oral toxicity, female rats received a single dose of 2000 mg/kg of AP and were observed for 14 days. In subchronic toxicity, doses of 150, 300, 600 mg/kg/day of AP were given per os to rats (males and females) for 28 days. No death and abnormal behaviors were observed in acute toxicity and the LD50 was estimated higher than 5000 mg/kg. In the subchronic study, AP induced no significant variation in body weight and relative weight of organs, whereas a delayed decrease of white blood cell count and granulocytes was observed. Inconsistent increase of the total cholesterol/high density lipoprotein was observed at 600 mg/kg in males. Such variation (not dose dependent) and without biological relevance indicate a wide margin of safety for the traditional use of AP.
1. Introduction
Medicinal plants remain the most used form of treatment in most African villages. According to the World Health Organization, about 80% of the world population, especially in developing countries, rely on traditional medicine for their primary health care [1]. Besides the deficiency and the elevated cost of modern medicine [2], the easy accessibility of herbal medicines and the belief that they are safe and harmless since they are natural and have been used for thousands of years account for this gained popularity of traditional medicine and encourage the public toward self-medication [3], [4]. Though several plants possess interesting pharmacological activities, the valorization of these medicinal plants is very often limited due to the lack of written records about their safety or toxicity [5]. Therefore, the acute and subchronic toxicological studies of Amphimas pterocarpoides were carried out.
Amphimas pterocarpoides Harms (Leguminoseae) is a sub-Saharan tree known by regional names such as bokanga, edjip, edzui, lati or yaya, and is distributed in Central and West Africa. Various preparations of root and stem bark of this plant are used in traditional medicine to treat dysenteria, anemia, hematuria, dysmenorrhoea, impotence, and to prevent spontaneous abortion [6], [7]. In previous studies, hydro-alcoholic and aqueous extracts of the bark of Amphimas pterocarpoides displayed antioxidant and antianemia activities in vitro and in vivo, respectively [8], [9]. Moreover, four isoflavonoids namely amphiisoflavone, isoformononetin, 8-methoxy-isoformononetin, and 6-methoxyisoformononetin with antioxidant and antimicrobial activities were isolated from the dichloromethane–methanol (50:50 v/v) extract of the root bark of Amphimas pterocarpoides [10]. In our previous report, we isolated 11 potential estrogenic isoflavonoids from a methanol extract of the stem bark of this plant [11]. Our ongoing investigation showed osteoprotective properties of this extract at the dose of 150 mg/kg BW in adult ovariectomized Wistar rats. Despite the wide application of this plant in the folkloric medical practice and its methodical published activities, no reports on toxicity and safety profile are yet available. Therefore, this study has been designed aiming to evaluate the acute and subchronic oral toxicity of the methanol extract of the stem bark of Amphimas pterocarpoides in Wistar rats.
2. Material and methods
2.1. Plant material and extraction
The stem bark of Amphimas pterocarpoides were collected at Mount Eloumdem, in the suburbs of Yaounde, Center Region of Cameroon, on August 10th 2013 (8:00–9:00 am). The plant was identified and authenticated by Mr. Victor Nana, a botanist at the Cameroon National Herbarium where a voucher specimen is deposited under the reference number 52563/HNC. After the drying and pulverization process, 3 kg of the powder was extracted with 10 L of methanol of analytical grade (Merk, Darmstadt, Germany) for a total duration of 1 week. The filtration and renewal of methanol occurred every 2 days. The collected solution was concentrated under reduced pressure at 40 °C and lyophilized to produce 60 g of extract (2% yield) called AP. The extract was kept at 2–8 °C and dissolved in distilled water prior to administration. The administered volume was 2 mL per 100 g BW.
As reported in our previous study, 20 isoflavonoids and 1 flavonoid were identified in the methanol extract of the stem bark of Amphimas pterocarpoides using a UHPLC-LTQ Orbitrap system, and 11 representative isoflavonoids were further isolated and structurally identified using 1 and 2D NMR techniques [11].
2.2. Animals
Young Wistar rats, aged 8–10 weeks (acute toxicity) and 5–6 weeks (subchronic study) obtained from the animal house of the Laboratory of Animal Physiology of the University of Yaounde I, Cameroon, were used. They were maintained in standard conditions of temperature (at around 25 °C), with approximately 12 h light/dark natural illumination cycle and a relative humidity of 45–55%. They had free access to standard rat chow and tap water ad libitum. The guidelines of the Institutional Ethic Committee of the Cameroon Ministry of Scientific Research and Technological Innovation, which has adopted the guidelines established by the European Union on Animal Care (CEE Council 86/609) were followed for all procedures.
2.3. Acute toxicity
The acute oral toxicity test was performed using the acute toxicity class method (ATC) in accordance with the Organization for Economic Cooperation and Development (OECD) guideline 423 adopted on December 17th, 2001 [12]. Based on a survey of tests done using the conventional LD50 that pointed out that female rats are generally more sensitive [13], [14] and according to the OECD recommendation, female rats were used for this experiment. Six healthy rats were allocated in two groups of 3 animals each. The first group (Control) received distilled water by gavage whereas the second group received a single dose of 2000 mg/kg BW of AP. Prior to administration, animals were weighted, marked, and fasted overnight without suppression of water. After dosing, food was withheld for a further 3–4 h while animals were observed individually during the first 30 min and then 2, 4, 6 h after treatment. Thereafter, observations were made once daily (5–10 min) for a total period of 14 days and the animals were weighed every 4 days. The experiment was repeated with the same dose and same number of animals according to the OECD flow charts [12]. The observation focused on mortality, changes in general behavior, skin, eyes, fur, and somatomotor activity. Attention was directed to observations of tremors, convulsions, salivation, diarrhea, lethargy, sleep, and coma. At the end of the 14th day, animals were sacrificed by decapitation under light anaesthesia (10 mg/kg BW diazepam and 50 mg/kg BW ketamine i.p.) and liver, lungs, kidneys, heart, stomach, spleen, and adrenals were removed, weighed and immediately observed for gross pathological changes (gross necropsy).
2.4. Subchronic toxicity
The repeated dose 28-day oral toxicity was carried out according to OECD guideline 407, adopted on October 3rd, 2008 [15]. Sixty Wistar rats were distributed in 6 groups of 10 animals each (5 females and 5 males). Animals were segregated according to their gender to avoid any chance of mating. After a 7-day adaptation, the animals were treated as follows: The first group received distilled water (Control); groups 2–4 received the AP extract at the doses of 150, 300, and 600 mg/kg BW, respectively; Groups 5–6 served as satellite in the control (Control SAT) and the top dose (600 SAT) groups, respectively. Animals were treated daily (12:00–13:00) by gavage for 28 days and observed once daily. The satellite groups were followed-up for 14 days after the end of the treatment for observation of reversibility, or persistence, or delayed occurrence of toxic effects. The observation focused on mortality, changes in general behavior, skin, eyes, fur, and somatomotor activity. Attention was directed to observations of tremors, convulsions, salivation, diarrhea, lethargy, sleep, and coma. Animals were weighed every 4 days during treatment and posttreatment periods. Twenty-four hours after the last administration (for groups 1–4) and after the posttreatment follow-up (satellite groups), animals were sacrificed by decapitation under light anaesthesia (10 mg/kg BW diazepam and 50 mg/kg BW ketamine i.p.) after a 10–12 h overnight fast. Blood samples were collected for hematological and biochemical parameters analyses. The portion used for hematological analyses was collected in EDTA-coated tubes and the remainder in dry tubes. The heart, liver, kidneys, stomach, spleen, adrenals, and lungs were dissected, weighed, and immediately observed for gross pathological changes (gross necropsy). Thereafter, liver, kidneys, and lungs were fixed for 4 days in 10% formaldehyde for histological analysis.
2.5. Measurement of hematological and biochemical parameters
Blood samples in dry tubes were centrifuged at 3500 g (15 min at 4 °C) and the supernatant (serum) was collected and introduced into new tubes. Triglycerides (TG), total cholesterol (TC), high density lipoprotein (HDL-C), alanine transaminase (ALT), and aspartate transaminase (AST) were measured using reagent kits from Fortress Diagnostics Limited (Muckamore, United Kingdom) whereas the total bilirubin (TB) concentration was determined using reagent kits from Cypress Diagnostics (Langdorp, Belgium). Total proteins (TP) were measured using the Biuret reagent.
For hematological analysis, white blood cell (WBC) count, lymphocytes, monocytes, granulocytes, red blood cell (RBC) count, hematocrit, hemoglobin, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelets count were evaluated using a HumaCount 30TS Automated Hematology Analyzer from Human Diagnostics Worldwide (Wiesbaden, Germany).
2.6. Histopathological examination of the liver and the kidney
Liver, lung, and kidneys were dehydrated by a series of ethanol solution and embedded in paraffin blocks before cutting into 5 μm sections. These sections were stained with hematoxylin and eosin (HE) and examined under an Axioskop 40 microscope connected to a computer where the image was transferred using MRGrab1.0 and Axio Vision 3.1 softwares (Zeiss, Hallbermoos, Germany).
2.7. Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Comparison and analysis were performed using the two way nonparametric Mann–Whitney U test using GraphPat InStat 3.05 statistical software. The results were considered to be significant at values p < 0.05.
3. Results
3.1. Acute toxicity
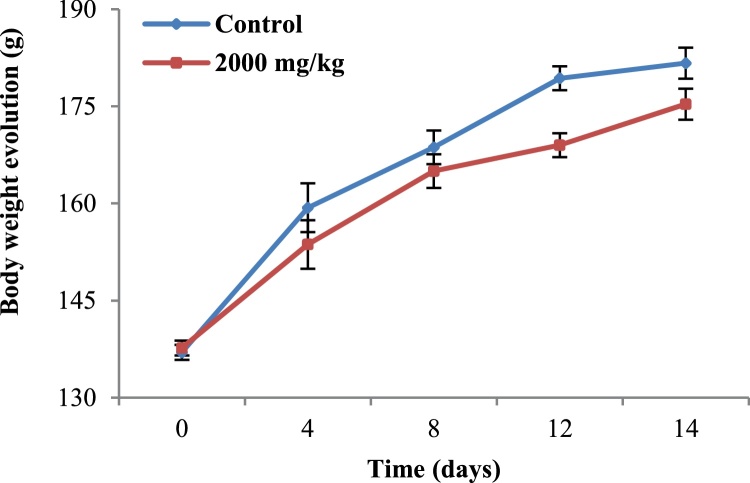
The single oral dose of AP at 2000 mg/kg BW, even after the repetition of the experiment did not cause mortality, changes in general behavior or any clinical signs of toxicity. In addition, between group significant differences were not observed with respect to body weight profile (Fig. 1), relative weight (Table 1), and gross pathological examinations of the selected organs. According to the OECD flow chart recommendations, the LD50 was estimated greater than 5000 mg/kg.
Fig. 1.
Body weight evolution of rats treated orally with the methanol extract of the stem bark of Amphimas pterocarpoides at the single dose of 2000 mg/kg BW. Each point represents the mean ± SEM (n = 6). No significant difference.
Table 1.
Relative weight of organs (mg/kg BW) in the control and AP-treated (2000 mg/kg BW) groups.
| Organs | Control | 2000 mg/kg |
|---|---|---|
| Heart | 3595 ± 79.0 | 3564 ± 107 |
| Liver | 34130 ± 126 | 31056 ± 976 |
| Stomach | 8473 ± 769 | 8068 ± 233 |
| Lungs | 6343 ± 40.0 | 6351 ± 739 |
| Spleen | 2790 ± 262 | 2949 ± 314 |
| Adrenals | 483 ± 32.0 | 395 ± 43.0 |
| Kidneys | 6862 ± 74.0 | 6849 ± 495 |
Data are represented as mean ± SEM (n = 6). No significant difference.
3.2. Subchronic toxicity
3.2.1. Observations
Throughout the experiment, no mortality or treatment-related signs of toxicity were recorded.
3.2.2. Body and organ weights
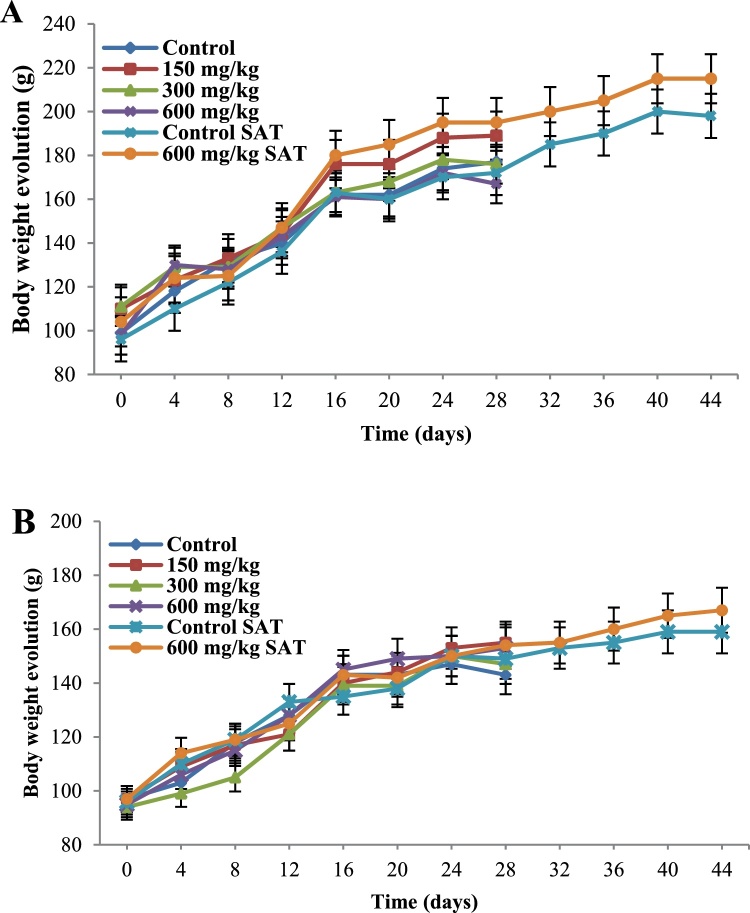
Compared to the control groups, no significant differences were observed in the body weight evolution (Fig. 2) or relative organ weights (Table 2, Table 3) at all the tested doses after the 28-day treatment and the 14-day posttreatment observation.
Fig. 2.
Body weight evolution of male (A) and female (B) rats during a 28-day treatment with the methanol extract of the stem bark of Amphimas pterocarpoides at 150, 300, and 600 mg/kg BW (n = 5). Each point represents the mean ± SEM. Control SAT: satellite control treated with vehicle; 600 mg/kg SAT: satellite of top dose treated with 600 mg/kg of extract. No significant difference.
Table 2.
Relative organ weights after a 28-day treatment with the methanol extract of stem bark of Amphimas pterocarpoides in male Wistar rats.
| Organs | Control |
A. pterocarpoides (mg/kg BW) |
Satellite groups |
|||
|---|---|---|---|---|---|---|
| 150 | 300 | 600 | Control SAT | 600 SAT | ||
| Liver | 37820 ± 1870 | 36542 ± 1932 | 37418 ± 2057 | 37340 ± 1870 | 40944 ± 5610 | 34287 ± 383 |
| Stomach | 9497 ± 624 | 9526 ± 387 | 10320 ± 634 | 9815 ± 1004 | 8728 ± 1116 | 7920 ± 277 |
| Kidneys | 6495 ± 249 | 6185 ± 225 | 6895 ± 251 | 7873 ± 817 | 6636 ± 881 | 5993 ± 148 |
| Lungs | 7160 ± 842 | 8053 ± 712 | 6579 ± 487 | 7770 ± 969 | 6975 ± 1115 | 5096 ± 182 |
| Spleen | 4628 ± 718 | 5375 ± 849 | 5003 ± 635 | 6175 ± 1360 | 2789 ± 393 | 2897 ± 256 |
| Heart | 3511 ± 105 | 3588 ± 217 | 4034 ± 231 | 3772 ± 343 | 3472 ± 469 | 3245 ± 62 |
| Adrenals | 264 ± 23 | 217 ± 15 | 235 ± 29 | 254 ± 52 | 382 ± 73 | 346 ± 25 |
Data are represented as mean ± SEM (n = 5). Control SAT: satellite control treated with vehicle; 600 SAT: satellite of top dose treated with the extract at 600 mg/kg BW. No significant difference.
Table 3.
Relative organ weights after a 28-day treatment with the methanol extract of stem bark of Amphimas pterocarpoides in female Wistar rats.
| Organs | Control |
A. pterocarpoides (mg/kg BW) |
Satellite groups |
|||
|---|---|---|---|---|---|---|
| 150 | 300 | 600 | Control SAT | 600 SAT | ||
| Liver | 32243 ± 1577 | 34944 ± 1105 | 36330 ± 2318 | 32457 ± 726 | 31156 ± 1188 | 34523 ± 1177 |
| Stomach | 9417 ± 520 | 8335 ± 217 | 9141 ± 226 | 9001 ± 327 | 8172 ± 324 | 9915 ± 215 |
| Kidneys | 6651 ± 141 | 6676 ± 186 | 6374 ± 312 | 6663 ± 93 | 6231 ± 93 | 6213 ± 120 |
| Lungs | 8665 ± 983 | 8438 ± 575 | 7635 ± 616 | 8374 ± 1166 | 7262 ± 309 | 7489 ± 682 |
| Spleen | 5121 ± 680 | 3427 ± 624 | 3799 ± 631 | 3100 ± 396 | 3076 ± 103 | 4059 ± 173 |
| Heart | 3845 ± 158 | 4180 ± 149 | 3816 ± 201 | 3812 ± 201 | 3916 ± 191 | 3725 ± 179 |
| Adrenals | 386 ± 19 | 543 ± 83 | 438 ± 100 | 383 ± 33 | 406 ± 37 | 545 ± 21 |
Data are represented as mean ± SEM (n = 5). Control SAT: satellite control treated with vehicle; 600 SAT: satellite of top dose treated with the extract at 600 mg/kg BW. No significant difference.
3.2.3. Hematological parameters
Table 4 shows that the 28-day oral administration of AP (once daily) induced a significant increase (p < 0.01) of platelets in males at the dose of 600 mg/kg. In addition, a significant and delayed decrease (p < 0.01) of white blood cell count (WBC), monocytes, granulocytes, and platelets as well as a significant increase (p < 0.01) of lymphocytes were noted in males after the 14-day posttreatment follow-up.
Table 4.
Hematological parameters after a 28-day treatment with the methanol extract of stem bark of Amphimas pterocarpoides in male Wistar rats.
| Parameters | Historical ranges | Control |
A. pterocarpoides (mg/kg BW) |
Satellite groups |
|||
|---|---|---|---|---|---|---|---|
| 150 | 300 | 600 | Control SAT | 600 SAT | |||
| WBC (×103 μL−1) | 5–16 | 15.58 ± 2.65 | 14.84 ± 2,18 | 15.96 ± 2.23 | 17.32 ± 4.78 | 13.38 ± 1.92 | 3.55 ± 0.35** |
| Lymphocytes (%) | 65–85 | 68.58 ± 2.27 | 68.04 ± 2,85 | 64.30 ± 2.23 | 70.18 ± 1.42 | 76.78 ± 1.19 | 89.33 ± 1.26** |
| Monocytes (%) | 0–20 | 19.66 ± 1.12 | 18.60 ± 1,18 | 20.96 ± 1.36 | 16.28 ± 1.27 | 14.10 ± 1.70 | 8.40 ± 0.45** |
| Granulocytes (%) | 0–27 | 11.78 ± 1.33 | 13.38 ± 1,55 | 14.74 ± 1.46 | 13.54 ± 1.53 | 11.60 ± 0.76 | 2.48 ± 0.53** |
| RBC (×103 μL−1) | 5–10 | 7.30 ± 0.31 | 6.71 ± 0,88 | 7.53 ± 0.30 | 7.65 ± 1.21 | 7.00 ± 0.48 | 5.47 ± 0.48 |
| Hematocrit (%) | 32–53 | 38.94 ± 1.26 | 35.66 ± 3,55 | 38.92 ± 1.33 | 39.82 ± 6.08 | 36.70 ± 4.46 | 27.03 ± 2.34 |
| MCV (fL) | 52–59 | 53.40 ± 0.81 | 54.80 ± 2,85 | 51.60 ± 0.81 | 52.20 ± 1.32 | 57.25 ± 3.95 | 49.80 ± 0.48 |
| Platelets (×103 μL−1) | 200–1100 | 317.60 ± 23.26 | 266.80 ±17,50 | 328.40 ± 15.07 | 419.60 ± 22.75** | 271.00 ± 24,46 | 72.67 ± 4.06** |
| MCH (pg) | 17–27 | 18.88 ± 0.27 | 21.12 ± 2,86 | 18.18 ± 0.40 | 17.74 ± 0.61 | 24.02 ± 2.23 | 19.13 ± 0.18 |
| Hemoglobin (g/dL) | 12–18 | 13.76 ± 0.48 | 13.16 ± 0,61 | 13.68 ± 0.48 | 13.68 ± 2.46 | 12.62 ± 1.49 | 10.40 ± 0.83 |
| MCHC (g/dL) | 32–45 | 35.34 ± 0.34 | 38.02 ± 0,97 | 35.18 ± 0.97 | 34.00 ± 0.77 | 41.34 ± 0.84 | 38.53 ± 0.26 |
WBC: white blood cells; RBC: red blood cells; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHM: mean corpuscular hemoglobin concentration. Control SAT: satellite control treated with vehicle; 600 SAT: satellite of top dose treated with AP at 600 mg/kg BW.
Data are represented as mean ± SEM (n = 5).
**p < 0.01 significantly different as compared to control groups.
In females, significant increases (p < 0.05) of RBC (at 150 and 600 mg/kg), along with hematocrit and hemoglobin (at 600 mg/kg) were observed. Moreover, significant increases (p < 0.01) of platelets were noted at the doses of 300 and 600 mg/kg after the 28-day treatment, whereas significant and delayed decreases of WBC (p < 0.05), granulocytes, and platelets (p < 0.01) were observed after a 14-day posttreatment (Table 5).
Table 5.
Hematological parameters after a 28-day treatment with the methanol extract of stem bark of Amphimas pterocarpoides in female Wistar rats.
| Parameters | Historical ranges | Control |
A. pterocarpoides (mg/kg BW) |
Satellite groups |
|||
|---|---|---|---|---|---|---|---|
| 150 | 300 | 600 | Control SAT | 600 SAT | |||
| WBC (×103 μL−1) | 5–16 | 11.90 ± 0.42 | 11.40 ± 0.49 | 12.94 ± 1.78 | 9.18 ± 2.35 | 14.92 ± 2.71 | 6.84 ± 0.82* |
| Lymphocytes (%) | 65–85 | 75.88 ±1.25 | 70.55 ± 2.53 | 72.68 ± 1.39 | 73.70 ± 1.82 | 79.06 ± 2.88 | 82.18 ± 2.57 |
| Monocytes (%) | 0–20 | 14.23 ± 0.70 | 14.98 ± 1.04 | 15.52 ± 0.88 | 16.46 ± 1.05 | 12.92 ± 0.59 | 11.80 ± 1.06 |
| Granulocytes (%) | 0–27 | 11.03 ± 0.66 | 16.58 ± 2.54 | 11.50 ± 0.84 | 9.88 ± 1.03 | 12.18 ± 1.45 | 6.28 ± 0.77** |
| RBC (×103 μL−1) | 5–10 | 6.86 ± 0.33 | 8.18 ± 0.37* | 7.47 ± 0.30 | 8.20 ± 0.21** | 5.51 ± 0.68 | 5.74 ± 0.37 |
| Hematocrit (%) | 32–53 | 37.22 ± 2.76 | 38.46 ± 3.97 | 40.50 ± 1.34 | 45.60 ± 1.30* | 25.76 ± 3.98 | 28.16 ± 1.72 |
| MCV (fL) | 52–59 | 58.00 ± 1.97 | 56.80 ± 4.72 | 54.40 ± 108 | 55.60 ± 1.20 | 54.60 ± 3.88 | 49.20 ± 0.20 |
| Platelets (×103 μL−1) | 200–1100 | 343.67 ± 9.39 | 390.33 ± 41.00 | 440.75± 41.02** | 488.00± 26.82** | 216.00 ± 82.47 | 105.00 ± 3.83** |
| MCH (pg) | 17–27 | 18.16 ± 0.26 | 17.03 ± 0.43 | 18.86 ± 0.49 | 18.12 ± 0.28 | 26.50 ± 3.26 | 19.56 ± 0.25 |
| Hemoglobin (g/dL) | 12–18 | 12.90 ± 0.45 | 13.73 ± 0.80 | 14.04 ± 0.48 | 14.92 ± 0.32** | 7.33 ± 1.51 | 11.26 ± 0.82 |
| MCHC (g/dL) | 32–45 | 32.50 ± 0.17 | 33.57 ± 0.93 | 34.66 ± 0.51 | 32.74 ± 0.78 | 45.60 ± 2.57 | 39.76 ± 0.54 |
WBC: white blood cells; RBC: red blood cells; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHM: mean corpuscular hemoglobin concentration. Control SAT: satellite control treated with vehicle; 600 SAT: satellite of top dose treated with AP at 600 mg/kg BW.
Data are represented as mean ± SEM (n = 5).
*p < 0.05 and **p < 0.01 significantly different from control groups.
3.2.4. Biochemical parameters
Table 6 shows a significant decrease (p < 0.01) of HDL-C levels at all tested doses and a significant increase (p < 0.01) of total bilirubin (TB) at 600 mg/kg in males. No significant variations were observed in TG and TC levels. On the other hand, significant increases (p < 0.05) of the TC/HDL-C ratio was observed at 150 mg/kg. Compared to the satellite control, the extract at the dose of 600 mg/kg induced a delayed and significant decrease (p < 0.01) of total proteins (TP) after a 14-day posttreatment.
Table 6.
Biochemical parameters after a 28-day treatment with the methanol extract of stem bark of Amphimas pterocarpoides in male Wistar rats.
| Parameters | Control |
A. pterocarpoides (mg/kg BW) |
Satellite groups |
|||
|---|---|---|---|---|---|---|
| 150 | 300 | 600 | Control SAT | 600 SAT | ||
| TG (mg/dL) | 95.92 ± 21.39 | 124.25 ± 9.14 | 79.64 ± 18.85 | 69.73 ± 11.98 | 105.06 ± 15.80 | 103.47 ± 3.91 |
| TC (mg/dL) | 76.36 ± 4.63 | 73.40 ± 5.06 | 74.38 ± 5.37 | 79.05 ± 9.19 | 77.13 ± 4.76 | 83.72 ±13.04 |
| HDL-C (mg/dL) | 31.86 ± 2.39 | 24.78 ± 0.70** | 22.36 ± 2.03** | 21.48 ± 2.27** | 13.92 ± 0.98 | 15.21 ± 1.77 |
| ALT (U/L) | 96.56 ± 6.64 | 93.23 ± 2.97 | 107.24 ± 4.58 | 116.96 ± 4.58 | 111.20 ± 4.58 | 99.16 ± 4.91 |
| AST (U/L) | 105.79 ± 6.39 | 106.54 ± 3.30 | 108.86 ± 1.26 | 115.91 ± 3.83 | 111.00 ± 2.92 | 108.67 ± 1.92 |
| TB (mg/dL) | 0.2 ± 0.02 | 0.19 ± 0.03 | 0.22 ± 0.03 | 0.45 ± 0.11** | 0.24 ± 0.02 | 0.24 ± 0.02 |
| TP (g/dL) | 1.39 ± 0.04 | 1.31 ± 0.02 | 1.35 ± 0.05 | 1.46 ± 0.07 | 1.43 ± 0.10 | 1.17 ± 0.02** |
| TC/HDL-C | 2.43 ± 0.18 | 2.97 ± 0.24 | 3.44 ± 0.37 | 3.70 ± 0.24* | 5.64 ± 0.47 | 5.45 ± 0.72 |
TG: triglycerides; TC: total cholesterol; HDL-C: high density lipoprotein cholesterol; ALT: alanine transaminase; AST: aspartate transaminase; TB: total bilirubin; TP: total protein. Control SAT: satellite control treated with vehicle; 600 SAT: satellite of top dose treated with AP at 600 mg/kg BW.
Data are represented as mean ± SEM (n = 5).
*p < 0.05 and **p < 0.01 significantly different compared to control groups.
In females, all biochemical parameters evaluated were not significantly different (Table 7).
Table 7.
Biochemical parameters after a 28-day treatment with the methanol extract of stem bark of Amphimas pterocarpoides in female Wistar rats.
| Parameters | Control |
A. pterocarpoides (mg/kg BW) |
Satellite groups |
|||
|---|---|---|---|---|---|---|
| 150 | 300 | 600 | Control SAT | 600 SAT | ||
| TG (mg/dL) | 68.65 ± 6.69 | 93.48 ± 4.96 | 92.96 ± 9.82 | 84.66 ± 8.72 | 66.64 ± 3.05 | 64.54 ± 8.00 |
| TC (mg/dL) | 60.87 ± 3.84 | 61.09 ± 8.91 | 60.20 ± 7.49 | 54.01 ± 3.55 | 63.92 ± 10.71 | 67.61 ± 12.63 |
| HDL-C (mg/dL) | 22.04 ± 1.19 | 18.80 ± 0.83 | 22.43 ± 1.37 | 22.33 ± 0.61 | 10.33 ± 0.87 | 10.71 ± 1.69 |
| ALT (U/L) | 105.4 ± 1.29 | 100.13 ± 3.70 | 104.73 ± 3.13 | 101.80 ± 2.58 | 115.72 ± 1.86 | 106.87 ± 4.04 |
| AST (U/L) | 121.2 ± 3.72 | 129.64 ± 5.71 | 114.34 ± 4.88 | 112.99 ± 2.98 | 108.46 ± 4.36 | 112.60 ± 3.93 |
| TB (mg/dL) | 0.3 ± 0.02 | 0.3 ± 0.02 | 0.3 ± 0.02 | 0.34 ± 0.02 | 0.26 ± 0.02 | 0.29 ± 0.03 |
| TP (g/dL) | 1.47 ± 0.05 | 1.52 ± 0.08 | 1.44 ± 0.05 | 1.48 ± 0.04 | 1.29 ± 0.07 | 1.07 ± 0.07 |
| TC/HDL-C | 2.78 ± 0.17 | 3.24 ± 0.45 | 2.71 ± 0.34 | 2.42 ± 0.13 | 6.71± 1.64 | 7.88 ± 3.01 |
TG: triglycerides; TC: total cholesterol; HDL-C: high density lipoprotein cholesterol; ALT: alanine transaminase; AST: aspartate transaminase; TB: total bilirubin; TP: total protein. Control SAT: satellite control treated with vehicle; 600 SAT: satellite of top dose treated with AP at 600 mg/kg BW.
Data are represented as mean ± SEM (n = 5).
*p < 0.05 and **p < 0.01 significantly different from control groups.
3.2.5. Histopathological assessment of the liver, kidney, and lung
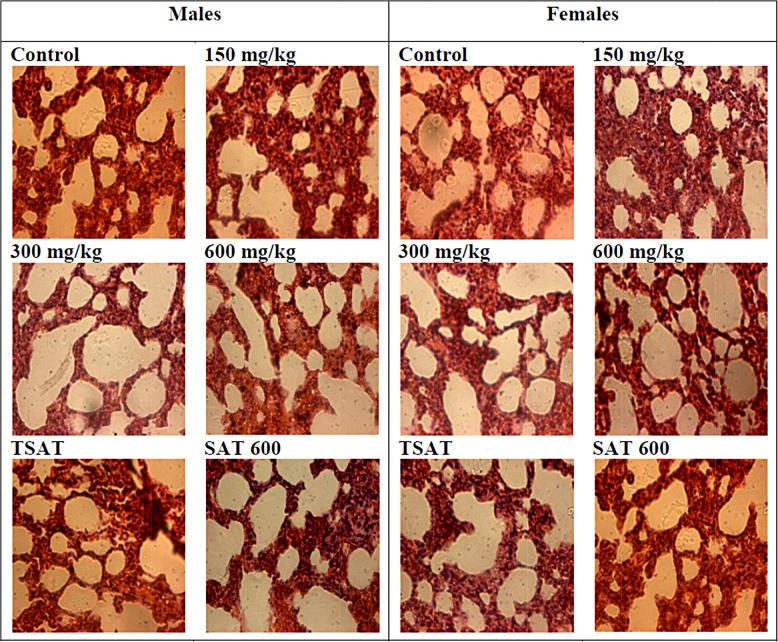
Compared to the control groups, histopathological features of the liver and the kidney of male and female Wistar rats showed normal structures after a subchronic (28 days) administration of AP at the doses of 150, 300, and 600 mg/kg. In males, a slight overdistention of air spaces was observed at the dose of 300 mg/kg (Fig. 3).
Fig. 3.
Microphotographs of hematoxylin/eosin (400×) stained sections of lungs in subchronic oral treatment with the methanol extract of stem bark of Amphimas pterocarpoides at 150, 300, and 600 mg/kg. TSAT: satellite control treated with the vehicle, SAT 600: Satellite of top dose treated with the extract at 600 mg/kg.
4. Discussion
Amphimas pterocarpoides is a plant widely used in African traditional medicine for the treatment of various ailments. The present work evaluates the acute and subchronic oral toxicity of the methanol extract of stem bark of this plant (AP) in order to determine its safety profile. In the acute toxicity study, neither mortality nor any signs of toxicity were observed in animals after the oral administration of AP at the dose of 2000 mg/kg BW and after a posttreatment period of 14 days. The results remained the same even after the repetition of the experiment and thus, the LD50 has been estimated greater than 5000 mg/kg. Such substances, according to Kennedy et al. are considered as practically nontoxic and resulted in classifying of AP in hazard category 5 or unclassified in accordance with the Globally Harmonized Classification System for Chemical Substances and Mixtures [12], [16], [17].
In the 28-day treatment, no significant differences in the relative weight of organs were noted compared to the control groups. Relative organ weight is an indicator of toxic effects of drugs [18], and any significant increase of relative weight of the organs might be due to the hypertrophy and/or hyperplasia. In line with this, our results suggest that at the tested doses, AP did not induce the hypertrophy or hyperplasia in the selected organs. Similarly, the change in body weight may be an important signal of toxicity [19]. No statistically significant difference in body weight of animals was observed suggesting that at the tested doses AP has no effect on animals’ growth.
The hematopoietic system is one of the most sensitive targets of xenobiotics [20]. Therefore, it constitutes an important marker of the physiopathological status [21] and provides informations on the vital state of the bone marrow activity and on the intra-vascular effects of xenobiotics [22]. Compared to the control group, significant increases in RBC count (at 150 and 600 mg/kg), hematocrit and hemoglobin (at 600 mg/kg) were noted in females. Although these slight variations were statistically significant, they remained within the normal physiological ranges [23] and therefore, cannot be considered as toxic effects. These variations are not observed in males, suggesting a higher sensitivity of females to the treatment. Platelets play an important role in hemostasis through the building of a platelet plug. Our results showed a significant increase albeit in the normal physiological limits, of platelet count at 300 mg/kg in females and 600 mg/kg in both sexes. However, satellites groups of both sexes treated with the top dose of extract (600 SAT) showed a significant decrease of platelet count compared to the Control SAT group. These results suggest that AP at the highest tested dose induced a delayed effect either by decreasing platelets production or by reducing the circulating levels of platelets (thrombocytopenia). Likewise, there was a delayed decrease of white blood cells count in both sexes at the dose of 600 mg/kg probably due to the action of AP on hematopoietic cells and/or on circulating white blood cells. On the other hand, delayed decreases in monocytes (in males) and granulocytes (both sexes) as well as a delayed increase in lymphocytes (in males) were observed suggesting that AP acts in opposite way on leucocyte subpopulations and has gender-related effects.
As far as biochemical parameters are concerned, the subchronic administration of AP induced no significant variation of ALT and AST. ALT, specific to hepatocytes and AST, found in liver, cardiac muscle, and kidney [24], [25] are well-known as markers of cell damage, especially hepatocyte necrosis [26], [27]. Therefore, the non variation of these two parameters indicated the absence of hepatocyte necrosis. Total bilirubin, product of hemoglobin degradation is a marker of hepatobiliary injury [28], [29]. In our study, subchronic treatment with AP at the dose of 600 mg/kg induced a significant increase of total bilirubin in males. Usually, the increase in serum bilirubin is related to an increase of hemolysis [30], liver injury or cholestasis [31]. Given the lack of any signs of necrosis following the histopathological examinations of the liver, no significant variation of ALT activity and RBC, the increase in total bilirubin observed in males not indicated any sign of toxicity since all values were within physiological ranges. Significant decrease of HDL-C levels were observed in males at all the tested doses while no statistically significant difference was noted in females, suggesting a gender-related effect in which males were more sensitive. It has long been known that a low HDL-C is a predictor of increased cardiovascular risk. However, over the last decade, it appeared that these lipids’ parameters taken individually are not always reliable indicators of the cardiovascular disease [32]. Therefore, the TC/HDL-C ratio is thought to be more predictive for the cardiovascular risks and its increase is correlated positively with the increase of cardiovascular risks. Increase of the TC/HDL-C ratio was only observed at the doses of 600 mg/kg in males. According to our results, the increase of TC/HDL-C appears to be mainly due to de decrease of HDL-C observed.
Histopathological examinations of the liver and the kidney of both sexes showed normal integrity of these tissues at all tested doses after a subchronic treatment of AP. However, a slight increase of the volume of pulmonary alveoli was noted in males at the dose of 300 mg/kg. Since there were no signs of lungs injury at the highest dose (600 mg/kg), this slight overdistention of alveoli air spaces was observed at 300 mg/kg appear to be an artifact or a biological variation with a lack of biological significance.
5. Conclusions
In conclusion, the single oral dose of 2000 mg/kg of the methanol extract of the stem bark of Amphimas pterocarpoides induced no mortality, no behavior changes, and any treatment-related signs of toxicity. However, in subchronic treatment, AP induced a delayed decrease of white blood cell and platelet count at 600 mg/kg. Therefore, the present investigation demonstrated that the methanol extract of stem bark of Amphimas pterocarpoides may be considered as relatively safe of toxicity. As the extract used differs from the traditional preparation (usually decoction), this could account for the inconsistent adverse effects observed. Therefore, further studies with the traditional way of preparation are needed to extrapolate the safety of this plant in human.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Transparency document
Acknowledgements
This work was supported by a grant from the International Foundation of Science (Reference: F/3336-2F) to Dieudonné Njamen.
Footnotes
Available online 16 October 2014
References
- 1.WHO . World Health Organization; Geneva: 2013. Traditional Medicine Strategy 2014–2023. [Google Scholar]
- 2.Zhu M., Lew K.T., Leung P.L. Protective effect of a plant formula on ethanol-induced gastric lesions in rats. Phytother. Res. 2002;16:276–280. doi: 10.1002/ptr.839. [DOI] [PubMed] [Google Scholar]
- 3.Fennell C.W., Light M.E., Sparg S.G., Stafford G.I., Van Staden J. Assessing African medicinal plants for efficacy and safety: agricultural and storage practices. J. Ethnopharmacol. 2004;95:113–121. doi: 10.1016/j.jep.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Haq I. Safety of medicinal plants. Pak. J. Med. Res. 2004;43:203–210. [Google Scholar]
- 5.WHO . World Health Organization; Geneva: 2002. Traditional Medicine Strategy 2002-2005. [Google Scholar]
- 6.Jiofack T., Ayissi I., Fokunang C., Guedje N., Kemeu V. Ethnobotany and phytomedicine of the upper Nyong valley forest in Cameroon. Afr. J. Pharm. Pharm. 2009;3:144–150. [Google Scholar]
- 7.Tchinda A.T., Tané P. Amphimas pterocarpoides Harms. In: Louppe D., Oteng-Amoako A.A., Brink M., editors. Plant Resources of Tropical Africa 7(1). Timber 1. PROTA Foundation, Wageningen/Backhuys Publishers; Leiden/CTA, Wageningen: 2008. pp. 72–75. [Google Scholar]
- 8.Biapa N.P.C., Agbor G.A., Oben J.E., Ngogang J.Y. Phytochemical studies and antioxidant properties of four medicinal plants used in Cameroon. Afr. J. Tradit. Complement. Altern. Med. 2007;4:495–500. doi: 10.4314/ajtcam.v4i4.31243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biapa N.P.C., Oben J.E., Ngogang J.Y. Scavenging radical kinetic and antianemic screening properties of some medicinal plants used in Cameroon. Int. J. Appl. Res. Nat. Prod. 2011;4:29–35. [Google Scholar]
- 10.Saah E.P.F., Sielinou V.T., Kuete V., Lacmata S.T., Nkengfack A.E. Antimicrobial and antioxidant isoflavonoid derivatives from the roots of Amphimas pterocarpoides. Zeitschrift fur Naturforschung. 2013;68:931–938. [Google Scholar]
- 11.Tchoumtchoua J., Njamen D., Mbanya J.C., Skaltsounis A.L., Halabalaki M. Structure-oriented UHPLC-LTQ Orbitrap-based approach as a dereplication strategy for the identification of isoflavonoids from Amphimas pterocarpoides crude extract. J. Mass Spectrom. 2013;48:561–575. doi: 10.1002/jms.3167. [DOI] [PubMed] [Google Scholar]
- 12.OECD . Acute oral toxicity—Acute Toxic Class Method. OECD; Paris: 2001. OECD Guidelines for testing of chemicals. [Google Scholar]
- 13.Bruce R.D. An up-and-down procedure for acute toxicity testing. Fundam. Appl. Toxicol. 1985;5:151–157. doi: 10.1016/0272-0590(85)90059-4. [DOI] [PubMed] [Google Scholar]
- 14.Lipnick R.L., Cotruvo J.A., Hill R.N., Bruce R.D., Stitzel K.A., Walker A.P., Chu I., Goddard M., Segal L., Springer J.A., Myers R.C. Comparison of the up-and-down, conventional LD50, and fixed-dose acute toxicity procedures. Food Chem. Toxicol. 1995;33:223–231. doi: 10.1016/0278-6915(94)00136-c. [DOI] [PubMed] [Google Scholar]
- 15.OECD . OECD; Paris: 2008. Guidelines for testing of chemicals: repeated dose 28-day oral toxicity in rodents. [Google Scholar]
- 16.Kennedy G.L., Ferenz R.L., Burgess B.A. Estimation of acute oral toxicity in rats by determination of the approximate lethal dose rather than the LD50. J. Appl. Toxicol. 1986;6:145–148. doi: 10.1002/jat.2550060302. [DOI] [PubMed] [Google Scholar]
- 17.OECD . OECD; Paris: 2001. Harmonised Integrated Classification System for Human Health and Environmental Hazards of Chemical Substances and Mixtures. [Google Scholar]
- 18.Piao Y., Liu Y., Xie X. Change trends of organ weight background data in Sprague Dawley rats at different ages. J. Toxicol. Pathol. 2013;26:29–34. doi: 10.1293/tox.26.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souza D.P.M., Paulino A.C., Maiorka P.C., Gorniak S.L. Administration Senna occidentalis seeds to adult and juvenile rats: effects on thymus, spleen and in hematological parameters. J. Pharm. Toxicol. 2010;5:46–54. [Google Scholar]
- 20.Harper H.A. Lange Medical Publications; Los Altos, California: 1973. Review of Physiological Chemistry. [Google Scholar]
- 21.Diallo A., Eklu G.K., Agbonon A., Aklikokou K., Creppy E.E., Gbeassor M. Acute and subchronic (28-day) oral toxicity studies of hydro-alcoholic extract of Lannea kerstingii Engl. and K. Krause (Anacardiaceae) stem bark. J. Pharm. Toxicol. 2010;5:343–349. [Google Scholar]
- 22.Voigt G.L. Anemia and polychythenias. In: Voigt G.L., editor. Hematology Techniques and Concepts for Veterinary Technicians. Iowa State University Press; Iowa: 2000. pp. 95–101. [Google Scholar]
- 23.Fallon M.T. Rats and Mice. In: Laber-Laird K., Swindle M.M., Flecknell P., editors. Handbook of Rodent and Rabbit Medicine. Elsevier Science Ltd.; Oxford: 1996. pp. 1–38. [Google Scholar]
- 24.Han Y., Song S., Lee J., Lee D., Yoon H. Multienzyme-modified biosensing surface for the electrochemical analysis of aspartate transaminase and alanine transaminase in human plasma. Anal. Bioanal. Chem. 2011;400:797–805. doi: 10.1007/s00216-011-4797-6. [DOI] [PubMed] [Google Scholar]
- 25.Witthawaskul P., Panthong A., Kanjanapothi D., Taesothikul T., Lertprasertsuke N. Acute and subacute toxicities of the saponin mixture isolated from Schefflera leucantha Viguier. J. Ethnopharmacol. 2003;89:115–121. doi: 10.1016/s0378-8741(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 26.Karthikeyan S., Gobianand K., Pradeep K., Raj Mohan C.V., Balasubramanian M.P. Biochemical changes in serum, lung, heart and spleen tissues of mice exposed to subacute toxic inhalation of mosquito repellent mat vapor. J. Environ. Biol. 2006;27:355–358. [PubMed] [Google Scholar]
- 27.Ramaiah S.K. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem. Toxicol. 2007;45:1551–1557. doi: 10.1016/j.fct.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Herlong H.F. Approach to the patient with abnormal liver enzymes. Hosp. Prac. 1994;29:32–38. doi: 10.1080/21548331.1994.11443103. [DOI] [PubMed] [Google Scholar]
- 29.McDonald G.B., Hinds M.S., Fisher L.D., Schoch H.G., Wolford J.L., Banaji M., Hardin B.J., Shulman H.M., Clift R.A. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann. Intern. Med. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 30.Orisakwe O.E., Afonne O.J., Chude M.A., Obi E., Dioka C.E. Subchronic toxicity studies of the aqueous extract of Boerhavia diffusa leaves. J. Health Sci. 2003;49:444–447. [Google Scholar]
- 31.Bearman S.I. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85:3005–3020. [PubMed] [Google Scholar]
- 32.Kastelein J.J.P., van der Steeg W.A., Holme I., Gaffney M., Cater N.B., Barter P., Deedwania P., Olsson A.G., Boekholdt S.M., Demicco D.A., Szarek M., LaRosa J.C., Pedersen T.R., Grundy S.M. Lipids, Apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation. 2008;117:3002–3009. doi: 10.1161/CIRCULATIONAHA.107.713438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.