Abstract
The increased pericardial fat which often accompanies overall obesity is thought to alter cardiac structure/function and increase the risk for atrial fibrillation. We hypothesized that chronic exposure to bisphenol A (BPA) would induce pericardial fat, cardiac hypertrophy or arrhythmia. C57bl/6n dams were exposed to BPA (25 ng/ml drinking water) beginning on gestation day 11 and progeny continued on 2.5 ng BPA/ml drinking water. The progeny of control dams (VEH) and dams treated with diethylstilbestrol (DES, 1 μg/kg/day, gestation days 11⿿14) had tap water. After weaning progeny were fed either a control (CD) or high fat diet (HFD) for 3 months. Pericardial fat was present in CD-BPA and CD-DES and not CD-VEH mice, and was increased in all HFD mice. Catecholamine challenge revealed no differences in males, but BPA-exposed females had longer P-wave and QRS complex duration. Only CD-BPA and CD-DES females developed cardiac hypertrophy which was independent of increased blood pressure. Calcium homeostasis protein expression changes in HFD-BPA and HFD-DES mice predict reduced SERCA2 activity in males and increased SERCA2 activity in females. Thus, chronic BPA exposure induced pericardial fat in the absence of HFD, and female-specific changes in cardiac hypertrophy development and cardiac electrical conduction after a catecholamine challenge.
Keywords: Bisphenol A, Diethylstilbestrol, High fat diet, Electrocardiograph, Echocardiograph, Calcium homeostasis
1. Introduction
Obesity is a risk factor for cardiovascular disease [27]. One reason may be because the increase in pericardial fat which often accompanies overall obesity alters cardiac structure/function and in some studies increased the risk for atrial fibrillation [2], [17], [18], [19]. Left ventricle mass (LVM) is greater in obese versus lean humans even when ancillary co-morbidities are absent [28]. Similarly in an animal model, high fat diet (HFD) fed male C57bl/6j mice became obese and developed increased LVM [10].
Epidemiological analyses correlate increased exposure to the estrogenic endocrine disruptor bisphenol A (BPA) suggesting that BPA may promote obesity and cardiovascular disease [29], [36], [37], [56]. Supporting the epidemiological results, early life exposure to BPA or the estrogenic compound diethylstilbestrol (DES) in mice and rats was correlated with increased body size and body weight in the adult progeny [26], [38], [51], [60]. In contrast, chronic exposure to BPA did not lead to increased body size [30], [42].
Acute BPA treatment of excised heart preparations has induced cardiac electrical conduction abnormalities and cardiac arrhythmia. In ex vivo rat heart preparations, BPA prolonged arrhythmia events after ischemic/reperfusion [63], decreased atrial contractility [41] and prolonged atria to ventricle conduction [46]. Moreover, BPA promoted premature ventricular beats and ventricular tachycardia when isolated cardiomyocytes were stimulated by catecholamines, and induced these effects by altering sarcoplasmic reticulum calcium handling [62].
Previously, we found gestational exposure to the estrogenic chemical diethylstilbestrol (DES) altered heart structure/function in male and female mice [24], [25]. The inclusion of a DES arm in studies of low dose estrogenizing endocrine disruptor activity has been suggested [50]. In essence, the physiological response to DES exposure is considered to afford a proof in principle of the potential for estrogenic endocrine disruptor impact. Accordingly, we used DES as a positive control and to test whether gestational exposure would alter response to a HFD. Based on the above evidence, we hypothesized that mice chronically exposed to oral BPA would develop increased pericardial fat, cardiac hypertrophy and overt arrhythmia. Here, we report on the cardiac impact of chronic oral BPA exposure in male and female mice fed a control diet (CD) or high fat diet (HFD) measuring pericardial fat deposition, cardiac structure/function, blood pressure and ECG, and expression of calcium handling proteins.
2. Methods
2.1. Animal manipulation and test chemicals
The animal care protocol was reviewed by the Lady Davis Institute Animal Care Committee and experiments were performed according to Canadian Council on Animal Care guidelines. C57bl/6n mice were purchased (Charles River, St. Constant, Que.). All dams were fed a natural ingredient all-purpose soy-based diet (Harlan Teklad Global 2018 diet, 3.1 Kcal/gm, 6.2% fat, 18% calories from fat) during gestation and lactation. All mice were housed in standard cages with 1/4⿿ corn cob bedding and a 12 h dark/light schedule. C57bl/n males and females, purchased from Charles River Canada (St. Constant, Quebec), were housed together. The females were examined daily for the presence of a vaginal plug and once detected, were separated from the males. A schematic showing the timing of treatments, diet changes and analyses is shown in Fig. 1A.
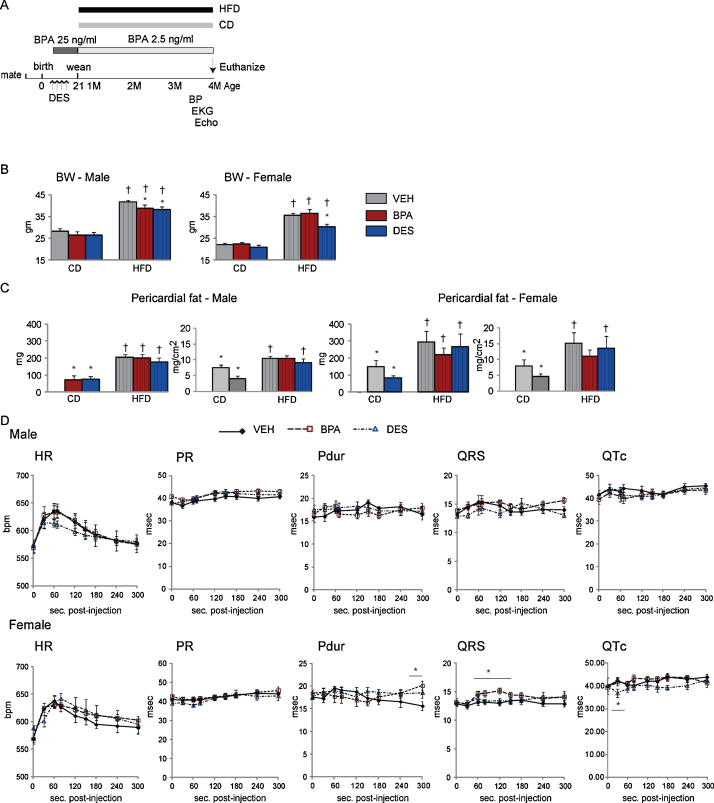
Fig. 1.
(A) A schematic indicating the timing of treatments, initiation of diet changes and analyses. (B and C) Mice, n = 6⿿9 mice/treatment/sex/diet, were euthanized and the BW and pericardial fat weighed. Body surface area was calculated from the BW at euthanasia. Data are expressed as mean ± SEM. Significance, indicated by p < 0.05 when compared with * VEH and control diet. (D) Mice, n = 8⿿9/treatment/sex, were anesthetized mice and ECG data collected after IP isoproterenol injection. Heart rate (HR), P wave duration (P-dur), QRS and QT corrected for HR (QTc) are plotted with time. The arrow indicates peak HR. Data are the mean ± SEM. Significance, indicated by p < 0.05 when compared with * VEH.
BPA (>99% pure, CAS 80-05-7, Sigma⿿Aldrich, Oakville, Ont.) was dissolved in ethanol, and added to glass-bottled drinking water. BPA exposed dams, n = 11, were treated with BPA-containing drinking water, 25 ng/ml, from gestation day 11.5 until pup weaning on post-natal day 21. Vehicle (VEH) treated dams, n = 10 and their progeny had an equal volume of ethanol, final concentration 0.1%, added to the drinking water. At weaning, BPA in the drinking water was reduced to 2.5 ng/ml and progeny continued on this treatment until euthanasia. At 4 months of age, spot urine was collected between 8 and 9 AM from BPA-exposed mice, n = 6. Animals were placed on a glass petri dish, urine collected using a glass pipet and placed in polypropylene tubes. Urine total BPA was quantified by the Institut National de Santé Publique du Québec using gas chromatography-tandem mass spectrophotometry [48]. The detection limit in this analytic method is 0.2 μg/L urine. Field blanks reported undetectable BPA.
Diethylstilbestrol (DES, 99% pure, CAS 56-53-1, Sigma⿿Aldrich, Oakville, Ont.) was dissolved in peanut oil and administered orally by a micropipettor to deliver 1.0 μg DES/kg body weight (BW)/day on gestation days 11.5⿿14.5 to n = 10 dams. This method was shown previously to increase adult BW [26].
At weaning on post-natal day 21, 2 mice of each sex/litter were randomly selected to one of two diets. The number of mice used for each analyses are presented in Supplementary Table 1. Mice were maintained on the diets until euthanasia at 4 months of age. These casein-based and purified component diets were either low (control diet, CD) (Teklad TD6416, 3.7 Kcal/g, 4.2% fat, 10% of calories from fat) or high in fat (HFD) (Teklad TD6414, 5.1 Kcal/g, 34% fat, 60% calories from fat). Body weight did not differ within the treatment groups when randomized. Diet components are presented in Supplementary data, Supplemental Tables 2 and 3. Two independent cohorts were analyzed. Water and food were ad libitum.
2.2. Cardiac function analyses
2.2.1. Surface electrocardiography (ECG)
At 15 weeks of age, mice, n = 9⿿11/treatment/sex/diet, were anesthetized with isoflurane. Surface ECG data was collected for 2 min using IOX2 software, (EMKA Technologies, Inc., Falls Church, VA). In a second set of CD mice, n = 8⿿10 mice/treatment/sex, baseline ECG data was obtained for 2 min, the mice injected with 0.5 μg isoproterenol/kg BW and data collected for 10 min. Recordings were collected once from each animal. Waves were analyzed using ecgAuto v2.8.1.11 software (EMKA Technologies, Inc., Falls Church, VA) and collated in 10 s blocks. Changes were considered significant if they persisted for greater than 30 s.
2.2.2. Tail cuff blood pressure
At 15 weeks of age, tail cuff blood pressure (BP) was measured in conscious mice, n = 6⿿10/treatment/sex/diet, using a Hatteras MC-4000 BP analysis system (Cary, NC). Mice were accustomed to the apparatus for 3 days. Measurements were recorded on days 4 and 5 when the heart rate was between 500 and 700 beats per minute.
2.2.3. Echocardiography
At ⿼16 weeks of age, echocardiography of anesthetized mice, n = 6⿿9/treatment/sex/diet, was performed using a VEVO 770 system (VisualSonics, Toronto, Ont) and analyzed with VisualSonics proprietary software as described previously [24], [25], [43]. Briefly, M-mode acquisitions were used to measure left ventricular (LV) dimensions and LV posterior wall (LVPW) and interventricular septal wall (IVS) thicknesses. Fractional shortening (FS) was calculated as [(LVIDd-LVIDs)/LVIDd] ÿ 100 where LVIDd is the LV internal diameter at diastole and LVIDs is the LV internal diameter in systole. Left ventricle mass (LVM) was calculated as 1.055[(LVPW + IVS + LVIDd)3 ⿿ (LVIDd3)]. Relative wall thickness (RWT) was calculated as (LVPW + IVS)/LVIDd. LV volume in diastole was calculated using the formula [7.0/(2.4 ÿ LVIDd)] ÿ LVIDd3. LV volume in systole was calculated using the same formula except that the LVIDs was used. Ejection fraction is the LV volume in systole divided by the LV volume in diastole. Stroke volume (SV) is the difference between the LV volume in diastole and the LV volume in systole. Cardiac output (CO) is stroke volume multiplied by the heart rate. Pulsed-wave Doppler images of the ascending aorta (Ao) and the pulmonary artery (PA) were used to measure the velocity time integral (VTI).
2.3. Physiological parameters
Body, heart, lung and pericardial fat weights were measured at euthanasia. Pericardial fat included the fat immediately surrounding the heart and thoracic cavity. Fat surrounding the aortic arch was not included. Hearts were placed in buffered formalin or immediately frozen and stored at ⿿80 °C. Body surface area (BSA) was calculated using the formula BSA = [K ÿ (3)] where K is 10.5 the species specific constant for mice, and W is the BW in grams [16].
2.4. Protein expression analysis
Ventricles, n = 5⿿7/treatment/sex/diet, were homogenized in RIPA buffer (1% NP-40, 50 mM Tris (pH 7.4), 0.5% deoxycholate, 159 mM NaCl, 0.1% SDS, 10 mM sodium metabisulfite, 1 mM sodium vanadate, proteinase inhibitor cocktail, PhosSTOP (Roche, Indianapolis, IN), and 1 mM PMSF). Protein was measured using the Bradford Protein Determination Assay (BioRad, Hercules, CA) as per the manufacturer⿿s instructions.
Protein expression was measured using standard immunoblotting methods. Primary antibodies to the cardiac calcium homeostasis proteins sodium calcium exchanger-1 (NCX1, Abcam Inc, Cambridge, MA ab6495); sarcoplasmic calcium ATPase 2a (SERCA2a, Santa Cruz Biotechnology, Santa Cruz, CA, sc-8095); cardiac calsequestrin 2 (CASQ2, Abcam Inc, Cambridge, MA, ab626662); phospholamban (PLB, Thermo Scientific, Nepean, ONT, MA3-922); and phospho-serine 16-specific PLB (pS16-PLB, Millipore, Temecula, CA, 07-052 1) were obtained. Secondary antibodies complexed to horseradish peroxidase and chemiluminescent detection kits were obtained from Pierce Chemical Co., (Rockford, IL). Several exposures from each membrane were collected onto X-ray film. After immunoblotting, membranes were washed, permanently stained with Coomassie Brilliant Blue, destained and scanned. The bands on the X-ray film and stained membrane were scanned and quantitated using Image J software.
2.5. Histology
Hearts, n = 5/treatment/sex/diet, were fixed with buffered formalin and paraffin embedded. Sections, 6 microns, were stained for Hematoxylin and Eosin (H&E) by the Lady Davis Institute Pathology Core or with Picric Acid Sirius Red (Polysciences, Warrington, PA) according to the manufacturer⿿s instruction. Stained sections were photographed using a Leica DM2000 microscope and Infinity Capture v4.6 software (Lumenera Corp. Ottawa, Ont).
2.6. Statistical analyses
The Kolmogorov⿿Smirnov test was used to establish equal variance about the group mean prior to ANOVA analyses. Significance was evaluated using two-way ANOVA, the statistical program SigmaStat 3.1 and the Student⿿Newman⿿Keuls post-hoc test. Significance for physiology parameters was also assessed by ANCOVA using litter size as a covariate and the SPSS version 20 Statistical package. A p-value of <0.05 was considered significant.
3. Results
3.1. BPA exposure, BW at euthanasia and pericardial fat deposition
Most human exposure to BPA is continuous as BPA leaches into consumed food and beverages [21]. Accordingly, we continuously exposed mice to oral BPA via their drinking water. To simulate the higher exposure of children versus adults [6], we treated the mice with a higher dose during gestation and lactation and reduced the dose of BPA at weaning. Using BW measurements to estimate water intake [3], BPA exposure of the adult progeny averaged ⿼0.55 ± 0.08 μg/kg/day. To verify this exposure, we collected early morning spot urine samples from 6 mice of 4 months of age and quantified urine BPA. The amount of BPA averaged 17.7 ± 3.7 ng/ml urine. Assuming a 2 ml urine output/day, the calculated [64] BPA exposure was 1.1 ± 0.3 μg/kg/day which corresponds to a human equivalent dose of 0.09 μg/kg/day [49]. This level is considered to be ⿿low dose⿿ and to approximate human exposure levels [54], [59].
To measure whether chronic BPA exposure increased BW, we measured BW at euthanasia, Fig. 1B. In males, treatments had no impact on BW of mice fed the CD, yet BPA and DES treatments reduced BW when fed the HFD. In females, the BW of CD-BPA and CD-DES females did not differ from that of CD-VEH females. In contrast, whereas the BW of HFD-BPA and HFD-VEH females were similar, the BW of HFD-DES females was significantly reduced. Thus, chronic BPA exposure had no impact on BW in mice fed the CD or HFD.
Pericardial fat was quantified at euthanasia to determine whether chronic BPA exposure would increase its deposition, Fig. 1C. Pericardial fat was present in male and female CD-BPA and CD-DES mice, and absent in CD-VEH mice. In mice fed the HFD, pericardial fat was increased similarly, regardless of treatment. When CD and HFD mice were compared, HFD-VEH and HFD-DES mice had increased indexed pericardial fat when compared with their CD cohorts. Thus, BPA- and DES-exposure induced pericardial fat deposition in mice that were not obese or fed the HFD, but did not enhance pericardial fat accumulation in HFD mice.
3.2. Electrocardiography
To establish whether chronic BPA exposure and increased pericardial fat deposition would alter cardiac electrical conduction, we performed surface ECG analysis. All ECG parameters measured were similar, regardless of treatment, Table 1. No ECG parameters differed when CD versus HFD mice were compared. Thus, BPA and DES exposure and the increased pericardial fat on the CD diet had no impact on cardiac electrical conduction in unstressed male or female hearts in vivo.
Table 1.
Electrocardiograph parameters.
| Male |
Female |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD |
HFD |
CD |
HFD |
|||||||||
| VEH | BPA | DES | VEH | BPA | DES | VEH | BPA | DES | VEH | BPA | DES | |
| PR | 37.5 ± .08 | 38.8 ± 0.7 | 38.1 ± 0.7 | 39.8 ± 1.1 | 37.4 ± 0.9 | 39.5 ± 0.9 | 42.3 ± 1.2 | 41.2 ± 1.3 | 40.0 ± 1.0 | 43.1 ± 1.5 | 40.0 ± 1.4 | 41.1 ± 1.4 |
| P-dur | 16.8 ± 0.8 | 17.2 ± 0.6 | 18.0 ± 0.6 | 16.4 ± 1.0 | 17.1 ± 0.9 | 17.7 ± 0.9 | 17.9 ± 0.7 | 18.4 ± 0.7 | 17.6 ± 0.6 | 18.8 ± 0.8 | 18.4 ± 0.7 | 17.6 ± 0.8 |
| QRS | 13.0 ± 0.4 | 13.4 ± 0.3 | 12.8 ± 0.3 | 13.8 ± 0.5 | 12.6 ± 0.4 | 12.6 ± 0.4 | 12.7 ± 0.4 | 12.9 ± 0.4 | 13.5 ± 0.3 | 12.8 ± 0.5 | 13.2 ± 0.4 | 12.3 ± 0.5 |
| QTc | 37.9 ± 1.3 | 38.1 ± 1.3 | 40.5 ± 1.2 | 37.6 ± 1.6 | 41.7 ± 1.4 | 39.6 ± 1.4 | 38.6 ± 1.1 | 39.1 ± 1.1 | 39.4 ± 0.9 | 38.8 ± 1.3 | 38.6 ± 1.2 | 38.0 ± 1.3 |
| HR | 579 ± 14 | 579 ± 8 | 539 ± 16 | 520 ± 25 | 546 ± 14 | 526 ± 11 | 546 ± 13 | 584 ± 17 | 545 ± 16 | 550 ± 21 | 577 ± 21 | 533 ± 12 |
To determine whether catecholamine stress would reveal changes in electrical conduction, we injected CD mice with the β-adrenergic agonist, isoproterenol, and gathered ECG data for the next 10 min, Fig. 1D. All mice increased HR similarly after catecholamine injection. In males, no differences were detected with time post-injection suggesting no change in the response to catecholamine stimulation. When the HR was maximal the QRS complex was prolonged in CD-BPA females and the QTc interval was reduced in CD-DES females compared with CD-VEH females. P-wave duration was longer in CD-BPA versus CD-VEH females as the HR returned to baseline levels. Thus, catecholamine challenge revealed female-specific alterations in P-wave control and QRS complex regulation with chronic BPA exposure.
3.3. Cardiac structure and blood pressure analyses
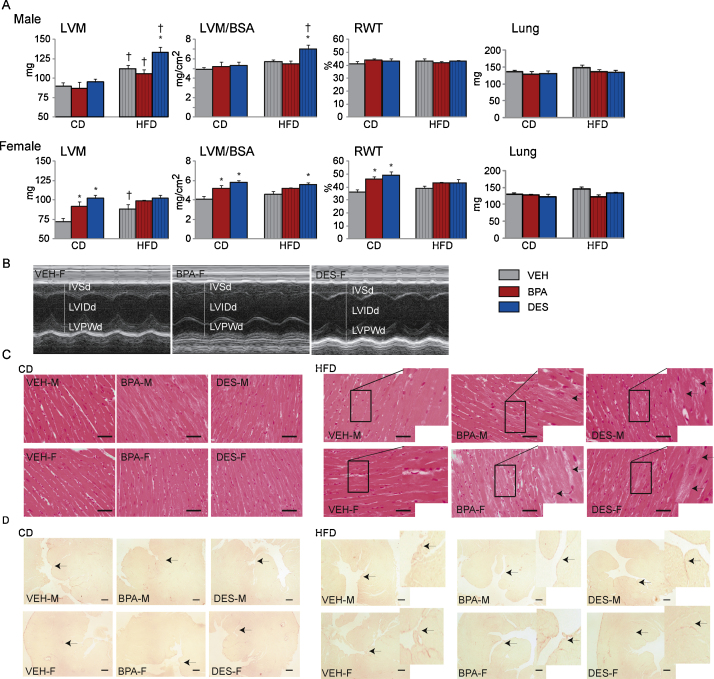
We performed echocardiography to measure cardiac structure, Fig. 2. LVM, LVM indexed to BSA and RWT were unaffected by treatment in males fed the CD, Fig. 2A. However, the increased LVM, indexed LVM and RWT in CD-BPA and CD-DES versus CD-VEH females suggests concentric hypertrophy development in treated females, Fig. 2A and B. The lack of any increase in lung weight in any group suggests no overt heart failure as a consequence of treatment or diet.
Fig. 2.
(A) Mice, n = 6⿿9/treatment/sex/diet, were anesthetized and echocardiography performed. Left ventricle mass (LVM), LVM indexed to body surface area (LVM/BSA) and relative wall thickness (RWT) were calculated. Mice were euthanized after echocardiography and lungs weighed. Data are the mean ± SEM. Significance, indicated by p < 0.05 when compared with * VEH and CD. (B) M-mode images from representative acquisitions of female VEH, BPA and DES mice are shown. The white lines indicate the interventricular wall in diastole (IVSd), left ventricle inner diameter in diastole (LVIDd) and left ventricle posterior wall in diastole (LVPWd), respectively. (C and D) Heart sections, n = 3/treatment/sex/diet, were stained with H&E (panel C) or sirius red (panel D). Photographs of representative sections are presented. The black bars in panels C and D represent 50 and 200 microns, respectively. Inserts show higher magnification of the highlighted area. Female (VEH-F, BPA-F and DES-F) and male (VEH-M, BPA-M and DES-M) sections are shown. In panel C, arrows point to cardiomyocytes demonstrating disorganized structure and vacuolation. In panel D, arrows point to sirius red stained collagen fibers.
To determine whether chronic BPA exposure increased BP and whether this increase might contribute to the increased LVM in female mice, we measured tail cuff BP, Table 2. We did not detect a difference in SBP among the male or female groups fed the CD suggesting that the increase in LVM in CD-BPA and CD-DES females is unlikely to be secondary to hypertension. H&E and Sirius Red staining of LV sections were essentially similar in CD mice regardless of treatment or sex suggesting no gross histological change, Fig. 2C and D.
Table 2.
Blood pressure and echocardiograph parameters.
| Males |
Females |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD |
HFD |
CD |
HFD |
|||||||||
| VEH | BPA | DES | VEH | BPA | DES | VEH | BPA | DES | VEH | BPA | DES | |
| SBP | 97 ± 7 | 105 ± 5 | 108 ± 5 | 95 ± 5 | 97 ± 6 | 113 ± 5a | 101 ± 5 | 100 ± 5 | 105 ± 5 | 95 ± 4 | 93 ± 5 | 99 ± 4 |
| DBP | 76 ± 5 | 86 ± 3 | 89 ± 5 | 79 ± 4 | 87 ± 7 | 94 ± 3a | 80 ± 6 | 85 ± 7 | 79 ± 5 | 79 ± 5 | 83 ± 5 | 86 ± 6 |
| HR | 636 ± 13 | 668 ± 12 | 668 ± 7 | 642 ± 14 | 669 ± 12 | 668 ± 11 | 618 ± 7 | 598 ± 22 | 645 ± 12 | 638 ± 17 | 661 ± 20 | 660 ± 13 |
| AoVTI | 5.11 ± 0.21 | 4.95 ± 0.27 | 4.42 ± 0.20 | 5.62 ± 0.14 | 5.54 ± 0.27b | 5.09 ± 0.16b | 4.60 ± 0.20 | 4.98 ± 0.13 | 4.53 ± 0.32 | 5.08 ± 0.14 | 5.18 ± 0.29 | 4.32 ± 0.23a |
| FS | 41.2 ± 1.6 | 41.2 ± 1.7 | 40.4 ± 0.6 | 44.5 ± 1.1 | 40.8 ± 1.5 | 36.0 ± 1.3a, b | 40.6 ± 1.7 | 38.2 ± 1.1 | 39.7 ± 2.0 | 42.8 ± 2.1 | 41.5 ± 1 | 39.9 ± 1.7 |
| EF | 72.7 ± 1.8 | 73.2 ± 2.2 | 72.1±0.8 | 76.3 ± 1.2 | 72.2 ± 1.7 | 66.1 ± 1.6a | 72.0 ± 2.0 | 69.4 ± 1.3 | 70.6 ± 1.7 | 74.4 ± 2.2 | 72.9 ± 1.3 | 71.2 ± 1.9 |
| SV | 38.9 ± 2.2 | 37.1 ± 3.6 | 38.1 ± 1.5 | 48.4 ± 2.6b | 43.6 ± 1.8 | 46.6 ± 2.4b | 38.0 ± 1.6 | 34.3 ± 2.8 | 33.6 ± 2.5 | 41.9 ± 2.0 | 41.1 ± 2.3b | 41.9 ± 2.3b |
| CO | 21.7 ± 1.1 | 21.7 ± 2.3 | 22.0 ± 1.2 | 28.4 ± 1.6b | 24.7 ± 1.2 | 27.2 ± 1.4b | 20.3 ± 0.9 | 19.4 ± 1.6 | 18.8 ± 1.5 | 23.2 ± 1.6 | 23.3 ± 0.7 | 23.1 ± 1.8b |
| LVIDd | 3.57 ± 0.09 | 3.52 ± 0.11 | 3.69 ± 0.11 | 3.83 ± 0.09 | 3.76 ± 0.10 | 4.00 ± 0.09b | 3.55 ± 0.09 | 3.44 ± 0.09 | 3.41 ± 0.09 | 3.65 ± 0.09 | 3.65 ± 0.09 | 3.70 ± 0.08 |
| LVIDs | 2.10 ± 0.10 | 2.08 ± 0.11 | 2.29 ± 0.13 | 2.14 ± 0.09 | 2.22 ± 0.07 | 2.57 ± 0.1a, b | 2.12 ± 0.11 | 2.13 ± 0.07 | 2.06 ± 0.11 | 2.09 ± 0.10 | 2.13 ± 0.04 | 2.23 ± 0.11 |
| LVvolD | 53.7 ± 3.4 | 51.0 ± 5.4 | 52.9 ± 2.2 | 63.7 ± 4.2b | 60.4 ± 2.2 | 70.9 ± 3.8b | 52.9 ± 3.3 | 49.4 ± 4.0 | 53.3 ± 1.9 | 56.5 ± 2.8 | 56.4 ± 1.0 | 59.0 ± 4.5b |
| LVvolS | 14.9 ± 1.6 | 13.9 ± 2.3 | 14.8 ± 0.9 | 15.3 ± 1.7 | 16.8 ± 1.3 | 24.3 ± 2.1 | 15.0 ± 1.9 | 15.1 ± 1.5 | 15.8 ± 1.2 | 14.6 ± 1.6 | 15.3 ± 0.8 | 17.1 ± 1.9 |
| PAVTI | 2.96 ± 0.11 | 2.71 ± 0.15 | 2.81 ± 0.16 | 2.60 ± 0.13 | 2.60 ± 0.13 | 2.88 ± 0.11 | 2.30 ± 0.11 | 2.37 ± 0.12 | 2.74 ± 0.08a | 2.36 ± 0.12 | 2.77 ± 0.03a, b | 2.68 ± 0.11a |
| HR | 560 ± 11 | 577 ± 18 | 581 ± 9 | 586 ± 17 | 581 ± 9 | 584 ± 11 | 541 ± 12 | 569 ± 22 | 561 ± 17 | 552 ± 13 | 563 ± 19 | 550 ± 12 |
Significance is when p < 0.05.
Compared with VEH within diet.
Compared with CD within treatment.
Within the HFD groups, LVM, indexed LVM and RWT, Fig. 2A, as well as SBP, Table 2, were similar in HFD-VEH and HFD-BPA males and females. However, HFD-DES males had increased LVM when compared with HFD-VEH males. Although it did not reach a level considered to be hypertensive, >140 mmHg, the slight increase in SBP to 113 ± 5 mmHg in HFD-DES males may have contributed to their LVM increase. H&E staining of LV sections from HFD-BPA and HFD-DES males and females revealed cardiomyocytes with some vacuolation and disorganized structure; however, the similar Sirius Red staining in heart sections from the HFD-fed groups indicates no increase in fibrosis, Fig. 2C and D.
When CD and HFD mice were compared, HFD-VEH males and females had increased LVM and no change in indexed LVM or RWT. This suggests that an increase in LVM is a normal accommodation to facilitate heart function in a heavier animal. HFD-BPA males remodeled similarly to HFD-VEH males. However, HFD-DES males showed an increase in LVM as well as indexed LVM when compared with their CD-DES cohort. In females, HFD-BPA and HFD-DES females had no increase in LVM, indexed LVM or RWT when compared with their CD-cohorts. Thus, in males, BPA and DES exposure did not induce a difference in cardiac structure in CD and chronic BPA exposure did not influence the response to a HFD. In females, chronic BPA exposure induced concentric hypertrophy in mice fed the CD which was not increased further with the HFD. We conclude that chronic BPA exposure preferentially influenced cardiac structure remodeling in females.
3.4. Echocardiograph-derived cardiac systolic function analyses
To determine whether BPA would induce cardiac systolic function, we measured LV and RV function by echocardiography, Table 2. In CD males, LV and RV function parameters were similar, regardless of treatment. In females, CD-VEH and CD-BPA females were also similar. In contrast, CD-DES females had increased PAVTI compared with CD-VEH females suggesting an increase in RV stroke volume. Thus, when fed the CD, chronic exposure to BPA had no impact on LV and RV function, and DES exposure influenced RV function only in females.
HFD-VEH and HFD-BPA males and females showed no differences in LV systolic function suggesting no impact of BPA exposure. In contrast, the reduced FS and EF as well as greater LVIDs in HFD-DES versus HFD-VEH males indicates functional deficits with DES exposure. In HFD females, no differences in LV parameters were detected with treatment. However, the increase in the RV indicator PAVTI in HFD-BPA and HFD-DES treated mice compared with HFD-VEH females, suggests an increase in RV SV was necessary to accommodate the HFD in these groups. Overall, BPA exposure did not worsen the impact of a HFD on LV systolic function, DES exposure reduced LV systolic function, and both treatments remodeled RV systolic function only in females.
When CD versus HFD males were compared, HFD-VEH males had increased SV and CO compared with CD-VEH males suggesting that these adaptations are necessary to accommodate a larger body size. HFD-BPA exposed males did not significantly increase SV or CO suggesting some impedance to LV function adaptation was present. The reduction in FS and increases in LVIDd and LVIDs as well as LV volume in diastole in HFD-DES males versus CD-DES males suggests reduced LV function and significant LV dilation with DES exposure.
When CD and HFD females were compared, HFD-VEH females were similar to their CD-VEH cohort suggesting that no additional adaptations were necessary to adapt to the change in diet or body size. HFD-BPA females had increased SV and increased PA VTI, but were otherwise similar to their CD-BPA exposed cohort implying no large change in LV function. Similar to the males, HFD-DES females had increased LV volume. Overall, cardiac function adaptations were more evident in DES-treated males and females than in BPA-exposed mice.
3.5. Calcium homeostasis protein expression
To assess the remodeling accompanying chronic BPA exposure and CD and HFD, we isolated protein homogenates and measured the expression levels of proteins involved in calcium homeostasis, Fig. 3. Controlled calcium cycling is crucial to normal heart function [1], [22]. Calcium is released from the sarcoendoplasmic reticulum (SER) to the cytosol for contraction and must be removed from the cytosol, either back into the SER or to the extracellular space, for relaxation with each heart beat. Cardiac relaxation is achieved by the activities of SERCA2a and the sodium-calcium exchanger (NCX1) which transport calcium from the cytosol to the SER or to the cell exterior, respectively [35]. SERCA2a activity is reduced when it is bound by unphosphorylated phospholamban (PLB). The ability to bind SERCA2a is lost, and thus SERCA2a activity is increased, when PLB is phosphorylated on serine-16 (pS16-PLB). Calsequestrin 2 (CASQ2) is a key calcium storage protein in the SER. Additionally, NCX1, SERCA2a and CASQ2 expression is affected by sex and sex hormones [23], [34] as well as by BPA [4], [62].
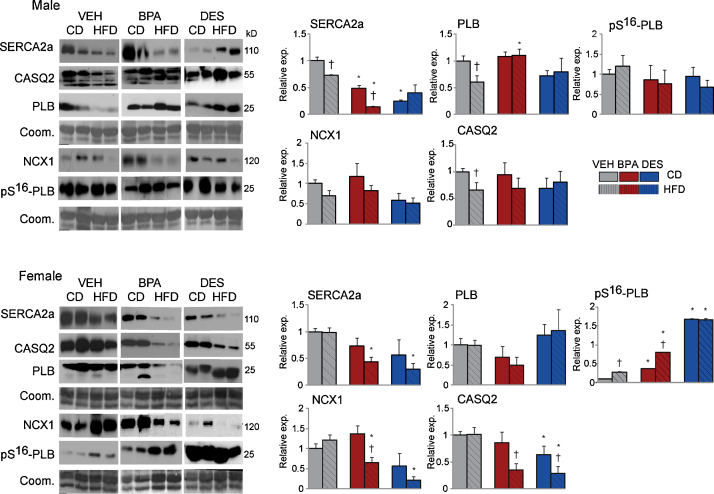
Fig. 3.
Ventricle heart, n = 5⿿7mice/treatment/sex/diet, was homogenized and immunoblots performed. Expression in VEH mice was set to 1.0. Representative immunoblots of pairs of samples are shown. Bar graphs represent the results of 3⿿5 repetitions for each protein on each mouse sample. Data are the mean ± SEM. Significance, indicated by p < 0.05 when compared with * VEH and CD.
The expression pattern in CD-BPA and CD-DES males versus CD-VEH mice, reduced SERCA2a coupled with no change in PLB or pS16-PLB expression, predicts reduced SERCA2a activity. Similarly within the HFD group, HFD-BPA males had reduced SERCA2a expression with no change in PLB or pS16-PLB expression suggesting the possibility for reduced SERCA2a activity. In females, the increased pS16-PLB with no change in PLB protein in CD-BPA and CD-DES versus CD-VEH females would be expected to increase SERCA2a activity. Reductions in NCX1 expression suggests reduced calcium efflux to the exterior in HFD-BPA and HFD-DES versus HFD-VEH females. Calcium storage capacity in CASQ2 is predicted to be reduced because of reduced CASQ2 protein in HFD-BPA and HFD-DES females.
When expression in CD versus HFD mice was compared, HFD-VEH males showed reductions in SERCA2a and PLB compared with CD-VEH males. This pattern was replicated in CD-BPA versus HFD-BPA males suggesting no deficit in remodeling with the HFD in the BPA exposed males. However, CD-DES versus HFD-DES males showed no expression changes suggesting an inability to remodel with HFD. In CD-VEH versus HFD-VEH females, only pS16-PLB was increased in HFD-VEH females. HFD-BPA and HFD-DES females had reduced NCX1 and CASQ2, and increased pS16-PLB when compared with CD-BPA females. We conclude that BPA and DES exposure modified the expression of proteins involved in calcium handling with greater effects observed in females and on a HFD.
4. Discussion
It is a paradox that whereas estrogen is generally beneficial and reduces cardiovascular disease [34], [57], increased exposure to the estrogenic endocrine disruptor BPA may enhance cardiovascular disease [29], [36], [37], [55], [56]. One major difference between estrogen and BPA exposures is that whereas women are exposed cyclically to estrogen, women and men are exposed chronically to BPA leaching from food and beverage containers. Here, we measured the impact of chronic oral exposure to BPA and gestational exposure to DES in male and female mice in the context of a CD or HFD and found: (1) similar BW, SBP and baseline ECG parameters in VEH, BPA and DES mice fed the CD or HFD; (2) female-specific alterations in P-dur and QRS after catecholamine challenge in BPA exposed mice; (3) induction of pericardial fat deposition in BPA- and DES-exposed mice in absence of obesity; (4) female-specific development of cardiac hypertrophy with BPA and DES; (5) reductions in LV systolic function in DES-, and not BPA-exposed mice; and (6) sex-specific changes in proteins important for calcium homeostasis. Our data are significant because they extend the results of acute exposure to BPA in ex vivo heart preparations to chronic exposure to BPA in mice. These data support the idea that other endocrine disrupting compounds might influence rodent cardiac electrical conduction and cardiac structure/function. Further, although there are multiple differences in cardiac physiology across species, these data strengthen the idea that cardiac conduction and cardiac structure/function in humans might be influenced by exposure to environmental chemicals, and that this exposure may increase pathology.
The coordination of cardiac contraction is accomplished by the cardiac conduction system. We detected no change in surface ECG parameters in either sex with chronic BPA or gestational DES exposure in unstressed mice. It is possible that extended exposure to BPA may be necessary to detect overt arrhythmia development in the absence of challenge. We found chronic exposure to BPA increased the susceptibility to aberrant ECG conduction changes with catecholamine challenge only in females. These data extend previous work which showed that the catecholamine response of cardiomyocytes isolated from female rats acutely exposed to BPA was greater than cardiomyocytes isolated from males [20], [62]. Further, this increased susceptibility to BPA may be most significant at times of sustained catecholamine release, such as during high intensity exercise or after an arrhythmia-promoting event such as a myocardial infarction. Heart rate returned to baseline within 5 min in all mice, indicating that catecholamine metabolism was likely unaffected by treatment. Whereas BPA- and DES-exposed male and female mice increased pericardial fat, we found no change in ECG parameters at baseline in either sex or after catecholamine challenge in males. This suggests that increased pericardial fat per se may not induce arrhythmia.
Prolonged QRS intervals and dropped beats at high BPA concentrations were detected in ex vivo heart preparations isolated from female rats using an optical mapping method to measure membrane potential [46]. Although they did not explore the impact of sex, these data support studies using isolated cardiac cells from female and male animals which showed that acute BPA treatments preferentially affected cardiac conduction in samples from female hearts. We extended these analyses to mice chronically exposed to BPA in vivo and examined the impact of sex. We found longer QRS intervals when the heart rate was maximal in catecholamine-stimulated BPA exposed females and no detectable impact in similarly treated males. Besides the impact of BPA on the QRS interval, we found a longer P-wave duration after catecholamine injection. P-wave duration is recognized as a non-invasive marker of atrial conduction and when it is prolonged has been associated with an increase in atrial fibrillation [45]. Together the data suggest that acute and chronic exposure to BPA impairs cardiac conduction, may promote arrhythmia and that this is particularly evident in exposed females.
BPA⿿s impact on cardiac conduction can be immediate as shown by acute exposure and long term as shown in in vivo exposure analyses. The potassium voltage-gated channel subfamily member 5 (KCN5) was identified as a downregulated gene in microarray analyses of fetal hearts exposed to BPA during gestation [12]. Inactivating mutations in KCN5 in humans are associated with atrial fibrillation, and pharmacological inhibition of KCN5 led to atrial fibrillation on catecholamine challenge in mice [40]. Thus, BPA exposure affected the expression of proteins important for control of cardiac conduction and that this impact begas in utero [6]. Besides influencing the expression of ion channel proteins, BPA directly binds and inactivates cardiac ion channels. In vitro studies showed BPA binding and blocking the activity of the cardiac sodium channel, hNAV1.5 [39] and the cardiac voltage-gated L-type calcium channel [13]. Although, the physiological relevance of BPA interactions with these ion channels remains unclear because the concentrations of BPA necessary to inhibit channel activities were several orders of magnitude greater than predicted serum BPA concentrations, these data highlight that BPA exposure may immediately increase the susceptibility to arrhythmia.
Our study shows no consequence to LV structure of chronic BPA or gestation-only DES exposure in males fed the CD, but significant LV structural adaptation leading to concentric hypertrophy in BPA- and DES-exposed females fed the CD. The protein base of a diet as well as the presence of phytoestrogens in the diet can profoundly influence cardiac outcomes in mice. In the current study, LVM and RWT were affected more in females than males when the BPA-exposed mice were fed a CD which was casein-based. Previously, we found LVM and RWT were more affected in males than females when the mice were exposed to similar, but not identical amounts of BPA, and fed a soy-based diet [43]. These differing outcomes support other work which found that male mice fed soy-based diets were particularly unable to adapt to stress whereas male mice fed a casein-based diet were able to adapt [33], [53]. In a direct comparison, males with an inherited form of hypertrophic cardiomyopathy developed dilation and heart failure when fed a soy-based diet, yet had normal cardiac function when fed a casein-based diet [53]. Together, these results show that sex, diet and estrogenizing compounds interact and have a large impact on the extent, and whether, cardiac pathology develops.
The increase in LVM in BPA- and DES-exposed females without an increase in SBP argues for a direct impact of BPA and DES to increase LVM. Subcutaneous BPA injection of pregnant outbred MF-1 mice induced a higher SBP in the adult male and female progeny [9]. The different outcomes on SBP may depend upon the mouse strain (inbred versus outbred), timing of exposure (chronic versus gestation-only) or dose (0.5 μg/kg/day versus 100 μg/kg/day) of BPA delivered. In our study, gestation-only treatment with DES did not increase SBP in CD-DES mice. This argues that estrogenic exposure during gestation may not always lead to increased SBP. We detected a small, non-hypertensive increase in SBP in HFD-DES males. We speculate that gestational estrogenic exposure of males may increase the sensitivity to develop higher SBP when under a cardiometabolic stress, such as a HFD. Overall, chronic BPA-mediated cardiac structure changes showed a strong female-preference.
Like the HFD-VEH males used in this study, HFD feeding to FVB [61] or C57bl/6 males [10] did not lead to demonstrable LV systolic dysfunction or an increase in blood pressure. Similarly, we did not detect cardiac LV systolic dysfunction in CD-BPA or HFD-BPA male or female mice. Preservation of normal cardiac structure/function in the face of challenge is of paramount importance and is often achieved by altered expression of calcium homeostasis proteins [35]. Expression of cardiac proteins, including those involved in calcium homeostasis, differs in intact versus gonadectomized rodents and differs in male and female mice [15], [23], [34], [52]. Previously, acute BPA exposure of ex vivo heart preparations sex-specifically altered SERCA2a expression and activity, as wells as phosphorylation of the SERCA2a inhibitor PLB [4], [62]. Thus, proteins important for calcium homeostasis are targets for estrogenic hormonal disruption. Our data extend these results; we found SERCA2a, NCX1 and CASQ2 expression and PLB phosphorylation were significantly and sex-specifically altered in male and female mice chronically exposed to BPA. Further, we found that the combination of BPA and the HFD accentuated the changes in calcium homeostasis protein expression in females. Together, these results suggest that acute and chronic exposure to BPA modify the expression and activity of cardiac calcium handling proteins. Also, our data suggest that the normalization of LV function with chronic exposure to BPA required sex-specific remodeling of calcium homeostasis proteins.
The ability of BPA to modulate the expression of these proteins may have long term consequences and be downstream of the estrogen receptor activation. Controlled expression of these proteins is important because their expression is altered with cardiac physiology or pathologies, such as heart failure. In heart failure, SERCA2a expression is decreased and NCX1 is increased whereas CASQ2 is generally unaffected [7]. An inability to change SERCA2a, CASQ2 or phospholamban expression in single gene gene-modifed mice has led to increased cardiac damage from various insults suggesting that changes in their expression is a key feature for successful cardiac remodeling when under stress [31], [32], [44], [47]. These data suggest that chronic BPA exposure may reduce the capability to effect these changes and reduce the ability to cardiac remodel with normal stresses such as exercise or pathological stress such as with catecholamine excess. Abnormalities in intracellular Ca2+ regulation also profoundly disrupt the electrophysiological properties of the heart. Mutations in CASQ2 as well as the ryanodine receptor lead to an inability to modulate calcium into and out of the SER and are associated with increased arrhythmogenesis [14], [47]. It is likely that BPA exposure impacts expression of a number of cardiac proteins. In other studies, outbred CD-1 mice sex-specific expression of a multitude of cardiac mRNAs was found when the mice were chronically exposed to a relatively high level of BPA [5]. Similar to estrogens, BPA activates nuclear and membrane estrogen receptors [8], [11]. Supporting a role for ER signaling as a mechanism for BPA effects in heart, ER blockade reduced BPA-mediated prolonged arrhythmia events after ischemic/reperfusion in acutely exposed ex vivo female hearts [58], [63]. Further, no spontaneous after contractions were detected in ERβ-deficient hearts [62]. Together these data show that acute and chronic BPA exposure induces cardiac conduction and expression changes, suggest that these changes are likely downstream of ERβ signaling.
5. Conclusions
Epidemiology studies have correlated increased BPA exposure with increased obesity. Obesity increases the risk for cardiac hypertrophy development and arrhythmia promotion. Our results show that chronic exposure to BPA from mid-gestation to euthanasia at 4 months of age did not substantially increase body weight, cardiac hypertrophy or induce overt arrhythmia in lean or obese male mice. In contrast, BPA⿿s impact was most evident in exposed females versus similar exposed males and was manifest as sex-specific changes in the expression of calcium homeostasis proteins and in an enhanced response to catecholamine stimulation in surface ECG analysis. These data suggest that chronic BPA exposure alters cardiac contraction and relaxation and alters coordination of this contraction by the cardiac conduction system. The enhanced responses in exposed female mice supports the idea that BPA-mediated activation of estrogen receptors is part of the mechanism for its adverse impact in heart and suggests the possibility that women experiencing a cardiac crisis may be particularly sensitive to BPA⿿s adverse effects.
Acknowledgements
This work was supported by grants from the Canadian Institutes for Health Research and the Heart and Stroke Foundation of Quebec to LEC and IAS.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2015.09.008.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Armand A.-S., deWindt L. Calcium cycling in heart failure: how the fast became too furious. Cardiovasc. Res. 2004;62:439–441. doi: 10.1016/j.cardiores.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Babcock M.J., Soliman E.Z., Ding J., Kronmal R.A., Goff D.C., Jr. Pericardial fat and atrial conduction abnormalities in the multiethnic study of atherosclerosis (MESA) Obesity. 2011;19:179–184. doi: 10.1038/oby.2010.121. [DOI] [PubMed] [Google Scholar]
- 3.Bachmanov A.A., Reed D.R., Beauchamp G.K., Tordoff M.G. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 2002;32:435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belcher S.M., Chen Y., Yan S., Wang H.-S. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17β-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology. 2012;153:712–720. doi: 10.1210/en.2011-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belcher S.M., Gear R., Kendig E.L. Bisphenol A alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology. 2015;156:882–895. doi: 10.1210/en.2014-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman ÿ., Heindel J.J., Jobling S., Kidd K.ÿ., Zoeller R.T., Jobling S.K. State of the science of endocrine disrupting chemicals 2012: an assessment of the state of the science of endocrine disruptors prepared by a group of experts for the United Nations Environment Programme and World Health Organization. World Health Organ. 2013 [Google Scholar]
- 7.Bernardo B.C., Weeks K.L., Pretorius L., McMullen J.R. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol. Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Birnbaum L.S. State of the science of endocrine disruptors. Environ. Health Perspect. 2013;121:a107. doi: 10.1289/ehp.1306695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cagampang F.R., Torrens C., Anthony F.W., Hanson M.A. Developmental exposure to bisphenol A leads to cardiometabolic dysfunction in adult mouse offspring. J. Dev. Origins Health Dis. 2012;3:287–292. doi: 10.1017/S2040174412000153. [DOI] [PubMed] [Google Scholar]
- 10.Calligaris S.D., Lecanda M., Solis F., Ezquer M., Gutiérrez J., Brandan E., Leiva A., Sobrevia L., Conget P. Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy. PLoS One. 2013;8:e60931. doi: 10.1371/journal.pone.0060931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casals-Casas C., Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu. Rev. Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 12.Chapalamadugu K.C., VandeVoort C.A., Settles M.L., Robison B.D., Murdoch G.K. Maternal bisphenol A exposure impacts the fetal heart transcriptome. PLoS One. 2014;9:e89096. doi: 10.1371/journal.pone.0089096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deutschmann A., Hans M., Meyer R., Häberlein H., Swandulla D. Bisphenol A inhibits voltage-activated Ca2+ channels in vitro: mechanisms and structural requirements. Mol. Pharmacol. 2013;83:501–511. doi: 10.1124/mol.112.081372. [DOI] [PubMed] [Google Scholar]
- 14.Faggioni M., Knollmann B.C. Calsequestrin 2 and arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1250–H1260. doi: 10.1152/ajpheart.00779.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fares E., Parks R.J., MacDonald J.K., Egar J.M.S., Howlett S.E. Ovariectomy enhances SR Ca2+ release and increases Ca2+ spark amplitudes in isolated ventricular myocytes. J. Mol. Cell. Cardiol. 2012;52:32–42. doi: 10.1016/j.yjmcc.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Farriol M., Rosselló J., Schwartz S. Body surface area in Sprague-Dawley rats. J. Anim. Physiol. Anim. Nutr. 1997;77:61–65. [Google Scholar]
- 17.Fitzgibbons T.P., Czech M.P. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J. Am. Heart Assoc. 2014;3:e000582. doi: 10.1161/JAHA.113.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman D.J., Wang N., Meigs J.B., Hoffmann U., Massaro J.M., Fox C.S., Magnani J.W. Pericardial fat is associated with atrial conduction: the framingham heart study. J. Am. Heart Assoc. 2014;3:e000477. doi: 10.1161/JAHA.113.000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaborit B., Abdesselam I., Dutour A. Epicardial fat: more than just an epi Phenomenon? Horm. Metab. Res. 2013;45:991–1001. doi: 10.1055/s-0033-1358669. [DOI] [PubMed] [Google Scholar]
- 20.Gao X., Liang Q., Chen Y., Wang H.-S. Molecular mechanisms underlying the rapid arrhythmogenic action of bisphenol A in female rat hearts. Endocrinology. 2013;154:4607–4617. doi: 10.1210/en.2013-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geens T., Aerts D., Berthot C., Bourguignon J.-P., Goeyens L., Lecomte P., Maghuin-Rogister G., Pironnet A.-M., Pussemier L., Scippo M.-L., Van Loco J., Covaci A. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012;50:3725–3740. doi: 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- 22.Goonasekera S.A., Molkentin J.D. Unraveling the secrets of a double life: contractile versus signaling Ca2+ in a cardiac myocyte. J. Mol. Cell. Cardiol. 2012;52:317–322. doi: 10.1016/j.yjmcc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Grohe C., Kahlert S., Lobbert K., Stimpel M., Karas R., Vetter H., Neyes L. Cardiac myocytes and fibroblasts contain function estrogen receptors. FEBS Lett. 1997;416:107–112. doi: 10.1016/s0014-5793(97)01179-4. [DOI] [PubMed] [Google Scholar]
- 24.Haddad R., Kasneci A., Mepham K., Sebag I.A., Chalifour L.E. Gestational exposure to diethylstilbestrol alters cardiac structure/function, protein expression and DNA methylation in adult male mice progeny. Toxicol. Appl. Pharmacol. 2013;266:27–37. doi: 10.1016/j.taap.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Haddad R., Kasneci A., Sebag I.A., Chalifour L.E. Cardiac structure/function, protein expression and DNA methylation are changed in adult female mice exposed to diethylstilbestrol in utero. Can. J. Physiol. Pharmacol. 2013;91:741–749. doi: 10.1139/cjpp-2013-0014. [DOI] [PubMed] [Google Scholar]
- 26.Hao C.-J., Cheng X.-J., Xia H.-F., Ma X. The endocrine disruptor diethylstilbestrol induces adipocyte differentiation and promotes obesity in mice. Toxicol. Appl. Pharmacol. 2012;263:102–110. doi: 10.1016/j.taap.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Haslam D.W., James W.P.T. Life expectancy. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 28.Iacobellis G., Leonetti F., Singh N., Sharma M.A. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int. J. Cardiol. 2007;115:272–273. doi: 10.1016/j.ijcard.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Inadera H. Developmental origins of obesity and type 2 diabetes: molecular aspects and role of chemicals. Environ. Health Prev. Med. 2013;18:185–197. doi: 10.1007/s12199-013-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi K., Ohtani K., Kubota H., Miyagawa M. Dietary exposure to low doses of bisphenol A: effects on reproduction and development in two generations of C57BL/6J mice. Congenit. Anom. 2010;50:159–170. doi: 10.1111/j.1741-4520.2010.00279.x. [DOI] [PubMed] [Google Scholar]
- 31.Kornyeyev D., Petrosky A.D., Zepeda B., Ferreiro M., Knollmann B., Escobar A.L. Calsequestrin 2 deletion shortens the refractoriness of Ca2+ release and reduces rate-dependent Ca2+-alternans in intact mouse hearts. J. Mol. Cell. Cardiol. 2012;52:21–31. doi: 10.1016/j.yjmcc.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kranias E.G., Hajjar R.J. Modulation of cardiac contractility by the phopholamban/SERCA2a regulatome. Circ. Res. 2012;110:1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luczak E.D., Barthel K.K.B., Stauffer B.L., Konhilas J.P., Cheung T.H., Leinwand L.A. Remodeling the cardiac transcriptional landscape with diet. Physiol. Genomics. 2011;43:772–780. doi: 10.1152/physiolgenomics.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luczak E.D., Leinwand L.A. Sex-based cardiac physiology. Annu. Rev. Physiol. 2009;71:1–18. doi: 10.1146/annurev.physiol.010908.163156. [DOI] [PubMed] [Google Scholar]
- 35.Marks A.R. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J. Clin. Invest. 2013;123:46–52. doi: 10.1172/JCI62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melzer D., Gates P., Osborn N.J., Henley W.E., Cipelli R., Young A., Money C., McCormack P., Schofield P., Mosedale D., Grainger D., Galloway T.S. Urinary bisphenol A concentration and angiography-defined coronary artery stenosis. PLoS One. 2012;7:e43378. doi: 10.1371/journal.pone.0043378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melzer D., Osborne N.J., Henley W.E., Cipelli R., Young A., Money C., McCormack P., Luben R., Khaw K.-T., Wareham N.J., Galloway T.S. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women/clinical perspective. Circulation. 2012;125:1482–1490. doi: 10.1161/CIRCULATIONAHA.111.069153. [DOI] [PubMed] [Google Scholar]
- 38.Newbold R., Padilla-Banks E., Snyder R.J., Phillips T.M., Jefferson W.N. Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod. Toxicol. 2007;23:290–296. doi: 10.1016/j.reprotox.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Reilly A.O., Eberhardt E., Weidner C., Alzheimer C., Wallace B.A., Lampert A. Bisphenol A binds to the local anesthetic receptor site to block the human cardiac sodium channel. PLoS One. 2012;7:e41667. doi: 10.1371/journal.pone.0041667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson T.M., Alekseev A.E., Liu X.K., Park S., Zingman L.V., Bienengraeber M., Sattiraju S., Ballew J.D., Jahangir A., Terzic A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum. Mol. Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 41.Pant J., Ranjan P., Deshpande S.B. Bisphenol A decreases atrial contractility involving NO-dependent G-cyclase signaling pathway. J. Appl. Toxicol. 2011;31:698–702. doi: 10.1002/jat.1647. [DOI] [PubMed] [Google Scholar]
- 42.Patel B.B., Di Iorio M., Chalifour L.E. Metabolic response to chronic bisphenol A exposure in C57bl/6n mice. Toxicol. Rep. 2014;1:522–532. doi: 10.1016/j.toxrep.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel B.B., Raad M., Sebag I.A., Chalifour L.E. Lifelong exposure to bisphenol A alters cardiac structure/function, protein expression and DNA methylation in adult mice. Toxicol. Sci. 2013;133:174–185. doi: 10.1093/toxsci/kft026. [DOI] [PubMed] [Google Scholar]
- 44.Pinz I., Tian R., Belke D., Swanson E., Dillmann W., Ingwall J.S. Compromised myocardial energetics in hypertrophied mouse hearts diminish the beneficial effect of overexpressing SERCA2a. J. Biol. Chem. 2011;286:10163–10168. doi: 10.1074/jbc.M110.210757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Platonov P.G. P-wave morphology: underlying mechanisms and clinical implications. Ann. Noninvasive Electrocardiol. 2012;17:161–169. doi: 10.1111/j.1542-474X.2012.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Posnack N.G., Jamies R., III, Asfour H., Swift L.M., Wengrowski A.M., Sarvazyan N., Kay M.W. Bisphenol A exposure and cardiac electrical conduction in excised rat hearts. Environ. Health Perspect. 2014;122:384–390. doi: 10.1289/ehp.1206157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Priori S.G., Chen S.R.W. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ. Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Provencher G., Bérubé R., Dumas P., Bienvenu J.-F., Gaudreau ÿ., Bélanger P., Ayotte P. Determination of bisphenol A, triclosan and their metabolites in human urine using isotope-dilution liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2014;1348:97–104. doi: 10.1016/j.chroma.2014.04.072. [DOI] [PubMed] [Google Scholar]
- 49.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 50.Richter C., Birnbaum L., Farabollini F., Newbold R., Rubin B., Talsness C., Vandenbergh J., Walser-Kuntz D., vom Saal F. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubin B.S., Soto A.M. Bisphenol A: perinatal exposure and body weight. Mol. Cell. Endocrinol. 2009;304:55–62. doi: 10.1016/j.mce.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sebag I.A., Gillis M.-A., Calderone A., Kasneci A., Meilleur M., Haddad R., Noiles W., Patel B., Chalifour L.E. Sex hormone control of left ventricular structure/function: mechanistic insights using echocardiography, expression, and DNA methylation analyses in adult mice. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1706–H1715. doi: 10.1152/ajpheart.00088.2011. [DOI] [PubMed] [Google Scholar]
- 53.Stauffer B.L., Konhilas J.P., Luczak E.D., Leinwand L.A. Soy diet worsens heart disease in mice. J. Clin. Invest. 2006;116:209–216. doi: 10.1172/JCI24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teeguarden J.G., Hanson-Drury S. A systematic review of bisphenol A low dose studies in the context of human exposure: a case for establishing standards for reporting low-dose effects of chemicals. Food Chem. Toxicol. 2013;62:935–948. doi: 10.1016/j.fct.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Trasande L. Further limiting bisphenol a in food uses could provide health and economic benefits. Health Aff. 2014 doi: 10.1377/hlthaff.2013.0686. [DOI] [PubMed] [Google Scholar]
- 56.Trasande L., Attina T., Blustein J. Association between urinary bisphenol a concentration and obesity prevalence in children and adolescents. JAMA: J. Am. Med. Assoc. 2012;308:1113–1121. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- 57.Vaccarino V., Badimon L., Corti R., de Wit C., Dorobantu M., Manfrini O., Koller A., Pries A., Cenko E., Bugiardini R. Presentation, management, and outcomes of ischaemic heart disease in women. Nat. Rev. Cardiol. 2013;10:508–518. doi: 10.1038/nrcardio.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vandenberg L.N., Colborn T., Hayes T.B., Heindel J.J., Jacobs D.R., Lee D.-H., Shioda T., Soto A.M., vom Saal F.S., Welshons W.V., Zoeller R.T., Myers J.P. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vandenberg L.N., Ehrlich S., Belcher S.M., Ben-Jonathan N., Dolinoy D.C., Hugo E.R., Hunt P.A., Newbold R.R., Rubin B.S., Saili K.S., Soto A.M., Wang H.-S., vom Saal F.S. Low dose effects of bisphenol A: an integrated review of in vitro, laboratory animal, and epidemiology studies. Endocr. Disruptors. 2013;1:0–1. [Google Scholar]
- 60.vom Saal F.S., Nagel S.C., Coe B.L., Angle B.M., Taylor J.A. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell. Endocrinol. 2012;354:74–84. doi: 10.1016/j.mce.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan J., Young M.E., Cui L., Lopaschuk G.D., Liao R., Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation. 2009;119:2818–2828. doi: 10.1161/CIRCULATIONAHA.108.832915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan S., Chen Y., Dong M., Song W., Belcher S.M., Wang H.-S. Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One. 2011;6:e25455. doi: 10.1371/journal.pone.0025455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan S., Song W., Chen Y., Hong K., Rubinstein J., Wang H.-S. Low-dose bisphenol A and estrogen increase ventricular arrhythmias following ischemia⿿reperfusion in female rat hearts. Food Chem. Toxicol. 2013;56:75–80. doi: 10.1016/j.fct.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye X., Pierik F.H., Angerer J., Meltzer H.M., Jaddoe V.W.V., Tiemeier H., Hoppin J.A., Longnecker M.P. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa) Int. J. Hygiene Environ. Health. 2009;212:481–491. doi: 10.1016/j.ijheh.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.