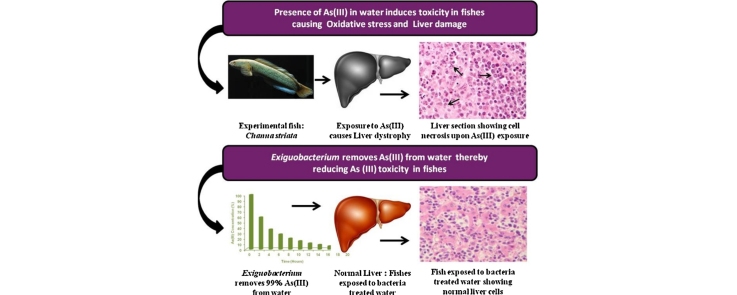
Graphical abstract
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BSM, basal salt medium; CAT, catalase; EDX, energy dispersive X-ray spectroscopy; FTIR, Fourier transform infrared spectrophotometer; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; GPx, glutathione peroxidase; HG-AAS, hydride generation atomic absorption spectrophotometer; MDA, malondialdehyde; SEM, scanning electron microscopy; SOD, superoxide dismutase
Keywords: Arsenic, Exiguobacterium, Arsenic removal, Oxidative damage, Antioxidative enzymes
Abstract
Arsenic is a toxic metalloid existing widely in the environment, and its removal from contaminated water has become a global challenge. The use of bacteria in this regard finds a promising solution. In the present study, Exiguobacterium sp. As-9, which is an arsenic resistant bacterium, was selected with respect to its arsenic removal efficiency. Quantification of arsenic in the water treated with bacterium showed that Exiguobacterium efficiently removed up to 99% of arsenic in less than 20 h. In order to reveal the possible effect of this bacterium in removal of arsenic from water and protecting fishes from the detrimental effects of arsenic, we initiated a range of studies on fresh water fish, Channa striata. It was observed that the fishes introduced into bacteria treated water displayed no symptoms of arsenic toxicity which was marked by a decreased oxidative damage, whereas the fishes exposed to arsenic revealed a significant (p < 0.05) increase in the oxidative stress together with the elevated levels of malondialdehyde. Determination of the bioaccumulation of arsenic in the liver tissues of C. striata using hydride generation atomic absorption spectrophotometry (HG-AAS) revealed an increased As(III) accumulation in the fishes exposed to arsenic whereas the arsenic level in the control and bacteria treated fishes were found below the detectable limit. In conclusion, this study presents the strategies of bacterial arsenic removal with possible directions for future research.
1. Introduction
Arsenic (As) is a metalloid and is one of the most hazardous substances released in the environment as a result of both natural and anthropogenic processes [1]. In nature, arsenic exists both in organic and inorganic forms, with the inorganic forms being more toxic occurring predominately in pentavalent arsenate [+5, As(V)] and trivalent arsenite [+3, As(III)] [2]. It has been observed that exposure to high concentrations of arsenic is lethal to most organisms and is associated with the increased risk of carcinogenic effects and other diseases [3].
Arsenic induces toxicological effects not only in humans, but also in aquatic life forms. Most studies to understand the toxicity of arsenic compounds were performed in mammalian cells [4], [5]. However, the study of the arsenic toxicity to the aquatic animal species, including fish, is limited. Arsenic has been reported to be immunotoxic and renders the host immunocompromised increasing its susceptibility to pathogen attacks [6]. Studies indicate that arsenic exposure promotes apoptotic and necrotic mediated cell death in fishes in concentration and time dependent manner in different phases of the cell cycle. It also leads to DNA fragmentation, alteration in mitochondrial membrane potential and formation of increased reactive oxygen species (ROS) [7]. Arsenic causes different types of anomalies [8] but liver is one of the target organs of arsenic toxicity. Reports suggest that chronic arsenic exposure causes abnormal liver function, hepatomegaly, liver fibrosis and cirrhosis in different fish species [9].
The dispersal of arsenic rich wastes generated by human activities leads to the contamination of water, which has increased the threat of chronic arsenic poisoning in aquatic animals [10]. The presence of arsenic in water is particularly dangerous for fish juveniles and may considerably reduce survival and growth of fish populations [11]. It may reduce the size of fish larvae or even cause the extinction of the entire fish population in polluted reservoirs. Fishes indwelling contaminated environments also accumulate high arsenic contents and their consumption may lead to serious health risk to humans, causing cancer and neurological disturbances [12].
Arsenic contamination of water bodies has raised important questions about the competing health benefits and risks of fish consumption. Many physical and chemical methods are currently available for the removal of arsenic from contaminated water [13], [14] but are not shown to be efficient. Recent investigations on the use of bacteria in mobilizing and removing arsenic from water bodies are gaining momentum [15]. Due to their potential for providing a clean and cost effective technology for arsenic removal, the use of bacteria is creating opportunities for developing systems for the treatment of arsenic contaminated water [16].
The present work is an attempt to evaluate the As(III) removal efficiency of the arsenic resistant Exiguobacterium and to understand its protective influence upon arsenic induced toxicity in Channa striata by studying different parameters like effect on blood cells, activity of antioxidative enzymes, liver marker enzymes and malondialdehyde level in tissues. The rationale behind selecting this fish was that, it serves as a popular food in India and has long been regarded as a valuable fish because of its considerable economic importance.
2. Materials and methods
2.1. Bacterial strain, culture conditions and arsenic removal
Exiguobacterium sp. As-9, an arsenic resistant bacterium (Gene Bank accession number: KC894600.1) isolated from the arsenic rich soil of Chhattisgarh, India was used in the present study. It is Gram positive, rod-shaped and an obligate aerobe which survived exceptionally high concentrations of As(III) (180 mM) [17]. The bacterium was cultured in Basal Salt Medium (BSM) (components per liter: yeast extract, 1.0 g; (NH4) 2SO4, 0.3 g; MgSO4·7H2O, 0.14 g; CaCl2·2H2O, 0.2 g; NaCl, 0.1 g; H3BO3, 0.6 mg; glucose, 10.0 g) and incubated at 30 °C for 48 h in a rotary shaker at 120 rpm. Cells were harvested by centrifugation at 5000 rpm for 10 min, washed twice with sterile saline solution (0.85% w/v NaCl) and immobilized in calcium alginate as described by Duarte et al. [18] to obtain a cell density of 109 cfu per cubic cm of suspension. The beads (2–3 mm in diameter) produced were left for 1 h at 22 ± 2 °C for curing and then washed with sterile saline solution. To determine the arsenic removal efficiency of the isolate, approximately 1.0 g of alginate bead was added to 250 ml water having sodium arsenite [(NaAsO2) (100 mg/l), SD Fine Chem. Ltd., Mumbai, India, PubChem CID: 443495]. As(III) concentration was monitored after every two hour interval using Hydride Generation Atomic Absorption Spectrophotometer (HG-AAS) (Shimadzu AA-7000) by the method described by Wang et al. [19]. A simultaneous arsenic removal assay was also carried out in the cell free control [water + As(III)] and in non bacterial alginate beads to monitor any abiotic removal. Percentage As(III) removal was calculated as:
| As(III) removal (%) = [(Ci − Cf) ÷ Ci] × 100 |
where, Ci = initial As(III) concentration and Cf = final As(III) concentration.
2.2. Scanning electron microscopy
The surface morphology of control alginate beads and those exposed to As(III) were studied with scanning electron microscope (JEOL JSM-6360, Japan) using the method described by Covarrubias et al. [20]. To investigate the diffusion of As(III) ions inside the alginate beads and to understand its effect on bacterium, SEM analysis was also carried out on a cross-sectioned As(III) treated alginate beads. All the samples were mounted on stub, sputtered with gold particle and observed under microscope operated at 20 kV. The elemental content of the samples was analyzed using energy dispersive X-ray spectroscopy (EDX) (INCA 250 EDS) equipped with X-MAX 20 mm Detector.
2.3. Fourier transform infrared analysis
The IR spectra of control and As(III) loaded alginate beads were analyzed with FTIR spectrophotometer (Shimadzu IRAffinity-1S, Japan). The samples were dried in room temperature and crushed with potassium bromide in 1:10 proportion. Spectral scanning was done in the range of 4000–400 cm−1.
2.4. Fish collection and acclimatization
C. striata (Class: Actinopterygii; Order: Perciformes; Family: Channidae), a striped snakehead, carnivorous freshwater fish was selected for the study. Fishes of almost the same size (length 17.0 ± 0.5 cm, weight 54.0 ± 0.8 g) were collected from a local fish farm and transported to the laboratory. They were immediately released into plastic tubs containing tap water (temperature, 27.4 ± 0.5 °C; pH, 6.83 ± 0.1; salinity, 0.081 ± 0.002%; dissolved oxygen, 6.52 ± 0.1 mg/l) and acclimated to laboratory conditions for about 6–7 days in a static condition. Fishes were fed twice daily ad libitum and the faecal matter was regularly siphoned off to reduce the ammonia content in water. After acclimatization, the healthy fishes were selected and used for the experiment.
2.5. Determination of LC50 and As(III) treatment
The lethal concentration (LC50) values of As(III) on C. striata were investigated as described by Ahmed et al. [21]. Fishes were randomly selected and exposed to five different concentrations of NaAsO2 (50, 100, 200, 300 and 500 mg/l) (n = 6 in each experimental group). The control group (n = 6) without As(III) was also maintained simultaneously and each experimental trial was carried out for a period of 96 h. The mortality of the fishes in all the groups was recorded at logarithmic time intervals (6, 12, 24, 48, 72 and 96 h) of exposure to determine the LC50 value. Percent mortality observed was 0, 20, 50, 75 and 90%, respectively, for 50, 100, 200, 300 and 500 mg/l As(III) concentrations. The 96 h-LC50 value of As(III) was calculated to be 200 mg/l for C. striata. Based on the LC50 value, 100 mg/l As(III) concentration was selected for the sub-lethal studies and was further used in the experiment.
The acclimatized fishes were divided into three groups: fishes of the first group were kept in tap water, which served as control (n = 6) while fishes of the second group [test (n = 6)] were exposed to 100 mg/l As(III) in water. The third group [treated (n = 6)] fishes were kept in the treated water [immobilized bacteria was added to the water amended with 100 mg/l As(III); after 24 h, bacteria was removed and same water was used as the treated water for the third group fishes]. At the end of the experiment (after 30 days), fishes were sacrificed and blood was collected by heart puncture. Also, livers were removed for biochemical and histological study. The experiments were performed as per the guidelines of Institutional Animal Ethical Committee (IAEC) of Guru Ghasidas Vishwavidyalaya and precaution was taken to avoid any cruelty.
2.6. Morphologic evaluation of blood cells
The effect of arsenic on the morphology of blood cells of C. striata was studied by simple staining procedures. Briefly, a single drop of blood was placed on the surface of a clean and grease free microscopic slide at a distance of 2 cm from one end and carefully extended to form a uniform smear. Once prepared, the smear was air dried and fixed in 95% ethanol for ten minutes. Staining was done with a freshly prepared mixture of Giemsa stain (HiMedia, India) (0.5 ml of commercial liquid stain diluted in 9.5 ml distilled water) for 30 min. The slides were carefully washed with distilled water, air dried and examined under light microscope equipped with a digital camera (Leica DM IL LED).
2.7. Measurement of liver antioxidative enzymes, lipid peroxidation and marker (function) enzymes
About 50 mg of liver tissue was homogenized in 2 ml of cold potassium phosphate buffer (50 mM, pH 7.0) containing 1 mM EDTA using chilled mortar and pestle. The homogenate was centrifuged at 15,000 rpm at 4 °C for 15 min and the clear supernatant was used for the enzyme assays. The activities of superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) were assayed according to the methods of Minami and Yoshikawa [22], Paglia and Valentine [23] and Cohen et al. [24], respectively. The concentration of malondialdehyde (MDA) in liver was determined by the method of Bloom and Westerfe [25]. The results were expressed as nmol MDA per mg protein. To analyze hepatic function, alanine aminotransferase (ALT) or glutamic pyruvic transferase (GPT) and aspartate aminotransferase (AST) or glutamic oxaloacetic transaminase (GOT) were measured according to the method of Reitman and Frankel [26].
2.8. Histological study
The liver tissue of the control, test and treated fishes were excised out and fixed in Bouin’s solution for 48 h. The tissues were dehydrated through graded alcohol series, cleared in xylene and embedded in paraffin. 5 μm thick paraffin sections were cut and stained with haematoxylin–eosin and examined under a light microscope for histological changes.
2.9. Arsenic accumulation in liver
The arsenic concentration in the liver of experimental fishes was determined according to the method described by Wang et al. [19]. Briefly, liver tissue (0.5 g) was digested in 5 ml acid mixture (H2SO4/HNO3, 3:1) at 100 °C for 30 min. After 24 h, few drops of 10% KI was added to convert any As(V) to As(III) and the excess iodine was destroyed by adding 1 ml ascorbic acid (5%). The sample was cooled and the arsenic concentration was determined by HG-AAS. The concentration was expressed as μg/mg weight of the tissue.
2.10. Statistical analysis
Values are given as means ± S.D. for six fishes in each group. Data were analyzed by one-way analysis of variance (ANOVA) using Minitab 17 software. Significance of differences was defined as p < 0.05.
3. Results and discussion
In the present study, we elucidated the toxicity effects of arsenic on freshwater fish, C. striata. The study shows for the first time a clear effect of applying arsenic resistant bacteria in removing arsenic from water thereby reducing its toxic manifestations in fishes.
3.1. Arsenic removal by immobilized bacteria
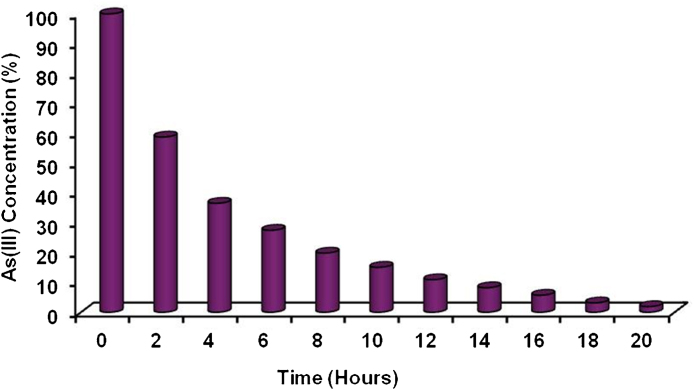
Immobilization of Exiguobacterium in calcium alginate beads showed efficient removal of As(III) from water in a short period of time (Fig. 1). The results indicated that the rate of As(III) removal was rapid in the first 4 h then decreased gradually. After 20 h of incubation, approximately 99% of As(III) was removed from the water. Therefore, incubation time of 20–24 h was used as the optimum time for As(III) removal for the rest of the experiments. Further, the experiments involving non bacterial beads showed no significant change in the arsenic concentration over the duration of incubation.
Fig. 1.
Percent removal of As(III) from water by immobilized Exiguobacterium.
Calcium alginate beads are one of the most commonly used supports for the immobilization of cells. The alginate bead is internally composed of many cavities which are formed after cross-linking and surface polymerization [27]. These cavities are located in every part of the bead where the bacteria reside and multiply after immobilization. This happens because the size of the pores on the surface is smaller than most bacteria which act as a physical barrier and keep them trapped inside the bead. But the pores are large enough to allow the diffusion of small molecules inside the cavity [20]. This facilitates easy diffusion of As(III) in the alginate beads which is further taken by the immobilized bacterium. It is also previously reported that alginate beads could act as a low cost carriers for the binding of other molecules (e.g. ferric hydroxide) for the efficient removal of arsenic [28].
As(III) removal has also been reported previously [29] but our study illustrates appreciable removal of As(III) in a short period of time. Arsenic removal and accumulation is a unique property of bacteria which is widely distributed in the environment [30], [31] and could be conveniently used for the removal of this toxic metalloid from the contaminated ecosystems.
3.2. Scanning electron microscopy
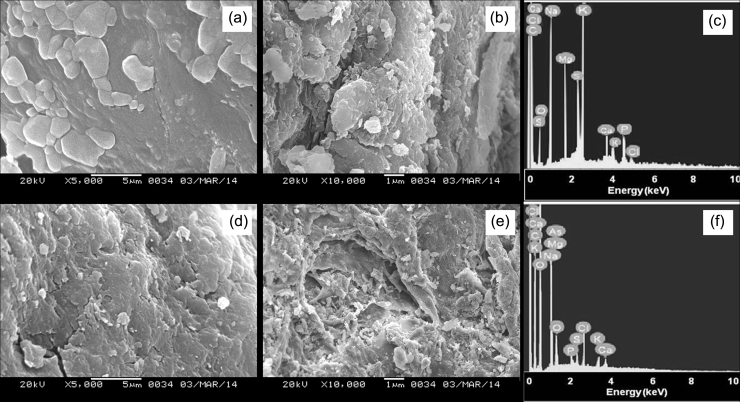
SEM was employed to determine the surface morphology of alginate beads before and after exposure to As(III). The control beads appeared normal and revealed a more compact structure while a visible change in the surface morphology was observed after interaction with As(III) (Fig. 2a and d). Smoothness of the beads decreased significantly and the beads appeared rougher with the uptake of As(III) when compared to control. Further, dissolution of the compact structure was observed due to As(III) exposure causing a more surface disturbance. This change in structure might be attributed to the cross-linking of some As(III) ions with alginate thereby altering surface properties of the beads [32].
Fig. 2.
SEM micrographs of (a) surface morphology of control alginate beads (b) sliced control beads showing the distribution of Exiguobacterium throughout the internal surface (c) EDX spectrum of control beads (d) change in the surface morphology of beads after As(III) exposure (e) internal surface of beads after exposure to As(III) depicting the aggregation of bacterial cells (f) EDX spectrum of As(III) loaded beads.
To understand the effect of As(III) upon bacteria, the control and As(III) loaded alginate beads were sliced and observed under microscope. When sliced open, the control alginate beads revealed a homogeneous distribution of Exiguobacterium covering the interior surface of beads (>80% of the surface) (Fig. 2b) while the bacterium tends to get aggregated (Fig 2e) when exposed to As(III). This depicts the mode of response of bacterial cells to As(III) stress, which could be attributed as a positive attitude to uptake arsenic from its surrounding.
The EDX spectrum of the cross sectioned beads [control and As(III) loaded] is shown in Fig. 2c and f. A clear appearance of As peak in the spectrum confirmed the uptake of As(III) by immobilized Exiguobacterium.
3.3. Fourier transform infrared analysis
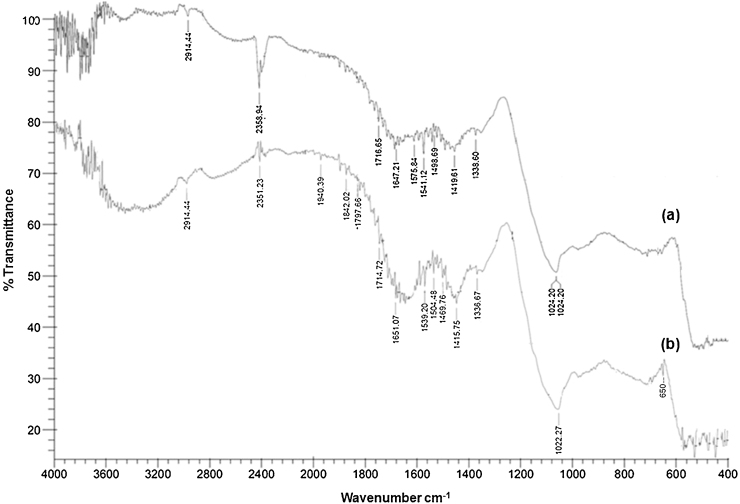
The IR spectra of control and arsenic loaded immobilized Exiguobacterium in calcium alginate beads are shown in Fig. 3. For alginate, a broad absorption peak around 3200–3600 cm−1 indicates the vibrational bands of —OH and confirms the presence of hydroxyl group [33]. The FTIR spectra also shows the characteristic peak of alginate at 1575 and 1419 cm−1 which corresponds to the COO— asymmetric and symmetric stretching [34], [35]. Further, a shift in the peaks to 1539 and 1415 cm−1 was observed in the As(III) loaded beads which indicate the participation of carboxyl group with arsenic [36], [37]. The FTIR spectra also revealed that the percent transmittance of peak at 2914 cm−1 corresponding to C—H bands decreased in As(III) loaded alginate beads as compared to control. Another important observation from the FTIR analysis was the appearance of sharp peak at about 650 cm−1 in As(III) loaded beads which is the characteristic of As—O stretch vibration [34], [36]. IR spectra of control beads showed the characteristic bands of —C C—, C O and —C C— stretching at 2260–2100, 1760–1665 and 1680–1640 cm−1, respectively, while the spectra obtained from As(III) loaded beads also indicated the presence of characteristic bands at almost the same wave number with lower intensities.
Fig. 3.
FTIR spectrum of (a) control beads and (b) As(III) loaded alginate beads.
3.4. Effect of As(III) on fishes
Various physical and behavioral anomalies were observed in the fishes of test group within a few days of As(III) exposure. The fishes became lethargic, tended to settle at the bottom with their mouth wide open. The falling of fins and scales were observed at a later stage of exposure with a significant decrease in locomoter activity. The test fishes also appeared to become slimy due to the secretion of excessive mucus. Similar effects of arsenic were observed by Ahmed et al. [21] in freshwater fish, tilapia. These findings suggest that the alteration in the behavioral parameters such as anxiety and locomotion might be due to the neurotoxic effects induced by arsenic [8]. Further, the present study also demonstrated that the control and the fishes of the treated groups remained normal and active throughout the experimental period.
3.5. Morphologic evaluation of blood cells
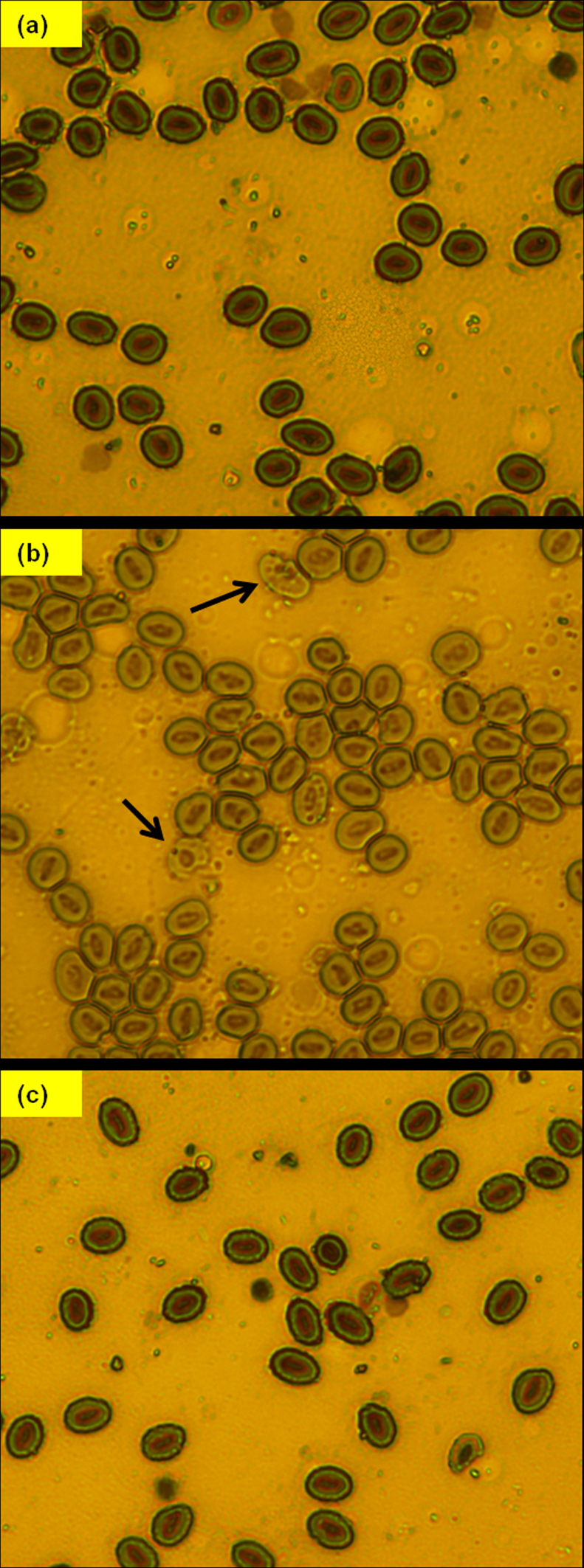
Arsenic exerts various toxicological effects on the hematological parameters of fishes [38], [39]. In the present study, the effect of As(III) on the morphology of blood cells of fresh water fish C. striata was evaluated. Results revealed that the blood cells of the control fishes were normal and appeared ellipsoidal in shape. The cell wall was intact and the nuclei stained dark purple in color. In contrast, the blood cells of the fishes exposed to As(III) lost their normal shapes and became more irregular.The other visible effects of As(III) was the lysis of red blood cells with the disintegration of the cell membrane and clumping of the nuclear material. As(III) exposure also resulted in the fragmentation of some red blood cells. Long term exposure (30 days) of As(III) also resulted in the appearance of stacked cells with nuclei losing some amount of central dye showing a sign of karyolysis. The stacked cells lost their original shape and acquired different shapes with the obvious disintegration of the cell membrane. Examination of blood cells of the treated fishes displayed normal morphology with no symptoms of toxicity which is consistent with the observations of the control group. This is in agreement with the hypothesis that Exiguobacterium facilitated complete removal of As(III) from the water, thereby reducing the toxic manifestations of As(III) in fishes (Fig. 4).
Fig. 4.
Effect of As(III) on the morphology of blood cells of Channa striata (a) control (b) test (c) treated.
3.6. Measurement of liver antioxidative enzymes, lipid peroxidation and marker (function) enzymes
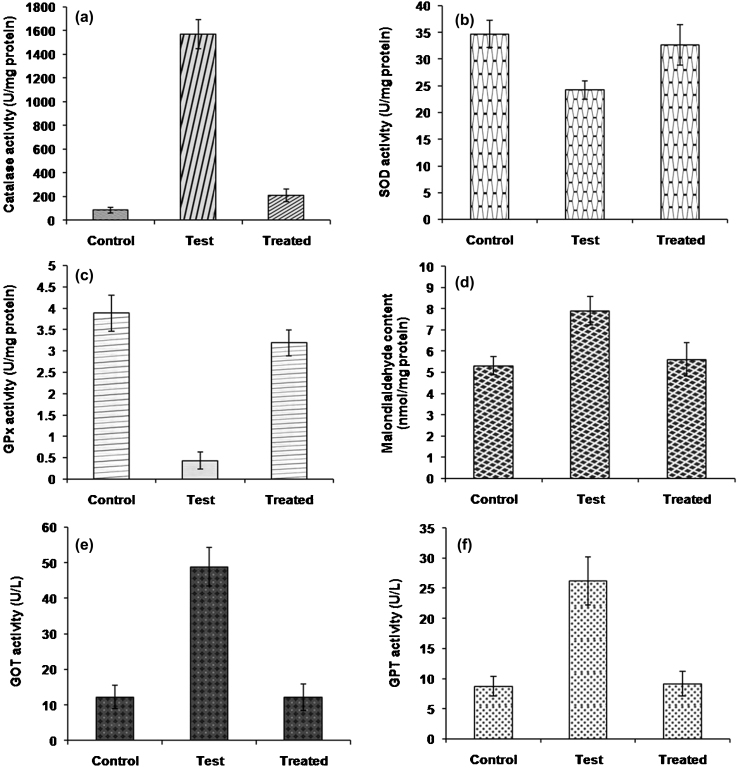
Arsenic exerts its toxicity by generating ROS such as superoxide, hydroxyl and peroxyl radicals and induces oxidative damage in different biomacromolecules that can lead to a critical failure of biological functions and ultimately cell death [40]. The enzymatic antioxidants such as superoxide dismutase, catalase and glutathione peroxidase play a vital role in the process of scavenging ROS to maintain a physiological balance in the cell [41]. In the present study, the effect of As(III) on hepatic antioxidative enzymes (SOD, CAT, GPx) of fish was investigated. Results revealed a significant (p < 0.05) decrease in the activities of SOD (30.5%) and GPx (88.91%) while increased CAT activity (94.44%) was observed in the fishes exposed to As(III) when compared to control. SOD and CAT activities are treated as important and reliable biomarkers for oxidative stress. SOD constitutes the first line of defense by dismutating the superoxide radical, a major ROS and produces hydrogen peroxide (H2O2) as an end product. The H2O2 is another ROS, which is further removed by the action of CAT. As SOD activity was decreased with As(III) treatment, it might be due to high concentration of H2O2 produced via an unknown pathway affecting SOD induction. Similar conclusions on SOD activities getting impaired at high arsenic concentration had also been reported previously by Altikat et al. [42]. The activity of SOD leads to the accumulation of H2O2 which increased the production of CAT to balance its level. GPx reduces lipid hydroperoxides into lipid alcohols and glutathione (GSH) serves as a substrate for GPx. In this study, the decreased GPx activities in liver might be due to the decrease in the levels of GSH or due to the interaction of trivalent arsenic with critical thiol groups in GPx molecules [43]. Moreover, the activities of these enzymes were found close to the control in the fishes of the treated group and displayed no symptoms of arsenic toxicity (Fig. 5a–c). In addition, the results provide evidence that monitoring the level of enzymes of the oxidative stress could be sensitive indicators of aquatic pollution [44].
Fig. 5.
Effect of As(III) on the liver antioxidative enzyme activity, malondialdehyde concentration and marker enzymes of Channa striata (a) CAT (b) SOD (c) GPx (d) MDA content (e) GOT and (f) GPT.
Increased level of ROS is also responsible for lipid peroxidation and increased concentration of MDA, a cytotoxic product of lipid peroxidation which is used as an indicator of membrane damage. Evaluation of the MDA content demonstrated a positive correlation between arsenic treatment and lipid peroxidation in liver of test fishes. Results revealed that As(III) treatment increased lipid peroxidation (Fig. 5d) with the MDA levels elevated by 1.4 fold in test fishes when compared to the control and treated groups. The increase in MDA content and the decrease in enzymatic activities in the liver confirmed that the fishes suffered oxidative damage upon exposure to inorganic arsenic. These results are in line with the findings of Altikat et al. [42] who reported considerable inhibition of antioxidative enzymes and increased MDA levels in liver of mirror carp in response to the arsenic toxicity.
GOT and GPT are two of the several enzymes that are measured to evaluate the liver function. In the present study, higher activities of AST and ALT have been found in response to arsenic induced oxidative stress. Results showed that the activities of GOT and GPT increased by 75.01 and 66.67%, respectively, in liver in response to arsenic in the test group fishes indicating serious hepatic damage and distress condition (Fig. 5e and f). Similar results were obtained by Vutukuru et al. [45] in Indian major carp, Labeo rohita. Further, treatment with Exiguobacterium alleviated the activity of both the enzymes in the treated group and the values were comparable to control.
3.7. Histological study
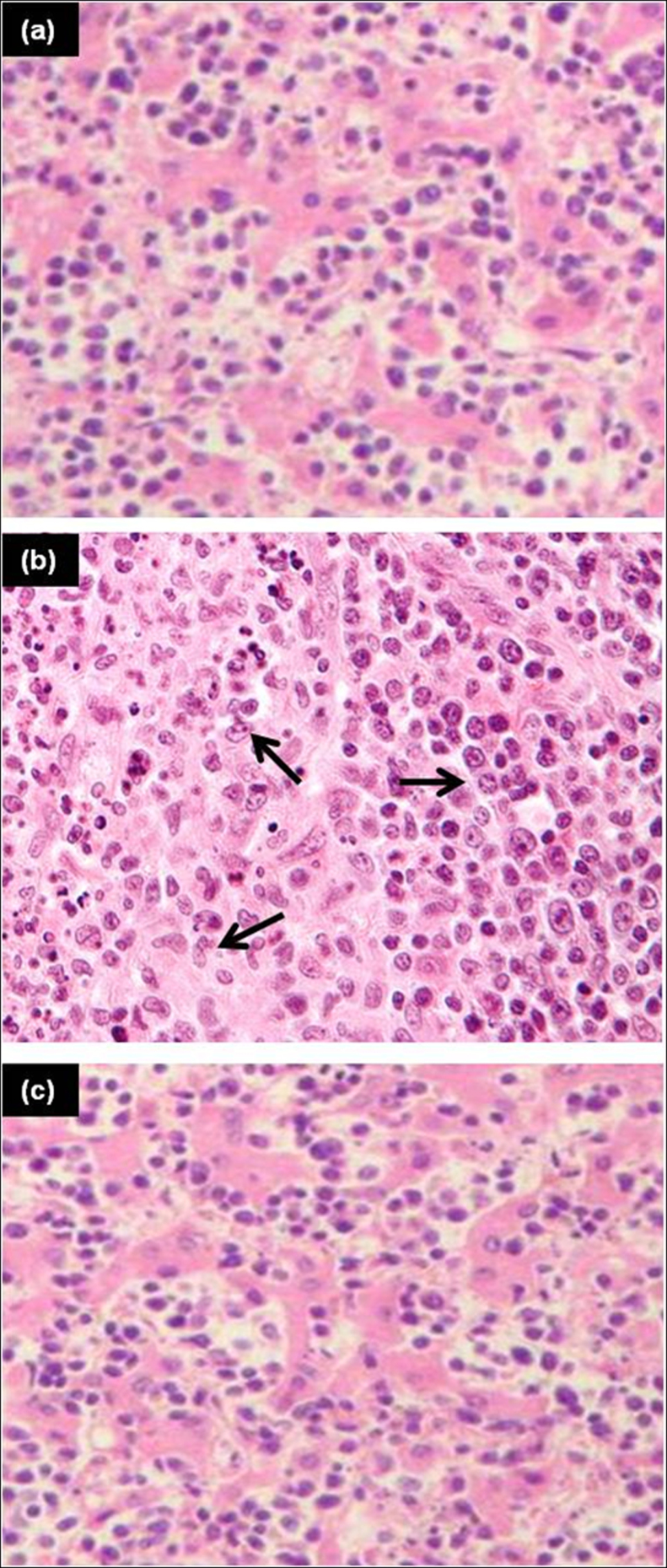
The detoxification of toxic substances takes place in liver and the histopathological results indicated that liver tissue was severely affected by As(III). In the control and treated group, no histopathologic lesions were observed when compared to the fishes exposed to As(III) which showed dystrophy in the form of hepatic necrosis. The histological changes in liver tissues of control, test and treated fishes are shown in Fig. 6. During the present investigation, intense degenerative changes in the liver of test group fishes were observed marked with irregular shaped hepatocytes, prominent cytoplasmic vacuolization and condensation of the nuclei (Fig. 6b). The most conspicuous changes with karyohexis and karyolysis were also observed in As(III) exposed fishes. Similar toxicity effects were noticed in freshwater fish, tilapia [21] and gibel carp Carassius auratus gibelio [46] when exposed to arsenic. The present study also revealed that the hepatocytes and other cells of the liver in control and bacteria treated groups were normal and systematically arranged with a centrally placed nucleus (Fig. 6a and c) indicating the effectiveness of Exiguobacterium in removing As(III) from water thereby reducing arsenic toxicity in fishes.
Fig. 6.
Liver sections of Channa striata (a) control (b) fish exposed to 100 mg/l As(III) showing liver cell necrosis (c) fish exposed to bacteria treated water displaying normal cells.
3.8. Arsenic accumulation in liver
Fishes execute detoxification mechanism, which operates principally in liver [47] and therefore possesses the chance of accumulating high arsenic concentrations. Previous studies proved that arsenic exposure causes adverse effects in aquatic biota due to the bioaccumulation of metalloid in tissues [48]. In the present study, As(III) accumulation in the liver tissues of C. striata was analyzed by HG-AAS. Significant (p < 0.05) accumulation was observed in the liver tissues of the fishes exposed to As(III) with the concentration of 6.21 ± 0.4 μg/mg biomass (Table 1). Similar results were obtained by Allen et al. [49] in Channa punctatus after exposure to inorganic arsenic. Such an increase in arsenic level in the tissues might be attributed to the availability of dissolved As(III) which contributed strongly to the metalloid bioaccumulation. However, results also indicated that the accumulation of As(III) in the liver tissues of the fishes of treated group was effectively reduced by the treatment of arsenic resistant bacteria which was below the detectable limit and comparable to control.
Table 1.
Accumulation of arsenic in the liver tissues of Channa striata.
| Groups | Control | Test | Treated |
|---|---|---|---|
| Arsenic concentration (μg/mg) | NDa | 6.21 ± 0.4 | ND |
ND corresponds to the concentration of arsenic below the detectable limit.
4. Conclusions
Aquatic organisms accumulate, retain, and transform arsenic species inside their bodies when exposed to it. Therefore, not only contaminated water, but also fishes and other aquatic foods containing arsenic may be potential sources of human health risks. The present study demonstrated the arsenic uptake capacity of arsenic resistant Exiguobacterium which may prove to be a promising candidate towards arsenic removal from the contaminated water. The study also revealed that the bacteria treated water helped the fishes to grow and survive in a normal way with no symptoms of toxicity while long term intake of arsenic altered the hematological and biochemical parameters and caused oxidative damage in C. striata. These results suggest that the present study might be useful in environmental biomonitoring of arsenic contamination and could serve as a model for the removal of toxicants from the contaminated ecosystems.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
We are thankful to the Department of Science and Technology (DST), New Delhi for funding the work through INSPIRE fellowship and to Sophisticated Test and Instrumentation Centre (STIC), Cochin for the FTIR analysis. Sophisticated Analytical Instrument Facility of North Eastern Hill University, Shillong is equally acknowledged for SEM facility.
References
- 1.Garelick H., Jones H., Dybowska A., Valsami-Jones E. Arsenic pollution sources. Rev. Environ. Contam. Toxicol. 2008;197:17–60. doi: 10.1007/978-0-387-79284-2_2. [DOI] [PubMed] [Google Scholar]
- 2.Oremland R.S., Stolz J.F. Arsenic, microbes and contaminated aquifers. Trends Microbiol. 2005;13:45–49. doi: 10.1016/j.tim.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Mandal B.K., Suzuki K.T. Arsenic round the world: a review. Talanta. 2002;58:201–235. [PubMed] [Google Scholar]
- 4.Garcia M.T.A., Duenas A.H., Pampliega J.P. Hematological effects of arsenic in rats after subchronical exposure during pregnancy and lactation: the protective role of antioxidants. Exp. Toxicol. Pathol. 2013;65:609–614. doi: 10.1016/j.etp.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Zhao X., Zhou W., Jian-jun L., Chen C., Ping-chuan Z., Lu D., Jing-hong C., Qun C., Xiao-tian Z., Zhi-lun W. Protective effects of selenium on oxidative damage and oxidative stress related gene expression in rat liver under chronic poisoning of arsenic. Food Chem. Toxicol. 2013;58:1–7. doi: 10.1016/j.fct.2013.03.048. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee S., Mitra T., Purohit G.K., Mohanty S., Mohanty B.P. Immunomodulatory effect of arsenic on cytokine and HSP gene expression in Labeo rohita fingerlings. Fish Shellfish Immunol. 2015;44:43–49. doi: 10.1016/j.fsi.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 7.Selvaraj V., Armistead M.Y., Cohenford M., Murray E. Arsenic trioxide (As(2)O(3)) induces apoptosis and necrosis mediated cell death through mitochondrial membrane potential damage and elevated production of reactive oxygen species in PLHC-1 fish cell line. Chemosphere. 2013;90:1201–1209. doi: 10.1016/j.chemosphere.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldissarelli L.A., Capiotti K.M., Bogo M.R., Ghisleni G., Bonan C.D. Arsenic alters behavioral parameters and brain ectonucleotidases activities in zebrafish (Danio rerio) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2012;155:566–572. doi: 10.1016/j.cbpc.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Guha Mazumder D.N. Effect of chronic intake of arsenic contaminated water on liver. Toxicol. Appl. Pharm. 2005;206:169–175. doi: 10.1016/j.taap.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Rahman M.A., Hasegawa H., Lim R.P. Bioaccumulation, biotransformation and trophic transfer of arsenic in the aquatic food chain. Environ. Res. 2012;116:118–135. doi: 10.1016/j.envres.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Erickson R.J., Mount D.R., Highland T.L., Russell Hockett J., Jenson C.T. The relative importance of waterborne and dietborne arsenic exposure on survival and growth of juvenile rainbow trout. Aquat. Toxicol. 2011;104:108–115. doi: 10.1016/j.aquatox.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Avigliano E., Schenone N.F., Volpedo A.F., Goessler W., Fernández Cirelli A. Heavy metals and trace elements in muscle of silverside (Odontesthes bonariensis) and water from different environments (Argentina): aquatic pollution and consumption effect approach. Sci. Total Environ. 2015;506–507:102–108. doi: 10.1016/j.scitotenv.2014.10.119. [DOI] [PubMed] [Google Scholar]
- 13.Torres-Perez J., Gerente C., Andres Y. Conversion of agricultural residues into activated carbons for water purification: application to arsenate removal. J. Environ. Sci. Health A Toxicol. Hazard. Substain. Environ. Eng. 2012;47:1173–1185. doi: 10.1080/10934529.2012.668390. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T., Moribe M., Okabe Y., Niinae M. A mechanistic study of arsenate removal from artificially contaminated clay soils by electrokinetic remediation. J. Hazard. Mater. 2013;254–255:310–317. doi: 10.1016/j.jhazmat.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Wang S., Zhao X. On the potential of biological treatment for arsenic contaminated soils and groundwater. J. Environ. Manage. 2009;90:2367–2376. doi: 10.1016/j.jenvman.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Lim K.T., Shukor M.Y., Wasoh H. Physical, chemical, and biological methods for the removal of arsenic compounds. Biomed. Res. Int. 2014;2014:503784. doi: 10.1155/2014/503784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey N., Bhatt R. Arsenic resistance and accumulation by two bacteria isolated from a natural arsenic contaminated site. J. Basic Microbiol. 2015 doi: 10.1002/jobm.201400723. [DOI] [PubMed] [Google Scholar]
- 18.Duarte J.C., Rodrigues J.A.R., Moran P.J.S., Valenca G.P., Nunhez J.R. Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. AMB Express. 2013;3:31. doi: 10.1186/2191-0855-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D.M., Feng J., Qin F.J., Mo Y.S. Determination of six heavy metal elements in Zanthoxylum nitidum in twelve habitats of guangxi by ICP-AES. Zhong Yao Cai. 2012;35:366–368. [PubMed] [Google Scholar]
- 20.Covarrubias S.A., de-Bashan L.E., Moreno M., Bashan Y. Alginate beads provide a beneficial physical barrier against native microorganisms in waste water treated with immobilized bacteria and microalgae. Appl. Microbiol. Biotechnol. 2012;93:2669–2680. doi: 10.1007/s00253-011-3585-8. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed M.K., Habibullah-Al-Mamun M., Parvin E., Akter M.S., Khan M.S. Arsenic induced toxicity and histopathological changes in gill and liver tissue of freshwater fish, tilapia (Oreochromis mossambicus) Exp. Toxicol. Pathol. 2013;65:903–909. doi: 10.1016/j.etp.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Minami M., Yoshikawa H. Simplified assay method of superoxide dismutase activity of clinical use. Clin. Chim. Acta. 1979;92:337–342. doi: 10.1016/0009-8981(79)90211-0. [DOI] [PubMed] [Google Scholar]
- 23.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidise. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 24.Cohen G., Dembuic D., Marcus J. Measurement of catalase activity in tissue extract. Anal. Biochem. 1970;34:30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 25.Bloom R.J., Westerfe W.W. The thiobarbituric acid reaction in relation to fatty livers. Arch. Biochem. Biophys. 1971;145:669–675. doi: 10.1016/s0003-9861(71)80027-9. [DOI] [PubMed] [Google Scholar]
- 26.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 27.Nokhodchi A., Tailor A. In situ cross-linking of sodium alginate with calcium and aluminum ions to sustain the release of theophylline from polymeric matrices. Farmaco. 2004;59:999–1004. doi: 10.1016/j.farmac.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar P., Pal P., Bhattacharyay D., Banerjee S. Removal of arsenic from drinking water by ferric hydroxide microcapsule-loaded alginate beads in packed adsorption column. J. Environ. Sci. Health A Toxicol. Hazard. Substain. Environ. Eng. 2010;45:1750–1757. doi: 10.1080/10934529.2010.513267. [DOI] [PubMed] [Google Scholar]
- 29.Aksornchu P., Prasertsan P., Sobhon V. Isolation of arsenic-tolerant bacteria from arsenic contaminated soil. Songklanakarin J. Sci. Technol. 2008;30:95–102. [Google Scholar]
- 30.Banerjee S., Datta S., Chattyopadhyay D., Sarkar P. Arsenic accumulating and transforming bacteria isolated from contaminated soil for potential use in bioremediation. J. Environ. Sci. Health A Toxicol. Hazard. Substain. Environ. Eng. 2011;46:1736–1747. doi: 10.1080/10934529.2011.623995. [DOI] [PubMed] [Google Scholar]
- 31.Rahman A., Nahar N., Nawani N.N., Jass J., Desale P., Kapadnis B.P., Hossain K., Saha A.K., Ghosh S., Olsson B., Mandal A. Isolation and characterization of a Lysinibacillus strain B1-CDA showing potential for bioremediation of arsenics from contaminated water. J. Environ. Sci. Health A Toxicol. Hazard. Substain. Environ. Eng. 2014;49:1349–1360. doi: 10.1080/10934529.2014.928247. [DOI] [PubMed] [Google Scholar]
- 32.Gotoh T., Matsushima K., Kikuchi K.I. Preparation of alginate–chitosan hybrid gel beads and adsorption of divalent metal ions. Chemosphere. 2004;55:135–140. doi: 10.1016/j.chemosphere.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Mahmoud G.A., Mohamed S.F. Removal of lead ions from aqueous solution using (sodium alginate/itaconic acid) hydrogel prepared by gamma radiation. Aust. J. Basic Appl. Sci. 2012;6:262–273. [Google Scholar]
- 34.Singh P., Singh S.K., Bajpai J., Bajpai A.K., Shrivastava R.B. Iron crosslinked alginate as novel nanosorbents for removal of arsenic ions and bacteriological contamination from water. J. Mater. Res. Technol. 2014;3:195–202. [Google Scholar]
- 35.Santana B.P., Nedel F., Piva E., de Carvalho R.V., Demarco F.F., Carreño N.L. Preparation, modification, and characterization of alginate hydrogel with nano-/microfibers: a new perspective for tissue engineering. Biomed. Res. Int. 2013;2013:307602. doi: 10.1155/2013/307602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim S.F., Zheng Y.M., Zou S.W., Chen J.P. Uptake of arsenate by an alginate-encapsulated magnetic sorbent: process performance and characterization of adsorption chemistry. J. Colloid Interface Sci. 2009;333:33–39. doi: 10.1016/j.jcis.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Torres E., Mata Y.N., Blazquez M.L., Munoz J.A., Gonzalez F., Ballester A. Gold and silver uptake and nanoprecipitation on calcium alginate beads. Langmuir. 2005;21:7951–7958. doi: 10.1021/la046852k. [DOI] [PubMed] [Google Scholar]
- 38.Kavitha C., Malarvizhi A., Senthil Kumaran S., Ramesh M. Toxicological effects of arsenate exposure on hematological, biochemical and liver transaminases activity in an Indian major carp, Catla catla. Food Chem. Toxicol. 2010;48:2848–2854. doi: 10.1016/j.fct.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Lavanya S., Ramesh M., Kavitha C., Malarvizhi A. Hematological, biochemical and ionoregulatory responses of Indian major carp Catla catla during chronic sublethal exposure to inorganic arsenic. Chemosphere. 2011;82:977–985. doi: 10.1016/j.chemosphere.2010.10.071. [DOI] [PubMed] [Google Scholar]
- 40.Sevcikova M., Modra H., Slaninova A., Svobodova Z. Metals as a cause of oxidative stress in fish: a review. Vet. Med. 2011;56:537–546. [Google Scholar]
- 41.Allen T., Rana S.V. Effect of arsenic (AsIII) on glutathione-dependent enzymes in liver and kidney of the freshwater fish Channa punctatus. Biol. Trace Elem. Res. 2004;100:39–48. doi: 10.1385/BTER:100:1:039. [DOI] [PubMed] [Google Scholar]
- 42.Altikat S., Uysal K., Kuru H.I., Kavasoglu M., Ozturk G.N., Kucuk A. The effect of arsenic on some antioxidant enzyme activities and lipid peroxidation in various tissues of mirror carp (Cyprinus carpio carpio) Environ. Sci. Pollut. Res. Int. 2015;22:3212–3218. doi: 10.1007/s11356-014-2896-6. [DOI] [PubMed] [Google Scholar]
- 43.Shen S., Li X.F., Cullen W.R., Weinfeld M., Le X.C. Arsenic binding to proteins. Chem. Rev. 2013;113:7769–7792. doi: 10.1021/cr300015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farombi E.O., Adelowo O.A., Ajimoko Y.P. Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int. J. Environ. Res. Public Health. 2007;4:158–165. doi: 10.3390/ijerph2007040011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vutukuru S.S., Prabhath N.A., Raghavender M., Yerramilli A. Effect of arsenic and chromium on the serum amino transferases activity in Indian major carp, Labeo rohita. Int. J. Environ. Res. Publ. Health. 2007;4:224–227. doi: 10.3390/ijerph2007030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Syasina I.G., Khlopova A.V., Chukhlebova L.M. Assessment of the state of the gibel carp Carassius auratus gibelio in the Amur River Basin: heavy-metal and arsenic concentrations and histopathology of internal organs. Arch. Environ. Contam. Toxicol. 2012;62:465–478. doi: 10.1007/s00244-011-9719-2. [DOI] [PubMed] [Google Scholar]
- 47.Sinaie M., Bastami K.D., Ghorbanpour M., Najafzadeh H., Shekari M., Haghparast S. Metallothionein biosynthesis as a detoxification mechanism in mercury exposure in fish, spotted scat (Scatophagus argus) Fish Physiol. Biochem. 2010;36:1235–1242. doi: 10.1007/s10695-010-9403-x. [DOI] [PubMed] [Google Scholar]
- 48.Shaw J.R., Jackson B., Gabor K., Stanton S., Hamilton J.W., Stanton B.A. The influence of exposure history on arsenic accumulation and toxicity in the killifish, Fundulus heteroclitus. Environ. Toxicol. Chem. 2007;26:2704–2709. doi: 10.1897/07-032.1. [DOI] [PubMed] [Google Scholar]
- 49.Allen T., Singhal R., Rana S.V. Resistance to oxidative stress in a freshwater fish Channa punctatus after exposure to inorganic arsenic. Biol. Trace Elem. Res. 2004;98:63–72. doi: 10.1385/BTER:98:1:63. [DOI] [PubMed] [Google Scholar]