Abstract
In Burkina Faso, as in most Sahelian countries, the failure to follow good agricultural practices coupled with poor soil and climate conditions in the locust control context lead to high environmental contaminations with pesticide residues. Thus, consumers being orally exposed to a combination of multiple pesticide residues through food and water intake, the digestive tract is a tissue susceptible to be directly exposed to these food contaminants. The aim of our work was to compare in vitro the impact of five desert locust control pesticides (Deltamethrin DTM, Fenitrothion FNT, Fipronil FPN, Lambda-cyalothrine LCT, and Teflubenzuron TBZ) alone and in combination on the human intestinal Caco-2 cells viability and function. Cells were exposed to 0.1–100 μM pesticides for 10 days alone or in mixture (MIX). Our results showed a cytotoxic effect of DTM, FNT, FPN, LCT, and TBZ alone or in combination in human intestinal Caco-2 cells. The most efficient were shown to be FPN and FNT impacting the cell layer integrity and/or barrier function, ALP activity, antioxidant enzyme activity, lipid peroxidation, Akt activation, and apoptosis. The presence of antioxidant reduced lipid peroxidation level and attenuated the pesticides-induced cell toxicity, suggesting that key mechanism of pesticides cytotoxicity may be linked to their pro-oxidative potential. A comparative analysis with the predicted cytotoxic effect of pesticides mixture using mathematical modeling shown that the combination of these pesticides led to synergistic effects rather than to a simple independent or dose addition effect.
Keywords: Pesticide, Mixture, Caco-2, Oxidative stress, Locust control, Antioxidant, Cell signaling
1. Introduction
Pesticides are useful tools against desert locust in various African and other world countries and several locust invasions need intensive use of these chemicals across millions of hectares [1]. As the results of the widespread use and the lack of safe management of pesticides in developing countries [2], [3], [4], various compartments of the environment are contaminated and exposure to pesticides is a concern toward the general population [5].
Epidemiological studies often established a positive correlation between occupational exposure to pesticides and the incidence of human chronic pathologies such as cancers, diabetes, neurodegenerative and reproductive disorders, birth defects and developmental toxicity, nephropathies, and respiratory, cardiovascular and autoimmune diseases (for a review see Mostafalou and Abdollahi [6]). Moreover, pesticides are known individually to induce toxicity at the cellular level throughout oxidant-mediated responses such as apoptotic or necrotic cells death, membrane lipids peroxidation, metabolic perturbation, deregulation of several signaling pathways [7] or alteration of tight junctions [8], [9].
Our previous investigations showed the presence of pesticide residues in water and plant samples from locust area in Burkina Faso several years after their use [10]. Elsewhere, pesticide residues in drinking water and plant [11], [12], [13] have been reported to be present at concentrations above Maximum Allowable Concentration (MAC) and Maximum Residue Levels (MRLs) respectively. High levels of pesticides in water and plants as sources of food commodities are a concern for consumers and may be at the onset of human health perturbations. Moreover, due to their use in a wide variety of consumer products it is likely that humans are exposed to several pesticides at any one time and it is well admitted that consumers are exposed through foodstuffs and water intake to cocktails of food contaminants. Although it was well admitted that the mixture is devoid of hazard when the concentration of each compound does not reach an health concern level, Kortenkamp et al. [14] demonstrated significant effects of combination of pesticides when present below their individual no observable adverse effect levels (NOAELs). Predicting the effects associated with simultaneous exposure to different food contaminants is often relying on two suitable assessment concepts, the concentration or dose addition (CA) and the concept of independent action (IA). CA is thought to be applicable to mixtures of chemicals that act on the same toxicological endpoint by a common mechanism of action, while IA is commonly applied to chemicals with dissimilar modes of action [15]. However, combined effects of pesticide mixtures have been assessed in various cell and animal model and results suggested that the effect of pesticide mixture cannot always be predicted from the effect of individual compounds [16], [17], [18], [19]. It thus appears the importance of assessing mixtures in toxicological studies. This has stimulated our interest in assessing the effects associated with simultaneous exposure to pesticides both trough toxicological studies and mathematical modeling.
We aimed to study the in vitro toxicological properties of five pesticides alone or in combination (Deltamethrin DTM, Fenitrothion FNT, Fipronil FPN, Lambda-cyalothrine LCT, and Teflubenzuron TBZ) and to compare the results to that predicted from a mathematical model in order to assess the role of modeling in predicting effects of mixture. These compounds are intensively used in Burkina Faso against the desert locust and have been identified as food contaminants found in edible plants and drinking water in this country. It is noteworthy that these insecticides are also largely used in the UE on various crops, vine and fruit farming excepted fenithrotion and diazinon which are not approved for utilization in the UE.
The oral route being the main way of consumers exposure, intestinal tract is a direct target organ of xenobiotics. Established cell lines, such as Caco-2 cells have proved to be the best model for studies of intestinal absorption and toxicity of xenobiotics [20], [21], [22]. These cells, despite their colonic origin, expressed in culture the main morphological and functional characteristics of intestinal cells [23]. The human colonic cell line Caco-2 cells was thus used as an in vitro model in our study.
2. Materials and methods
2.1. Chemicals
The pesticides deltamethrin (CAS No. 52918-63-5), fenitrothion (CAS No. 122-14-5), fipronil (CAS No. 120068-37-3), lambda-cyhalothrin (CAS No. 91465-08-6), and teflubenzuron (CAS No. 83121-18-0) were purchased from Fluka (Riedel-de-Haën®, France). Purity of each compound was ranged from 95.2 to 99.6% according to manufacturer specification. Dulbecco's modified Eagle's medium (DMEM), Roswell Park Memorial Institute medium (RPMI), l-glutamine, Dimethyl sulfoxide (DMSO), non essential amino acids (NEAA), Penicillin and Streptomycin, Phosphate buffer saline (PBS), Trypsin-EDTA, p-nitrophenol, p-nitrophenyl-phosphate, hydrogen peroxides (H2O2), Reduced glutathione, glutathione reductase, α-NADPH, 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCF-DA), Hank's balanced salt solution (HBSS), Hoechst 33342, propidium iodide (PI), thiobarbituric acid (TBA), 1,1,3,3-tetramethoxypropane (TMOP), and Bicinchoninic Acid (BCA) were purchased from Sigma–Aldrich (France). Fetal bovine serum (FBS) and the sterile material used for culture (flasks and culture plates) were from Dutscher (France). When not specified chemicals were purchased from Sigma–Aldrich (France).
2.2. Cell culture
Caco-2 cells were obtained from the American Type Culture Collection (ATCC, USA) and cultured in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Culture medium (DMEM) was supplemented with 1% l-glutamine, 1% NEAA, 1% Penicillin/Streptomycin and 10% of heat-inactivated FBS. When the cell culture reached 80% confluence, cells were dispersed with 0.025 M trypsin-EDTA and reseeded in new flask. The passage number of the cells used in the experiments was between 25 and 39. The culture medium was replaced every 48 h.
2.3. Pesticide treatments
Pesticides concentrations assessed in our study were ranged from 0.1 to 100 μM. The chosen doses were based on the LMR values adapted to our in vitro study as follows: LMR values expressed in mg/kg of vegetables were converted into μg of active substance supposed to be present in the digestive tract by considering a total assimilation of the LMR amount of pesticide in vivo upon a dietary intake of 100 g of vegetables. LMRs for DTM, FNT, TBZ, FNT, and LCT vary respectively from 0.01 to 2; 0.05 to 6; 0.05 to 1; 0.002 to 5; and 0.02 to 3 mg/kg vegetables (according to Codex Alimentarius http://www.codexalimentarius.org/).Stock solutions of pesticides (100 mM) were prepared in DMSO. One day after seeding, cell layers were washed with serum free medium and then incubated with 4% FBS containing medium supplemented with the indicated concentrations of pesticides. The freshly mixture solution was made by adding individual pesticides directly in medium containing 4% FBS at equimolar proportion respecting the same range concentration of single agents. Treatments with pesticides were renewed every 48 h. Control cells were exposed to 0.1% DMSO alone. Each experiment was repeated independently 3 times in quadruplicate (MTT assay) or in triplicate.
2.4. Evaluation of cell viability by MTT assay
The effects of pesticides on Caco-2 cells proliferation and viability were monitored during a 10 days-period of exposure. Reduction of the permeant tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide (MTT) was monitored as described by Mosmann [24], with modifications. Briefly cells were seeded in 24-well plates (5 × 104 cells/well). After 20 h of incubation, the cells were treated with various concentrations (0.1–100 μM) of pesticides alone and in combination. At the end of the incubation time (3, 7 and 10 days of exposure), culture medium was discarded and the monolayer washed twice with PBS. Cells were then incubated with MTT (500 μl per well at a concentration of 0.5 mg/ml) in RPMI for 4 h at 37 °C. The reaction was stopped using 500 μl of a solution containing 10% SDS and 1 N NaOH at 37 °C. Then, 200 μl of formazan solution was transferred into 96-well plates and absorbances were measured by a microplate reader (Tecan, Lyon, France) at 570 and 690 nm. The relative cell viability was expressed as the ratio (%) of the absorbance in the experimental wells to that of the control wells (cells treated with DMSO). The EC50 (cytotoxic concentration for 50% cell death) was determined from the dose-response curve.
2.5. Observation of morphologic changes
Caco-2 cells were seeded in 12-well plates at a density of 1.125 × 105 cells/well and allowed to grow for 20 h in 500 μl medium. Thereafter, cells were exposed to pesticides (25 μM) for 72 h and morphological changes were analyzed using a phase contrast microscope (Olympus).
2.6. Evaluation of pesticide effects on cell monolayer integrity
2.6.1. Transepithelial electrical resistance (TEER) measurement
Cells were seeded at 2.5 × 105 cells/well in 6-well Transwell culture plate coated with collagen and allowed to grow for 4 days. Then 4% FBS-supplemented medium containing pesticides (1, 5, 10 or 25 μM) or DMSO (0.1%) were introduced in both apical and basolateral compartments. Treatment was renewed every 2 days. Monolayer integrity was measured at day 0, 3, 7 and 10 using the Millicell Electrical Resistance System (Millipore, Molsheim, France). Results are expressed as electrical resistance of the monolayer (Ω cm2) and were the mean ± SEM of 3 independent experiments in which each treatment was done in triplicate.
2.6.2. Phenol red diffusion
Caco-2 cells culture conditions were the same as described above. At last day of exposure (day 10), the culture medium was discarded and apical and basolateral sides were rinsed twice with 2 ml of PBS (37 °C). Two milliliters of phenol red containing medium were introduced in apical side of monolayer. In the basolateral side, phenol red containing medium was replaced by equivalent medium without phenol red. Diffusion of phenol red from apical to basolateral (A–B) compartment was evaluated for 4 h. Quantification of phenol red in the basal side was performed by measuring medium absorbance at 560 nm and percent diffusion was calculated.
2.7. Alkaline phosphatase essay for cell differentiation
Caco-2 cells were seeded into 12-well plates (1.125 × 105 cells/well). Next day cells were treated with pesticides (1, 5, 10, and 25 μM) or vehicle (DMSO 0.1%) for 10 days. Then cell layers were washed twice with 1 ml of cold NaCl 9‰ and harvested by scraping with a rubber policeman in 500 μl of cold TRIS buffer (50 mM, pH = 7.5). Cell suspensions were then homogenized by five passages through a 25G needle fitted with a 1 ml syringe. Alkaline phosphatase activity, marker of enterocytic cells differentiation [25], was measured in whole cell lysates according to Walter and Schütt [26] with modifications. Briefly, ALP splits paranitrophenyl-phosphate (pNPP), liberating yellow colored p-nitrophenol (pNP) under basic condition. Quantification of p-nitrophenol (μmoles per mg of proteins) was performed at 405 nm upon an incubation time of 30 min at pH 10.4 and 37 °C. Results are expressed as percent of ALP activity in treated cells compared to control. The total protein concentration was determined by BCA protein assay according to the manufacturer-suggested protocol.
2.8. Pesticide-induced oxidative stress assessment
2.8.1. Lipids peroxidation measurement
Caco-2 cells were seeded in 24-well plates (1.25 × 105 cells/well) and were treated as described above upon a 48 h-period of exposure with the indicated concentration of pesticides. Lipid peroxidation was quantified in cell medium by using the thiobarbituric acid reactive substances (TBARs) assay [27], which measures malondialdehyde (MDA) equivalents. Briefly, 50 μl of cell medium were mixed with 5 μl of 0.5 N chlorhydric acid (HCl) and 50 μl of TBA buffer (sodium hydroxide 0.12 M TBA, pH 7). Samples were then boiled for 10 min at 95 °C. After ice-cooling in dark, 200 μl of n-butanol were added, samples were mixed and centrifuged for 10 min at 2000 rpm at 4 °C, 150 μl of supernatant from each sample were transferred into 96-well plate and the absorbance was recorded at 532 nm. The TBARs content was calculated from a calibration curve by using TMOP as MDA precursor.
2.8.2. Antioxidant enzyme assays
Caco-2 cells were seeded in 6-well plates (1.5 × 105 cells/well) and were exposed next day to pesticides (1, 5, 10, and 25 μM) or vehicle as described above. At day 10, cells were harvested by scraping on ice. After centrifugation (900 rpm, 4 °C), cell pellets were washed twice with ice cold PBS and suspended in phosphate buffer (50 mM, pH = 7) supplemented with 1 mM EDTA. Cell membranes were disrupted using ultrasounds three times for 10 s on ice. After ultracentrifugation at 105,000 × g for 1 h at 4 °C, supernatants were used to determine the catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) activities. The total protein concentration of individual sample was determined by BCA protein assay kit according to the manufacturer-suggested protocol.
CAT activity was monitored according to the method described by Aebi [28]. Briefly, hydrogen peroxide enzymatic decomposition by sample catalase was measured for 30 s at 240 nm on a spectrophotometer. The GPx activity was determined according to the method developed by Lawrence and Burk [29]. Briefly, GPx reacts with H2O2 to generate glutathione disulfide (GSSG) which is reduced by glutathione reductase (GR) in the presence of NADPH. The disappearance of NADPH was monitored at 340 nm on a spectrophotometer as indirect measure of GPx activity. The SOD activity was determined following the method of Oberley and Spitz [30]. Briefly, xanthine–xanthine oxidase system in the presence of water generates the superoxide anion (O2•¯) which reacts with Nitroblue Tetrazolium (NBT) reducing it to Formazan dye. Sample SOD and NBT compete for O2•¯. The percent inhibition of NBT reduction for different protein concentrations of each sample was measured as the amount of SOD present in sample. One unit of SOD was defined as the amount of enzyme which inhibits by 50% the formation of formazan blue observed at 560 nm. Inhibition of NBT reduction was kinetically monitored for 5 min using Tecan® microplate reader. Specific enzymatic activities were reported as percent of activity in treated cells compared to control.
2.8.3. Intracellular ROS measurement
Intracellular ROS were measured according to the method described by Wang and Joseph [31] with or without pretreatment with several antioxidants (vitamin E, vitamin C or Trolox). Cells plated in 48-well dishes (5 × 104 cells/well) were allowed to grow for 48 h in complete medium supplemented with 10% FBS. Then cell layers were washed twice with PBS and treated for 6 h at 37 °C with 25 or 100 μM of pesticides (0.025% of DMSO as control cells) in HBSS medium containing 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCFDA 5 μM). At the end of the 6 h incubation, medium was removed and the cell layers washed twice with PBS. Cell fluorescence was measured with Tecan® microplate reader at the excitation and emission wavelength of 485 and 530 nm respectively.
2.9. Determination of pesticides-induced cell death mechanism
2.9.1. Detection of apoptosis and necrosis by Hoechst 33342 and PI staining
Caco-2 cells were seeded in 12-well plates at a density of 1.25 × 105 cells/well. After 20 h incubation, the cells were treated with 25 μM of pesticides alone or in combination for 48 h. At the end of the treatment the culture medium was discarded and the monolayer was stained with 5 μg/ml of Hoechst 33342 and 1 μg/ml of PI solution in the dark for 30 min at 37 °C. The cell layer was then washed with PBS. The morphological features of apoptosis, such as cellular nucleus shrinkage, chromatin condensation, and nuclear fragmentation, were showed via Hoechst staining in PI negative cells using fluorescent microscope with standard excitation filters. Necrotic cells were stained in red by IP. Each experiment was repeated independently three times in triplicate. To record the number of normal, apoptotic and necrotic cells by treatment, four random microscopic fields per well were considered. Each experiment was repeated three times in triplicate.
2.9.2. Western blot analysis of Akt
Cells were seeded at a density of 1.5 × 105 cells/well into 6-well plates and were exposed 20 h after incubation to 25 μM of each individual pesticide or mixture for 10 days. At the end of pesticide treatments, Caco-2 cells were washed several times in cold phosphate-buffered saline, were scraped off the plate and lysed in modified RIPA lysis buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% triton X-100, 5 mM EDTA pH 8.0) containing 1× protease inhibitor cocktail (HALT, Thermo scientific), and phosphatase inhibitor cocktail (1 mM NaVO4, 50 nM NaF), for 30 min on ice. Cell lysates were cleared by centrifugation (10,000 rpm, 15 min at 4 °C). Protein concentrations were determined using Bradford reagent (sigma). Twenty five micrograms (25 μg) samples of extracted protein were resolved on SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were incubated in the presence of 1/2000 phospho-Akt ser473 (Cell signaling, 4060) or 1/1000 Akt 1/2/3 (santa cruz biotechnology, sc-8312) antibodies at 4 °C overnight. Antibody binding was detected using an infrared fluorescent dye conjugated antibodies absorbing at 800 nm (Biotium, CF 770 conjugated antibodies, 20078). Immunoblots were visualized using an Odissey Infrared imaging scanner (Li-Cor, Science Tec, les Ulis, France). Relative fluorescence units allowed a quantitative analysis. Each experiment was done independently 3 times.
2.10. Data analysis of combined cytotoxic effects
Combined effects of pesticides were analyzed according to Takakura et al. [32]. The total concentration of mixture at which an effect is generated can be calculated on the basis of the concentration–response curves of individual pesticides using the CA concept according to the follow equation:where ECxmix is the total concentration of the cocktail provoking x% effect, ECxi the concentration of component i provoking the x% effect when applied singly, and Pi denotes the relative proportions of the total mixture concentrations.
The IA prediction concept is used to explicitly calculate combined effects according to:
E(Cmix) denotes the effect provoked by the total mixture at concentration Cmix, while E(Picmix) are the effects that the individual cocktail component i would cause if applied singly at the same concentration at which it is present in the cocktail.
2.11. Statistical analysis
Values are representative of the means ± SEM (standard error of the mean) of three independent experiments performed at least in triplicate. Statistical analyses were performed with GraphPad PRISM 5 (GraphPad Software Inc., San Diego, CA, USA). Comparisons have used one-way or two-way analysis of variance (ANOVA) followed respectively by Dunnett's and Bonferroni multiple comparison posttests. The level of significance was set at p < 0.05.
3. Results
3.1. Effect of pesticides on cell proliferation and viability
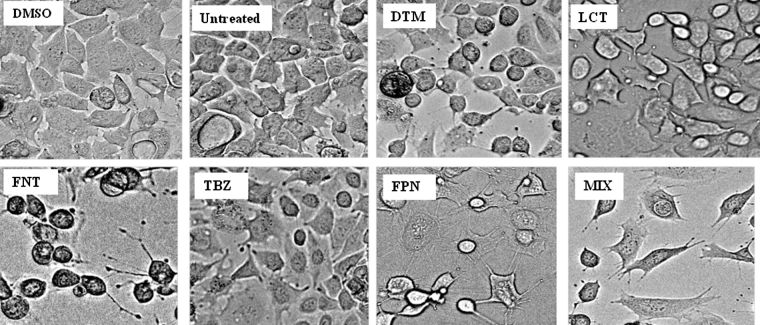
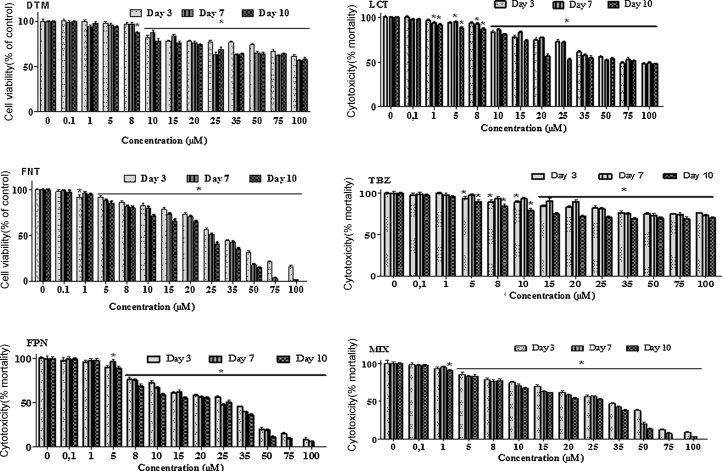
Cell proliferation and viability were assessed by measuring MTT reduction assay. Overall, microscopy observations of Caco-2 cells layer revealed that exposure to pesticides for 3, 7 and 10 days led to morphological changes compared to control cells (Fig. 1). Furthermore, as shown in Fig. 2, each pesticide alone or in mixture, exerted an impact on cell viability in a time and concentration dependent manner. Their observed toxicity (EC50 values of Table 1) in Caco-2 cells could be ranked in the following decreasing order: FPN > MIX > FNT > LCT > DTM > TBZ. FNT and TBZ toxicity was observed upon a 3 days-period of exposure to 1 and 5 μM respectively. The impact of exposure to LCT (1 μM), DTM (8 μM) or FPN (5 μM) appeared later (J7).
Fig. 1.
Pesticides-induced morphological changes in Caco-2 cells. Caco-2 cells were seeded in 12-well plates and allowed to grow for 20 h. Thereafter, cells were exposed to pesticides (25 μM) for 72 h and morphological changes were analyzed using Olympus inverted microscope. Images selected are representative of three independent experiments. Photographs were performed with a magnification of ×20.
Fig. 2.
Dose response curves of the impact of pesticides on Caco-2 cells viability. Cells were treated 20 h after seeding with the indicated pesticide alone or in combination at increasing doses of 0.1–100 μM. Treatments were repeated each 48 h. MTT test was performed on 3, 7 and 10 days old cells as described in Section 2. The data are normalized to the viability of control cells (100%). Results are the mean ± SEM of quadruplets from three repeated independent experiments. Asterisk indicates level of significance at p ≤ 0.05.
Table 1.
EC50 values (μM) of pesticides alone on Caco-2 cells upon 3, 7, and 10 days-period of exposure. Caco-2 cells were exposed to DTM, FNT, FPN, LCT, and TBZ either alone or as a mixture of the five for 3, 7 and 10 days at concentrations ranging from 0.1 to 100 μM. After exposure, cell viability was assessed by MTT assay and effect concentrations 50% (EC50) were calculated according to the dose response curves in Fig. 1 as described in Section 2.
| Time (days) | EC50 (μM)a |
||||
|---|---|---|---|---|---|
| DTM | FNT | FPN | LCT | TBZ | |
| 3 | 191.0 ± 5.5 | 31.6 ± 0.7 | 24.1 ± 0.4 | 72.9 ± 2.7 | 571.1 ± 51.0 |
| 7 | 136.4 ± 10.7 | 25.6 ± 0.8 | 21.6 ± 0.5 | 74.3 ± 4.8 | 471.3 ± 37.6 |
| 10 | 111.9 ± 3.3 | 21.3 ± 0.5 | 18.1 ± 0.5 | 59.5 ± 2.3 | 429.4 ± 24.5 |
Mean ± SEM of three independent experiments.
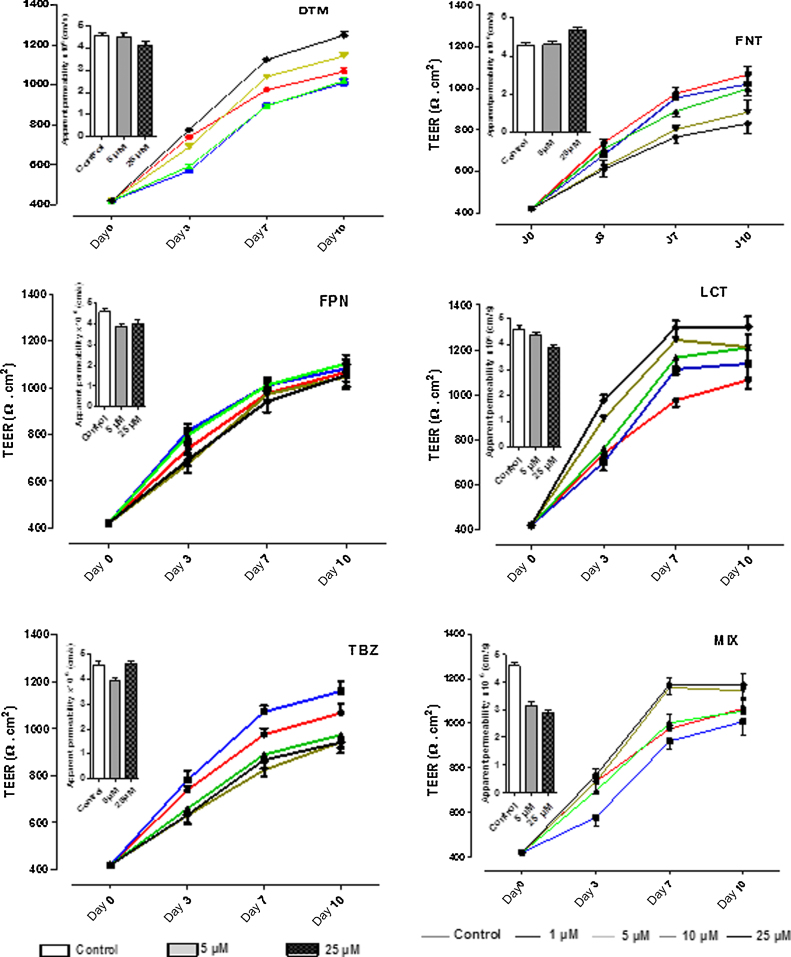
3.2. Effect of pesticides on cell monolayer integrity
Impact of pesticides alone or in combination on the cell monolayer integrity was assessed by analyzing, along a 10 days-period of treatment, the TEER (Ω cm2) (Fig. 4) and the passage of phenol red across the cell monolayer (Fig. 4, inset figures). Control cells, treated with the vehicle alone, showed a continuously increase of TEER until the end of the treatment (from 420.00 ± 15.14 to 1010.30 ± 39.96 Ω cm2 at day 10, Fig. 4). Our results showed that exposure to FNT or TBZ led to a net decrease of membrane resistance associated with an increase of the apparent permeability of the cell layer (Fig. 4). On the other hand, exposure of Caco-2 cells to LCT, DTM, and MIX induced a significant increase of membrane resistance associated with a decrease of membrane permeability suggesting a net reinforcement of cellular integrity. Treatment of Caco-2 cells with FPN failed to produce a clear and noticeable change in TEER values relatively to control. We next checked whether the observed changes in viability and cell layer integrity upon exposure to pesticides could be linked to a change in the cellular differentiation process of Caco-2 cells.
Fig. 4.
Effect of pesticides on cell layers integrity. Caco-2 cells were allowed to grow on permeable membrane filter supports in presence of pesticides at concentrations ranging from 1 to 25 μM for 10 days. Transepithelial electrical resistance (TEER) was measured at days 0, 3, 7, and 10 following proceeding described in Section 2 and its value is expressed as percent of control. In parallel, phenol red passage through cell layer was assessed at day 10 (inset) as described by Section 2. Both TEER and PR diffusion values are expressed in means ± SEM of 3 independent experiences done in triplicate. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
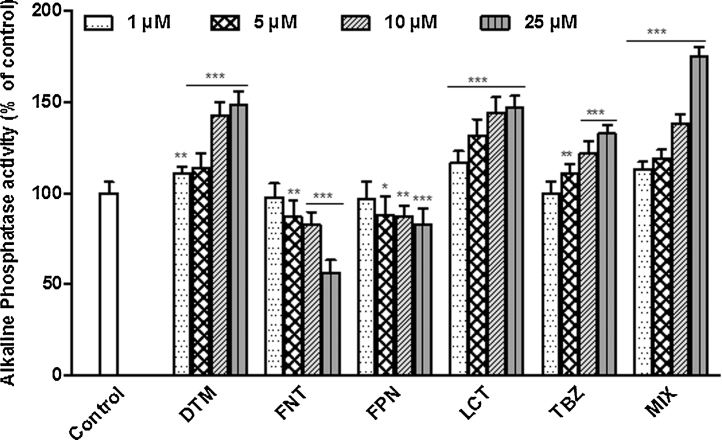
3.3. Effect of pesticides on alkaline phosphatase activity
Caco-2 cells differentiation was characterized by domes formation and cells polarization with apical brush border bearing specific enzymes. Differentiation of non-treated Caco-2 cells was accompanied with a graduate increase of ALP activity (Fig. 5) culminant at day 10. Our results showed a clearly dose dependent perturbation of the enzyme activity upon exposure to pesticides alone or in combination. FNT and FPN exposure of Caco-2 cells led to a decreased ALP activity compared to control whereas the opposite was observed upon exposure to DTM, LCT, TBZ, or MIX.
Fig. 5.
Effect of pesticides on Caco-2 cells differentiation. Cells were exposed to single or mixture of pesticides at concentrations ranging from 1 to 25 μM for 10 days. Cell differentiation was evaluated by measuring ALP activity as described in Section 2. Data are expressed as the percentage of ALP activity in treated compared to control cells (exposed to the vehicle only DMSO) and are the mean ± SEM of three independent experiences in which each treatment was performed in triplicate. Asterisk indicate level of significance at p < 0.05 (*), p < 0.01 (**), p < 0.001 (***).
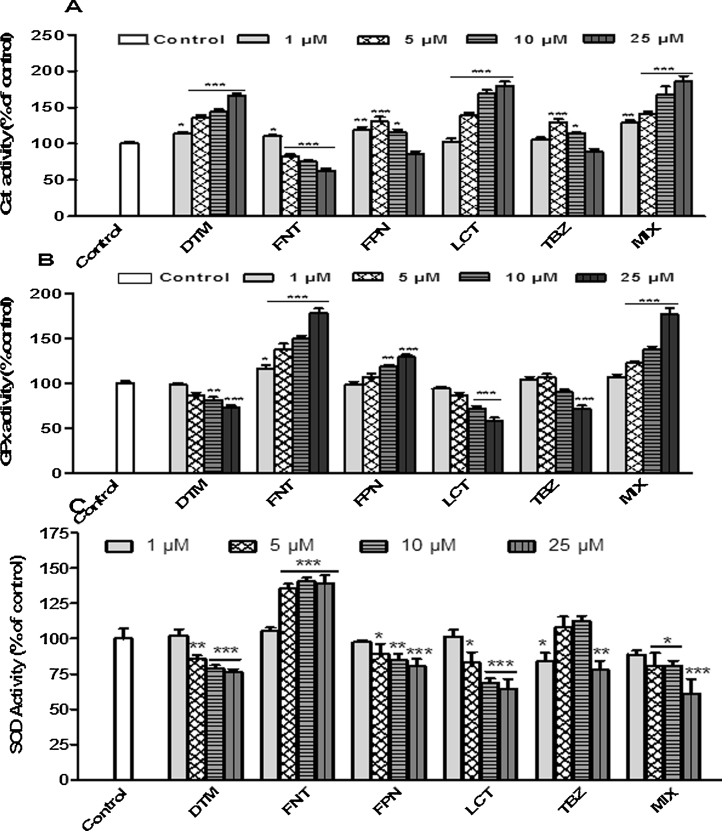
3.4. Pesticides-induced oxidative stress in Caco-2 cells
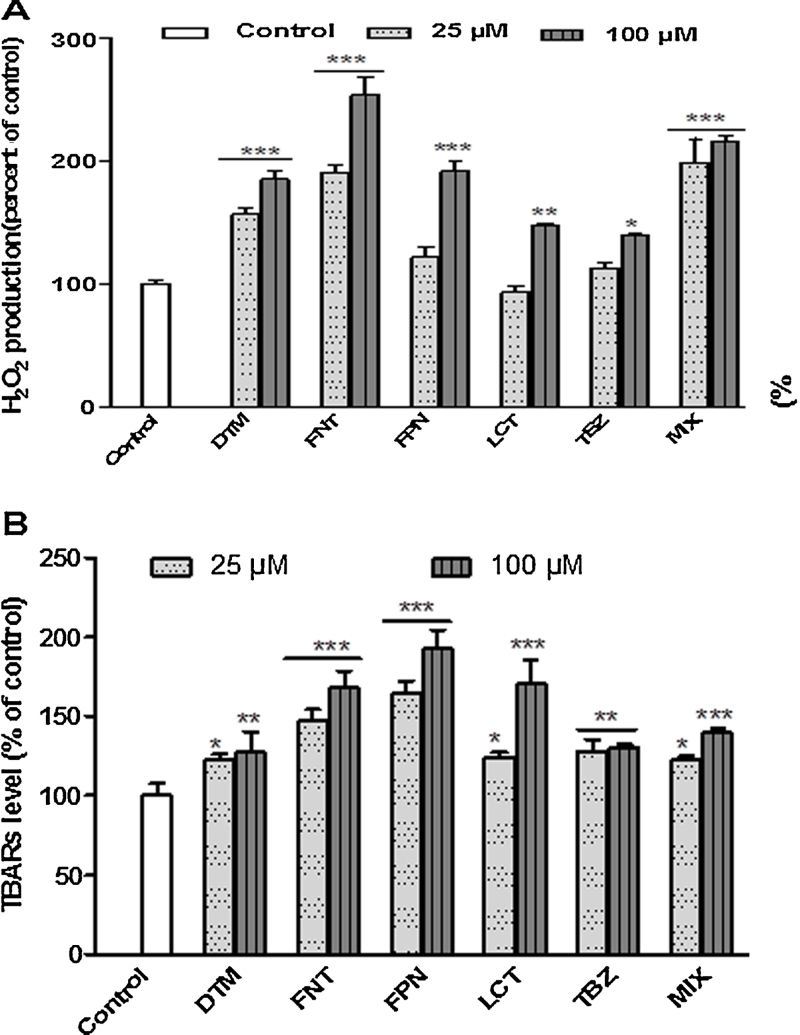
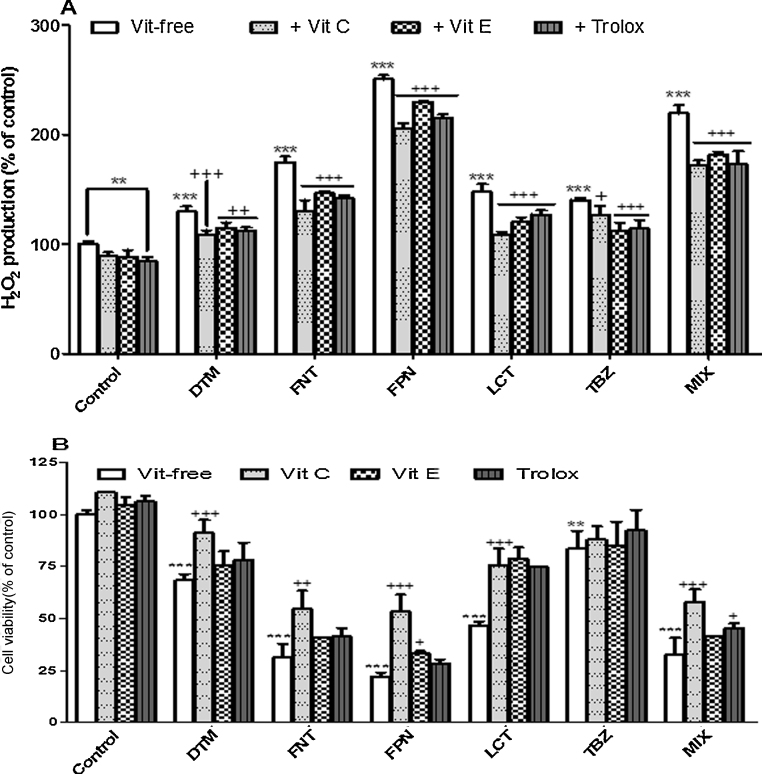
Oxidative status in Caco-2 cells upon exposure to pesticides was investigated by measuring the activities of the main enzymes involved in the control of ROS level (CAT, GPx, and SOD). As shown in Fig. 6, a 10 days-period of treatment of Caco-2 cells with pesticides alone or in mixture (1, 5, 10, and 25 μM) induced in a dose dependent manner an increase in CAT (DTM/LCT/MIX), GPx (FNT/FPN/MIX), and SOD (FNT) activities suggesting a change in oxidative status of Caco-2 cells upon exposure to these compounds (Fig. 6A–C). The increased SOD activity upon exposure to FNT suggests a downstream production of O2•−. The activation of GPx or CAT observed upon exposure to FNT, FPN, DTM, LCT, and Mix suggested an increase in level of H2O2. On the other hand TBZ did not appear to induce noticeable changes in oxidative status since no change in enzyme activity was observed. In order to confirm the prooxidative activities of pesticides in Caco-2 cells we next evaluated H2O2 production in cells by using specific fluorescent probe. As showed in Fig. 7A, FPN, FNT, DTM, and MIX treatments induced the strongest effect on intracellular H2O2 generation evidenced by the increased fluorescence staining. These results were well correlated with the increased activity of CAT and GPx as described above. We also determined the lipid peroxidation status by examining the TBARs concentrations upon a short term period of exposure (48 h) to pesticides (25 and 100 μM). Results clearly indicated a significant increase of TBARS level upon exposure to each pesticide alone or in mixture. FPN and FNT were found to be the most efficient with respectively 192.45 and 167.66% with respect to control at 25 μM (Fig. 7B). Furthermore, pretreatment with 100 μM of Vitamin C for 4 h or 10 μM of Vitamin E or Trolox for 24 h before exposure of Caco-2 cells to pesticides partially reversed the production of H2O2 (Fig. 8A) and led to a net decrease of pesticide cytotoxicity specially in Caco-2 cells exposed to the most cytotoxic compounds DTM, FPN, FNT, LCT, and MIX (Fig. 8B). These results suggest that oxidative stress could be at least in part involved in Caco-2 cells death induced by these pesticides treatment.
Fig. 6.
Effects of pesticides on the activity of antioxidant enzymes in Caco-2. Cells were exposed for 10 days to pesticides individually and in mixture at concentrations ranging from 1 to 25 μM. Catalase (A), GPx (B), and Cu/Zn SOD (C) activities were measured in cytosolic fractions of cell lysates as described in Section 2. Data are expressed as the ratio of the activity in treated cells compared to control cells (DMSO). Results are the mean ± SEM for 3 independent experiments where each treatment was done in triplicate. Statistical differences are shown as *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 7.
Effects of pesticides exposure on lipid peroxidation and on the level of hydroxide peroxide in Caco-2. Cells were exposed for 6 and 48 h (lipid peroxidation and H2O2 dosage respectively) to FPN, FNT, LCT, DTM, and TBZ either alone or as a mixture of the five at 25 and 100 μM. (A) H2O2 production was measured using H2-DCF-DA. (B) Lipid peroxidation was assessed by TBARs quantification in the incubation medium. Data are expressed as the ratio of fluorescence in treated cells compared to untreated cells (DMSO exposed). Results are the mean ± SEM for 3 independent experiments in which each treatment were done in triplicate. Difference is statistically significant for p ≤ 0.05 (*), p ≤ 0.01 (**), and p ≤ 0.001 (***).
Fig. 8.
Effects of antioxidant on H2O2 production and promotion of cell viability. Twenty hours after seeding, Caco-2 cells were pretreated with Vitamin C (4 h at 100 μM) and Vitamin E or Trolox (10 μM for 24 h). Cells were then exposed to pesticides at 100 μM for 6 h in the presence of H2-DCF-DA (H2O2 dosage) or 72 h (cell viability assay). H2-DCF-DA fluorescence (A) and cells viability (B) were assessed as described in Section 2. Data represent the ratio of the value in treated cells compared to untreated one (DMSO). Results are the mean ± SEM for 3 independent experiments in which each treatment was done in triplicate. Asterisk indicate level of significance at p < 0.05 (*), p < 0.01 (**), p < 0.001 (***) for comparison between pesticide-treated results and untreated control one. Statistical differences between pesticide-treated results in presence and without antioxidant are shown as +for p < 0.05, ++for p < 0.01, and +++for p < 0.001).
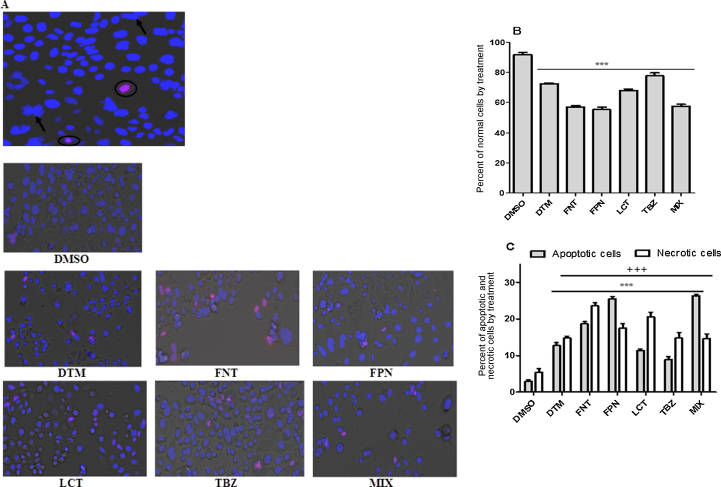
3.5. Pesticides-induced cell death mechanism on Caco-2 cells
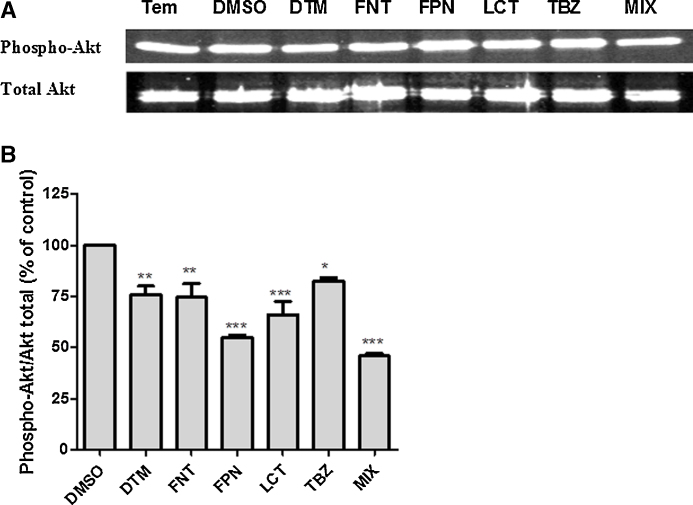
To elucidate the mechanism of pesticide-induced cell death in Caco-2 cells, we performed a double staining of cell layer with Hoechst 33342 and PI followed by a microscopic observation. Cells exhibiting a condensed nuclei and no PI staining could be classified as apoptotic whereas cells stained with both Hoechst and PI could be considered as necrotic cells (Fig. 9A). Results showed an increased of cell death through both apoptotic and necrotic mechanisms (Fig. 9B and C). It is noteworthy that only FPN and mixture treatments led to a huge increased of apoptotic whereas the percentage of necrotic cells increased to a lesser extent. In order to confirm apoptosis in cells exposed to pesticides we next examine the level of expression of the active form of Akt (phospho-Akt), a key cell signaling enzyme involved in the inhibition of apoptosis and induction of cell proliferation. Results showed a reduced level of phospho-Akt in cells exposed to pesticides especially in FPN and MIX exposed cells compared to control (Fig. 10A and B) confirming the role of apoptosis at least in part in the mechanism of pesticide induced cell death in our model.
Fig. 9.
Impact of pesticides alone or in combination on Caco-2 cells death. Cells were treated with pesticides (25 μM) for 48 h. At the end of the incubation period, cells were labeled with Hoechst 33342 and IP for 30 min. Cell layer was then observed using fluorescence microscope to record normal viable (uncondensed nuclei and no PI staining), apoptotic (condensed nuclei and no PI staining), and necrotic cells (uncondensed nuclei and PI staining). (A) Shows the image of stained cells indicating necrotic (encircled) and apoptotic cells. Photographs were performed with a magnification of ×20. (B) Shows the percentage of nonapoptotic nor necrotic cells in treated and untreated assays, and (C) shows the percentage of apoptotic and necrotic cells. Statistical differences with untreated result are shown as ***p < 0.001 (normal/apoptotic cells) and +++p < 0.001 (necrotic cells).
Fig. 10.
Mechanism of pesticide induced cell death. One day after seeding, cells were exposed for 48 h to pesticide (DTM, FNT, FPN, LCT, and TBZ) alone or in combination (MIX) (25 μM). Control corresponds to cells treated with the vehicle only (DMSO 0.1%). (A) Shows the western blot analysis of total and phospho-Akt (Ser473). (B) Represents the ratio Phospho-Akt/Total-Akt expressed as the percentage of the control. Results are the mean ± SEM of three independent experiments in triplicate. Statistical differences are shown as *p < 0.05, **p < 0.01, ***p < 0.001.
3.6. Comparison of the observed effect versus the predicted effect of pesticide mixture
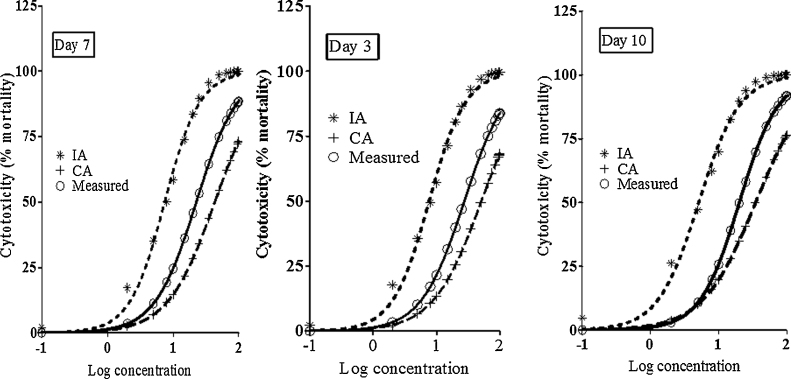
The experimentally measured effect of pesticides mixture on cell viability (Fig. 1) was compared with the predicted effect calculated using CA and IA models from the impact of single pesticide. Results are presented in Table 2. The 95% CI from the measured EC50 failed to overlap the EC50 obtained in the predicted model at each tested dose (Table 2 and Fig. 3). These results suggest that the combined toxicological effects of the 5 pesticides resulted from complex interactions different from that observed in independent or dose addition situation.
Table 2.
Statistical comparison between measured and predicted combined effect concentrations (EC50). Caco-2 cells were exposed to the mixture of the five for 3, 7 and 10 days at concentrations ranging from 0.1 to 100 μM. After exposure, cell viability was assessed by MTT assay and predicted effect concentrations 50% (EC50) were calculated according to Section 2. IA, independent action model; CA, concentration addition; CI, confidence interval.
| Time (days) | Measured |
IA |
CA |
|||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| 3 | 27.5 | 26.1–29.0 | 7.7 | 7.4–8.0 | 51.3 | 51.0–51.5 |
| 7 | 22.6 | 21.5–23.8 | 7.5 | 7.2–7.8 | 43.3 | 42.8–43.8 |
| 10 | 20.1 | 19.1–21.2 | 5.1 | 4.9–5.4 | 35.1 | 34.9–35.3 |
Fig. 3.
Comparison between the measured and predicted effects of the mixture of pesticides. The measured effects of the mixture were assessed by analyzing the dose–response curves of its cytotoxicity determined by the MTT assay after a 3, 7, and 10 days-period of treatment in Caco-2 cells as described in Section 2. Cytotoxicity is expressed as the mean ± SEM of three independent experiments in quadruplet. The combined effects of pesticides were also predicted using an independent action (IA) and a concentration addition (CA) models as described in Section 2.
4. Discussion
In Burkina Faso, as in most Sahelian countries, the failure to follow good agricultural practices (GAP) coupled with poor soil and climate conditions in the locust control context lead to high environmental contaminations with pesticide residues [10]. Consumers being orally exposed to a combination of multiple pesticide residues through food and water intake, the digestive tract is thus a tissue susceptible to be directly exposed to these food contaminants mixtures. The aim of our work was to compare in vitro the impact of five desert locust control pesticides (DTM, FNT, FPN, LCT, and TBZ) commonly found in food and water in Burkina Faso alone and in combination on the human intestinal Caco-2 cells viability and function. Pesticides concentrations ranges were chosen according to their LMR and adapted to the in vitro study as described in material and method.
Our results which are summarized in Table 3 clearly showed a cytotoxic effect of DTM, FNT, LCT, and TBZ alone or in combination in human intestinal Caco-2 cells at dose ranging from 1 to 8 μM (1 μM being equivalent to 0.7–1.3 μg/well according to the pesticide). These doses could be considered as relevant to human exposure. Indeed based on the LMR values of each compound, pesticides could be present in the digestive tract at dose ranging from 0.2 to 300 μg, when considering a total assimilation of the LMR amount of pesticide in vivo upon a dietary intake of 100 g of vegetables.
Table 3.
Summary of the effect of pesticides alone or in combination on cell viability and transepithelial membrane resistance (TEER), apparent permeability (phenol red diffusion) and alkaline phosphatase activity (ALP) at J10, on lipid peroxidation and H2O2 production (upon 48 and 6 h, respectively) and on the ration of the percentage of apoptotic or necrotic cells in treated versus untreated cells (48 h). In control cells (DMSO treated cells) the ratio of the apototic cell percentage versus necrotic cell percentage was equal to 0.5.
| Cell viability (J10)* | TEER (J10) | Apparent permeability (J10) | ALP activity (J10)** | Lipid peroxidation** | H2O2 production* | Percent of apoptosis in treated vs control cells | Percent of necrosis in treated vs control cells | |
|---|---|---|---|---|---|---|---|---|
| Deltamethrin | ↘ | ↗ | ↘ | ↗ | ↗ | ↗ | 4.3 | 2.7 |
| 8 μM | 10 μM | 25 μM | 1 μM | 100 μM | 25 μM | |||
| Fenitrothion | ↘ | ↘ | ↗ | ↘ | ↗ | ↗ | 6.3 | 4.4 |
| 5 μM | 10 μM | 25 μM | 5 μM | 25 μM | 25 μM | |||
| Fipronil | ↘ | ↔ | ↘ | ↘ | ↗ | ↗ | 8.6 | 3.2 |
| 5 μM | 5 μM | 10 μM | 25 μM | 100 μM | ||||
| Teflubenzuron | ↘ | ↘ | ↗ | ↗ | ↗ | ↗ | 3.9 | 3.8 |
| 5 μM | 5 μM | 25 μM | 5 μM | 25 μM | 100 μM | |||
| Lambda cyalothrin | ↘ | ↗ | ↘ | ↗ | ↗ | ↗ | 3.0 | 2.7 |
| 1 μM | 25 μM | 25 μM | 1 μM | 100 μM | 100 μM | |||
| Mixture | ↘ | ↗ | ↘ | ↗ | ↗ | ↗ | 8.9 | 2.7 |
| 1 μM | 10 μM | 5 μM | 1 μM | 100 μM | 25 μM |
p < 0.05.
p < 0.01.
The most cytotoxic compounds were FPN and FNT that impacted the cell layer integrity, ALP activity, oxidative status, Akt activation, and apoptosis (data summarized in Table 3). These results are observed for the first time in a human intestinal cell line (excepted FPN) and are in agreement with their observed cytotoxicity in other in vitro models. Cytotoxicity of DTM was indeed observed in cortical neurons [33], in rat spermatozoa [34] and in SH-SY5Y cells [35]. FNT was shown to exert cytotoxic effect in rat hepatocytes [36]. FPN has been shown to display toxic effect in SH-SY5Y cells [37], [38], [39], in mouse N2a neuroblastoma [40]. LCT exerted cytotoxic effect in human lymphocytes [41].
The alteration of the cell layer integrity which increased with the time of exposure to pesticides, was characterized by either a decrease in TEER with a proportional increase of the passage of PR (FNT and TBZ), or a rise in TEER with a reduced passage of PR from the apical to the basal side of the epithelium (DTM and LCT). It is well admitted that free radicals may directly damage cell membrane through the oxidation of Poly Unsaturated Fatty acids within the phospholipids structure of the membrane itself and thus alter the membrane resistance or permeability. However, the differential effect of DTM/LCT on one hand and of FPN and TBZ on the other hand on TEER in our model cannot be explained by their pro-oxidative properties because it is an almost universal property of pesticides. The differential impact between DTM and FNT on TEER may be due to their specific activity on cytoskeleton and membrane fluidity respectively. Indeed FTN is known to alter membrane fluidity and as suggested by Gonzales-Baro et al. [42] it is possible that the toxic properties of FNT could be related to an increased permeability of membranes. DTM effect on TEER may be linked to its impact on the expression of a transcriptional factor involved in the expression of genes related to the cytoskeleton organization (NFATC1/cathepsin/c-SRC) [43]. FPN treatment that induced oxidative stress in our model and the highest cytotoxicity (EC50 = 18.06 μM) led to changes in the apparent permeability to phenol red without altering the TEER. This is in agreement with the hypothesis formulated by Karczewski and Groot [44] according to which an alteration of permeability can occur without noticeable change in electrical resistance.
As suggested by Sambuy et al. [22], the alteration of the permeability and the integrity of the cell monolayer suggest perturbation of the intestinal barrier function. Modification of the epithelium integrity is considered as a critical toxicokinetic parameter that influences the fate and consequently the systemic toxicity of chemicals in the organism. Previous findings have shown the involvement of tight junction's failure and cytoskeleton disorganization in chemicals induced-cell monolayer permeability alteration [45]. Oxidative stress is also known to disturb intestinal and renal epithelium or blood brain barrier integrity by altering tight junction molecules [8], [9], [46]. In the present study, pesticides-induced functional alteration of the epithelial Caco-2 cell layer was well correlated with the prooxidative activity of these chemicals.
In parallel our results showed a decreased activity of ALP in cells exposed to FPN and FNT, while exposure to DTM, LCT, and TBZ, increased its activity. ALP is a marker of intestinal cell differentiation and is also involved in lipid absorption across the apical membrane of enterocytes, in regulation of duodenal surface pH, in bacterial transepithelial passage limitation, and in detoxification of intestinal lipopolysaccharide [47]. The decreased ALP activity in Caco-2 cells exposed to FPN and FNT could be correlated with their cytoxicity. The involvement of oxidative stress in the regulation of ALP activity has also been demonstrated [48], [49]. It may be indeed suggested that pesticide induced Caco-2 cells toxicity and ALP activity disturbance could be linked to their prooxidative properties.
Exposure of Caco-2 cells to individual pesticides in our model also led to a decreased adaptive capacity of cells suggested by a time dependent diminution of the EC50 values of individual compound. Moreover morphological changes as well as a reduction of cell density upon exposure to FNT, FPN, and MIX suggested that cells did not reach confluence. Although the induction of cell cycle arrest or increased susceptibility of dividing cells upon pesticide exposure has been shown in various cell models [50], [51], [52], the increased number of apoptotic and necrotic cells upon exposure to pesticides alone or in combination in our model (summarized in Table 3) supported the hypothesis that these compounds affected rather the cell viability processes than the cycle progression. Our results are in agreement with previous studies showing proapoptotic property of FPN and DTM [33], [37], [39]. Here we also showed that exposure to each individual pesticide is associated with a decreased level of phosphorylated Akt (Ser 473), FPN and LCT leading to the greatest impact, confirming the start-up of an apoptotic mechanism [53], [54] upon exposure to each compound. However from our results it appears that apoptosis was not the one mechanism involved in cell death induced by pesticides since the percent of necrotic cells was also increased. Moreover our data showed that FPN and to a lesser extent DTM and FNT were stronger apoptotic compounds than LCT and TBZ suggesting dissimilar mechanism of action of these pesticides.
From our overall data summarized in Table 3, DTM and LCT which belong to the same chemical family (pyrethroid), appeared to share the same mechanism of action in the induced Caco-2 cells toxicity, although DTM was more pro apoptotic than LCT (Table 3). On the opposite FPN, FNT and TBZ showed differential impacts on Caco-2 cells compared from each other. FNT and TBZ differentially affected ALP activity, and FPN did not affect TEER and exerted clearly the strongest apoptotic effect with minor changes of necrotic cell number compared to control.
We also investigated the joint effects of the five pesticides on various cell functions by testing a mixture of pesticides in which each compound was present at equimolar concentration. Although the mixture diluted the most toxic compounds when combined, pesticides led to a higher effect than that expected regarding to the effect of each single compound suggesting a synergistic interaction of the compounds. The mixture induced a dose dependent cytotoxicity characterized by cells death according to apoptotic and necrotic mechanisms, the disturbance of TEER values, the increase of PAL activity, an alteration of oxidative stress enzymes activity, and an increased lipid peroxidation. When comparing the effect of the mixture with its predicted theoretical effect our results pointed out a difference between the measured impact of the mixture and the theoretically calculated one. In the analysis of combined effects two reference concepts are currently applied for the assessment of mixture toxicities, i.e. the Loewe additivity (based on the dose addition (CA) concept) and the Bliss independence (based on the independent action (IA) concept) [55], [56]. They have been widely applied to predict the joint toxicity of mixtures [19], [32], [57], [58]. Evaluation of the effects associated with simultaneous exposure to chemical contaminants has to rely on suitable assessment concepts, but it is not immediately obvious which of the two models, CA or IA, should be employed (according to Ermler et al. [15]). The CA model is suitable for predicting the combined toxicity of mixtures with compounds sharing the same mechanism of action (inhibition of cholinesterases by organophosphorus for example). For this type of mixture, the prediction is reliable. The IA model is meanwhile indicated in predicting the combined toxicity of mixtures whose constituents do not share the same mechanism of action (strictly distinct mechanisms of action). As already underlined by Rajapakse et al. [59], the difference found in our model between the predicted and the observable effect of the mixture is materialized on one hand by the shift of the observed and predicted concentration–effect relationship curves, and on the other hand by the lack of an overlap between the measured and the predicted 95% CI of the dose response. Such deviations are currently observed since the predicted toxicity by the IA and CA concept can deviate by a factor n from the observed values, n being the number of mixture components [60]. Furthermore, a deviation less than twofold has been generally applied as a threshold which shows a correlation between predicted and observed mixture toxicity [61], [62]. In our study using the CA concept we observed a deviation factor of 1.87, 1.92, and 1.75 at day 3, 7, and 10, respectively. Whereas a factor of 3.57, 3.01, and 3.94 was found at the same time applying the IA concept (data not show). We can thus presume that CA concept could be considered as a more accurate model than the IA concept to predict the effect of the combined pesticides in our study. However although the similarity or dissimilarity of the mechanisms of action of the mixture components are the governing factor for the prediction quality of both CA and IA concepts it is assumed that CA overestimates the mixture toxicity of strictly dissimilar acting substances, and IA underestimates the toxicity of strictly similar acting substances [60]. Ermler et al. [15] also reported that the assessment of chemical mixtures composed of substances that do not act via the same mechanism is more complicated. Rider et al. [63] have recently presented an analysis of their own mixture studies with anti-androgens where they investigated the effects of in utero exposures on male reproductive tract development in rats. CA predicted the mixture effects better than IA, even with mixtures composed of anti-androgens with diverse mechanisms of action. IA consistently underestimated the effects of these anti-androgen mixtures. Pesticides from a specific chemical class (organophosphorus, organochlorine, etc.) could induce similar phenomenological effects, and such similarity of action would support the choice of CA as an assessment concept, irrespective of the finer details of molecular mechanisms of the compounds. In this case the similarity assumption of CA could not entirely be met, because of subtle differences in the ways in which the chemicals in the component mixtures interacted at the cellular and molecular level. Moreover fewer compounds can be expected to qualify for inclusion in a common mixture/effects assessment and this may lead to underestimation of the risk [15]. In the light of our results and in agreement with the conclusions of Junghans et al. [60] and Ermler et al. [15], we can make the following assumptions: (i) the mixture effect could be due to dissimilarly acting components since the toxicity of mixture assessed using IA concept is higher than the measured toxicity; (ii) in the mixture, compounds interact together leading to a mixture toxicity that is higher than the predicted effect by CA.
In conclusion, locust control that generates environmental and subsequent food commodities and water contamination with pesticides mixture is a public health concern. In our study, we clearly showed that pesticides often found in water and vegetables in Burkina Faso damage human intestinal cells in culture individually or in mixture at dose closed to the real exposure, by modifying cellular growth, survival and homeostasis suggesting subsequent disorders of the intestinal epithelium. Moreover, from the comparison of our measured and predicted results it appears clearly that CA model is the most suitable for predicting interaction of pesticide mixture in our model; however it does not correlate with the observed synergistic effect which suggests dissimilar acting substance. Our in vitro experiments serve the purpose to highlight the need for further evaluation of the in vivo toxicity of chemical mixture.
Conflict of interest
The authors declare that they do not have any conflict of interest.
Transparency document
Acknowledgment
This study was supported by a grant from the French Ministry of Foreign Affairs (Grant No. 745249G) through the SCAC (Service de Coopération et d’Action Culturelle) of French Embassy in Burkina Faso.
Footnotes
Available online 25 July 2014
References
- 1.Lecoq M. Vers une solution durable au problème du criquet pèlerin? Sécheresse. 2004;15(3):217–224. [Google Scholar]
- 2.Jeyaratnam J. Acute pesticide poisoning: a major global health problem. World Health Stat. Q. 1990;43(3):139–144. [PubMed] [Google Scholar]
- 3.Ouédraogo .M., Tankoano A., Ouédraogo T.Z., Guissou I.P. Étude des facteurs de risques d’intoxications chez les utilisateurs de pesticides dans la région cotonnière de Fada N’Gourma au Burkina Faso. Environ. Risques Santé. 2009;8(4):343–347. [Google Scholar]
- 4.Arias A.H., Buzzi N.S., Pereira M.T., Marcovecchio J.E. Pesticides reaching the environment as a consequence of inappropriate. In: Stoytcheva M., editor. Agricultural Practices in Argentina, Pesticides – Formulations, Effects, Fate. InTech; 2011. ISBN: 978-953-307-532-7. Available from: http://www.intechopen.com/books/pesticides (accessed 30.05.14) [Google Scholar]
- 5.Salako A.A., Sholeye O.O., Dairo O.O. Beyond pest control: a closer look at the health implication of pesticides usage. J. Toxicol. Environ. Health Sci. 2012;4(2):37–42. [Google Scholar]
- 6.Mostafalou S., Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol. Appl. Pharm. 2013;268:157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Tebourbi O., Sakly M., Ben Rhouma K. 2011. Molecular Mechanisms of Pesticide Toxicity Pesticides in the Modern World – Pests Control and Pesticides Exposure and Toxicity Assessment. www.researchgate.net/ (accessed 30.05.14) [Google Scholar]
- 8.Lee H.S., Namkoong K., Kim D.H., Kim K.J., Cheong Y.H., Kim S.S., Lee W.B., Kim K.Y. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc. Res. 2004;68(3):231–238. doi: 10.1016/j.mvr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto K., Oshima T., Tomita T., Kim Y., Matsumoto T., Joh T., Miwa H. Oxidative stress induces gastric epithelial permeability through claudin-3. Biochem. Biophys. Res. Commun. 2008;376:154–157. doi: 10.1016/j.bbrc.2008.08.140. [DOI] [PubMed] [Google Scholar]
- 10.Ilboudo S., Toe A.M., Ouedraogo R., Ouedraogo M., Guissou I.P. Ecological risk assessment of pesticide residues in water from desert locust area in Burkina Faso. Res. Res. J. Environ. Earth Sci. 2014;6(4):227–232. [Google Scholar]
- 11.Islam A., Farrukh M.A., Rahman A., Qureshi F.A., Ahmed S. Residue analysis of an organophosphate pesticide in wild plants in Lahor area. Am. Eurasian J. Agric. Environ. Sci. 2010;9(5):514–518. [Google Scholar]
- 12.Hernández-Borges J., Cabrera C.J., Rodríguez-Delgado M.A., Hernández-Suárez E.M., Saúco V.G. Analysis of pesticide residues in bananas harvested in the Canary Islands (Spain) Food Chem. 2009;113:313–319. [Google Scholar]
- 13.Knezevic Z., Serdar M. Screening of fresh fruit and vegetables for pesticide residues on Croatian market. Food Control. 2009;20:419–422. [Google Scholar]
- 14.Kortenkamp A., Michael F., Martin S., Thomas B. Low-level exposure to multiple chemicals: reason for human health concerns? Environ. Health Perspect. 2007;115(1):106–114. doi: 10.1289/ehp.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ermler S., Scholze M., Kortenkamp A. The suitability of concentration addition for predicting the effects of multi-component mixtures of up to 17 anti-androgens with varied structural features in an in vitro AR antagonist assay. Toxicol. Appl. Pharm. 2011;257(2):189–197. doi: 10.1016/j.taap.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Moser V.C., Padilla S., Simmons J.E., Haber L.T., Hertzberg R.C. Impact of chemical proportions on the acute neurotoxicity of a mixture of seven carbamates in preweanling and adult rats. Toxicol. Sci. 2012;129(1):126–134. doi: 10.1093/toxsci/kfs190. [DOI] [PubMed] [Google Scholar]
- 17.Rouimi P., Zucchini-Pascal N., Dupont G., Razpotnik A., Fouché E., De Sousa G., Rahmani R. Impacts of low doses of pesticide mixtures on liver cell defence systems. Toxicol. In Vitro. 2012;26(5):718–726. doi: 10.1016/j.tiv.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Demur C., Métais B., Canlet C.M., Tremblay-Franco M., Gautier R., Blas-Y-Estrada F., Sommer C., Gamet-Payrastre L. Dietary exposure to a low dose of pesticides alone or as a mixture: the biological metabolic fingerprint and impact on hematopoiesis. Toxicology. 2013;308:74–87. doi: 10.1016/j.tox.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Hernández A.F., Parrón T., Tsatsakis A.M., Requena M., Alarcón R., López-Guarnido O. Toxic effects of pesticide mixtures at a molecular level: their relevance to human health. Toxicology. 2013;307:136–145. doi: 10.1016/j.tox.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Eisenbrand G., Pool-Zobel B., Baker V., Balls M., Blaauboer B.J., Boobis A. Methods of in vitro toxicology. Food Chem. Toxicol. 2002;40:193–236. doi: 10.1016/s0278-6915(01)00118-1. [DOI] [PubMed] [Google Scholar]
- 21.Zucco F., Batto A.F., Bises G., Chambaz J., Chiusolo A., Consalvo R. An inter-laboratory study to evaluate the effects of medium composition on differentiation and barrier function of Caco-2 cell lines. Altern. Lab. Anim. 2005;33:603–618. doi: 10.1177/026119290503300618. [DOI] [PubMed] [Google Scholar]
- 22.Sambuy Y., De Angelis I., Ranaldi G., Scarino M.L., Stammati A., Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 23.Le Ferrec E., Chesne C., Artusson P., Brayden D., Fabre G., Gires P. In vitro models of the intestinal barrier. The report and recommendations of ECVAM workshop 46. Altern. Lab. Anim. 2001;29:649–668. doi: 10.1177/026119290102900604. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 25.Singh V.K. Department of Zoology, Dr B.R. Ambedkar University; Agra: 1996. Response of liver and serum phosphatases to pyrethroid and synergize pyrethroid in Rattus norvegicus. (M.Phil. dissertation) [Google Scholar]
- 26.Walter K., Schütt C. In: 2nd ed. Bergmeyer H.U., editor. vol. II. Academic Press, Inc.; New York: 1974. pp. 860–864. (Methods of Enzymatic Analysis). [Google Scholar]
- 27.Van Ye T.M., Roza A.M., Pieper G.M., Henderson J.J., Johnson C.P., Adams M.B. Inhibition of intestinal lipid peroxidation does not minimize morphologic damage. J. Surg. Res. 1993;55(5):553–558. doi: 10.1006/jsre.1993.1183. [DOI] [PubMed] [Google Scholar]
- 28.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence R.A., Burk R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 30.Oberley L.W., Spitz D.R. Assay of superoxide dismutase activity in tumor tissue. Methods Enzymol. 1984;105:457–464. doi: 10.1016/s0076-6879(84)05064-3. [DOI] [PubMed] [Google Scholar]
- 31.Wang H., Joseph J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999;27(5–6):612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 32.Takakura N., Sanders P., Fessard V., Le Hégarat L. In vitro combined cytotoxic effects of pesticide cocktails simultaneously found in the French diet. Food Chem. Toxicol. 2013;52:153–162. doi: 10.1016/j.fct.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Wu A., Li L., Liu Y. Deltamethrin induces apoptotic cell death in cultured cerebral cortical neurons. Toxicol. Appl. Pharm. 2003;187:50–57. doi: 10.1016/s0041-008x(02)00032-7. [DOI] [PubMed] [Google Scholar]
- 34.Ben Abdallah F., Hamden K., Galeraud-Denis I., El Feki A., Keskes-Ammar K. An in vitro study on reproductive toxicology of Deltamethrin on rat spermatozoa. Andrologia. 2010;42:254–259. doi: 10.1111/j.1439-0272.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 35.Romero A., Ramos E., Castellano V., Martínez M.A., Ares I., Martínez M. Cytotoxicity induced by deltamethrin and its metabolites in SH-SY5Y cells can be differentially prevented by selected antioxidants. Toxicol. In Vitro. 2012;26:823–830. doi: 10.1016/j.tiv.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 36.El-Shenawy N.S. Effects of insecticides fenitrothion, endosulfan and abamectin on antioxidant parameters of isolated rat hepatocytes. Toxicol. In Vitro. 2010;24:1148–1157. doi: 10.1016/j.tiv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Lee J.E., Kang J.S., Ki Y.W., Lee S.H., Lee S.J., Lee K.S., Koh H.C. Akt/GSK3β signaling is involved in fipronil-induced apoptotic cell death of human neuroblastoma SH-SY5Y cells. Toxicol. Lett. 2011;202:133–141. doi: 10.1016/j.toxlet.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 38.Ki Y.W., Lee J.E., Park J.H., Shin I.C., Koh H.C. Reactive oxygen species and mitogen-activated protein kinase induce apoptotic death of SH-SY5Y cells in response to fipronil. Toxicol. Lett. 2012;211:18–28. doi: 10.1016/j.toxlet.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 39.Vidau C., Brunet J.L., Badiou A., Belzunces L.P. Phenylpyrazole insecticides induce cytotoxicity by altering mechanisms involved in cellular energy supply in the human epithelial cell model Caco-2. Toxicol. In Vitro. 2009;23:589–597. doi: 10.1016/j.tiv.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Sidiropoulou E., Sachana M., Flaskos J., Harris W., Hargreaves A.J., Woldehiwet Z. Fipronil interferes with the differentiation of mouse N2a neuroblastoma cells. Toxicol. Lett. 2011;201:86–91. doi: 10.1016/j.toxlet.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Naravaneni R., Jamil K. Evaluation of cytogenetic effects of lambda-cyhalothrin on human lymphocytes. J. Biochem. Molecular. Toxicol. 2005;19(5):304–310. doi: 10.1002/jbt.20095. [DOI] [PubMed] [Google Scholar]
- 42.González-Baró M.R., Garda H., Pollero R. Effect of fenitrothion on dipalmitoyl and 1-palmitoyl-2-oleoylphosphatidylcholine bilayers. Biochim. Biophys. Acta. 2000;1468:304–310. doi: 10.1016/s0005-2736(00)00269-8. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto H., Sakai E., Fumimoto R., Yamaguchi Y., Fukuma Y., Nishishita K. Deltamethrin inhibits osteoclast differentiation via regulation of heme oxygenase-1 and NFATc1. Toxicol. In Vitro. 2012;26(6):817–822. doi: 10.1016/j.tiv.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Karczewski J., Groot J. Molecular physiology and pathophysiology of tight junctions III. Tight junction regulation by intracellular messengers: differences in response within and between epithelia. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:660–665. doi: 10.1152/ajpgi.2000.279.4.G660. [DOI] [PubMed] [Google Scholar]
- 45.Ferruzza S., Scacchi M., Scarino M.L., Sambuy Y. Iron and copper alter tight junction permeability in human intestinal Caco-2 cells by distinct mechanisms. Toxicol. In Vitro. 2002;16:399–404. doi: 10.1016/s0887-2333(02)00020-6. [DOI] [PubMed] [Google Scholar]
- 46.Rao R.K., Baker R.D., Baker S.S., Gupta A., Holycross M. Oxidant-induced disruption of intestinal epithelial barrier function: role of protein tyrosine phosphorylation. Am. J. Physiol. 1997;273:812–823. doi: 10.1152/ajpgi.1997.273.4.G812. [DOI] [PubMed] [Google Scholar]
- 47.Lallès J.P. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr. Rev. 2010;68(6):323–332. doi: 10.1111/j.1753-4887.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 48.Sánchez de Medinaa F., Martínez-Augustinb O., González R., Ballester I., Nieto A., Gálvez J., Zarzuelo A. Induction of alkaline phosphatase in the inflamed intestine: a novel pharmacological target for inflammatory bowel disease. Biochem. Pharmacol. 2004;68:2317–2326. doi: 10.1016/j.bcp.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 49.López-Posadas R., González R., Ballester I., Martínez-Moya P., Romero-Calvo I., Dolores Suárez M. Tissue-nonspecific alkaline phosphatase is activated in enterocytes by oxidative stress via changes in glycosylation. Inflamm. Bowel Dis. 2011;17:543–556. doi: 10.1002/ibd.21381. [DOI] [PubMed] [Google Scholar]
- 50.Carbonell E., Puig M., Xamena N., Creus A., Marcos R. Mitotic arrest induced by fenvalerate in human lymphocyte cultures. Toxicol. Lett. 1989;48:45–48. doi: 10.1016/0378-4274(89)90184-7. [DOI] [PubMed] [Google Scholar]
- 51.Hadnagy W., Seemayer N.H., Kühn K.H., Leng G., Idel H. Induction of mitotic cell division disturbances and mitotic arrest by pyrethroids in V79 cell cultures. Toxicol. Lett. 1999;107:81–87. doi: 10.1016/s0378-4274(99)00034-x. [DOI] [PubMed] [Google Scholar]
- 52.Hreljac I., Zajc I., Lah T., Filipič M. Effects of model organophosphorous pesticides on DNA damage and proliferation of HepG2 cells. Environ. Mol. Mutagen. 2008;49:360–367. doi: 10.1002/em.20392. [DOI] [PubMed] [Google Scholar]
- 53.Kennedy S.G., Wagner A.J., Conzen S.D., Jordan J., Bellacosa A., Tsichlis P.N., Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimoto T., Uchino H., He Q.P., Li P.A., Siesjo B.K. Cyclosporin A, but not FK506, prevents the downregulation of phosphorylated Akt after transient focal ischemia in the rat. Brain Res. 2001;899:148–158. doi: 10.1016/s0006-8993(01)02220-x. [DOI] [PubMed] [Google Scholar]
- 55.Greco W., Bravo G., Parsons J.C. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 56.Altenburger R., Boedekers W., Faust M. Regulations for combined effects of pollutants: consequences from risk assessment in aquatic toxicology. Food Chem. Toxicol. 1996;34:1155–1157. doi: 10.1016/s0278-6915(97)00088-4. [DOI] [PubMed] [Google Scholar]
- 57.Backhaus T., Faust M. Predictive environmental risk assessment of chemical mixtures: a conceptual framework. Environ. Sci. Technol. 2012;46:2564–2573. doi: 10.1021/es2034125. [DOI] [PubMed] [Google Scholar]
- 58.González-Pleiter M., Gonzalo S., Rodea-Palomares I., Leganés F., Rosal R., Boltes K., Marco E., Fernández-Pinãs F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: implications for environmental risk assessment. Water Res. 2013;47:2050–2064. doi: 10.1016/j.watres.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 59.Rajapakse N., Silva E., Scholze M., Kortenkamp A. Deviation from additivity with estrogenic mixtures containing 4-nonylphenol and 4-tert-octylphenol detected in the E-SCREEN assay. Environ. Sci. Technol. 2004;38:6343–6352. doi: 10.1021/es049681e. [DOI] [PubMed] [Google Scholar]
- 60.Junghans M., Backhaus T., Faust M., Scholze M., Grimme L.H. Application and validation of approaches for the predictive hazard assessment of realistic pesticide mixtures. Aquat. Toxicol. 2006;76:93–110. doi: 10.1016/j.aquatox.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 61.Deneer J.W. Toxicity of mixtures of pesticides in aquatic systems. Pest Manage. Sci. 2000;56:516–520. [Google Scholar]
- 62.Belden J.B., Gilliom R.J., Lydy M.J. How well can we predict the toxicity of pesticide mixtures to aquatic life? Integr. Environ. Assess. Manage. 2007;3:364–372. [PubMed] [Google Scholar]
- 63.Rider C.V., Furr J.R., Wilson V.S., Gray L.E., Jr. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int. J. Androl. 2010;33(2):443–462. doi: 10.1111/j.1365-2605.2009.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.