Highlights
-
•
NOAEL for general toxicity was 1.875 (male) or 3.75 (female) mg of TPT/kg bw/d po.
-
•
Reproductive toxicity occurred only at doses of TPT that also caused general toxicity.
-
•
NOAEL for harmful effects on male reproduction was 1.875 mg of TPT/kg bw/d po.
-
•
NOAEL for harmful effects on female reproduction was 3.75 mg of TPT/kg bw/d po.
-
•
TPT-caused decrease in spermatid and sperm count was reversed after treatment.
-
•
Exposure to TPT during pre/pubertal period did not impair adult mice fertility.
Keywords: Triphenyltin, TPTH, Organotin compounds, Puberty, Postnatal exposure, Fertility
Abstract
This study investigated the effects of pre- and peripubertal exposure (PND 15–45) to triphenyltin hydroxide (TPT: 0, 1.875, 3.75, 7.5 and 15 mg/kg bw/d po) on mouse sexual maturation and fertility. Half of the mice were euthanized on PND 46 and the remaining mice were submitted to fertility tests on PND 65–75. TPT caused a transient decrease of weight gain at 3.75 mg/kg bw/d, and deaths and body weight deficits at higher doses. Delays of testes descent (TD), vaginal opening (VO) and first estrus (FE) occurred at doses ≥3.75 (TD) and ≥7.5 mg/kg bw/d (VO, FE), respectively. Body weight on the days of TD, VO and FE did not differ among groups. TPT at doses ≥3.75 mg/kg decreased sperm and spermatid counts at the end of treatment (PND 46) but no alteration was noted later on PND 75. Testicular histopathology (PND 46) showed a dose-dependent reduction of seminiferous tubules diameter, a greater degree of vacuolation in Sertoli cells and germ cell degeneration and necrosis in TPT-treated mice. TPT did not affect the outcome of fertility tests. Study-derived NOAEL was 1.875 mg TPT/kg bw/d for males and 3.75 mg TPT/kg bw/d for females. The detrimental effects of TPT on spermatogenesis were reversed after treatment discontinuation.
1. Introduction
Triphenyl (TPT) and tributyl (TBT) tins are powerful biocides (algicides, acaricides, insecticides, molluscicides, miticides, fungicides, slimicides, bactericides) that have a diversity of applications such as agricultural pesticides, disinfectants, preservatives for wood and antifouling agents in paints for ship hulls and fish farm nets and cages. The marked toxicity and putative endocrine disrupting properties of organotin compounds have raised concerns about the impact of their widespread use on the environment and human health.
Marine contamination by tri-substituted organotins has been associated with increased occurrence of imposex, or the superimposition of male type genital organs (penis and vas deferens), in female bivalve and gastropod mollusks [25]. Since TBT and TPT act as competitive inhibitors of a cytochrome P450-aromatase that converts androgen into estrogen, it has been hypothesized that imposex might result from elevated concentrations of unconverted androgens in organotin-exposed mollusks [9]. Some authors, however, pointed out that while organotin compounds are potent inducers of imposex, their effective concentrations as aromatase inhibitors are high. Along this line, hypotheses about modes of action other than inhibition of aromatase have been proposed for imposex [9].
A variety of potential deleterious effects of tri-organotins on mammals have been described including a marked toxicity to mammalian development [20] and reproduction. Nonetheless, the critical molecular targets for organotin induced reproductive toxicity remain unclear. Within this context, it has been debated whether aromatase inhibition plays a role in mediating developmental toxic effects of tri-organotin compounds on mammals. Nakanishi [15], for instance, reported that in human choriocarcinoma cells, organotins instead of inhibiting estradiol biosynthesis substantially enhanced it along with an increase of both aromatase activity and 17β-hydroxysteroid dehydrogenase type I activity, an enzyme that converts estrone (a weak estrogen) into the biologically active estrogen 17β-estradiol at the same low concentrations.
Several studies including those by Kanayama et al. [10] Hiromori et al. [8], and Le Maire et al. [12] demonstrated that TBT and TPT are nanomolar agonists for retinoid X receptor alpha (RXR-α) and peroxisome proliferator-activated receptor (PPAR) gamma. Along this line, it has been suggested that some toxic effects (and potential anticancer effects) of organotin compounds are mediated via actions on the RXR and PPAR-γ nuclear receptors [15].
Although a number of studies have found detrimental effects of tri-organotins (TBT and TPT) on rodent reproductive organs and function, mostly in rats, reports in the literature are far being consistent among compounds, doses and routes of exposure, species and toxic responses. A study by Podratz et al. [11], for instance, described that TBT given orally to adult rats, at a dose as low as 100 ng/kg bw/d for two weeks, disrupted cycling regularity, markedly reduced ovary weights and 17β-estradiol concentrations in the serum, increased progesterone concentrations and produced histological changes in the ovary. Similar histological findings (increased number of atretic tertiary and preovulatory follicles) were noted by Watermann et al. [26] in rats receiving TPTCl at an oral dose as high as 6 mg/kg bw/d from PND 23 until 53, while only minor changes were found at a lower dose (2 mg/kg bw/d). Moreover, Grote et al. [5] reported that in female rats TPT (6 mg/kg bw/d po) administered on PND 23–53 retarded the puberty onset (day of vaginal opening) and increased 17β-estradiol blood serum concentrations. A previous study by the same authors [6] had found that oral treatment of male rats with TBT (15 mg/kg bw/d) or TPT (6 and 12 mg/kg bw/d) during the pre- and peripubertal periods (PND 23–53) also delayed puberty onset (preputial separation), and decreased testosterone and luteinizing hormone (LH) serum concentrations.
The reproductive toxicity of tri-organotin compounds was less studied in mice than in rats. Nonetheless, Si et al. [24] described that TBT (1, 10, 100 μg/kg bw/d) given orally to female mice during gestation and lactation retarded testes descent and acquisition of cliff-avoidance reflex in the exposed offspring (100 μg/kg bw/d). A subsequent study by the authors found that a similar treatment with TBT (gestation + lactation) advanced the day of vaginal opening and the day of the first estrus and that exposed female offspring (10 and 100 μg/kg bw/d) also exhibited altered cycling regularity in adulthood [22]. Data on the toxicity of orally administered TPT (fentin) to mice during postnatal development have been provided as well. Delgado et al. [4] treated pregnant mice (GD7–17) with TPT (7.5, 15 and 30 mg TPT/kg bw/d po) and noticed that the two highest doses produced a marked perinatal mortality. Postnatal growth, days of vaginal opening and testes descent, and other landmarks of somatic development, however, remained unaffected in the offspring exposed in utero to TPT. In a further study, the authors treated female mice with TPT (1.875, 3.75, or 7.5 mg/kg bw/d po) during pregnancy and lactation (GD6–PND 21) and evaluated offspring somatic development and fertility [21]. Results showed no discernible effect of TPT at any tested dose on offspring development and fertility.
This study was undertaken to investigate whether exposure to TPT beginning before puberty and extending over pubertal onset period would adversely affect sexual maturation and fertility in Swiss mice. Since half of the treated mice were evaluated at the end of treatment and the remaining animals one month later, data were also obtained on the reversibility of TPT induced detrimental effects.
2. Materials and methods
2.1. Animals
Female Swiss Webster mice, from the FIOCRUZ Central Animal House (CECAL) breeding stock, were used. Mice were housed individually in standard plastic cages with stainless-steel covers and autoclaved wood shavings as bedding and kept under controlled temperature (23 ± 2 °C), relative humidity (approximately 70%) and day/night cycle (lights on from 8:00 a.m. to 8:00 p.m.). A pelleted diet (Nuvital®, for laboratory rats and mice, Nuvilab Ltd., Curitiba, PR, Brazil) and filtered tap water were provided ad libitum. All procedures were performed in accordance with Brazilian animal protection and welfare laws. The study protocol was approved by the Ethics Committee on the Use of Animals of Oswaldo Cruz Foundation (CEUA-FIOCRUZ, License Nr 0077-01).
2.2. Mating, pregnancy, parturition and culling
Two females were placed into the cage of one male for 2 h at the end of the dark period (6:00–8:00 a.m.) and copulation was confirmed by the presence of a vaginal plug. The day on which mating was confirmed was designated as pregnancy day ‘0’. From pregnancy day 18 onwards cages were inspected twice a day (8:00 a.m. and 5:00 pm) for deliveries. The first 24 h after birth was considered as postnatal day (PND) 1. On PND 5, litter size was adjusted by culling to 10 pups (five females and five males, whenever possible). Natural litters with 10 or fewer pups were not standardized.
2.3. Treatment, weaning and euthanasia
Triphenyltin hydroxide (TPT, purity ≥96.0%) was from Aldrich, Inc. On PND 15, all pups were weighed and allocated at random (within their litters) to one of five treatment groups (0, 1.875, 3.75, 7.5 and 15 mg TPT/kg bw/d). Male and female mice (N = approximately 40 per gender and treatment group) from 20 litters were daily treated from PND 15 through to 45. After weaning on PND 21, dams were euthanized and up to 5 pups of a same gender and litter were housed in one cage. TPT or its vehicle alone (pharmaceutical grade corn oil, Sigma Chemical Co.) was administered by gavage at a fixed volume of 10 mL/kg bw/d. A control group received the vehicle only. Males and females from 10 litters were killed on the day following that of last dose of TPT or its vehicle (PND 45). Pups from the remaining litters received no further treatment until euthanasia on PND 80–90. Fertility tests took place on PND 65–70.
2.4. General toxicity
During the period of treatment (PND 15–45) body weights were determined on PNDs 15, 21, 25, 30, 35 and 45 while cages were inspected for deaths and clinical signs of toxicity on a daily basis. Cage-side observations of clinical signs of toxicity included, but were not limited to: behavioral abnormalities, CNS depression symptoms, slow or irregular breathing, appearance of diarrhea, bleeding, changes in skin and fur, eyes and mucous membranes, tumoral masses, edema and other abnormalities.
2.5. Vagina opening and testis descent
Occurrence of testes descent (by scrotum palpation) or vaginal opening (visual inspection) was evaluated every morning (8:00–10:00 am) from PND 15 onwards.
2.6. Estrous cycle
From the day of VO onwards, for 20 consecutive days, vaginal smears were prepared every morning (8:00–9:00 h) to determine the day on which the first estrus took place and characterize the estrous cycle. Cytological findings for staging the estrous cycle were as follows: pro-estrus, predominance of round pro-nucleated epithelial cells; estrous, presence of cornified epithelial cells; meta-estrous, presence of both cornified epithelial cells and leukocytes and mucus, diestrus, predominance of leukocytes.
2.7. Euthanasia
On the day following the last dose of TPT or its vehicle, pups from 10 litters selected at random were killed by cervical dislocation. A blood sample was collected by cardiac puncture. Liver, spleen, thymus, uterus, ovaries, testis, epididymis and seminal vesicle with coagulating glands (without fluid) were removed and weighed. Pups of the remaining 10 litters received no further treatment.
2.8. Measurement of the number of spermatids in the testis and epididymal sperm count
Following removal of the tunica albuginea, the right testis from each male mouse was minced and homogenized in 1 mL of 0.9% NaCl containing 0.5% Triton X-100 for manual homogenization. After dilution in 0.9% NaCl, the number of homogenization-resistant spermatids of each testis was counted with a Neubauer chamber. Similarly, the cauda of epididymis was cut into small pieces, minced and homogenized, and the sperm (spermatozoa) were counted as described above [2].
2.9. Sperm morphology
To assess the percentage of morphologically abnormal sperm (detected in the head or tail) the deferens ducts were rinsed with 0.3 mL 0.9% NaCl and a sperm suspension was obtained. The smear on the slide (lamina) was prepared with an aliquot of sperm suspension carefully stained with Congo red and Gencian violet. Two hundred sperm per animal were examined at 1000× magnification and morphologically normal and abnormal sperm were scored according to the presence or absence of defects in the head (e.g., double-headed, amorphous head, reduced hook, banana head, no hook head, detached head, dwarf head, giant head, pin head) or tail (crooked flagellum, broken flagellum, coiled flagellum, tip coiled flagellum, bent flagellum tip) of the sperm [18].
2.10. Histopathology and morphometry
The left testis from each male mouse was fixed with Bouin's solution that was further replaced with Millonig's phosphate buffered formalin as modified by Carson. After fixation, testes were embedded in paraffin, cut and mounted on slides and subsequently stained with hematoxylin/eosin. One hundred seminiferous tubules in randomly selected cross sections of the testis from each mouse were identified regarding the stages of spermatogenesis. Twenty essentially round seminiferous tubules per testis were examined to assess the mean tubule diameter. The height of the germinal epithelium was measured in stages VII/VIII seminiferous tubules.
2.11. Testosterone level
Plasma testosterone concentrations were determined (samples in duplicate) using a commercially available competitive immunoassay kit (Free Testosterone ELISA, ARP, Inc™).
2.12. Fertility test
Fertility tests were carried out with half of mice from each treatment group when they were approximately 65 day old. One male and 3 females from the same treatment group and different litters (to avoid brother–sister mating), chosen at random, were housed in the same cage for 15 d. Females were examined every day in the morning for the presence of a vaginal plug. On the day a vaginal plug was found (GD 0), females were transferred to individual cages. Females that had not been impregnated by males during the first cohabitation period were placed again into a male cage for a second mating test. The second mating test was similar to the first, except that untreated males of proven fertility were used. Males that did not impregnate at least 2 females during the first mating test had a second 15-d cohabitation period with 3 untreated females. On day 16 of pregnancy females were killed by CO2 inhalation. The gravid uteri were weighed with their contents. The number of dead and living fetuses and resorptions were recorded. The number of implantation sites was determined by the Salewski's method [19]. Liver, spleen, thymus, gravid uterus and ovaries were removed and weighed.
Male mice that took part in the fertility test were killed by cervical dislocation on PND 75. Liver, spleen, thymus, testis, epididymis and seminal vesicle with coagulating glands (without fluid) were removed and weighed. Collection of epididymal sperm and sperm counting was performed as described in a previous section.
2.13. Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test, or by the Kruskal–Wallis test followed by the Mann–Whitney U test, whenever the data did not fit a normal distribution. Proportions were evaluated by the chi-square test or by the Fisher exact test. Statistical evaluation was performed using a SPSS® program and differences were considered as statistically significant whenever p < 0.05.
3. Results
3.1. Effects of TPT on pup survival and body weight gain
No clinical sign of toxicity was noted at the two lowest doses (1.875 or 3.75 mg/kg bw/d) of TPT. A number of deaths, however, occurred among mice treated with the second highest (7.5 mg/kg bw/d: 7.4% of males and 3.7% of females) and the highest (15 mg/kg bw/d: 80.8% of males and 80.9% of females) doses of TPT. All deaths of pups treated with TPT took place during the second week of treatment within a few days of weaning (PND 21–25) (Table 1).
Table 1.
Survival of mice treated orally with TPT (0, 1.875, 3.75, 7.5, 15 mg/kg bw/day) on postnatal days (PND) 15–45.
| Pups alive | TPT (mg/kg bw/day po) |
||||
|---|---|---|---|---|---|
| 0 | 1.875 | 3.75 | 7.5 | 15 | |
| Females | |||||
| PND 15, N (%) | 23 (100) | 22 (100) | 21 (100) | 27 (100) | 21 (100) |
| PND 45, N (%) | 23 (100) | 22 (100) | 21 (100) | 26 (96.3)a | 4 (19)a,b |
| Males | |||||
| PND 15, N (%) | 26 (100) | 26 (100) | 24 (100) | 27 (100) | 26 (100) |
| PND 45, N (%) | 26 (100) | 26 (100) | 24 (100) | 25 (92.6)a | 5 (19.2)a,b |
All deaths occurred between PND 21 (weaning) and PND 25.
Differ (chi-square test, p < 0.05) from other group survival rates.
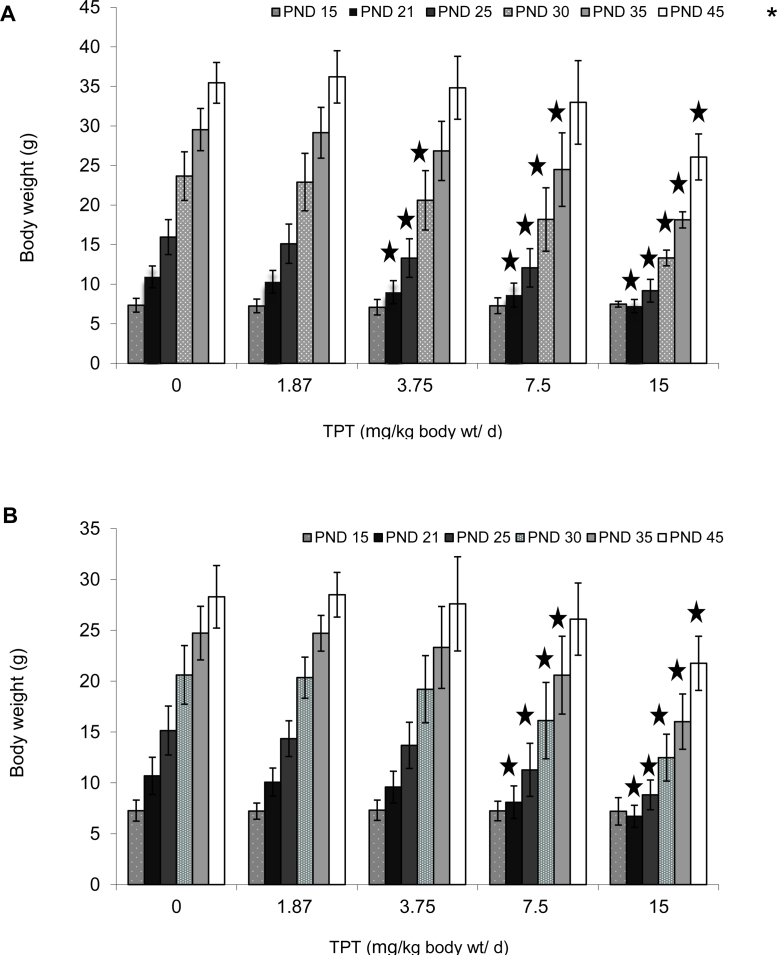
The body weight changes of mouse pups treated with TPT are shown in Fig. 1. The treatment caused a dose-dependent reduction of body weight gain in males (Fig. 1A) at TPT doses ≥3.75 mg/kg bw/d while weight gain of females (Fig. 1B) was decreased only at the two highest doses (7.5 and 15 mg/kg bw/d). It is of note that the weight gain deficit during treatment with TPT was transient. Except for the highest dose group (15 mg/kg bw/d) no differences in body weights between control and TPT treated mice were noted at the end of treatment (PND45). Except for body weight gain deficit no other clinical signs of toxicity were noted.
Fig. 1.
Body weight gain (g) of male (A) and female (B) mice treated orally (gavage) with triphenyltin hydroxide (0, 1.875, 3.75, 7.5 or 15 mg/kg bw/d) suspended in corn oil from postnatal day 15 through to 45. The height of the histogram bar corresponds to mean ± SD. A star indicates that the mean body weights differ (p < 0.05, ANOVA followed by Bonferroni's post hoc test) from the mean body weight of controls (0 mg/kg bw/d) at the same postnatal day.
3.2. Effect of treatment with TPT on the onset of puberty
As shown in Table 2, the day on which landmarks of puberty onset appear in female mice was not affected by treatment with the lowest doses of TPT. At the two highest doses (7.5 and 15 mg/kg bw/d), however, TPT delayed the day of vaginal opening and the day of the first estrus. The time (days) between vaginal opening and the first estrus remained unaltered at all doses of TPT (Table 2). The day of testes descent was delayed in male pups treated with doses of TPT ≥3.75 mg/kg bw/d. At first sight, these findings seem to indicate that TPT at the highest doses tested delayed puberty onset in both male and female mice. Nonetheless, TPT-treated pups did not differ from controls regarding pup body weight on the days of vaginal opening, first estrus and testes descent (Table 2). Taken together, these results suggest that delays of puberty onset reflect a general retardation of growth and somatic maturation rather than specific effects on male and female endocrine-sensitive endpoints.
Table 2.
Landmarks of puberty onset in mice treated orally with TPT (0, 1.875, 3.75, 7.5, 15 mg/kg bw/day) from postnatal day (PND) 15–45.
| Landmarks | TPT (mg/kg bw/day po) |
||||
|---|---|---|---|---|---|
| 0 | 1.875 | 3.75 | 7.5 | 15 | |
| Female pups, N | 23 | 22 | 21 | 26 | 4 |
| Vaginal opening (VO) day | 27 (22–30) | 27.5 (23–32) | 28 (24–38) | 31 (24–44)a | 39.5 (31–42)a |
| Body weight on VO day (g) | 17.0 ± 2.4 | 17.0 ± 2.6 | 16.7 ± 3.0 | 17.6 ± 2.6 | 17.9 ± 0.9 |
| First estrus (FE) day | 40 (29–48) | 38 (26–53) | 41 (31–50) | 48.5 (31–53)a,b | 51.5 (51–52)a |
| Body weight on FE day (g) | 24.8 ± 3.5 | 24.4 ± 3.1 | 26.0 ± 2.0 | 25.0 ± 2.4 | 24.8 ± 1.6 |
| Time between VO and FE (days) | 12.0 ± 4.2 | 11.8 ± 6.5 | 13.0 ± 5.2 | 11.6 ± 4.4 | 11.5 ± 2.1 |
| Male pups, N | 26 | 26 | 24 | 25 | 5 |
| Testes descent (TD) day | 21 (19–25) | 21 (20–25) | 22.5 (20–28)a | 23 (21–29)a | 29 (27–30)a |
| Body weight on TD day (g) | 11.4 ± 1.1 | 11.0 ± 1.1 | 10.8 ± 1.1 | 10.6 ± 1.1 | 11.6 ± 1.8 |
Kruskal–Wallis test and Mann Whitney U test:
≠ control (0 mg/kg bw/d) group.
≠ lowest dose (1.875 mg/kg bw/d) group.
3.3. Effects of TPT on male reproductive organs, testosterone concentrations, and sperm parameters at the end of treatment period
On the day following the last dose of TPT (PND 46) mice that had been treated with doses of TPT ≥ 3.75 mg/kg bw/d exhibited dose-dependent reductions of the number of spermatids (testes) and spermatozoa (epididymis) compared to controls (Table 3). A small and statistically non-significant increase in the proportion of sperm with an abnormal morphology was also noted at the two highest doses of TPT. A decrease of male reproductive organs (testis, epididymis and seminal vesicle) weight, consistent with a nearly 10% body weight reduction, was detected in male mice treated with the highest dose of TPT (15 mg/kg bw/d), but not among those mice that received 7.5 mg/kg bw/d or lower doses (Table 3). On PND 46, free testosterone concentrations in the plasma of TPT-treated mice did not differ from the concentrations measured in control group mice (Table 3).
Table 3.
Reproductive organ weights, sperm parameters, and testosterone concentrations in male mice treated orally with TPT (0, 1.875, 3.75, 7.5, 15 mg/kg bw/day) from postnatal day (PND) 15–45 and killed at the end of treatment period (PND 46).
| Treatment on PND 15–45: Reproductive parameters on PND 46 | TPT (mg/kg bw/day po) |
||||
|---|---|---|---|---|---|
| 0 | 1.875 | 3.75 | 7.5 | 15 | |
| Male pups, N | 14 | 14 | 12 | 13 | 4 |
| Reproductive organ weights (g) | |||||
| Testis right | 0.12 ± 0.02 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.12 ± 0.02 | 0.10 ± 0.01b |
| left | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.01 |
| Epididymis | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01a,b |
| Seminal vesicle | 0.16 ± 0.03 | 0.16 ± 0.03 | 0.15 ± 0.02 | 0.13 ± 0.01a,b | 0.08 ± 0.03a,b,c,d |
| Spermatid count (× 106/testis), N | 8.9 ± 1.7 | 8.8 ± 2.1 | 6.9 ± 1.7 | 6.1 ± 2.1a,b | 4.1 ± 1.0a,b |
| Sperm count (× 106/cauda epididymis), N | 16.7 ± 3.9 | 15.0 ± 3.3 | 12.4 ± 2.0a | 10.3 ± 3.1a,b | 3.0 ± 0.9a,b,c,d |
| Sperm with abnormal morphology, % | 12.70 | 13.32 | 10.50 | 14.50 | 15.00 |
| Seminiferous tubule diameter, μm | 231.1 ± 11.70 | 227.0 ± 8.0 | 219.4 ± 7.80a | 218.6 ± 11.31a | 215.0 ± 12.8f |
| Germinal epithelium heighte, μm | 68.8 ± 5.9 | 68.8 ± 6.6 | 62.2 ± 3.9a,b | 64.2 ± 3.9 | 63.7 ± 1.5 |
| Plasma free testosterone level, pg/mL | 16.7 ± 21.8 | 34.2 ± 32.8 | 14.4 ± 22.9 | 18.2 ± 20.7 | 0.12 ± 0.09 |
| Male pup body weight on PND 46 (g) | 40.8 ± 3.1 | 41.9 ± 2.3 | 41.9 ± 3.6 | 41.6 ± 4.2 | 35.5 ± 3.2a,b,c,d |
≠ 0 mg/kg bw/d.
≠ 1.875 mg/kg bw/d.
≠ 3.75 mg/kg bw/d.
≠ 7.5 mg/kg bw/d.
Germinal epithelium height was measured in stage VII/VIII seminiferous tubules. ANOVA and Bonferroni's post hoc test:
Data from three mice were evaluated because no stage VII–VIII was found in the slide from one mouse testis.
The morphometrical analysis showed that mice that received doses of TPT ≥ 3.75 mg/kg bw/d presented a smaller seminiferous tubule diameter and a reduced germinal epithelium height (Table 3), a finding suggestive that treatment with this tri-organotin compound impaired mouse spermatogenesis.
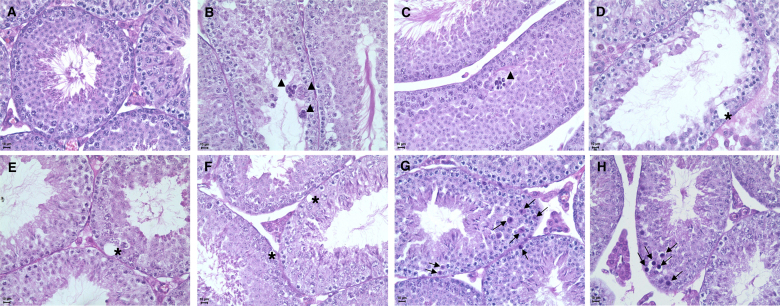
Histopathology of testicular tissue on PND 46 revealed sparse alterations of slight to moderate severity such as increased degree of vacuolation in Sertoli cells (Fig. 2, panels E and F), presence of immature germ cells (round spermatids) and cellular debris in the tubule lumen and signs of degeneration and necrosis of germ cells in the seminiferous tubules of TPT-treated mice (Fig. 2). No alteration of Leydig cell morphology was noted in the testis of TPT-treated mice.
Fig. 2.
Testicular histology changes in mice treated with triphenyltin hydroxide (0, 1.875, 3.75, 7.5 or 15 mg/kg bw/d po) from PND 15–45. Mice were euthanized at the end of treatment (PND 46). Testes were fixed in Bouin's solution and Millonig's buffered formalin as modified by Carson and stained with hematoxylin–eosin. Typical histology of a control mouse testis is shown in panel A, while all remaining panels illustrate histological changes found in TPT-treated animals (B, C–E: 1.875 mg/kg bw/d; G: 3.75 mg/kg bw/d; D, F, H: 7.5 mg/kg bw/d), such as an increased degree of vacuolation of Sertoli cells (panels E, F, asterisk *), vacuole apparently associated with germ cell deaths (panel D, asterisk *), acidophilic cells with pyknotic nuclei and hypercondensation of chromatin (panels G and H, arrows), multinucleated cell aggregates (panels B and C, arrow head). Magnification: 400×.
3.4. Effect of TPT on male liver and female liver, ovary and uterus (PND 46)
Post-mortem examination after euthanasia on PND 46 revealed no abnormality and no alteration of reproductive organ (ovary and uteri) weight in female mice treated with TPT. The relative weights of liver (%; [liver wt/body wt] × 100), however, were increased (Kruskal Wallis test and Mann–Whitney U test, p < 0.05) in males treated with TPT doses ≥7.5 mg/kg bw/d (mg TPT/kg bw/d; 0: 5.6 ± 0.4, 1.785: 5.7 ± 0.3, 3.75: 5.8 ± 0.4, 7.5: 6.2 ± 0.7, 15.0: 6.6 ± 0.3) and females treated with doses ≥3.75 mg/kg bw/d (mg TPT/kg bw/d; 0: 5.0 ± 0.4, 1.785: 5.4 ± 0.6, 3.75: 5.7 ± 0.6, 7.5: 6.0 ± 0.6, 15.0: 6.3 ± 0.4).
3.5. Effects of TPT on fertility tests and sperm parameters 30 days after treatment discontinuation
The outcome of fertility tests carried out approximately one month after discontinuation of treatment indicated that pre/peripubertal exposure to TPT in the dose range tested did not affect reproductive performance of male and female mice. As shown in Table 4, ratios of pregnant females per mated females and of males that copulated per males that took part in the fertility test did not differ between controls and TPT-treated mice and, in all dose-groups percentages of pregnant females were higher than 90% and in most cases 100%. One male that did not copulate, and two females that did not become pregnant in the test were subsequently mated with an untreated female or male. In this second mating, females were not successfully impregnated by their male partners, a result that confirmed that the male and two females that failed in the first test were in fact infertile. Moreover, data from C-section (performed on GD 16) of females that were impregnated in the fertility test revealed no difference between control and TPT-treated groups regarding gravid uteri weight (with their contents), and numbers of dead and living fetuses, resorptions and implantations. Post-mortem examination at the C-section revealed no gross pathology or weight change of maternal organs and no externally visible abnormality in the recovered fetuses.
Table 4.
Outcome of fertility test carried out with male and female mice treated orally with TPT (0, 1.875, 3.75, 7.5, 15 mg/kg bw/day) from postnatal day (PND) 15–45 approximately 20 days after the end of treatment (PND 65).
| Treatment on PND 15–45: | TPT (mg/kg bw/day po) |
||||
|---|---|---|---|---|---|
| 0 | 1.875 | 3.75 | 7.5 | 15 | |
| First mating | |||||
| Copulating males/Mated males (%) | 12/12 (100%) | 12/12 (100%) | 12/12 (100%) | 11/12 (91.6%) | 2/2 (100%) |
| Males impregnating a female/Mated males | 12/12a (100%) | 10/10 (100%) | 9/10 (90%) | 11/12 (91.6%) | 2/2 (100%) |
| Pregnant females/Mated females (%) | 12/12a (100%) | 10/10 (100%) | 9/10 (90%) | 11/12 (91.6%) | 2/2 (100%) |
| Second mating | |||||
| Copulating males/Mated males (%) | – | – | – | 0/1 (0%) | – |
| Pregnant females/Mated females (%) | – | – | 0/1 (0%) | 0/1 (0%) | – |
| First plus second mating | |||||
| Copulating males/Mated males (%) | 12/12 (100%) | 12/12 (100%) | 12/12 (100%) | 11/12 (91.6%) | 2/2 (100%) |
| Males impregnating a female/Mated males | 12/12a (100%) | 10/10 (100%) | 9/10 (90%) | 11/12 (91.6%) | 2/2 (100%) |
| Pregnant females/Mated females (%) | 12/12a (100%) | 10/10 (100%) | 9/10 (90%) | 11/12 (91.6%) | 2/2 (100%) |
| Implantation sites (N, total) per litter (mean ± SD) |
149 13.6 ± 1.9 |
140 14.0 ± 2.2 |
123 13.7 ± 3.2 |
150 13.6 ± 1.6 |
27 13.5 ± 3.5 |
| Live fetuses (N, total) per litter (mean ± SD) |
143 13.0 ± 2.0 |
135 13.5 ± 2.0 |
110 12.2 ± 3.4 |
145 13.2 ± 1.5 |
23 11.5 ± 0.7 |
One pregnant female died during gestation. All other females were pregnant and survived to scheduled C-section (GD16). Statistical analysis revealed no difference between control and TPT-treated groups.
TPT-treated male mice euthanized after fertility test mating period (approx PND 75) exhibited no discernible difference from controls regarding body weight and reproductive organs (testes, epididymis and seminal vesicle) weight, spermatid count, sperm count, proportion of sperm with an abnormal morphology, and plasma free testosterone concentrations (Table 5).
Table 5.
Reproductive organ weights, sperm parameters, and testosterone concentrations in mice treated orally with TPT (0, 1.875, 3.75, 7.5, 15 mg/kg bw/day) from postnatal day (PND) 15–45 and evaluated nearly 30 days after the last administered dose (PND 76).
| Treatment on PND 15–45: | TPT (mg/kg body wt/day po) |
||||
|---|---|---|---|---|---|
| Reproductive parameters on PND 76: | 0 | 1.875 | 3.75 | 7.5 | 15 |
| Male pups, N | 12 | 12 | 12 | 12 | 1 |
| Reproductive organ weights (g) | |||||
| Testis right | 0.13 ± 0.02 | 0.12 ± 0.02 | 0.13 ± 0.02 | 0.13 ± 0.02 | 0.15 |
| left | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.13 ± 0.02 | 0.12 ± 0.02 | 0.13 |
| Epididymis | 0.09 ± 0.01 | 0.09 ± 0.02 | 0.09 ± 0.01 | 0.09 ± 0.02 | 0.10 |
| Seminal vesicle | 0.19 ± 0.03 | 0.19 ± 0.04 | 0.20 ± 0.03 | 0.20 ± 0.03 | 0.25 |
| Spermatid count (× 106/testis), N | 10.0 ± 3.8 | 8.3 ± 3.7 | 8.2 ± 3.0 | 6.4 ± 2.8 | 10.0 |
| Sperm count (× 106/cauda epididymis), N | 30.2 ± 6.6 | 30.6 ± 8.1 | 29.9 ± 6.0 | 26.9 ± 6.5 | 20.0 |
| Sperm with abnormal morphology, % | 17.8 | 19.4 | 18.6 | 19.4 | 19.0 |
| Plasma free testosterone level, pg/mL | 27.7 ± 30.2 | 40.0 ± 34.9 | 38.6 ± 24.6 | 38.5 ± 34.7 | – |
| Male pup body weight on PND 76 (g) | 45.4 ± 3.6 | 44.3 ± 5.4 | 44.4 ± 3.8 | 43.7 ± 5.4 | 45.4 |
Statistical analysis (0, 1.875, 3.75 and 7.5 mg/kg bw/d) revealed no difference between dose groups.
4. Discussion
Data from this study showed that treatment of mice with oral doses of TPT ≥ 7.5 mg/kg/d from PND 15 onwards resulted in a dose-dependent reduction of pup weight gain and high mortality rates. All deaths occurred during the second week of treatment within a few days of weaning (PND 21–25). No death occurred at the two lowest doses of TPT tested in this study. A small and transient reduction of weight gain was noted at 3.75 mg/kg bw/d while no toxic effect of TPT was detected among mice treated with 1.875 mg/kg bw/d (Table 1, Fig. 1). Therefore, under the conditions of this study, the NOAELs for general toxicity findings (weight gain reductions and mortality) were 1.875 mg/kg bw/d (males) and 3.75 mg/kg bw/d (females). The same NOAEL was set for male reproductive endpoints (testes descent delay, sperm count and seminiferous tubule diameter reduction) while 3.75 mg/kg bw/d was the study-derived NOAEL for female reproductive endpoints.
Delays in the day of vaginal opening completion (VO) and of day of the first estrus (FE) indicated that TPT at doses ≥7.5 mg/kg bw/d retarded puberty onset in females. TPT at doses ≥3.75 mg/kg bw/d also delayed the day on which testes descent occurred. Since the body weight on the day on which these landmark events occurred did not differ between control and TPT-treated mice, it seems fair to conclude that delays in puberty onset in males and females were associated with a general retardation of growth and somatic maturation. Notwithstanding this fact, study results lend support to the interpretation that in rodents the testis is a target organ for TPT toxicity. Soon after treatment discontinuation (PND 46) both spermatid and sperm counts were decreased in a dose-dependent manner in males treated with TPT at doses ≥3.75 mg/kg bw/d. It is of note that spermatid and sperm count reductions were consistent with histological examination findings that revealed shorter diameters of seminiferous tubules and a number of signs of degeneration and necrosis of germ cells (Fig. 2) in the testis of males treated with TPT at doses ≥3.75 mg/kg bw/d (Table 3). Fertility tests undertaken nearly 30 days after discontinuation of TPT administration revealed no decline of fertility in treated animals (Table 4). Since fertility of males on PND 65–75 was unaffected by doses of TPT that caused testicular injury and decreased sperm count on PND 46, either the reduction of sperm count was insufficient to decrease the reproductive performance, or a recovery of TPT adverse effects on sperm parameters was reversed during the 30 days that elapsed between discontinuation of treatment and the fertility test. In rats and mice, the sperm count of a normal ejaculate is far in excess of that number required for 100% of impregnation success. Therefore, if sperm quality and motility are good, a substantial decline of sperm count is required to affect the outcome of rodent fertility tests. Since no decline in sperm count was detected in TPT-treated males on PND 75, it seems fair to conclude that adverse effects of TPT on mouse spermatogenesis were reversed with treatment discontinuation.
Two previous studies from our laboratory had found no effect of TPT on the puberty onset (VO and testes descent) of the offspring of mice treated (same dose range) during pregnancy [4] or pregnancy and lactation [21]. Our previous negative results are not at odds with the present study findings. In this study, pups were directly exposed during prepubertal and pubertal periods while in the other investigations offspring were exposed via placenta (pregnancy) or via placenta and maternal milk (pregnancy and lactation). Kinetic investigations showed that, albeit passing through the placenta and accumulating in rodent embryos, only minimal amounts of organotin compounds are transferred via maternal milk to suckling pups [3], [14]). Therefore, our previous findings consistently demonstrated that prenatal exposure to TPT did not affect puberty onset in mice. In this study pups were exposed directly (by gavage) to TPT and exposure extended over post-weaning pre- and pubertal periods.
To the authors’ knowledge, there is no other study on the effects of similar pre- and pubertal exposures to TPT on sexual maturation of mice. Reddy et al. [17] reported that spermatid and sperm counts were reduced in adult (sexually mature) male mice that received three ip injections of 25 μg/kg bw of TPT. They also found decreased activities of testicular 3β- and 17β-hydroxysteroid dehydrogenases in treated mice and suggested that TPT impaired spermatogenesis due to the inhibition of testosterone production.
As mentioned previously we found a disruption of seminiferous tubules morphology along with unaltered serum concentrations of free testosterone in mice treated orally with TPT during pre- and pubertal periods.
In male rats, Grote et al. [6], [7] observed that orally administered TPT (6 mg/kg bw/d) for 30 days (from PND 23 onwards) [6], or TPT (2 mg/kg bw/d) during pregnancy, lactation (maternal treatment), and post-weaning period (given directly to pups) until PND 64 [7], decreased testosterone serum concentrations. Grote et al. [7], however, detected no change in serum testosterone concentrations in mature male rats (PND 64) the treatment of which with TPT (2 mg/kg/bw/d, administered to mothers) had been discontinued at weaning. Grote et al. [6] found that TPT (6 mg/kg/d po, PND 23–53), albeit decreasing testosterone serum concentrations, did not alter the day of preputial separation (PS). Nonetheless, a further study by the same authors [7] revealed that puberty onset (PS) was delayed in rats treated with TPT (2 mg/kg bw/d po) until weaning (PND 21) (no effect on testosterone) and in those that continued to be treated until PND 64 (decrease of testosterone concentrations). Data by Grote et al. [6], [7] therefore suggested that, as far as male rats are concerned, the retardation of puberty onset did not hinge on the depression of testosterone serum concentrations caused by TPT.
Grote et al. [5], [7] also described the effects of TPT on female rats. A study by Grote et al. [5] found that TPT administered orally from PND 23 onwards had a biphasic effect on puberty onset; at a dose as high as 6 mg/kg bw/d TPT delayed VO while at a lower dose (2 mg/kg bw/d) it advanced VO. A subsequent study by the same authors [7], involving pre- and postnatal (until PND 21 or PND 64) exposure to TPT (2 mg/kg bw/d), found an advancement of puberty onset (VO) that was more pronounced in the group the treatment of which was discontinued at weaning (PND 21). Based on the foregoing Grote et al. [7] proposed that the prenatal period would be a developmental window of susceptibility to effects of TPT on puberty onset and that males would be more susceptible than females to TPT-induced disruption of sexual maturation.
Contrasting to the foregoing results by Grote et al. [5] we did not observe a treatment-related advancement of female mouse puberty onset (VO) at any dose level. The retardation of VO and FE in mice treated with 7.5 mg/kg bw/d was consistent with Grote et al.’s [5] results showing VO retardation in rats treated with 6 mg/kg bw/d. No alteration of female puberty onset, however, was noted in mice treated with the two lowest doses (1.875 and 3.75 mg/kg bw/d), whereas Grote et al. [5] reported that VO was advanced in rats treated with 2 mg/kg bw/d. For a similar period of treatment (PND 23 onwards), Grote et al. [6] found no effect of TPT on rat preputial separation (6 mg/kg bw/d) while we detected a delay of testes descent and reduction of sperm count in mice treated with 3.75 and 7.5 mg/kg bw/d.
The effects of TBT on sexual maturation were investigated by Si et al. in the offspring of female mice treated orally (0, 1, 10, 100 μg/kg bw/d) during pregnancy (from GD6 on) and throughout lactation until weaning (PND 21). The authors reported results for males [23], [24] and females [22] in separate publications. Both testes descent and acquisition of cliff-drop avoidance reflex were slightly, albeit significantly retarded in males exposed to the highest dose (100 μg/kg bw/d) [24] while a similar marked advancement of VO and FE, with a shortening of VO to FE time interval was noted in females treated with all tested doses [22]. Moreover, Si et al. [22] also demonstrated that TBT-treated females exhibited body weights lower than those of untreated controls on the day of VO and FE and that in addition to an early puberty onset TBT also disrupted normal cycling in mature female mice. Nonetheless, the effect of 1 μg/kg bw/d was almost identical to that of a 100-fold higher dose (100 μg/kg bw/d). The absence of any change of the magnitude of the toxic response over such a broad range of doses tested by Si et al. [22] is an intriguing finding.
At any rate, a distinction should be made between “programming effects” of exposure to organotin compounds during prenatal and or neonatal periods affecting further sexual maturation, and effects on puberty onset somatic landmarks arising from exposures that take place later (i.e., pre/pubertal exposures) when the process is going on. Effects of TBT on female mouse puberty onset estrous cyclicity and reported by Si et al. [22] possibly arise from a epigenetic “programming” effect elicited during in utero and neonatal periods and appear to be persistent. The effects reported in the present study, on the other hand, were transient effects of doses of TPT that were also associated to non-reproductive toxicity.
Recently, Mitra et al. [13] reported that, in in vitro germ-cell Sertoli cell co-cultures, TBT (300–1000 nM concentration range) induced stress proteins and mitochondrial depolarization leading to caspase-3-activation and apoptotic (at lower concentrations) and necrotic (at higher concentrations) cell deaths. They also noted that Sertoli cells were more susceptible than germ cells. Moreover, the authors demonstrated that, in rats, an oral dose as high as 50 mg/kg bw disrupted the blood-testicular-barrier. Mitra et al. [13] suggested that damage to Sertoli cells plays a pivotal role in tri-organotin compounds-mediated testicular toxicity. The histopathological changes in the seminiferous tubules of TPT treated mice (vacuolation in Sertoli cells and degeneration and necrosis of germ cells) seem to be consistent with Mitra et al.’s hypothesis.
In conclusion, repeated exposure of mice to TPT by the oral route during pre- and pubertal periods caused in addition to non-reproductive toxic effects, a high mortality and a delay of puberty onset in females (vaginal opening and first estrus) and in males (testes descent), a decline of spermatid and sperm counts, and slight to moderate damage to testicular Sertoli and germ cells. There was an overlap between doses that cause general toxicity (deaths and weight gain deficits) and reproductive toxicity in males (NOAEL 1.875 mg/kg bw/d) and females (NOAEL 3.75 mg/kgbw/d). TPT-induced decline of spermatid/sperm counts were reversed after treatment discontinuation. No effect of pre-pubertal/pubertal treatment with TPT was detected on the outcome of fertility tests performed with mature mice. It is of note that NOAELs for reproductive toxicity determined in this rodent study are orders of magnitude much higher than estimated human exposures. Notwithstanding the fact that data on human exposure to organotins (OTCs) are scanty, a Finnish study estimated that the average daily intake of ΣOTCs through fish consumption (a main dietary source of organotins) was 3.2 ng/kg bw/d, which corresponds to 1.3% from tolerable daily intake of 250 ng/kg bw/d set by the European Food Safety Authority [1]. A study by Rantakokko et al. [16] determined blood concentrations of TPTs in Finnish fishermen and their relatives and found that in only 27 out of 300 samples analyzed TPT concentrations were higher than limit of quantification of the method (0.04 ng/mL). The maximum TPT blood concentration found by Rantakokko et al. [16] was 0.56 ng/mL.
Transparency document
.
Acknowledgements
The research project was funded by the Brazilian National Research Council (CNPq), FAPERJ, and FIOCRUZ (PAPES-III). This study is part of MSCM doctoral thesis (INCQS-FIOCRUZ). FJRP, IFD and WGK are recipients of research fellowships from CNPq.
References
- 1.Airaksinen R., Rantakokko P., Turunen A.W., Vartiainen T., Vuorinen P.J., Lappalainen A., Vihervuori A., Mannio J., Hallikainen A. Organotin intake through fish consumption in Finland. Environ. Res. 2010;110(6):544–547. doi: 10.1016/j.envres.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Amann R.P. Detection of alterations in testicular and epididymal function in laboratory animals. Environ. Health Perspect. 1986;70:149–158. doi: 10.1289/ehp.8670149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke G.M., Forsyth D.S., Bondy G.S., Tachon R., Tague B., Coady L. Organotin speciation and tissue distribution in rat dams, fetuses, and neonates following oral administration of tributyltin chloride. J. Toxicol. Environ. Health A. 2008;71(6):384–395. doi: 10.1080/15287390701801653. [DOI] [PubMed] [Google Scholar]
- 4.Delgado I.F., Viana V.G., Sarpa M., Paumgartten F.J. Postnatal development and resistance to Plasmodium yoelii infection of mice prenatally exposed to triphenyltin hydroxide. Environ. Toxicol. 2009;24(6):629–635. doi: 10.1002/tox.20465. [DOI] [PubMed] [Google Scholar]
- 5.Grote K., Andrade A.J., Grande S.W., Kuriyama S.N., Talsness C.E., Appel K.E., Chahoud I. Effects of peripubertal exposure to triphenyltin on female sexual development of the rat. Toxicology. 2006;222(1–2):17–24. doi: 10.1016/j.tox.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Grote K., Stahlschmidt B., Talsness C.E., Gericke C., Appel K.E., Chahoud I. Effects of organotin compounds on pubertal male rats. Toxicology. 2004;202(3):145–158. doi: 10.1016/j.tox.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Grote K., Hobler C., Andrade A.J., Grande S.W., Gericke C., Talsness C.E., Appel K.E., Chahoud I. Sex differences in effects on sexual development in rat offspring after pre- and postnatal exposure to triphenyltin chloride. Toxicology. 2009;260(1–3):53–59. doi: 10.1016/j.tox.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Hiromori Y., Nishikawa J., Yoshida I., Nagase H., Nakanishi T. Structure-dependent activation of peroxisome proliferator-activated receptor (PPAR) gamma by organotin compounds? Chem. Biol. Interact. 2009;180(2):238–244. doi: 10.1016/j.cbi.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Horiguchi T. Masculinization of female gastropod mollusks induced by organotin compounds, focusing on mechanism of actions of tributyltin and triphenyltin for development of imposex. Environ. Sci. 2006;13(2):77–87. [PubMed] [Google Scholar]
- 10.Kanayama T., Kobayashi N., Mamiya S., Nakanishi T., Nishikawa J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway? Mol. Pharmacol. 2005;67(3):766–774. doi: 10.1124/mol.104.008409. [DOI] [PubMed] [Google Scholar]
- 11.Lang-Podratz P., Delgado Filho V.S., Lopes P.F., Cavati Sena G., Matsumoto S.T., Samoto V.Y., Takiya C.M., de Castro Miguel E., Silva I.V., Graceli J.B. Tributyltin impairs the reproductive cycle in female rats. J. Toxicol. Environ. Health A. 2012;75(16–17):1035–1046. doi: 10.1080/15287394.2012.697826. [DOI] [PubMed] [Google Scholar]
- 12.Le Maire A., Grimaldi M., Roecklin D., Dagnino S., Vivat-Hannah V., Balaguer P., Bourguet W. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep. 2009;10(4):367–373. doi: 10.1038/embor.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitra S., Srivastava A., Khandelwal S. Tributyltin chloride induced testicular toxicity by JNK and p38 activation, redox imbalance and cell death in sertoli-germ cell co-culture. Toxicology. 2013;314(1):39–50. doi: 10.1016/j.tox.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Moser V.C., McGee J.K., Ehman K.D. Concentration and persistence of tin in rat brain and blood following dibutyltin exposure during development. J. Toxicol. Environ. Health A. 2009;72(1):47–52. doi: 10.1080/15287390802445582. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi T. Endocrine disruption induced by organotin compounds; organotins function as a powerful agonist for nuclear receptors rather than an aromatase inhibitor? J. Toxicol. Sci. 2008;33(3):269–276. doi: 10.2131/jts.33.269. [DOI] [PubMed] [Google Scholar]
- 16.Rantakokko P., Turunen A., Verkasalo P.K., Kiviranta H., Männistö S., Vartiainen T. Blood levels of organotin compounds and their relation to fish consumption in Finland? Sci. Total Environ. 2008;399(1–3):90–95. doi: 10.1016/j.scitotenv.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Reddy P.S., Pushpalatha T., Reddy P.S. Reduction of spermatogenesis and steroidogenesis in mice after fentin and fenbutatin administration. Toxicol. Lett. 2006;166(1):53–59. doi: 10.1016/j.toxlet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Robb G.W., Amann R.P., Killian G.J. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J. Reprod. Fertil. 1978;54(1):103–107. doi: 10.1530/jrf.0.0540103. [DOI] [PubMed] [Google Scholar]
- 19.Salewski E. Faerbemethoden zum Makroskopischen Nachweis von Implantationsstellen am Uterus der Ratte. Naunyn-Schmiedebergs Archiv fuer Experimentelle Pathologie und Pharmakologie. 1964;247:367. doi: 10.1007/BF00245415. [DOI] [PubMed] [Google Scholar]
- 20.Sarpa M., De-Carvalho R.R., Delgado I.F., Paumgartten F.J. Developmental toxicity of triphenyltin hydroxide in mice. Regul. Toxicol. Pharmacol. 2007;49(1):43–52. doi: 10.1016/j.yrtph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Sarpa M., Tavares Lopes C.M., Delgado I.F., Paumgartten F.J. Postnatal development and fertility of offspring from mice exposed to triphenyltin (fentin) hydroxide during pregnancy and lactation. J. Toxicol. Environ. Health A. 2010;73(13–14):965–971. doi: 10.1080/15287391003751752. [DOI] [PubMed] [Google Scholar]
- 22.Si J., Han X., Zhang F., Xin Q., An L., Li G., Li C. Perinatal exposure to low doses of tributyltin chloride advances puberty and affects patterns of estrous cyclicity in female mice. Environ. Toxicol. 2012;27(11):662–670. doi: 10.1002/tox.21756. [DOI] [PubMed] [Google Scholar]
- 23.Si J., Li P., Xin Q., Li X., An L., Li J. Perinatal exposure to low doses of tributyltin chloride reduces sperm count and quality in mice. Environ. Toxicol. 2013 doi: 10.1002/tox.21892. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Si J., Li J., Zhang F., Li G., Xin Q., Dai B. Effects of perinatal exposure to low doses of tributyltin chloride on pregnancy outcome and postnatal development in mouse offspring. Environ. Toxicol. 2012;27(10):605–612. doi: 10.1002/tox.20753. [DOI] [PubMed] [Google Scholar]
- 25.Titley-O’Neal C.P., Munkittrick K.R., Macdonald B.A. The effects of organotin on female gastropods. J. Environ. Monit. 2011;13(9):2360–2388. doi: 10.1039/c1em10011d. [DOI] [PubMed] [Google Scholar]
- 26.Watermann B., Grote K., Gnass K., Kolodzey H., Thomsen A., Appel K.E., Candia-Carnevali D., Schulte-Oehlmann U. Histological alterations in ovaries of pubertal female rats induced by triphenyltin? Exp. Toxicol. Pathol. 2008;60(4–5):313–321. doi: 10.1016/j.etp.2008.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
.