Abstract
Betel quid chewing is associated with various pathologic alterations in oral mucosa. However, the molecular mechanism behind so many contradictory alterations remains unclear. Here we aimed to build a model to facilitate the related studies in cultured cells. In our results, areca nut extract (ANE) was found to exert different effects in oral cells depending on the supplemented serum level. ANE strongly induced DNA damage, necrotic ballooning, and inflammatory cytokines under lower serum concentration while might convert to facilitate deregulated growth of serum-supplemented cells via modulating the activity/expression of factors such as E-cadherin and Snail. Despite ANE significantly activated NF-κB, a mediator critical for inflammation, inhibition of NF-κB did not prevent the activation of IL8 promoter. We further discovered Y705-dephosphorylated STAT3 might enhance IL8 transcription. Since necrosis and the inflammatory cytokines could cause massive inflammation, infiltration of interstitial fluid might potentiate cellular resistance against the acute cytotoxicity of ANE and further support the proliferation of transforming cells. Induction of VEGF and angiogenesis under lower serum condition also paved the way for cell growth and subsequent metastasis. Accordingly, we concluded that in correlation with serum infiltration ANE caused particular effects in oral cells and possibly the various clinicopathological alterations in vivo.
Keywords: Areca nut extract (ANE), Necrosis, Inflammation, Serum, STAT3
1. Introduction
Oral cancer is one of the most common cancers. About 275,000 cases are reported annually worldwide [1]. Oral squamous cell carcinoma (OSCC) accounts for more than 90% of oral cancer incidence and is frequently found at tongue, buccal, and gingival areas [2]. Compared to normal tissues, several proteins with aberrant regulation and/or expression have been found in oral cancer, including epidermal growth factor receptor (EGFR), Akt, STAT3, cyclin D1 (CCND1), GSK3β, and possibly p21 [3], [4], [5], [6].
Consisted of areca nut, inflorescence or leaf of Piper betle, and slaked lime, betel quid has been implicated in the high occurrence of oral malignance in south-east Asia [7]. In addition to the premalignant lesions such as leukoplakia, chewing of betel quid was reported to be highly associated with the so-called “betel chewer's mucosa” featuring with pseudomembranous wrinkle, thickened epithelium, brownish discoloration, ulcer, and submucosal fibrosis [8], [9], [10]. Histologically, ballooning and vacuolated cells, round nuclear remnants or pyknotic nuclei, massive inflammatory infiltration, and deregulated epithelial growth are frequently observed in betel quid chewers [8], [9], [10], [11].
Areca nut, the major component of betel quid, is considered carcinogenic [12]. Treatment of areca nut extract (ANE) increased reactive oxygen species (ROS) and caused morphological alterations such as retraction and autophagosome-like vacuoles in cultured cells [13], [14]. In contrast, we recently discovered that ANE caused ballooning and pyknosis under serum starvation [15]. By inducing miR-23a, ANE reduced Fanconi anemia group G protein (FANCG) and impeded double-strand break (DSB) DNA repair [16]. ANE also impaired cytokinesis and induced micronuclei in Chinese hamster ovary (CHO) cells [17]. Induction of cytokines interleukin-6 (IL-6) and interleukin-8 (IL-8) by ANE in peripheral blood mononuclear cells might partially contribute to the mucosa inflammatory infiltration [18]. Among the identified compounds of areca nut, arecoline had been proven genotoxic and might contribute to oral carcinogenesis by facilitating error-prone DNA replication [19]. Areca nut-derived oligomericprocyanidins had also been demonstrated to induce apoptosis in human lymphocytes [20].
Betel quid chewing is associated with various alterations in oral mucosa. It remains obscure how so many different alterations such as deregulated epithelial growth and the adjacent ulcerative inflammation are induced. Under normal condition (10% FBS), however, these alterations could not be easily simulated in cultured cells. In this study we aim to build a model for studying the cytopathic effects of ANE in oral cells that may facilitate mechanism research in the future.
2. Materials and methods
2.1. Cell culture
OC2, an oral squamous cell carcinoma cell line derived from a Taiwanese man with habits of drinking, smoking, and areca nut chewing, was maintained in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The other oral cancer cell line SAS were maintained in DMEM with similar supplements. Cells were routinely kept in a 37 °C incubator supplied with 5% CO2 and subcultured every two to three days. Twelve to sixteen hours after seeding, experiments were performed soon after medium refreshing when cell confluence was about 70–90% except for the morphological tests (30–40%). For low serum culture, cells were washed twice with and cultured in medium containing no FBS or 1% FBS immediately before treatment.
2.2. Areca nut extract
Areca nut extract (ANE) was prepared from fresh nuts. In brief, the nuts were chopped into about 0.5–1 cm3 dices by a blender and the water-soluble ingredients were extracted at 4 °C overnight. The supernatant was harvested and concentrated by −70 °C lyophilization. The powder derived from water as prepared from fresh nuts. In brief, the nuts were chopped into about 0.5–1 cm3 dices by a blender and the water-soluble ingredients were extracted at 4 °C overnight. The supernatant was harvested and concentrated by −70 °C lyophilization. The powder derived from water extract was weighed, re-dissolved in ddH2O, and stored at −20 °C before experimental use.
2.3. Reagents and antibodies
Wortmannin, N-acetylcysteine (NAC), acridine orange (AO), propidium iodide (PI), and dimethyl sulfoxide (DMSO) were purchased from Sigma–Aldrich (St. Louis, MO, USA). NF-κB inhibitor quinazoline (QNZ) was from Cayman (Ann Arbor, MC, USA). STAT3 inhibitor NSC74859 was from Selleckchem (Houston, TX, USA). JAK inhibitor I was from Merck (Billerica, MA, USA). Antibodies against GSK3β, phosphorylated Akt (T308), phosphorylated p70S6K (T389), and epidermal growth factor receptor (EGFR) were from Cell Signaling Technology (Danvers, MA, USA). Antibodies of phosphorylated GSK3β (S9), p21, p16, phosphorylated histone H3 (S10), and cleaved PARP (24 kDa) were from Epitomics (Burlingame, CA, USA). Antibodies of fibronectin, snail, STAT3, phosphorylated STAT3 (Y705), and MCP1 were from Abcam (Cambridge, UK). The antibody of cyclin D1 (CCND1) was from Santa Cruz (Dallas, TX, USA). Antibodies of E-cadherin and p27 were from BD Biosciences (San Jose, CA, USA). The antibody of γH2AX was from Abnova (Walnut, CA, USA). The IL-8 promoter reporter was kindly provided by Dr. Yueh-Hsin Ping (National Yang-Ming University, Taiwan). The COX2 promoter reporter and the NF-κB activity reporter were kindly provided by Dr. Shih-Ming Huang (National Defense Medical Center, Taiwan). Human sera were collected from two healthy 20 to 30 years old Taiwanese males without habits of smoking, alcohol drinking, and betel quid chewing. Before collection, the donors were completely informed about the experimental procedures and agreed on paper consent. The independently collected sera in different tubes with no personal information were stored at 4 °C and used in experiments within three days. All the procedures were under supervision of the donors and the review board in Buddhist Dalin Tzu Chi General Hospital, Chia-Yi, Taiwan.
2.4. Acridine orange (AO)/ethidium bromide (EtBr) staining
For AO/EtBr staining, AO/EtBr mixture was added to the medium to a final concentration of 10 μg/ml. Ten minutes later, cells were washed, kept in PBS and observed immediately under the fluorescence microscope.
2.5. Cell lysate preparation and Western blot
Cell lysate preparation and Western blot were performed as described [19]. The results were the representatives from at least two independent experiments. The photometric intensity was determined using the software ImageJ.
2.6. Cell fractionation
After washing three times with PBS, cells in 10 cm culture dishes were scraped into 1 ml ice-cold fractionation buffer composed of 250 mM sucrose, 20 mM HEPES (pH 7.4), 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, and the freshly added 1 mM DTT and protease inhibitor cocktail (Roche, Basel, Switzerland). After incubation on ice for about 5–10 min, cells were passed through gauge 26 needles equipped with 1 ml syringes 10 times. The passing-through was centrifuged at 800 × g for 10 min. The supernatant was harvested as the cytoplasmic fraction and mixed with corresponding amount of 4× Laemmli loading dye. The pellet, or the nuclear fraction, was washed twice with fractionation buffer by centrifugation and directly dissolved in 300 μl 4× Laemmli loading dye. After boiling, samples in equal amount were run for Western blot.
2.7. Luciferase reporter assay
OC2 cells were transfected with reporter vectors using Turbofect according to manufacturer's instruction. Six hours after transfection in the case of NF-κB or 24 h in all other cases, cells were washed twice and continuously maintained in fresh medium containing indicated concentrations or 1% FBS. After ANE treatment, luciferase activity was determined using Dual-Luciferase Reporter Assay kit (Promega, Madison, WI, USA) 42 or 24 h after initiation of the experiments for NF-κB or the other reporters. The used doses of NSC74859 and JAK I are 50 and 1 μM, respectively. For RNA silencing, cells were previously transfected with control or NF-κB p65 dsRNAs (Cell Signaling Technology, Danvers, MA, USA) using Lipofectamine 2000 for 24 h. Cells were then washed and continuously transfected with IL-8 or NF-κB reporter and treated with ANE as described above.
2.8. Viability analysis
Cells at 90% confluence were treated with the indicated reagents. One day later, MTT reagent (Sigma, St. Louis, MO, USA) with a final concentration of 1 mg/ml was added into each well. Plates were swirled gently for a few seconds and the cells were cultured continuously for 3 h. After incubation, the cells were washed twice with PBS and MTT metabolic product was resuspended in 500 μl DMSO. After swirling for seconds, 50 μl supernatant from each well was transferred to optical plates for detection at 595 nm.
2.9. Real-time PCR
Cells were harvested for RNA extraction using TriPure reagent (Roche, Basel, Switzerland) 24 h after ANE treatment. After cDNA synthesis, reaction was conducted using BioRad SYBR green kit. Primers for transcripts quantification are: E-cadherin: 5′-CCTGGGACTCCACCTACAGA-3′ and 5′-AGGAGTTGGGAAATGTGAGC-3′, vimentin: 5′-GGCTCAGATTCAGGAACAGC-3′and 5′-CTGAATCTCATCCTGCAGGC-3′, IL6: 5′-GAACTCCTTCTCCACAAGCGCCTT-3′ and 5′-CAAAAGACCAGTGATGATTTTCACCAGG-3′, IL8: 5′-TCTGCAGCTCTGTGTGAAGG-3′ and 5′-ACTTCTCCACAACCCTCTGC-3′, RANTES: 5′-CGCTGTCATCCTCATTGCTA-3′ and 5′-GCACTTGCCACTGGTGTAGA-3′, VEGF: 5′-CTTGCTGCTGTACCTCCACCAT-3′ and 5′-TGTTGTGCTGTAGGAAGCTCATCT-3′.
2.10. Statistical analysis
The data were analyzed using t-test and the results with p value less than 0.05 were considered significant.
3. Results
3.1. Areca nut extracts exert different effects in oral cells depending on serum concentration
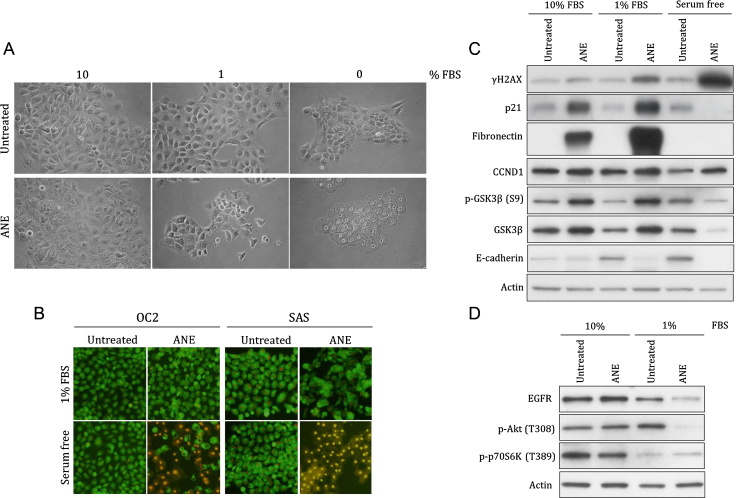
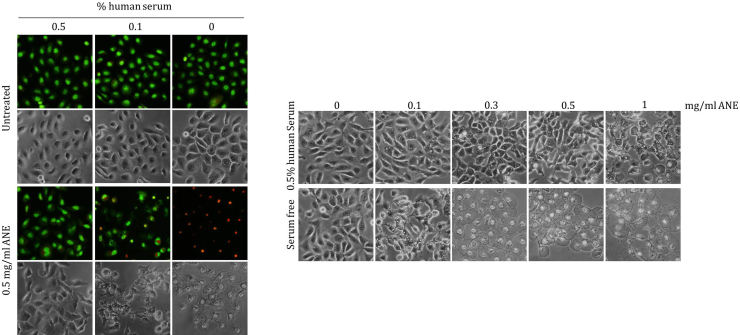
Betel quid chewing is associated with various morphological alterations in oral cavity. However, several alterations could not be simulated in normally cultured cells. High concentration of ANE even caused cell retraction, a phenomenon rarely reported in clinical histology. In this study, we discovered that ANE could exert particular effects on morphology and cellular signaling in oral cells under different serum concentrations. ANE evidently caused ballooning and pyknotic nuclei in serum starved cells (Fig. 1A). The increased membrane permeability and the evidences including ROS- and Ca2+-dependence in our previous study suggested ANE induced pyknotic necrosis (Fig. 1B) [15]. In contrast, most serum-supplemented cells remained intact after treatment of lower doses of ANE although cells supplemented with 1% FBS had more autophagosome-like vacuoles. The sera from two healthy adult males similarly antagonized the ANE-induced ballooning (Fig. S1).
Fig. 1.
Serum concentration influences the effects of ANE on cell morphology and various proteins. OC2 or the indicated SAS cells cultured with various concentrations of FBS were treated with 0.5 mg/ml ANE for 24 h. Cells were then directly photographed (A) or stained with AO/EtBr for evaluating membrane damage (B). The other panels of cells were harvested for monitoring the changes of indicated proteins by Western blot (C, D). The results are the representative figures from at least two independent experiments.
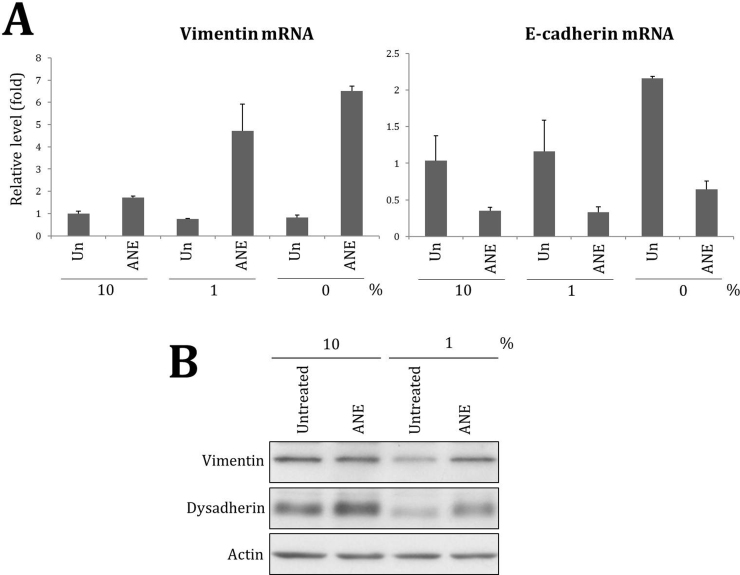
Besides the morphology alterations, ANE significantly induced DNA damage in cells without sufficient supply of FBS as evidenced by the increased γH2AX (Fig. 1C). Induction of several proteins such as p21 and fibronectin was also increased after reducing serum although these two proteins were barely detectable under serum free condition possibly due to loss of cytoplasmic components after membrane damage or the other unknown mechanisms (Fig. 1C and Fig. S2). In addition, Akt phosphorylation or the level of epidermal growth factor receptor (EGFR) was downregulated by ANE only in cells supplemented with 1% FBS (Fig. 1D). As a control, the phosphorylation of a mTOR complex 1 activity indicator p70S6K had detectably decreased at 1% FBS condition. Regulation of other proteins like GSK3β and cyclin D1 (CCND1), however, was not obviously affected by serum concentration except in necrotic cells (Fig. 1C). Taken together, these results suggest that ANE has different physiological effects in oral cells depending on serum concentration.
3.2. Areca nut extracts enhance expression of inflammatory cytokines particularly under lower serum condition
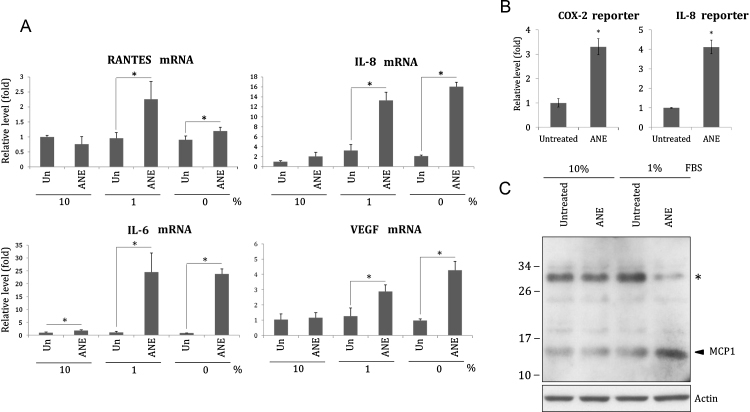
An important characteristic of betel chewer's mucosa is the massive inflammatory infiltration. In our results, ANE significantly increased transcripts of several inflammatory cytokines including IL6, IL8, and RANTES in cells supplemented with less or no serum (Fig. 2A). Under 1% FBS condition, ANE also obviously increased the promoter activity of IL8 and COX2 (Fig. 2B). Interestingly, we discovered that ANE increased monocyte chemotactic protein 1 (MCP1) in cells supplemented with 1% FBS (Fig. 2C). However, it is possible that ANE enhanced deglycosylation rather than expression of MCP1 since Western blotting showed that the increase of MCP1 after ANE treatment was correlated with significant reduction of a high-molecular-weight form of MCP1. Given that deglycosylated MCP1 has been shown to possess higher chemoattractant ability [21], this result has further confirmed ANE-induced inflammatory infiltration under low serum condition.
Fig. 2.
ANE under lower serum condition induces inflammatory cytokines more efficiently. (A) OC2 cells cultured with indicated concentrations of FBS were treated with 0.5 mg/ml ANE. The cells were harvested for measurement of IL6, IL8, RANTES or VEGF transcripts 24 h later. The value of the untreated, 10% FBS-supplemented cells was defined as 1. (B) The cells transfected with IL8 or COX2 promoter reporter were cultured in medium containing 1% FBS. The luciferase activity was determined 24 h after ANE treatment. (C) Similarly treated cells were harvested for Western blot. The upper signal marked with asterisk might be a glycosylated form of MCP1.
Since under lower serum concentration ANE is apt to induce necrosis and inflammatory cytokines, infiltration of interstitial fluid during massive inflammation might potentiate cellular resistance against the acute cytotoxicity of ANE and further support the proliferation of transforming cells. Induction of VEGF and angiogenesis under lower serum condition also paved the way for cell growth and subsequent metastasis (Fig. 2A).
3.3. Areca nut extracts activate NF-κB under 1% FBS condition
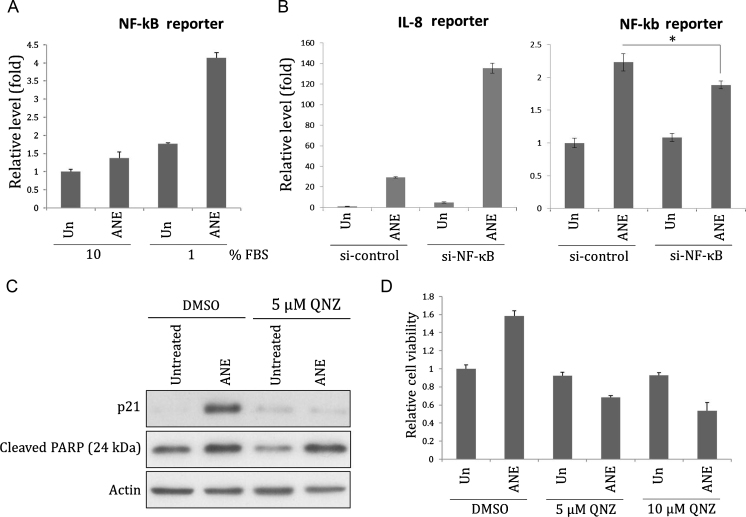
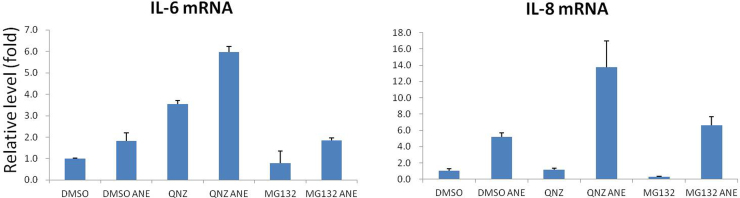
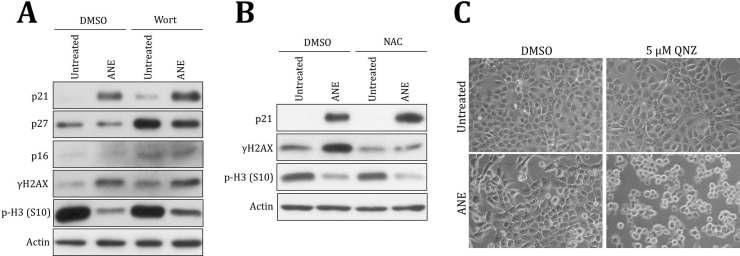
The previous results indicated serum concentration influenced the effects of ANE on cell appearance and the levels of transcripts or proteins. To further confirm the impact on cell signaling, we investigated the effects of serum and ANE on the activity of NF-κB, a known inflammation mediator [22]. By NF-κB reporter assay, we showed that ANE efficiently enhanced NF-κB activity under 1% serum (Fig. 3A). Surprisingly, knock-down of NF-κB p65 had reduced the corresponding reporter activity while conversely enhanced ANE-mediated IL8 reporter activation (Fig. 3B). Using a NF-κB inhibitor quinazoline (QNZ) or MG132 also could not counteract this effect even in the case of IL6 (Fig. S3). In comparison, inhibition of NF-κB by the same dose of QNZ significantly prevented the induction of p21 by ANE, confirming the validity of the above experiments (Figs. 3C and S4A). Because the induction is independent of reactive oxygen species-mediated DNA damage, ANE may upregulate NF-κB signaling to directly increase p21 (Fig. S4B). NF-κB inhibition also obviously increased ANE cytotoxicity but not PARP cleavage, an indicator of apoptosis (Figs. 3D and S4C). Although all these results suggested ANE indeed activated NF-κB to modulate cell functions, NF-κB is not directly involved in the upregulation of IL8 transcription. ANE might also induce a few inflammatory cytokines via a mechanism in addition to NF-κB.
Fig. 3.
ANE increases NF-κB activity. (A) OC2 cells transfected with NF-κB activity reporter were maintained with indicated concentrations of FBS. Cells were harvested for luciferase assay 24 h after treatment of 0.5 mg/ml ANE. (B) Cells sequentially transfected with NF-κB p65 siRNA and IL8 promoter reporter or NF-κB activity reporter were cultured in medium supplemented with 1% FBS. Cells were harvested for evaluating the luciferase activity 24 h after treatment. (C, D) Cells treated with ANE in combination or not with QNZ were harvested for Western blot or for MTT assay 24 h later.
3.4. ANE induces inflammatory signaling at least via enhancing dephosphorylation of STAT3
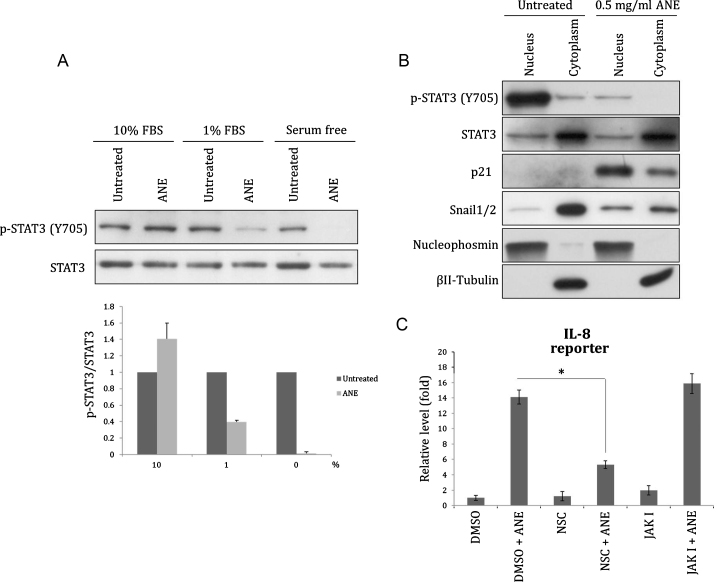
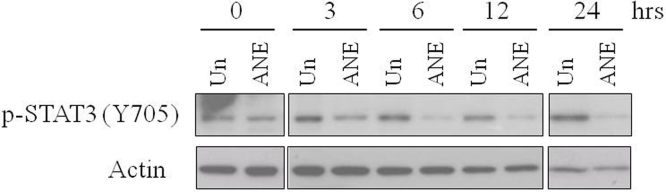
Like Akt, phosphorylation of STAT3 (Y705) was also decreased by ANE under lower serum condition (Figs. 4A and S5). Despite that ANE treatment significantly reduced the phosphorylation of total STAT3 (Y705), the ratio of nuclear to cytoplasmic localization of unphosphorylated STAT3 was not altered (Fig. 4B). As a control, ANE enhanced nuclear translocation of Snail proteins. Moreover, inhibition of STAT3 dimerization by NSC74859, which reduces DNA-binding STAT3 with IC50 of 86 μM, reduced the activation of IL8 promoter (Fig. 4C) [23]. In contrast, inhibition of STAT3 phosphorylation by JAK inhibitor I, a pan JAK inhibitor with IC50 value between 1 and 15 nM, did not detectably downregulate the reporter activity (Fig. 4C) [24]. This result suggests STAT3 is required for ANE-induced IL8 transcription but JAK-mediated Y705 phosphorylation is dispensable. Similar effects also could be seen in the transcripts level of IL6 although the case of IL8 was inconsistent possibly because the mRNA stability may be independently regulated (data not shown) [25]. These results increase a possibility that ANE enhances inflammation in oral mucosa at least via facilitating dephosphorylation of nuclear STAT3. Activated STAT3 is associated with inflammation during tumor progress [26]. However, ANE may modulate the transcription of a few inflammatory cytokines by enhancing Y705 dephosphorylation of STAT3 since un- and phosphorylated STAT3 had been reported to differently regulate several downstream targets [27].
Fig. 4.
ANE induces dephosphoylation of STAT3. OC2 cells cultured with various concentrations of or 1% FBS were treated with 0.5 mg/ml ANE. Twenty-four hours after treatment, cells were directly harvested for Western blot (A) or fractionated for evaluating the localization of STAT3 (B). The effect of ANE on STAT3 phosphorylation in two independent experiments was demonstrated after normalization to each untreated sample (A, lower panel). The value of each untreated sample under different FBS conditions was defined as 1. (C) Cells transfected with IL8 promoter reporter were treated with ANE in combination with NSC74859 or JAK inhibitor I. The used doses of NSC74859 and JAK I are 50 and 1 μM, respectively. The luciferase activity was determined 24 h later.
4. Discussion
In this study, we provided a few examples to prove serum concentration influenced the effects of ANE in cultured cells. The effects of ANE under different serum condition give a rational explanation to the various alterations in betel quid chewers. In serum-starved cells, ANE caused cell ballooning and nuclear pyknosis. Theoretically, the environment that oral epithelial cells reside in is impossible to be stringently serum free. However, epithelial tissue normally is avascular and less accessible to the circulating nutrients in blood stream. A previous research indicated the epidermis in average has lower ratio of interstitial fluid than dermis [28]. Because in our results even 0.5% human serum could not completely circumvent necrosis, these alterations may hence occasionally occur in vivo especially in the superficial cells. The repeated stimuli of highly concentrated ANE during chewing may further increase the chance of pyknotic necrosis.
According to our results, ANE may enhance deregulated cell growth via multiple mechanisms. Both dysadherin and snail lead to decrease of E-cadherin [29], [30]. Besides, ANE slightly increased CCND1, a protein critical for cell cycle progress [31]. ANE also constantly inhibited GSK3β regardless of serum concentration. Because GSK3β is a negative regulator of proteins including snail and β-catenin, hyperphosphorylation of GSK3β is common in several tumors [32], [33]. However, it remains unanswered why inflammation and ulcer frequently exist underneath or close to hyperplasia lesion in betel quid chewers. A previous study proved that during carcinogenesis the hyperplasia has had higher interstitial fluid pressure (IFP) due to abnormal, compressed vasculature system regardless of the increased permeability of blood vessels [34]. Elevated IFP hinders transport and tumors which similarly have higher IFP hence are less accessible to therapeutic chemicals. In contrast, inflammation stimuli reduce IFP and result in infiltration of interstitial fluid and edema [35]. Our previous study had proven insulin is a key component in serum to counteract ANE-induced ballooning [15]. Since the half-life of insulin in circulation is only minutes, it is highly possible that ANE could strongly induce inflammation and ulcer in the region where insulin is insufficient [36]. Significant increase of fibronectin under lower serum condition also possibly enhances fibrosis.
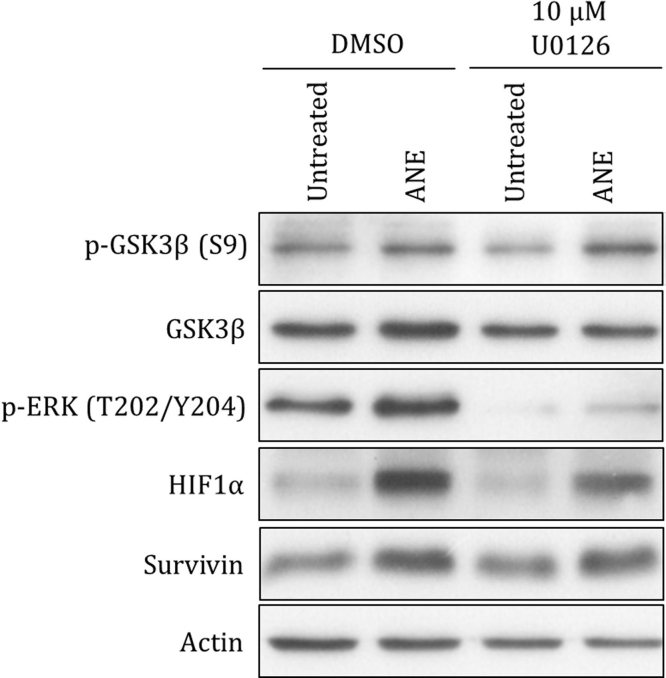
Interestingly, the survival rate of ANE-treated cells was obviously increased in the presence of higher serum concentration. In contrast to inhibition of STAT3 dimerization, in our results inhibition of NF-κB weakly impeded the induction of IL6 and IL8 by ANE. ANE possibly induce inflammation in part by reducing STAT3 Y705 phosphorylation in cells supplemented with less serum. Because un- and phosphorylated STAT3 had been reported to differently regulate several downstream targets, ANE may thus modulate the activity of particular genes depending on serum conditions [27]. However, it should not be ruled out that ANE may oppositely regulate the phosphorylation of STAT3 S727. Given that ANE is apt to induce necrosis and inflammatory cytokines under low serum condition, the resulted massive inflammation and infiltration of interstitial fluid in oral mucosa may increase cellular resistance against the acute cytotoxicity of ANE. Considering that hyperplasia is frequently accompanied with inflammatory infiltrate, it is possible that ANE may exacerbate oral carcinogenesis after massive inflammation or angiogenesis [8]. Although it is widely accepted that ANE can cause cytotoxic effects in cultured cells, we found that ANE could upregulate some prosurvival signaling, which increased the possibility of malignant transformation. Supporting this speculation is the result that survivin was detectably increased by ANE in OC2 cells (Fig. S6). ANE also obviously induced HIF1α, the master regulator of hypoxia adaptation, via activating ERK (Fig. S6). In addition, activation of NF-κB appeared to favor cell survival during ANE treatment in spite of the potential side effect, cell cycle retardation. As a cyclin-dependent kinase (CDK) inhibitor, p21 is well known as a negative regulator of cell proliferation [37]. However, increasing evidence has suggested that nuclear p21 may not simply induce cell cycle arrest. Accumulation of p21 in the nucleus has been shown to be correlated with poor prognosis and disease progress in OSCC [38], [39]. Interestingly, p21 may facilitate G1/S transition after assembling into CCND1/Cdk2/p21/PCNA complex unless cyclin E/Cdk2 is sequestered by excessive p21 proteins [40], [41]. Given that ANE-induced p21 retards cell cycle, cells may continue proliferation once areca nut is removed after chewing.
In surviving cells, ANE possibly triggers transformation via mechanisms besides the ROS-mediated DNA damage. In the shown examples, however, it is unclear how and why EGFR and Akt were downregulated by ANE at lower serum concentration. Although Akt is commonly known as an oncoprotein, accumulating evidence has suggested that like Ras, overactivated Akt may induce senescence under specific circumstances [42]. Because Akt could sensitize cells to ROS-mediated apoptosis, downregulation of Akt activity might facilitate early carcinogenesis induced by ANE [43]. Once nutrients and serum are sufficiently available especially after angiogenesis, activation of EGFR and Akt signaling possibly accelerates the progression of OSCC.
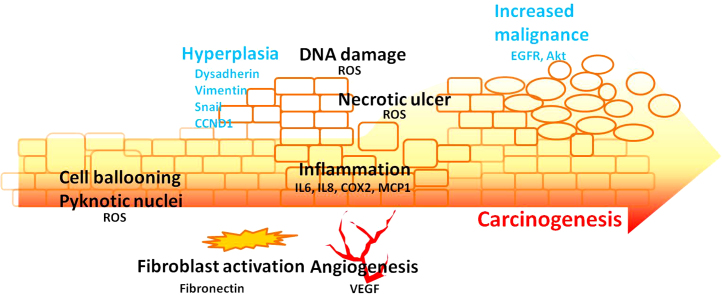
Taken together, by manipulating FBS concentration we discovered that ANE differentially determined cellular destiny, thus delineating a possible progression of ANE-mediated oral carcinogenesis (Fig. 5). Without the interference from exogenous growth factors, the effects of ANE on epithelial-mesenchymal transition are also easier to observe in cells supplemented with less FBS. Our results give a potential model for the simulation of ANE-mediated pathogenesis in culture cells.
Fig. 5.
A proposed model for ANE-mediated carcinogenesis and various alterations. ANE steadily enhances deregulated cell growth and the resulted hyperplasia lesion gradually suffers from shortage of supplements including insulin. Under such condition, ANE obviously induces inflammation, necrosis, and ROS. Subsequently, infiltration and angiogenesis support survival of transforming cells. Finally, recovered activity of Akt and EGFR may exacerbate the carcinogenesis.
Author contributions
W.T. Ji initiated this project, executed most of the experiments, and wrote the manuscript. Y.C. Chuang provided related resources. H.P. Chen was responsible for morphology photos. C.C. Lee provided the comments of clinical observations. Jeff Y.F. Chen conceived the plan and corrected the manuscript. S.R. Yang was responsible for independent Western blot and morphology photos. J.H. Chen and C.J. Wang were responsible for RT-PCR and reporter assay, respectively. H.R. Chen conceived the plan and corrected the manuscript. All authors read and approved the final manuscript.
Transparency document
Acknowledgement
This work was partially supported by National Science Council (97-2311-B-194-001-MY3) and no additional external funding was received for this study.
Footnotes
Available online 4 November 2014
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.10.018.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y.K., Huang H.C., Lin L.M., Lin C.C. Primary oral squamous cell carcinoma: an analysis of 703 cases in southern Taiwan. Oral Oncol. 1999;35:173–179. doi: 10.1016/s1368-8375(98)00101-8. [DOI] [PubMed] [Google Scholar]
- 3.Daniel F.I., Fava M., da Rocha Hoffmann R., Campos M.M., Yugel L.S. Main molecular markers of oral squamous cell carcinoma. Appl. Cancer Res. 2010;30:279–288. [Google Scholar]
- 4.Hong K.O., Kim J.H., Hong J.S., Yoon H.J., Lee J.I., Hong S.P., Hong S.D. Inhibition of Akt activity induces the mesenchymal-to-epithelial reverting transition with restoring E-cadherin expression in KB and KOSCC-25B oral squamous cell carcinoma cells. J. Exp. Clin. Cancer Res. 2009;28:1. doi: 10.1186/1756-9966-28-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol. Cancer. 2010;9:144. doi: 10.1186/1476-4598-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah N.G., Trivedi T.I., Tankshali R.A., Goswami J.A., Jetly D.H., Kobawala T.P., Shukla S.N., Shah P.M., Verma R.J. Stat3 expression in oral squamous cell carcinoma: association with clinicopathological parameters and survival. Int. J. Biol. Markers. 2006;21:175–183. doi: 10.1177/172460080602100307. [DOI] [PubMed] [Google Scholar]
- 7.Chiba I. Prevention of betel quid chewers’ oral cancer in the Asian-Pacific area. Asian Pac. J. Cancer Prev. 2001;2:263–269. [PubMed] [Google Scholar]
- 8.Chiu C.T., Lee S.Y., Wang D.J., Liu Y.C., Chang W.F., Yen C.Y., Lee C.H., Liu S.Y. Matrix metalloproteinase-1 expression in betel quid-associated oral cancers. J. Dent. Sci. 2008;3:75–82. [Google Scholar]
- 9.Reichart P.A., Phillipsen H.P. Betel chewer's mucosa – a review. J. Oral Pathol. Med. 1998;27:239–242. doi: 10.1111/j.1600-0714.1998.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 10.Trivedy C.R., Craig G., Warnakulasuriya S. The oral health consequences of chewing areca nut. Addict. Biol. 2002;7:115–125. doi: 10.1080/13556210120091482. [DOI] [PubMed] [Google Scholar]
- 11.Rajendran R., Sivapathasundharam B. 6th ed. Elsevier; 2009. Shafer's Textbook of Oral Pathology; p. 96. [Google Scholar]
- 12.Jeng J.H., Chang M.C., Hahn L.J. Role of areca nut in betel quid-associated chemical carcinogenesis: current awareness and future perspectives. Oral Oncol. 2001;37:477–492. doi: 10.1016/s1368-8375(01)00003-3. [DOI] [PubMed] [Google Scholar]
- 13.Chang M.C., Ho Y.S., Lee P.H., Chan C.P., Lee J.J., Hahn L.J., Wang Y.J., Jeng J.H. Areca nut extract and arecoline induced the cell cycle arrest but not apoptosis of cultured oral KB epithelial cells: association of glutathione, reactive oxygen species and mitochondrial membrane potential. Carcinogenesis. 2001;22:1527–1535. doi: 10.1093/carcin/22.9.1527. [DOI] [PubMed] [Google Scholar]
- 14.Lu H.H., Kao S.Y., Liu T.Y., Liu S.T., Huang W.P., Chang K.W., Lin S.C. Areca nut extract induced oxidative stress and upregulated hypoxia inducing factor leading to autophagy in oral cancer cells. Autophagy. 2010;6:725–737. doi: 10.4161/auto.6.6.12423. [DOI] [PubMed] [Google Scholar]
- 15.Ji W.-T., Lee C.-I., Chen J.Y.-F., Cheng Y.-P., Yang S.-R., Chen J.-H., Chen H.-R. Areca nut extract induces pyknotic necrosis in serum-starved oral cells via increasing reactive oxygen species and inhibiting GSK3b: an implication for cytopathic effects in betel quid chewers. PLOS ONE. 2013;8:e63295. doi: 10.1371/journal.pone.0063295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai Y.S., Lin C.S., Chiang S.L., Lee C.H., Lee K.W., Ko Y.C. Areca nut induces miR-23a and inhibits repair of DNA double-strand breaks by targeting FANCG. Toxicol. Sci. 2011;123:480–490. doi: 10.1093/toxsci/kfr182. [DOI] [PubMed] [Google Scholar]
- 17.Lin C.C., Chang M.C., Chang H.H., Wang T.M., Tseng W.Y., Tai T.F., Yeh H.W., Yang T.T., Hahn L.J., Jeng J.H. Areca nut-induced micronuclei and cytokinesis failure in Chinese hamster ovary cells is related to reactive oxygen species production and actin filament deregulation. Environ. Mol. Mutagen. 2009;50:367–374. doi: 10.1002/em.20463. [DOI] [PubMed] [Google Scholar]
- 18.Chang L.Y., Wan H.C., Lai Y.L., Liu T.Y., Hung S.L. Enhancing effects of areca nut extracts on the production of interleukin-6 and interleukin-8 by peripheral blood mononuclear cells. J. Periodontol. 2006;77:1969–1977. doi: 10.1902/jop.2006.060039. [DOI] [PubMed] [Google Scholar]
- 19.Ji W.T., Yang S.R., Chen J.Y., Cheng Y.P., Lee Y.R., Chiang M.K., Chen H.R. Arecoline downregulates levels of p21 and p27 through the reactive oxygen species/mTOR complex 1 pathway and may contribute to oral squamous cell carcinoma. Cancer Sci. 2012;103:1221–1229. doi: 10.1111/j.1349-7006.2012.02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C.C., Huang P.L., Liu T.Y., Jan T.R. Highly oligomeric procyanidins from areca nut induce lymphocyte apoptosis via the depletion of intracellular thiols. Toxicol. In Vitro. 2009;23:1234–1241. doi: 10.1016/j.tiv.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z.G., Haelens A., Wuyts A., Struyf S., Pang X.W., Proost P., Chen W.F., van Damme J. Isolation of a lymphocyte chemotactic factor produced by the murine thymic epithelial cell line MTEC1: identification as a 30 kDa glycosylated form of MCP-1. Eur. Cytokine Netw. 1996;7:381–388. [PubMed] [Google Scholar]
- 22.Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddiquee K., Zhang S., Guida W.C., Blaskovich M.A., Greedy B., Lawrence H.R., Yip M.L.R., Jove R., McLaughlin M.M., Lawrence N.J., Sebti S.M., Turkson J. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc. Natl. Acad. Sci. U.S.A. 2007;104:7391–7396. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsunaga Y., Inoue H., Fukuyama S., Yoshida H., Moriwaki A., Matsumoto T., Matsumoto K., Asai Y., Kubo M., Yoshimura A., Nakanishi Y. Effects of a Janus kinase inhibitor, pyridone 6, on airway responses in a murine model of asthma. Biochem. Biophys. Res. Commun. 2011;404:261–267. doi: 10.1016/j.bbrc.2010.11.104. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann E., Dittrich-Breiholz O., Holtmann H., Kracht M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- 26.Yu H., Pardoll D., Jove R. STAT3 in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J., Chatterjee-Kishore M., Staugaitis S.M., Nguyen H., Schlessinger K. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65:939–947. [PubMed] [Google Scholar]
- 28.Groenendaal W., von Basum G., Schmidt K.A., Hilbers P.A., van Riel N.A. Quantifying the composition of human skin for glucose sensor development. J. Diabetes Sci. Technol. 2010;4:1032–1040. doi: 10.1177/193229681000400502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ino Y., Gotoh M., Sakamoto M., Tsukagoshi K., Hirohashi S. Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc. Natl. Acad. Sci. U.S.A. 2002;99:365–370. doi: 10.1073/pnas.012425299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peinado H., Ballestar E., Esteller M., Cano A. Snail mediates E-cadherin repression by the recruitment of the sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol. Cell. Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eeckhoute J., Carroll J.S., Geistlinger T.R., Torres-Arzayus M.I., Brown M. A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev. 2006;20:2513–2526. doi: 10.1101/gad.1446006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo J. Glycogen synthase kinase 3β (GSK3β) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273:194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou B.P., Deng J., Xia W., Xu J., Li Y.M., Gunduz M., Hung M. Dual regulation of snail by GSK3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 34.Hagendoorn J., Tong R., Fukumura D., Lin Q., Lobo J., Padera T.P., Xu L., Kucherlapati R., Jain R.K. Onset of abnormal blood and lumphatic vessel function and interstitial hypertension in early stages of carcinogenesis. Cancer Res. 2006;66:3360–3364. doi: 10.1158/0008-5472.CAN-05-2655. [DOI] [PubMed] [Google Scholar]
- 35.Wigg H. Pathophysiology of tissue fluid accumulation in inflammation. J. Physiol. 2011;589:2945–2953. doi: 10.1113/jphysiol.2011.206136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duckworth W.C., Bennett B.G., Hamel F.G. Insulin degradation: progress and potential. Endocr. Rev. 1998;19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 37.Gartel A.L., Serfas M.S., Tyner A.L. p21 – negative regulator of the cell cycle. Proc. Soc. Exp. Biol. Med. 1996;213:138–149. doi: 10.3181/00379727-213-44046. [DOI] [PubMed] [Google Scholar]
- 38.Nemes J.A., Nemes Z., Marton I.J. p21WAF1/CIP1 expression is a marker of poor prognosis in oral squamous cell carcinoma. J. Oral Pathol. Med. 2005;34:274–279. doi: 10.1111/j.1600-0714.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- 39.Schoelch M.L., Regezi J.A., Dekker N.P., Ng I.O., McMillan A. Cell cycle proteins and the development of oral squamous cell carcinoma. Oral Oncol. 1999;35:333–342. doi: 10.1016/s1368-8375(98)00098-0. [DOI] [PubMed] [Google Scholar]
- 40.Law M., Forrester E., Chytil A., Corsino P., Green G. Rapamycin disrupts cyclin/cyclin-dependent kinase/p21/proliferating cell nuclear antigen complexes and cyclin D1 reverses rapamycin action by stabilizing these complexes. Cancer Res. 2006;66:1070–1080. doi: 10.1158/0008-5472.CAN-05-1672. [DOI] [PubMed] [Google Scholar]
- 41.Masamha C.P., Benbrook D.M. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res. 2009;69:6565–6572. doi: 10.1158/0008-5472.CAN-09-0913. [DOI] [PubMed] [Google Scholar]
- 42.Astle M.V., Hannan K.M., Ng P.Y., Lee R.S., George A.J. AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy. Oncogene. 2012;31:1949–1962. doi: 10.1038/onc.2011.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nogueira V., Park Y., Chen C.C., Xu P.Z., Chen M.L. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.