Highlights
-
•
NaF induced DNA-lesions interact with radiation and probably repaired rapidly.
-
•
Co-exposure to NaF and radiation increased exchange aberrations.
-
•
NaF and radiation together induces more apoptosis in cancer cells.
-
•
Co-exposure to NaF and radiation downregulates inhibitor of apoptosis proteins.
Keywords: Fluoride, Chromosome aberrations, Inhibitor of apoptosis proteins, Radiation
Abstract
Fluoride is an essential trace element but also an environmental contaminant with major sources of exposure being drinking water, food and pesticides. Previous studies showed that sodium fluoride (NaF) at 5 mM or more is required to induce apoptosis and chromosome aberrations and proposed that DNA damage and apoptosis play an important role in toxicity of excessive fluoride. The aim of this study is directed to understand the nature of DNA-lesions induced by NaF by allowing its interaction with radiation induced DNA-lesions. NaF 5 mM was used after observing inability to induce DNA damages and apoptosis by single exposure with 50 μM or 1 mM NaF. Co-exposure to NaF and radiation significantly increased the frequency of aberrant metaphases and exchange aberrations in human lymphocytes and arrested the cells in G1 stage instead of apoptotic death. Flow cytometric analysis, DNA fragmentation and PARP-cleavage analysis clearly indicated that 5 mM NaF together with radiation (1 Gy) induced apoptosis in both U87 and K562 cells due to down regulation of expression of anti-apoptotic proteins, like Bcl2 in U87 and inhibitors of apoptotic proteins like survivin and cIAP in K562 cells. This study herein suggested that single exposure with extremely low concentration of NaF unable to induce DNA lesions whereas higher concentration induced DNA lesions interact with the radiation-induced DNA lesions. Both are probably repaired rapidly thus showed increased interactive effect. Coexposure to NaF and radiation induces more apoptosis in cancer cell lines which could be due to increased exchange aberrations through lesions interaction and downregulating anti-apoptotic genes.
1. Introduction
Fluoride (F) is an essential trace element for all mammalian species for prevention of caries and enamel fluorosis. On the other hand, F is also considered an environmental contaminant with major sources of exposure being drinking water, food, dental products and pesticides. Excessive consumption of F causes fluorosis, a slow, progressive degenerative disorder which not only affects the skeletal systems and teeth but also damages soft tissues like, kidney, liver and brain [1], [2].
There are conflicting reports regarding the genotoxic effects of F. One study found that NaF induced chromosome aberrations (CA) in cultured human lymphocytes [3] but others did not find such effects [4], [5]. Chaurasia et al. [6] observed dose dependent increase in CA in bone marrow cells of Swiss albino mice. Similar trend was noted in in vitro experiments in HL-60 cells where reduced cell viability, decreased DNA and protein biosynthesis, and enhanced apoptosis were observed upon exposure to high concentrations of F (100–250 mg/L) but with no such effects at lower concentrations (0–50 mg/L) [7]. Comet assay revealed increased DNA damage in all NaF-treated rat hippocampal neurons [8] and in liver, kidney, and bone marrow cells of mice treated with NaF [9].
On the whole it appears that DNA damage plays an important role in toxicity of excessive F [10]. However, the nature of DNA lesions induced by NaF is not known. Therefore, in the present study an attempt was made for the first time in human peripheral blood lymphocytes (HPBL) since this system is well established and suitable for the assessment of cytogenetic effects [11] to investigate the nature of NaF-induced DNA lesions in concert with induction of DNA damage induced by radiation. Preston [12] demonstrated that if the DNA damage produced by two agents is repaired at very different rates, the probability of producing a synergistic effect on aberration frequency is low. However, if the damage is repaired rapidly, there is a high probability of a synergistic or interactive effect. The present study considered these possibilities and selected γ-rays and NaF as two interacting mutagenic agents.
Apoptosis has been demonstrated to play an important role in toxicity of excessive F [13]. However, the underlying mechanism by which F induces apoptosis is not clear. Induction of DNA-damage by F-toxicity is closely associated with the ability of F to induce oxidative stress [14], which elicits a wide variety of cellular events, such as cell-cycle arrest, apoptosis and necrosis [15], [16]. A close association between F-toxicity and extensive oxidative stress through increased lipid-peroxidation and reduced antioxidant enzyme activities has been reported in human cells [17], [18] although some other studies did not find comparable impairment of antioxidant system by F [19], [20].
It was observed earlier that 1 mM NaF failed to induce stress-response RNAs or initiate apoptosis in mouse odontoblast cell line M06-G3 [21] but not in rat primary hippocampal neurons [22]. In general, it was observed that exposure to NaF at 5–10 mM concentration is required to induce apoptosis in rat thymocytes and human gingival fibroblasts, rat primary lung cells, and in the odontoblast cell line MDPC-23 [1], [23], [24]. Therefore, we used 50 μM, 1 and 5 mM (2, 42 and 210 mg/L) NaF in this study. 50 μM is lower in concentration than the US Environmental Protection Agency recommended upper limit of F-level in drinking water (4 mg/L) [25]. In India the upper limit is recommended to be 1.5 mg/L [2]. In the present study, we used sodium fluoride (NaF) which is used as a source of fluoride ions in diverse applications. This investigation was undertaken with the aim to understand the nature of DNA-lesions induced by NaF and investigate the underlying mechanism by which NaF induces apoptosis. To this end, we evaluated the extent of apoptosis induced by NaF alone and in combination with radiation treatment in HPBL and human cancer cell line and also analyzed expression of genes like Bcl2 and inhibitors of apoptosis proteins (IAPs).
2. Materials and methods
2.1. Chemicals
Sodium fluoride (NaF), Hoechst 33258, 5-bromodeoxyuridine (BrdU), phytohemagglutinin (PHA), Nonidet P-40, sodium dodecylsulfate and aprotinin were obtained from Sigma Chemical Company (St Louis, MO, USA). Fetal calf serum (FCS), penicillin and streptomycin and l-glutamine were used from Biological Industries, Israel. Giemsa stain was obtained from BDH chemicals, UK.
Other chemicals used in this study were of analytical grade from reputed manufacturers.
2.2. Different types of cells and their cultivation
Heparinized peripheral blood from three healthy male donors (HPBL) was used soon after its collection. Lymphocyte cultures were set up in RPMI 1640 medium supplemented with 10% heat inactivated FCS. Penicillin (100 U/ml) and streptomycin (100 mg/ml) and 2 mM l-glutamine were added to the medium. Lymphocytes were stimulated with PHA. 5-Bromodeoxyuridine (final concentration 5 μg/ml) was added in each culture during initiation. All cultures were incubated at 37 °C and were harvested at 56 and 72 h. The study was performed with full compliance of the “Ethical Guidelines for Biomedical Research on Human Subjects” formulated by the Indian Council of Medical Research, India.
K-562 (human immortalized myelogenous leukemia line) and U87 (human malignant glioma cell line) were obtained from the National Center for Cell Science (Pune, India). Cells were grown in 25 cm2 culture flasks (T25) in Dulbecco's minimal essential medium supplemented with 10% FCS. K-562 cells grow in suspension. Penicillin 100 U/ml, streptomycin 100 mg/ml and 2 mM l-glutamine were added to the medium.
2.3. Treatment with NaF/γ-rays, culture procedures and cell fixation
Heparinized peripheral blood from three healthy male donors was used immediately after venipuncture. An aliquot (1 ml) of whole blood was taken in a sterilized flat bottom 25 ml glass beaker. Fresh solution of NaF was prepared in double distilled water. NaF 50 μM, 1 and 5 mM were added into the blood and kept at 37 °C for 24 h. In case of radiation treatment, the samples were irradiated to 1, 2 and 4 Gy in a 60Co γ-chamber (dose rate 0.6 Gy/min) and were kept at 37 °C for 1 h after irradiation so as to allow normal cellular repair before setting up cultures. In case of combined treatment, radiation was given to NaF-treated cells 23 h after NaF treatment and then kept for 1 more hour. All the treated and untreated samples were washed twice with prewarmed medium after 24 h of NaF-treatment and cultures were set up. All the three concentrations of NaF were used in 56 h harvested cultures and only 5 mM NaF was used also for 72 h harvested cultures. Colcemid was added at a concentration of 0.01 μg/ml during the last 3 h in all the cultures. Hypotonic treatment was for 18 min and the cells were fixed in acetic acid:methanol (1:3) before slides preparation.
2.4. Differential staining of sister chromatids
The method of Goto et al. [26] was followed for differential staining of sister-chromatids in relation to their numbers of division cycles in presence of BrdU. Slides were treated for 10 min with Hoechst 33258 (50 mg/ml) at room temperature in dark, rinsed in distilled water, mounted in 2× SSC (NaCl–sodium-citrate, pH 6.8) and kept in sunlight for 30–40 min, depending upon the intensity of sunlight. After rinsing in distilled water, slides were stained with 2% Giemsa for 4 min.
2.5. Flow cytometric analysis of cells
Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque (Sigma Diagnostics, St. Louis, MO) density gradient centrifugation (specific gravity 1.077 g/ml) for 30 min at 400 × g from the freshly drawn heparinized whole blood from three individuals. These lymphocytes were grown in culture for 24 h with and without NaF (5 mM) and then fixed with 70% ethanol. In case of K-562 and U87cells, cells were fixed with 70% ethanol at 24 h after 5 mM NaF exposure. For radiation treatment, it was given 6 h before ethanol-fixation. In case of combined treatment, NaF 5 mM was given for 24 h and radiation was given on 18th hour i.e. 6 h before cells were fixed with 70% ethanol.
The fixed cells were washed in PBS and resuspended in 500 μl of propidium iodide solution (50 μg/ml propidium iodide, 0.2 mg/ml RNase) for 1 h at room temperature in dark. 10,000 cells were acquired for each sample and analyzed with a FACS Calibur (Becton-Dickinson). CELLQuest Pro software was used to quantify cell cycle compartments to estimate the percentage of cells distributed in the different cell cycle phases.
2.6. Annexin V labeling studies
Apoptotic cell death was evaluated using annexinV–fluorescein isothiocyanate method in the untreated, NaF (5 mM) and 1 Gy radiation alone and in combination treated HPBL, K562 and U87 cells. Ficoll-Hypaque mediated isolated human lymphocytes were grown in culture for 24 h with and without NaF (5 mM). In case of K-562 and U87 cells, cells were treated with 5 mM NaF for 24 h. For radiation treatment, cells were irradiated after 18 h of culture. In case of combined treatment, NaF 5 mM was given for 24 h and radiation was given on 18th hour i.e. 6 h before cultures were terminated.
The cell pellet was resuspended in PBS. Cells were stained with propidium iodide and Annexin-V-FITC using BD PharmingenTM Annexin V: FITC Apoptosis Detection Kit (BD-Pharmingen Biosciences, San Diego, CA) as per manufacturer's instruction. Briefly, after collecting and washing twice with PBS, cells were resuspended in the binding buffer (500 μl). FITC-Annexin-V (5 μl) was added to the cells followed by addition of 5 μl PI according to the protocol. The samples were then incubated for 15 min in the dark at room temperature and subjected to flow cytometry evaluation.
2.7. Flow cytometric analysis of mitochondrial membrane potential
During apoptosis, engagement of the mitochondrial pathway involves the permeabilization of the outer mitochondrial membrane, which leads to the release of proteins such as cytochrome c and Smac/DIABLO [27]. Mitochondrial membrane potential (ΔΨm) was measured qualitatively using the lipophilic fluorescent probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetra-ethyl-benzimidazol-carbocyanine iodide according to the manufacturer's protocol (JC-1; BD Mitoscreen JC-1 kit; Cat 51302). In brief, JC-1 working solution was prepared in 1xAssay buffer and added 0.5 ml JC-1 stain to 1 × 106 K562 or U87 cells for 10 min at 37 °C in CO2 incubator. The cells were washed twice with 1× Assay buffer at room temperature and finally 0.5 ml cell suspension was analyzed by fluorescence-activated cell sorter (FACS). JC-1 fluorescence was measured using a Becton Dickinson FACScalibur analytical flow cytometer (BD Biosciences, San Jose, CA). The percentage of cells of green (530 nm) and red (590 nm) fluorescence of JC-1 was analyzed.
2.8. Immunoblotting
Treated with NaF and radiation alone and in combination (as it was mentioned before) and untreated K-562 and U87 cells were lysed in radioimmuno-precipitation buffer (0.1% SDS, 2 mM EDTA, 1% NP-40, 1% sodium deoxycholate and 100 U/ml aprotinin). The amount of protein was determined using the bicinchoninic acid protein assay. Equal amount of protein (40 μg) from each sample was loaded in each well; equal loading was further verified by immunoblotting with actin antibodies. Samples were loaded in Novex Tris-Glycine 4–20% gradient gels and electrophoresis was performed in NuPAGE electrophoresis system (Invitrogen, USA). Proteins were transferred to a Polyvinylidene difluoride (PVDF) membrane (Sigma) following standard protocol. The membranes were probed with a 1:1000 dilution of a mouse monoclonal antibody against p53 (PAb 240; ab26; Abcam, UK), rabbit monoclonal antibody against PARP (46D11; Cell Signaling Technology, USA) and β-actin (AC-15; ab6276; Abcam, UK). Blots were washed 3 times for 10 min each in TBST buffer pH 7.6 (1 M Tris Cl, 5 M NaCl and 0.05% Tween 20) and incubated with secondary antibody (alkaline–phosphatase conjugated anti-mouse IgG or alkaline–phosphatase conjugated antirabbit IgG 1:2000; Abcam, UK) for 1 h at room temperature. After extensive washing, the blot was immersed in 4 ml substrate solution of BCIP/NBT (Bangalore Genei, India). Sufficient staining was obtained within 15 min. Each immunoblotting was performed three times per-point.
2.9. Semiquantitative RT-PCR (reverse transcription polymerase chain reaction)
Total RNA was isolated from K562 and U87 cells using RNAeasy kit (Qiagen GmbH, Hilden Germany) from untreated and treated with NaF and radiation alone or in combination. The treatment details are similar as it was mentioned above. Reverse transcription reaction was performed with 1 μg of total RNA from each sample using Quantiscript Reverse Transcriptase, Quantiscript-RT-buffer and RT-primer-mix of QuantiTect Reverse Transcription kit (Qiagen GmbH, Hilden Germany) according to the manufacturer's protocol.
Amplification of cDNA was carried out in 20 μl solution containing 2 μl cDNA, 10 pmol primer pairs for Bcl2, Survivin, XIAP, cIAP and GAPDH and 10 μl of RT qPCR Master mix (Qiagen GmbH, Hilden, Germany). The PCR consisted of initial denaturation at 94 °C for 5 min, followed by 25 reaction cycles (30 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C) and a final cycle at 72 °C for 10 min. GAPDH was used as internal control. All PCR products were electrophoretically separated on ethidium bromide-stained agarose gel and visualized with UV light.
2.10. DNA fragmentation assay
U87 cells treated with NaF (1 and 5 mM) and radiation alone or in combination (details about the treatment were mentioned above) were considered for this assay. Cells were washed once with ice-cold PBS, and resuspended in 250 μl lysis buffer (10 mM Tris–HCl, pH7.6, 20 mM EDTA, pH 8.0 and 0.5% (w/v) Triton X-100). After centrifugation at 12,000 rpm for 5 min, the supernatant was extracted once with phenol/chloroform (1:1) and once with chloroform/isoamyl alcohol (24:1). DNA was precipitated with sodium acetate (pH5.2) at −20 °C overnight. The DNA was then pelleted and subsequently digested with DNase-free RNAase (Amersham Biosciences UK Ltd., Little Chalfont, Buckinghamshire, UK) at 37 °C for 20 min. The extracted genomic DNA fragments were fractionated by 2% agarose gel and were further visualized by ethidium bromide under UV-light.
2.11. Scoring and statistical analysis
Slides were randomly coded. For scoring cell cycle kinetics, metaphases from human lymphocytes were categorized as in first, second and subsequent division cycles based on their differential staining patterns. Chromosome aberrations (CAs) were scored from first cycle metaphases (M1) only and they were: exchanges including dicentrics and rings (with or without fragments); deletions and chromatid breaks. The fragments associated with exchange aberrations were subtracted from the total deletion aberrations scored. Statistical significance of the difference between radiation and NaF + radiation groups for the frequency of aberrant metaphases and between untreated and NaF or radiation-treated group for the frequency of M1 (first-cycle metaphases) cells was evaluated using Fischer's exact test (2 × 2) and for the frequency of exchange aberrations Fischer's exact test (2 × 3) was used. Values of protein and mRNA levels are expressed as mean ± SEM and statistical analysis were performed by Student's t-test (paired) with GraphPad software Prism 5.1.
3. Results
3.1. NaF induces very low frequency of CAs
The induction of CA by NaF (50 μM, 1 and 5 mM) was low consistently. In fact 50 μM and 1 mM NaF did not show any increase in the frequency of CA with respect to untreated control. Even coexposure to 50 μM NaF and radiation did not increase the frequency of aberrations (Table 1). Since 5 mM NaF induced low frequency of CAs, more detailed studies were undertaken subsequently with 5 mM NaF. The frequency of CA was significantly enhanced when 1, 2 and 4 Gy γ-rays was given to 5 mM NaF treated samples (Table 2). The data in Table 2 were pooled from three donors. NaF alone mostly induced chromatid breaks without any deletions and exchange aberrations whereas radiation alone predominantly induced deletions and exchanges in a dose-dependent manner. Interestingly, the frequency of aberrant metaphases and exchanges was enhanced significantly in samples treated with both NaF and radiation than radiation alone (Table 2). The frequency of aberrations and aberrant metaphases induced by NaF and radiation alone or in combination was marginally more in 56 h fixed samples than 72 h.
Table 1.
Induction of CAs by NaF or γ-rays alone or in combination in HPBLs.
| Experimental condition | TM | Percentage |
TM for cell cycle | Fixation hour | Donor No. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Aberrant M | Chromatid break | Deletion | D + R | M1 | |||||
| Untreated | 115 | 2 | 2 | 0 | 0 | 87 | 150 | 56 | 1 |
| F-50 μM | 110 | 2 | 2 | 0 | 0 | 90 | 150 | ||
| F-1 mM | 107 | 2 | 2 | 1 | 0 | 98 | 150 | ||
| F-5 mM | 127 | 9 | 8 | 1 | 0 | 96 | 150 | ||
| 1 Gy | 112 | 28 | 4 | 8 | 17 | 95 | 150 | ||
| F-50 μM + 1 Gy | 156 | 25 | 6 | 10 | 19 | 98 | 150 | ||
| 2 Gy | 116 | 42 | 6 | 18 | 28 | 98 | 150 | ||
| F-50 μM + 2 Gy | 112 | 45 | 4 | 24 | 32 | 98 | 150 | ||
| Untreated | 119 | 3 | 4 | 0 | 0 | 80 | 150 | 56 | 2 |
| F-50 μM | 161 | 2 | 2 | 0 | 0 | 84 | 150 | ||
| F-1 mM | 167 | 2 | 2 | 1 | 0 | 88 | 150 | ||
| F-5 mM | 130 | 8 | 7 | 2 | 0 | 93 | 150 | ||
| 1 Gy | 116 | 35 | 4 | 15 | 15 | 90 | 138 | ||
| F-50 μM + 1 Gy | 128 | 31 | 6 | 16 | 14 | 94 | 148 | ||
| 2 Gy | 111 | 53 | 2 | 25 | 23 | 92 | 142 | ||
| F-50 μM + 2 Gy | 128 | 50 | 2 | 28 | 20 | 98 | 180 | ||
TM, total metaphase; M1, first cycle metaphases; D, dicentric; R, ring.
Table 2.
Pooled data on Induction of CAs by NaF or γ-rays alone or in combination in HPBLs from 3 donors.
| Experimental condition | TM | Percentage ± SEM |
TM for cell cycle | Fixation hour | ||||
|---|---|---|---|---|---|---|---|---|
| Aberrant M | Chromatid break | Deletion | D + R | M1 cells | ||||
| Untreated | 348 | 3 ± 0.5 | 3 | 0 | 0 | 82 ± 2.5 | 444 | 56 |
| F-5 mM | 391 | 9 ± 0.5 | 7 | 2 ± 0.5 | 0 | 94 ± 1.0$ | 448 | |
| 1 Gy | 330 | 33 ± 3.5 | 4 | 12 ± 2.2 | 17 ± 1.4 | 89 ± 3.8$ | 428 | |
| F-5 mM +1 Gy | 327 | 47 ± 3.0* | 7 | 14 ± 1.7 | 31 ± 2.7# | 96 ± 1.0 | 421 | |
| 2 Gy | 337 | 47 ± 3.3 | 4 | 24 ± 3.5 | 26 ± 1.5 | 93 ± 2.9$ | 437 | |
| F-5 mM +2 Gy | 341 | 58 ± 3.7* | 7 | 32 ± 1.8 | 39 ± 1.4# | 97 ± 1.2 | 421 | |
| 4 Gy | 324 | 80 ± 1.2 | 2 | 42 ± 2.2 | 70 ± 6.3 | 97 ± 2.1$ | 410 | |
| F-5 mM +4 Gy | 323 | 90 ± 1.5* | 6 | 45 ± 4.4 | 87 ± 4.4# | 100 ± 0 | 386 | |
| Untreated | 326 | 2 ± 0.5 | 3 | 0 | 0 | 58 ± 4.6 | 458 | 72 |
| F-5 mM | 327 | 7 ± 1.4 | 6 | 1 ± 0.5 | 0 | 86 ± 2.6$ | 432 | |
| 1 Gy | 367 | 28 ± 4.9 | 2 | 13 ± 1.3 | 12 ± 2.6 | 74 ± 5.2$ | 420 | |
| F-5 mM +1 Gy | 330 | 40 ± 5.1* | 6 | 22 ± 3.6 | 21 ± 4.6# | 93 ± 3.1 | 391 | |
| 2 Gy | 330 | 41 ± 3.4 | 2 | 30 ± 4.9 | 23 ± 1.0 | 82 ± 3.2$ | 420 | |
| F-5 mM +2 Gy | 336 | 52 ± 1.8* | 7 | 41 ± 7.3 | 36 ± 1.0# | 96 ± 2.0 | 377 | |
| 4 Gy | 308 | 78 ± 0.9 | 0 | 49 ± 3.2 | 64 ± 6.1 | 89 ± 4.0$ | 392 | |
| F-5 mM +4 Gy | 321 | 89 ± 0.3* | 5 | 49 ± 2.5 | 76 ± 4.6# | 100 ± 0 | 361 | |
TM, total metaphase; M1, first cycle metaphases; D, dicentric; R, ring.
p < 0.001; significant using Fischer's exact test between radiation and F + radiation.
p < 0.001; significant using Fischer's exact test between untreated control and treatment either with F or radiation.
p < 0.001; significant using Freeman-Halton extension of the Fisher exact probability test for a two-rows by three-columns contingency table between radiation and F + radiation data.
3.2. NaF induces delay in cell cycle kinetics
Cell cycle progression and CA were scored simultaneously and the data are presented in Table 1, Table 2. For scoring cell cycle kinetics in human PBLs, metaphases were identified to have undergone different numbers of cell cycle on basis of their differential staining of sister chromatids since BrdU labeling permits unequivocal identification of the number of divisions in the presence of this base analog. Delay in cell cycle progression was measured in terms of increase in the frequency of first-cycle metaphases (M1) following treatment in comparison to that of untreated controls.
Basic cell cycle progression varied among all the three donors and therefore, the percentage of delay induced by either NaF or radiation in each experiment was measured from the untreated control specimens carried out in parallel. The data presented in Table 1, Table 2 clearly showed higher frequency of first cycle metaphases in NaF or radiation treated samples than untreated sample. The extent of delay induced by 5 mM of NaF was higher than 50 μM, 1 mM NaF. Significant delay in cell cycle was observed after treatment with either NaF or radiation and it was also noted that the extent of delay was more at 72 h than in the 56 h samples (Table 2). The samples treated with NaF and radiation in combination showed higher frequency of M1 cells than the samples treated with either NaF or radiation alone.
3.3. Flow cytometric analysis of human lymphocytes, K562 and U87 cells
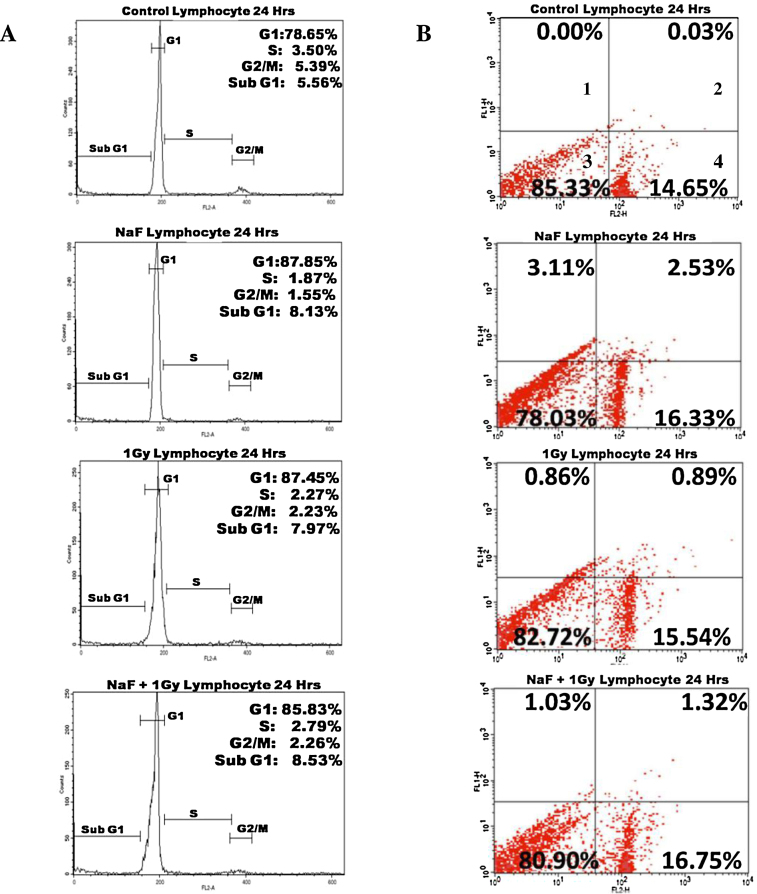
Flow cytometric analysis (Fig. 1A) after 5 mM of NaF-treatment for 24 h and 1 Gy radiation alone or in combination did not show any increase in sub-G1 cells in human lymphocytes. However, increase in cells in G1 stage was observed after the treatment indicating G1-arrest.
Fig. 1.
Effect of NaF with or without radiation on human lymphocytes. (A) Analysis of cell cycle after treated with NaF alone or in combination with 1 Gy γ-rays. This analysis were repeated twice. (B) Representative cytograms of Annexin V versus PI fluorescence intensities as determined by flow cytometric analysis in human lymphocytes after treated with NaF alone or in combination with 1 Gy γ-rays. Within a cytogram, quadrant 1 and 2 represent early and late apoptotic cells, respectively; quadrant 3, viable cells; quadrant 4, dead cells. This analysis was repeated twice.
To validate the apoptotic induction by 5 mM NaF alone and in combination with radiation, dual staining with Annexin V and PI was performed and Fig. 1B indicate no increase in positive staining with Annexin-V in quadrant 2 and 3 in human lymphocytes.
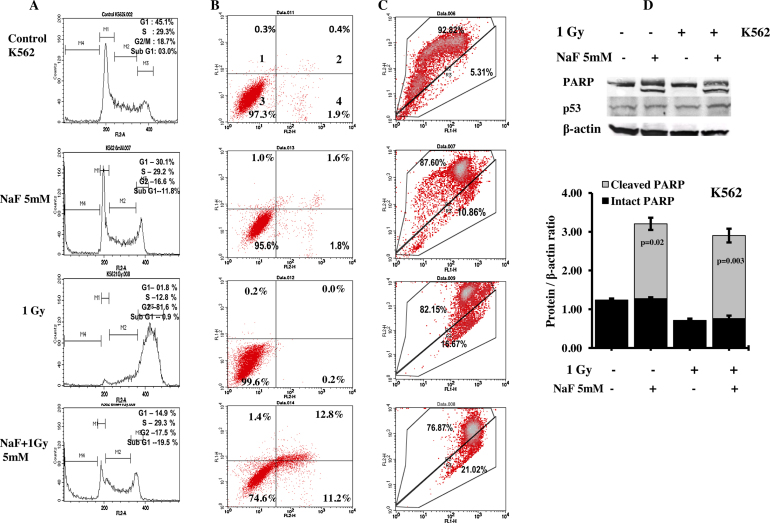
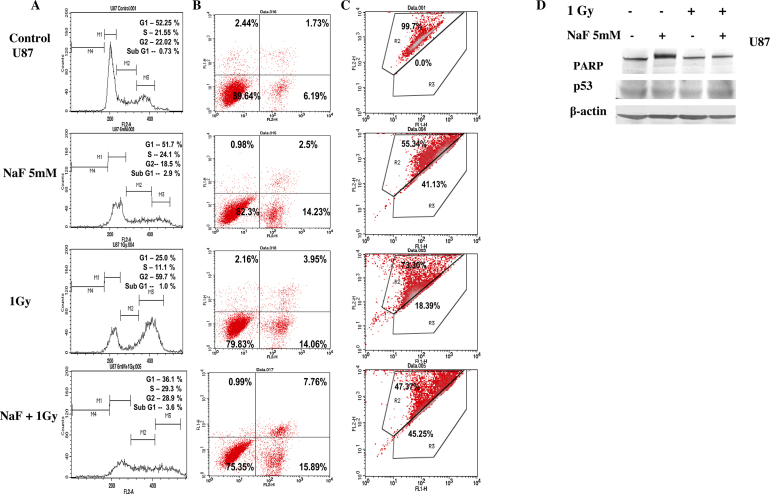
Interestingly, flow cytometric analysis of K562 cells after treatment with NaF alone revealed reduction in G1 cells with a concomitant rise in sub-G1 cells (Fig. 2A). Similar increase in sub-G1 was not observed after the treatment with radiation alone rather a significant arrest at G2-stage was observed. While 5 mM NaF treatment was combined with radiation, cells in sub-G1 were increased with respect to NaF-treatment alone. Dual staining (Fig. 2B) indicates that sub-G1 cells were mostly apoptotic (quadrant 2, 3 and 4). Flow cytometric analysis of U87 cells (Fig. 3A) after 5 mM of NaF-treatment alone or in combination of radiation (1 Gy) did not show any increase in sub-G1 cells. Dual staining with Annexin V and PI data (Fig. 3B) indicate marginal increase in apoptosis and dead cells after combined treatment with NaF and radiation. In both the cell lines, G2-arrest was clear after radiation exposure (Fig. 2, Fig. 3).
Fig. 2.
Effect of NaF with or without radiation on K562 cell lines. (A) Analysis of cell cycle after treated with NaF alone or in combination with 1 Gy γ-rays. (B) Analysis of apoptosis induced by NaF alone or in combination with radiation is shown. Representative cytograms of Annexin V versus PI fluorescence intensities as determined by flow cytometric analysis in K562 cells. Within a cytogram, quadrant 1 and 2 represent early and late apoptotic cells, respectively; quadrant 3, viable cells; quadrant 4, dead cells. (C) Analysis of apoptosis by measuring the mitochondrial membrane potential after JC1 staining in untreated and treated with NaF or radiation alone or in combination in K562 cells. The upper part indicates the percentage of cells shows polarization of mitochondrial membrane and the lower part shows the percentage of cells having mitochondrial membrane depolarization. All these experiments were repeated twice. (D) Upper panel: representative western blotting detection of p53, PARP and β-actin after exposure to 5 mM NaF and 1 Gy radiation alone or in combination. Lower panel-Quantitative densitometric analysis of the level of PARP with and without cleavage in the treated and untreated K562 cells. The values are the mean ± SEM of three independent experiments. The values are normalized to respective β-actin values.
Fig. 3.
Effect of NaF with or without radiation on U87 cell lines. (A) Analysis of cell cycle after treated with NaF alone or in combination with 1 Gy γ-rays. (B) Analysis of apoptosis induced by NaF alone or in combination with radiation is shown. Representative cytograms of Annexin V versus PI fluorescence intensities as determined by flow cytometric analysis in U87 cells. Within a cytogram, quadrant 1 and 2 represent early and late apoptotic cells, respectively; quadrant 3, viable cells; quadrant 4, dead cells. (C) Analysis of apoptosis by measuring the mitochondrial membrane potential after JC1 staining in untreated and treated with NaF or radiation alone or in combination in U87 cells. The upper part indicates the percentage of cells shows polarization of mitochondrial membrane and the lower part shows the percentage of cells having mitochondrial membrane depolarization. All these experiments were repeated twice. (D) Immunoblotting analysis of p53, PARP and β-actin after exposure to 5 mM NaF and 1 Gy radiation alone or in combination.
Analysis of the mitochondrial membrane potential by JC1 labeling revealed that percentage of polarized cells was significantly reduced in U87 as well as K562 cells treated with NaF and radiation (Fig. 2, Fig. 3). While the untreated cells mostly exhibited red fluorescence, indicating an intact mitochondrial membrane potential. Upon treatment with either NaF or radiation alone enhanced the number of cells showing green fluorescence; a combination of NaF and radiation treatment resulted in maximum increase in cells showing green fluorescence.
3.4. Immunoblotting
For further validation of cell death by apoptosis, we measured the cleavage of PARP in cells collected after 24 h NaF treatment, with or without radiation (Fig. 2, Fig. 3). PARP cleavage was detected in K562 cells treated with either NaF alone or in combination with radiation. Quantitative densitometric analysis of the level of PARP cleavage indicated higher cleavage of PARP in samples treated with NaF and radiation than in only NaF-treated samples. PARP cleavage was not seen in U87 cells. No appreciable change in the level of p53 protein was noticed in U87 cells whereas in K562 cells slight increase in the level of p53 protein was observed in the treated samples.
3.5. DNA fragmentation analysis
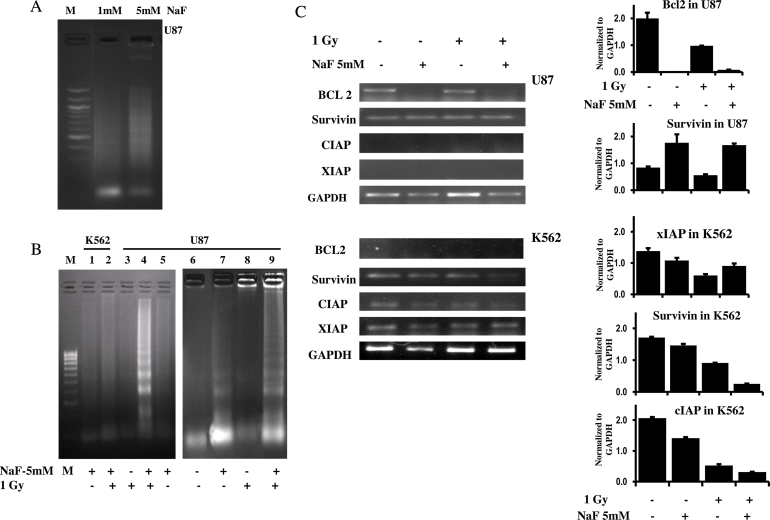
To validate the induction of apoptosis by 1 and 5 mM NaF treatment for 24 h, DNA fragmentation analysis was performed with the U87 cells (Fig. 4A). A weak DNA laddering pattern was observed with 5 mM NaF-treatment. Therefore, 5 mM NaF was used for DNA fragmentation analysis in U87 and K562 cells. A typical laddering pattern, characteristic of later stage of apoptosis, is shown in Fig. 4B. It was observed that treatment with 5 mM NaF with or without radiation showed DNA laddering in U87 cells, however, the DNA laddering was much more prominent in samples treated with NaF and radiation in combination. K562 cells did not display such laddering pattern.
Fig. 4.
DNA-laddering assay and the expression pattern of anti-apoptotic proteins after treated with NaF and radiation alone and in combination. (A) DNA-laddering assay in U87 cells after exposure to NaF (1 and 5 mM). (B) DNA-laddering assay shows the typical pattern of oligonucleosomal-sized fragments of about 200 base pair length in lane 4 and 9 after coexposure to NaF and radiation and in lane 7 after treatment with NaF alone to U87 cells. Lane 1 and 2 are from K562 cells treated with NaF with and without radiation. M-indicates marker. These experiments were repeated twice. (C) Expression pattern of Bcl2, survivin, XIAP and cIAP using semiquantitative RT-PCR in K562 and U87 cells treated with NaF and radiation alone or in combination. Right-side panel – quantitative densitometric analysis of the expression profile of genes mRNA level. The values are the mean ± SEM of two independent experiments and are normalized to respective GAPDH values.
3.6. NaF downregulates apoptotic inhibitor proteins
The expression profile of four apoptosis inhibitor genes was investigated by semi-quantitative RT-PCR after treatment with NaF with and without radiation in U87 and K562 cells (Fig. 4A). Expression of Bcl2 was downregulated in U87 cells by both NaF alone or NaF in combination with radiation. In K562 cells, the expression of survivin, cIAP and XIAP was downregulated with maximum downregulation observed in NaF + 1 Gy treated sample. Expression of cIAP and XIAP genes in U87 cells and Bcl2 gene in K562 cells was not seen in untreated as well as in treated samples.
4. Discussion
In the present study, we noted that single exposure with 5 mM but not 50 μM and 1 mM NaF induced CAs in HPBL although the frequency was low. Besides normal human lymphocytes, we have chosen K562 cells which were the first human immortalized myelogenous leukemia line. U87 cells were also used, which are extremely aggressive and most common primary malignancy in human central nervous system where NaF showed partial protection from Metformin-triggered apoptosis [28]. Since 50 μM and 1 mM NaF failed to induce CAs and apoptosis, all subsequent studies was pursued with 5 mM NaF. In addition to CAs, 5 mM NaF also induced apoptosis in cancer cell lines (U87 and K562) but not in HPBL. Combined treatment of NaF (5 mM) with radiation increased the frequency of DNA damages and induced more apoptosis in cancer cell lines by downregulating the inhibitors of apoptosis proteins (IAPs). In fact, the present study has two aspects: (a) to understand the nature of DNA-lesions induced by NaF and (b) to know the mechanism by which it induces apoptosis. In order to address the first aspect we used radiation and when we found that combined exposure increased the exchange aberrations we tempted to see the influence of coexposure on apoptosis and compared with the NaF treatment alone.
Most of the CA studies carried out with NaF showed mainly chromatid gaps and chromatid breaks [5], [19]. However, reports are not available regarding the nature of DNA lesions induced by NaF and therefore, allowing an interaction of DNA lesions induced by NaF with the lesions induced by radiation. Our results indicate that DNA lesions induced by both NaF and radiation are interacted and elevated the frequency of exchange aberrations. Exchange aberrations, like dicentrics and rings, are thought to arise as a consequence of illegitimate reunion (misrejoining) of free ends from different DNA dsbs [20]. Such misrejoining may be expected to depend on the number and proximity of the breaks. Therefore, it seems that many NaF-induced DNA single strand breaks (ssbs) are converted to DNA double strand breaks (dsbs) due to induction of another ssbs by radiation at the opposite site on the other strand of DNA. Such converted DNA dsbs probably contributed further in the misrejoining process and enhanced the frequency of exchange aberrations in the samples treated with both the agents. It was proposed earlier [12] that if the DNA-lesions induced by two agents are repaired rapidly, then there is a high probability of a synergistic or interactive effect. The present result indicates that the DNA-lesions induced by both these agents can interact and therefore their repair-kinetics is probably of similar in nature.
Both the cytogenetic and flow-cytometric data here showed that NaF and radiation delays lymphocytes in their passage through the cell cycle. Both 50 μM and 1 mM NaF induced delay in cell cycle without inducing DNA-lesions. The extent of delay induced by 5 mM of NaF was almost equal to 2 Gy of radiation. The delay was more in 72 h fixed samples than in 56 h fixed ones in this study and similar trend was observed earlier where higher level of delay was induced in 72 h than 56 h fixed cells [29], [30]. Bender and Brewen [31] proposed the existence of two subpopulations of PHA-stimulated lymphocytes that differ in the rate of progression from PHA stimulation through cell division. It may be possible that late-arising first divisions are delayed in their responsiveness to PHA or progression through the S and G2 stages to mitosis, and if these cells are aberrant, then their responsiveness to PHA would be delayed further. It has been suggested that first-division lymphocytes fixed at different time periods show similar radiosensitivity (determined by chromosome aberrations induction) [32], whereas differences were reported in other studies [33]. By classical cytogenetic analysis, the present study has shown almost similar frequencies of aberrations (from the pooled data) in both 56 and 72 h fixed samples.
Literature survey revealed that F genotoxicity induced CAs in cultured cells [21] and reduced cell viability in HL-60 cells [7]. In the present study, 5 mM NaF induced very low frequency of CA without reducing any significant cell viability. In general, it was observed that exposure to NaF at 5–10 mM range are required to induce apoptosis in rat thymocytes and human gingival fibroblasts, rat primary lung cells, and in the odontoblast cell line MDPC-23 [1], [24], [25]. Flow cytometric analysis with annexin/PI staining and JC1 labeling revealed that 5 mM NaF alone failed to induce any apoptosis in human lymphocytes but induced weakly in both the cell lines. However, radiation alone induced higher frequency of CA and subsequently arrested cell cycle at G1 stage for lymphocytes and G2 stage for cell lines instead of inducing apoptosis. The rationale behind this combined treatment is that it induces higher DNA damages including exchange aberrations and such aberrations carrying cells may die apoptotically [34]. Interestingly, it was observed that co-exposure to NaF and radiation (1 Gy) induced more apoptosis in both U87 and K562 cells than NaF alone. Such co-exposure to NaF and radiation likely to induce higher DNA damages as it was shown in human lymphocytes but the induction of apoptosis was observed only in cancer cell lines and not in normal lymphocytes. Rather in lymphocytes G1 arrest was observed. The induction of apoptosis in both resting as well as proliferating lymphocytes is evident. Excessive exposure to oxidative stress facilitates the programmed death of lymphocytes [35]. Both resting and activated primary human lymphocytes showed decreased survival and increased apoptosis after exposure to 10–100 μM Cr6+ [36]. Therefore, apoptotic death of cancer cell lines but not human lymphocytes by combined treatment with NaF and radiation in this study is interesting.
Consistent to this observation, mild DNA ladder was observed in U87 cells after treatment with 5 mM NaF alone and such DNA fragmentation was increased significantly in the samples treated with NaF and radiation together. On the other hand, DNA ladder was not found in K562 cells, however, western blot analysis confirmed higher poly-ADP-ribose polymerase (PARP) cleavage after NaF and radiation combined treatment. Both DNA fragmentation and proteolytic cleavage of PARP are caspase-dependent events and are hallmark of apoptosis. In U87 cells, PARP-level was increased after the treatment with NaF with or without radiation but no proteolytic cleavage of PARP was observed. PARP cleavage is an universal phenomenon observed during programmed cell death induced by a variety of apoptotic stimuli. However, it was also demonstrated that PARP−/− cells exhibit a normal apoptotic response to various stimuli, including TNF-α and anti-Fas treatment, suggesting that PARP itself is dispensable in various apoptotic pathways [37], [38].
Present semi-quantitative RT-PCR revealed that mRNA expression of Bcl2 in U87 and survivin and cIAP in K562 cells was downregulated compared to untreated control. Such down-regulation was more prominent in the samples treated with NaF and radiation in combination. Since NaF and radiation failed to induce any apoptosis in lymphocytes, further study with RT-PCR was not pursued. Inhibitor of apoptosis proteins (IAPs) are a family of proteins that can block apoptosis in normal cells and have been suggested to cause resistance to apoptosis in cancer [39]. Among the family of IAPs, survivin has received special attention because it is highly expressed in cancer tissues and cancer cell lines [40]. In the present study, we did not see any expression of XIAP and CIAP in U87 cells and Bcl2 in K562 cells. XIAP is the most potent caspase inhibitor in the IAP family: it binds to and inhibits active caspases 3, 7 and 9, and ubiquitinates them [41], [42]. Another IAP, cIAP also binds caspases but do not directly inhibit them, instead inducing their proteasomal degradation [43]. Survivin, as a IAP, plays a key role in the regulation of apoptosis and cell division [44]. An increased apoptosis was observed where the level of Bcl2 showed an irregular pattern in spite of increased expression of p53 and caspase-3 proteins in the group of rats that received a 50-ppm dose of NaF for 8 weeks [45]. Earlier studies indicate that fluoride induces apoptosis through mechanisms of oxidative stress, caspase and PKC activation, MAPK signal pathway and DNA damage [46], [47]. Present study demonstrated that NaF induces apoptosis by downregulating Bcl2 or IAPs in cancer cell lines and such action was more prominent while treatment with NaF combined with radiation.
5. Conclusion
Present study showed that single exposure with extremely low concentration of NaF unable to induce DNA lesions. DNA lesions induced by higher concentration of NaF interact with the DNA lesions induced by radiation and both are probably repaired rapidly and thereby showed increased interactive effect. Coexposure to NaF and radiation induces more apoptosis in cancer cell lines which could be attributed due to increased exchange aberrations through lesions interaction and downregulating either Bcl2 or some IAPs.
Transparency document
Acknowledgements
This work was supported by grants from University Grants Commission, India (No. 13-494/2011) and Department of Science and Technology, Govt. of India (No. SR/SO/HS-0028/2010); UGC-Dr. DS Kothari Postdoctoral Fellowship to SP.
References
- 1.Thrane E.V., Refsnes M., Thoresen G.H., Lag M., Schwarze P.E. Fluoride-induced apoptosis in epithelial lung cells involves activation of MAP kinases p38 and possibly JNK. Toxicol. Sci. 2001;61:83–91. doi: 10.1093/toxsci/61.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Susheela A.K., Bhatnagar M. Reversal of fluoride induced cell injury through elimination of fluoride and consumption of diet rich in essential nutrients and antioxidants. Mol. Cell. Biochem. 2002;234:335–340. [PubMed] [Google Scholar]
- 3.Jachimczak D., Skotarczak B. The effect of fluorine and lead ions on the chromosomes of human leukocytes in vitro. Genet. Pol. 1978;19:353–357. [Google Scholar]
- 4.Kram D., Schneider E.L., Singer L., Martin G.R. The effects of high and low fluoride diets on the frequencies of sister chromatid exchanges. Mutat. Res. 1978;57:51–55. doi: 10.1016/0027-5107(78)90233-6. [DOI] [PubMed] [Google Scholar]
- 5.Thompson E.J., Kilanowski F.M., Perry P.E. The effect of fluoride on chromosome aberrations and sister chromatid exchange frequencies in cultured human lymphocytes. Mutat. Res. 1985;144:89–92. doi: 10.1016/0165-7992(85)90008-9. [DOI] [PubMed] [Google Scholar]
- 6.Chaurasia O.P., Kumari C., Sangita A. Genotoxic effects of ground water salts rich in fluoride. Cytologia (Tokyo) 2007;72:141–144. [Google Scholar]
- 7.Song J.S., Lee H.Y., Lee E., Hwang H.J., Kim J.H. Cytotoxicity and apoptosis induction of sodium fluoride in human promyelocytic leukemia (HL-60) cells, Environ. Toxicol. Pharmacol. 2002;11:85–91. doi: 10.1016/s1382-6689(01)00108-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M., Wang A., Xia T., He P. Effects of fluoride on DNA damage, S-phase cell-cycle arrest and the expression of NF-kB in primary cultured rat hippocampal neurons. Toxicol. Lett. 2008;179:1–5. doi: 10.1016/j.toxlet.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Manivannan J., Sinha S., Ghosh M., Mukherjee A. Evaluation of multi-endpoint assay to detect genotoxicity and oxidative stress in mice exposed to sodium fluoride. Mutat. Res. 2013;751:59–65. doi: 10.1016/j.mrgentox.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 10.He L.F., Chen J. GDNA damage, apoptosis and cell cycle changes induced by fluoride in rat oral mucosal cells and hepatocytes, World J. Gastroenterol. 2006;12:1144–1148. doi: 10.3748/wjg.v12.i7.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adler I.D. Cytogenetic tests in mammals. In: Venitt S., Perry J.M., editors. Mutagenecity Testing. IRL Press; London: 1984. pp. 275–305. [Google Scholar]
- 12.Preston R.J. DNA repair and chromosome alteration: interactive effects radiation and chemicals. Prog. Mutat. Res. 1982;4:25–35. [Google Scholar]
- 13.Chen J., Chen X., Yang K., Xia T., Xie H. Studies on DNA damage and apoptosis in rat brain induced by fluoride. Chin. J. Prevent. Med. 2002;36:222–224. [PubMed] [Google Scholar]
- 14.Valko M., Izakovic M., Mazur M., Rhodes C.J., Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 15.Roos W.P., Kaina B. DNA damage-induecd cell death by apoptosis. Trends Mol. Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Barzilai A. The contribution of the DNA damage response to neuronal viability. Antioxid. Redox Signal. 2007;9:211–218. doi: 10.1089/ars.2007.9.211. [DOI] [PubMed] [Google Scholar]
- 17.Podder S., Chattopadhyay A., Bhattacharya S., Ray M.R. Differential in vivo genotoxic effects of lower and higher concentrations of fluoride in mouse bone marrow cells. Fluoride. 2008;41:301–307. [Google Scholar]
- 18.Chouhan S., Flora S.J.S. Effects of fluoride on the tissue oxidative stress and apoptosis in rats: biochemical assays supported by IR spectroscopy data. Toxicology. 2008;254:61–70. doi: 10.1016/j.tox.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Chulubek. D. Fluoride and oxidative stress. Fluoride. 2003;36:217–228. [Google Scholar]
- 20.Hasan H.A., Abdel-Aziz. A.F. Evaluation of free-radical scavenging and anti-oxidant properties of black berry against fluoride toxicity in rats. Food Chem. Toxicol. 2010;48:1999–2004. doi: 10.1016/j.fct.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Wurtz T., Houari S., Mauro N., MacDougall M., Peters H., Berdal A. Fluoride at non-toxic dose affects odontoblast gene expression in vitro. Toxicology. 2008;249:26–34. doi: 10.1016/j.tox.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M., Wang A., He W., He P., Xu B., Xia T. Effects of fluoride on the expression of NCAM, oxidative stress, and apoptosis in primary cultured hippocampal neurons. Toxicology. 2007;236:208–216. doi: 10.1016/j.tox.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Matsui H., Morimoto M., Horimoto K., Nishimura Y. Some characteristics of fluoride-induced cell death in rat thymocytes: cytotoxicity of sodium fluoride. Toxicol. In Vitro. 2007;21:1113–1120. doi: 10.1016/j.tiv.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Karube H., Nishitai G., Inageda K., Kurosu H., Matsuoka M. NaF activates MAPKs and induces apoptosis in odontoblast-like cells. J. Dent. Res. 2009;88:461–465. doi: 10.1177/0022034509334771. [DOI] [PubMed] [Google Scholar]
- 25.EPA, Basic information about Fluoride in drinking water, US, Environmental Protection Agency, May 2012. Website: http://water.epa.gov/drink/contaminants/basic information/fluoride.cfm.
- 26.Goto K., Avenatsu T., Silmagu H., Suigiama T. Simple differential Giemsa staining of sister chromatids after treatment with photosensitive dyes and exposure to light and the mechanism of staining. Chromosoma. 1975;53:223–230. doi: 10.1007/BF00329173. [DOI] [PubMed] [Google Scholar]
- 27.Green D.R., Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 28.Isakovic A., Harhaji L., Stevanovic D., Markovic Z., Sumarac-Dumanovic M., Starcevic V., Micic D., Trajkovic V. Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol. Life Sci. 2007;64:1290–1302. doi: 10.1007/s00018-007-7080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann G.R., Sayer A.M., Littlefield L.G. Higher frequency of chromosome aberrations in late-arising first-division metaphases than in early-arising metaphases after exposure of human lymphocytes to X-rays in G 0. Int. J. Radiat. Biol. 2002;78:765–772. doi: 10.1080/09553000210152962. [DOI] [PubMed] [Google Scholar]
- 30.Purrot R.J., Vulpis N., Lloyd D.C. The use of harlequin staining to measure delay in human lymphocyte cell cycle induced by in vitro irradiation. Mutat. Res. 1980;69:275–284. doi: 10.1016/0027-5107(80)90092-5. [DOI] [PubMed] [Google Scholar]
- 31.Bender M.A., Brewen J.G. Factors influencing chromosomes aberration yields in the human peripheral leukocyte system. Mutat. Res. 1969;8:383–390. doi: 10.1016/0027-5107(69)90016-5. [DOI] [PubMed] [Google Scholar]
- 32.Scott D., Lyons C.Y. Homogenous sensitivity of human peripheral blood lymphocytes to radiation induced chromosome damage. Nature. 1979;278:756–758. doi: 10.1038/278756a0. [DOI] [PubMed] [Google Scholar]
- 33.Beek B., Obe G. The human leukocyte test system: higher sensitivity to X-irradiation in the Go stage of the cell cycle of early as compared to late replicating cells. Hum. Genet. 1976;35:57–70. doi: 10.1007/BF00295619. [DOI] [PubMed] [Google Scholar]
- 34.Bassi L., Carloni Meschini M.R., Fonti E., Palitti F. X-irradiated human lymphocytes with unstable aberrations and their preferential elimination by p53/surviving-dependent apoptosis. Int. J. Radiat. Biol. 2003;79:1–12. doi: 10.1080/09553000310001632930. [DOI] [PubMed] [Google Scholar]
- 35.Larbi A., Kempf J., Pawelec G. Oxidative stress modulation and T cell activation. Exp. Gerontol. 2007;42:852–858. doi: 10.1016/j.exger.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Moeed Akbar, Brewer James M., Helen Grant M. Effect of chromium and cobalt ions on primary human lymphocytes in vitro. J. Immunotoxicol. 2011;8:140–149. doi: 10.3109/1547691X.2011.553845. [DOI] [PubMed] [Google Scholar]
- 37.Leist M., Single B., Kunstle G., Volbracht C., Hentze H., Nicotera P. Apoptosis in the absence of poly-(ADP-ribose) polymerase. Biochem. Biophys. Res. Commun. 1997;233:518–522. doi: 10.1006/bbrc.1997.6491. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z.-Q., Stingl L., Morrison C., Jantsch M., Los M., Schulze-Osthoff K., Wagner E.F. PARP is important for genomic stability but dispensable in apoptosis. Genes Dev. 1997;11:2347–2358. doi: 10.1101/gad.11.18.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed J.C., Doctor K.S., Godzik A. The domains of apoptosis: a genomics perspective. Sci. STKE. 2004;239 doi: 10.1126/stke.2392004re9. re9. [DOI] [PubMed] [Google Scholar]
- 40.Ambrosini G., Adida C., Altieri D.C. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 41.Scott F.L., Denault J.B., Riedl S.J., Shin H., Renatus M., Salvesen G.S. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs. EMBO J. 2005;24:645–655. doi: 10.1038/sj.emboj.7600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki Y., Nakabayashi Y., Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eckelman B.P., Salvesen G.S. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J. Biol. Chem. 2006;281:3254–3260. doi: 10.1074/jbc.M510863200. [DOI] [PubMed] [Google Scholar]
- 44.Deveraux Q.L., Reed J.C. IAP family proteins-suppressor of apoptosis. Genes Dev. 1999;13:1253–1262. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 45.Gutiérrez-Salinas J., Morales-González J.A., Madrigal-Santillán E., Esquivel-Soto J., Esquivel-Chirino C., García-Luna M., González-Rubio Y., Suástegui-Domínguez S., Valadez-Vega C. Exposure to sodium fluoride produces signs of apoptosis in rat leukocytes. Int. J. Mol. Sci. 2010;11:3610–3622. doi: 10.3390/ijms11093610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Refsnes M., Schwarze P.E., Holme J.A., Låg M. Fluoride-induced apoptosis in human epithelial lung cells (A549 cells): role of different G protein-linked signal systems. Hum. Exp. Toxicol. 2003;22:111–123. doi: 10.1191/0960327103ht322oa. [DOI] [PubMed] [Google Scholar]
- 47.Thrane E.V., Refsnes M., Thoresen G.H., Låg M., Schwarze P.E. Fluoride-induced apoptosis in epithelial lung cells involves activation of MAP kinases p38 and possibly JNK. Toxicol. Sci. 2001;61:83–91. doi: 10.1093/toxsci/61.1.83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.